Abstract
The superficial layers II and III of the entorhinal cortex, which form the main cortical input to the hippocampus, receive a large serotonergic projection from the raphe nuclei and express 5-HT receptors at high density. Here, we studied the effects of serotonin on the intrinsic properties and excitatory synaptic transmission of the superficial medial entorhinal cortex.
Intracellular and patch clamp recordings revealed that serotonin hyperpolarized only one-third of the cells, approximately, through a potassium conductance via a GTP-dependent process.
Serotonin depressed mixed as well as isolated α-amino-3-hydroxy-5-methyl-4-isoxazole- propionic acid receptor (AMPAR)- and N-methyl-d-aspartic acid receptor (NMDAR)-mediated excitatory postsynaptic potentials/currents (EPSPs/EPSCsapproximately 40 % reduction with 1 μm serotonin).
The effect of serotonin on EPSPs/EPSCs was similar in whole-cell versus intracellular recordings; it did not require intracellular GTP and was not visible in glutamate applications to excised patches. Miniature EPSCs recorded in the presence of tetrodotoxin and bicuculline were reduced in frequency, but not altered in amplitude.
The effects of serotonin on intrinsic properties and EPSPs were partially mimicked by 5-HT1A receptor agonists (±)-8-hydroxy-2-(di-n-propylamino)tetralin hydrobromide (8-OH-DPAT) and 5-carboxamido-tryptamine maleate (5-CT), and reduced by 5-HT1A receptor antagonists S-(-)-5-fluoro-8-hydroxy-DPAT hydrochloride (S-UH-301), 1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine hydrobromide (NAN-190) and spiperone.
We conclude that serotonin potently suppresses excitatory synaptic transmission via 5-HT1A receptors in layers II and III of the medial entorhinal cortex by a presynaptic mechanism.
The entorhinal cortex (EC) receives sensory information from various sources which is then passed to and received back from the hippocampus (Alonso & Köhler, 1984). Far from simply acting as a funnel for these inputs and outputs, the EC has complex intrinsic properties which contribute to signal processing (Jones, 1993). The superficial cells form the two branches of the perforant path input to the hippocampus (Steward & Scoville, 1976). Layer II cells project to the full transverse extent of the dentate gyrus and CA3 regions. Layer III cells project to CA1 and the subiculum (Steward & Scoville, 1976).
The cells of the superficial layers of the EC receive a strong serotonergic projection from the raphe nuclei and also express a high density of 5-HT receptors (Bobillier, Petitjean, Salvert, Ligier & Seguin, 1975; Pazos, Cortés & Palacios, 1985; Pazos & Palacios, 1985). Interestingly, this region shows characteristic lesions in certain forms of schizophrenia, Alzheimer's and Korsakow's disease which are associated with changes in serotonergic transmission and serotonin turnover in the EC (Langlais, Mair, Anderson & McEntee, 1987; Tejani-Butt, Yang & Pawlyk, 1995). Moreover, recent evidence suggests that the serotonergic system is involved in the modulation of slow rhythmic activity and its function in spatial learning (Vanderwolf, 1988; Richter-Levin & Segal, 1991).
Here, we show, using intracellular and patch clamp recordings from layer II and III cells in the medial EC, that the main action of serotonin is to reduce excitatory synaptic transmission in the superficial EC, most probably through a presynaptic mechanism.
METHODS
Slice preparation
Horizontal slices containing the hippocampus, entorhinal, perirhinal and temporal cortex were prepared from adult, mostly male, Wistar rats (180–250 g), as previously described (Schmitz, Empson & Heinemann, 1995b). In brief, the animals were deeply anaesthetized with ether, decapitated and the brain removed. Tissue blocks containing the temporal cortex and hippocampus were mounted on a Vibratome (Campden Instruments, Loughborough, UK) in a chamber filled with cold (approximately 4°C) artificial cerebrospinal fluid (ACSF), containing (mm): NaCl, 124; NaHCO3, 26; KCl, 3; NaH2PO4, 1.25; CaCl2, 1.6; MgSO4, 1.8; and glucose, 10; saturated with 95 % O2-5 % CO2, pH 7.4. Horizontal slices were cut at 300–400 μm thickness and transferred to an interface chamber where they were maintained at 35°C and perfused with ACSF at a rate of 1.5–1.8 ml min−1. The slices were allowed to rest for at least 1 h after the preparation, before recording.
Electrophysiological recordings
Intracellular electrodes were pulled from borosilicate glass (o.d., 1.2 mm) and filled with 2 m potassium or caesium acetate. In some recordings, 50 mm 2(triethylamino)-N-(2,6-dimethylphenyl)acetamide (QX-314) was also included. Electrode resistance was 40–120 MΩ. Intracellular recordings were performed in an interface chamber at 35°C using a Neuro Data IR 183 (Neuro Data Instruments Corp., New York, USA) or a SEC10L (NPI Electronic, Tamm, Germany) amplifier. Cells were impaled and then allowed to rest for 5–10 min before recording. Only cells with resting potentials more negative than −50 mV and with overshooting action potentials were accepted. Resting membrane potentials of the cells were corrected after the recording by subtraction of the remaining tip potential after withdrawal of the pipette.
Patch clamp electrodes were pulled from borosilicate glass (o.d., 2.0 mm; i.d., 1.0 mm). When filled with the internal solution (see below), they had resistances of 1.5–5 MΩ. The pipettes were fire polished, and for experiments on excised patches they were coated with Sylgard (Dow Corning) to reduce capacitive noise. Evoked EPSPs/EPSCs were recorded in whole-cell configuration with a ‘switched’ discontinuous voltage clamp amplifier (SEVC, SEC10L, NPI Electronic), whereas for miniature EPSCs and excised patch recordings we used continuous feedback patch clamp amplifiers (EPC-7, List or EPC-9, HEKA, Lambrecht, Germany). The passive parameters of each cell and patch were recorded as responses to 10 mV hyperpolarizing voltage steps with nominally no low-pass filtering. Membrane resistance was estimated as Rm= dV/Ioffset, series resistance as Rser= dV/Imax and effective series resistance as Rser,comp=Rser× (1 - percentage compensation/100) (when using continuous feedback patch clamp amplifiers). Access resistances were Rser= 14 ± 2 MΩ (variation between 4 and 20 MΩ) and Rser,comp= 6 ± 1 MΩ. Access resistance was repeatedly checked during the experiment, and recordings showing an increase of more than 20 % were rejected.
Miniature EPSCs and stimulus-evoked EPSPs/EPSCs were recorded in an interface chamber at 35°C in the ‘blind’ version of the slice patch clamp technique (Blanton, Lo Turco & Kriegstein, 1989). Compound polysynaptic potentials were evoked by electrical stimulation (0.05 ms duration, 1–30 V) at 0.1–0.05 Hz via a bipolar insulated stimulation electrode placed mostly in the lateral EC and sometimes also in the deep medial EC or the subiculum. However, we could not find any differences for particular synaptic pathways. Fast application of glutamate was performed on isolated patches from visualized and morphologically identified neurons using infrared video microscopy and differential interference contrast (IR-DIC) in a submerged recording chamber at room temperature (20–22°C) (Stuart, Dodt & Sakmann, 1993). The rapid application of agonists was achieved by lateral movements of a double-barrelled pipette (Jonas, 1995). The double-barrelled application pipette was fabricated from theta glass (o.d., 2.0 mm; wall thickness, 0.3 mm; septum, 0.12 mm: Hilgenberg, Malsfeld, Germany) driven by a low-voltage-operated piezoelectric element (P-810.20, Physik Instrumente, Waldbronn, Germany). The exchange time (20–80 %) was 500–1000 μs as measured with an open patch electrode during a change between 140 mm K+ and 14 mm K+. Glutamate pulses were applied to outside-out patches every 6 s for activation of AMPA receptors (AMPARs) and every 10–15 s for activation of NMDA receptors (NMDARs).
The signals were filtered at 3 kHz, digitized at 8–10 kHz by a CED 1401 (Cambridge Electronic Design (CED), Cambridge, UK) or an ITC-16 (Instrutech Corp., Great Neck, NY, USA) interface, and subsequently stored on an IBM-compatible computer.
‘Switched’ single electrode voltage clamp recordings were performed with conventional sharp microelectrodes and also with patch pipettes (see above) using a SEVC amplifier (SEC10L, NPI Electronic). After clamping the cell close to the resting membrane potential, we optimized the gain, capacitance compensation and switching frequency (8–35 kHz). The headstage voltage was continuously monitored and always decayed sufficiently before the next current injection (1/4 duty cycle). Clamp efficiency, as estimated from the difference between the clamped and unclamped EPSP, ranged from 70 to 90 %.
Drugs and solutions
Most drugs were bath applied at the concentrations indicated. Bicuculline methiodide (5 μm) and 5-hydroxytryptamine creatine sulphate complex (5-HT) were purchased from Sigma. (±)-2-Amino-5-phosphonopentanoic acid (APV, 30–60 μm), 1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine hydrobromide (NAN-190, 10–50 μm), spiperone (5 μm), (±)-8-hydroxy-2-(di-n-propylamino)tetralin hydrobromide (8-OH-DPAT, 0.1, 1, 10 and 50 μm), CGS-12066A maleate (10 μm), 2-methyl-5-hydroxytryptamine maleate (10 and 50 μm), α-methyl-5-hydroxytryptamine maleate (10 and 50 μm), 5-carboxamido-tryptamine maleate (0.1, 1 and 10 μm), S-(-)-5-fluoro-8-hydroxy-DPAT hydrochloride (S-UH-301, 1 and 10 μm), ritanserin (10 μm), clozapine (10 μm) and methysergide maleate (10 μm) were all from Research Biochemicals International. 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 μm) was from Tocris Cookson (Bristol, UK). 1,2,3,4-Tetrahydro-6-nitro-2,3-dioxo-benzol(f)quinoxaline-7-sulphonamide (NBQX, 10 μm) was a kind gift from Novo Nordisk (Denmark) and CGP 55845A (2–5 μm) was a kind gift from Ciba Geigy.
In order to isolate EPSPs or EPSCs, we applied bicuculline methiodide and CGP 55845A together with either NBQX, CNQX, or APV. For miniature EPSCs, we applied TTX at high concentrations (1–5 μm) to avoid activation of the Na+ current with low TTX affinity, which has been described in EC neurons (White, Alonso & Kay, 1993).
Serotonin was also applied, in some experiments, to the surface of the slice from a glass pipette with a 10–20 μm tip diameter placed close to the cell from which we recorded. The substance was dissolved at 0.5–1 mm concentration in ACSF, and a blue or green food dye (McCormick, Baltimore, MD, USA) was included to monitor the spread of the applied drug. Control experiments with ACSF and the dye did not elicit any effects. The area covered by the applied drugs was estimated to be approximately 100 μm in diameter. These kinds of applications were mostly done for testing the effects of serotonin on intrinsic properties of the neurons.
The Hepes-buffered external solution used for the fast application of agonists (one barrel without and the other barrel with 10 mm glutamate) to excised patches contained (mm): NaCl, 130; KCl, 3; MgCl2, 1; CaCl2, 2; Hepes, 10; and glucose, 25; pH adjusted to 7.3 with NaOH. To study glutamate-activated currents on AMPARs in isolation, APV was added to both barrels. To study NMDARs in isolation, NBQX and glycine (10 μm) were added to both barrels, and the external Mg2+ was omitted. When studying the effects of serotonin on glutamate-activated currents, 50 μm 5-HT and 50 μm Na2S2O5 (an antioxidant) were added to both barrels of the theta glass. The pipette solution in whole-cell and excised patch recordings contained (mm): KCl, 140; MgCl2, 1; CaCl2, 1; Hepes, 10; and EGTA, 10; pH adjusted to 7.3 with KOH. To study the postsynaptic effects of 5-HT, 0.5 mm Tris-GTP (Sigma) was added to the K+-rich solution. Alternative pipette solutions contained (mm): CsCl, 140; MgCl2, 1; CaCl2, 1; Hepes, 10; and EGTA, 10; pH adjusted to 7.3 with CsOH. In some experiments we used the following internal solution (mm): CsF, 140; NaCl, 10; MgCl2, 1; CaCl2, 1; Hepes, 10; and EGTA, 10; pH adjusted to 7.3 with CsOH.
Data analysis and statistics
Data were analysed off-line using Sigavg (CED, UK) or Wintida (HEKA). Amplitudes of evoked synaptic potentials and currents were measured from averaged (3–10) sweeps. Spontaneous synaptic currents were recorded on video tape at 36 kHz digitization rate and subsequently filtered at 3 kHz with an eight-pole Bessel filter, redigitized at 8–10 kHz and then analysed using an event detection program (CDR and SCAN; Strathclyde Electrophysiology Software, courtesy of Dr J. Dempster). The amplitude distributions were compared by using a Kolmogorov-Smirnov test (SPSS program). The kinetics of the EPSCs were described as 20–80 % rise time and the decay was approximated by monoexponential or the sum of two exponential fits (Koh, Geiger, Jonas & Sakmann, 1995; Silver, Colquhuon, Cull-Candy & Edmonds, 1996). Data are expressed as means ±s.e.m. Drug effects were analysed with Student's t test (SigmaPlot, Jandel, Corte Madera, USA) for paired data, and an error probability of P < 0.05 was regarded as significant.
RESULTS
We performed intracellular and patch clamp recordings from different types of projection neurons of layers II and III of the medial entorhinal cortex. We could identify different projection neurons on the basis of their morphological (typical illustration of a layer III projection neuron in Fig. 5A) and electrophysiological characteristics. Therefore, we performed recordings from stellate and non-stellate cells of layer II (Alonso & Klink, 1993) and two types of pyramidal cells within layer III (type 1 and 2; see Gloveli, Schmitz, Empson, Dugladze & Heinemann, 1997). Since we could not find differences in the reaction towards serotonin within the group of projection neurons, we pooled the data.
Figure 5. Effects of serotonin on AMPAR- and NMDAR-mediated currents activated by fast application of glutamate to outside-out patches.
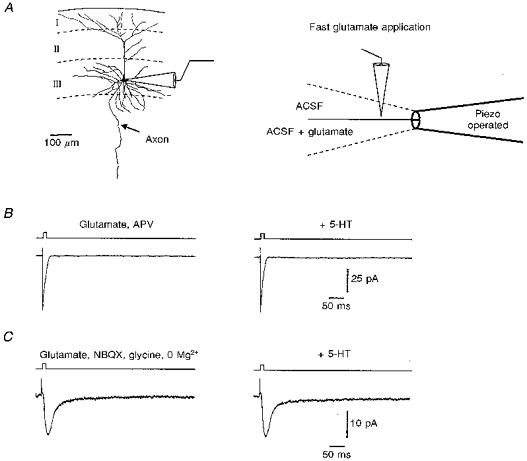
A, schematic drawings of the methodological principle of the fast application system (representative camera lucida drawing of a layer III projecton neuron). B, 5-HT (50 μm) had no effect on isolated AMPAR-mediated currents when elicited by a fast application of glutamate (10 mm) in the presence of 60 μm APV in both barrels. C, glutamate-activated currents in the presence of 20 μm NBQX and 10 μm glycine, while the external Mg2+ was omitted (both barrels). Again, 5-HT did not show any effects on the NMDAR-mediated currents.
Effects of serotonin on intrinsic properties of projection neurons of the superficial layers of the entorhinal cortex
Only one-third of the projection neurons (15 out of 42 cells recorded with sharp microelectrodes without K+ channel blockers) responded to the application of 5-HT with a small hyperpolarization of -2.5 ± 0.4 mV (Fig. 1A). The corresponding outward currents were measured in single-electrode voltage clamp recordings with sharp microelectrodes and had an amplitude of 42.6 ± 14 pA (not shown). In two of these cells, the initial hyperpolarization was followed by a small (1–2 mV) depolarization, which brought none of the cells to the threshold for action potential generation. The 5-HT-induced hyperpolarization was associated with a decrease in input resistance from 54 ± 7 to 47 ± 6 MΩ (P < 0.05) and reversed at about −90 mV (n= 3). Recordings with CsCl-filled electrodes or with QX-314 in the electrode solution (n= 5 each) strongly reduced or abolished the hyperpolarization. These effects of Cs+ and QX-314 developed gradually over time following impalement (10–40 min), and for QX-314 they paralleled the time course of the blockade of the action potentials. Extracellularly applied Cs+ (n= 3) or the 5-HT1A receptor antagonists NAN-190 (n= 4) and spiperone (n= 3) were also able to block the 5-HT-induced hyperpolarization. Moreover, in whole-cell recordings, the serotonin-induced hyperpolarization was absent or vanished quickly after breaking through the cell membrane (n= 40, experiments on evoked EPSPs/EPSCs and miniature EPSCs included). However, the response persisted in recordings with GTP in the internal patch solution (n= 5).
Figure 1. Effects of serotonin on membrane potential and input resistance.
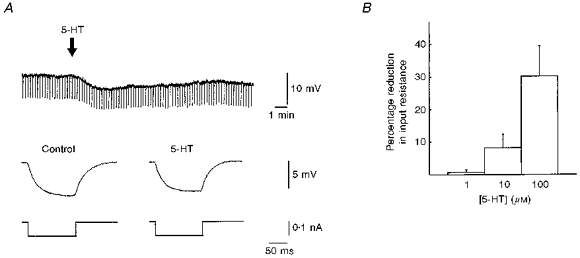
A, the downward deflections in the upper trace represent the changes in membrane potential during negative current pulses. Following 5-HT application, there was a small hyperpolarization which was associated with a decrease in the input resistance, as shown in the lower traces. However, this effect of serotonin was only observed in one-third of the cells, and the amplitudes of the hyperpolarization were quite small (see Results). B summarizes the concentration dependency of the reduction in input resistance in 6 cells (out of the one-third of cells which showed a hyperpolarization following the application of 5-HT).
Serotonin reduces synaptically evoked EPSPs/EPSCs independent of the effects on membrane properties
EPSPs were mostly evoked by electrical stimulation of the lateral entorhinal cortex and sometimes also in the deep medial EC or the subiculum. However, we could not find any differences for particular synaptic pathways. Serotonin reduced the amplitude of these mixed EPSPs in a dose-dependent manner with high potency (40 % depression at 1 μm; see Fig. 2A). In most cases (approximately two-thirds of the cells), the effects of 5-HT on EPSPs/EPSCs were independent of any effects of 5-HT on intrinsic properties of the cell, suggesting that the effects of 5-HT on excitatory synaptic transmission were independent of the properties of the postsynaptic cell (see also Fig. 3A). Moreover, we also observed reductions in the synaptic responses in experiments in which we blocked the 5-HT-induced conductance increase by intracellular potassium channel blockers (Cs+, QX-314).
Figure 2. Effects of serotonin, 5-HT receptor agonists and antagonists on mixed EPSPs.
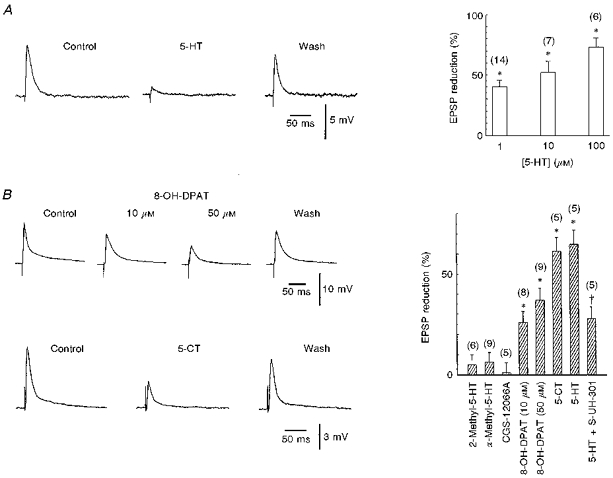
A, 5-HT suppressed the EPSPs in a concentration-dependent manner. Statistical diagram of pooled data from 27 experiments. The asterisks indicate that the mean ratios are significantly different from control (P < 0.01, paired t tests). The number of experiments is given in parentheses. B, the 5-HT1A receptor agonists 8-OH-DPAT and 5-CT (10 μm) could both mimick the effect of serotonin on mixed EPSPs in two different cells. Histograms summarizing the lack of effect (2-methyl-5-HT, α-methyl-5-HT, CGS-12066A) and effects (8-OH-DPAT, 5-CT) of different 5-HT receptor agonists. * Mean ratios significantly different from control (P < 0.01, paired t tests). The last two bars demonstrate the antagonistic effect of the 5-HT1A receptor antagonist S-UH-301. Whilst 50 μm 5-HT depressed the EPSPs by 64.8 ± 7 %, in the same 5 cells the effect was reduced to 27.8 ± 5.7 when 5-HT was co-applied with S-UH-301. † Significantly different mean ratios (P < 0.01, paired t test). Numbers of experiments are given in parentheses.
Figure 3. Influence of serotonin on AMPAR- and NMDAR-mediated EPSCs.
Serotonin depressed both AMPAR- and NMDAR-mediated EPSCs in two different cells of the entorhinal cortex, when measured with a sharp microelectrode and an unchanged intracellular milieu. Note the differences in amplitude and time scales. A, 5-HT reduced the AMPAR-mediated EPSCs (in APV, bicuculline and CGP 55845A) in a layer III projection neuron of the medial entorhinal cortex. Note that there was no outward current (upper trace, collected with a chart recorder) and no change in the total conductance of the cell (as shown by the current response to voltage step command, prior to the synaptically evoked EPSC) following the application of 5-HT. This cell was voltage clamped at −79 mV with a gain of 10.6 nA mV−1 and a switching frequency of 13 kHz. Clamp efficiency during the EPSCs was estimated from the difference between the clamped and the unclamped EPSP; in this cell the clamp efficiency was 76.9 % (clamped EPSP, 4 mV; unclamped EPSP, 17.3 mV). B, 5-HT also reduced the NMDAR-mediated EPSCs (in NBQX, bicuculline and CGP 55845A) in another cell of the superficial layers of the entorhinal cortex. This cell was voltage clamped at −69 mV with a gain of 10.4 nA mV−1 and a switching frequency of 20 kHz. Clamp efficiency in this cell was 90.3 % (clamped EPSP, 0.6 mV; unclamped EPSP, 6.2 mV). Following the 5-HT application, a small outward current of 50 pA was noted in this cell (not shown).
In order to identify the subtype of the 5-HT receptor mediating the depression of EPSPs, we applied various specific agonists: 8-OH-DPAT and 5-CT (both agonists on the 5-HT1A and 5-HT7 receptors), CGS-12066A (5-HT1B), α-methyl-5-HT (5-HT2C) and 2-methyl-5-HT (5-HT3). However, only 8-OH-DPAT and 5-CT mimicked the effect of 5-HT on EPSPs (Fig. 2B). In contrast, all other specific agonistic drugs (CGS-12066A, α-methyl-5-HT, 2-methyl-5-HT) were ineffective in mimicking the serotonin effect (see also histograms in Fig. 2B). This result suggests that 5-HT1A and/or 5-HT7 receptors are involved in the effect of serotonin. The 5-HT7 receptor is a recently identified 5-HT receptor subtype (Lovenberg et al. 1993; Ruat et al. 1993), which has an agonist profile closely related to that of the 5-HT1A receptor. Binding profiles of the 5-HT7 receptor revealed high binding affinities for several antagonists, for example ritanserin and methysergide (Lovenberg et al. 1993; Ruat et al. 1993). Therefore, we tested the possible antagonistic effect of these drugs. Neither drug could antagonize the effects of serotonin on EPSPs (n= 4 for each substance; P≥ 0.1). On the other hand, co-application of S-UH-301 (10 μm), a highly potent and selective 5-HT1A receptor antagonist, with 5-HT reduced the effects of serotonin (see also the last two bars in the histogram in Fig. 2B). However, the effect of serotonin on EPSPs could only be antagonized when S-UH-301 was applied in high concentrations (see above).
We then isolated AMPAR- and NMDAR-mediated components of the EPSPs/EPSCs by addition of bicuculline (5 μm) and CGP 55845A (2–5 μm) together with either APV (30 μm) or NBQX (10 μm). In recordings with conventional intracellular electrodes (leaving the intracellular metabolism largely unaltered) serotonin depressed isolated AMPAR- as well as isolated NMDAR-mediated EPSPs/EPSCs to the same extent (1 μm: 43 ± 5 %, n= 9, P < 0.05, and 42 ± 5 %, n= 9, P < 0.05, respectively (see Fig. 3); 10 μm: 53.3 ± 10.1 %, n= 7, P < 0.05, and 52.9 ± 10.0 %, n= 7, P < 0.05, respectively).
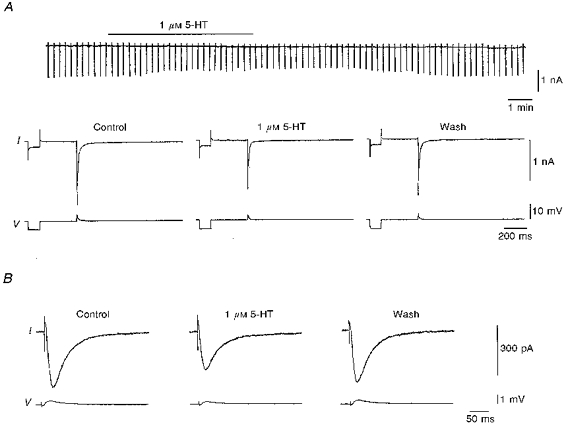
Any effect on postsynaptic responses mediated by the action of serotonin receptors on pertussis-sensitive G-proteins will be abolished by intracellular perfusion of the cell in patch clamp recordings (Andrade, 1992). However, both glutamatergic EPSP/EPSC components (measured either with voltage or current clamp) were still strongly reduced by serotonin under these conditions (51 ± 10 % (n= 13) and 54 ± 9 % (n= 6) reduction of isolated AMPA- and NMDA-mediated EPSPs/EPSCs, respectively, Fig. 4). However, serotonin did not change the kinetics of the AMPAR-mediated EPSCs. The rising phase (time to peak, 20–80 %) was 1.7 ± 0.2 ms under control and unchanged (1.8 ± 0.2 ms) after application of serotonin (P= 0.1). The decay phases were 14.4 ± 1.4 ms under control and 13.9 ± 1.5 ms with serotonin when monoexponentially fitted (n= 9, P= 0.2). When fitted with the sum of two exponentials, the decay phases were τ1= 8.1 ± 0.5 ms and τ2= 59.8 ± 10 ms under control, and τ1= 8.1 ± 0.4 ms and τ2= 60.8 ± 11 ms after application of serotonin (n= 4). The kinetics of the NMDAR-mediated EPSCs were also not affected by serotonin. The rising phases were 7.6 ± 0.5 ms under control and 7.4 ± 0.3 ms with serotonin (P= 0.6). The decay phases were 43.8 ± 3.1 ms under control and 43.5 ± 2.7 ms with serotonin (P= 0.6, n= 6).
Figure 4. Effects of serotonin on AMPAR- and NMDAR-mediated EPSCs.
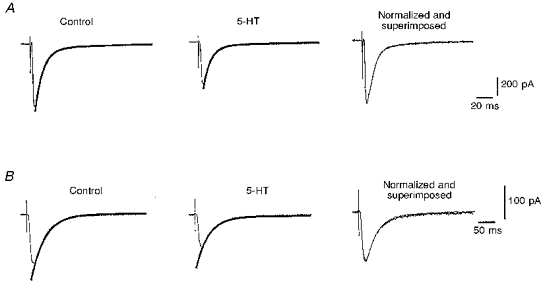
Serotonin depressed both AMPAR-mediated (in APV, bicuculline and CGP 55845A) and NMDAR-mediated (in NBQX, bicuculline and CGP 55845A) EPSCs in 2 different cells, when measured with a patch electrode, where the intracellular milieu was changed by perfusion with the internal solution of the patch pipette. A, 5-HT reduced the AMPAR-mediated EPSCs in a layer II neuron of the entorhinal cortex, while the kinetics of the event were unchanged (right). There was no detectable slow outward current following the application of 5-HT. B, 5-HT also reduced the NMDAR-mediated EPSCs in another cell of the superficial layers of the entorhinal cortex, while the kinetics of the event were again unchanged (right).
Serotonin had no effect on the response of cells to fast glutamate applications
Serotonin can interact directly with glutamate receptors (Murase, Randic, Shirasaki, Nakagawa & Akaike, 1990), and this could be the mechanism operating here. To address this possibility we performed fast applications of glutamate on outside-out patches (Jonas, 1995) from the somata or dendrites of visualized and morphologically identified cells of the superficial layers of the entorhinal cortex (Fig. 5). To study glutamate-activated currents on AMPARs in isolation, APV was added to both barrels of the application pipette. To study NMDARs in isolation, NBQX and glycine were added to both barrels and the external Mg2+ was omitted. After recording control responses to glutamate (10 mm), 50 μm 5-HT and 50 μm Na2S2O5 (antioxidants) were added to both barrels of the theta glass. In control experiments, the glutamate-activated currents on AMPARs peaked at 0.7 ± 0.2 ms (20–80 % rise time) and had a mean amplitude of 13.0 ± 3.3 pA (n= 9). However, serotonin did not influence the AMPAR-mediated currents (rise time, 0.7 ± 0.2 ms; amplitude, 13.1 ± 3.3 pA; see Fig. 5B). The decay time constants (control, 10.8 ± 1.8 ms; serotonin, 11.3 ± 1.9 ms) were also not changed by serotonin (P= 0.3, n= 9). Moreover, glutamate-activated currents through NMDARs, which had a rise time of 5.2 ± 0.6 ms and a mean amplitude of 13.83 ± 2.76 (n= 9), were also not affected by serotonin (rise time, 5.2 ± 0.6 ms; amplitude, 13.76 ± 2.83 pA; see also Fig. 5C). The decay time constants (control, 32.8 ± 9.5 ms; serotonin, 30.8 ± 10.0 ms) were also not changed by serotonin (P= 0.2, n= 6).
Serotonin did not alter the amplitude of miniature EPSCs, but did alter their frequency
To address the question of whether serotonin acts by a modulation of presynaptic terminals, we investigated the effects of serotonin on spontaneous miniature EPSCs in cells of the superficial layers of the entorhinal cortex. Whole-cell recordings under TTX (1–5 μm) and bicuculline (5 μm) at -60 to −80 mV revealed spontaneous synaptic inward currents. These currents were blocked by glutamate receptor antagonists, suggesting that they reflect the spontaneous release of glutamate from presynaptic terminals. The frequency of the events varied considerably between cells (minimum, 0.85 Hz; maximum, 11.1 Hz; mean, 2.8 ± 0.6 Hz, n= 16), but was stable within the same cell. We did not attempt to differentiate between spontaneous miniature AMPAR- or NMDAR-mediated EPSCs (Berretta & Jones, 1996). Following the application of 5-HT (50 μm) for short periods of 3–10 min, there was a drastic reduction in the frequency of the events (reduction from 2.8 ± 0.6 to 1.0 ± 0.3 Hz; n= 16, P < 0.01, Fig. 6), while the mean amplitudes (24.2 ± 2.2 to 24.6 ± 2.2 pA; P= 0.5) and amplitude distributions (Kolmogorov-Smirnov test, P > 0.3) did not change.
Figure 6. Effects of serotonin on miniature EPSCs.
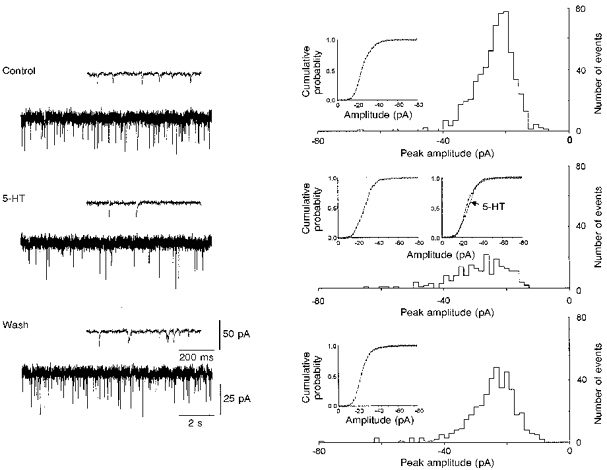
Left, effects of 5-HT in original recordings on 2 different time scales. The events were recorded at −60 mV in the presence of 5 μm TTX and 5 μm bicuculline. The spontaneous synaptic currents were recorded on video tape at 36 kHz digitization rate and subsequently filtered at 3 kHz with an eight-pole Bessel filter, redigitized at 8–10 kHz and then analysed using an event detection program (see Methods). The amplitude distributions were compared by using a Kolmogorov-Smirnov test. Right, corresponding frequency histograms of miniature EPSC amplitude before and after bath application of 50 μm 5-HT for a short period of 5 min. Serotonin clearly reduced the frequency (number of the events) of the spontaneous miniature EPSCs, while the mean amplitude was not significantly changed (from -26.8 ± 0.4 to -27.7 ± 0.6 pA). When analysing the amplitude distribution with a cumulative probability (insets), a small shift to the right (bigger amplitudes) could be observed, but these changes were not significant (Kolmogorov-Smirnov test, P= 0.5). The histograms were plotted with a bin width of 1.6 pA and were composed of 668 events under control, 209 events with serotonin and 418 events after washout.
DISCUSSION
Here we show that serotonin induces changes of the intrinsic and excitatory synaptic properties of EC layer II and III cells. Since these neurons of the EC form the main input to the hippocampus proper for transfer of sensoric information (Steward & Scoville, 1976), the modulation by serotonin will markedly alter the information flow to the hippocampus.
Influence on intrinsic properties
Serotonin hyperpolarizes neurons through the activation of 5-HT1A receptors. The effect is mediated by a pertussis toxin-sensitive G-protein and the opening of a Ca2+-independent K+ channel (Clarke, De Vivo, Beck, Maayani & Goldfard, 1987; Colino & Halliwell, 1987; Kelly, Larkman, Penington, Rainnie, McAllister-Williams & Hodgkiss, 1991; Penington, Kelly & Fox, 1993). Our findings in the EC neurons are consistent with an intrinsic mechanism as described by others (Segal, 1980; Andrade & Nicoll, 1987; Colino & Halliwell, 1987; Andrade, 1991). First, the 5-HT-induced hyperpolarization reversed at −90 mV and was blocked by both extra- and intracellular Cs+ and by the lidocaine derivative QX-314, which strongly suggests that the hyperpolarization results from the opening of K+ channels. Second, the hyperpolarization was abolished when recorded with a patch pipette, which suggests that the effect is dependent on a factor in the intracellular milieu. Third, by including GTP in the internal solution of the patch pipette, the response could be restored. Additionally, the 5-HT-induced hyperpolarization could be blocked by 5-HT1A-receptor antagonists NAN-190 and spiperone. These results suggest that the cellular mechanism responsible for the 5-HT-induced hyperpolarization is the same in the entorhinal cortex as in other regions such as the hippocampus.
Serotonin selectively decreases excitatory synaptic transmission in the EC
The most potent effect of serotonin in EC layers II and III is the general depression of excitatory transmission. This effect contrasts with data from hippocampal pyramidal cells where high concentrations of 5-HT are necessary to reduce EPSPs (Jahnsen, 1980; Schmitz et al. 1995a), and where lower concentrations can even transiently increase the EPSP amplitude (Segal, 1990; Schmitz et al. 1995a,b).
The 5-HT1A, 5-HT1B, 5-HT1C, 5-HT2C and 5-HT3 receptor subtypes show the highest densities of all serotonin receptors in the entorhinal cortex (Pazos et al. 1985; Pazos & Palacios, 1985; Palacios, Waeber, Hoyer & Mengod, 1990). Therefore, we tested the most commonly used selective agonists/antagonists of these receptor subtypes. The 5-HT1A receptor agonists 8-OH-DPAT and 5-CT could both mimick the effect of serotonin on EPSPs, while the specific agonists for 5-HT1B, 5-HT1C, 5-HT2C and 5-HT3 receptors were ineffective. Since 8-OH-DPAT and 5-CT bind with high affinity to 5-HT1A and 5-HT7 receptors, and the limbic structures express significant levels of 5-HT7 receptors, we tested both 5-HT1A and 5-HT7 receptor antagonists. However, the effects of serotonin on EPSPs were only antagonized by the 5-HT1A receptor antagonist (see Results). These results suggest that activation of the 5-HT1A receptor is most probably responsible for the depression of EPSPs. However, since the effect of serotonin was only partially antagonized by the 5-HT1A receptor antagonist, and the 5-HT1A receptor agonists 8-OH-DPAT and 5-CT were only effective in high concentrations, it is feasible that the effect is mediated by an additional 5-HT1A-receptor subtype (Liu & Albert, 1991) which is only partially sensitive to the commonly used agonists and antagonists. This would be in agreement with our observation that the 5-HT-induced hyperpolarization was much more sensitive to the 5-HT1A receptor antagonists (not shown).
Several mechanisms might account for the effect of serotonin. It is conceivable that postsynaptic ion channels are modulated indirectly via the activation of G-proteins and second-messenger pathways. Most postsynaptic effects of serotonin can be blocked by intracellular perfusion with a patch pipette (Andrade, 1992). However, the effects of serotonin on EPSPs/EPSCs were identical in sharp microelectrode recordings and in patch clamp experiments. Therefore, a postsynaptic G-protein-mediated cellular pathway is unlikely to underly the depression of excitation. Alternatively, serotonin might modulate glutamate receptors by competitive interaction with binding sites (Murase et al. 1990). We addressed this possibility by mimicking the postsynaptic AMPAR- and NMDAR-mediated responses with a fast application system (Jonas, 1995). However, serotonin had no effect on the amplitude and the kinetics of the glutamate-activated currents. Moreover, serotonin did not influence the amplitudes of miniature EPSCs either. These experiments exclude a direct interaction of 5-HT with postsynaptic glutamate receptors. Therefore, our data can largely exclude any postsynaptic mechanism underlying the EPSP/EPSC modulation by serotonin. In contrast, our experiments on spontaneous synaptic currents in the presence of tetrodotoxin and bicuculline revealed that serotonin reduced the frequency of miniature EPSCs. Thus, it is likely that serotonin suppresses the release of glutamate from synaptic terminals. Moreover, the blockade of post- and presynaptic GABAB receptors with CGP 55845A could not prevent the serotonin effects and, therefore, we can exclude an interaction of serotonin with GABAB autoreceptors on presynaptic terminals. There is evidence that serotonin can reduce Ca2+ currents (Penington & Kelly, 1990; Fraser & MacVicar, 1991) and thereby depress excitatory synaptic transmission (Schmitz et al. 1995a). Furthermore, it has been shown recently that serotonin inhibits glutamatergic transmission onto rat motoneurons by a presynaptic mechanism (Singer, Bellingham & Berger, 1996). In contrast to our findings, fluorometric enzyme assay experiments in the entorhinal cortex showed no effects of serotonin on the glutamate release (Sizer, Kilpatrick & Roberts, 1992), probably due to a different experimental design.
The increase in total cell conductance induced by 5-HT is important for the interpretation of the depressant effects on synaptic responses, since a general membrane conductance increase can reduce the amplitude of synaptic events (Ginsborg, 1973). However, in two-thirds of the projection neurons we could observe a significant reduction in synaptic responses in the absence of any intrinsic conductance changes. In addition, we also observed reductions in the synaptic responses in experiments where we blocked the 5-HT-induced conductance increase with intracellular potassium channel blockers or by diluting the intracellular solution with patch pipettes. One might still argue that the depression of EPSPs is due to postsynaptic conductance changes in remote dendritic regions, which we could not control with our intracellular or patch electrodes. However, at least three further arguments exclude such a postsynaptic shunting effect. First, extracellularly applied 5-HT1A receptor antagonists blocked the 5-HT-induced hyperpolarization at low concentrations, but failed to block the effect of serotonin on EPSPs. Only higher concentrations of the antagonist S-UH-301 could partially antagonize the effect of serotonin on EPSPs. Second, if serotonin reduced the EPSPs/EPSCs only by shunting them in the dendrites, one would expect that the kinetics of the synaptic current would be changed, but this was not the case. Third, one would expect the miniature EPSC amplitudes to be reduced by serotonin, which was also not the case. We, therefore, conclude that the main effects of serotonin in the superficial layers of the EC are not due to alterations of intrinsic neuronal properties, but are specifically linked to a selective depression of excitatory transmission via a presynaptic mechanism that requires further investigation.
Acknowledgments
This work was supported by the BMBF, a Royal Society Exchange Program Fellowship to R. M. E., and DFG grant INK21/A1-1. We thank A. Duerkop for help with the manuscript and H. Tetsch, Dr H. Gabriel and Dr H. Siegmund for technical help.
References
- Alonso A, Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. Journal of Neurophysiology. 1993;70:128–143. doi: 10.1152/jn.1993.70.1.128. [DOI] [PubMed] [Google Scholar]
- Alonso A, Köhler C. A study of the reciprocal connections between the septum and the entorhinal area using anterograde and retrograde axonal transport methods in the rat brain. Journal of Comparative Neurology. 1984;225:327–343. doi: 10.1002/cne.902250303. [DOI] [PubMed] [Google Scholar]
- Andrade R. Blockade of neurotransmitter-activated K+ conductance by QX-314 in the rat hippocampus. European Journal of Pharmacology. 1991;199:259–262. doi: 10.1016/0014-2999(91)90467-5. 10.1016/0014-2999(91)90467-5. [DOI] [PubMed] [Google Scholar]
- Andrade R. Whole-cell and perforated-patch recording of serotonin responses in the rat hippocampus. Journal of Chemical Neuroanatomy. 1992;5:339–341. doi: 10.1016/0891-0618(92)90023-j. 10.1016/0891-0618(92)90023-J. [DOI] [PubMed] [Google Scholar]
- Andrade R, Nicoll RA. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. Journal of Physiology. 1987;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta N, Jones RSG. A comparison of spontaneous EPSCs in layer II and layer IV-V neurons of the rat entorhinal cortex in vitro. Journal of Neurophysiology. 1996;76:1089–1100. doi: 10.1152/jn.1996.76.2.1089. [DOI] [PubMed] [Google Scholar]
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. Journal of Neuroscience Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Bobillier P, Petitjean F, Salvert D, Ligier M, Seguin S. Differential projections of the nucleus Raphe dorsalis and nucleus Raphe centralis as revealed by autoradiography. Brain Research. 1975;85:205–210. doi: 10.1016/0006-8993(75)90071-2. [DOI] [PubMed] [Google Scholar]
- Clarke WP, De Vivo M, Beck SG, Maayani S, Goldfarb J. Serotonin decreases population spike amplitude in hippocampal cells through a pertussis toxin substrate. Brain Research. 1987;410:357–361. doi: 10.1016/0006-8993(87)90338-6. [DOI] [PubMed] [Google Scholar]
- Colino A, Halliwell JV. Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature. 1987;328:73–77. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- Fraser DD, MacVicar BA. Low-threshold transient calcium current in rat hippocampal lacunosum-moleculare interneurons: Kinetics and modulation by neurotransmitters. Journal of Neuroscience. 1991;11:2812–2820. doi: 10.1523/JNEUROSCI.11-09-02812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg BL. Electrical changes in the membrane in junctional transmission. Biochimica et Biophysica Acta. 1973;300:289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Gloveli T, Schmitz D, Empson RM, Dugladze T, Heinemann U. Morphological and electrophysiological characterisation of layer III cells of the medial entorhinal cortex of the rat. Neuroscience. 1997;77:629–648. doi: 10.1016/s0306-4522(96)00494-0. 10.1016/S0306-4522(96)00494-0. [DOI] [PubMed] [Google Scholar]
- Jahnsen H. The action of 5-hydroxytryptamine on neuronal membranes and synaptic transmission in area CA1 of the hippocampus in vitro. Brain Research. 1980;197:83–94. doi: 10.1016/0006-8993(80)90436-9. 10.1016/0006-8993(80)90436-9. [DOI] [PubMed] [Google Scholar]
- Jonas P. Single-Channel Recording. New York, London: Plenum Press; 1995. [Google Scholar]
- Jones RSG. Entorhinal-hippocampal connections: A speculative view of their function. Trends in Neurosciences. 1993;16:58–64. doi: 10.1016/0166-2236(93)90018-h. 10.1016/0166-2236(93)90018-H. [DOI] [PubMed] [Google Scholar]
- Kelly JS, Larkman P, Penington NJ, Rainnie DG, McAllister-Williams H, Hodgkiss J. Serotonin receptor heterogeneity and the role of potassium channels in neuronal excitability. Advances in Experimental Medicine and Biology. 1991;287:177–191. doi: 10.1007/978-1-4684-5907-4_15. [DOI] [PubMed] [Google Scholar]
- Koh D-S, Geiger JRP, Jonas P, Sakmann B. Ca2+-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. Journal of Physiology. 1995;485:383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlais PJ, Mair RG, Anderson CD, McEntee WJ. Monoamines and metabolites in cortex and subcortical structures: normal regional distribution and the effects of thiamine deficiency in the rat. Brain Research. 1987;421:140–149. doi: 10.1016/0006-8993(87)91284-4. 10.1016/0006-8993(87)91284-4. [DOI] [PubMed] [Google Scholar]
- Liu YF, Albert PR. Cell-specific signaling of the 5-HT1A receptor. Journal of Biological Chemistry. 1991;266:23689–23697. [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, De Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danileson PE, Sutcliffe JG, Erlander MG. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. 10.1016/0896-6273(93)90149-L. [DOI] [PubMed] [Google Scholar]
- Murase K, Randic M, Shirasaki T, Nakagawa T, Akaike N. Serotonin suppresses N-methyl-d-aspartate responses in acutely isolated spinal dorsal horn neurons of the rat. Brain Research. 1990;525:84–91. doi: 10.1016/0006-8993(90)91323-9. 10.1016/0006-8993(90)91323-9. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Waeber C, Hoyer D, Mengod G. Distribution of serotonin receptors. Annals of the New York Academy of Sciences. 1990;600:36–52. doi: 10.1111/j.1749-6632.1990.tb16871.x. [DOI] [PubMed] [Google Scholar]
- Pazos A, Cortés R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2-receptors. Brain Research. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Research. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. 10.1016/0006-8993(85)90856-X. [DOI] [PubMed] [Google Scholar]
- Penington NJ, Kelly JS. Serotonin receptor activation reduces calcium current in an acutely dissociated adult central neuron. Neuron. 1990;4:751–758. doi: 10.1016/0896-6273(90)90201-p. [DOI] [PubMed] [Google Scholar]
- Penington NJ, Kelly JS, Fox AP. Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. Journal of Physiology. 1993;469:387–405. doi: 10.1113/jphysiol.1993.sp019819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G, Segal M. The effects of serotonin depletion and Raphe grafts on hippocampal electrophysiology and behavior. Journal of Neuroscience. 1991;11:1585–1596. doi: 10.1523/JNEUROSCI.11-06-01585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang J-M, Schwartz J-C. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proceedings of the National Academy of Sciences of the USA. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Empson RM, Heinemann U. Serotonin and 8-OH-DPAT reduce excitatory transmission in rat hippocampal area CA1 via reduction in presumed presynaptic Ca2+ entry. Brain Research. 1995a;701:249–254. doi: 10.1016/0006-8993(95)01005-5. 10.1016/0006-8993(95)01005-5. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Empson RM, Heinemann U. Serotonin reduces inhibition via 5-HT1A receptors in area CA1 of rat ventral hippocampal slices in vitro. Journal of Neuroscience. 1995b;15:7217–7225. doi: 10.1523/JNEUROSCI.15-11-07217.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. The action of serotonin in the rat hippocampal slice preparation. Journal of Physiology. 1980;303:423–439. doi: 10.1113/jphysiol.1980.sp013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Serotonin attenuates a slow inhibitory postsynaptic potential in rat hippocampal neurons. Neuroscience. 1990;36:631–641. doi: 10.1016/0306-4522(90)90006-p. 10.1016/0306-4522(90)90006-P. [DOI] [PubMed] [Google Scholar]
- Silver RA, Colquhoun D, Cull-Candy SG, Edmonds B. Deactivation and desensitization of non-NMDA receptors in patches and the time course of EPSCs in rat cerebellar granule cells. Journal of Physiology. 1996;493:167–173. doi: 10.1113/jphysiol.1996.sp021372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. Journal of Neurophysiology. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- Sizer AR, Kilpatrick GJ, Roberts MHT. A post-synaptic depressant modulatory action of 5-hydroxytryptamine on excitatory amino acid responses in rat entorhinal cortex in vitro. Neuropharmacology. 1992;31:531–539. doi: 10.1016/0028-3908(92)90184-q. 10.1016/0028-3908(92)90184-Q. [DOI] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. Journal of Comparative Neurology. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt H-U, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Archiv. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Yang J, Pawlyk AC. Altered serotonin transporter sites in Alzheimer's disease raphe and hippocampus. NeuroReport. 1995;6:1207–1210. doi: 10.1097/00001756-199505300-00033. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Cerebral activity and behavior: control by central cholinergic and serotonergic systems. International Review of Neurobiology. 1988;30:225–340. doi: 10.1016/s0074-7742(08)60050-1. [DOI] [PubMed] [Google Scholar]
- White JA, Alonso A, Kay AR. A heart-like Na+ current in the medial entorhinal cortex. Neuron. 1993;11:1037–1047. doi: 10.1016/0896-6273(93)90217-f. [DOI] [PubMed] [Google Scholar]


