Abstract
The objective of this study was to investigate the mechanism of PGE2 regulation of Cl− transport across glandular endometrial cells grown in primary culture.
Most of the basal short circuit current (Isc) was inhibited by luminal addition of 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) or glibenclamide, suggesting the presence of a basally active Cl− conductance in the apical membrane.
Basolateral addition of 10 μm PGE2 increased Isc by 41 ± 3 μA. A similar response was observed when cells were treated with 8-(4-chlorophenylthio) adenosine 3′,5′-cyclic monophosphate (CPT-cAMP). Pretreatment of monolayers with NPPB and glibenclamide blocked the PGE2 and cAMP-mediated increase in Isc, suggesting that the effects of PGE2 and cAMP were dependent on the activity of an apical NPPB- and glibenclamide-sensitive conductance.
Addition of 50 nm antiPGE2 antibody to the basolateral bathing solution decreased basal Isc by 20 % and shifted the threshold response to exogenous PGE2. This result suggests autocrine regulation of electrogenic Cl− transport by PGE2.
Experiments with amphotericin B-permeabilized monolayers revealed that the apical PGE2-activated, NPPB- and glibenclamide-sensitive conductance was Cl− dependent and that the current-voltage relationship and anion permeation properties (SCN−>Br− > Cl− > I−) were characteristic of the cystic fibrosis transmembrane conductance regulator (CFTR).
Cultured porcine endometrial epithelial cells were specifically labelled with an antibody to a peptide sequence within the regulatory domain of CFTR.
The effect of PGE2 was blocked by basolateral addition of bumetanide and furosemide at concentrations that are selective for inhibition of Na+-K+-2Cl−cotransport activity. The effect of bumetanide on Isc was Cl− dependent, suggesting a role for the bumetanide-sensitive transport pathway in Cl− secretion.
PGE2 and cAMP also activated an outwardly rectifying basolateral K+ channel which presumably sustains the driving force for electrogenic Cl− efflux across the apical membrane.
The concentration-conductance and concentration-Isc response relationships for PGE2 showed that basolateral K+ permeability was rate limiting with respect to transepithelial anion secretion and that activation of a basolateral K+ channel by PGE2 was necessary to achieve maximum rates of Cl− secretion.
The endometrial epithelium consists of a surface epithelium which faces the uterine lumen and a glandular epithelium which invaginates into the endometrial stroma (Davis & Blair, 1993). The endometrium is responsible for the transfer of nutrients, fluid and electrolytes between the circulatory system and the uterine lumen. The transport-related activity of the surface and glandular epithelium provides an optimal intrauterine environment for implantation and embryo development. Previous studies of uterine fluid ionic composition from pigs, humans and rats have revealed that the K+ concentration is 4- to 5-fold higher than that in plasma, and the Na+ concentration is significantly lower than its concentration in plasma (Clemetson, Kim, Mallikarjuneswara & Wilds, 1972; Iritani, Sato & Nishikawa, 1974; Casslen & Nilsson, 1984). Regulation of Na+ and K+ concentrations within uterine and oviduct fluids appears to be important for reproductive events including sperm capacitation, acrosomal reaction, sperm-egg fusion and implantation of the developing embryo (Clemetson et al. 1972; Boldt, Casas, Whaley, Creazzo & Lewis, 1991; Fraser, 1992, 1995). Other important functions of endometrial epithelial cells include regulation of luminal pH and uterine fluid volume. Active secretion of Cl− and HCO3− ions has been shown to establish a driving force for fluid secretion and to regulate luminal fluid pH in the intestine (Wenzl, Sjaastad, Weintraud & Machen, 1989; MacLeod, Redican, Lembessis, Hamilton & Field, 1996) and airways (Smith & Welsh, 1992). In rabbit, fluid secretion into the oviduct lumen is driven by active secretion of Cl− across the oviduct epithelium (Leese, 1988).
Previous studies using primary cultures of human endometrial epithelial cells grown on permeable supports demonstrated that amiloride-sensitive Na+ absorption was accompanied by K+ secretion across the cell monolayers (Matthews, Thomas, Redfern & Hirst, 1993). Other studies with primary cultures of rodent endometrial epithelial cells grown on permeable supports showed that the short circuit current was stimulated by forskolin, an activator of adenylyl cyclase (Rochwerger, Dho, Parker, Foskett & Buchwald, 1994; Leung, Wong, Gabriel, Yankaskas & Boucher, 1995). In cultured mouse endometrial cells, adrenergic agonists were shown to produce an increase in Isc that was consistent with anion secretion. This response was mediated by β-adrenergic receptors and was blocked by apical addition of either glibenclamide or diphenylamine 2,2′-dicarboxylic acid (DPC) (Chan, Fong, Chung & Wong, 1997). These experiments indicate that endometrial epithelial cells in culture are capable of both Na+ absorption and anion secretion.
Prostaglandins are produced by epithelial and stromal endometrial cells in human and other species (Alecozay, Harper, Scheken & Hanahan, 1991; Zhang, Paria & Davis, 1991; Chen, Yang, Hilsenrath, Le & Harper, 1995). They act in both an endocrine and paracrine manner to increase uterine blood flow, vascular permeability and to regulate menstruation and implantation (Ford, Reynolds & Magness, 1982; Keys, King & Kennedy, 1986; Chaudhuri, 1991; Yee & Kennedy, 1993). Prostaglandins, specifically PGE2, have been shown to regulate electrolyte transport function in rabbit cortical collecting duct (Sakairi & Jacobson, 1995), prairie dog gallbladder (Saunders-Kirkwood, Cates & Roslyn, 1993) and porcine distal colon epithelium (Traynor, Brown & O'Grady, 1993). However, little is known about the transport-related actions of prostaglandins on endometrial epithelial cells. Recent studies with porcine surface endometrium using amphotericin B-permeabilized tissues showed that PGF2α increased Na+ absorption by activation of K+ channels in the basolateral membrane (Vetter & O'Grady, 1996). The aim of this study was to characterize the transport properties of porcine glandular epithelial cells and to investigate the effect of PGE2 on regulation of anion transport.
METHODS
Materials
5-Nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) and 8-(4-chlorophenylthio) adenosine 3′,5′-cyclic monophosphate (CPT-cAMP) were purchased from Research Biochemicals International. Prostaglandin (PG) E2 and F2α were purchased from Biomol Research Laboratories (Plymouth, PA, USA). Furosemide (frusemide) was purchased from Aldrich Chemicals and bumetanide from Hoffmann La Roche. Glibenclamide, indomethacin, insulin, non-essential amino acids and high purity grade salts were purchased from Sigma. Dulbecco's modified Eagle's medium (DMEM), Dulbecco's phosphate buffer saline (DPBS), fetal bovine serum (FBS), collagenase (type 1), kanamycin, penicillin- streptomycin and fungizone were purchased from Gibco. Charcoal-stripped FBS was obtained from Cocalico Biologicals (Reams Powns, PA, USA). Mouse antiPGE2 antibody (2B5 mAb) was kindly provided by Dr Joseph Portanova (Monsanto Company, St Louis, MO, USA).
Cell isolation and culture
Porcine uterine tissue was collected from 4- to 5-month-old (reproductively immature) Yorkshire × Pietrain cross pigs purchased from stock herds maintained by the University of Minnesota College of Agriculture. Uterine tissue from reproductively immature animals was used to minimize variability in electrolyte transport properties at different stages of the oestrus cycle. Uteri were obtained from pigs that were killed at the University of Minnesota Meat Sciences Laboratory by the captive bolt euthanasia procedure approved by the University Animal Care Committee and supervised by a USDA certified veterinarian. The tissue was placed in ice-cold porcine Ringer solution containing (mm): 130 NaCl, 6 KCl, 3 CaCl2, 0.7 MgCl2, 20 NaHCO3, 0.3 NaH2PO4, 1.3 Na2HPO4, pH 7.4. After removal of the serosal muscle layer, the endometrial tissue was minced into small pieces (∼1 mm3) in Ca2+- and Mg2+-free PBS. The tissue fragments were incubated overnight (15–20 h) at 37°C in DMEM with 0.2 % collagenase. After collagenase digestion, the tissue was dissociated with gentle pipetting through a Pasteur pipette. The undigested tissue was removed by filtration through a mesh screen. In suspension, the stromal cells were dissociated to single cells whereas the epithelial glands remained intact. The epithelial glands were isolated by size using gravitational sedimentation. After 10 min, the supernatant (stromal-rich fraction) was removed. The pellet (epithelial-rich fraction) was resuspended twice in PBS and allowed to settle. The resultant pellet containing primarily epithelial glands was suspended in DMEM supplemented with 3.7 g l−1 sodium bicarbonate, 10 % FBS, 10 μg ml−1 insulin, 1 % non-essential amino acids, 5 μg ml−1 fungizone, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 100 μg ml−1 kanamycin (standard media). The epithelial cells were then plated onto cell culture dishes and incubated at 37°C in a humidified atmosphere of 5 % CO2 in air. Culture medium was changed after 24 h and then every 2–3 days. The epithelial cells became confluent monolayers within 3–4 days. The remaining stromal cells were removed by adding 0.02 % collagenase in serum-free medium for 24 h. The epithelial cells were then trypsinized and placed on 24 mm (4.5 cm2) transparent permeable supports (Costar, Cambridge, MA, USA). To study steroid hormone regulation, the epithelial cells were grown on permeable supports in DMEM supplemented with 10 % FBS for 7 days to allow complete attachment and formation of a monolayer. The medium was then replaced with Phenol Red-free DMEM supplemented with 10 % charcoal-stripped FBS for 7 days.
Measurement of electrical parameters
Transepithelial resistance was measured using the EVOM epithelial voltohmmeter coupled to Ag-AgCl ‘chopstick’ electrodes (World Precision Instruments). After 10 days, the confluent culture inserts were mounted in Ussing Chambers, bathed on both sides with standard porcine Ringer solution which was maintained at 37°C and bubbled with 95 % O2- 5 % CO2. Transepithelial potential difference, tissue conductance and short circuit current (Isc) were measured with the use of voltage-clamp circuitry from JWT Engineering (Overland Park, KS, USA). The data from the voltage clamp experiments was digitized, stored and analysed using Workbench data acquisition software (Kent Scientific Corporation, Litchfield, CT, USA) and recorded with a Compaq pentium microcomputer. All cells were pretreated with indomethacin (10 μm) added to both apical and basolateral solutions at least 10 min before the beginning of the experiment except the experiments using antiPGE2 antibodies. In anion substitution experiments, gluconate salts were used to replace chloride and Hepes was used to replace HCO3− and bubbled with 100 % O2.
To measure membrane permeability, current-voltage relationships were determined using amphotericin B-permeabilized monolayers mounted in Ussing chambers. The intracellular solution contained (mm): 120 KMeSO4, 30 mannitol, 10 NaCl, 2 calcium gluconate, 1 MgSO4, 20 KHCO3, 0.3 KH2PO4, 10 D-glucose; pH 7.4. Varying intracellular chloride concentrations were obtained by replacement of 10 and 25 mm KMeSO4 with equimolar KCl. Amphotericin B (10 μm) was added to the KMeSO4 Ringer solution to permeabilize the membrane. For anionic selectivity experiments, 50 mm KMeSO4 in the basolateral solution was replaced with 50 mm KCl. The composition of the extracellular (apical) solution was similar to the intracellular solution where 50 mm KCl, KSCN, KBr or KI was used to replace 50 mm KMeSO4, and amphotericin B (10 μm) was added to permeabilize the basolateral membrane. A World Precision Instruments epithelial voltage clamp was used to voltage clamp the monolayers. A Compaq pentium microcomputer with a Dagan LM-12 A/D interface was used to record the data. Data were acquired using pCLAMP software (Axon Instruments). Current-voltage relationships were obtained from voltage steps indicated in the figure legends. The compound-sensitive components of the current were obtained by subtracting the resultant currents before and after addition of the compound. The anion permeability ratios PX/PCl where X is SCN−, Br− or I− were calculated from reversal potential (Erev) measurements using the Goldman-Hodgkin-Katz equation (Erev=RT/zF ln (PX[X])/(PCl[Cl−])).
Immunocytochemistry
Epithelial cells were allowed to grow on Lab-tek chamber slides (Nunc, Naperville, IL, USA) or permeable filter supports. After a week, the cell monolayers were washed twice in DPBS (pH 7.4), fixed in 4 % paraformaldehyde at 37°C for 10 min, permeabilized with 0.5 % Triton X-100 in DPBS at room temperature for 10 min and then fixed in methanol at −20°C for 10 min. After fixation, filters were cut from their supports. The cells were then washed with DPBS and incubated with DPBS containing 0.5 % bovine serum albumin and 10 % normal goat serum to block non-specific binding at room temperature for 1 h. Then the cells were incubated overnight with primary antibodies (anticytokeratin peptide 18, 1: 400; anti-cystic fibrosis transmembrane conductance regulator (antiCFTR), 1: 80) at 4°C. Anticytokeratin was a mouse monoclonal IgG1 raised against cytokeratin peptide 18 (Sigma). AntiCFTR was a rabbit polyclonal IgG raised against a synthetic peptide (RKTTASTRKVSL) corresponding to amino acids 785–796 within the R-domain of human CFTR obtained as a gift from Dr Catherine Fuller and Dr Dale Benos. For absorption control, CFTR synthetic peptide (1 mg ml−1) at a dilution of 1:40 was pre-incubated with CFTR antibody for 1 h before being applied to the cell monolayers. After washing, the cells were incubated with secondary antibodies (indocarbocyanine (Cy3)-labelled goat anti-rabbit antibody for antiCFTR and Cy3-labelled goat anti-mouse antibody for anticytokeratin, 1: 400 (Jackson ImmunoResearch Laboratories, PA, USA) for 1 h at room temperature. After the final wash, the filters were placed on a glass slide. The cells and filters were embedded in fluoromount (Gallard Schlessinger, Carle Place, NY, USA) and examined by confocal microscopy, using a Bio-Rad MRC1024 laser confocal microscope equipped with krypton-argon lasers.
To verify that the cells in culture were of epithelial origin, we used anticytokeratin antibody to label the epithelial cells. Briefly, a cell suspension (1 × 105 cells) was washed with DPBS and fixed with 2 % paraformaldehyde-0.2 % Triton X-100 on ice for 20 min. After washing, the cells were incubated with anticytokeratin antibody and followed by Cy3-labelled anti-mouse antibody as previously mentioned. Then, a 20 μl aliquot of cells was applied to a poly-l-lysine-coated slide and observed under fluorescent microscopy.
Statistics
All values are presented as means ±s.e.m., n is the number of monolayers and N is the number of animals in each experiment. The differences between means of control and treatment data were analysed using a Student's t test for paired or unpaired means where appropriate. A value of P < 0.05 was considered statistically significant. The IC50 values for NPPB, glibenclamide, bumetanide and furosemide and the EC50 value for PGE2 and PGF2α were determined using a four parameter logistic function to fit the data. The concentration of each compound at 50 % maximal effect was determined from the equation.
RESULTS
Cell characterization and basal electrical properties
Endometrial glands appeared as tubular structures following isolation (Fig. 1A). After 24 h in culture, a monolayer of epithelial cells migrated radially away from the glandular explants. These monolayers presumably consisted mostly of glandular epithelial cells with some contribution of surface epithelial cells. The cultured cells exhibited polyhedral shapes and grew in a whorl-like pattern around the glandular fragments (Fig. 1B). Immunocytochemical studies of these cells showed that greater than 90 % of the cells were labelled with a monoclonal antibody to cytokeratin. The antibody labelling was present in the cytoplasm and did not appear when primary antibody was absent (Fig. 1C and D).
Figure 1. Isolation and identification of glandular endometrial epithelial cells using cytokeratin antibody.
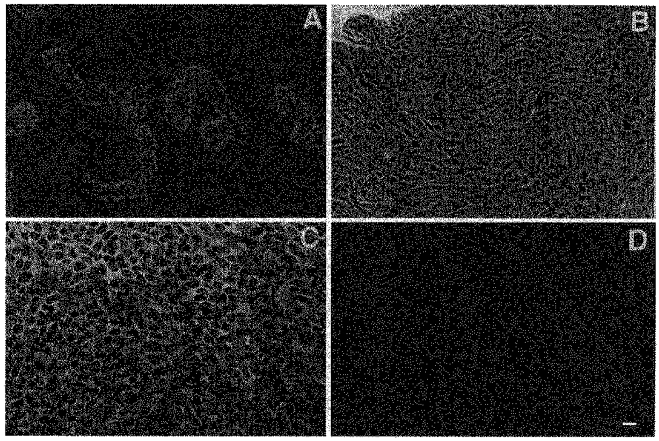
A and B, phase-contrast micrographs of endometrial epithelial glands after isolation and primary culture of epithelial cells grown on plastic after 4 days. C and D, localization of cytokeratin antibody labelling using laser confocal immunofluorescence microscopy of cultured glandular endometrial epithelial cells. The cells were grown on lab-tek slides in standard media for 5 days. Monolayers were fixed, permeabilized and incubated with mouse antibody against cytokeratin peptide 18 and goat antimouse Cy3-labelled secondary antibody (C). D, cells incubated with Cy3-labelled secondary antibody alone. Scale bar, 25 μm; applies to all panels.
Endometrial epithelial cells were seeded on permeable supports and became confluent within 3–4 days. The transepithelial resistance of the monolayers reached a maximum of 2870 ± 54 Ω cm2 3 days after seeding (Fig. 2). High resistance was sustained for at least 20 days after seeding. Monolayers after 10 days in culture were mounted in Ussing chambers and bathed in standard porcine Ringer solution. Pretreatment with indomethacin decreased the basal current by 10 ± 0.6 μA (37 ± 2 % basal Isc, n= 80, N= 18). After a restabilization period, the mean basal Isc was 28 ± 1 μA and the mean potential difference was 30 ± 1 mV (luminal solution as reference; n= 200, N= 25).
Figure 2. Time course showing changes in transepithelial resistance of the cell monolayers over a period of 20 days.
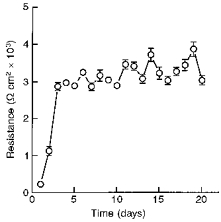
After a week in culture, the cells were seeded on permeable supports. The transepithelial resistance was measured using the EVOM epithelial voltohmmeter coupled to Ag-AgCl ‘chopstick’ electrodes. The transpithelial resistance reached a maximum value of 2870 ± 54 Ω cm2 after 3 days and was sustained up to 20 days (n= 100, N= 10).
Effects of NPPB and glibenclamide on Isc
Figure 3A shows the effect of NPPB on basal Isc of monolayers mounted in Ussing chambers and bathed on both sides with standard porcine Ringer solution. Addition of 100 μm NPPB to the apical solution produced a sustained 79 ± 5 % decrease in basal Isc. Addition of glibenclamide (100 μm) to the apical solution produced a similar decrease in basal Isc (50 ± 4 % of basal Isc, n= 7, N= 4). Subsequent addition of 200 μm glibenclamide after pretreatment with 200 μm NPPB had no effect on basal Isc. These results indicate that the effects of NPPB and glibenclamide on Isc are non-additive, suggesting that they act on the same population of channels in the apical membrane. The concentration-response relationships for NPPB and glibenclamide showed that the threshold response for both compounds was 0.5 μm and the IC50 values were 19 μm for NPPB and 52 μm for glibenclamide (Fig. 3B). In addition, apical addition of 200 μm 4,4′-diisothiocyanatostilbene 2,2′-disulphonic acid (DIDS) had no effect on basal Isc (n= 5, N= 4) and a small effect after PGE2 stimulation (4 ± 2 % of PGE2-stimulated Isc, n= 5, N= 3).
Figure 3. Effects of Cl− channel inhibitors on basal Isc.
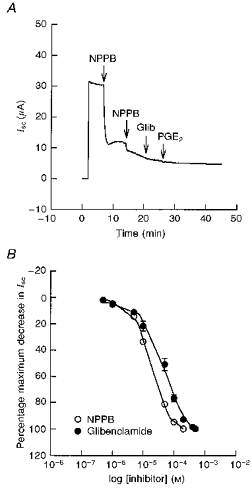
A, a representative Isc tracing showing the effect of NPPB on basal Isc. Addition of 100 μm NPPB to the apical solution produced a rapid and sustained decrease in basal Isc by 79 ± 5 %. The subsequent addition of 100 μm NPPB further decreased basal Isc whereas the addition of 200 μm glibenclamide (Glib) produced no further response. Addition of 3 μm PGE2 to the basolateral solution had no stimulatory effect in the presence of NPPB and glibenclamide in the apical solution (n= 9, N= 4). B, concentration-response relationships showing the decrease in Isc following treatment with various concentrations of NPPB and glibenclamide to the apical solution. The IC50 value was 19 μm for NPPB and 52 μm for glibenclamide (n= 8, N= 4 for each compound). NPPB was found to be 2.7-fold more potent than glibenclamide.
Effects of PGE2, PGF2α and CPT-cAMP on Isc
Basolateral addition of 3 μm PGE2 to cell monolayers grown in Phenol Red-free medium containing charcoal-stripped FBS resulted in a rapid increase in Isc from 12 ± 2 μA to a peak value of 65 ± 4 μA, followed by a rapid decrease to a new sustained plateau of 36 ± 2 μA (n= 13, N= 5, Fig. 4). The PGE2-stimulated Isc was blocked by the apical additions of either 200 μm NPPB or glibenclamide. The increase in Isc produced by PGE2 was similar in character and magnitude to data obtained from the cells grown in standard media. (data not shown). In addition, pretreatment with NPPB or glibenclamide abolished the response to PGE2 in standard media (Fig. 3A). PGE2 had a small effect when added to the apical solution (peak value, 9 ± 3 μA; plateau value, 5 ± 1 μA; n= 6, N= 4). A concentration-response curve for PGE2 and PGF2α is shown in Fig. 5A. Analysis of the concentration-response relationship for PGE2 showed that the threshold response was 3 nm and the EC50 value was 72 nm. The threshold response for PGF2α was 30 nm and the EC50 value was > 7.5 μm. PGE2 was estimated to be at least 100 times more potent than PGF2α.
Figure 4. Representative Isc tracings showing the effect of PGE2 on basal Isc.
Addition of 3 μm PGE2 to the basolateral solution produced an increase in Isc followed by a sustained plateau greater than that of the basal Isc. The PGE2-stimulated Isc was blocked by apical additions of 200 μm NPPB (n = 7, N = 4) (A) and 200 μm glibenclamide (Glib) (n = 5, N = 4) (B). In these experiments the cells were grown under standard media conditions for 7 days followed by Phenol Red-free medium containing charcoal-stripped serum for 7 days (see Methods).
Figure 5. Concentration-response relationships for PGE2 and PGF2α and effect of CPT-cAMP on basal Isc.
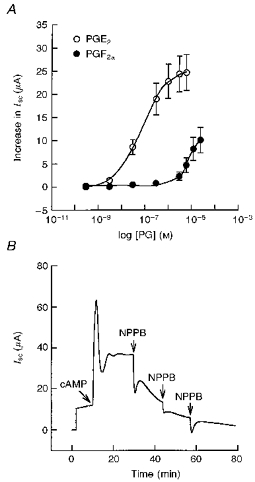
A, concentration-response relationships showing the increase in Isc following treatment with various concentrations of PGE2 and PGF2α to the basolateral solution. The EC50 value was 72 nm for PGE2 (n= 10, N= 5) and > 7.5 μm for PGF2α (n= 11, N= 4). B, a representative Isc tracing showing the effect of CPT-cAMP on basal Isc. Addition of 100 μm CPT-cAMP to the basolateral solution produced an immediate increase in Isc followed by a sustained plateau greater than that of the basal Isc. The cAMP-stimulated Isc was blocked by apical additions of 200 μm NPPB (n= 6, N= 3).
Addition of 100 μm CPT-cAMP to the basolateral solution produced a rapid increase in Isc followed by a sustained plateau similar to the PGE2 response (Fig. 5B). The effects of PGE2 and CPT-cAMP when added sequentially were non-additive (data not shown). Similar to PGE2, the cAMP-stimulated Isc was blocked by the apical additions of 200 μm NPPB or glibenclamide (data not shown).
Effect of ion substitution on the PGE2 response
Ion substitution experiments were performed to determine the ionic basis for the increase in Isc produced by PGE2. Figure 6A shows that in standard porcine Ringer solution the basolateral addition of 10 μm PGE2 resulted in an increase in Isc with a final plateau value of 41 ± 3 μA. Replacement of Cl− or HCO3− in the solutions bathing both apical and basolateral surfaces inhibited the maximal Isc response produced by PGE2 to 9 ± 2 μA and 2 ± 0.9 μA (79 and 96 % of control), respectively. The PGE2 response was completely abolished by the replacement of both Cl− and HCO3− ions (-2 ± 1 μA).
Figure 6. Effects of anion substitution and antiPGE2 antibody on Isc.
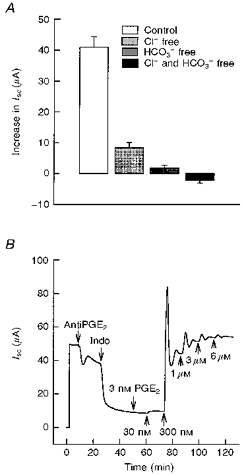
A, effect of anion substitution on the maximal Isc response to PGE2. In standard porcine Ringer solution, PGE2 at a concentration of 10 μm produced a mean increase in Isc of 41 ± 3 μA (n= 15, N= 3). Replacement of Cl− and HCO3− in both the apical and basolateral solutions significantly inhibited the maximal response to PGE2 by 79 % and 96 %, respectively (P < 0.001 compared with control). The PGE2 response was completely abolished following replacement of both Cl− and HCO3− (n= 5, N= 3 for each condition). B, a representative Isc tracing showed the effect of antiPGE2 antibody on basal Isc. Addition of 50 nm antiPGE2 antibody to the basolateral solution inhibited basal Isc by 21 ± 7 % (n= 5, N= 2). Addition of 10 μm indomethacin (Indo) to both the apical and the basolateral solution further inhibited the basal current. Pretreatment with antiPGE2 antibody blocked the response to exogenous PGE2 at concentrations of 3 and 30 nm.
Effect of antiPGE2 antibody
To investigate whether cultured porcine endometrial epithelial cells release PGE2 under basal conditions, we examined the effect of antiPGE2 antibody on Isc before adding indomethacin. Mouse antiPGE2 antibody was previously generated against a PGE2-thyroglobulin conjugate in BALB/c mice (Mnich et al. 1995). It is one of the highest affinity and specific antiPGE2 antibodies reported to date with a Kd of approximately 300 pm and cross-reactivity towards other eicosanoids of less that 1 %. Addition of 50 nm antiPGE2 antibody to the basolateral solution produced a rapid decrease in basal Isc from 45 ± 4 to 35 ± 6 μA (21 ± 7 % basal Isc) as shown in Fig. 6B. Subsequent addition of a second 50 nm dose to the basolateral solution produced no further inhibition. Addition of indomethacin to both the apical and the basolateral solutions caused a further decrease in the remaining Isc. Pretreatment of monolayers with antiPGE2 antibody abolished PGE2 responses at concentrations of 3 and 30 nm and thus shifted the threshold response to exogenous PGE2.
Apical membrane permeability
To characterize the properties of the apical NPPB-/ glibenclamide-sensitive permeability, the basolateral membrane of the epithelium was permeabilized with amphotericin B and bathed with KMeSO4 Ringer solution containing varying Cl− concentrations. The apical surface of the cells was bathed in standard porcine Ringer solution. Current-voltage relationships were obtained using a voltage step protocol ranging from -90 to +120 mV (15 mV steps) at a holding potential of 0 mV. The resultant currents before and 10 min after apical addition of NPPB or glibenclamide were subtracted to obtain the NPPB- and glibenclamide-sensitive currents shown in Fig. 7.
Figure 7. Current-voltage (I-V) relationships for the NPPB- and glibenclamide-sensitive conductances in the apical membrane.
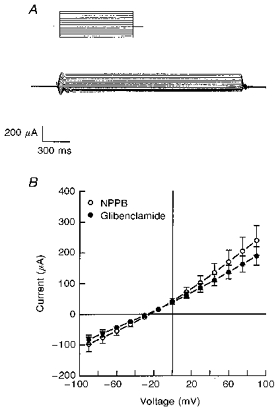
A, a representative tracing of the glibenclamide-sensitive current obtained from amphotericin B-permeabilized monolayers in response to a voltage step protocol from -90 to +120 mV (15 mV step increments) at a holding potential of 0 mV. Glibenclamide (200 μm) was added to the apical solution. B, NPPB- and glibenclamide-sensitive components of the apical membrane current were plotted as a function of voltage. Experiments were performed under conditions where the basolateral surface was permeabilized with amphotericin B and bathed in KMeSO4 Ringer solution with 10 mm NaCl. Standard Ringer solution was used to bathe the apical surface of the epithelium. NPPB or glibenclamide, at a concentration of 200 μm, was added to the apical solution. Mean reversal potentials for NPPB- and glibenclamide-sensitive currents were -28 ± 3 and -27 ± 1 mV, respectively (n= 7, N= 4 for each experiment).
Figure 7A shows a representative tracing of the glibenclamide-sensitive component of the apical membrane current where 10 mm NaCl was present in the basolateral solution. The reversal potentials for the NPPB- and glibenclamide-sensitive currents were -28 ± 3 and -27 ± 1 mV, respectively (Fig. 7B). Increasing Cl− concentration in the basolateral solution from 10 mm to 35 mm produced a shift in reversal potential for the NPPB- and glibenclamide-sensitive currents to -16 ± 2 mV (n= 6, N= 4) and -17 ± 1 mV (n= 9, N= 4), respectively. The NPPB- and glibenclamide-sensitive currents under PGE2-stimulated conditions are shown in Fig. 8. The PGE2-sensitive current before and 10 min after basolateral addition of 3 nm PGE2 had a reversal potential of -28 ± 1 mV which was not significantly different from that of NPPB- (-30 ± 2 mV) and glibenclamide-sensitive currents (-31 ± 2 mV) following PGE2 stimulation. The reversal potentials for the PGE2-sensitive currents and for the NPPB- and glibenclamide-sensitive currents under PGE2-stimulated conditions were also dependent on Cl− concentration (Fig. 9), indicating selectivity for Cl− ions.
Figure 8. Current-voltage (I-V) relationships for the PGE2-, NPPB- and glibenclamide-sensitive conductances in the apical membrane.
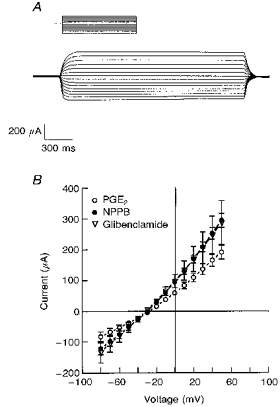
A, a representative tracing of the glibenclamide-sensitive component of the PGE2-stimulated current. Current measurements were obtained from amphotericin B-permeabilized monolayers which were held at 0 mV and stepped through a series of voltages from -90 to +50 mV (10 mV step increments). PGE2 (3 nm) was added to the basolateral surface and NPPB or glibenclamide, at a concentration of 200 μm, was subsequently added to the apical solution. B, PGE2-sensitive, NPPB-sensitive and glibenclamide-sensitive currents were plotted as a function of voltage. Experiments were performed using amphotericin B-permeabilized monolayers (basolateral surface) with KMeSO4 Ringer solution containing 10 mm NaCl bathing the basolateral surface and standard Ringer solution bathing the apical surface. Mean reversal potentials for PGE2-sensitive, NPPB-sensitive and glibenclamide-sensitive currents were -28 ± 1 mV (n= 13, N = 4), -30 ± 2 mV (n= 6, N = 3) and -31 ± 2 mV (n= 7, N = 4), respectively.
Figure 9. The reversal potential for PGE2-sensitive, NPPB-sensitive and glibenclamide-sensitive currents at various basolateral Cl− concentrations.
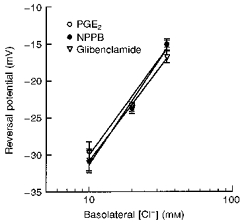
The data were fitted using linear regression analysis with correlation coefficients (R) of 0.991, 0.993 and 0.999 for PGE2-, NPPB- and glibenclamide-sensitive responses, respectively (n= at least 5, N= 4 for each experiment).
Amphotericin permeabilization experiments also demonstrated that the reversal potential of the apical cAMP-sensitive current was -29 ± 1 mV as shown in Fig. 10. The cAMP-activated current response was blocked by either NPPB or glibenclamide. The reversal potentials of NPPB- and glibenclamide-sensitive currents after CPT-cAMP stimulation were -38 ± 2 mV (P < 0.05) and -30 ± 1 mV, respectively.
Figure 10. Current-voltage (I-V) relationships for the CPT-cAMP, NPPB- and glibenclamide-sensitive conductances in the apical membrane.
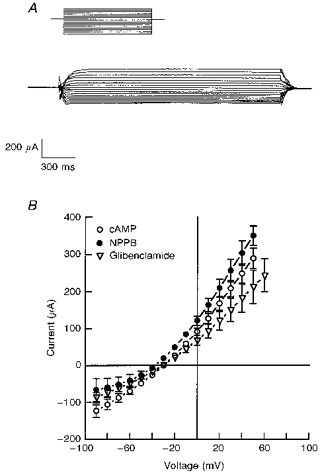
A, a representative tracing of glibenclamide-sensitive current after CPT-cAMP stimulation. The current was obtained from amphotericin B-permeabilized monolayers which were held at 0 mV and stepped through a series of voltages from -100 to +70 mV (10 mV increments). CPT-cAMP (1 μm) was added to the basolateral solution and NPPB or glibenclamide at a concentration of 200 μm was subsequently added to the apical solution. B, cAMP-sensitive, NPPB-sensitive and glibenclamide-sensitive currents were plotted as a function of voltage. Experiments were performed using amphotericin B-permeabilized monolayers (basolateral surface) with KMeSO4 Ringer solution containing 10 mm NaCl bathing the basolateral surface and standard Ringer solution bathing the apical surface of the epithelium. Average reversal potentials for CPT-cAMP activation, NPPB inhibition and glibenclamide inhibition were -29 ± 1 mV (n= 10, N= 3), -38 ± 2 mV (n= 5, N= 3) and -30 ± 1 mV (n= 5, N= 3), respectively.
Anion permeability of the PGE2-stimulated current
To investigate the anion permeability of the apical Cl− conductance, we measured the current after permeabilizing the basolateral membrane with amphotericin. The reversal potentials of PGE2-sensitive current for intracellular KCl and extracellular KSCN, KBr, KCl and KI were -23 ± 2, -5 ± 0.5, 0 ± 0.2 and 18 ± 1 mV, respectively (Fig. 11). The permeability ratios PX/PCl were calculated to be 2.4 ± 0.2 for SCN−, 1.2 ± 0.03 for Br−, 1.0 ± 0.009 for Cl− and 0.5 ± 0.02 for I−. The anion permeability sequence of PGE2-stimulated Cl− current was SCN− > Br− > Cl− > I− which was consistent with that previously reported for T84 cells and for cloned CFTR Cl− channels expressed in HeLa cells and 3T3 fibroblasts (Anderson et al. 1991). These results suggest that cAMP-activated Cl− channels in endometrial epithelial cells have selectivity properties similar to that of the CFTR Cl− channel.
Figure 11. Current-voltage (I-V) relationships of PGE2-sensitive current in the presence of 50 mm KCl in the intracellular (basolateral) solution and 50 mm KCl, KSCN, KBr or KI in the extracellular (apical) solution.
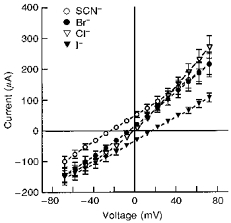
Experiments were performed using amphotericin B to permeabilize the basolateral membrane. PGE2 was added to the basolateral solution at 3 μm for KSCN and KI experiments and at 3 nm for KBr and KCl experiments. Average reversal potentials for KSCN, KBr, KCl and KI were -23 ± 2 mV (n= 5, N= 3), -5 ± 0.5 mV (n= 6, N= 3), 0 ± 0.2 mV (n= 6, N= 3) and 18 ± 1 mV (n= 5, N= 3), respectively.
Immunocytochemistry
Figure 12 shows the summation of immunofluorescence images 4–6 obtained at 3 μm steps as the microscope was focused from the permeable support towards the apical membrane of glandular epithelial cell monolayers. The cell monolayers were labelled using a polyclonal antibody to a peptide sequence in the R-domain of human CFTR. The peptide sequence used in this study contains putative protein kinase A and protein kinase C phosphorylation sites which show a high degree of conservation between species (Hart et al. 1996). This antibody has been previously used to identify CFTR protein in T84 cells and cloned CFTR transfected into CHO cells, HEp-2 cells, Bsc-40 cells and HeLa cells (Fuller, Howard, Bedwell, Frizzell & Benos, 1992). The cells grown in both standard media and Phenol Red-free medium containing charcoal-stripped serum exhibited CFTR-like immunoreactivity. The staining pattern of CFTR for the cells grown on slides was similar to the cells grown on filters. Intense CFTR labelling was detected in sections 5–6 with some scattered labelling in section 4 and very little labelling in the first four sections, suggesting localization of CFTR in the apical membrane, presumably within subapical vesicles. No labelling was observed in control cells labelled with CFTR antibody pre-incubated with the CFTR peptide antigen (Fig. 12E and F).
Figure 12. Localization of CFTR-like immunoreactivity using laser confocal immunofluorescence microscopy of cultured glandular endometrial epithelial cells.
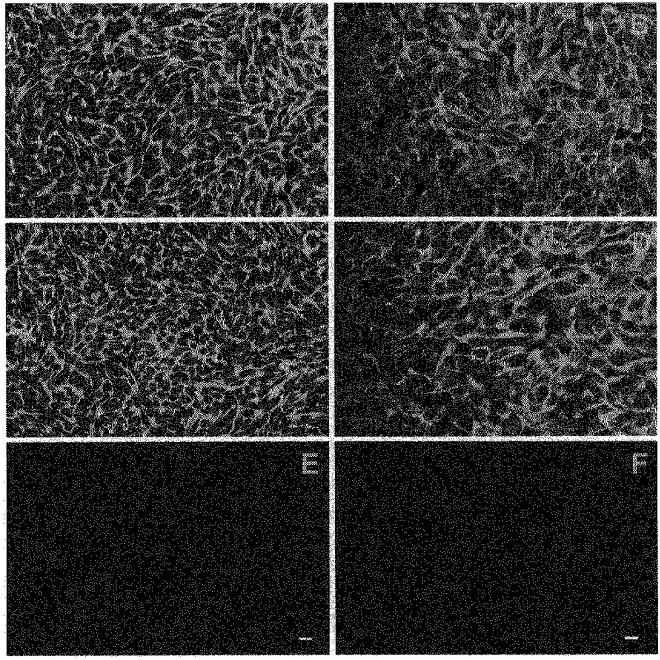
The results shown are the summation of images (4–6) starting from the permeable support towards the apical membrane in 3 μm increments. The cells were grown on Lab-tek slides (A, C and E; scale bar, 25 μm) or permeable filter supports (B, D and F; scale bar, 12.5 μm) in standard media (A and B) and Phenol Red-free medium containing charcoal-stripped serum (B and F). Monolayers were fixed, permeabilized and labelled with rabbit antibody against a peptide sequence within the R-domain of human CFTR and goat antirabbit Cy3-labelled secondary antibody (A and D. E) and F show the results when CFTR antibody was pre-incubated with CFTR peptide antigen.
Effect on basolateral membrane permeability
To characterize the effect of PGE2 on basolateral membrane permeability, amphotericin B was used to permeabilize the apical membrane. The apical surface of the epithelium was bathed with KMeSO4 Ringer solution supplemented with 10 mm NaCl while the basolateral surface was bathed with standard porcine Ringer solution. I-V relationships were obtained under basal conditions using a voltage step protocol ranging from -100 to +70 mV (10 mV steps) at a holding potential of 0 mV. Figure 13A shows a representative tracing of the PGE2-sensitive current 10 min after addition of 3 μm PGE2 to the basolateral solution. The current was outwardly rectifying with a mean reversal potential of -59 ± 2 mV (Fig. 13B). Increasing the basolateral K+ concentration from 6 to 140 mm significantly shifted the reversal potential for the PGE2-sensitive current to -5 ± 2 mV (n= 6, N= 3, not significantly different from 0 mV). The I-V relationship for the basolateral cAMP-sensitive current was also found to be outwardly rectifying with a mean reversal potential of -61 ± 4 mV (Fig. 14). The IC50 for CPT-cAMP activation of this basolateral conductance was 21 μm.
Figure 13. Current-voltage (I-V) relationships of the PGE2-sensitive permeability in the basolateral membrane.
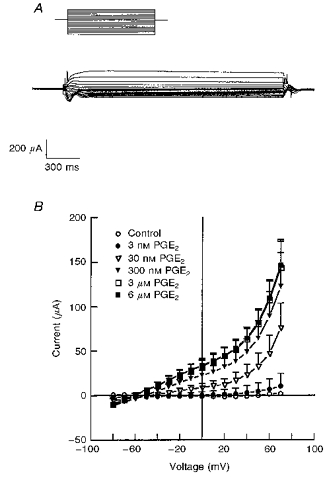
A, a representative tracing of PGE2-sensitive current obtained from amphotericin B-permeabilized monolayers in response to step commands from -100 to +70 mV (10 mV increments) at a holding potential of 0 mV. PGE2 (3 μm) was added to the basolateral solution. B, effects of PGE2 concentrations on PGE2-sensitive currents obtained from amphotericin B-permeabilized monolayers in response to step commands from -100 to +70 mV in 10 mV increments at a holding potential of 0 mV. Experiments were performed using amphotericin B-permeabilized monolayers (apical surface) with KMeSO4 Ringer solution containing 10 mm NaCl bathing the apical surface and standard Ringer solution bathing the basolateral surface. The mean reversal potential was -59 ± 2 mV (n= 5, N= 3 for each experiment).
Figure 14. Current-voltage (I-V) and concentration-response relationships for the cAMP-sensitive conductance in the basolateral membrane.
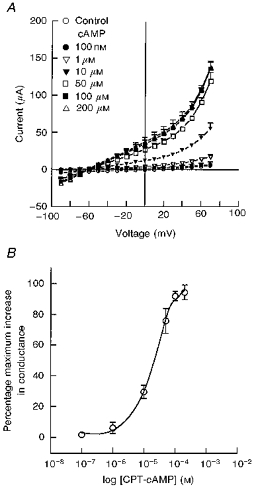
A, effects of increasing CPT-cAMP concentrations on the basolateral outwardly rectifying K+ conductance. The mean reversal potential was -61 ± 4 mV (n= 5, N= 3). B, concentration-response relationship showing the increase in basolateral membrane conductance following treatment with increasing concentrations of CPT-cAMP added to the basolateral solution. The EC50 value was 21 μm (n= 5, N= 3).
The apical and basolateral membrane conductances following PGE2 stimulation were plotted as a function of PGE2 concentration (Fig. 15). The EC50 values for PGE2 were calculated to be 14 nm for the apical membrane conductance and 81 nm for the basolateral membrane conductance. The concentration-conductance relationship of the basolateral membrane was not significantly different from the concentration-Isc relationship for PGE2.
Figure 15. Concentration-Isc and concentration-conductance response relationships following addition of increasing concentrations of PGE2 to the basolateral solution.
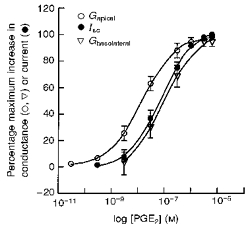
The EC50 values were 14 nm for the apical conductance (n= 6, N= 3), 73 nm for Isc (n= 10, N= 5) and 81 nm for the basolateral membrane conductance (n= 5, N= 3).
Effects of bumetanide and furosemide on Isc
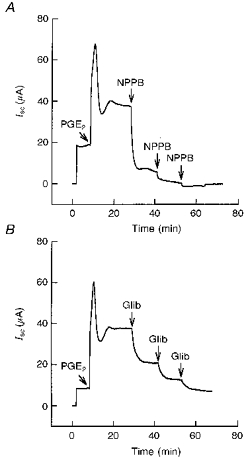
Figure 16A shows the effect of bumetanide on basal Isc when added to the basolateral solution. Addition of 100 μm bumetanide produced a sustained decrease of 41 ± 5 % in basal Isc. Basolateral addition of 100 μm furosemide produced a similar decrease in Isc with a mean percentage decrease of 37 ± 2 %. Maximum concentrations of bumetanide and furosemide inhibited 85–90 % of the PGE2-stimulated increase in Isc (Fig. 17). Analysis of the concentration- response relationship showed that the IC50 values for bumetanide and furosemide were 1.5 and 75 μm, respectively (Fig. 16B). Replacement of Cl− reduced the inhibitory effect of bumetanide on the basal Isc from 14 ± 2 to 0.8 ± 0.6 μA (n= 5, N= 3, P < 0.001) and on the PGE2-stimulated Isc from 18 ± 3 to 2 ± 0.9 μA (n= 5, N= 3, P < 0.001). Similar to bumetanide, chloride replacement reduced the inhibitory effect of furosemide on the basal Isc from 9 ± 1 to 1 ± 0.6 μA (n= 5, N= 3, P < 0.001) and on the PGE2-stimulated Isc from 11 ± 1 to 3 ± 0.6 μA (n= 5, N= 3, P < 0.001).
Figure 16. Effects of bumetanide and furosemide on basal Isc.
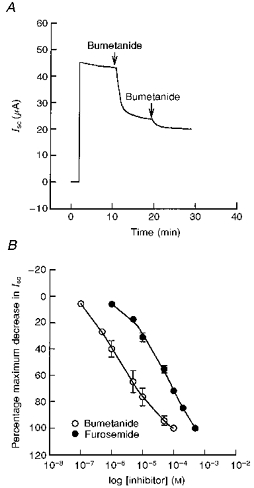
A, a representative Isc tracing showing the effect of bumetanide on basal Isc. Addition of 100 μm bumetanide to the basolateral solution produced a sustained decrease of 41 ± 5 % in basal Isc (n= 7, N= 4). B, concentration-response relationships showing the decrease in Isc following treatment with various concentrations of bumetanide and furosemide to the basolateral solution. The IC50 values were 1.5 μm for bumetanide and 75 μm for furosemide (n= 5, N= 3 for each compound). Bumetanide was found to be at least 50-fold more potent than furosemide.
Figure 17. Histogram illustrating the percentage decrease of bumetanide and furosemide on basal and PGE2-stimulated Isc in standard porcine Ringer solution.

Addition of 100 μm bumetanide to the basolateral solution decreased the basal Isc by 41 ± 5 % (n= 7, N= 4) and inhibited 88 ± 13 % of PGE2-stimulated Isc response (n= 5, N= 3, P < 0.001). Basolateral addition of 100 μm furosemide decreased the basal Isc by 37 ± 2 % (n= 4, N= 4) and inhibited 83 ± 11 % of PGE2-stimulated Isc response (n= 8, N= 3, P < 0.001).
DISCUSSION
Endometrial epithelial cells, grown from isolated endometrial glands, form confluent monolayers on membrane filters. The overall morphology of the cells, and the observation that nearly all of the cells were specifically labelled with cytokeratin antibody suggested that they were of epithelial origin. Time course experiments showed that these cells form high resistance monolayers within 3–4 days. The transepithelial resistance remained high for several weeks indicating that stable tight junctions had formed between the cells. Transport experiments performed with confluent monolayers showed that anion secretion was regulated by PGE2 and CPT-cAMP. Under basal conditions, a significant portion of the Isc was inhibited by pretreatment with indomethacin, an inhibitor of cyclooxygenase activity. Most of the basal Isc was blocked by NPPB and glibenclamide. Analysis of the current-voltage relationships for NPPB- and glibenclamide-sensitive currents at two different intracellular Cl− concentrations using amphotericin B-permeabilized monolayers showed that these compounds block anion channels present in the apical membrane. The observation that NPPB and glibenclamide had non-additive effects on Isc and that the reversal potentials for NPPB- and glibenclamide-sensitive currents were nearly identical indicates that these compounds block the same population of basally active Cl− channels.
Previous studies using porcine and guinea-pig colon as well as T84 cells showed that PGE2 stimulates Cl− secretion (Weymer, Huott, Lui, McRoberts & Dharmsathaphorn, 1985; Yajima, Suzuki & Suzuki, 1988; Traynor et al. 1993). Treatment of porcine endometrial epithelial cell monolayers with PGE2 produces a concentration-dependent increase in Isc which is blocked by pretreatment with NPPB or glibenclamide and by Cl− replacement. The relative potency of PGE2 was approximately 100 times greater than PGF2α, indicating that prostaglandin receptors present in the basolateral membrane were selective for PGE2. Experiments with amphotericin B-permeabilized monolayers demonstrated that the I-V relationships for the NPPB- and glibenclamide-sensitive currents after PGE2 stimulation had the same reversal potential as the basal NPPB-/glibenclamide-sensitive current and the PGE2-sensitive current. These currents were dependent on basolateral Cl− concentration, indicating that the PGE2-sensitive current was also Cl− dependent. These findings support the conclusion that NPPB and glibenclamide block a population of basally active Cl− channels that increase their conductance in response to PGE2. The effects of exogenous cAMP were similar to those reported for PGE2 with the exception that the reversal potential for the NPPB-sensitive I-V relationship (-38 ± 2 mV) was significantly more negative than the reversal potentials for CPT-cAMP alone (-29 ± 1 mV) or for glibenclamide (-30 ± 1 mV). One possible explanation for this effect could be that NPPB at maximum concentrations inhibits other ion channels that reverse at more hyperpolarized voltages. For example, NPPB might block a population of K+ channels which could result in the 8 mV shift in reversal potential observed in this experiment. It is interesting to note, however, that the actions of NPPB were not significantly different from those of glibenclamide following PGE2 stimulation. This would imply that increasing intracellular cAMP by exogenous addition of cAMP to the bathing solution produces activation of at least one other population of channels in the apical membrane that exhibits sensitivity to NPPB. At this time, the reason for the shift in reversal potential following CPT-cAMP but not PGE2 stimulation is not understood.
The cystic fibrosis transmembrane conductance regulator (CFTR) has been shown to mediate apical Cl− efflux in a variety of epithelia present in airways, intestine, pancreas and sweat glands (Cliff & Frizzell, 1990; Petersen, 1990; Anderson & Welsh, 1991; Fuller & Benos, 1992). The functional and immunocytochemical data presented in this study suggest that CFTR mediates apical Cl− efflux in response to PGE2 and exogenous cAMP stimulation in epithelial cells from porcine uterus. Evidence supporting this conclusion includes (1) activation of apical Cl− permeability with exogenous cAMP, (2) the inhibitory effects of glibenclamide and NPPB, (3) lack of inhibition by the disulphonic stilbene compound DIDS, (4) the near-linear current-voltage relationship of the PGE2-activated current observed under symmetric Cl− conditions, (5) the anion selectivity of the PGE2-activated current which matches that of CFTR and (6) the specific labelling of the apical region of cultured epithelial cells with an antibody to a peptide sequence present within the regulatory domain of CFTR. This conclusion is also consistent with previous studies demonstrating that CFTR mRNA is differentially expressed in glandular and surface epithelia of human uterus (Trizzano, Silver, Chitayat, Benichou & Buchwald, 1993), rat uterus and rat oviduct (Trezise et al. 1993). More recently, CFTR expression has been shown to be regulated by steroid hormones (Rochwerger & Buchwald, 1993; Mularoni, Beck, Sadir, Adessi & Nicollier, 1995). Experiments using immature and ovariectomized mature rats demonstrated that uterine CFTR mRNA could not be detected by in situ hybridization or by Northern blot analysis but was present after administration of 17 β-oestradiol (Rochwerger & Buchwald, 1993). Moreover, studies using primary cultures of rat uterine epithelial cells showed that CFTR mRNA was detected in the cells cultured in media supplemented with 17 β-oestradiol but not in Phenol Red-free medium containing charcoal-stripped serum (Rochwerger et al. 1994). These experiments indicate that CFTR expression in rat uterus is regulated by oestrogen. However, the data presented in this study showed that anion secretion and antibody labelling of cultured porcine endometrial cells were not affected by the use of Phenol Red-free medium containing charcoal-stripped serum after 7 days of exposure to these conditions. Thus, species differences may exist with respect to oestrogen-dependent regulation of CFTR in endometrial epithelial cells.
In addition to the effects of PGE2 on Cl− permeability in the apical membrane, experiments using amphotericin to permeabilize the apical membrane revealed the activation of an outwardly rectifying current in the basolateral membrane. The reversal potential of this PGE2-dependent current was −59 mV. Increasing the basolateral K+ concentration from 6 to 140 mm shifted the reversal potential to 0 mV, indicating that K+ was the current carrying ion. Activation of this conductance was dependent on PGE2 concentration. A comparison of the effects of PGE2 on Isc, apical Cl− conductance and basolateral K+ conductance indicates that significant increases in apical Cl− permeability occur at PGE2 concentrations that produce relatively modest changes in Isc. The data show that increases in Isc more closely correspond to increases in basolateral K+ conductance. We conclude from this observation that basolateral K+ permeability is rate limiting with respect to Cl− secretion. In a previous study of native porcine endometrium, we observed that PGF2α and cAMP produce increases in Na+ absorption across surface epithelial cells by activation of basolateral K+ channels. Inhibition of these channels with barium significantly blocked the effects of PGF2α and cAMP on Na+ transport (Vetter & O'Grady, 1996). In that study it was suggested that electrogenic K+ efflux across the basolateral membrane would provide an increase in driving force for Na+ uptake through amiloride-sensitive Na+ channels in the apical membrane. It seems reasonable to speculate that driving force may also be limiting for Cl− efflux across the apical membrane of cultured endometrial epithelial cells and that increases in basolateral membrane K+ permeability provide the necessary driving force to support maximum rates of Cl− secretion.
Previous studies of Cl− secretion in airway and intestinal epithelia have shown that Cl− uptake across the basolateral membrane is mediated by a bumetanide-sensitive Na+- K+-2Cl− cotransport mechanism (Haas, McBrayer & Yankaskas, 1993; Matthews et al. 1994). The inhibitory effects of bumetanide and furosemide on Isc when added to the basolateral surface of cultured endometrial epithelial cells also suggests the involvement of Na+-K+-2Cl− cotransport as a mechanism for Cl− uptake. This conclusion is supported by the IC50 determinations and order of potency for bumetanide and furosemide which are similar to those previously reported for the Na+-K+-2Cl− cotransporter (Widdicombe, Nathanson & Highland, 1983; O'Grady, Palfrey & Field, 1987). In addition, we demonstrated that the effects of bumetanide and furosemide on Isc were Cl− dependent, providing further evidence that the basolateral loop diuretic sensitive transport mechanism is involved in Cl− secretion.
In this study, indomethacin produced a significant decrease in basal Isc, suggesting that release of arachidonic acid metabolites from the epithelium modulates transepithelial ion transport. To investigate whether cultured porcine endometrial epithelial cells release PGE2 under basal conditions, we examined the effects of an antiPGE2 antibody on Isc. Our results show that this antibody at a concentration of 50 nm inhibits 20 % of the basal Isc and about 25 % of the indomethacin-sensitive portion of the Isc. Higher concentrations of the antibody did not produce any additional decrease in Isc (data not shown). Moreover, addition of increasing concentrations of exogenous PGE2 in the presence of 50 nm antiPGE2 antibody resulted in a 10-fold shift in the threshold response to exogenous PGE2. This decrease in threshold is consistent with the presence of excess unbound antibody in the basolateral solution available for immunoneutralization of exogenously added PGE2. This experiment suggests that PGE2 functions as an autocrine regulator of anion transport across the porcine glandular endometrium. The observation that indomethacin produces an additional Isc decrease in the presence of excess antiPGE2 antibody suggests that there are other arachidonic acid metabolites released from the epithelium that affect transepithelial electrolyte transport.
The stimulatory effect of PGE2 on Cl− secretion appears to be mediated by cAMP. This conclusion is supported by the observation that Isc responses to basolateral addition of PGE2 and CPT-cAMP were qualitatively and quantitatively similar. In addition, we demonstrated that the increase in Isc produced by CPT-cAMP was blocked by NPPB and glibenclamide. Experiments with amphotericin B-permeabilized monolayers also demonstrated that rectification characteristics and reversal potentials for the apical PGE2-activated Cl− conductance and the basolateral K+ conductance were nearly identical to the results obtained using CPT-cAMP. PGE2 has been shown to stimulate cAMP production in other epithelia (Sakairi & Jacobson, 1995). Our findings suggest that in cultured endometrial epithelial cells, PGE2 activates adenylyl cyclase resulting in an increase in intracellular cAMP. Cyclic AMP could increase apical Cl− and basolateral membrane K+ conductances either through protein phosphorylation, mediated by cAMP-dependent protein kinase, or perhaps by some direct effect of cAMP on the channels themselves.
A model summarizing the mechanism and regulation of Cl− secretion by PGE2 in porcine endometrial epithelial cells is presented in Fig. 18. Stimulation of cultured endometrial epithelial cells with PGE2 produces activation of Cl− secretion as a result of increasing apical Cl− permeability and basolateral K+ permeability. We propose that uptake of Cl− across the basolateral membrane is accomplished by Na+-K+-2Cl− cotransport activity which is blocked by the diuretic compounds bumetanide and furosemide. Na+ that enters the cell with Cl− is recycled across the basolateral membrane by the Na+-K+-ATPase. Basolateral potassium recycling is mediated in part by cAMP-activated K+ channels in response to PGE2 stimulation. Chloride that enters the cell by means of the cotransporter exits across the apical membrane through cAMP-activated Cl− channels which possess functional, immunological and pharmocological properties similar to that of CFTR.
Figure 18.
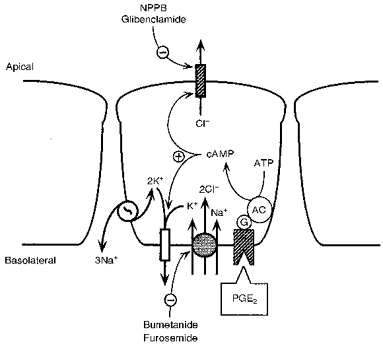
Proposed model explaining the mechanism of action of PGE2 on Cl− secretion across endometrial epithelial cells (see Discussion). AC, adenylyl cyclase; G, G protein.
Acknowledgments
The authors thank Dr Catherine Fuller and Dr Dale Benos for providing the antibody to CFTR and CFTR synthetic peptide used in this study and Dr Joseph Portanova for providing us with the antiPGE2 antibody. We wish to thank David Plath for his help in obtaining porcine endometrial tissue. The authors acknowledge the helpful comments and suggestions of Dr Alisen Vetter and Dr David Brown. This work was supported by NIH RO1 DA 10200 and by a grant from the Minnesota Agriculture Experiment Station.
References
- Alecozay AA, Harper MJK, Scheken RS, Hanahan DI. Paracrine interactions between platelet-activating factor and prostaglandins in hormonally-treated human luteal phase endometrium in vitro. Journal of Reproduction and Fertility. 1991;91:301–312. doi: 10.1530/jrf.0.0910301. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Welsh MJ. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proceedings of the National Academy of Sciences of the USA. 1991;88:6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt J, Casas A, Whaley E, Creazzo T, Lewis JB. Potassium dependence for sperm-egg fusion in mice. Journal of Experimental Zoology. 1991;257:245–251. doi: 10.1002/jez.1402570215. [DOI] [PubMed] [Google Scholar]
- Casslen B, Nilsson B. Human uterine fluid examined in undiluted samples for osmolarity and the concentrations of inorganic ions, albumin glucose and urea. American Journal of Obstetrics and Gynecology. 1984;150:877–881. doi: 10.1016/0002-9378(84)90466-6. [DOI] [PubMed] [Google Scholar]
- Chan HC, Fong SK, Chung YW, Wong PYD. Stimulation of anion secretion by β-adrenoreceptors in the mouse endometrial epithelium. Journal of Physiology. 1997;501:517–525. doi: 10.1111/j.1469-7793.1997.517bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri G. Biosynthesis and function of eicosanoids in the uterus. In: Carsten ME, Miller ID, editors. Uterine Function—Molecular and Cellular Aspects. New York: Plenum Press; 1991. pp. 423–448. [Google Scholar]
- Chen DB, Yang ZM, Hilsenrath R, Le SP, Harper MJK. Stimulation of prostaglandin (PG) F2α and PGE2 release by tumour necrosis factor-α in cultured human luteal phase endometrial cells. Human Reproduction. 1995;10:2773–2780. doi: 10.1093/oxfordjournals.humrep.a135790. [DOI] [PubMed] [Google Scholar]
- Clemetson CAB, Kim JK, Mallikarjuneswara VR, Wilds JH. The sodium and potassium concentrations in the uterine fluid of the rat at the time of implantation. Journal of Endocrinology. 1972;54:417–423. doi: 10.1677/joe.0.0540417. [DOI] [PubMed] [Google Scholar]
- Cliff WH, Frizzell RA. Separate Cl− conductances activated by cAMP and Ca2+ in Cl−-secreting epithelial cells. Proceedings of the National Academy of Sciences of the USA. 1990;87:4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DL, Blair RM. Studies of uterine secretions and products of primary cultures of endometrial cells in pigs. Journal of Reproduction and Fertility Supplement. 1993;48:143–155. [PubMed] [Google Scholar]
- Ford SP, Reynolds LP, Magness RR. Blood flow to the uterine and ovarian vascular beds of gilts during the estrous cycle and early pregnancy. Biology of Reproduction. 1982;27:878–885. doi: 10.1095/biolreprod27.4.878. [DOI] [PubMed] [Google Scholar]
- Fraser LR. Requirements of successful mammalian sperm capacitation and fertilization. Archives of Pathology Laboratory Medicine. 1992;116:345–350. [PubMed] [Google Scholar]
- Fraser LR. Ionic control of sperm function. Reproduction, Fertility and Development. 1995;7:905–925. doi: 10.1071/rd9950905. [DOI] [PubMed] [Google Scholar]
- Fuller CM, Benos DJ. CFTR! American Journal of Physiology. 1992;263:C267–286. doi: 10.1152/ajpcell.1992.263.2.C267. [DOI] [PubMed] [Google Scholar]
- Fuller CM, Howard MB, Bedwell DM, Frizzell RA, Benos DJ. Antibodies against the cystic fibrosis transmembrane regulator. American Journal of Physiology. 1992;262:C396–404. doi: 10.1152/ajpcell.1992.262.2.C396. [DOI] [PubMed] [Google Scholar]
- Haas M, McBrayer DG, Yankaskas JR. Dual mechanisms for Na+-K+-2Cl− cotransport regulation in airway epithelial cells. American Journal of Physiology. 1993;264:C189–200. doi: 10.1152/ajpcell.1993.264.1.C189. [DOI] [PubMed] [Google Scholar]
- Hart P, Warth JD, Levesque PC, Collier ML, Geary Y, Horowitz B, Hume JR. Cystic fibrosis gene encodes a cAMP-dependent chloride channel in heart. Proceedings of the National Academy of Sciences of the USA. 1996;93:6343–6348. doi: 10.1073/pnas.93.13.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iritani A, Sato E, Nishikawa Y. Secretion rates and chemical composition of oviduct and uterine fluids in sows. Journal of Animal Science. 1974;39:582–588. doi: 10.2527/jas1974.393582x. [DOI] [PubMed] [Google Scholar]
- Keys JL, King GL, Kennedy TG. Increased uterine vascular permeability at the time of embryonic attachment in the pig. Biology of Reproduction. 1986;34:405–411. doi: 10.1095/biolreprod34.2.405. [DOI] [PubMed] [Google Scholar]
- Leese HJ. The formation and function of oviduct fluid. Journal of Reproduction and Fertility. 1988;82:843–856. doi: 10.1530/jrf.0.0820843. [DOI] [PubMed] [Google Scholar]
- Leung A-YH, Wong PYD, Gabriel SE, Yankaskas JR, Boucher RC. cAMP- but not Ca2+-regulated Cl− conductance in the oviduct is defective in mouse model of cystic fibrosis. American Journal of Physiology. 1995;268:C708–712. doi: 10.1152/ajpcell.1995.268.3.C708. [DOI] [PubMed] [Google Scholar]
- MacLeod RJ, Redican F, Lembessis P, Hamilton JR, Field M. Sodium-bicarbonate cotransport in guinea pig ileal crypt cells. American Journal of Physiology. 1996;270:C786–793. doi: 10.1152/ajpcell.1996.270.3.C786. [DOI] [PubMed] [Google Scholar]
- Matthews CJ, Thomas EJ, Redfern CPF, Hirst BH. Ion transport by human endometrium in vitro. Human Reproduction. 1993;8:1510–1575. doi: 10.1093/oxfordjournals.humrep.a137893. [DOI] [PubMed] [Google Scholar]
- Matthews JB, Smith JA, Tally KJ, Awtrey CS, Nguyen H, Rich J, Madara JL. Na+-K+-2Cl− cotransport in intestinal epithelial cells. Journal of Biological Chemistry. 1994;269:15703–15709. [PubMed] [Google Scholar]
- Mnich SJ, Veenhuizen AW, Monahan JB, Sheehan KC, Lynch KR, Isakson PC, Portanova JP. Characterization of a monoclonal antibody that neutralizes the activity of prostaglandin E2. Journal of Immunology. 1995;155:4437–4444. [PubMed] [Google Scholar]
- Mularoni A, Beck L, Sadir R, Adessi GL, Nicollier M. Downregulation by progesterone of CFTR expression in endometrial epithelial cells: A study by competitive RT-PCR. Biochemical and Biophysical Research Communications. 1995;217:1105–1111. doi: 10.1006/bbrc.1995.2883. [DOI] [PubMed] [Google Scholar]
- O'Grady SM, Palfrey HC, Field M. Characteristics and functions of Na+-K+-2Cl− cotransport in epithelial tissues. American Journal of Physiology. 1987;253:C177–192. doi: 10.1152/ajpcell.1987.253.2.C177. [DOI] [PubMed] [Google Scholar]
- Peterson PS. Chloride permeability regulation via a cyclic AMP pathway in cultured human sweat duct cells. Journal of Physiology. 1990;421:379–397. doi: 10.1113/jphysiol.1990.sp017950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochwerger L, Buchwald M. Stimulation of the cystic fibrosis transmembrane regulator expression by estrogen in vivo. Endocrinology. 1993;133:921–930. doi: 10.1210/endo.133.2.7688293. [DOI] [PubMed] [Google Scholar]
- Rochwerger L, Dho S, Parker L, Foskett JK, Buchwald M. Estrogen-dependent expression of the cystic fibrosis transmembrane regulator. Journal of Cell Science. 1994;107:2439–2448. doi: 10.1242/jcs.107.9.2439. [DOI] [PubMed] [Google Scholar]
- Sakairi Y, Jacobson HR. Luminal prostaglandin E2 receptors regulate salt and water transport in rabbit cortical collecting duct. American Journal of Physiology. 1995;269:F257–265. doi: 10.1152/ajprenal.1995.269.2.F257. [DOI] [PubMed] [Google Scholar]
- Saunders-Kirkwood K, Cates JA, Roslyn JJ. Prostaglandin E2 stimulates ion transport in prairie dog gallbladder. Digestive Disease and Sciences. 1993;38:167–172. doi: 10.1007/BF01296791. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. Journal of Clinical Investigation. 1992;89:1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor TR, Brown DR, O'Grady SM. Effects of inflammatory mediators on electrolyte transport across the porcine distal colon epithelium. Journal of Pharmacology and Experimental Therapeutics. 1993;264:61–66. [PubMed] [Google Scholar]
- Trezise AO, Linder CC, Grieger D, Thompson EW, Meunier H, Griswold MD, Buchwald M. CFTR expression is regulated during both the cycle of the seminiferous epithelium and the estrous cycle of rodents. Nature Genetics. 1993;3:157–164. doi: 10.1038/ng0293-157. [DOI] [PubMed] [Google Scholar]
- Trizzano EF, Silver MM, Chitayat D, Benichou J, Buchwald M. Differential cellular expression of cystic fibrosis transmembrane regulator in human reproductive tissues. American Journal of Pathology. 1994;144:906–914. [PMC free article] [PubMed] [Google Scholar]
- Vetter AE, O'Grady SM. Mechanisms of electrolyte transport across the endometrium I. Regulation by PGF2α and cAMP. American Journal of Physiology. 1996;270:663–672. doi: 10.1152/ajpcell.1996.270.2.C663. [DOI] [PubMed] [Google Scholar]
- Wenzl E, Sjaastad MD, Weintraud WH, Machen TE. Intracellular pH regulation in IEC-6 cells, a cryptlike intestinal cell line. American Journal of Physiology. 1989;257:G732–740. doi: 10.1152/ajpgi.1989.257.5.G732. [DOI] [PubMed] [Google Scholar]
- Weymer A, Huott P, Lui W, McRoberts JA, Dharmsathaphorn K. Chloride secretory mechanism induced by prostaglandin E1 in a colonic epithelial cell line. Journal of Clinical Investigation. 1985;76:1828–1836. doi: 10.1172/JCI112175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JH, Nathanson IT, Highland E. Effects of ‘loop’ diuretics on ion transport by dog tracheal epithelium. American Journal of Physiology. 1983;245:C388–396. doi: 10.1152/ajpcell.1983.245.5.C388. [DOI] [PubMed] [Google Scholar]
- Yajima T, Suzuki T, Suzuki Y. Synergism between calcium-mediated and cyclic AMP-mediated activation of chloride secretion in isolated guinea pig distal colon. Japanese The Journal of Physiology. 1988;38:427–443. doi: 10.2170/jjphysiol.38.427. [DOI] [PubMed] [Google Scholar]
- Yee GM, Kennedy TG. PGE2, cAMP and cAMP-dependent protein kinase isozymes during decidualization of rat endometrial stromal cells. Prostaglandins. 1993;46:117–138. doi: 10.1016/0090-6980(93)90038-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Paria BC, Davis DL. Pig endometrial cells in primary culture: morphology, secretion of prostaglandins and proteins, and effects of pregnancy. Journal of Animal Science. 1991;69:3005–3015. doi: 10.2527/1991.6973005x. [DOI] [PubMed] [Google Scholar]


