Abstract
The properties of the 5-HT-sensitive K+ conductance of neonatal rat facial motoneurones were examined in brainstem slices using whole-cell patch-clamp techniques.
In a small proportion of motoneurones, 5-hydroxytryptamine (5-HT) evoked an inward current mediated solely by a decrease in K+ conductance. The reversal potential (V5-HT) was dependent on the external K+ concentration and the 5-HT-evoked current (I5-HT) displayed a linear current–voltage (I–V) relationship.
In the remaining motoneurones, the 5-HT-evoked decrease in K+ conductance could only be observed in isolation once a concomitant 5-HT-mediated enhancement of the hyperpolarization-activated current, Ih, had been abolished with the Ih blocker, ZD-7288.
External Cs+ also abolished the Ih-mediated component of I5-HT but, in addition, blocked part of the 5-HT-sensitive K+ current. At potentials hyperpolarized to V5-HT, Cs+ voltage dependently blocked I5-HT while at potentials depolarized to V5-HT, I5-HT was largely unaffected. Ba2+ and Rb+ had identical actions to Cs+ on the 5-HT-sensitive K+ current.
The Ba2+-, Rb+- and Cs+-sensitive component of the 5-HT-sensitive K+ current inwardly rectified with a reversal potential that was dependent on the K+ equilibrium potential (EK).
Replacing external Na+ with N-methyl-D-glucamine, blocking Ca2+ entry, or preventing an increase in intracellular [Ca2+] with BAPTA, all failed to alter I5-HT at potentials depolarized to EK.
I5-HT at depolarized potentials was reversibly blocked by 4-aminopyridine (4 mm) but not tetraethylammonium chloride (30 mm) and did not show inactivation during depolarizing voltage pulses (1.5 s duration).
The results suggest that, in addition to enhancing Ih, 5-HT modulates two distinct K+ conductances in neonatal rat facial motoneurones. The actions of Cs+, Ba2+ and Rb+ support the involvement of a member of the inwardly rectifying family of K+ channels while the other K+ channel may belong to the voltage-gated family.
5-Hydroxytryptamine (5-HT, serotonin) is widespread in the mammalian central nervous system and its cellular actions are mediated by up to fourteen distinct, cell membrane-located, receptor subtypes (Hoyer & Martin, 1997). Release of 5-HT leads to either inhibition or excitation depending on the neurone type and the receptor subtype(s) activated. Several 5-HT receptors mediate a change in neuronal excitability by modulating K+ channel activity. In many central neurones, 5-HT, acting through a 5-HT1A receptor, activates an inwardly rectifying K+ current (IK(ir)) leading to hyperpolarization and inhibition (reviewed by Anwyl, 1990; Penington, Kelly & Fox, 1993; Oh, Ho & Kim, 1995). Conversely, a reduction in a resting K+ conductance has been implicated in some of the excitatory actions of 5-HT. In nucleus accumbens neurones, reduction of IK(ir), through a 5-HT2 receptor, led to depolarization and an increase in excitability (North & Uchimura, 1989). Elsewhere, however, the nature of the K+ conductance inhibited by 5-HT has rarely been characterized.
Among the many neurones that receive dense synaptic contacts from 5-HT-containing cells are motoneurones of the cranial nerve motor nuclei (Takeuchi, Kojima, Matsuura & Sano, 1983). The consensus view is that 5-HT acts in a predominantly excitatory fashion on motoneurones and that this contributes, in vivo, to a tonic facilitatory regulation of motoneuronal excitability (Jacobs & Fornal, 1993). In vitro, exogenously applied 5-HT depolarizes motoneurones in the facial, hypoglossal and spinal motor nuclei of adult and neonatal rats (Aghajanian & Rasmussen, 1989; Larkman, Penington & Kelly, 1989; Takahashi & Berger, 1990; Wang & Dun, 1990; Berger, Bayliss & Viana, 1992; Elliott & Wallis, 1992). The 5-HT-evoked depolarization of adult rat facial motoneurones can involve two interacting and reinforcing ionic mechanisms (Larkman & Kelly, 1992; Garratt, Alreja & Aghajanian, 1993). 5-HT inhibits a resting, or ‘leak’, K+ current (IK(leak)) and enhances the hyperpolarization-activated, cationic inward rectifier, Ih.
In neonatal rat facial and spinal motoneurones a 5-HT-evoked inward current mediated by an increase in conductance and probably due to an enhancement of Ih has been characterized in detail (Takahashi & Berger, 1990; Larkman, Kelly & Takahashi, 1995; Larkman & Kelly, 1997). In addition, both neonatal spinal and facial motoneurones possess a second, distinct, component of 5-HT-evoked outward current in high external K+ solutions, assumed to be a manifestation of the decrease in IK(leak) seen in adult motoneurones (Takahashi & Berger, 1990; Larkman & Kelly, 1995). In both adult and neonatal facial motoneurones the conductance increase associated with the 5-HT-mediated enhancement of Ih dominates the overall response at hyperpolarized potentials making it difficult to characterize the 5-HT-sensitive K+ conductance in isolation.
Recently, a novel bradycardic agent, ZD-7288 (4-(N-ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino) pyrimidinium chloride), has been shown to selectively block If, a hyperpolarization-activated current regarded as the cardiac cell equivalent of neuronal Ih (BoSmith, Briggs & Sturgess, 1993). In this study, we have used ZD-7288 to help isolate the 5-HT-sensitive K+ conductance of neonatal rat facial motoneurones and thereby undertake a more detailed characterization of its properties. Our results with a range of K+ channel blockers suggest that the 5-HT-sensitive K+ conductance may be the result of either 5-HT modulating more than one type of K+ channel or modulation of a single channel type with properties different from the range of characterized K+ channels. Some of this work has been presented in preliminary form (Larkman & Kelly, 1996).
METHODS
Slice preparation and recording methods were similar to those described previously (Larkman et al. 1995). Male or female rats, 3–14 days old, were decapitated without anaesthesia. The hindbrain was rapidly isolated and placed in ice-cold (∼4°C) artificial cerebrospinal fluid (ACSF) containing (mm): NaCl, 57; sucrose, 114; KCl, 3; NaH2PO4, 1; NaHCO3, 25; D-glucose, 11; CaCl2, 1; MgCl2, 5; lactate, 4. The pH of the solution was 7.4 when continuously bubbled with a 95 % oxygen, 5 % carbon dioxide gas mixture. The cerebellum was then removed and the brainstem mounted on the cutting stage of a tissue slicer (DTK-1000; Dosaka Co., Kyoto, Japan) where it was supported by a block of agar (2 % w/v). Slices were cut at approximately 120 μm thickness and incubated at 30°C for 60 min in a total volume of 50 ml ACSF. During this time the sucrose in the ACSF was slowly replaced by exchanging the ACSF, at a rate of ∼1 ml min−1, with ACSF in which sucrose was replaced by NaCl (57 mm).
Prior to recording, the slices were transferred to a maintaining chamber and perfused at room temperature (∼23°C) with the same ACSF but with [MgCl2] lowered to 1 or 2 mm and [CaCl2] raised to 2 mm. Individual slices were transferred, as required, to a recording chamber and continuously perfused (3–5 ml min−1) with standard ACSF without lactate. Motoneurones were visualized using a water-immersion objective lens and Nomarski differential interference optics. The surface of a motoneurone was cleared of debris and connective tissue prior to introduction of a recording pipette into the chamber. Recording pipettes were made from thin-walled borosilicate glass capillaries (Clark Electromedical; o.d., 1.5 mm) using a patch-pipette puller (Narishige PP-83). The patch pipette was coated with Sylgard to reduce capacitance. The normal internal solution was (mm): potassium gluconate, 122.5; KCl, 17.5; NaCl, 9; MgCl2, 1; EGTA, 0.2; Hepes, 10; guanosine 5′-triphosphate (sodium salt; GTP), 0.3; adenosine 5′-triphosphate (magnesium salt; ATP), 3; neutralized to pH 7.3 with KOH (4 mm). Occasionally this was diluted to 90 % to improve maintenance of the cell condition. When BAPTA was included, the internal solution was modified to (mm): potassium gluconate, 42.5; KCl, 17.5; NaCl, 9; MgCl2, 1; K4BAPTA, 20; Hepes, 10; sucrose, 60; GTP, 0.3; ATP, 3; neutralized to pH 7.3. A gigaohm seal was obtained in the cell-attached configuration and compensation of the stray capacitance was performed prior to rupture of the patch membrane for whole-cell recording. Series resistance (range, 14–25 MΩ) was monitored with the current response to a repetitive voltage step (−10 mV, 20 ms) and was compensated up to 70 % if necessary. All recorded potentials using standard internal solution were corrected for a junction potential of 8 mV. Whole-cell currents were recorded with a patch-clamp amplifier (EPC 7B; List-Medical, Germany) filtered at 10 kHz and stored on a digital audiotape recorder (Biologic DTR-1204; Intracel, Royston, UK). Off-line analysis was performed using a CED1401 Plus interface (Cambridge Electronic Design Ltd (CED), Cambridge, UK), personal computer and patch- and voltage-clamp software v6.0 (CED). Membrane current responses to voltage steps were digitized at 1–3 kHz depending on signal speed while those evoked by voltage ramps were digitized at 0.2–1 kHz.
Unless otherwise stated, whole-cell recordings were obtained in ACSF from which Ca2+ had been removed and [Mg2+] raised to 5 mm to minimize trans-synaptic actions of applied drugs and prevent activation of Ca2+-activated K+ channels. In some experiments [Ca2+] was lowered to 0.5 mm and Mn2+ (2 mm) included to fulfil the same aims. Tetrodotoxin (TTX; 0.3 μm) was routinely included in all external solutions to block voltage-gated Na+ channels. Changes in the external K+ concentration and the addition of Cs+, tetraethylammonium chloride (TEA) or 4-aminopyridine (4-AP) were compensated for by equimolar alterations in the external Na+ concentration. When Rb+ was included in the ACSF it replaced equimolar K+ and when N- methyl-D-glucamine chloride (NMDG) was included in the ACSF it replaced external NaCl. 5-HT was routinely bath applied at 5 or 10 μm. These concentrations are nearly maximal in the dose-response curve (Takahashi & Berger, 1990; Larkman & Kelly, 1995). Conductance changes were measured using either voltage step pulses of varying amplitude or ramp pulses (−40 to +70 mV from the holding potential, 2 s duration). Drugs were applied in the superfusing ACSF unless otherwise stated.
4-AP, 5-hydroxytryptamine HCl (5-HT), BAPTA, barium chloride, caesium chloride, NMDG, rubidium chloride, TEA and TTX were all from Sigma. ZD-7288 was from Tocris Cookson (Bristol, UK).
Results are given as means ±s.e.m.
RESULTS
Whole-cell recordings were obtained from 212 visually identified facial motoneurones in neonatal rat brainstem slices in vitro. The mean input conductance of a representative sample, obtained from the slope of the linear portion of the current–voltage (I–V) relationship measured before the onset of activation of Ih, was 7 ± 0.4 nS at −58 mV in ACSF containing 3 mm K+ (holding current, 7 ± 10 pA; n= 40).
Effects of 5-HT on neonatal rat facial motoneurones
Bath application of 5-HT (5 or 10 μm), to facial motoneurones voltage clamped at potentials between −58 and −80 mV in 3 mm K+ ACSF, predominantly evoked an inward current (98 % of motoneurones tested, n= 130; Fig. 1Aa). This response was reproducible on repeated 5-HT application and showed little run-down over the course of whole-cell recording (∼60 min; Figs 2C and 10B). In the majority of facial motoneurones, depolarizing and hyperpolarizing step voltage commands evoked current responses that indicated a decrease in conductance was associated with the 5-HT-evoked inward current (49/55; Fig. 1B). 5-HT-evoked current (I5-HT) at different potentials could be observed by subtraction of I–V relationships obtained in the absence of 5-HT from those produced in its presence (Fig. 1C). In a small proportion of facial motoneurones (27 %, 15/55), I5-HT varied linearly with voltage and reversed polarity close to the predicted K+ equilibrium potential (EK) in 3, 7 and 12 mm K+ ACSF (EK(3 mm)= −99.5 mV, reversal potential of I5-HT (V5-HT) = −95 ± 1.6 mV, n= 15; EK(7 mm)= −74 mV, V5-HT= −72 mV, n= 2; EK(12 mm)= −61 mV, V5-HT= −58.7 ± 1.5 mV, n= 12; Fig. 1C). Thus, in this subset of neonatal facial motoneurones, the 5-HT-evoked current can be attributed solely to a decrease in K+ conductance as described in adult rat facial motoneurones (Larkman & Kelly, 1992).
Figure 1. Properties of the 5-HT-evoked current (I5-HT) in neonatal rat facial motoneurones.
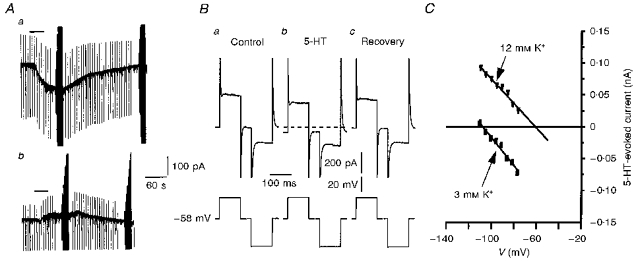
A, chart records showing the response of an 11-day-old facial motoneurone voltage clamped at −80 mV to bath application of 5-HT (10 μm, 30 s, horizontal bar) in 3 mm K+ ACSF (a) and 12 mm K+ ACSF (b). Holding current was −217 pA in a and −544 pA in b. Vertical deflections reflect the current responses to repetitive −10 mV, 200 ms voltage steps. At the peak of, and after recovery from, each 5-HT-evoked response, voltage commands of varying amplitude were imposed to allow measurement of the 5-HT-evoked current at different potentials. B, representative whole-cell current responses (upper traces) to voltage step commands (lower traces) obtained before (a), during (b) and after (c) 5-HT application to a 10-day-old facial motoneurone clamped at −60 mV in 3 mm K+ ACSF. The dashed line represents the control holding current level of 99 pA. C, plots of I5-HT at different potentials for the chart records shown in A. In 3 mm K+ (▪) I5-HT was linear with a reversal potential (V5-HT) of −106 mV. In 12 mm K+ (▾) I5-HT was also linear and the V5-HT determined by linear extrapolation was −61 mV.
Figure 2. 5-HT evoked an inward current that did not reverse at EK.
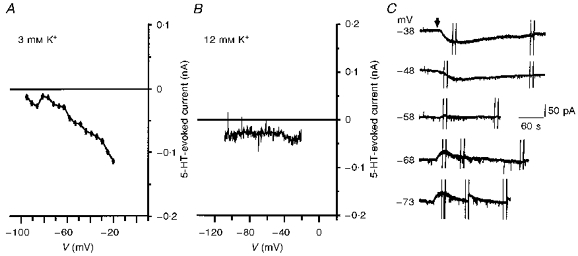
A, plot of I5-HT at different potentials from a 10-day-old facial motoneurone in 3 mm K+ ACSF. Note the deviation of the current from linearity at negative potentials and its failure to reverse polarity. B, 5-HT still evoked an inward current when the external [K+] was increased to 12 mm. Records obtained from an 8-day-old facial motoneurone voltage clamped at −70 mV. Note that I5-HT was inward over the whole voltage range tested. C, chart records from a 6-day-old facial motoneurone voltage clamped at the indicated potentials in 12 mm K+ ACSF. Bath application of 5-HT (10 μm, 10 s, arrow) evoked an inward current at potentials depolarized to EK (−61 mV). At potentials negative to EK, 5-HT evoked an outward current that decayed more rapidly as a slower inward current developed. The outward current was abolished close to −58 mV. 5-HT was applied at 6 min intervals.
Figure 10. Actions of 4-AP on I5-HT.
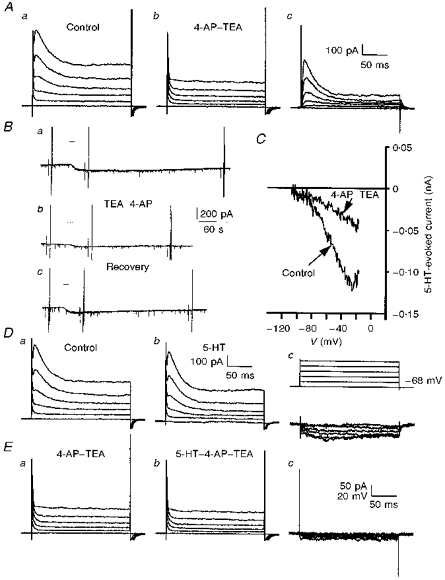
A, superimposed whole-cell current records obtained using depolarizing voltage steps shown in Dc (upper traces) prior to (a), and during (b), application of 4-AP (4 mm) with TEA (30 mm). Subtraction of the records in a and b gave the 4-AP-TEA-sensitive current (c). Note the block of both a transient and sustained outward component of current by 4-AP–TEA. Holding current was −111 pA before and −132 pA during 4-AP–TEA application. B, chart records showing the effects of bath application of 5-HT (10 μm, 10 s, horizontal bar, 8 min intervals) before (a), during (b) and after (c) application of 4-AP–TEA. 5-HT evoked a control inward current of −74 pA which was reduced to −32 pA in 4-AP–TEA and recovered to −84 pA after washout of the channel blockers. Holding current was −46, −98 and −54 pA in a, b and c, respectively. C, superimposed plots of I5-HT in the absence and presence of 4-AP–TEA taken from the same records as in B. Control is an average of I5-HT derived from Ba and c. D, whole-cell current records obtained as described in A, in the absence (a) and presence (b) of 5-HT (10 μm). Dc, 5-HT-evoked current (lower traces) obtained by subtraction of the records in a and b. Note the lack of a transient component to the 5-HT-evoked current even at potentials at which the transient outward current was activated. E, whole-cell current records obtained in the presence of 4-AP–TEA before (a) and during (b) application of 5-HT (10 μm). Ec shows that the subtracted 5-HT-evoked currents were greatly reduced in the presence of 4-AP. All recordings were obtained from the same 9-day-old facial motoneurone in 3 mm K+ ACSF, voltage clamped at −68 mV in A, D and E, and at −58 mV in B and C.
In the remaining facial motoneurones the 5-HT-evoked current did not reverse polarity at the predicted EK in 3 mm K+ ACSF (62 %, 34/55). This indicated that the overall decrease in whole-cell conductance was less than would be expected for a mechanism involving K+ channel closure alone (Fig. 2A). In these motoneurones, raising external [K+] to 12 mm and clamping at potentials hyperpolarized to EK resulted in 5-HT evoking either a small outward current followed by an inward current (30/75; Fig. 2C), or only an inward current (24/75; Figs 2B and 3Aa). The inward current has previously been suggested to be mediated by an enhancement of the hyperpolarization-activated current, Ih, in both adult (Larkman & Kelly, 1992) and neonatal rat motoneurones (Takahashi & Berger, 1990; Larkman et al. 1995). The outward current might be a manifestation of the K+-mediated event observed in isolation above (Fig. 1) as the net flow of K+ at this holding potential would be inward. In support of this idea we observed that the outward component was abolished at potentials close to the EK (Fig. 2C). Nevertheless, under these conditions, conductance changes associated with the two components of 5-HT-evoked current were difficult to quantify. We suspected that this may be the result of a temporal overlap of the two, distinct, 5-HT-induced conductance changes and thus attempted to isolate the effects of 5-HT on the K+ conductance by blocking Ih.
Figure 3. Properties of I5-HT in the presence of ZD-7288.
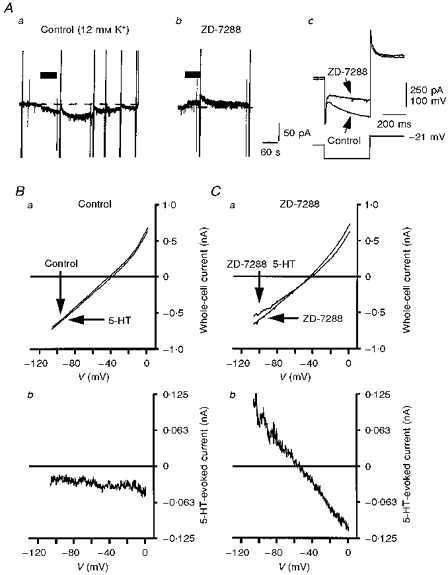
A, chart records showing the response of an 8-day-old facial motoneurone voltage clamped at −68 mV in 12 mm K+ ACSF to bath application of 5-HT (10 μm, 60 s, horizontal bar) before (a), and during (b), superfusion with ZD-7288 (10 μm). Holding current was −304 pA in a and −276 pA in b. Vertical deflections are attenuated current responses to various voltage commands. Note that the 5-HT-evoked inward current observed prior to ZD-7288 application was replaced by an outward current in its presence. Ac, superimposed current responses (upper traces) evoked by a voltage command protocol (lower trace) before, and in the presence of, ZD-7288. Note the selective block of Ih over the currents evoked by the depolarizing component of the command voltage. Holding current was −26 pA before and −6 pA after addition of ZD-7288. B and C, current-voltage (I–V) relationships, generated using a voltage ramp protocol (see Methods), obtained before and during 5-HT application in the absence (Ba) and the presence (Ca) of ZD-7288 (3 μm). Subtracted I5-HT in the absence and presence of ZD-7288 (3 μm) is shown in Bb and Cb, respectively. All records shown in B and C are taken from the same 7-day-old facial motoneurone voltage clamped at −68 mV in 12 mm K+ ACSF. Note that in the absence of ZD-7288, I5-HT was inward over the voltage range tested while in its presence, I5-HT was linear over the whole voltage range with a clear reversal potential at −58 mV.
Effects of ZD-7288 on 5-HT-induced modulation of facial motoneurone excitability
We examined the actions of ZD-7288 on facial motoneurones and their response to 5-HT. Bath application of ZD-7288 (1–10 μm) led to a time- (10–20 min) and concentration-dependent block of Ih that could not be reversed within the normal lifetime of whole-cell recording (up to 90 min). The block was specific for Ih over currents generated by depolarizing voltage commands (Fig. 3Ac). At holding potentials of −68 to −70 mV, within the activation range of Ih, ZD-7288 evoked a slow outward current (45 ± 15 pA) and a decrease in conductance (10.1 ± 1.2 to 8.6 ± 1 nS, n= 8).
Figure 3Aa shows a chart recording from a facial motoneurone voltage clamped at −68 mV in 12 mm K+ ACSF. 5-HT evoked an inward current associated with a rightward shift in the whole-cell I–V relationship (Fig. 3Aa and Ba). The subtracted I5-HT was inward over the whole voltage range but indicated a small, 5-HT-induced decrease in conductance (Fig. 3Bb). After block of Ih by ZD-7288, bath-applied 5-HT, to the same facial motoneurone, evoked an outward current (Fig. 3Ab). In the presence of ZD-7288 the 5-HT-evoked outward current was associated with a clear decrease in slope conductance (8.9 ± 0.8 to 7.6 ± 0.7 nS, n= 6) and intersected with the control I–V plot at −55.3 ± 1.9 mV (n= 6), close to the predicted EK (Fig. 3Ca). The subtracted I5-HT was linear over the voltage range −20 to −110 mV (Fig. 3Cb). At −30 mV, inward I5-HT was −33.8 ± 7.7 pA and at −90 mV, outward I5-HT was 49.6 ± 11.9 pA (n= 6).
The selective block of Ih by ZD-7288 isolates the 5-HT-evoked outward current confirming mediation through a decrease in K+ conductance. Furthermore, it unequivocally asserts that modulation of Ih mediates the 5-HT-evoked inward current seen at this holding potential in 12 mm K+ ACSF.
Differential effect of Cs+ on 5-HT-induced modulation of facial motoneurone excitability
In common with other cell types, bath application of Cs+ (5 mm) completely inhibited the hyperpolarization-activated current of neonatal rat facial motoneurones (Fig. 4A, inset). In contrast with the action of ZD-7288 on Ih, the effects of Cs+ were both rapid in onset (30 s) and fully reversible (not shown). In addition, a large component of instantaneous current, seen in response to hyperpolarizing voltage steps, was also blocked. It has been shown previously that Cs+ also blocks the 5-HT-induced inward current seen at hyperpolarized potentials in high external K+ ACSF (Takahashi & Berger, 1990; Larkman et al. 1995). We therefore compared the action of 5-HT on facial motoneurones in the presence of Cs+ with that seen in the presence of ZD-7288.
Figure 4. Actions of external Cs+ on I5-HT.
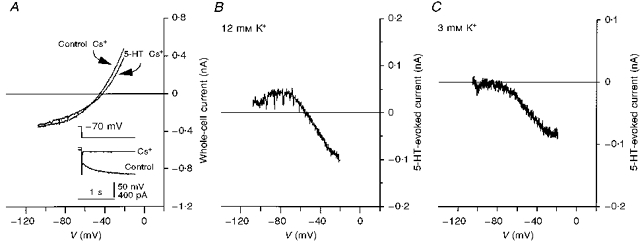
A, I–V relationships, obtained from an 8-day-old facial motoneurone voltage clamped at −70 mV in ACSF containing 12 mm K+ and 5 mm Cs+ in the presence (5-HT-Cs+) and absence (Control-Cs+) of 5-HT. Inset, superimposed whole-cell current responses (lower traces) from the same facial motoneurone evoked by −40 mV, 1.5 s voltage commands (upper trace) before (Control) and in the presence of Cs+. Note the block of both Ih and a large component of instantaneous current. Holding current was -299 pA before, and -230 pA after, the addition of Cs+. B, plot of I5-HT obtained by subtraction of the plots in A. Note the linearity of the current at depolarized potentials and the pronounced deviation at potentials negative to the reversal potential (V5-HT= −61 mV). C, I5-HT obtained from a 5-day-old facial motoneurone voltage clamped at −58 mV in ACSF containing 3 mm K+ and 5 mm Cs+. Note that I5-HT was abolished close to −80 mV and that no outward I5-HT was observed.
Figure 4A and B, respectively, shows whole-cell I–V relationships and the subtracted I5-HT from a facial motoneurone voltage clamped at −70 mV in 12 mm K+ ACSF containing Cs+ (5 mm). Previously, in the absence of Cs+, 5-HT had evoked a parallel shift in the I–V relationship and the subtracted I5-HT was inward over the whole voltage range (Fig. 2B). In the presence of Cs+, 5-HT decreased the slope of the I–V relationship and a clear point of intersection close to the predicted EK was observed (Fig. 4A). The mean V5-HT obtained in the presence of Cs+ was -54.9 ± 1.3 mV (n= 9). Examination of I5-HT in the presence of Cs+ showed that, at potentials depolarized to V5-HT, I5-HT varied linearly with voltage. However, at potentials negative to V5-HT, I5-HT deviated from linearity resulting, overall, in an outwardly rectifying relationship (Fig. 4B). At −30 mV, I5-HT was -35.2 ± 7.8 pA while at −90 mV, it was 11.1 ± 4.8 pA (n= 8). This decreased further to 5.4 ± 2.8 pA at −110 mV (n= 7). In the absence of Cs+, I5-HT at −90 mV was 51.4 ± 6.3 pA (n= 18, pooled data ± ZD-7288) and 73.6 ± 10.3 pA at −110 mV (n= 12).
These results indicate that, in addition to abolishing 5-HT-mediated enhancement of Ih, Cs+ also, voltage dependently, blocked a component of the 5-HT-sensitive K+ conductance. The inhibition of outward 5-HT-evoked, whole-cell current, at potentials negative to EK, reflects a block of K+ influx, through 5-HT-sensitive K+ channels, by Cs+. At potentials depolarized to EK, K+ efflux is apparently unaffected by Cs+ thus leaving modulation by 5-HT, and hence inward 5-HT- evoked current, largely unaltered.
Cs+ had essentially the same action on I5-HT in 3 mm K+ ACSF. Outward current was blocked while inward current was largely unaltered (Fig. 4C). Interestingly, in the presence of Cs+, a value of −82 ± 1.3 mV (n= 9) was obtained for V5-HT, more positive than the predicted EK and significantly different from the V5-HT in the absence of Cs+ (P < 0.001, Student's t test).
The actions of barium and rubidium
Cs+, in addition to blocking Ih, also blocks inwardly rectifying K+ (Kir) channels. Ba2+ ions selectively and potently blocked IK(ir) without affecting Ih (Fig. 5D, inset). Addition of Ba2+ (100 μm to 2 mm) to facial motoneurones clamped at −68 to −70 mV in 12 mm K+ ACSF led to a marked reduction in conductance at hyperpolarized potentials with less effect at depolarized potentials (Fig. 5A). The subtracted Ba2+-sensitive current showed marked inward rectification and reversed at −53.2 ± 1.5 mV (n= 10), close to the predicted EK (Fig. 5B). In 7 mm K+ ACSF, the Ba2+- sensitive current reversed at −77 mV; however, as seen for V5-HT in Cs+, lowering [K+] to 3 mm resulted in a reversal potential of −80 ± 2.8 mV (n= 5) for the Ba2+-sensitive current, more positive than the predicted EK. These actions of Ba2+ were independent of the block of Ih by ZD-7288 (compare Fig. 5C and D, insets). Under conditions in which a control application of 5-HT evoked a linear I5-HT (12 mm K+ ACSF with or without ZD-7288), the presence of Ba2+ led to a block of I5-HT at potentials negative to V5-HT while at potentials depolarized to V5-HT, I5-HT was only slightly affected (Fig. 5C and D). At −30 mV, inward I5-HT was −57 ± 24 pA (97 ± 8 % of control) while at −90 mV outward I5-HT was 12 ± 9 pA (28 ± 8 % of control; n= 5). V5-HT was unaltered by the presence of Ba2+ (−58.5 ± 1.4 mV, n= 5). Altering the external K+ concentration from 12 to 7 mm shifted the reversal potential but not the block of outward compared with inward I5-HT. Thus, the combined actions of ZD-7288 and Ba2+ appeared identical to that of Cs+ alone indicating that Ba2+ ions also selectively block K+ influx through 5-HT-sensitive K+ channels. Indeed, on facial motoneurones displaying only K+ channel-mediated responses to 5-HT, the actions of Cs+ and Ba2+ were identical (n= 2, not shown).
Figure 5. Actions of Ba2+ on facial motoneurones and I5-HT.
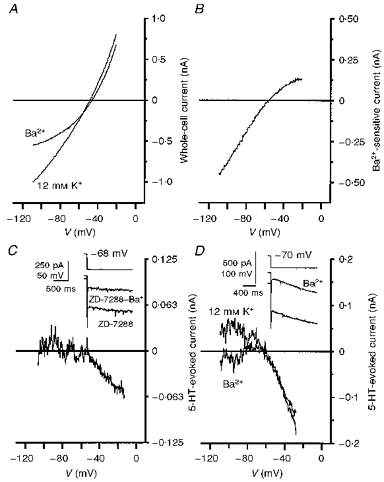
A, I–V relationships obtained using a voltage ramp command before and after the addition of Ba2+ (2 mm) to the superfusing ACSF. Records were taken from a 9-day-old facial motoneurone voltage clamped at −68 mV in 12 mm K+ ACSF. Ba2+ preferentially blocked current at negative potentials. B, plot of the Ba2+-sensitive current obtained by subtraction of the records in A. The Ba2+-sensitive current reversed at −56 mV and showed strong inward rectification suggesting block of an inwardly rectifying K+ conductance. C, I5-HT in the presence of ZD-7288 (3 μm) and Ba2+ (100 μm). Records taken from the same facial motoneurone as in Fig. 3B and C. Note that outward I5-HT, negative to V5-HT, was blocked by Ba2+. Inset, the inhibition by Ba2+, in the presence of ZD-7288, of the current response (lower traces) to a hyperpolarizing voltage command (upper trace). Holding current was -232 pA in ZD-7288 and -190 pA after addition of Ba2+. D, superimposed plots of I5-HT obtained in the absence of ZD-7288, before and after the addition of Ba2+ (2 mm). Records are from a different 9-day-old facial motoneurone maintained under the same experimental conditions as in A. As in C, Ba2+ selectively blocked the outward component of I5HT. Inset, taken from the same facial motoneurone, shows the inhibition by Ba2+ of the instantaneous current but not Ih (lower traces), evoked by a hyperpolarizing voltage command (upper trace). Holding current was -354 pA before, and -212 pA after, addition of Ba2+ at a holding potential of −70 mV.
A low permeability to Rb+ is a characteristic of some Kir channels. Replacement of external K+ (7 mm) with Rb+ (7 mm) led to an outward shift in holding current at −60 mV. Current responses to voltage steps showed a decrease in conductance, preferentially at negative potentials, in the presence of Rb+ (Fig. 6A and B). At more positive potentials, activation of the transient outward current was not affected by Rb+ (Fig. 6Aa and Ab). Longer duration hyperpolarizing pulses revealed a decrease in the amplitude of Ih in Rb+ (Fig. 6Ac). Rb+ is a less potent blocker of Ih than Cs+ and may also permeate the channel (reviewed by Pape, 1996). The I–V relationship in the presence of Rb+ intersected with the control plot at -55 ± 2.7 mV (n= 9; Fig. 6B). As seen with Ba2+ and Cs+, Rb+ selectively inhibited I5-HT at potentials more negative than V5-HT (Fig. 6C n= 4).
Figure 6. Effects of substituting Rb+ for K+ on I5-HT.
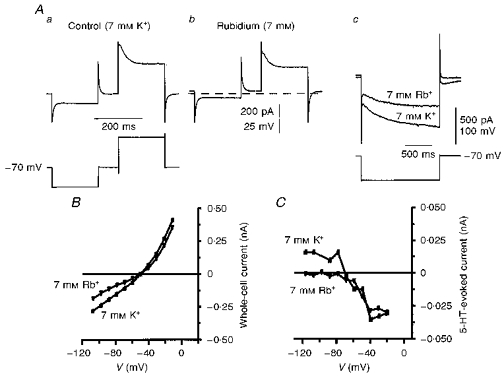
A, representative whole-cell current responses (upper traces) to voltage commands (lower traces) taken from a 7-day-old facial motoneurone voltage clamped at −70 mV in ACSF containing 7 mm K+ (a) and 7 mm Rb+ (b). Holding current was -111 pA before, and -61 pA after, the switch to Rb+. The dashed line represents the control holding current level of -111 pA. Ac, longer hyperpolarizing commands to the same facial motoneurone show the activation of Ih and its reduction after replacement of K+ with Rb+. B, I–V relationships in 7 mm K+ (▪) and 7 mm Rb+ (▾) obtained from the traces in A, by measuring current amplitude 100 ms into each pulse. Note the point of intersection at −48 mV. C, superimposed plots of I5-HT in 7 mm K+ (▪) and 7 mm Rb+ (▾) recorded from a different 7-day-old facial motoneurone voltage clamped at −58 mV. Rb+ selectively blocked I5-HT at potentials negative to V5-HT.
When the control (linear) I5-HT reversed at the EK, subtraction of I5-HT in the presence of either Cs+, Ba2+ or Rb+ gave the ion-sensitive component of I5-HT. With all three cations, the ion-sensitive I5-HT inwardly rectified (Fig. 7). The reversal potential of the ion-sensitive I5-HT was -55 ± 2.7 mV (n= 5) and -64 ± 1 mV (n= 2) in 12 and 7 mm K+ ACSF, respectively. Thus, despite the linear properties of the control I5-HT, at least part of the response to 5-HT may be attributable to an action on Kir channels.
Figure 7. Cs+, Ba2+ and Rb+-sensitive I5-HT shows inward rectification.
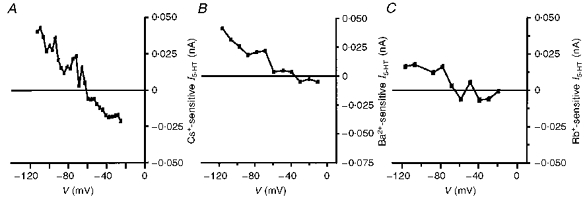
Subtraction of the I5-HT remaining in the presence of each ion from the control I5-HT gave plots of the current sensitive to block by Cs+ (5 mm; A), Ba2+ (200 μm; B) and Rb+ (7 mm; C). In all cases, the ion-sensitive current showed strong inward rectification and a reversal potential close to the predicted EK. The external K+ concentration was 12 mm in A, and 7 mm in B and C.
Effects of NMDG and Ca2+ channel blockers on the 5-HT-evoked current
As can be seen from Figs 4B and C, 5C and D and 6C, the remaining 5-HT-evoked current in the presence of Ba2+, Cs+, or Rb+ was approximately linear and tended to zero at the EK. Replacing the external Na+ with the impermeant cation NMDG failed to alter significantly the properties of this component of I5-HT, supporting a role of K+ channels in its mediation (Fig. 8A; n= 3). Including Cd2+ (500 μm; n= 3), Ni2+ (100 μm; n= 4), or Mn2+ (2 mm; n= 11) in the ACSF or removing external Ca2+ and raising [Mg2+] to 5 or 10 mm (n= 20) all failed to alter the ability of 5-HT to evoke this inward current (not shown). Likewise, including BAPTA (20 mm) in the internal pipette solution failed to alter the K+ conductance-mediated response to 5-HT up to 30 min after whole-cell access was achieved (Fig. 8B; n= 5).
Figure 8. I5-HT is not altered by intracellular BAPTA or the replacement of external Na+ with NMDG.
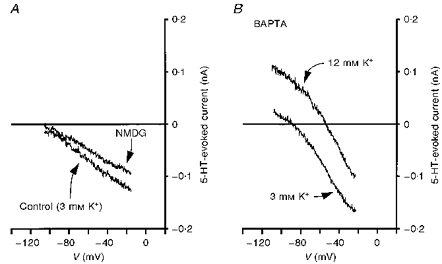
A, replacement of the external NaCl with NMDG failed to abolish I5-HT. Recording obtained from an 11-day-old facial motoneurone voltage clamped at −58 mV in 3 mm K+ ACSF. B, including BAPTA (20 mm) in the patch pipette solution failed to alter the properties of I5-HT in 3 or 12 mm K+ ACSF. Recordings were obtained from a 9-day-old facial motoneurone 10 min (3 mm K+) and 20 min (12 mm K+) after achieving the whole-cell configuration.
Effects of TEA on the 5-HT-evoked current
We further investigated the sensitivity of the inward component of I5-HT to other classes of K+ channel blocker. TEA blocks a range of K+ channel types. Addition of TEA (30 mm) to the ACSF blocked a sustained component of current activated by depolarizing voltage commands without significantly altering the transient outward current or current evoked by negative voltage steps (Fig. 9A). Subtracted I–V plots showed marked outward rectification of the TEA-sensitive current (Fig. 9B). The presence of TEA, however, failed to alter the amplitude of the 5-HT-evoked current at potentials depolarized to V5-HT (n= 7; Fig. 9Ca, Cb and D).
Figure 9. Action of TEA on I5-HT.
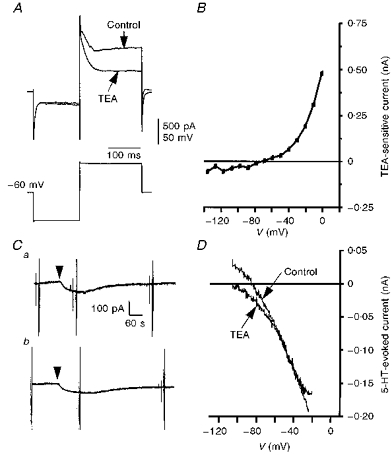
A, superimposed whole-cell current responses (upper traces) to voltage commands (lower trace) obtained in the presence and absence of TEA (30 mm). The recordings were made from a 4-day-old facial motoneurone voltage clamped at −60 mV in 3 mm K+ ACSF with nominally zero Ca+ and 5 mm Mg2+. TEA selectively inhibited a delayed component of outward current evoked by depolarizing voltage steps. B, plot of the TEA-sensitive current obtained by subtraction of records similar to those in A, and measuring current amplitude at the end of the pulse. C, chart records taken from a 10-day-old facial motoneurone voltage clamped at −58 mV in 3 mm K+ ACSF in the absence (a), and presence (b), of TEA (30 mm). Bath application of 5-HT (10 μm, 30 s, arrowhead) evoked an inward current of -57 pA in control (a) and -52 pA in TEA (b). D, superimposed plots of I5-HT in the absence and presence of TEA taken from the records illustrated in C. TEA failed to alter the inward I5-HT.
Effects of 4-AP on the 5-HT-evoked current
Addition of 4-AP (4 mm) in the continued presence of TEA (30 mm) evoked an inward current in facial motoneurones voltage clamped at −58 to −68 mV in 3 mm K+ ACSF. This application of 4-AP led to the suppression of the transient outward current evoked by a depolarizing voltage step (Fig. 10Aa and Ab). In addition, subtraction of step currents evoked in the presence of 4-AP from those in its absence identified a second sustained component of 4-AP-sensitive current that showed little inactivation over the course of the command pulse (Fig. 10Ac). Under these conditions, the 5-HT-evoked current at potentials depolarized to V5-HT was suppressed in a reversible manner by 4-AP in all but one facial motoneurone tested (n= 8; Fig. 10Ba, Bb, Bc, Dc and Ec). I–V relationships showed that 4-AP suppressed I5-HT without altering the V5-HT (-96 ± 4.5 mV, n= 6; Fig. 10C). At −30 mV, 4-AP reduced I5-HT from a control value of -110 ± 19 pA to -50 ± 14 pA (43 ± 7 % of control; n= 7). Interestingly, the block by 4-AP was more pronounced when TEA was present (33 ± 2 % of control; n= 5) than when applied alone (68 % of control; n= 2).
It is unlikely that modulation of the transient outward current contributed to the inward current evoked by 5-HT. Subtraction of current responses evoked in the absence of 5-HT from those evoked in its presence gave roughly rectangular 5-HT-evoked currents despite the presence of the transient outward current in the whole-cell records (Fig. 10Da, Db and Dc). In addition, leak subtraction of whole-cell currents before and during the application of 5-HT showed identical transient outward currents indicating that this current was not modulated by 5-HT (not shown). Thus, a sustained component of 4-AP-sensitive current is the target of modulation by 5-HT in neonatal rat facial motoneurones.
In addition to the fast transient outward current, a slowly decaying outward current could also be activated by depolarizing voltage commands after a priming hyperpolarizing prepulse (Fig. 11A). The threshold for activation of this current was more depolarized than that for the fast transient outward current (Fig. 11B). Unlike the transient outward current, the slowly decaying current was preferentially blocked by low concentrations of 4-AP (100 μm; Fig. 11C). It is unlikely that inhibition of this current mediates the actions of 5-HT at depolarized potentials for two reasons. Firstly, such low concentrations of 4-AP were ineffective against the 5-HT-evoked inward current. Secondly, the amplitude of I5-HT at depolarized potentials was independent of the priming hyperpolarizing prepulse (Fig. 11D).
Figure 11. Depolarizing voltage steps evoke fast and slowly decaying outward currents.
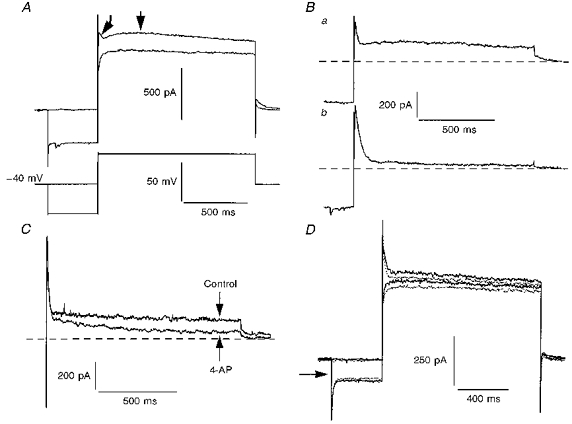
A, superimposed whole-cell current records (upper traces) obtained from a 7-day-old facial motoneurone voltage clamped at −40 mV and stepped to 0 mV for 1.2 s with or without a 400 ms prepulse to −80 mV (lower traces). Note the appearance of both fast and slowly decaying transient outward currents after the prepulse (arrows). External ACSF contained (mm): K+, 3; Ca2+, 0.5; Mg2+, 2; Mn2+, 2; and ZD-7288, 0.01. Holding current was 54 pA. Ba, subtraction of traces in A clearly shows the two components of voltage-sensitive outward current. Bb, subtracted current record obtained using the same voltage protocol as described in A but from a holding potential of −60 mV. Note only the fast transient outward current is present. Dashed lines represent the baseline current. C, superimposed, subtracted current records from a different facial motoneurone (8 days old) under the same conditions as in A. 4-AP (100 μm) preferentially suppressed the slowly decaying outward current. Holding current was 237 pA. D, superimposed whole-cell current records from another 8-day-old facial motoneurone voltage clamped at −40 mV in the presence (interupted lines) and absence (continuous lines) of 5-HT (10 μm). The amount of current inhibited by 5-HT was independent of the prepulse. Holding current was 51 pA.
DISCUSSION
The aim of this study was to characterize the K+ conductance that contributes to the 5-HT-evoked depolarization of neonatal rat facial motoneurones. ZD-7288 selectively blocked Ih in neonatal rat facial motoneurones. This allowed isolation of the 5-HT-sensitive K+ conductance in those motoneurones where 5-HT also enhanced Ih. ZD-7288 has been shown to block Ih selectively in guinea-pig substantia nigra neurones (Harris & Constanti, 1995) and hippocampal interneurones (Maccaferri & McBain, 1996). In common with those reports, the actions of ZD-7288 and Cs+, a commonly used blocker of Ih, showed some significant differences. Compared with the block of Ih by Cs+, the onset of the effects of ZD-7288 on facial motoneurones was slow and apparently irreversible. However, unlike other cell types, Cs+, but not ZD-7288, evoked an additional large decrease in instantaneous current in response to rectangular hyperpolarizing voltage commands. Cs+ is known to also block Kir channels and this observation suggests that a similar current is blocked in facial motoneurones by Cs+ but not ZD-7288.
As seen in adult rat facial motoneurones, in the absence of a block of Ih, the 5-HT-evoked current in the majority of neonatal rat facial motoneurones failed to reverse polarity at or near the EK. Evidence suggests that this is not a property of the K+ conductance itself but a result of the concomitant modulation of Ih described in detail elsewhere (Larkman et al. 1995; Larkman & Kelly, 1997). In facial motoneurones where 5-HT failed to enhance Ih, or when Ih was blocked by ZD-7288, I5-HT varied linearly with voltage and displayed a clear point of reversal at the predicted EK. Thus, the 5-HT-sensitive K+ current appears to possess a linear I–V relationship over a wide voltage range around the reversal potential similar to the IK(leak) described in adult motoneurones (Larkman & Kelly, 1992).
When Cs+ was used to block Ih the 5-HT-evoked current possessed different properties. At potentials positive to V5-HT the 5-HT-evoked current was similar to that seen in the presence of ZD-7288, varying linearly with voltage. However, at potentials negative to V5-HT, I5-HT was markedly inhibited by Cs+. Interestingly, in the presence of Cs+, while the V5-HT in 7 and 12 mm K+ ACSF was close to the predicted EK, in 3 mm K+ it was significantly different from the predicted EK. This difference was also seen in Ba2+-containing ACSF. Reasons for this are unclear but it could reflect the voltage dependence of the Cs+- and Ba2+-insensitive I5-HT or imperfect selectivity of the K+ channel. The ability of Ba2+ and Rb+ to block the same component as Cs+ of the K+-mediated, 5-HT-evoked current supports the idea that a Kir channel may be involved. Both ions have greater selectivity for Kir channels over other K+ channels and while Rb+ partially reduces Ih, this current is unaffected by Ba2+. Subtraction procedures showed prominent inward rectification in the voltage dependence of the component of I5-HT blocked by these ions and a reversal potential dependent on the external K+ concentration. This is consistent with the fact that activation of Kir channels is related to the external K+ concentration rather than only to voltage (Hille & Schwarz, 1978). Thus, the shift in the ability of these ions to block the K+ influx that is inhibited by 5-HT with changing external [K+] might be expected, if indeed a Kir channel is involved.
In other cell types, the ability of Cs+ to block IK(ir) shows voltage dependence, being more effective at hyperpolarized potentials (Hagiwara, Miyazaki & Rosenthal, 1976; Hille & Schwarz, 1978). In cultured rat nucleus basalis neurones, substance P (SP) inhibits Kir channels (Yamaguchi, Nakajima, Nakajima & Stanfield, 1990). In common with the 5-HT-sensitive K+ current in facial motoneurones, external Cs+ and Ba2+ voltage dependently blocked the SP-sensitive current at potentials negative to the reversal potential (VSP) without significantly affecting the current at potentials positive to VSP. However, unlike the 5-HT-sensitive K+ current in facial motoneurones, the SP-sensitive current was completely blocked at all potentials by Rb+ (Stanfield, Nakajima & Yamaguchi, 1985). Unlike the effects of Ba2+ in facial motoneurones, this ion completely blocked, at all potentials, the inwardly rectifying 5-HT-sensitive K+ current in nucleus accumbens neurones (North & Uchimura, 1989) and the 5-HT1A-activated inwardly rectifying K+ current in dorsal raphe neurones (Penington et al. 1993). Thus, the inability of Cs+, Ba2+ and Rb+ to block the 5-HT-sensitive current at potentials depolarized to V5-HT in facial motoneurones may not be consistent with the sole involvement of a Kir channel.
Internal Mg2+ and Na+ both mediate mild rectification of Kir channels by blocking the pore at depolarized potentials (Matsuda, Saigusa & Irisawa, 1987; Matsuda, 1993), while strong rectification is the result of pore block by internal polyamines such as spermine and spermidine (Lopatin, Makhina & Nichols, 1994). It is unlikely that the linearity of I5-HT in facial motoneurones is due to loss of internal Mg2+ and Na+ as both were included in the pipette solution at physiological levels. Whole-cell dialysis of polyamines could have occurred; however, linear I5-HT was also seen in intracellular recordings obtained with sharp electrodes where dialysis does not take place (Larkman & Kelly, 1992).
Inwardly rectifying K+ channels (Kir) are expressed in a wide range of cells where they play a major role in maintaining resting potential as well as being implicated in the regulation of action potential duration. mRNA for the Kir2.3 and Kir3.4 inward rectifier channel proteins has been shown to be present in adult rat facial motoneurone cell bodies (Falk et al. 1995; Iizuka, Tsunenari, Momota, Akiba & Kono, 1997). Nevertheless, the subunit composition of a 5-HT-sensitive Kir channel in facial motoneurones is unknown.
The 5-HT-sensitive current not blocked by inhibitors of IK(ir) may be the result of an inhibition of a distinct, non-inactivating, voltage-sensitive K+ channel. This component of the 5-HT-sensitive current was not blocked by TEA but could be blocked by high concentrations of 4-AP. 4-AP suppressed three components of whole-cell current: a fast transient outward current, a sustained component of membrane current displaying a linear I–V relationship, and a more slowly decaying outward current only activated at depolarized potentials similar to that described in guinea-pig facial and trigeminal motoneurones (Nishimura, Schwindt & Crill, 1989; Chandler, Hsaio, Inoue & Goldberg, 1994). The involvement of the fast transient outward current in the action of 5-HT can be ruled out because leak subtraction of currents evoked in the absence of 4-AP showed the amplitude of this current to be unaltered by 5-HT. Subtracted 5-HT-sensitive currents from rectangular voltage commands showed a rectangular activation with little inactivation over the course of the command suggesting that the sustained component of 4-AP-sensitive current is inhibited by 5-HT. It is unlikely that the slowly decaying outward current is responsible for the actions of 5-HT because it could be blocked by concentrations of 4-AP (100 μm) that were ineffective against the 5-HT-evoked current and the amplitude of I5-HT at depolarized potentials was independent of priming hyperpolarizing prepulses.
The lack of effect of Ca2+ channel blockers or internal BAPTA eliminates the possibility that the channels closed by 5-HT are Ca2+ sensitive. I5-HT was not blocked by high concentrations of external Ba2+ ruling out involvement of an M-type K+ channel. Replacement of external NaCl with the impermeant NMDG slightly reduced I5-HT but the V5-HT was unchanged in both 3 and 12 mm K+ ACSF. The small inhibitory effect on I5-HT could have been due to a direct effect by NMDG on the channel, a depressive effect of intracellular protons due to the suppression of Na+-H+ exchange, or the block of an additional Na+-mediated component of I5-HT.
Characterization of the K+ channels mediating excitatory actions of 5-HT on central neurones is noticeably scarce. A Ba2+-sensitive IK(ir) is inhibited by 5-HT in nucleus accumbens neurones (North & Uchimura, 1989). In unidentified cultured spinal neurones of large soma diameter, the depolarization (inward I5-HT) at the resting potential was insensitive to Ba2+, TEA, Cd2+ and also 4-AP (10 mm) (Legendre, Guzman, Dupouy & Vincent, 1989). The voltage dependence of the Ba2+-insensitive I5-HT in facial motoneurones is similar to that of the 5-HT-sensitive or S-current seen in Aplysia neurones (Klein, Camardo & Kandel, 1982). The S-current, while being insensitive to Ba2+ and TEA, is, however, not blocked by 4-AP. It has been reported that a 5-HT1A-activated outward current in guinea-pig nucleus prepositus hypoglossi neurones involves two distinct K+ conductances resulting in a relatively linear relationship between current amplitude and voltage (Bobker & Williams, 1995). As seen in facial motoneurones, an inwardly rectifying component of this current, which reverses at the predicted EK, is preferentially blocked by low concentrations (200 μm) of Ba2+. However, unlike facial motoneurones, the remaining outwardly rectifying component of 5-HT-activated current in these cells was insensitive to 4-AP as well as TEA but could be blocked by higher concentrations of Ba2+ (2 mm). Moreover it was abolished by external Cd2+ or when the cells were loaded with a high concentration of BAPTA, suggesting the involvement of a Ca2+-activated conductance.
In conclusion, our results indicate that neonatal rat facial motoneurones possess a 5-HT-sensitive K+ conductance that at hyperpolarized potentials is sensitive to blockers of Kir channels and possesses strong inwardly rectifying characteristics. 5-HT-sensitive current, at depolarized potentials, can be blocked by 4-AP but is insensitive to block by external Cs+, Ba2+, Rb+ or TEA and does not involve modulation of a Ca2+-sensitive channel. Thus, either two distinct K+ channels, one of the Kir type and the other an unidentified voltage-sensitive channel, contribute to the excitatory actions of 5-HT on facial motoneurones, or a single channel type, with properties not currently attributed to any of the known K+ channels, is involved. Studies at the single channel level will be needed to decide between the two possibilities.
Acknowledgments
This work was funded by MRC grant number G9222376 N awarded to P. M. L.
References
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurones in adult rat brain slices. Synapse. 1989;3:331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Neurophysiological actions of 5-hydroxytryptamine in the vertebrate nervous system. Progress in Neurobiology. 1990;35:451–468. doi: 10.1016/0301-0082(90)90031-b. 10.1016/0301-0082(90)90031-B. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neuroscience Letters. 1992;143:164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Bobker DH, Williams JT. The serotonergic inhibitory postsynaptic potential in prepositus hypoglossi is mediated by two potassium currents. Journal of Neuroscience. 1995;15:223–229. doi: 10.1523/JNEUROSCI.15-01-00223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. British Journal of Pharmacology. 1993;110:343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler SH, Hsaio C-F, Inoue T, Goldberg LJ. Electrophysiological properties of guinea pig trigeminal motoneurones recorded in vitro. Journal of Neurophysiology. 1994;71:129–145. doi: 10.1152/jn.1994.71.1.129. [DOI] [PubMed] [Google Scholar]
- Elliott P, Wallis DI. Serotonin and L-norepinephrine as mediators of altered excitability in neonatal rat motoneurons studied in vitro. Neuroscience. 1992;47:533–544. doi: 10.1016/0306-4522(92)90163-v. 10.1016/0306-4522(92)90163-V. [DOI] [PubMed] [Google Scholar]
- Falk T, Meyerhof W, Corrette BJ, Schäfer J, Bauer CK, Schwarz JR, Richter D. Cloning, functional expression and mRNA distribution of an inwardly rectifying potassium channel protein. FEBS Letters. 1995;367:127–131. doi: 10.1016/0014-5793(95)00527-g. 10.1016/0014-5793(95)00527-G. [DOI] [PubMed] [Google Scholar]
- Garratt JC, Alreja M, Aghajanian GK. LSD has high efficacy relative to serotonin in enhancing the cationic current Ih: Intracellular studies in rat facial motoneurons. Synapse. 1993;13:123–134. doi: 10.1002/syn.890130205. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Miyazaki S, Rosenthal NP. Potassium current and the effect of caesium on this current during anomalous rectification of the egg cell membrane of a starfish. Journal of General Physiology. 1976;67:621–638. doi: 10.1085/jgp.67.6.621. 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NC, Constanti A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea-pig substantia nigra neurons in vitro. Journal of Neurophysiology. 1995;74:2366–2378. doi: 10.1152/jn.1995.74.6.2366. [DOI] [PubMed] [Google Scholar]
- Hille B, Schwarz W. Potassium channels as multi-ion single-file pores. Journal of General Physiology. 1978;72:409–442. doi: 10.1085/jgp.72.4.409. 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Martin G. 5-HT receptor classification and nomenclature: towards a harmonization with the human genome. Neuropharmacology. 1997;36:419–428. doi: 10.1016/s0028-3908(97)00036-1. 10.1016/S0028-3908(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Iizuka M, Tsunenari I, Momota Y, Akiba I, Kono T. Localization of a G-protein-coupled inwardly rectifying K+ channel, CIR, in the rat brain. Neuroscience. 1997;77:1–13. doi: 10.1016/s0306-4522(96)00460-5. 10.1016/S0306-4522(96)00460-5. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends in Neurosciences. 1993;16:346–352. doi: 10.1016/0166-2236(93)90090-9. 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- Klein M, Camardo J, Kandel ER. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proceedings of the National Academy of Sciences of the USA. 1982;79:5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. Ionic mechanisms mediating 5-hydroxytryptamine- and noradrenaline-evoked depolarization of adult rat facial motoneurones. Journal of Physiology. 1992;456:473–490. doi: 10.1113/jphysiol.1992.sp019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. The use of brain slices and dissociated neurones to explore the multiplicity of actions of 5-HT in the central nervous system. Journal of Neuroscience Methods. 1995;59:31–39. doi: 10.1016/0165-0270(94)00191-i. 10.1016/0165-0270(94)00191-I. [DOI] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. Characterization of the 5-HT- and noradrenaline-sensitive potassium conductance in neonatal rat facial motoneurones in vitro. Journal of Physiology. 1996;495.P:55P. doi: 10.1111/j.1469-7793.1998.067br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. Modulation of Ih by 5-HT in neonatal rat motoneurones in vitro: Mediation through a phosphorylation independent action of cAMP. Neuropharmacology. 1997;36:721–733. doi: 10.1016/s0028-3908(97)00021-x. 10.1016/S0028-3908(97)00021-X. [DOI] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS, Takahashi T. Adenosine 3′:5′-cyclic monophosphate mediates a 5-hydroxytryptamine-induced response in neonatal rat motoneurones. Pflügers Archiv. 1995;430:763–769. doi: 10.1007/BF00386174. [DOI] [PubMed] [Google Scholar]
- Larkman PM, Penington NJ, Kelly JS. Electrophysiology of adult rat facial motoneurones: the effects of serotonin (5-HT) in a novel in vitro brainstem slice. Journal of Neuroscience Methods. 1989;28:133–146. doi: 10.1016/0165-0270(89)90018-6. [DOI] [PubMed] [Google Scholar]
- Legendre P, Guzman A, Dupouy B, Vincent JD. Excitatory effect of serotonin on pacemaker neurons in spinal cord cell culture. Neuroscience. 1989;28:201–209. doi: 10.1016/0306-4522(89)90244-3. [DOI] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat hippocampal stratum oriens-alveus interneurones. Journal of Physiology. 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Effects of internal and external Na+ ions on inwardly rectifying K+ channels in guinea-pig ventricular cells. Journal of Physiology. 1993;460:311–326. doi: 10.1113/jphysiol.1993.sp019473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inwardly rectifying K+ channel and blocking by internal Mg2+ Nature. 1987;325:156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Schwindt PC, Crill WE. Electrical properties of facial motoneurones in brainstem slices from guinea pig. Brain Research. 1989;502:127–142. doi: 10.1016/0006-8993(89)90468-x. [DOI] [PubMed] [Google Scholar]
- North RA, Uchimura N. 5-Hydroxytryptamine acts at 5-HT2 receptors to decrease potassium conductance in rat nucleus accumbens neurones. Journal of Physiology. 1989;417:1–12. doi: 10.1113/jphysiol.1989.sp017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh U, Ho Y-K, Kim D. Modulation of the serotonin-activated K+ channel by G protein subunits and nucleotides in rat hippocampal neurons. Journal of Membrane Biology. 1995;147:241–253. doi: 10.1007/BF00234522. [DOI] [PubMed] [Google Scholar]
- Pape H-C. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annual Review of Physiology. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Penington NJ, Kelly JS, Fox AP. Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. Journal of Physiology. 1993;469:387–405. doi: 10.1113/jphysiol.1993.sp019819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield PR, Nakajima Y, Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985;315:498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. Journal of Physiology. 1990;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Kojima M, Matsuura T, Sano Y. Serotonergic innervation on the motoneurones in the mammalian brainstem. Anatomy and Embryology. 1983;167:321–333. doi: 10.1007/BF00315670. [DOI] [PubMed] [Google Scholar]
- Wang MY, Dun NJ. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. Journal of Physiology. 1990;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Nakajima Y, Nakajima S, Stanfield PR. Modulation of inwardly rectifying channels by substance P in cholinergic neurones from rat brain in culture. Journal of Physiology. 1990;426:499–520. doi: 10.1113/jphysiol.1990.sp018151. [DOI] [PMC free article] [PubMed] [Google Scholar]


