Abstract
Cardiac vagal receptors are chemically and/or mechanically sensitive but it is unknown if this information is preserved centrally within the nucleus of the solitary tract (NTS). The present study had two aims: first, to investigate qualitatively whether both mechanically and chemically sensitive cardiac vagal encoding were preserved within the NTS, and second, to determine the patterns of convergence from other cardiorespiratory afferents to NTS neurones receiving cardiac vagal inputs.
The extracellular activity of single NTS neurones was investigated during stimulation of both chemically and mechanically sensitive cardiac vagal receptors in a working heart-brainstem preparation of mouse. Chemically sensitive cardiac receptors were stimulated using intra-left ventricular injections of either veratridine (1–3 μg kg−1), bradykinin (0.25–1 μg) or prostaglandin E2 (100–200 ng), whereas the left ventricle was distended to activate cardiac mechanoreceptors.
Forty-three NTS neurones were activated both synaptically by electrical stimulation of the ipsilateral vagus nerve (latency, 35 ± 3 ms), and by intra-left ventricular injection of veratridine and also, in some cases, by bradykinin and/or PGE2. These NTS neurones were delineated into two populations based on their response to left ventricular distension and convergence properties. Left ventricular distension-insensitive neurones (n= 30) were excited by stimulation of carotid body chemoreceptors (81 %) but not arterial baroreceptors (3 %; i.e. n= 1 neurone), whereas distension-sensitive cells (n= 13) were activated mainly by baroreceptors (86 %) rather than peripheral chemoreceptors (14 %; i.e. n= 1 neurone).
The data reveal two distinct populations of NTS neurones receiving cardiac vagal inputs: (a) cells responsive to veratridine stimulation only, and (b) neurones activated by both veratridine and mechanical stimuli. The specific convergence pattern of baroreceptors and chemoreceptors to these cardioreceptive NTS neurones is discussed in relation to a common afferent modality integration within the NTS.
In cats and dogs, stimulation of cardiac vagal receptors with unmyelinated axons evokes a pronounced reflex bradycardia, hypotension (Daly, 1991; Al-Timman, Drinkhill & Hainsworth, 1993), dilatation of capacitance vessels (McGregor, Hainsworth & Ford, 1986; Tutt, McGregor & Hainsworth, 1988), and respiratory depression (Crisp, Tutt, McGregor & Hainsworth, 1989). Recordings from single fibres demonstrated that cardiac vagal receptor endings were excited by veratridine, capsaicin, nicotine, phenylbiguanide (a 5-HT3 receptor agonist) or acetylstrophanthidin applied topically to the myocardium or injected into either the coronary arteries or pericardial sac (Coleridge, Coleridge & Kidd, 1964; Sleight & Widdicombe, 1965; Sleight, Lall & Muers, 1969; Öberg & Thorén, 1972; Drinkhill, Moore & Hainsworth, 1993). In addition, exterior probing or distension of the left ventricle produced trains or bursts of action potentials (Coleridge et al. 1964; Sleight & Widdicombe, 1965; Öberg & Thorén, 1972; Gupta & Thames, 1983; Drinkhill et al. 1993). It was concluded that there was a spectrum of cardiac receptors ranging from pure chemically to pure mechanically sensitive (Sleight et al. 1969; Öberg & Thorén, 1972; Kaufman, Baker, Coleridge & Coleridge, 1980). A recent estimate based on recordings from nodose ganglion cells in dogs indicates a small multimodal contribution of 10 % (Armour, 1994). It follows, therefore, that cardiac receptors may provide the central nervous system with information containing different sensory modalities. Consistent with this interpretation is the finding that selective activation of chemically versus mechanically sensitive cardiac receptors produced different reflex effects on the cardiovascular system (compare McGregor et al. 1986 with Tutt et al. 1988). This raises the question of whether chemically and mechanically sensitive cardiac receptors innervate different central target neurones that have separate central reflex pathways.
Anatomical and electrophysiological evidence indicates that vagal afferents from the left side of the heart terminate within restricted regions of the nucleus of the solitary tract (NTS) located in the dorsomedial medulla. Intracardiac injections of horseradish peroxidase resulted in dense afferent labelling in the dorsolateral subnuclei around the level of the obex and within the commissural NTS in the cat (Kalia & Mesulam, 1980). In agreement with these observations were the recording sites of NTS neurones responding to electrical stimulation of the cardiac branch of the vagus nerve in the cat (Donoghue, Fox, Kidd & Koley, 1981; Bennett, Goodchild, Kidd & McWilliam, 1985, 1988).
To date, no attempts have been made to stimulate cardiac receptors using stimuli to excite both chemically and mechanically sensitive cardiac receptors selectively while recording from NTS neurones. This would resolve the question as to whether the peripheral encoding of chemically and mechanically sensitive cardiac receptors is preserved centrally. Furthermore, there are only preliminary data describing the convergence characteristics of characterized NTS neurones receiving inputs from cardiac receptors (Jones, Wang & Jordan, 1995; Paton 1996a) with other cardiorespiratory afferents (e.g. baro- and peripheral chemoreceptors). A knowledge of any convergence patterns would further enhance our understanding of the central integration of cardiorespiratory afferents within the NTS.
The present experiments demonstrate the existence of two distinct populations of NTS neurones driven by cardiac vagal receptors. The pattern of convergence from other cardiorespiratory afferents to these NTS neurones lends support to an organization based on sensory modality.
Preliminary findings of part of this study have been communicated to The Physiological Society (Paton, 1996a).
METHODS
A working heart-brainstem preparation (WHBP; Paton 1996b) was developed originally to overcome some of the technical difficulties of physiologically stimulating cardiac receptors without co-activation of other reflexogenic areas in the systemic circulation and while recording brainstem neurones simultaneously.
Surgical procedures
All experiments were performed in an arterially perfused WHBP of mice (3–6 weeks old) which preserves cardiorespiratory reflex function (Paton & Butcher, 1997). A full description of the working heart-brainstem preparation was reported previously (Paton, 1996b) and only brief details are given here.
Mice (P21–56; NIH and MF1; 15–28 g) of either sex were placed in a saturated atmosphere of either ether or halothane vapour contained in a dessicator. Once the respiratory rate and depth were greatly reduced and the mouse failed to respond to a noxious pinch of a paw or the tail, it was immediately bisected sub-diaphragmatically. The thorax and head were placed in ice-chilled artificial cerebrospinal fluid (ACSF) gassed with 95 % oxygen and 5 % carbon dioxide (carbogen). Mice were decerebrated at the precollicular level, skinned and the cerebellum removed to expose the dorsal surface of the medulla before transferring to a recording chamber. Once transferred, the cranium was fixed using custom-built ear bars mounted on the chamber. The dura was reflected and the head tilted to expose the caudal-most region of the dorsal medulla. The descending aorta was cannulated and perfused retrogradely at constant flow (18–22 ml min−1) with carbogen-gassed solution (see below) using a roller pump. The perfusate was warmed to 31°C, filtered (100 μm and 40 μm pore sizes) and passed through two bubble traps which also dampened pulsations originating from heart.
Recording cardiovascular variables
Perfusion pressure was monitored close to the tip of the perfusion cannula (o.d., 0.8–1.0 mm). In six experiments to determine the effect of changing left ventricular pressure on systemic pressure and vice versa, a common carotid artery was cannulated to monitor arterial pressure directly (o.d., 0.5 mm). In all experiments the right atrium was cannulated (o.d., 0.63 mm) via the inferior vena cava for drug injection. A second cannula (o.d., 0.63 mm) was placed into the right atrium to record pressure in many preparations. A transmyocardial cannula (25 g hypodermic needle) was placed through the apex of the heart and was equipped with a side arm placed close to its tip to monitor left ventricular pressure and inject drugs. Pressure signals were transduced (Gould Statham), amplified and displayed (Gould TA11). The ECG was recorded via silver wires placed on the thoracic cage; signals were amplified and filtered (Neurolog modules; NL 104 and 125).
Electrical stimulation of the cervical vagus and the left ventricle
In all central recording experiments the ipsilateral vagus nerve was isolated in the neck and a fine insulated silver wire (0.15 mm diameter; California Fine Wire Co., USA), bared at one end, wrapped around the intact nerve before electrically isolating in low melting point wax. An indifferent electrode was placed in nearby muscle. In addition, in some experiments the outer surface of the left ventricle was electrically stimulated directly using a five-branched wire electrode manufactured from fine insulated silver wire. The end of each branch was bared of insulation and positioned in different regions of the left ventricle to enhance recruitment. Both the vagus nerve and left ventricle were stimulated (0.1–0.5 ms, 1 Hz, 0.5–20 V) using a pulse generator (Neurodata 4000) and isolated stimulator (Digitimer DS2A).
Stimulation of cardiorespiratory receptors and controlling for selectivity
Cardiac, baro- and carotid body chemoreceptors (referred to as chemoreceptors) were stimulated. Procedures of stimulation and methods for controlling against simultaneous co-activation of other cardiorespiratory receptors are described below. Since cardiac mechanoreceptors are sensitive to changes in left ventricular end-diastolic pressure (LVEDP; Öberg & Thorén, 1972; Gupta & Thames, 1983), this variable only is quoted.
Stimulation of cardiac receptors
It should be emphasized that veratridine is non-selective in stimulating unmyelinated vagal afferents and is a foreign substance which does not discriminate between chemically and mechanically sensitive fibres. Thus, NTS neurones activated by distending the left ventricle may also be driven by veratridine. However, cardiac mechanoreceptors may not necessarily be responsive to naturally occurring chemicals released from the myocardium such as bradykinin.
Both chemically and mechanically sensitive cardiac receptors were stimulated. Relatively small volumes (10–50 μl) of veratridine (1–3 μg kg−1), bradykinin (0.25–1 μg) and prostaglandin E2 (100–200 ng) solutions were injected slowly (over 1–2 s) into the left ventricle to stimulate chemically sensitive cardiac receptors, and produced minimal effects in left ventricular pressure. Any change in left ventricular pressure caused by the injection was mimicked by injection of perfusate to control for co-activation of mechanically sensitive cardiac receptors. The route of injection of veratridine was based on previous reports (Daly, 1991). Doses of bradykinin and prostaglandin E2 were derived from levels measured during myocardial ischaemia (Ustinova & Schultz 1994a), and distension of the left ventricle (Block, Poole & Vane, 1974), respectively. To control for the site of action of chemical cardiac receptor stimulants, injections of doses identical to those used intraventricularly were made into the systemic circulation via the perfusion cannula.
Stimulation of mechanically sensitive cardiac receptors was achieved by increasing LVEDP using volumes of perfusate (50–400 μl) injected over 0.5–2 s. Maintained distensions (i.e. > 2 s) were not performed. Due to the technical restraints of the size of the mouse heart, it was only possible to insert one transmyocardial cannula without disruption of cardiac performance. Thus, all drugs and perfusate were injected via a side arm tube connected to the transmyocardial cannula. It was therefore not possible to measure simultaneously the exact pressure change within the left ventricle during injections, due to the resistance imposed by the transmyocardial cannula. Because of this, a quantitative analysis of the transfer function of NTS neurones responding to changes in left ventricular pressure was not carried out.
The effect of raising LVEDP on carotid artery pressure was measured. The data were fitted using non-linear regression analysis (Fig. 1). From this equation it could be estimated what the change in arterial pressure would be for a given change in LVEDP. As a control for this, perfusion pressure was elevated to the same level as, or slightly higher than that induced by the change in left ventricular pressure.
Figure 1. Dynamic relationship between pressures within the left ventricle and systemic circulation in the working heart-brainstem preparation.
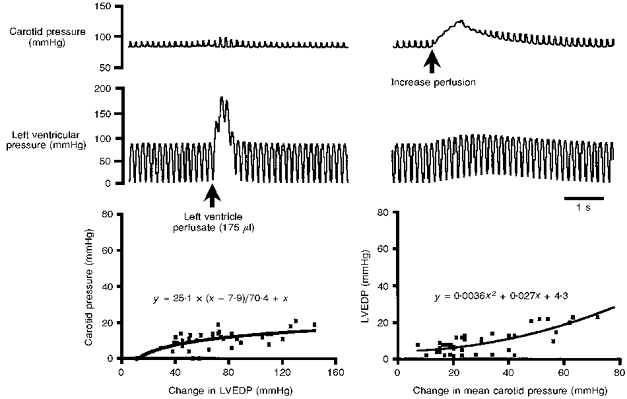
The effect of changing pressure within the left ventricle on arterial pressure and vice versa is shown (top). The graphs plot the relationships between left ventricle end-diastolic pressure (LVEDP) and carotid artery pressure when LVEDP (left) and systemic pressure (right) are increased, respectively. The equations allow calculations of the effect on systemic and LVEDP that would be induced by increasing LVEDP and arterial pressure, respectively. The fact that the relationship between the pressures was not equal permitted the origin of the reflexogenic region responsible for the synaptically evoked responses in the NTS to be delineated. Data are means ±s.e.m. from 6 preparations and > 5 tests per preparation.
Stimulation of baroreceptors
Perfusion pressure was raised by either increasing the resistance on a bypass line of the perfusion circuit or increasing pump flow rate (see Paton & Butcher, 1997). Controls against mechanical stimulation of neurones by the microelectrode were as published previously (Rogers, Paton & Schwaber, 1993): briefly, during pressure excursions, only neuronal responses that showed no change in spike width with a minimal change in action potential height (± 10 %) during the pressor test, and were repeatable after withdrawing the recording electrode 5–15 μm were accepted. The effect of raising perfusion pressure on LVEDP was measured (Fig. 1) and plotted. From this it could be predicted what the change in LVEDP would be for a given rise in perfusion pressure. To control for a possible co-activation of cardiac receptors during baroreceptor stimulation, LVEDP was raised to similar levels to those caused by the elevation in perfusion pressure (Fig. 1) during recordings of NTS neurones.
Stimulation of peripheral chemoreceptors
Injection of sodium cyanide (0.05 %, 50–200 μl) into the perfusion cannula was used to activate carotid body chemoreceptors. Since arterial flow is retrograde in the descending aorta of the WHBP, sodium cyanide will be carried towards the carotid bodies. Sodium cyanide injection produced small (2–10 mmHg) rises in perfusion pressure. Since these could potentially co-activate baroreceptors, control elevations in perfusion pressure to levels evoked by sodium cyanide injections were made.
Right atrial stretch receptors
Perfusate (50–300 μl) was injected over 1–1.5 s via a right atrial catheter to increase right atrial pressure and stimulate right atrial stretch receptors. This was routinely done to control against co-activation of right atrial receptors caused by changes in perfusion pressure and LVEDP. It also permitted an assessment of any convergence from right atrial stretch receptors.
Recording phrenic nerve activity
In all central recording experiments, WHBP were paralysed (see below), the left phrenic nerve cut distally at the level of the diaphragm, and discharge recorded from the central end using a glass suction electrode (tip diameter, 150–225 μm). Central respiratory activity was recorded in all experiments to continuously monitor preparation viability. All data in this study were from preparations that displayed a ramping phrenic nerve discharge pattern characteristic of eupnoeic activity of a spontaneously breathing anaesthetized mouse (Paton, 1996b, c) and indicative of a well-oxygenated brainstem. Respiratory activity was amplified and filtered (Neurolog modules 104 and 125; 8 Hz to 3 kHz), displayed on an oscilloscope (Gould DSO 400) and/or computer monitor (Spike2 CED) and connected to both an audio amplifier (Grass AM8) and a thermal chart recorder (Gould TA 11).
Recording central neuronal activity
All preparations were paralysed with either gallamine or vecuronium (see ‘Solutions and drugs’, below). NTS neurones were recorded extracellularly using glass microelectrodes filled with 3 m NaCl (0.9–8 MΩ) or 1 m sodium acetate with Pontamine Sky Blue (2 %) to mark recording sites ionophoretically (-1 to -5 μA, 5 min). Signals were amplified (Neurolog 104), filtered (8 Hz to 3 kHz; Neurolog 125) and displayed on an oscilloscope and/or computer monitor. Recording electrodes were placed into the NTS under visual guidance using a binocular microscope, and driven into the tissue with a stepping motor (1–2 μm steps). Surface landmarks of the dorsal medulla (e.g. mid-line and area postrema) were used for orientation. Microelectrodes were driven into the dorsal medulla at an angle of approximately 60 deg at rostrocaudal sites corresponding to area postrema and 1–1.5 mm caudal to it.
Experimental protocol and NTS neurone classification
The protocol followed was to test all NTS neurones receiving a relatively long-latency synaptic input following vagus nerve stimulation (i.e. > 18 ms) for their response to both intraventricular injection of veratridine and, after a > 5 min period, mechanical distension of the left ventricle. Subsequently, some cells were also tested with other chemical agents such as bradykinin and PGE2. Cells responding to veratridine were termed veratridine driven. Veratridine-driven units that failed to respond to mechanical distension of the left ventricle were termed distension insensitive (DIS) and assumed to receive input from one or more cardiac chemically sensitive vagal receptors. Neurones driven by veratridine and distension of the left ventricle were named distension-sensitive (DS) NTS neurones. Since veratridine activates chemically and mechanically sensitive cardiac vagal unmyelinated fibres non-selectively, it should not be dismissed, nor assumed, that some DS neurones may also be chemically sensitive.
Analysis
All recorded variables were digitized (Instrutech VR100; sampling rate, 26 kHz) and stored on VCR tape (Panasonic) for off-line analysis. The reflex changes in cardiac rate, phrenic frequency and amplitude during stimulation of cardiorespiratory receptors in the WHBP are reported elsewhere (Paton & Butcher, 1998). Non-linear regression analysis was performed on data from experiments determining the dynamic relationship between LVEDP and systemic pressure in the WHBP (Prism Graphpad). Neuronal firing frequency and response durations of single units were quantified either on- or off-line using a CED 1401 interface and Spike2 software. Post-stimulus time histograms were constructed for synaptically evoked spikes following electrical stimulation of both the vagus nerve and left ventricle. R wave-triggered histograms were made from data obtained from veratridine-driven NTS neurones with on-going activity. All data are expressed as the means ±s.e.m. Student's t test was used to test statistical significance using paired data. N= number of preparations, and n= number of neurones.
Histological procedures
Following extracellular recordings, either the tip of the microelectrode was left in the medulla or Pontamine Sky Blue was deposited ionophoretically (-1 to -5 μA, 5 min) to mark recording sites. The brainstem was removed and fixed in 2 % paraformaldehyde overnight and then placed into 2 % paraformaldehyde with 20 % sucrose for > 12 h. Tissue was sectioned transversely (50 μm), stained with Neutral Red and recording sites documented using a microscope fitted with a camera lucida.
Solutions and drugs
The arterial perfusate constituents were (mm): 10 dextrose, 125 NaCl, 24 NaHCO3, 5 KCl, 2.5 CaCl2, 1.25 MgSO4, 1.25 KH2PO4, plus 2.0–2.2 % dextran (Sigma) and an antibiotic cocktail containing penicillin (50 u l−1), streptomycin (0.05 mg l−1) and neomycin (0.1 mg l−1; Sigma) and either gallamine triethiodide (0.1 mg ml−1; Sigma) or vecuronium bromide (0.04 μg ml−1; Norcuron Organon Teknika): the latter agents blocked neuromuscular transmission. Perfusate osmolality was 298 ± 5 mosmol kg−1. After gassing with carbogen, the pH was 7.35 ± 0.05. Injections of sodium cyanide and chemicals to stimulate cardiac receptors (see above) were warmed to preparation temperature (i.e. 31°C).
RESULTS
The experiments described here also formed part of the data describing cardiorespiratory reflexes in mice which were published elsewhere (Paton & Butcher, 1998).
Baseline cardiovascular variables in the working heart-brainstem preparation (WHBP; N= 43)
Mean cardiac rate was 353 ± 6 beats min−1 (at 31°C) and perfusion pressure was 87 ± 5 mmHg. Right atrial pressure was pulsatile (see Paton 1996b, and Fig. 4) between 4 and 8 mmHg. This was achieved by a ‘natural’ venous return of perfusate via the superior vena cava. The right side of the heart pumped perfusate through the pulmonary circulation and provided adequate filling of the left ventricle as observed by an end-systolic left ventricular pressure of 75–90 mmHg (see Figs 1 and 3–6). The left ventricle was ejecting as deduced from the pulse-related deflections in perfusion and common carotid artery pressure (Fig. 1). These pulse deflections were 4.5 ± 0.9 mmHg.
Figure 4. Characterization of a distension-sensitive (DS) and veratridine-driven NTS neurone.
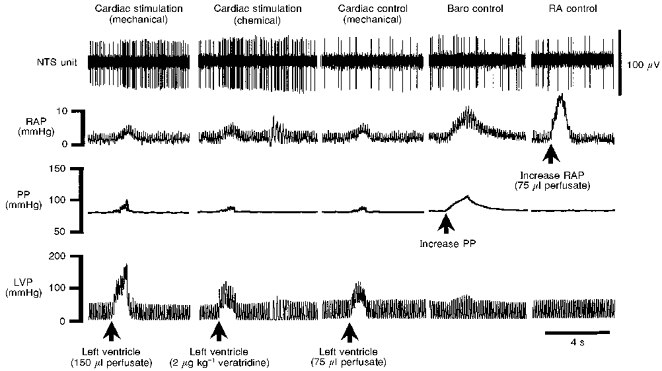
DS NTS neurones were characterized by an excitatory synaptic response following mechanical distension of the left ventricle (Cardiac stimulation (mechanical)) by increasing left ventricular pressure (LVP). This DS neurone also responded to an intraventricular injection of veratridine (Cardiac stimulation (chemical)). To control against the increase in LVP caused by the injection of veratridine, LVP was raised to similar levels, but failed to activate the cell (Cardiac control (mechanical)). This stimulus was subthreshold for activating the neurone. Since increasing LVP produced small increases in perfusion pressure (PP; see Fig. 1 and Cardiac stimulation (mechanical)) and right atrial pressure (RAP; see Cardiac stimulation (mechanical)), control elevations in these latter pressures were performed to rule out a contribution from co-activation of baroreceptors and/or right atrial stretch receptors, respectively. Raising either PP (Baro control) or RAP (RA control) with injections of perfusate was without effect, indicating that the synaptic response observed during left ventricular distension originated from receptors within the left side of the heart. This cell also received a synaptic input following electrical stimulation of the vagus nerve (not shown).
Figure 3. Characterization of a distension-insensitive (DIS) and veratridine-driven NTS neurone.
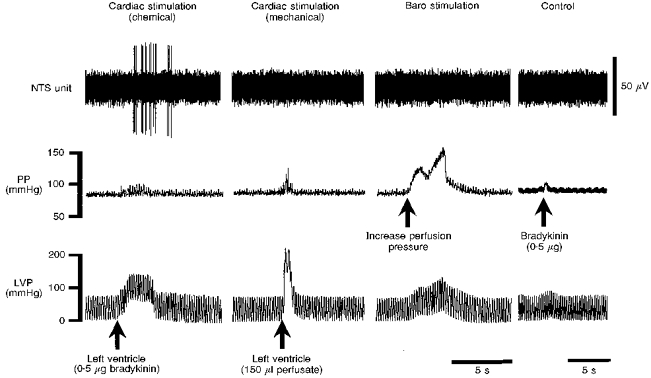
This DIS NTS cell was characterized by its excitatory response following stimulation of chemically sensitive cardiac receptors with an intraventricular injection of bradykinin (Cardiac stimulation (chemical)), but was not driven by increasing left ventricular pressure (LVP) to activate mechanically sensitive cardiac receptors (Cardiac stimulation (mechanical)). The small increase in perfusion pressure (PP) caused by the injection of bradykinin was not responsible for the discharge of the NTS unit since increasing perfusion pressure (to stimulate baroreceptors) was without effect (Baro stimulation). Injection of bradykinin into the systemic circulation (via the perfusion cannula) failed to elicit firing of the cell (Control), indicative of a relatively specific action on cardiac receptors. Note that the time scale in the Control panel is reduced compared with the other three panels. Left ventricular distension was achieved by a bolus injection of perfusate via a transmyocardial cannula. This cell also received a synaptic input following electrical stimulation of the vagus nerve (not shown). The preparation was paralysed with gallamine.
Figure 6. Convergence characteristics of distension-sensitive (DS) NTS neurones.
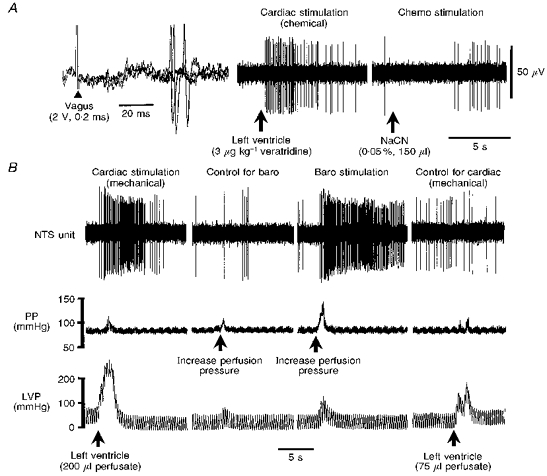
A and B are recordings from the same NTS neurone. DS NTS neurones were identified by an action potential barrage following left ventricular distension to stimulate mechanically sensitive cardiac receptors (B, Cardiac stimulation (mechanical)). This neurone also received a relatively long-latency excitatory synaptic response following ipsilateral vagus nerve stimulation (A, left; 3 consecutive superimposed traces) and was typically excited by a left ventricular injection of veratridine (A, Cardiac stimulation (chemical)) but not bradykinin (not shown). DS neurones were also activated by baroreceptor stimulation (B, Baro stimulation), achieved by relatively large elevations in perfusion pressure (PP), but not by chemoreceptor stimulation (A, Chemo stimulation). To control against co-activation of baroreceptors during left ventricular distension, perfusion pressure was elevated to a comparable level but failed to evoke a response (B, Control for baro), indicative of selective stimulation of mechanically sensitive cardiac receptors. To control for the rise in left ventricular pressure (LVP) during baroreceptor stimulation, LVP was increased to levels recorded during the induced increases in perfusion pressure, but was without effect, i.e. subthreshold (B, Control for cardiac (mechanical)). Increasing right atrial pressure to levels caused by increasing left ventricular pressure (LVP) and perfusion pressure (see Fig. 4) did not alter the firing of any DS NTS neurone (not shown).
Dynamic relationship of pressures within the left ventricle and systemic circulation in the WHBP (n= 6)
It was critically important to quantify the dynamic relationship between left ventricular end-diastolic pressure (LVEDP) and systemic pressure in the WHBP so that the origin of the reflexogenic area (i.e. cardiac receptors and/or baroreceptors) responsible for synaptically driving NTS neurones could be delineated. Figure 1 depicts the group data of six control experiments (> 5 tests per preparation) showing (i) the evoked changes in carotid artery pressure that were induced by raising LVEDP 10–135 mmHg above control, and (ii) the effect of increasing after-load by 10–80 mmHg from baseline on LVEDP. In both cases the responses were well below that expected in an intact in vivo animal where an almost equal change in left ventricular and aortic pressure would be expected (Pasipoularides, Murgo, Bird & Craig, 1984). Figure 1 also describes equations used to predict pressure changes in LVEDP or systemic circulation following manipulations of arterial pressure and LVEDP, respectively. These equations were tested for goodness of fit using non-linear regression analysis, and were significant (r2 > 0.50). There were also small increases in arterial pulse pressure during elevations in LVEDP. For example, an increase in LVEDP of 80–100 mmHg produced an increase in pulse pressure of 5.2 ± 0.2 mmHg above control.
Experimental protocol to delineate baroreceptor versus mechanically sensitive cardiac receptor inputs to NTS
The protocol adopted to delineate synaptic inputs from mechanically sensitive cardiac receptors and/or baroreceptors to NTS neurones was to measure the change in LVEDP during baroreceptor stimulation and vice versa. From the equations in Fig. 1 the calculated rise in LVEDP during an increase in systemic pressure of, for example, 45 mmHg was 12.8 mmHg. To rule out the possibility that this increase in LVEDP produced a synaptic response in the nucelus of the solitary tract (NTS), LVEDP was raised to approximately 12.8 mmHg. The latter produced a minimal effect on arterial pressure (Fig. 1) due to the unequal relationship between the two pressures (see above).
Characterization of cardiorespiratory NTS neurones in the WHBP
A total of 267 neurones were recorded, of which 181 received an excitatory synaptic input from the ipsilateral vagus nerve (latency range, 3–75 ms). Of these 181 neurones, 89 were characterized physiologically by their synaptic response following stimulation of cardiorespiratory receptors.
Central encoding of cardiac vagal receptor activity by NTS neurones
NTS neurones were excited by chemical and/or mechanical stimulation of cardiac receptors. With two exceptions, NTS neurones were activated by intraventricular injection of veratridine but not by systemic administration (e.g. Figs 3 and 5); these NTS neurones are described as veratridine driven.
Figure 5. Convergence characteristics of distension-insensitive (DIS) NTS neurones.
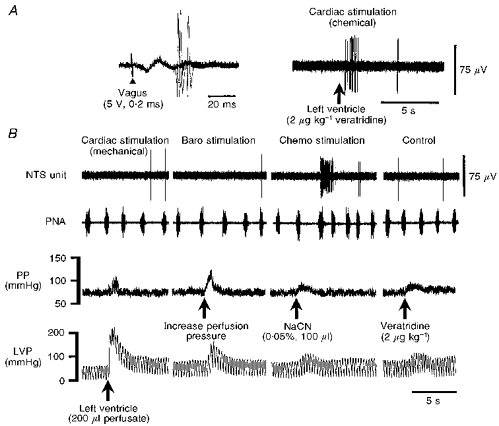
A and B are recordings from the same NTS neurone. A, in all DIS NTS cells a relatively long-latency synaptic potential was evoked following stimulation of the ipsilateral vagus nerve (left, 3 consecutive, superimposed sweeps). DIS NTS neurones were further characterized by an excitatory synaptic response following left ventricular injection of veratridine (Cardiac stimulation (chemical)) and/or bradykinin (not shown) to stimulate chemically sensitive cardiac receptors. B, in contrast, stimulation of mechanically sensitive cardiac receptors (Cardiac stimulation (mechanical)) by raising left ventricular pressure (LVP) was without effect. DIS neurones consistently received a convergent input from peripheral chemoreceptors (Chemo stimulation; activated by injection of sodium cyanide (NaCN) into the descending aorta) but not baroreceptors (Baro stimulation). As a control, veratridine injected into the arterial circulation was without effect (Control). Note the reflex activation of phrenic nerve discharge (PNA) frequency during chemoreceptor stimulation. The latency of the chemoreceptor reflex-induced respiratory response is due to the diffusion time of sodium cyanide (see Methods). The preparation was paralysed using gallamine, which blocked reflex vagal effects on heart rate.
Veratridine-driven NTS neurones (n= 43)
Of forty-one veratridine-driven NTS neurones, seventeen out of the eighteen tested were also driven by bradykinin, but only four out of thirteen were responsive to intraventricular PGE2. Additionally, two neurones responsive to mechanical distension of the left ventricle did not respond to any of the chemical stimulants (see below).
All forty-three veratridine-driven NTS neurones received an excitatory synaptic input following ipsilateral vagus nerve stimulation, which consisted of either a single spike or (more usually) multiple spikes (Fig. 2). In twenty neurones, the synaptic latency was relatively invariant between stimuli (mean variance, 1.2 ± 0.5 ms; range, 0.4–2 ms), indicative of a relatively direct input. Two-point electrical stimulation (i.e. of the left ventricle and cervical vagus nerve) produced a similar number and pattern of evoked spikes (Fig. 2) with a mean latency difference of 15 ± 2 ms in five out of five veratridine-driven NTS cells tested. Since the distance between these stimulation sites was approximately 9–10 mm, these vagally evoked events were conducted at a velocity of 0.6–0.66 m s−1, indicative of unmyelinated vagal fibres. In another twenty-three veratridine-driven NTS neurones, the synaptic latency following vagus nerve stimulation varied by 3.6 ± 0.3 ms (range, 2.0–7.0 ms). Finally, an additional nine NTS neurones with on-going activity were inhibited following veratridine stimulation of the left ventricle and by vagus nerve stimulation (onset latency, 23–43 ms). Although attempted, it was not technically possible to identify the receptive field of the cardiac receptor by probing the heart and recording from an NTS neurone simultaneously.
Figure 2. Distribution of synaptic latencies of veratridine-driven NTS neurones evoked following electrical stimulation of the vagus nerve.
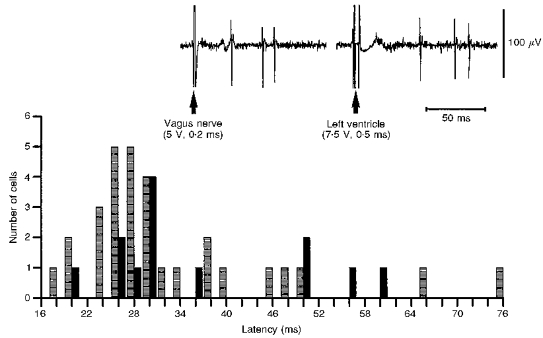
A distribution plot of the latencies of synaptic inputs from the ipsilateral cervical vagus nerve to distention-insensitive (DIS;  , n= 30, see Fig. 3) and distenstion-sensitive (DS;
, n= 30, see Fig. 3) and distenstion-sensitive (DS;  , n= 13, see Fig. 4) NTS neurones. Inset, comparison of the response pattern and latency of a DIS NTS neurone following electrical stimulation of the vagus nerve and left ventricle (a single sweep each). The latencies of synaptic inputs from the heart were on average 15 ± 2 ms longer (n= 5) than the vagus nerve. The computed conduction velocity was in the C fibre range (i.e. 0.6–0.66 m s−1). Note the similar firing pattern evoked following vagus nerve and left ventricular stimulation.
, n= 13, see Fig. 4) NTS neurones. Inset, comparison of the response pattern and latency of a DIS NTS neurone following electrical stimulation of the vagus nerve and left ventricle (a single sweep each). The latencies of synaptic inputs from the heart were on average 15 ± 2 ms longer (n= 5) than the vagus nerve. The computed conduction velocity was in the C fibre range (i.e. 0.6–0.66 m s−1). Note the similar firing pattern evoked following vagus nerve and left ventricular stimulation.
Veratridine-driven NTS neurones could be delineated into two groups depending on whether they responded to distension of the left ventricle; these comprised (i) distension-insensitive (DIS; Fig. 3), and (ii) distension-sensitive neurones (DS; Fig. 4).
DIS NTS neurones (n= 30)
DIS neurones were characterized by: (i) an absence of response during an increase in LVEDP (Fig. 3); (ii) a prompt (i.e. within 2 s) excitatory response following intraventricular injection of veratridine, bradykinin and/or PGE2 (Fig. 3); (iii) an absence of response following veratridine or bradykinin injection into the arterial circulation (Fig. 3), indicative that the recepive field was likely to be of cardiac origin; and (iv) when tested, a synaptic input following electrical stimulation of the left ventricle muscle (Fig. 2).
The majority of DIS NTS neurones displayed on-going activity ranging from 0.1 to 9 Hz which consisted of either irregular single spiking (n= 13), which in three cells was intermixed with 200–300 ms duration bursts, or tonic firing (n= 7). The remaining DIS neurones were silent (n= 10). No DIS neurones showed a correlation in discharge with either the cardiac cycle or phrenic nerve activity. Intraventricular injection of veratridine excited all thirty DIS neurones (Figs 3 and 5). The on-going activity of an additional four DIS NTS neurones was inhibited with veratridine. Central responses occurring 1–2 s from the start of the injection were analysed; longer-latency effects were attributed to non-specific actions. Veratridine-induced excitatory responses had a peak discharge rate of 14.7 ± 1 Hz which lasted between 1 and 25 s (Table 1). In twelve out of thirteen neurones tested, subsequent injection of bradykinin produced a similar response to that observed with veratridine. In contrast, only one of nine DIS NTS neurones was responsive to left ventricular injection of PGE2. Electrical stimulation of the ipsilateral vagus nerve evoked synaptic discharge in all DIS neurones, comprising between 1 and 10 spikes per stimulus (mean, 2.7 ± 0.4 spikes) at a latency of 33 ± 2.4 ms (range, 17–75 ms; Figs 2 and 5A).
Table 1.
Evoked discharge characteristics of cardiorespiratory NTS neurones
| Cardiorespiratory receptor stimulated | Peak firing frequency (Hz) | Firing frequency range (Hz) | Mean duration (s) | Range of duration (s) |
|---|---|---|---|---|
| Cardiac chemo (n= 30) | 14.7 ± 1 | 6–32 | 7.3 ± 0.9 | 1–25 |
| Cardiac mechano (n= 13) | 14.3 ± 2.5 | 4–40 | 4.3 ± 1.2 | 2–5 |
| Baroreceptor (n= 23) | 14.0 ± 2.1 | 4–38 | 2.7 ± 1.1 | 0.2–5 |
| Chemoreceptor (n= 34) | 18.2 ± 1.8 | 8–37 | 7.7 ± 1.2 | 1–10 |
| Right atrial stretch (n= 5) | 14.7 ± 0.7 | 9–28 | 2.8 ± 0.6 | 1–3 |
The mean (±s.e.m.) peak frequency and duration of firing responses of neurones within the NTS during stimulation of cardiac receptors (either chemically with 1–3 μg kg−1 veratridine or by increasing left ventricular pressure by 70–85 mmHg), baroreceptors (by increasing perfusion pressure by 55–70 mmHg), peripheral chemoreceptors (using 1–5 μg sodium cyanide) and right atrial stretch receptors (increasing right atrial pressure by 5–6 mmHg from control levels) are shown. Note that the data are pooled and so any one neurone may be activated by more than one afferent type.
DS NTS neurones (n= 13)
Despite extensive searching and use of microelectrodes with varying resistances, DS NTS neurones were less commonly found than DIS neurones. Injection of perfusate into the left ventricle to increase left ventricular pressure resulted in a brisk activation of all DS NTS unit activity occurring during the rise in ventricular pressure (Fig. 4; Table 1). The peak firing response frequency was 14.3 ± 2.5 Hz lasting between 2 and 5 s (Table 1), and either extended beyond the stimulus (n= 5) or terminated during the pressure rise. The pattern of on-going discharge and absence of correlation with both the cardiac and phrenic cycle were similar to the active DIS neurones. Unlike DIS neurones, all DS cells displayed on-going activity (0.2–4 Hz). In an additional five cells, inhibition of on-going activity was also evoked by left ventricular distension, and lasted for up to 5 s.
To control against possible co-activation of baroreceptors that could occur due to the smaller rise in perfusion pressure that resulted from raising intraventricular pressure (see above and Fig. 4), perfusion pressure was raised to a similar level, but in all neurones there was no detectable change in firing rate (n= 7; Fig. 4). Furthermore, mimicking the changes in right atrial pressure that occurred during left ventricle distension also failed to alter the activity of all DS NTS neurones (Fig. 4). Thus, in the WHBP it was possible to control against possible co-stimulation of baroreceptors and right atrial stretch receptors during increases in left ventricular pressure.
With the exception of two DS NTS neurones, both the excitatory and inhibitory synaptic responses of left ventricular distension were mimicked by subsequent injection of veratridine (n= 15/17), bradykinin (n= 5/5) or PGE2 (n= 3/4); many neurones were responsive to all three stimulants. Neurones excited by left ventricular distension also received an excitatory synaptic response evoked from the ipsilateral vagus nerve. The latency and patterns of evoked response were not different from DIS neurones (i.e. 39 ± 4 ms; range, 20–60 ms; 1–7 spikes per stimulus; Figs 2 and 6A). In contrast, four of the five NTS neurones inhibited by distension of the left ventricle were either inhibited (n= 2) or failed to respond to vagus nerve stimulation (n= 2).
NTS encoding of baro-, peripheral chemo- and right atrial receptor inputs
Baroreceptors (n= 23)
Raising arterial pressure by 15–85 mmHg induced volleys of discharge ranging in peak frequency (4–38 Hz) and duration (0.2–5 s; Fig. 6; Table 1). In some cases it was evident that there was an arterial pressure threshold at which the NTS neurone became activated (n= 6). The activity of other baroreceptive neurones either terminated during the stimulus (n= 4) or remained above baseline for several seconds after the stimulus (n= 6). In neurones with on-going discharge, R wave-triggered histograms were generally unconvincing in demonstrating a pulse relationship (n= 20/23). Baroreceptor stimulation also reduced or abolished the on-going activity in seven NTS neurones. As a control, LVEDP was raised to a level that was either equal to, or just above that caused by the induced increase in perfusion pressure; in all cases this was ineffective in changing the firing of NTS cells.
Peripheral chemoreceptors (n= 34)
Stimulation of peripheral chemoreceptors with sodium cyanide produced a volley of action potentials with a peak discharge rate of 18 ± 1.8 Hz lasting between 1 and 10 s (Fig. 5; Table 1). None of these neurones showed respiratory modulation in their on-going activity during control conditions. The NTS responses occurred either before, or coincident with, increases in phrenic motor outflow, and are therefore unlikely to be secondary to a change in central respiratory activity. However, the responses of six NTS neurones were modulated by changes in central respiratory activity, as seen by an augmentation during the post-inspiratory and/or inspiratory phase. In addition, six NTS neurones with on-going activity were inhibited by chemoreceptor stimulation. In all cases tested, raising perfusion pressure to a level induced by sodium cyanide injection was without effect (n= 10).
Right atrial stretch receptors (n= 8)
NTS neurones were encountered that responded when right atrial pressure was elevated. Five cells were excited (Table 1) and three neurones with on-going activity were inhibited. Excitatory responses were typically of 10 ± 0.7 Hz (range, 6–15 Hz) and relatively short in duration (1–3 s).
Convergence from cardiorespiratory receptors to veratridine-driven NTS neurones
Twenty-seven of the thirty DIS and seven of the thirteen DS NTS neurones were tested for convergence from other cardiorespiratory receptors. A distinct pattern of convergence from baro- and chemoreceptors was found to DS and DIS NTS neurones (Figs 5-7).
Figure 7. Common afferent modality convergence in the NTS.
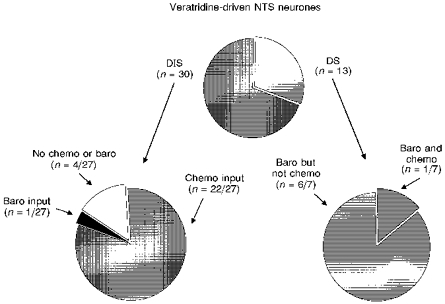
Distension-insensitive (DIS) NTS neurones typically received convergent inputs from peripheral chemoreceptors but not baroreceptors. Conversely, distension-sensitive neurones (DS) failed to respond to chemoreceptor activation but were consistently driven following baroreceptor stimulation. All DIS and all but 2 DS neurones could also be driven by veratridine stimulation of cardiac receptors. Not all DIS and DS cells were tested for synaptic inputs from chemo- and baroreceptors.
Convergence from baroreceptors
There was a paucity of convergence from baroreceptors to DIS NTS neurones: of the twenty-seven DIS NTS neurones tested, only one showed a convergent input following baroreceptor stimulation (i.e. 4 %; Figs 5 and 7). Furthermore, three of four NTS neurones inhibited by chemical stimulation of the left side of the heart also failed to respond to baroreceptor activation; notably, the one converging input was also inhibitory. In contrast, all DS NTS neurones tested (n= 7) exhibited convergence from baroreceptors (Figs 6 and 7). Three of these cells were tested but failed to respond to intraventricular injections of either bradykinin or PGE2. In addition, three out of four neurones inhibited by left ventricular distension were also inhibited by baroreceptor stimulation.
Convergence from peripheral chemoreceptors
Eighty-one per cent (i.e. 22/27) of DIS NTS neurones received a convergent excitatory input from chemoreceptors (Figs 5 and 7). In addition, three out of four neurones inhibited by chemical stimulation of cardiac receptors were also inhibited by chemoreceptor stimulation. Conversely, only one out of seven DS NTS neurones tested received a convergent excitatory input from chemoreceptor afferents (Fig. 7). Furthermore, the on-going discharge of NTS neurones inhibited by left ventricular distension was not influenced by chemoreceptor stimulation (n= 0/4).
Right atrial stretch receptors
In twelve veratridine-driven NTS neurones (ten DIS and two DS), raising right atrial pressure failed to produce a response. This suggests that inputs from the left and right sides of the heart do not converge within the NTS.
Convergence patterns of baro- and chemoreceptors in the WHBP
The following analysis describes the presence and absence of convergence from receptors other than those activated by chemical or distension stimuli applied to the left ventricle. The population of NTS neurones considered included cells receiving inputs from cardiac receptors.
There was limited convergence between baro- and chemoreceptors in the NTS: in neurones excited by chemoreceptors, only 20 % were influenced by baroreceptor stimulation (n= 10/49), including both excitatory (n= 5) and inhibitory (n= 5) inputs. In addition, distension of the right atrium either excited (n= 3) or inhibited (n= 3) NTS neurones that were also excited by baroreceptor stimulation, but failed to respond to chemoreceptors. Finally, NTS cells were found that failed to show convergence, including neurones driven only by baroreceptors (n= 10), chemoreceptors (n= 4) and cardiac receptors (n= 7; i.e. four DIS, three DS).
Recording sites of veratridine-driven NTS neurones
The recording sites of twenty-one physiologically characterized veratridine-driven NTS neurones were recovered. Figure 8 illustrates that the location of recording sites of DIS and DS NTS neurones were in regions of the commissural subdivision. These neurones were located 0.6–1.2 mm caudal to the obex, 0.1–0.4 mm lateral to mid-line and 0.15–0.48 mm below the dorsal surface. Many neurones outside this region (i.e. extending 1.5 mm rostral to 1 mm caudal to the obex, and 0–1.5 mm lateral to mid-line) were tested for their response to intraventricular injection of veratridine but failed to respond.
Figure 8. Recording locations of veratridine-driven NTS neurones.
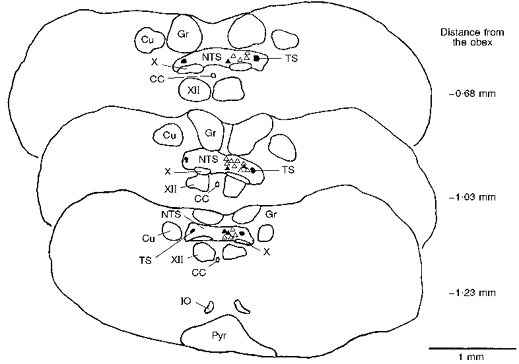
Both distension-insensitive (DIS; ▵, n= 16) and 5 distension-sensitive (DS; ▴) NTS neurones are depicted. The recording sites were drawn on representative transverse sections of the caudal medulla using a camera lucida. The DIS neurones responded to intraventricular injection of either veratridine (n= 11) or bradykinin (n= 5), whereas DS neurones were also driven synaptically following distension of the left ventricle. All neurones were located approximately 0.6–1.2 mm caudal to the obex. CC, central canal; Cu, cuneate nucleus; Gr, gracile nucleus; IO, inferior olive; Pyr, pyramid; TS, solitary tract; X, dorsal vagal motor nucleus; XII, hypoglossal motor nucleus.
DISCUSSION
The present experiments in a working heart-brainstem preparation (WHBP) of mouse have demonstrated, first, two populations of veratridine-driven NTS neurones delineated by their response following distension of the left ventricle into (i) distension-insensitive (DIS), and (ii) distension-sensitive neurones (DS). Second, distinct convergence patterns from baro- and chemoreceptors were found, such that DIS units responded preferentially to chemoreceptor stimulation whereas DS neurones were activated predominantly by baroreceptors.
Specificity of cardiac receptor and baroreceptor stimulation
In an in vivo animal with an intact arterial system, any changes in left ventricular pressure will produce a near-equal change in arterial pressure (e.g. Pasipoularides et al. 1984). This relationship depends, in part, on peripheral vascular resistance, ventricular and arterial compliance and venous return. Because of the open arterial system in the WHBP, both peripheral resistance and venous return are relatively low compared with an intact animal. Thus, the relationship between left ventricular end-diastolic pressure (LVEDP) and systemic pressure is far from being equal (see Fig. 1), and the WHBP offers an opportunity for permitting changes in LVEDP with minimal effect on arterial pressure. With appropriate controls it is possible to determine the origin of the pressure receptor responsible for the synaptic response in the NTS (see Figs 3-6) following increases in LVEDP. Differential stimulation of baroreceptors versus cardiac receptors using pressure stimuli can be performed in vivo but requires the use of large mammals and complex bypass perfusion systems (e.g. Al-Timman et al. 1993; McMahon, Drinkhill & Hainsworth, 1996), and simultaneous central neurophysiological recordings have never been attempted. Finally, although probing the heart has been a method used in peripheral fibre recording studies to identify the receptive fields of cardiac afferents (see Introduction) it was not technically possible to do this while maintaining a central neuronal recording in the present study.
It should be emphasized that intraventricularly injected chemical stimulants will have access to receptors within the myocardial tissues as well as coronary baroreceptors (Brown, 1965; Al-Timman et al. 1993; McMahon et al. 1996). Further, raising LVEDP will also elevate pressure in the left atrium and activate left atrial mechanoreceptors. The approach used in the present study cannot delineate between left ventricular receptors, coronary baroreceptors or left atrial receptors. In addition, part of the afferent information from the heart may be conveyed by cardiac sympathetic afferents activated by chemical and mechanical stimuli (see Armour, 1994, and Hainsworth, 1991, for reviews). However, the presence of a synaptic input evoked following electrical stimulation of the ipsilateral vagus nerve to all DIS and DS NTS neurones suggests that the evoked central responses were, at least in part, mediated by vagal afferents (see below). Additionally, in WHBP with spinal cord section at the cervical level, both DIS (n= 3) and DS neurones (n= 2) were found together with comparable convergence patterns from baroreceptors and chemoreceptors to those in the present study (J. F. R. Paton, unpublished observations).
It is acknowledged that substances injected into the left ventricle will be distributed within the arterial circulation and could potentially activate other cardiorespiratory receptors (e.g. baroreceptors, chemoreceptors). In the present study, the route of administration of veratridine was similar to previous studies investigating cardiac reflexes (e.g. Daly, 1991). As a control, in the present study arterial injections of similar doses of stimulants were made, but failed to excite DIS or DS NTS neurones, indicating a specific action from the heart. For instance, the absence of synaptic inputs to DIS neurones following rises in perfusion pressure, to activate baroreceptors, demonstrates that intraventricular veratridine did not co-activate baroreceptors. Likewise, there was no reflex increase in phrenic nerve activity after intraventricular injection of veratridine, bradykinin or PGE2, suggesting that peripheral chemoreceptors were not stimulated by these chemical stimulants.
Veratridine-driven NTS neurones in the mouse
The present data demonstrate the presence of both DIS and DS veratridine-driven NTS neurones, and support previous peripheral fibre recordings of two distinct populations of cardiac receptors - chemically and mechanically sensitive (Sleight et al. 1969; Öberg & Thorén, 1972; Kaufman et al. 1980). In addition, the finding of both DIS and DS NTS neurones is consistent with the results of Hines, Toney & Mifflin (1994), who described two populations of NTS neurones responding to either chemical stimulation of cardiopulmonary afferents or mechanical distension of the right atrium.
Previous NTS studies were unable to characterize DIS and DS NTS neurones as they only employed electrical stimulation of a cardiac branch of the vagus nerve (Donoghue et al. 1981; Bennett et al. 1985, 1988; Jones et al. 1995). Indeed, this study is one of the first (see Hines et al. 1994) to selectively stimulate both chemically and mechanically sensitive cardiac receptors to characterize NTS neurones, and, in this regard, the WHBP appears to be a viable preparation for studying the central integration of cardiac vagal afferent inputs.
The finding of smaller numbers of DS than DIS (13 vs. 30) NTS neurones gives a ratio of 30 : 70 %, which is consistent with the numbers of distension- and chemically activated cardiac nodose ganglion cells estimated recently in dogs (Armour, 1994) and rats (Ustinova & Schultz, 1994b). Further, it has been reported that most mechanically sensitive cardiac receptors are also stimulated by veratridine (Brown, 1965; Sleight et al. 1969; Öberg & Thorén, 1972), which is in agreement with the present finding of all but two DS NTS neurones being driven by this agent. The non-selectivity of veratidine must not be misinterpreted as meaning that all mechanically sensitive cardiac afferents are also chemically sensitive. Recent estimates indicate that only 10 % of cardiac vagal afferents show multimodality (i.e. chemically and mechanically sensitive; Armour, 1994). Based on this low number of multimodal cardiac vagal afferents, it is unlikely that all DS NTS neurones would be sensitive to chemical stimulants. Indeed, cardiac vagal mechanoreceptors are not necessarily responsive to naturally occurring chemicals released from the myocardium. However, since an exhaustive test of all possible cardiac chemical stimulants on all DS neurones was not performed, it cannot be ruled out that some DS neurones were also sensitive to chemical stimulants.
The fact that many veratridine-driven DIS NTS neurones were also activated by both bradykinin and PGE2 is not surprising, since these are known to stimulate cardiac receptors (Kaufman et al. 1980; Smith & Thames, 1994; Ustinova & Schultz, 1994a). Unlike veratridine, bradykinin may be a more physiological means of driving both chemically sensitive and multimodal cardiac receptors, since it is released during myocardial ischaemia (Kaufman et al. 1980; Ustinova & Schultz, 1994a). The finding that mechanical distension of the left side of the heart (i.e. an increase in LVEDP) also increased PGE2 (Block et al. 1974) is of interest regarding the present data indicating that three out of four DS versus only one out of nine DIS NTS neurones were also driven by an intraventricular injection of PGE2.
All veratridine-driven NTS neurones received a synaptic input following electrical stimulation of the ipsilateral vagus nerve and, when tested, from the left ventricle itself. The relatively long latency of the vagally evoked synaptic response (Fig. 2) together with the latency difference between evoked synaptic potentials from the vagus nerve and left ventricle is indicative of unmyelinated or C fibre inputs with an estimated conduction velocity of around 0.6 m s−1 (see Results).
Previous studies in the cat demonstrated that electrical stimulation of a cardiac branch of the vagus nerve evoked excitatory responses in NTS neurones which were mediated by either myelinated or unmyelinated vagal afferents (Donoghue et al. 1981; Bennett et al. 1985). The finding that the innervation of the left ventricle is predominantly by unmyelinated vagal fibres in the cat (see Hainsworth, 1991, for review) is consistent with the present observations in mice. Since the cardiac vagal branches in the cat contain afferents from both the ventricles and atria as well as the oesophagus and lungs (Bennett et al. 1985), the inputs to NTS described by Bennett et al. (1985) and Donoghue et al. (1981) might originate from receptors of non-cardiac origin.
There are no previous reports describing the response characteristics of NTS neurones during chemical or mechanical stimulation of the left side of the heart. However, the responses observed during chemical stimulation are consistent with those during chemical stimulation of unmyelinated vagal pulmonary C fibres (Hines et al. 1994; Wilson, Zhang & Bonham, 1996). The finding that the response of DS NTS neurones terminated abruptly during the pressure stimulus is indicative of a pressure threshold as reported for baroreceptive NTS neurones (Rogers et al. 1993). In other DS neurones (and some baroreceptive cells), firing remained elevated after the pressure stimulus, which may reflect the in vitro findings of prolonged increased excitability of commissural NTS neurones following solitary tract stimulation (Champagnat, Denavit-Saubie, Grant & Shen, 1986). Indeed, the latter slice preparation and the WHBP share common features such as an absence of anaesthesia and descending inhibitory connections, which may both potentiate and prolong these excitatory responses.
Common modality convergence to veratridine-driven NTS neurones
A novel finding of the present study was the preferential convergence of chemoreceptor inputs to DIS NTS neurones and baroreceptor inputs to DS NTS neurones. A clear trend was that DIS units were excited by stimulation of chemo- but not baroreceptors, whereas DS NTS neurones were driven by baro- but not chemoreceptor stimulation (Fig. 7). Although this may indicate a separation of peripheral inputs of different modality to NTS neurones, it must be recognized that not all veratridine-driven DS neurones were tested for their response to naturally occurring cardiac stimulants (e.g. bradykinin, PGE2 and adenosine). This would have permitted a test of chemical sensitivity of the mechanically sensitive cardiac vagal fibre(s) stimulated during left ventricular distension. It is conceivable, therefore, that some of the DS NTS neurones may have responded to naturally occurring cardiac stimulants. However, the absence of effect following intraventricular injection of bradykinin and PGE2 on the DS neurones receiving convergence from baroreceptors (3 out of 3 neurones tested), and the relatively low multimodal contribution of cardiac vagal afferents (i.e. 10 %; Armour, 1994), suggests that these cardiac inputs were from mechanoreceptors and supports the notion of a common afferent modality integration in the NTS (i.e. afferents relaying mechano- and chemo-information project to different NTS neurones). This notion may still be valid even if the cardiac mechanoreceptor is also driven by chemicals released from the myocardium during distension (see Block et al. 1974). In the latter case, while the mode of afferent transduction relies on liberation of a chemical, the stimulus is clearly mechanical. In this context, it was suggested that chemically sensitive cardiac receptors are not chemoreceptors in the way that aortic or carotid bodies are (Hainsworth, 1991). Rather, they respond to substances released during ischaemia and in this sense are relaying information regarding tissue concentration of these mediators which depends on myocardial blood gas content and/or distension.
An organization of peripheral afferents based on sensory modality was noted previously: Dawid-Milner, Silva-Carvalho, Goldsmith & Spyer (1995) demonstrated convergence of non-mechanically sensitive laryngeal receptors and chemoreceptors, whereas laryngeal receptors responding to mechanical stimulation converged onto NTS neurones driven by baroreceptors. All told, it is suggested that there may be a channeling of different afferent modalities of information within the NTS. This information may then be relayed to distinct outflows either controlling cardiac vagal motor or sympathetic pre-motor neurones, for example. With regard to reflexes evoked from cardiac receptors, stimulation of chemically sensitive cardiac receptors produced bradycardia/depressor in both mouse (Paton & Butcher, 1997) and dog (McGregor et al. 1986; Tutt et al. 1988). Since the common reflex response of both chemically sensitive cardiac receptors and chemoreceptors is a vagally mediated bradycardia, an NTS neurone receiving convergence from these afferents may, therefore, indicate a role in controlling cardiac vagal motor neurones. By analogy, since stimulation of mechanically sensitive cardiac receptors produced a depressor effect but failed to produce a bradycardia consistently (Tutt et al. 1988; Paton & Butcher, 1997), NTS neurones excited by these cardiac receptors and baroreceptors may act to depress sympathetic activity. Since pulse-modulated activity was only seen in cardiac vagal afferents during high and maintained levels of LVEDP (e.g. Öberg & Thorén, 1972; Gupta & Thames, 1983), this may explain the absence of pulse-modulated discharge in DS NTS neurones. In contrast, the lack of pulse modulation of NTS neurones receiving baroreceptor inputs probably reflects the small amplitude of the pulse pressure in the WHBP (see Methods and Results). The converging excitatory inputs to NTS neurones from baro- and chemoreceptors (Lipski, McAllen & Trzebski, 1976; Mifflin, 1992) as well as baroreceptor and unidentified vagal afferents (see, Spyer, 1994, for review) have both been reported before.
The latency and fibre-type mediating converging inputs from baro- and chemoreceptors to veratridine-driven NTS neurones were not identified in the present study. However, based on the absence of a mixed convergence from both myelinated and unmyelinated vagal inputs to NTS neurones driven orthodromically by electrical stimulation of the cardiac branch of the vagus (Donoghue et al. 1981; Bennett et al. 1985), it is suggested that the converging baro- and chemoreceptor inputs may be predominantly unmyelinated.
Cardiac receptors and cardiovascular regulation
Physiological stimuli to cardiac vagal receptors remain uncertain (Hainsworth, 1991) and this has raised the question of whether they play a role in controlling circulation during physiological conditions. Armour (1994) argued that since the majority of cardiac receptors are sensitive to chemical mediators rather than ventricular distension, and since chemically sensitive cardiac receptors respond more vigorously than distension-sensitive endings, it will be the chemical milieu of the heart that may exert a greater influence on NTS neurones. Prostaglandins, bradykinin and adenosine, which all activate cardiac receptors, are released during coronary occlusion, ischaemia and/or over-distension of the left ventricle which may occur during severe exercise. The latter are all examples of pathophysiological conditions which may, via reflex pathways, reduce both pre- and after-load, thereby reducing cardiac load to provide a protective mechanism against, for example, sudden cardiac death. In contrast, the convergence of baroreceptor and mechanically sensitive cardiac receptors within the NTS, together with the resultant tachycardia and hypertension following section of cardiac vagal afferents in sinoaortic denervated animals (Persson, Ehmke, Kirchheim & Seller, 1988), suggests that cardiac receptors play an important role in controlling circulatory homeostasis (see McMahon et al. 1996).
Acknowledgments
I wish to express my sincere thanks to Dr S. Kasparov for helpful discussions during the course of this study. The research was funded by the British Heart Foundation (BS/93003) and Royal Society (grant no. 14349).
References
- Al-Timman JKA, Drinkhill MJ, Hainsworth R. Reflex responses to stimulation of mechanoreceptors in the left ventricle and coronary arteries in anaesthetized dogs. Journal of Physiology. 1993;472:769–783. doi: 10.1113/jphysiol.1993.sp019972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour JA. Peripheral autonomic neuronal interactions in cardiac regulation. In: Armour JA, Ardell JL, editors. Neurocardiology. New York and Oxford: Oxford University Press; 1994. pp. 219–244. [Google Scholar]
- Bennett JA, Goodchild CS, Kidd C, McWilliam PN. Neurones in the brainstem of the cat excited by vagal afferent fibres from the heart and lungs. Journal of Physiology. 1985;369:1–15. doi: 10.1113/jphysiol.1985.sp015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JA, Goodchild CS, Kidd C, McWilliam PN. Inhibition of brain stem neuronal activity by cardiac and pulmonary vagal afferent fibres in the cat. Quarterly Journal of Experimental Physiology. 1988;73:959–972. doi: 10.1113/expphysiol.1988.sp003230. [DOI] [PubMed] [Google Scholar]
- Block AJ, Poole S, Vane JR. Modification of basal release of prostaglandins from rabbit isolated hearts. Prostaglandins. 1974;7:473–486. doi: 10.1016/0090-6980(74)90092-6. [DOI] [PubMed] [Google Scholar]
- Brown AM. Mechanoreceptors in or near the coronary arteries. Journal of Physiology. 1965;177:203–214. doi: 10.1113/jphysiol.1965.sp007586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagnat J, Denavit-Saubie M, Grant K, Shen KF. Organization of synaptic transmission in the mammalian solitary complex, studied in vitro. Journal of Physiology. 1986;381:551–573. doi: 10.1113/jphysiol.1986.sp016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG, Kidd C. Cardiac receptors in the dog, with particular reference to two types of afferent ending in the ventricular wall. Journal of Physiology. 1964;174:323–329. doi: 10.1113/jphysiol.1964.sp007490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp AJ, Tutt SM, McGregor KH, Hainsworth R. The effects of changes in left ventricular pressure on respiratory activity in anaesthetized dogs. Quarterly Journal of Experimental Physiology. 1989;74:291–300. doi: 10.1113/expphysiol.1989.sp003272. [DOI] [PubMed] [Google Scholar]
- Daly M de Burgh. Some cardioinhibitory responses in the cat and their modulation by central inspiratory neuronal activity. Journal of Physiology. 1991;439:559–577. doi: 10.1113/jphysiol.1991.sp018682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid-Milner MS, Silva-Carvalho L, Goldsmith GE, Spyer KM. Hypothalamic modulation of laryngeal reflexes in the anaesthetized cat: role of the nucleus tractus solitarii. Journal of Physiology. 1995;487:739–749. doi: 10.1113/jphysiol.1995.sp020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue S, Fox RE, Kidd C, Koley BN. The distribution in the cat brain stem of neurones activated by vagal non-myelinated fibres from the heart and lungs. Quarterly Journal of Experimental Physiology. 1981;66:391–404. doi: 10.1113/expphysiol.1981.sp002582. [DOI] [PubMed] [Google Scholar]
- Drinkhill MJ, Moore J, Hainsworth R. Afferent discharges from coronary arterial and ventricular receptors in anaesthetized dogs. Journal of Physiology. 1993;472:785–799. doi: 10.1113/jphysiol.1993.sp019973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta BN, Thames MD. Behavior of left ventricular mechanoreceptors with myelinated and nonmyelinated afferent vagal fibres in cats. Circulation Research. 1983;52:291–301. doi: 10.1161/01.res.52.3.291. [DOI] [PubMed] [Google Scholar]
- Hainsworth R. Reflexes from the heart. Physiological Reviews. 1991;71:617–658. doi: 10.1152/physrev.1991.71.3.617. [DOI] [PubMed] [Google Scholar]
- Hines T, Toney GM, Mifflin SW. Responses of neurons in the nucleus tractus solitarius to stimulation of heart and lung receptors in the rat. Circulation Research. 1994;74:1188–1196. doi: 10.1161/01.res.74.6.1188. [DOI] [PubMed] [Google Scholar]
- Jones JFX, Wang Y, Jordan D. Dorsal medullary neurones activated by stimulation of the cardiac vagal branch of the anaesthetized rat, and their behaviour during the pulmonary chemoreflex. Journal of Physiology. 1995;483.P:89–90P. [Google Scholar]
- Kalia M, Mesulam M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II Laryngeal, tracheobronchial, pulmonary, cardiac and gastrointestinal branches. Journal of Comparative Neurology. 1980;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Baker DG, Coleridge HM, Coleridge JCG. Stimulation by bradykinin of afferent vagal C-fibres with chemosensitive endings in the heart and aorta of the dog. Circulation Research. 1980;46:476–484. doi: 10.1161/01.res.46.4.476. [DOI] [PubMed] [Google Scholar]
- Lipski J, McAllen RM, Trzebski A. Carotid baroreceptor and chemoreceptor inputs onto single medullary neurones. Brain Research. 1976;107:132–135. doi: 10.1016/0006-8993(76)90101-3. 10.1016/0006-8993(76)90101-3. [DOI] [PubMed] [Google Scholar]
- McGregor KH, Hainsworth R, Ford R. Hindlimb vascular responses in anaesthetised dogs to aortic root injections of veratridine. Quarterly Journal of Experimental Physiology. 1986;71:577–587. doi: 10.1113/expphysiol.1986.sp003018. [DOI] [PubMed] [Google Scholar]
- McMahon NC, Drinkhill MJ, Hainsworth R. Vascular responses to stimulation of carotid, aortic and coronary artery baroreceptors with pulsatile and non-pulsatile pressures in anaesthetized dogs. Experimental Physiology. 1996;81:969–981. doi: 10.1113/expphysiol.1996.sp003997. [DOI] [PubMed] [Google Scholar]
- Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. American Journal of Physiology. 1992;263:R368–375. doi: 10.1152/ajpregu.1992.263.2.R368. [DOI] [PubMed] [Google Scholar]
- Öberg B, Thorén P. Studies on left ventricular receptors, signalling in non-medullated vagal afferents. Acta Physiologica Scandinavica. 1972;85:145–163. doi: 10.1111/j.1748-1716.1972.tb05246.x. [DOI] [PubMed] [Google Scholar]
- Pasipoularides A, Murgo JP, Bird JJ, Craig WE. Fluid dynamics of aortic stenosis: mechanisms for the presence of subvalvular pressure gradients. American Journal of Physiology. 1984;246:H542–550. doi: 10.1152/ajpheart.1984.246.4.H542. [DOI] [PubMed] [Google Scholar]
- Paton JFR. Convergence characteristics of solitary tract neurones receiving cardiac receptor inputs revealed in a working heart-brainstem preparation of the mature mouse. Journal of Physiology. 1996a;497.P:76P. [Google Scholar]
- Paton JFR. A working heart-brainstem prepartion of the mouse. Journal of Neuroscience Methods. 1996b;65:63–68. doi: 10.1016/0165-0270(95)00147-6. 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JFR. The ventral respiratory network of the mature mouse studied in a working heart-brainstem preparation. Journal of Physiology. 1996c;493:819–831. doi: 10.1113/jphysiol.1996.sp021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR, Butcher JW. Cardiorespiratory reflexes in mice. Journal of the Autonomic Nervous System. 1998 doi: 10.1016/s0165-1838(97)00125-2. in the Press. [DOI] [PubMed] [Google Scholar]
- Persson P, Ehmke H, Kirchheim H, Seller H. Effect of sino-aortic denervation in comparison to cardipulmonary deafferentation on long-term blood pressure in conscious dogs. Pflügers Archiv. 1988;411:160–166. doi: 10.1007/BF00582309. [DOI] [PubMed] [Google Scholar]
- Rogers RF, Paton JFR, Schwaber JS. NTS neuronal responses to arterial pressure and pressure changes in the rat. American Journal of Physiology. 1993;265:R1355–1368. doi: 10.1152/ajpregu.1993.265.6.R1355. [DOI] [PubMed] [Google Scholar]
- Sleight P, Lall A, Muers MF. Reflex cardiovascular effects of epicardial stimulation by acetylstrophanthindin in dogs. Circulation Research. 1969;25:705–711. doi: 10.1161/01.res.25.6.705. [DOI] [PubMed] [Google Scholar]
- Sleight P, Widdicombe JG. Action potentials in fibres from receptors in the epicardium and myocardium of the dog's left ventricle. Journal of Physiology. 1965;181:235–258. doi: 10.1113/jphysiol.1965.sp007758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Thames MD. Cardiac receptors: Discharge characteristics and reflex effects. In: Armour JA, Ardell JL, editors. Neurocardiology. New York and Oxford: Oxford University Press; 1994. pp. 19–52. [Google Scholar]
- Spyer KM. Central nervous mechanisms contributing to cardiovascular control. Journal of Physiology. 1994;474:1–19. doi: 10.1113/jphysiol.1994.sp019997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutt SM, McGregor KH, Hainsworth R. Reflex vascular responses to changes in left ventricular pressure in anaesthetized dogs. Quarterly Journal of Experimental Physiology. 1988;73:425–437. doi: 10.1113/expphysiol.1988.sp003158. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Schultz HD. Activation of cardiac vagal afferents in ischemia and reperfusion. Circulation Research. 1994a;74:904–911. doi: 10.1161/01.res.74.5.904. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Schultz HD. Activation of cardiac vagal afferents by oxygen-derived free-radicals in rats. Circulation Research. 1994b;74:895–903. doi: 10.1161/01.res.74.5.895. [DOI] [PubMed] [Google Scholar]
- Wilson CG, Zhang Z, Bonham AC. Non-NMDA receptors transmit cardiopulmonary C fibre input in nucleus tractus solitarii of rats. Journal of Physiology. 1996;496:773–785. doi: 10.1113/jphysiol.1996.sp021726. [DOI] [PMC free article] [PubMed] [Google Scholar]


