Abstract
Recombinant ATP-sensitive K+ channels (KATP channels) were heterologously expressed in the NIH3T3 mouse cell line, and the electrophysiological properties were studied using patch-clamp techniques.
The NIH3T3 cell lines transfected with the inwardly rectifying K+ channel Kir6.1 alone or with both Kir6.1 and cystic fibrosis transmembrane conductance regulator (CFTR) exhibited time-independent K+ currents with weak inward rectification. In contrast, no measurable K+ conductance was observed in mock-transfected cells or in cells transfected with CFTR alone. Regardless of co-transfection with Kir6.1, the transfection with CFTR produced a Cl− conductance that was activated by cell dialysis with cAMP (1 mm). The conductance was reversibly suppressed by glibenclamide (30 μm).
Whole-cell currents at +60 mV were blocked in a concentration-dependent manner by Ba2+ ions with similar IC50 values: 89.3 ± 23.3 μm (Kir6.1 alone) and 67.3 ± 24.9 μm (Kir6.1-CFTR).
The currents recorded from Kir6.1-transfected cells were not affected by glibenclamide, whereas glibenclamide did inhibit the conductance expressed in cells co-transfected with CFTR (IC50= 35.9 ± 6.6 μm).
In the cell-attached mode with a 150 mm K+ pipette solution, both Kir6.1- and Kir6.1-CFTR-transfected cells displayed a class of K+ channels showing weak inward rectification and a slope conductance of 50.7 ± 1.0 and 52.4 ± 4.9 pS, respectively.
In the inside-out mode, the single-channel currents recorded from both types of cells were not inhibited by intracellular ATP (1 mm). However, glibenclamide was found to block the single-channel activities in the co-transfected cells.
The cystic fibrosis transmembrane conductance regulator (CFTR) belongs to the ATP-binding cassette (ABC) superfamily and forms a cAMP-dependent protein kinase A (PKA)-regulated Cl− channel, the function of which is impaired in patients with cystic fibrosis (Riordan et al. 1989; Welsh et al. 1992). Besides serving as a Cl− channel itself, CFTR has been shown to be a multifunctional protein (Higgins, 1995), regulating the activity of several other ion channels (Stutts et al. 1995; Johnson, Boyles, Wilson & Boucher, 1995; Schwiebert et al. 1995). CFTR has significant sequence homology with the sulphonylurea receptor (SUR) (Aguilar-Bryan et al. 1995). More recently, CFTR was shown to be sensitive to sulphonylurea drugs (SU) (Sheppard & Welsh, 1992; Tominaga, Horie, Sasayama & Okada, 1995), and to confer sensitivity to SU on ROMK2 (Kir1.2), a renal inward rectifier K+ channel with relatively low sensitivity to SU (McNicholas, Guggino, Schwiebert, Hebert, Giebisch & Egan, 1996). We report here that co-expression of CFTR confers sensitivity to SU, but not to ATP, on the Kir6.1 channel, a ubiquitous type of inward rectifier K+ channel that is primarily insensitive to both SU and ATP (Inagaki et al. 1995b; Ämmälä, Moorhouse & Ashcroft, 1996a; Yamada et al. 1997). Co-expression with CFTR also modified the single-channel kinetics of Kir6.1. Since both CFTR and Kir6.1 are widely expressed in various organs (Riordan et al. 1989; Inagaki et al. 1995b), the two molecules might be expected to interact with each other under physiological conditions.
METHODS
Cell culture and transfection
The coding sequences of Kir6.1 and CFTR were subcloned into the vectors pRcCMV neo (Invitrogen) and pMAM2-BSD (Kaken Chemicals Co., Japan), respectively. NIH3T3 cells were transfected with these plasmids using the Mammalian Transfection Kit (Stratagene) followed by selection and propagation in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10 % fetal bovine serum (Gibco), 100 u ml−1 penicillin, 100 μg ml−1 streptomycin, 1 mg ml−1 G418 (Gibco) and 8 μg ml−1 brasticidin S (Gibco). The pooled transfectants were frozen at early passages. For electrophysiological experiments, the transfectants (5 × 103) were plated onto coverslips (4 mm × 15 mm) placed in plastic dishes, and after 12–72 h, individual coverslips were transferred to a test chamber (with 0.5 ml of medium) on the stage of an inverted microscope (Nikon TMD type II, Japan). Recordings were made with randomly selected cells (n > 100).
Electrophysiology
In whole-cell voltage-clamp experiments, the pipette solution used for recording K+ currents contained (mm): 110 L-aspartic acid, 30 KCl, 2 MgCl2, 5 Na2CrP, 5 Hepes (pH 7.2 with KOH; total [K+] 140 mm) and 3 K2ATP. The pipette solution used for recording Cl− currents contained (mm): 85 L-aspartic acid, 10 EGTA, 20 TEA-Cl, 0.5 MgCl2, 5 Na2CrP, 10 Hepes, 5.5 glucose (pH 7.2 with CsOH) and 10 MgATP. The bathing solution for K+ currents contained (mm): 143 NaCl, 5.4 KCl, 0.5 MgCl2, 0.3 NaH2PO4, 5 Hepes (pH 7.4); and for Cl− currents (mm): 150 NaCl, 5 Hepes, 0.5 MgCl2, 5.5 glucose (pH 7.4). Single-channel recordings were performed in either the cell-attached or inside-out mode. Both pipette and standard extracellular solutions contained (mm): 145 KCl, 2 CaCl2, 5 Hepes and 0.5 EGTA (pH 7.4). In the inside-out mode, CaCl2 was eliminated from the extracellular solution which faced the internal surface of the patch membrane. The channel activities are expressed as mean patch current (I=nPoi) at −60 mV, where n, Po and i represent the number of open channels, open probability and unit amplitude, respectively. All experiments were performed at room temperature (22–25°C). The threshold for judging the open state was set at half the channel amplitude. Concentration- response curves were fitted to the Hill equation: I/IC= 1/(1 + ([X]/IC50)h), where IC is the mean patch current in control, [X] is the concentration of BaCl2 or glibenclamide, IC50 is the half-maximally inhibitory concentration, and h is the Hill coefficient. The data are presented as means ±s.d.
RESULTS
Kir6.1-transfected NIH3T3 cells displayed a time-independent conductance showing weak inward rectification when recorded using a 140 mm K+ pipette solution and an extracellular K+ concentration of 5.4 mm. This inwardly rectifying current was not affected by co-expression with CFTR. On the other hand, control (mock-transfected cells) and CFTR alone-transfected cells showed no measurable conductance (Fig. 1A-D). Current amplitudes at the −40 mV holding potential were measured in a representative group of cells and are summarized in Fig. 1E.
Figure 1. Whole-cell currents from cells transfected with Kir6.1 and/or CFTR.
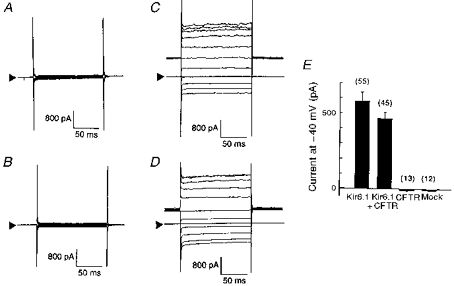
Four sets of whole-cell current traces recorded from mock- (A), CFTR- (B), Kir6.1- (C), and Kir6.1-CFTR-transfected (D) cells in response to voltage steps from −140 to +60 mV from a holding potential of −40 mV (20 mV steps). Extracellular K+ concentration was 5.4 mm in all experiments. Arrowheads indicate the zero current level. E, bar-graphs showing current amplitude measured at −40 mV holding potential from each group of cells. Error bars indicate the standard deviation, and the numbers in parentheses the number of observations.
The inwardly rectifying conductance of Kir6.1-transfected cells was dependent on the extracellular K+ concentration ([K+]o), as would be expected of a predominantly K+-selective conductance. Co-expression with CFTR did not affect the sensitivity of the conductance to [K+]o (Fig. 2). In a single cell, [K+]o was successively increased from 2.7 to 20 mm, and then to 140 mm. Three sets of original Current Traces Are Shown in Fig. 2Aa and Ba. Figure 2Ab and Bb summarizes the current-voltage (I-V) relationships thus obtained in a single cell transfected with either Kir6.1 (A) or Kir6.1-CFTR (B). Raising [K+]o produced a steeper slope conductance measured at the negative limbs of the I-V relationships with a positive shift of the reversal potential as predicted by the Nernst equation (Kir6.1-transfected cells, 22.4 ± 0.16 mV; Kir6.1-CFTR-transfected cells, 21.8 ± 0.18 mV, per e-fold change in [K+]o; Fig. 2Ac and Bc). This confirms that the expressed currents are carried by K+ channels and that Kir6.1 encodes an inwardly rectifying K+ channel as previously reported (Inagaki et al. 1995b; Ämmäläet al. 1996b; Yamada et al. 1997).
Figure 2. Whole-cell currents and reversal potentials (Vrev) measured from either Kir6.1- or Kir6.1-CFTR-transfected cells in the presence of various [K+]o.
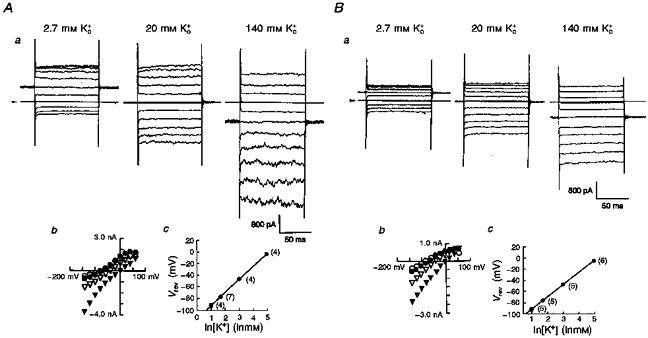
Two sets of traces from either Kir6.1- (Aa) or Kir6.1-CFTR-transfected cells (Ba) at three different extracellular [K+]o. Arrowheads indicate the zero current level. Amplitudes of membrane currents were plotted against test potentials between −140 mV and +60 mV (Ab and Bb; 2.7 mm, ^; 5.4 mm, •; 20 mm, ▿; and 140 mm, ▾). Vrev values were measured as the x-intercept and are plotted against [K+]o (Ac and Bc; means ±s.d.). Straight lines were drawn by fitting to the equation: Vrev= (RT/F)ln([K+]o/[K+]i), where R is the universal gas constant, T is absolute temperature and F is Faraday's constant. Numbers in parentheses indicate the number of observations.
Cells transfected with CFTR under voltage-clamp recording conditions in which all K+ ions had been replaced with Cs+ displayed no measurable conductance (Fig. 3A). However, dialysis with cAMP (1 mm) in the CFTR-transfected cells produced a time-independent outwardly rectifying conductance whether or not the cells had been co-transfected with Kir6.1 (Fig. 3Ba). In this series of experiments, we used a holding potential of 0 mV. Whole-cell currents showed a strong outward rectification and reversed at −43 mV, which is close to the equilibrium potential for Cl− ions (ECl) under these recording conditions (ECl= −51 mV; 21 mm Cl− pipette solution, 151 mm extracellular Cl− concentration). Since reducing the extracellular Cl− concentration ([Cl−]o) shifted the reversal potential of the cAMP-elicited conductance according to ECl (data not shown), CFTR Cl− channels were thought to be successfully expressed in our preparation.
Figure 3. Glibenclamide inhibited cAMP-induced whole-cell currents in the CFTR-transfected cells in a concentration-dependent manner.
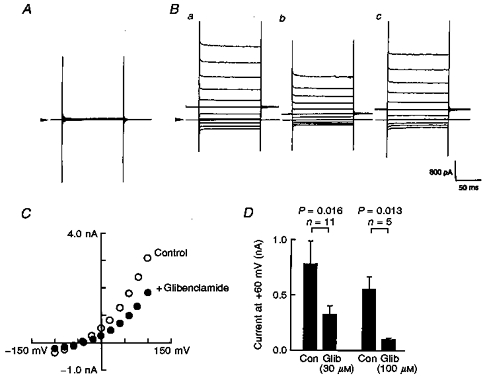
In order to isolate the Cl− conductance, all the K+ ions were replaced with Cs+ ions. A and B, representative whole-cell current traces measured in the absence (A) or presence (B) of cAMP (1 mm) in the pipette solution. Holding potential was 0 mV, and step pulses of ± 100 mV with 20 mV steps were applied. Arrowheads indicate the zero current level. Note that the holding potential was different from that employed in the experiments of Figs 1 and 2. Glibenclamide (30 μm) reversibly inhibited the intrapipette cAMP-induced Cl− conductance (Bb and c). C, two I-V relationships taken from the experiment shown in B (^, before, and •, after application of glibenclamide). D, bar-graphs showing the effect of glibenclamide (Glib; 30 μm and 100 μm) on the cAMP-induced whole-cell curents. Membrane current amplitudes were measured at +60 mV test potential and were compared before (control, Con) and after application of glibenclamide. Vertical bars indicate the standard deviation. n, number of observations. P values were calculated by Student's paired t test.
Glibenclamide (30 μm) reversibly blocked the Cl− conductance (Fig. 3B and C). The inhibitory action of glibenclamide was concentration dependent, and half-maximal block was obtained with ∼30 μm at +60 mV (Fig. 3D). This concentration of glibenclamide was close to the IC50 values previously reported for the inhibition of CFTR Cl− currents expressed in NIH3T3 cells (Sheppard & Welsh, 1992) and of the cardiac isoform of native CFTR Cl− conductance in guinea-pig ventricular myocytes (Tominaga et al. 1995).
In cells transfected with Kir6.1, regardless of co-transfection with CFTR, exposure to Ba2+ ions (100 μm) reversibly reduced the K+ conductance (Fig. 4A and B). Current amplitudes were measured during the depolarization to +60 mV before (IC) and 5 min after application of Ba2+ ions (IBa). Current ratios (IBa/IC) were calculated in every experiment (one trial for each Ba2+ concentration) and are plotted against Ba2+ concentration (Fig. 4C). Ba2+ IC50 values were similar: 89.3 ± 23.3 μm for Kir6.1-transfected cells and 67.3 ± 24.9 μm for Kir6.1-CFTR-transfected cells. In contrast, glibenclamide (30 μm) was found to block the K+ current only in the cells that were co-transfected with CFTR (Fig. 4D and E). In these co-transfected cells, the inhibitory action of glibenclamide was concentration dependent. The glibenclamide dose-inhibition relationship was constructed in the same fashion as in the experiment with Ba2+ ions and yielded an IC50 of 35.9 ± 6.6 μm and a Hill coefficient (h) of 0.72 ± 0.10 (n= 33; Fig. 4F). The IC50 values were similar to those reported previously for block of the CFTR Cl− current (Sheppard & Welsh 1992; Tominaga et al. 1995).
Figure 4. Co-transfection with CFTR conferred sensitivity to glibenclamide on Kir6.1 channels.
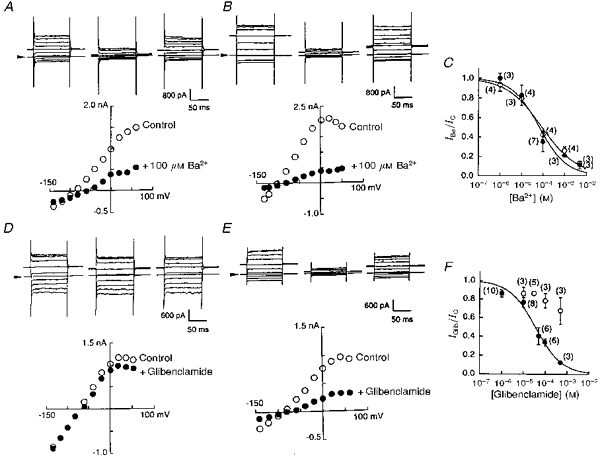
A and B, whole-cell curent traces recorded before, during and after application of Ba2+ (100 μm) to a Kir6.1-transfected cell (A) or to a cell co-transfected with Kir6.1 and CFTR (B). Arrowheads indicate the zero current level. Graphs below the traces show I-V relationships measured from original current traces in control (^) and in the presence of Ba2+ (•). C, Ba2+ concentration-inhibition relationships in Kir6.1-transfected (^) and co-transfected (•) cells. Smooth lines were drawn by fitting to the Hill equation with h= 0.53 ± 0.07 (^) and h= 0.68 ± 0.15 (•). Numbers in parentheses indicate the number of observations. D and E, whole-cell curent traces recorded before, during and after application of glibenclamide (30 μm) to a Kir6.1-transfected cell (D) or to a cell co-transfected with Kir6.1 and CFTR (E). Graphs indicate I-V relationships measured from the original current traces indicated above in control (^) and in the presence of glibenclamide (•). F, glibenclamide concentration-inhibition relationships for Kir6.1-transfected (^) and co-transfected (•) cells. Mean values and standard deviations are shown. Numbers in parentheses indicate the number of observations.
In the cell-attached recording mode, we failed to record the single-channel activity from mock-transfected cells (Fig. 5A). However, both Kir6.1- and Kir6.1-CFTR-transfected cells displayed a class of single K+ channels with weak inward rectification (Fig. 5B and C). The percentage appearance of single-channel openings was calculated for every patch; this was obtained as 100 × (number of channels observed)/(number of trials), and is shown as a bar-graph in Fig. 5D.
Figure 5. Single-channel properties measured from three types of transfected cells.
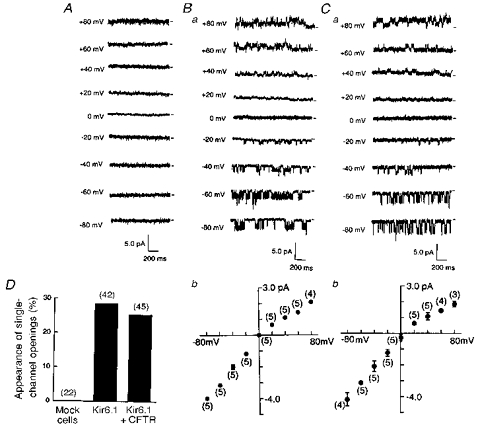
Single-channel current traces were recorded in the cell-attached patches with 140 mm K+ pipette solution, for cells superfused with a 140 mm solution. A, mock-transfected cell. B, Kir6.1-transfected cell. C, cell co-transfected with Kir6.1 and CFTR. Short horizontal bars to the right of each trace indicate the zero current level and numbers to the left of each trace the test potential. Bb and Cb show single-channel I-V relationships. Means ±s.d. Numbers in parentheses are the number of observations. D, percentage appearance of single-channel openings was estimated in each set of cells (mock-, Kir6.1- and Kir6.1-CFTR-transfected) and is summarized in the bar-graphs. Numbers in parentheses indicate the number of trials.
Co-transfection with CFTR per se did not change the single-channel conductance (from 50.7 ± 1.0 to 52.4 ± 4.9 pS, n= 5) when measured as the inward current of the I-V relationship (Fig. 5Bb and Cb). However, the long bursting kinetics were significantly altered at potentials negative to −60 mV (Fig. 5Ba and Ca). Single-channel currents from cells transfected with Kir6.1 alone showed longer bursting periods. Open-time histograms constructed at −60 mV could be fitted by the sum of two exponentials in both types of channel; co-expression with CFTR reduced the longer burst time from 157 ± 26.9 to 12.0 ± 1.8 ms (n= 4). In contrast, the kinetics of the intraburst flicker remained unchanged (mean dwell times, 3.4 ± 0.5 vs. 3.2 ± 0.3 ms; n= 4).
Application of glibenclamide (100 μm) to the outside of the recording pipettes failed to affect the channel activity in the cells transfected with Kir6.1 alone (n= 4; Fig. 6Aa and Ac). In contrast, glibenclamide reduced the mean patch currents to 13.9 ± 2.8 % of the control (n= 5) in co-transfected cells (Fig. 6Ab and Ac), supporting the results of the whole-cell experiment. In the inside-out mode recordings, MgATP (1 mm) applied to the internal surface of the cell membrane was without effect in both types of transfected cells (Fig. 6B).
Figure 6. The effect of extracellular glibenclamide and internal ATP on single-channel activites.
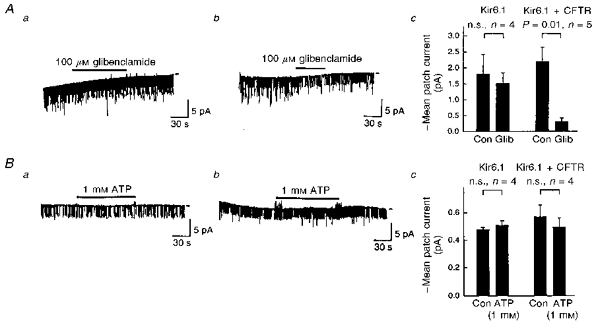
A, the effect of glibenclamide (100 μm) on the single-channel activities measured in the cell-attached mode from Kir6.1- (a) or Kir6.1-CFTR-transfected cells (b). The recording conditions were the same as those used in the experiment of Fig. 5. The test potential was −60 mV. Bar-graphs in Ac summarize the effect of glibenclamide (Glib). Con, control. Error bars indicate the standard deviation. n, number of observations. P values were estimated by Student's paired t test. n.s., not significant. B, two representative traces of single-channel currents recorded from inside-out patches of Kir6.1- (a) or Kir6.1-CFTR-transfected (b) cells. Bars indicate the internal application of ATP (1 mm). K+ concentrations were 140 mm in both the pipette solution and internal solution that superfused the internal side of the patch membrane. The test potential was −60 mV. Bar-graphs in Bc summarize the effect of ATP, as in Ac. Note that mean current was reduced in the inside-out condition.
DISCUSSION
The present study shows that co-expression of Kir6.1 channels with CFTR modifies the single-channel kinetics, and confers sensitivity to SU on the K+ channel. Notably, sensitivity to ATP at the intracellular surface of the patch membrane was not conferred on Kir6.1 channels by co-expression with CFTR. Functional interaction between CFTR and channel proteins has been reported in epithelial outwardly rectifying Cl− channels and Na+ channels (Stutts et al. 1995; Johnson et al. 1995; Schwiebert et al. 1995; for review, see Higgins, 1995). More recently, other members of the ABC transporters, SURs, have been shown to form ATP-sensitive K+ channels in association with Kir6.1 or Kir6.2 (Inagaki et al. 1995a; Ämmälä et al. 1996a, b; Yamada et al. 1997). Changes in the pharmacological properties and gating kinetics of the K+ channels have been noted in Kir6.2-transfected cells with different types of SURs (Inagaki et al. 1996). Co-expression of Kir6.1 with SURs confers sensitivity to both ATP and SU on Kir6.2 (Inagaki et al. 1995a) and Kir6.1 channels (Ämmälä et al. 1996a, b; Yamada et al. 1997).
Unlike SURs, since CFTR could not confer sensitivity to ATP on Kir6.1 channels, but did confer sensitivity to SU, the mechanism by which SU produces inhibition of Kir6.1- CFTR channels appears to differ from that of the ATP-dependent inhibition observed in Kir6.1-SUR or Kir6.2- SUR channels (Ämmäläet al. 1996a). Direct evidence that the primary site for ATP sensitivity is located on K+ channels was shown by an elegant experiment from the same laboratory, using truncated Kir6.2 channels (Tucker, Gribble, Zhao, Trapp & Ashcroft, 1997). On the other hand, SU appears to bind to ABC superfamily proteins, i.e. CFTR or SUR, directly, via intramembrane pathways as has been demonstrated in β-cell membranes (Zünkler, Trube & Panten, 1989).
The Kir6.1 channel expressed alone in HEK 293 cells had a unitary conductance of 70 ± 2 pS (Inagaki et al. 1995b), whereas Kir6.1-SUR2B expressed in HEK 293T had a conductance of 32.9 pS (Yamada et al. 1997). The difference in single-channel conductance might be due to differences in the cells used for channel expression, and/or to other molecules in each cell membrane that might interact with the Kir6.1 channel. More recently, it has been shown that CFTR functionally associates with Kir1.x channels, which are members of the inward-rectifier K+ channel family, thereby conferring sensitivity to both ATP and SU (McNicholas et al. 1996; Ruknudin, Shulze, Sullivan, Lederer & Welling, 1997). Our data support the idea that CFTR has direct protein-protein interaction and hetero-oligomerization, rather than indirect interaction, with Kir6.1. Such a functional communication between CFTR and Kir6.1 may take place in native cell membranes, since both are widely distributed in many organs (Riordan et al. 1989; Inagaki et al. 1995b).
Acknowledgments
We are grateful to Dr S. Seino (Chiba University, Japan) and Dr J. Rommens (Toronto Children's Hospital, Canada) for generous gifts of Kir6.1 and CFTR, and to Dr Y. Okada (National Institute of Physiological Sciences, Japan), Dr Andrew F. James and Dr Bruce M. Hendry (King's College London, UK) for critical comments. This work was supported by grants-in-aid for Priority Areas of ‘Channel-Transporter Correlation’ from the Japan Ministry of Education, Science and Culture.
References
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, Boyd AE, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Ämmälä C, Moorhouse A, Ashcroft FM. The sulphonylurea receptor confers diazoxide sensitivity on the inwardly rectifying K+ channel Kir6.1 expressed in human embryonic kidney cells. Journal of Physiology. 1996a;494:709–714. doi: 10.1113/jphysiol.1996.sp021526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ämmälä C, Moorhouse A, Gribble F, Ashfield R, Proks P, Smith PA, Sakura H, Coles B, Ashcroft SJH, Ashcroft FM. Promiscuous coupling between the sulphonylurea receptor and inwardly rectifying potassium channels. Nature. 1996b;379:545–548. doi: 10.1038/379545a0. 10.1038/379545a0. [DOI] [PubMed] [Google Scholar]
- Higgins CF. The ABC of channel regulation. Cell. 1995;82:693–696. doi: 10.1016/0092-8674(95)90465-4. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995a;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, IV, Wang C-Z, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. 10.1016/S0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, Seino Y, Mizuta M, Seino S. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. Journal of Biological Chemistry. 1995b;270:5691–5694. doi: 10.1074/jbc.270.11.5691. 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- Johnson LG, Boyles SE, Wilson J, Boucher RC. Normalization of raised sodium absorption and raised calcium-mediated chloride secretion by adenovirus-mediated expression of cystic fibrosis transmembrane conductance regulator in primary human cystic fibrosis airway epithelial cells. Journal of Clinical Investigation. 1995;95:1377–1382. doi: 10.1172/JCI117789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas CM, Guggino WB, Schwiebert EM, Hebert SC, Giebisch G, Egan ME. Sensitivity of a renal K+ channel (ROMK2) to the inhibitory sulfonylurea compound glibenclamide is enhanced by coexpression with the ATP-binding cassette transporter cystic fibrosis transmembrane regulator. Proceedings of the National Academy of Sciences of the USA. 1996;93:8083–8088. doi: 10.1073/pnas.93.15.8083. 10.1073/pnas.93.15.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J-L, Drumm ML, Iannuzzi MC, Collins FS, Tsui L-C. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Ruknudin A, Shulze DH, Sullivan SK, Lederer WJ, Welling PA. Novel subunit composition in a renal epithelial KATP channel (Kir1.1 + CFTR) Biophysical Journal. 1997;72:A33. doi: 10.1074/jbc.273.23.14165. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Egan ME, Hwang T-H, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. Journal of General Physiology. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Horie M, Sasayama S, Okada Y. Glibenclamide, an ATP-sensitive K+ channel blocker, inhibits cardiac cAMP-activated Cl− conductance. Circulation Research. 1995;77:417–423. doi: 10.1161/01.res.77.2.417. [DOI] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Anderson MP, Rich DP, Berger HA, Denning GM, Ostedgaard LS, Sheppard DN, Cheng SH, Gregory RJ, Smith AE. Cystic fibrosis transmembrane conductance regulator: A chloride channel with novel regulation. Neuron. 1992;8:821–829. doi: 10.1016/0896-6273(92)90196-k. 10.1016/0896-6273(92)90196-K. [DOI] [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. Journal of Physiology. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zünkler BJ, Trube G, Panten U. How do sulfonylureas approach their receptor in the B-cell plasma membrane? Naunyn-Schmiedeberg's Archives of Pharmacology. 1989;340:328–332. doi: 10.1007/BF00168518. [DOI] [PubMed] [Google Scholar]


