Abstract
Line-scan analyses of spontaneous Ca2+ sparks, non-propagating local rises in Ca2+ concentration, and the early phase of Ca2+ transients in cardiomyocytes were performed with a rapid-scanning laser confocal microscope (Nikon RCM8000) and fluo-3.
On electrical stimulation, points at which rise in Ca2+ began earliest were observed at regular spacings of 1.82 ± 0.26 μm (mean ± s.d.) along the longitudinal axis of the cell. The points were heavily stained with di-2-ANEPEQ, which stains the T-tubules, indicating that they were at the Z-line.
The points where spontaneous Ca2+ sparks originated coincided with the points which showed faster Ca2+ elevation, i.e. the Z-line.
In some cases where a Ca2+ spark had occurred within about 30 ms before the evoked Ca2+ transient, fast elevation of Ca2+ was not observed at the corresponding Z-line, indicating the presence of a refractory period in Ca2+ release from the SR.
The present results provide visual evidence for Ca2+ release from the junctional sarcoplasmic reticulum in cardiomyocytes. The presence of a refractory period in Ca2+ release after Ca2+ sparks provided new evidence that the normal Ca2+ transient may be the summation of Ca2+ sparks.
In cardiomyocytes, non-propagating local increases in Ca2+ concentration called Ca2+ sparks have been detected in the cytoplasm with confocal microscopy, which was shown to be the result of spontaneous Ca2+ release from the sarcoplasmic reticulum (SR; Cheng, Lederer & Cannell, 1993). When cardiomyocytes were electrically stimulated in the presence of Ca2+ channel antagonists, spatial inhomogeneity in Ca2+ concentration with properties similar to those of Ca2+ sparks was observed during the initial phase of the Ca2+ transients. Further analyses led to the hypothesis that Ca2+ sparks are the elementary events underlying excitation-contraction coupling; the normal myocardial Ca2+ transient was suggested to be a summation of Ca2+ sparks triggered by trans-sarcolemmal Ca2+ influx through L-type Ca2+ channels (Cannell, Cheng & Lederer, 1995; Cheng, Cannell & Lederer, 1995; Lopez-Lopez, Shacklock, Balke & Wier, 1995). This hypothesis leads to the expectation that during the initial phase of normal Ca2+ transients, spatial non-uniformity in Ca2+ resembling Ca2+ sparks would be observed. Direct visual observation of such intrasarcolemmal inhomogeneity and analyses of the spatio-temporal relation with Ca2+ sparks requires confocal microscopes with time resolution in the millisecond range.
In the present study, we obtained line-scan confocal images of Ca2+ sparks with a rapid scanning confocal microscope (RCM8000, Nikon Corporation; Kawanishi et al. 1994) which we have previously shown to be useful in the millisecond analyses of spatio-temporal patterns of myocardial Ca2+ movements (Tanaka, Kawanishi, Kato, Nakamura & Shigenobu, 1996a; Tanaka, Kawanishi, Matsuda Takahashi & Shigenobu, 1996b) including Ca2+ sparks (Tanaka et al. 1997). We used the line-scan mode of the microscope where a single line across the cell was scanned once every 69 μs and observed inhomogeneity in Ca2+ during the initial several milliseconds of the normal Ca2+ transient. The waveform of the initial rise in Ca2+, which occurred at the Z-line, resembled those of Ca2+ sparks. Further, we found that Ca2+ sparks occurring immediately prior to the Ca2+ transients result in deletion of the initial rising phase of corresponding Z-line Ca2+ transients, which was considered to reflect the presence of a refractory period in SR Ca2+ release.
METHODS
Wistar strain rats weighing 250–300 g, anaesthetized with pentobarbitone (100 mg kg−1, i.p.) were killed by rapid removal of the heart. Ventricular myocytes were obtained by Langendorff perfusion of the heart with collagenase solution as previously described (Takahashi, Keto, Adachi, Agata, Tanaka, Shigenobu, 1995). To load the myocardial cells with fluo-3, they were incubated for 30 min with fluo-3 AM (10 μm). Cells were placed in a chamber on the stage of the Nikon RCM8000 inverted microscope and perfused with the Tyrode solution of the following composition (mm) at 35 °C: NaCl, 143; KCl, 4; MgCl2, 0.5; CaCl2, 1.8; NaH2PO4, 0.33; glucose, 5.5; and Hepes, 5. Ca2+ transients were elicited by field stimulation through a pair of platinum wire electrodes. In our previous study, we found that isoprenaline (isoproterenol) increases the amplitude of spontaneous Ca2+ sparks without affecting its frequency or time course of Ca2+ rise and decay (Tanaka et al. 1997). Therefore, in the present study, data were obtained in the presence of 1 μm isoprenaline because it enabled us to obtain line-scan images of Ca2+ sparks with lower laser power and less cell damage due to laser irradiation. In some experiments, suction electrodes were attached to the cells under the whole-cell configuration. The electrodes had a resistance of 2–4 MΩ and contained the following solution (mm): KCl, 130; Hepes, 5; MgCl2, 1; K2ATP, 5; and fluo-3, 0.2; pH 7.2. Action potentials were elicited under current clamp mode with current pulses 3 ms in duration with a patch clamp amplifier (PC-one, Dagan), a personal computer (Deskpro 386s Compaq) and pCLAMP software (Axon Instruments, Inc.). Imaging experiments were performed at about 15 min after rupture of the cell membrane.
The rapid scanning confocal microscope used in the present study (RCM8000, Nikon Corporation; Kawanishi et al. 1994) is a point laser scan type, by which the highest spatial resolution can be obtained, and uses a resonant galvanometer scanner for rapid horizontal scan. The principle of the system is essentially the same as the system developed by Tsien and Bacskai (Tsien, 1990; Tsien & Bacskai, 1994). The objective lens used was a Nikon Fluor × 40, 1.15 numerical aperture (water immersion). For the imaging of Ca2+ transients and Ca2+ sparks, fluo-3 fluorescence in the cells was excited at 488 nm from a high-power Ar + laser, and the emission, at wavelengths longer than 515 nm, was detected by a photomultiplier. A pinhole 0.33 mm in diameter was used to remove out-of-focus light. Under the line-scan mode of the microscope, 175 μm horizontal lines were scanned in 512 pixels at the speed of one line per 69 μs. In some cases, laser scanning transmission images were obtained before and after observation of Ca2+ transients. For direct visualization of the sarcolemmal membrane and T-tubules, the cells were incubated in extracellular solution containing 5 μm di-2-ANEPEQ, a dye known to accumulate selectively in the surface membrane, and excited at 514 nm.
Estimation of Ca2+ concentration from fluorescence intensity ratios was performed as described earlier (Cheng et al. 1993) with the equation [Ca2+]= KR/[(K/[Ca2+]rest + 1) - R], where K is the dissociation constant for the indicator fluo-3, and R is the fluorescence intensity ratio which was obtained by dividing the fluorescence intensity at a point corresponding to the Z-line or M-line by the basal fluorescence intensity at the same point before the occurrence of Ca2+ sparks or Ca2+ transients. Values of 400 nm and 100 nm were used for K and [Ca2+]rest, respectively (Cheng et al. 1993). Values of fluorescence intensity ratios and Ca2+ concentrations as well as of latencies were presented as means ± standard error of the mean (s.e.m.). Statistical significance of difference in means was evaluated by Student's paired or unpaired t tests and P values less than 0.05 were considered significant.
RESULTS
Images of resting rat cardiac myocytes obtained by scanning the confocal spot along the longitudinal axis of the cell showed spontaneous Ca2+ sparks with a characteristic ‘comet tail’ appearance (Fig. 1). During the initial phase of the Ca2+ transient evoked by field stimulation, non-uniformity in Ca2+ fluorescence was observed (Fig. 1). Points at which a rise in Ca2+ began several milliseconds earlier than other regions were spaced at regular intervals of 1.82 ± 0.26 μm (mean ±s.d. from 120 measurements in 8 cells) along the longitudinal axis of the cell. The spatial non-uniformity lasted for several milliseconds resulting in a characteristic ‘teeth of a comb’ appearance. The points where Ca2+ sparks originated coincided with the points which showed faster Ca2+ elevation on a Ca2+ transient (Fig. 1, arrows in lower panel). The spatial non-uniformity was also observed when cardiomyocytes were loaded with non-acetoxymethyl fluo-3 and stimulated under current clamp condition via patch electrodes (data not shown). In transverse line-scan images, where the confocal spot was scanned at 90 degrees to the longitudinal axis, the points of faster Ca2+ elevation were not regularly distributed although non-uniformity was observed (Fig. 2).
Figure 1. Typical longitudinal line-scan images of a stimulation-evoked Ca2+ transient preceded by a spontaneous Ca2+ spark.
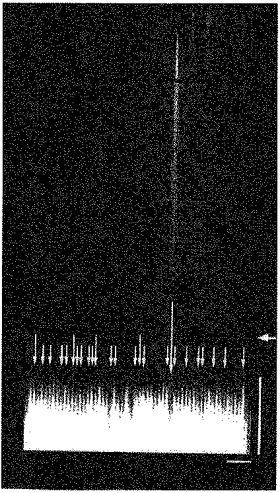
Distance along the scan line was depicted horizontally and successive scan lines at 69 μs intervals stacked vertically. The horizontal arrow on the right indicates the moment of field stimulation. Points at which rise in Ca2+ began several milliseconds earlier than other regions were spaced at regular intervals of about 2 μm along the longitudinal axis of the cell. The vertical arrows in the lower panel indicate the points where Ca2+ sparks occurred during a 3 s scanning period preceding the stimulation. Short arrows indicate the points where a single spark occurred and intermediate arrows twice. Note that these points coincide with the points where a rapid increase in fluorescence was observed on stimulation. In contrast, the initial rapid rise in fluorescence was not observed at the point where a spontaneous Ca2+ spark occurred about 30 ms before the stimulation as can be seen in the upper and middle panels (long arrow). Vertical scale bar, 10 ms; horizontal bar, 10 μm.
Figure 2. Transverse line-scan images of stimulation-evoked Ca2+ transients.
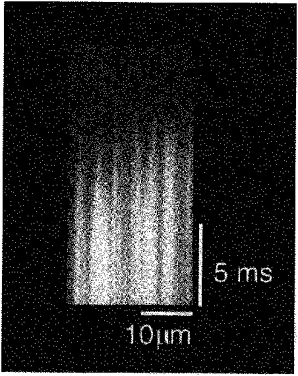
Points at which the rise in Ca2+ began several milliseconds earlier than other regions were recorded but no periodicity was observed.
To clarify the spatial relation of the non-uniformity in the rising phase of Ca2+ transients with sarcomere structure, line-scan images of Ca2+ were compared with laser transmission images of a corresponding area of the cell (data not shown). A transmission image of striations showed periodic presence of light and dark bands, that is the I-band and A-band, respectively. Comparison with line-scanned Ca2+ images revealed that points where the rise in Ca2+ was fast aligned to the centre of the I-band (Z-line), and the points where the rise in Ca2+ was slow aligned to the centre of the A-band (M-line). Further, cells were stained with the membrane-selective probe di-2-ANEPEQ after line-scan analysis of Ca2+ transients (Fig. 3). The fluorescence images of cells after staining with di-2-ANEPEQ showed the presence of T-tubules at about 1.8 μm intervals in the longitudinal direction. The points showing faster Ca2+ elevation coincided with the points heavily stained with the membrane probe indicating that they corresponded to the T-tubules located at the level of the Z-line. Consequently, we will call the fluorescence signals from the points showing faster Ca2+ elevation ‘Z-line transients’, and the signals from the points showing slower Ca2+ elevation ‘M-line transients’.
Figure 3. Relationship of rapidly rising Ca2+ transients to sarcomere structure.
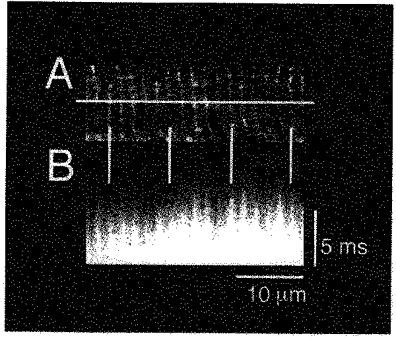
Images of di-2-ANEPEQ fluorescence (A) were compared with the longitudinal line-scan images of the Ca2+ transient in the same cell (B). Horizontal bars in A indicate the position of the scanned line for the image in B. Vertical bars indicate that the points where rise in Ca2+ is fast coincide with the region heavily stained with di-2-ANEPEQ (T-tubules; Z-line).
Fluorescence intensity of Z-line transients and M-line transients was quantified and presented as a function of time (Fig. 4). At the Z-line, a latency was observed between the electrical stimulation and the onset of the Ca2+ transient (Table 1). At 2 ms after the onset of Z-line Ca2+ transients, the fluorescence increased to reach 240 % of basal values, which corresponds to 370 nm Ca2+. The onset of M-line transients lagged behind those of adjacent Z-line transients, resulting in non-uniformity for several milliseconds. In some cases where a Ca2+ spark had occurred within 30 ms before stimulation, the initial rising phase of the corresponding Z-line Ca2+ transient was absent: the rise in Ca2+ at the Z-line after the spark was slower than that at the adjacent M-lines. Examples of stimulation evoked Ca2+ transients at a Z-line where a spark had occurred 30 ms in advance, at the adjacent Z-line where no sparks were observed and at the M-line in between are shown in Fig. 4A. The Ca2+ concentration of the Z-line without a spark at 2 ms after the onset of the Ca2+ transient was about 500 nm. At the same moment, that at the Z-line where a spark had occurred was about 150 nm, which means that the increase in Ca2+ concentration was about 13 % of that at the adjacent Z-line. At the same moment, the Ca2+ at the M-line in between was 300 nm. Thus, the increase in Ca2+ at the Z-line following a spark was smaller than that at the M-line, suggesting lack of Ca2+ release at this point. The increase in fluorescence at this point could be attributed to diffusion of Ca2+ and the optical contamination of fluorescence from the neighbouring release sites. When the cell was stimulated again and the same line was scanned, no sparks were observed. This time, the Ca2+ transients at the two Z-lines were almost identical indicating that Ca2+ release occurred at both Z-lines (Fig. 4B).
Figure 4. Time course of typical Z-line and M-line Ca2+ transients following field stimulation.
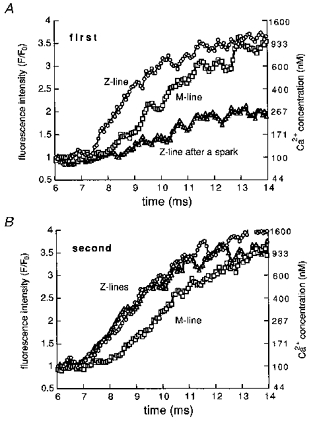
Quantification of fluorescence was performed at points corresponding to the Z- and M-lines. The fluorescence was averaged over two pixels (680 nm) and expressed as a ratio against the basal fluorescence value obtained before the occurrence of Ca2+ sparks or Ca2+ transients. At one of the Z-lines, a spontaneous Ca2+ spark had occurred 30 ms before the first stimulation (▵ in A). The Ca2+ concentration of the Z-line after a spark was lower than those at the adjacent Z-line (^ in A) and M-line (□ in A). At the same points, such difference was not observed in the second Ca2+ transient, which was not preceded by a spark (B).
Table 1.
Properties of Z-line and M-line Ca2+ transients
| Z-line transients | M-line transients | |
|---|---|---|
| Latency (ms) | from stimulation | from onset at Z-line |
| 3.6 ± 0.4 | 0.8 ± 0.04 * | |
| Normalized fluorescence intensity (F/F0) | ||
| 1 ms after Z-line onset | 1.8 ± 0.1 | 1.4 ± 0.04 * |
| 2 ms after Z-line onset | 2.4 ± 0.2 | 1.7 ± 0.09 * |
| Ca2+ concentration (nm) | ||
| 1 ms after Z-line onset | 227 ± 17 | 152 ± 7.8 * |
| 2 ms after Z-line onset | 369 ± 131 | 231 ± 23 * |
Values were obtained from Z-line Ca2+ transients which were not preceded by spontaneous Ca2+ sparks within 67 ms before stimulation and the adjacent M-line Ca2+ transients. Onset of Ca2+ transients was defined here as the time point where the normalized fluorescence intensity (F/F0) reached 120 % of the basal value. Data are means ±s.e.m. from 30 Z-line-M-line pairs in 15 cells.
Significant difference from corresponding values for Z-line transients evaluated by Student's paired t test.
Summarized results of fluorescence measurements at Z-lines where sparks had occurred before the Ca2+ transient are presented in Fig. 5. The initial rise in Ca2+ concentration at the Z-line following a spark was compared with that at the adjacent Z-line not preceded by a spark. The fluorescence at two Z-lines was measured at 2 ms after the onset of the Ca2+ transient at the Z-line not preceded by a spark. The Ca2+ concentration at the Z-line following a spark showed an ‘all-or-nothing’ behaviour; it was either higher than 90 % or lower than 30 % of that at the adjacent Z-line. In other words, the Ca2+ concentration at the Z-line following a spark was either almost the same as that at the adjacent Z-line or lower than that at the M-line in between. Plotting the data against the interval between Ca2+ sparks and Ca2+ transients (Fig. 5) revealed that the initial rapid rise of the Z-line transients were either present or absent, irrespective of the interval, and no intermediate response was observed.
Figure 5. Intensity distribution of Z-line fluorescence preceded by a spontaneous Ca2+ spark plotted against the interval.
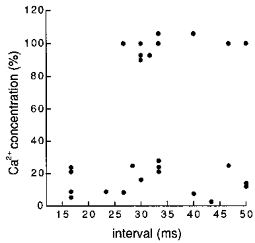
Increase in fluorescence intensity at 2 ms after the onset of a Ca2+ transient at the Z-line preceded by a Ca2+ spark was expressed as a percentage of the corresponding increase at the adjacent Z-line, and plotted against the interval between the preceding Ca2+ spark and stimulation. Note that the Ca2+ concentration at the Z-line preceded by a Ca2+ spark was either higher than 90 % or lower than 30 % of that at the adjacent Z-line: no intermediate values were observed.
We next examined whether the incidence of rapidly rising Z-line transients was influenced by the interval between the preceding Ca2+ spark and cell stimulation. Z-line transients observed after a Ca2+ spark were grouped by the interval and the ratio of rapidly rising Z-line transients to the total number of Z-line transients was calculated for each group. The results (Fig. 6A) showed that the occurrence of rapidly rising Z-line transients decreases at intervals shorter than 50 ms. In other words, a refractory period in Ca2+ release from the SR was observed in intact cardiomyocytes. As mentioned in Methods, we performed most of our experiments in the presence of 1 μm isoprenaline to minimize cell damage due to laser irradiation. To examine whether the presence of the refractory period depends on the presence of isoprenaline, we performed the same analysis in the absence of isoprenaline (Fig. 6B). The incidence of rapidly rising Z-line transients decreased at intervals shorter than 50 ms. No substantial difference was observed in the probability-interval relationship obtained in the presence and absence of isoprenaline.
Figure 6. Incidence of rapidly rising initial phase of Z-line Ca2+ transients preceded by a spontaneous Ca2+ spark.
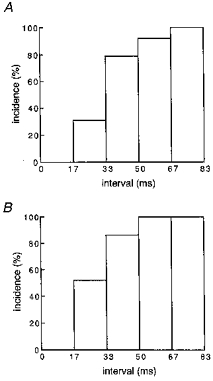
Analysis of the fluorescence intensity at the Z-line as presented in Fig. 5 was performed with Z-line transients preceded by a Ca2+ spark with various intervals. The Z-line transients were grouped by the interval, and the ratio of rapidly rising Z-line transients to the total number of Z-line transients was calculated for each interval range. A, in the presence of 1 μm isoprenaline with 86 Z-line transients from 36 cells. B, in the presence of 1 μm isoprenaline with 41 Z-line transients from 15 cells.
DISCUSSION
It is generally accepted that, during normal myocardial contraction, the major fraction of Ca2+ responsible for the Ca2+ transient is released from the SR. Intracellular localization of the ryanodine receptor to the junctional SR which is in close contact with the T-tubules located at the Z-lines suggests that the Ca2+ release occurs at this region (Jorgensen, Shen, Arnold, McPherson & Campbell, 1993). This would possibly result in small gradients of Ca2+ within each sarcomere early during the Ca2+ transient (Wier & Yue, 1986). The Ca2+ concentration at the restricted space between the T-tubular membrane and the adjacent junctional SR membrane is considered to change more drastically than that at other regions of the cytoplasm, and to influence the activity of various ion channels and transporters involved in excitation-contraction coupling (Lederer, Nigglli & Hardley, 1990; Carmeliet, 1992). Nevertheless, there is little functional information of such intrasarcolemmal non-uniformity in Ca2+ during normal myocardial Ca2+ transients. There is a report using fluorescence microscopy and deconvolution techniques which showed that Ca2+ transients initiate from localized regions of the sarcomere corresponding to the Z-line (Isenberg et al. 1996). Although the length of a single sarcomere is about 1.8 μm, which is within the spatial resolution range of laser-scanning confocal microscopes, direct visualization of such non-uniformity with confocal microscopy had not been reported probably due to the insufficient time resolution of most currently available confocal microscopes.
In the present study, we could observe periodic non-uniformity in Ca2+ during early Ca2+ transient of intact cardiomyocytes: the points where rise in Ca2+ began first were spaced at regular intervals of about 1.8 μm along the longitudinal axis of the cell (Fig. 1). The points coincided with the staining by the membrane dye di-2-ANEPEQ (Fig. 3) and also coincided with the centre of the I-band, i.e. the Z-line. Thus, we obtained direct visual evidence with line-scan confocal microscopy that, during normal Ca2+ transients, the primary site of Ca2+ release is localized at the junctional SR located at the level of the Z-lines. This agrees with the conclusion of the earlier report with fluorescence microscopy and deconvolution techniques (Isenberg et al. 1996). The characteristic ‘teeth of a comb’ appearance was observed on stimulation both when the fluorescent probe was loaded from the extracellular solution using its acetoxymethyl derivative and when the non-acetoxymethyl probe was injected into the cell via patch electrodes (data not shown). Thus, the observed spatial non-uniformity could not be an artifact due to distortion of intracellular environment or localization of the probe in intracellular micro-organisms.
The Z-line Ca2+ transients initiated at about 5 ms after electrical stimulation of the myocyte (Fig. 3, Table 1). The latency could be explained by the time required for activation of the trans-sarcolemmal Ca2+ current and the subsequent release of Ca2+ from the SR ryanodine receptor channel. The onset of M-line Ca2+ transients lagged behind that of Z-line transients and the difference in fluorescence intensity between the Z-line and M-line lasted for several milliseconds (Fig. 4, Table 1). Escobar, Monck, Fernandez & Vergara (1994) performed confocal spot analyses of electrically stimulated skeletal muscle, and showed that Ca2+ fluorescence at the Z-line was greater than that at the M-line during several milliseconds after stimulation. The authors pointed out that there was no significant delay in the onset of M-line transient from that at the Z-line, and raised the possibility that a broad band of SR might participate in the Ca2+ release process. This possibility was supported by the presence of extra-junctional ryanodine receptor channels in skeletal muscle (Dulhunty, Junankar & Stanhope, 1992). Such extra-junctional ryanodine receptor channels have not been observed in cardiac muscle (Jorgensen et al. 1993).
The present observation of intrasarcomere non-uniformity in Ca2+ during the early phase of myocardial excitation- contraction coupling supports earlier theoretical (Wohlfart & Noble, 1982; Wier & Yue, 1986) and experimental (Jorgensen et al. 1993; Isenberg et al. 1995) work suggesting that Ca2+ release is initiated at the junctional SR. The rapid increase in Ca2+ concentration at the restricted space between the T-tubular and junctional SR membranes during the initial phase of excitation-contraction coupling may allow activation of various Ca2+ sensitive processes in that region when the Ca2+ concentration around the contractile proteins is still low. In the mammalian ventricular myocardium, the dihydropyridine binding sites (L-type Ca2+ channels; Wibo, Bravo & Godfraind, 1991), as well as the Na+-Ca2+ exchanger (Frank, Mottino Reid Molday & Philipson, 1992; Chen, Mottino, Klitzner, Philipson & Frank, 1995), are reported to be localized along the T-tubules. Rapid inactivation of the trans-sarcolemmal Ca2+ influx through L-type Ca2+ channels and activation of inward current via the Na+-Ca2+ exchange system could be explained by rapid increase in Ca2+ concentration in the restricted area (Adachi-Akahane, Cleeman & Morad, 1996).
In longitudinal line-scan images, lack of the initial rising phase of Z-line transients on depolarization was observed at points where a Ca2+ spark had occurred within about 30 ms in advance (Figs 1 and 4). This indicates that common mechanisms are involved in the generation of spontaneous Ca2+ sparks and Ca2+ transients, and provides new evidence that Ca2+ sparks are the functional units of Ca2+ release from the junctional SR. In agreement with our results, it was demonstrated in earlier studies that Ca2+ sparks evoked by electrical stimulation arise at the T-tubules (Shacklock, Weir & Balke, 1995) and that spontaneous Ca2+ spark sites were regularly spaced longitudinally but not in transverse planes (Parker, Zang, & Weir, 1997). The refractory period in Ca2+ release from the SR would counter the inherent positive feedback of Ca2+-induced Ca2+-release and serve as a mechanism to avoid uncontrolled release of Ca2+ from the SR. It would enable Ca2+ release to terminate on its own when the Ca2+ concentration at the restricted space between the T-tubules and the junctional SR membrane is still high.
The mechanisms for the refractoriness of Ca2+ release remain to be investigated. One explanation may be inactivation of ryanodine receptor channels by Ca2+ from the cytoplasmic side, which has been observed in skinned myocardial fibres (Fabiato, 1985) and with ryanodine receptor channels incorporated into lipid bilayers (Laver, Roden, Ahern, Eager, Junankar & Dulhunty, 1995; Sitsapesan, Montgomery & Williams, 1995; Shiefer, Meissner & Isenberg, 1995). Ca2+ sparks are also reported to be influenced by intraluminal Ca2+ of the SR (Lukyanenko, Gyorke & Gyorke, 1996). Translocation of SR Ca2+ to a releasable pool (Wier & Yue, 1985) within the Ca2+ release unit at each Z-line might also explain the refractory period. In this case, the Ca2+ release from the release unit would be graded depending on the length of the interval between Ca2+ release events. Our present results, however, showed that the Z-line transients following Ca2+ sparks were ‘all or nothing’ rather than graded; the initial rise of Ca2+ concentration at the Z-line following Ca2+ sparks was either almost the same as the full size Z-line Ca2+ transient or less (slower) than that at the M-line (Fig. 4; Table 1). The possibility of the rapidly rising Z-line transients occurring, rather than the fluorescence intensity, changed depending on the interval between the preceding Ca2+ spark and cell stimulation (Figs 5 and 6). Thus, the influence of spark-induced change in luminal Ca2+ on subsequent Z-line transients, if any, would be through some regulatory process, rather than a simple decrease in the amount of releasable Ca2+ in the SR.
The refractory period was observed both in the presence and absence of isoprenaline, and the length of the refractory period was not influenced by the drug (Fig. 6). At present, the mechanisms for the refractory period are not clear and it is difficult to explain clearly the lack of isoprenaline effects. In our previous study, we found that although the amplitude of Ca2+ sparks was increased by isoprenaline, the time course of decay was not influenced: the time for half-decay was 10–15 ms (Tanaka et al. 1997). This means that the Ca2+ concentration at the cytoplasmic side of the ryanodine receptor channel at tens of milliseconds after the Ca2+ spark may not be greatly increased by isoprenaline. As for the luminal Ca2+ of the SR, it is possible that the amount of Ca2+ released during a single Ca2+ spark does not substantially affect the total releasable Ca2+ within the Ca2+ release unit or that the amount of releasable Ca2+ is not changed by isoprenaline as a result of a balance of increased Ca2+ release and uptake.
In summary, the present results provide visual evidence that Ca2+ release occurs at the junctional SR in cardiomyocytes. Further, the present finding of a refractory period in Ca2+ release following a Ca2+ spark provides new evidence that Ca2+ sparks are the functional units of Ca2+ release from the SR and that the normal Ca2+ transient may be the summation of Ca2+ sparks.
Acknowledgments
The authors express their thanks to Dr Satomi Adachi-Akahane for her advice on the staining method of T-tubules. This study was supported in part by a grant-in-aid for Drug Innovation Science Project (to T. K. and K. S.) and a grant-in-aid (to T. K. and R. N.) from the Japan Health Science Foundation, and a grant-in-aid (to H. T., T. K. and K. S.) for General Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
References
- Adachi-Akahane S, Cleeman L, Morad M. Cross-signaling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. Journal of General Physiology. 1996;108:435–454. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M, Cheng H, Lederer W. Control of calcium release in heart muscle. Science. 1995;268:1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. A fuzzy subsarcolemmal space for intracellular Na+ in cardiac cells. Cardiovascular Research. 1992;26:433–442. doi: 10.1093/cvr/26.5.433. [DOI] [PubMed] [Google Scholar]
- Chen F, Mottino G, Klitzner T, Philipson K, Frank J. Distribution of the Na+/Ca2+ exchange protein in developing rabbit myocytes. American Journal of Physiology. 1995;268:C1126–1132. doi: 10.1152/ajpcell.1995.268.5.C1126. [DOI] [PubMed] [Google Scholar]
- Cheng H, Cannell M, Lederer W. Partial inhibition of Ca2+ current by methoxyverapamil (D600) reveals spatial nonuniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Circulation Research. 1995;76:236–241. doi: 10.1161/01.res.76.2.236. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Junankar PR, Stanhope C. Extra-junctional ryanodine receptors in the terminal cisternae of mammalian skeletal muscle fibers. Proceedings of the Royal Society. 1992;B 247:69–75. doi: 10.1098/rspb.1992.0010. [DOI] [PubMed] [Google Scholar]
- Escobar A, Monck J, Fernandez J, Vergara J. Localization of the site of Ca release at the level of a single sarcomere of skeletal muscle fibers. Nature. 1994;367:739–741. doi: 10.1038/367739a0. 10.1038/367739a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. Journal of General Physiology. 1985;85:247–290. doi: 10.1085/jgp.85.2.247. 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Mottino G, Reid D, Molday R, Philipson K. Distribution of the Na+/Ca2+exchange protein in mammalian cardiac myocytes: an immunofluorescence and immunocolloidal gold-labelling study. Journal of Cell Biology. 1992;117:337–345. doi: 10.1083/jcb.117.2.337. 10.1083/jcb.117.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G, Etter E, Wendt-Gallitelli M, Schifer A, Carrington W, Tuft R, Fay F. Intrasarcomere Ca2+ gradients in ventricular myocytes revealed by high speed digital imaging microscopy. Proceedings of the National Academy of Sciences of the USA. 1996;93:5413–5418. doi: 10.1073/pnas.93.11.5413. 10.1073/pnas.93.11.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A, Shen A, Arnold W, McPherson P, Campbell K. Ca2+-release channel/ryanodine receptor is localized in junctional and corbular sarcoplasmic reticulum in cardiac muscle. Journal of Cell Biology. 1993;120:969–980. doi: 10.1083/jcb.120.4.969. 10.1083/jcb.120.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi T, Asou H, Kato T, Uneyama C, Toyoda K, Ohata H, Momose K, Takahashi M. Ratio-imaging of calcium waves in cultured hepatocytes using rapid scanning confocal microscope and indo-1. Bioimages. 1994;2:7–14. [Google Scholar]
- Laver D, Roden L, Ahern G, Eager K, Junankar P, Dulhunty A. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. Journal of Membrane Biology. 1995;147:7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- Lederer W, Nigglli E, Hardley R. Sodium-calcium exchange in excitable cells. Science. 1990;248:283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez J, Shacklock P, Balke C, Gil Wier W. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995;268:1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Gyorke I, Gyorke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflügers Archiv. 1996;432:1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- Parker IP, Zang WJ, Wier WG. Ca2+ sparks involving multiple Ca2+ release sites along Z-lines in rat heart cells. Journal of Physiology. 1996;497:31–38. doi: 10.1113/jphysiol.1996.sp021747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer A, Meissner G, Isenberg G. Ca2+ activation and Ca2+ inactivation of canine reconstituted cardiac sarcoplasmic reticulum Ca2+ release channels. Journal of Physiology. 1995;489:337–348. doi: 10.1113/jphysiol.1995.sp021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R, Montgomery R, Williams A. New insights into the gating mechanisms of cardiac ryanodine receptors revealed by rapid changes in ligand concentration. Circulation Research. 1995;77:765–772. doi: 10.1161/01.res.77.4.765. [DOI] [PubMed] [Google Scholar]
- Sutko J, Airey J. Ryanodine receptor Ca2+ release channel: does diversity in form equal diversity in function? Physiological Reviews. 1996;76:1027–1071. doi: 10.1152/physrev.1996.76.4.1027. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kato Y, Adachi M, Agata N, Tanaka H, Shigenobu K. Effects of cyclopiazonic acid on rat myocardium: inhibition of calcium uptake into sarcoplasmic reticulum. Journal of Pharmacology and Experimental Therapeutics. 1995;272:1095–1100. [PubMed] [Google Scholar]
- Tanaka H, Kawanishi T, Kato Y, Nakamura R, Shigenobu K. Restricted propagation of cytoplasmic Ca2+ oscillation into the nucleus guinea pig cardiac myocytes as revealed by rapid scanning confocal microscopy and indo-1. Japanese Journal of Pharmacology. 1996a;70:235–242. doi: 10.1254/jjp.70.235. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Kawanishi T, Matsuda T, Takahashi M, Shigenobu K. Intracellular free Ca2+ movements in cultured cardiac myocytes as shown by rapid scanning confocal microscopy. Journal of Cardiovascular Pharmacology. 1996b;27:761–769. doi: 10.1097/00005344-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Nishimaru K, Sekine T, Kawanishi T, Nakamura R, Yamagaki K, Shigenobu K. Two-dimensional millisecond analysis of intracellular Ca2+ sparks in cardiac myocytes by rapid scanning confocal microscopy: increase in amplitude by isoproterenol. Biochemical and Biophysical Research Communications. 1997;233:413–418. doi: 10.1006/bbrc.1997.6470. [DOI] [PubMed] [Google Scholar]
- Tsien RY. Laser scanning confocal fluorescence microscopy at video rate (30 frames/sec) with dual-wavelength emission rationing for quantitative imaging of intracellular messages. Proceedings of the Royal Microscope Society. 1990;25:S53. [Google Scholar]
- Tsien RY, Bacskai BJ. Handbook of Biological Confocal Microscopy. 2. New York: Plenum Press; 1994. Video-rate confocal microscopy; pp. 459–478. [Google Scholar]
- Wibo M, Bravo G, Godfraind T. Post-natal maturation of excitation-contraction coupling in rat ventricle in relation to the subcellular localization and surface density of 1,4-dihydropyridine and ryanodine receptors. Circulation Research. 1991;68:662–673. doi: 10.1161/01.res.68.3.662. [DOI] [PubMed] [Google Scholar]
- Wier W, Yue D. Intracellular calcium transients underlying the short-term force-interval relationship in ferret ventricular myocardium. Journal of Physiology. 1986;376:507–530. doi: 10.1113/jphysiol.1986.sp016167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfart B, Noble M. The cardiac excitaion-contraction cycle. Pharmacology and Therapeutics. 1982;16:1–43. doi: 10.1016/0163-7258(82)90030-4. [DOI] [PubMed] [Google Scholar]


