Abstract
The spinal volleys evoked by single transcranial magnetic or electric stimulation over the cerebral motor cortex were recorded from a bipolar electrode inserted into the cervical epidural space of three conscious human subjects. These volleys were termed direct (D) and indirect (I) waves according to their latency.
We measured the size and number of volleys elicited by magnetic stimulation at various intensities with subjects at rest and during 20 or 100% maximum contraction of the contralateral first dorsal interosseous muscle (FDI). Surface EMG activity was also recorded.
Electrical stimulation evoked a D-wave volley. Magnetic stimulation at intensities up to about 15% of stimulator output above threshold evoked only I-waves. At higher intensities, a D-wave could be seen in two of the three subjects.
At all intensities tested, voluntary contraction increased the number and size of the I-waves, particularly during maximum contractions. However, there was only a small effect on the threshold for evoking descending activity. Voluntary contraction produced large changes in the size of EMG responses recorded from FDI.
Because the recorded epidural activity is destined for muscles other than the FDI, it is impossible to say to what extent increased activity contributes to voluntary facilitation of EMG responses. Indeed, our results suggest that the main factor responsible for enhancing EMG responses in the transition from rest to activity is likely to be increased excitability of spinal motoneurones, rather than increases in the corticospinal volley. The latter may be more important in producing EMG facilitation at different levels of voluntary contraction.
In their original description of transcranial electrical stimulation of the motor cortex, Merton & Morton (1980) noted that EMG responses were enhanced considerably if stimulation was applied during voluntary contraction of the target muscle. One accepted reason for facilitation is that voluntary contraction increases the excitability of the spinal motoneurone pool, so that a given descending volley evokes a larger motoneuronal discharge than if the subject had been at rest. The main point of debate is whether voluntary contraction also increases cortical excitability so that a given size of stimulus evokes a larger corticospinal volley. The question is important because several authors have used the assumption that cortical excitability can change the size of descending volleys to make inferences about the role of the motor cortex in different types of movement.
Until recently most experiments relied on indirect arguments. Day et al. (1987) investigated facilitation of electrically evoked responses. They used H-reflex excitability as an indirect monitor of corticospinal input to motoneurones and showed that voluntary contraction had no effect on the threshold intensity needed to evoke corticospinal activity. In a more recent study, Mazzocchio, Rothwell, Day & Thompson (1994) employed the same technique using magnetic stimulation and showed that voluntary contraction lowered the threshold for evoking H-reflex facilitation by about 6%. The difference between the results was thought to be due to differences in the way electric and magnetic stimulation activate corticospinal output. Responses to the former were assumed to be unaffected by voluntary activity because electrical stimulation activates corticospinal axons within the white matter where they are relatively unaffected by changes in cortical excitability. In contrast, voluntary activation of the cortex was thought to facilitate responses to magnetic stimulation because magnetic stimulation over the hand area of the motor cortex (with an antero-posterior coil orientation as used by Mazzocchio et al. 1994), preferentially activates corticospinal neurones trans-synaptically. Using a different approach, Maertens de Noordhout, Pepin, Gerard & Delwaide (1994) used high voltage electrical stimulation over the spinal cord to activate descending motor tracts directly, and compared the amount of voluntary facilitation of EMG responses in tibialis anterior with that seen after magnetic stimulation of the motor cortex. There was no difference between the two, suggesting that voluntary activity had had no effect on the amount of descending activity produced by the cortical shock. However, this was probably because magnetic stimulation over the leg area tends to produce direct activation of corticospinal axons (Priori et al. 1993), and hence the results are similar to those seen after electrical stimulation of the arm area.
More recent studies have employed direct recording of the descending activity evoked by magnetic stimulation. In two separate reports, Kaneko, Kawai, Fuchigami, Shiraishi & Ito (1996a, b) recorded through electrodes implanted in the epidural space of the cervical cord in conscious patients prior to spinal surgery. They found that although voluntary facilitation increased the size of evoked EMG responses, there was no significant effect on the amplitude or number of descending volleys produced by each stimulus. They concluded that voluntary activity did not facilitate the cortical response to magnetic stimulation. In contrast, a recent experiment in a behaving monkey gave the opposite result. Direct recordings of corticospinal activity showed that the size of volley evoked by a given magnetic stimulus was dependent on the task that the monkey was performing at the time of stimulation (Baker, Olivier & Lemon, 1995).
The present report is an attempt to resolve this controversy. We have made use of the opportunity to record directly the descending corticospinal volleys from three conscious patients who had a stimulator implanted in the cervical cord for the treatment of intractable pain. Following surgery, the electrode connections are externalized for several days in order to allow them to be tested before final implantation in a second operation. During this time, it is possible to record both the descending activity in the cord and also the EMG responses produced by transcranial stimulation of the motor cortex. We could therefore investigate directly how the size, latency and number of descending volleys was related to the amplitude and latency of EMG responses during different amounts of voluntary activity. The epidural electrode may record from many fibre tracts in the cord. However, since the latency of the volleys and their recruitment at different stimulus intensities is similar to that described in animal experiments in which electrodes have been placed directly on the corticospinal tract (Edgley, Eyre, Lemon & Miller, 1990), we shall assume that the majority of the activity is conducted in corticospinal fibres. In discussing these results, we acknowledge the possibility that facilitation at an interneurone level exists in addition to the cortical and motoneuronal mechanisms discussed above. However, if we confine ourselves to responses in small hand muscles, which are generally thought to be predominantly monosynaptic in origin, then this is the least likely explanation (Pierrot-Deseilligny, 1996).
METHODS
Corticospinal volleys evoked by transcranial magnetic and electrical stimulation of the motor cortex were recorded from the high cervical cord in three subjects with no abnormality of the central nervous system (two females and one male; aged 56, 68 and 48 years). All three had a spinal cord stimulator implanted for treatment of intractable dorso-lumbar pain. The electrode array (model Quad 3487A, Medtronic, Minneapolis, MN, USA) was implanted percutaneously in the epidural space at C1-C2 level, with the recording sites aligned vertically along the dorsum of the cord. Recordings of descending activity were made 2-3 days after implantation during the trial screening period when the electrode connections are externalized. The subjects gave informed consent to the study that was performed with the approval of the appropriate Institutional Ethics Committee.
Recordings were made simultaneously from the epidural electrode and from the first dorsal interosseous muscle (FDI) of the left hand. Epidural potentials were recorded between the most proximal and most distal of the four electrode contacts on each implant. These had a surface area of 2.54 mm2 and were equally spaced in a row spanning 30 mm. The distal contact was connected to the reference input of the amplifier, with an earth electrode around the neck. Surface EMGs were obtained via two 9 mm diameter Ag-AgCl electrodes with the active electrode over the motor point of the muscle and the reference on the metacarpophalangeal joint of the index finger. EMGs and the corticospinal volleys were amplified and filtered (bandwidth, 3 Hz to 3 kHz) by D150 amplifiers (Digitimer, Welwyn Garden City, UK). Data were collected on a computer with a sampling rate of 5 kHz per channel and stored for later analysis using a CED 1401 A/D converter (Cambridge Electronic Design, Cambridge, UK).
Magnetic stimulation was performed with a high power Magstim 200 (Magstim Co., Whitland, Dyfed, UK). A figure-of-eight coil with external loop diameters of 9 cm was held over the right motor cortex at the optimum scalp position to elicit motor responses in the contralateral FDI with the induced current flowing in a postero-anterior direction. Intensities were expressed as a percentage of the maximum output of the stimulator. Active motor threshold (AMT) was defined as the minimum stimulus intensity that produced a liminal motor evoked response (about 50 μV in 50% of trials) during isometric contraction of the tested muscle at about 20% maximum. Descending volleys and FDI EMG responses were recorded under three experimental conditions: at rest, during 20% isometric contraction, and during maximum voluntary contraction. A constant level of voluntary contraction was maintained with reference to an oscilloscope display of EMG in front of the subject. Each contraction lasted 1 min or so, after which subjects had a short rest (30 s or more). Auditory feedback of the EMG activity was also provided. We used increasing intensities of magnetic stimulation in steps of 3% of the stimulator output, starting from a value equal to AMT minus 3% rising to AMT plus 30% in two subjects, and AMT plus 21% in the remaining subject. Ten sweeps were averaged at each intensity of stimulation.
Anodal stimulation of the motor cortex was used to identify the latency of the earliest (probable D-wave) descending volley. Electrical stimulation was performed with a Digitimer D180 stimulator, with a 50 μs time constant. The cathode was located at the vertex and the anode 7 cm laterally. The responses to ten liminal stimuli at an intensity of 5% of maximum stimulator output above AMT were averaged during a 20% maximum contraction.
The latency of each component of the descending volley was measured to its peak because the precise onset was often difficult to define for all but the first component. Amplitudes were measured from the peak to the next trough in order to minimize distortions due to stimulus artefact. Only consistent deflections with a mean amplitude over ten responses of > 2 μV occurring within the time period of interest were analysed.
Linear regression analysis was used to determine the correlation between stimulus intensity and response size. The size of the descending volleys recorded under the three different experimental conditions was compared using one-way analysis of variance. Post hoc comparisons were made using Student's t test.
RESULTS
Epidural volleys: electrical stimulation
In order to minimize subject discomfort, only a single intensity (5% of stimulator output above active threshold) of electrical stimulation was used. Responses were recorded during active contraction at 20% MVC. In all three subjects electrical (anodal) stimulation of the motor cortex evoked a single negative wave. It had a latency of 2.6 ms in two subjects and 2.4 ms in the third subject (Fig. 1).
Figure 1. Descending volleys evoked by magnetic stimulation using increasingly strong stimulus intensities and with different degrees of voluntary contraction in three subjects.
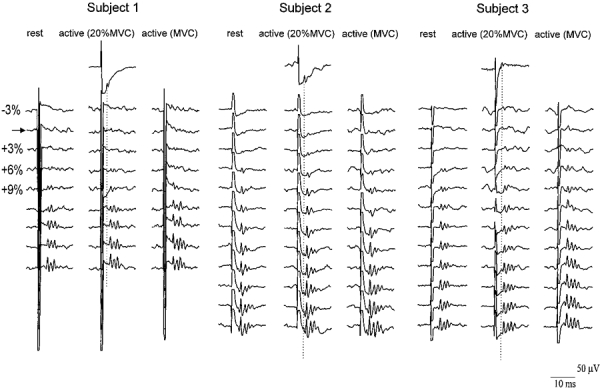
The traces show averaged (10 sweeps each) epidural recordings from the high cervical cord. The vertical columns of traces are arranged in order of increasing magnetic stimulus intensity, from 3% of stimulator output below the threshold for evoking EMG responses in active muscle (horizontal row marked by arrow) to 21% (subject 1) or 30% (subjects 2 and 3) above threshold in steps of 3%. Each subject contributes 3 columns of results, recorded at rest (left), 20% maximum contraction (middle) and 100% maximum contraction (right) of FDI muscle. The single trace for each subject in the top row shows the potentials recorded after transcranial electrical stimulation of the motor cortex. The stimulus intensity in this trace was 5% of stimulator output above the threshold for evoking EMG responses in active muscle, and the responses were recorded during a voluntary contraction of 20% maximum. The peak latency of the first volley evoked by electrical stimulation (D-wave) is indicated by the vertical dotted line. In all three subjects and in all recording conditions the size and number of waves increased as the stimulus intensity was increased. The waves recruited by low intensity magnetic stimulation are probably I-waves. At high intensities, a D-wave can be seen in subjects 2 and 3. Voluntary contraction, particularly at maximum strength, slightly reduces the threshold intensity at which magnetic stimulation evokes I-waves, and increases their amplitude and number at higher intensities.
Epidural volleys: magnetic stimulation
Figure 1 shows the epidural volleys recorded in all three subjects at different stimulus intensities and contraction levels. Active motor threshold was equal to 29% of stimulator output in subject 1, 28% in subject 2 and 23% in subject 3. The mean amplitude of each volley at each contraction level is shown in Fig. 2.
Figure 2. Mean amplitude of the epidural volleys evoked by magnetic stimulation using increasingly strong stimulus intensities and with different degrees of voluntary contraction.
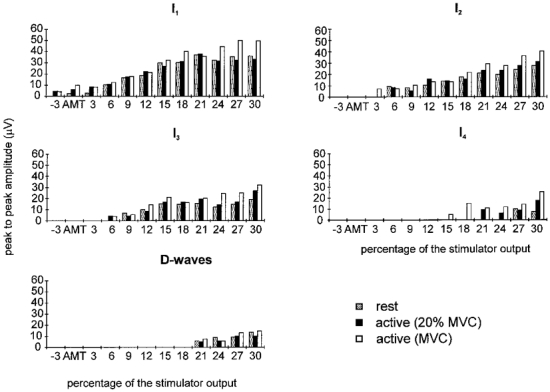
Histograms showing the mean amplitude of the different epidural volleys recorded in the three patients during stimulation at different intensities and with different levels of background contraction. The amplitude of the volleys is larger during contraction, and the threshold for evoking the later I-waves and the D-wave is higher than that for the early waves. The average standard error limit on these bars was 11 μV.
At all levels of contraction, the lowest threshold volley recruited by magnetic stimulation had a latency of 3.6-4 ms, which was 1-1.6 ms longer than the volley recruited by electrical stimulation. This volley increased in size, and was followed by later volleys as the intensity of stimulation was increased (Figs 1 and 2). The mean interpeak interval between these waves was 1.4 ms (s.d., 0.1 ms; range, 1-1.6 ms). At a stimulus intensity 21% of the stimulator output above active motor threshold, an earlier small wave appeared in subjects 2 and 3 which had the same latency as the volley recorded following electrical stimulation.
This pattern of recruitment is the same as that described by Kaneko et al. (1996a, b). At liminal stimulus intensities, the earliest volley elicited by electrical stimulation is probably a direct wave (D-wave; Patton & Amassian, 1954). We have therefore termed the later volleys recruited by magnetic stimulation indirect waves (I-waves), numbered in order of their appearance. The early volley seen at high magnetic stimulus intensities is probably a D-wave.
Comparison of responses recorded when FDI was relaxed or active
The intensity of stimulation was defined with respect to the threshold for evoking EMG responses in active muscle (AMT). When subjects were at rest, magnetic shocks at this intensity only produced a recognizable volley in subject 2; higher intensities were needed to evoke activity in the other two subjects. In subject 3 this first wave was recognizable only with a stimulus of 3% of the stimulator output above active EMG threshold whilst in subject 1 a stimulus of 6% above threshold was needed (Fig. 1).
Voluntary contraction had two effects on the volleys evoked by magnetic stimulation. First, it appeared that the threshold for evoking recognizable activity was reduced in one of the three subjects, subject 3 (Figs 1 and 2). During contraction, an I1 wave was seen at 3% of stimulator output below motor threshold whereas 3% above threshold had been needed at rest. The effect on threshold was not clear in the other two subjects. (It should be noted that because our electrodes are some distance from the spinal cord, the threshold for identification of a volley in epidural recordings may not be the same as the absolute threshold for evoking activity in corticospinal fibres. However, this does not affect the comparison of thresholds in relaxed and active conditions.)
The second effect of contraction was that at virtually all intensities, the amplitude of each individual I wave was slightly higher during activity (particularly during maximum contractions) than when at rest (Fig. 2). Figure 3A shows how the grand mean amplitude of the total epidural volley (the sum of the amplitudes of all the individual waves) over all strengths of stimulation was affected by the strength of contraction. An ANOVA (taking into account the repeated measurements made on each individual) showed that there was a significant (P < 0.01) effect of contraction strength. Post hoc analysis revealed that the volleys recorded during 100%, but not at 20%, maximum contraction were larger than at rest (paired t test, P < 0.05). During maximum contractions the effect was quite large: the total amplitude of the volley increased by almost 50%.
Figure 3. Effect of contraction strength on the amplitude of epidural volleys.
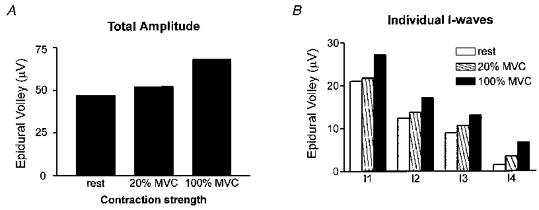
A shows how the grand mean amplitude of the epidural volleys varied with contraction strength. In B these data have been separated for each of the individual I-waves. The average standard error on these bars was 3.4 μV.
The same analysis was conducted on each individual I-wave (Fig. 3B) with similar results. There was a significant effect of contraction strength on each I-wave (ANOVA, P < 0.05), but post hoc analysis revealed that this was limited to the comparison of rest and 100% MVC. There was a significant (P < 0.05) difference between rest and 20% MVC only for the I4 wave. We were unable to test the effect of voluntary contraction on the D-wave since magnetic stimulation evoked this wave in only two of the three subjects. Finally, regression analysis showed that the total amplitude of the epidural volley was linearly related to the stimulus intensity (Fig. 4A) at each contraction level. The slope of the relationship was steeper during 100% maximum contraction than at rest, but not at 20% maximum. The total amplitude of the volleys did not appear to saturate over the range of intensities studied.
Figure 4. Relationships between amplitude of epidural volley peak-to-peak size of the evoked EMG response in FDI, and the intensity of magnetic stimulation.
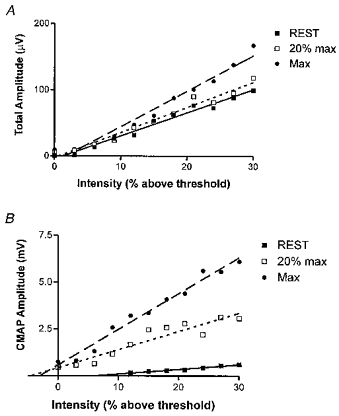
A, intensity of stimulation (x-axis) plotted against the total amplitude of the epidural volley (y-axis). The values at each intensity have been averaged across subjects and plotted as a single point at each contraction level. Linear regression lines are plotted and have the following slopes: rest = 3.45, r2 = 0.98; 20% max = 3.80, r2 = 0.97; 100% max = 5.36, r2 = 0.97. Only the slope at 100% maximum contraction is significantly different from that at rest (P < 0.05). B, intensity of stimulation (x-axis) plotted against the peak-to-peak size of the EMG response in FDI. The values at each intensity have been averaged across subjects and plotted as a single point. The regression slopes are: rest = 0.025, r2 = 0.97; 20% max = 0.097, r2 = 0.89; 100% max = 0.19, r2 = 0.98. There is a significant difference in slope between all three lines (P < 0.05).
Relationship of epidural volleys to surface EMG responses in FDI
Volleys of activity recorded from the epidural space may be destined for other muscles in addition to the FDI that was recorded. Indeed, during maximal hand muscle contraction and with high stimulus intensities, it was obvious that muscle twitches occurred in many muscles other than the target FDI. Thus the relationship between volley size and EMG response can be described only in qualitative terms.
One point, however, seems very clear. Changing from rest to voluntary contraction had more effect on the amplitude of EMG responses than on epidural volleys (Fig. 4). The slope of the relationship between stimulus intensity and amplitude of epidural volley was only slightly higher during 20% maximum contraction than at rest. However, the slope of the relationship between intensity and EMG amplitude more than tripled. This is even more dramatic if we accept that some of the increase in volley size could have been due to activity that was not destined for the FDI. The result is that the same size of EMG response can be obtained with a much smaller amount of descending activity during contraction than at rest.
Figure 5 shows some raw data examples of the effect. In Fig. 5A, the intensity of magnetic stimulation was adjusted to produce the same size of EMG response at rest and during 20 or 100% maximum contraction. At rest, three I-waves and a small D-wave were needed to evoke the response. During contraction at 20% maximum, the three I-waves were much smaller, and there was no D-wave. The EMG onset occurred about 1.5 ms earlier suggesting that less time was needed to discharge the motoneurones than at rest. Finally, at maximum contraction strength, only a single I-wave was evident in the epidural recording.
Figure 5. Relationship of epidural volleys to surface EMG responses in FDI.
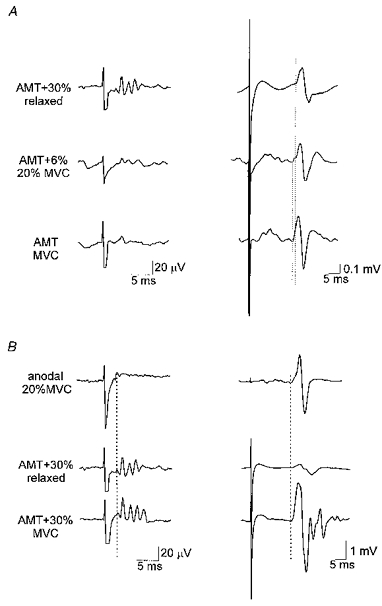
Averaged recordings from the epidural electrode are shown on the left; EMG responses from the FDI are shown on a longer time scale on the right. A, similar sized EMG responses evoked at different contraction strengths. At rest, a magnetic stimulus 30% of stimulator output above threshold is needed, whereas at 20% maximum contraction the intensity can be reduced to 6% above threshold, whilst at maximum contraction, only a threshold stimulus is needed. Note the corresponding reduction in the size and number of epidural volleys, and the longer latency of the EMG response recorded at rest (vertical dotted line). B, comparison of responses to anodal and magnetic stimulation. Responses to anodal stimulation were recorded during a 20% maximum contraction (20% MVC), and show a D-wave in the epidural recording (vertical dotted line, left trace), with a biphasic EMG response. At rest, a large magnetic stimulus (second pair of traces from top) evokes a small D-wave and three I-waves, but the resulting EMG response is small. However, when the subject contracts maximally (MVC), a fourth I-wave becomes visible, and the EMG response is much bigger and more complex.
Another example of the relationship between epidural volleys and EMG response is shown in Fig. 5B. Electrical stimulation evoked a D-wave that in an active muscle evoked a simple biphasic EMG potential with short onset latency. It was not possible to evoke an EMG response of the same amplitude in resting muscle using magnetic stimulation. At the highest intensity that we used (30% above motor threshold), magnetic stimulation evoked only a small EMG potential, despite the fact that the epidural volley was highly complex (a D-wave and 3 I-waves). During maximum contraction, the same intensity of magnetic stimulation resulted in a slightly more complex epidural volley (an extra I-wave), but the corresponding EMG potential was highly polyphasic. It had the same latency as that seen after electrical stimulation, presumably due to the D-wave that this high intensity stimulation evoked.
DISCUSSION
The present results provide clear evidence that voluntary contraction increases the size and number of descending volleys recorded epidurally over C1-C2 evoked by transcranial magnetic stimulation over the motor cortex in conscious humans. The effect can be substantial: maximum contraction can increase the total amplitude of the volleys by 50%. This differs from the two previous reports of Kaneko et al. (1996a, b) who concluded that there was no significant influence of voluntary activity on epidural activity. However, their data show a tendency for voluntary contraction to facilitate the volleys, and it may be that if they had used stronger contractions, rather than limited themselves to 20-30% maximum, they would have seen effects similar to those described here.
Characteristics of epidural volleys in conscious humans
The responses we recorded had characteristics that were very similar to those recorded previously in conscious humans by Nakamura, Kitagawa, Kawaguchi & Tsuji (1996) and Kaneko et al. (1996a, b). (1) Transcranial electrical stimulation evokes a short latency volley that is probably equivalent to the D-wave recorded in animal experiments (Patton & Amassian, 1954). Since the wave is present even at threshold intensities, it is presumably due to activation of corticospinal axons in the cortex, or just sub-cortical white matter. It is not known whether the latency of the volley decreases if the intensity of the electrical stimulus is raised substantially. However, Burke, Hicks & Stephen (1990) have described such behaviour in anaesthetized patients. (2) Using an antero-posterior coil orientation, magnetic stimulation over the arm area of motor cortex tends to produce volleys that occur about 1.5 ms later than the D-wave. (3) If the intensity of magnetic stimulation is increased, then it is possible in some subjects to recruit an earlier volley with the same latency as the D-wave.
These features are consistent with the ‘D- and I-wave’ hypothesis of transcranial stimulation that was outlined by Day et al. (1989), on the basis of single motor unit data. They differ from the results described by Burke, Hicks, Gandevia, Stephen, Woodforth & Crawford (1993) in anaesthetized patients using a circular coil in that the relative threshold for I-wave generation using magnetic stimulation is much lower than that needed to recruit D-waves (using an antero-posterior coil). This is presumably because anaesthetic suppresses the excitability of cortical synaptic transmission. The results are also different from those initially described in the monkey by Edgley, Eyre, Lemon & Miller (1990), who found that D-waves were readily recruited by magnetic stimulation using a circular coil. However, in their most recent work, in which they report the recordings from single corticospinal axons, the same group now accept that electrical stimulation is more likely to activate D-waves than magnetic stimulation (Edgley, Eyre, Lemon & Miller 1997), especially at intensities close to threshold. Any remaining differences between man and monkey may be due to the anatomical differences in size and folding of the monkey and human brain.
Voluntary facilitation of epidural volleys
The most important result of the present experiments was the direct demonstration that voluntary contraction can decrease the threshold and increase the size and number of epidural volleys evoked by a given intensity of magnetic stimulation. The effect on threshold was small and only clear in one of the three subjects, but is compatible with indirect evidence obtained from H-reflex studies in intact subjects by Mazzocchio et al. (1994). They estimated that voluntary contraction could reduce the threshold for evoking epidural activity by about 6% of the intensity needed to evoke a response in active muscle. This corresponds to about 2% of stimulator output, a value very similar to that seen in the present data. The comparatively small effect of voluntary contraction on threshold contrasts with the pronounced effect on volley amplitude. This suggests that the elements activated by magnetic stimulation are not very sensitive to the changes in excitability produced by voluntary contraction. Therefore the elements activated would not be the cortical neurones but their axons at some distance from the cell body where they are insensitive to the level of cortical excitability. These axons cannot belong to corticospinal neurones since no D-wave is elicited at low intensities. Presumably they are axons of cortico-cortical or thalamo-cortical neurones which can release EPSPs onto corticospinal neurones. Discharge of the latter then evokes I-waves in epidural records.
The effect of voluntary contraction on the amplitude of the volley could be much greater than its effect on the threshold. With contractions of 20% maximum, stimuli could evoke about 10% extra descending activity compared with that seen at rest. During maximum voluntary contractions, there was 50% extra activity. On the model above, such facilitation could be the result of increased excitability of post-synaptic neurones. This could involve both corticospinal neurones themselves and any cortical interneurones involved in producing the series of late I-waves.
Finally, it should be remembered that it is unlikely that all of this extra activity was destined for the muscle that was the focus of the voluntary contraction (FDI). The epidural electrode can record activity from axons projecting to all muscles of the body. Large contractions of one muscle are inevitably accompanied by both supra- and subthreshold activation of other muscles, so that some of the extra descending activity could reflect changes in corticospinal excitability to these muscles rather than the target muscle. However, this does not detract from the conclusion that voluntary contraction (whether of the target or of associated muscles) can increase the amplitude of corticospinal volleys elicited by transcranial magnetic stimulation.
Effects of changes in spinal cord excitability on EMG responses
As pointed out in the Results, we do not know how much of the descending activity recorded from the epidural electrodes was destined for the FDI muscle. Indeed, some of the descending activity may have inhibitory as well as excitatory effects on spinal motoneurones. This means that although we can say that voluntary activity increases the size of both descending volley and EMG response, we cannot say how much of the latter is explained by the former. Nevertheless, even in qualitative terms we can say that there is at least one circumstance in which spinal cord excitability has a very important effect on the size of EMG responses. This occurs when subjects go from a state of rest to tonic voluntary activity. A moderate muscle contraction could more than triple the slope of the relationship between stimulus intensity and EMG amplitude whereas it had a much smaller effect on the slope of the relationship between intensity and amplitude of the descending volley. Presumably the effect of voluntary activation in bringing the resting potential of spinal motoneurones nearer to threshold explains the predominant role of spinal excitability.
Conclusions
Voluntary contraction increases the size and number of descending volleys evoked by transcranial magnetic stimulation in conscious humans. We suggest that this is the result of increased excitability of elements post-synaptic to the site of stimulation in motor cortex. The large increase in size of EMG responses which is seen when going from rest to activity is caused more by changes in spinal than cortical excitability.
References
- Baker S N, Olivier E, Lemon R N. Task-related variation in corticospinal output evoked by transcranial magnetic stimulation in the macaque monkey. Journal of Physiology. 1995;488:795–801. doi: 10.1113/jphysiol.1995.sp021011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia S C, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. Journal of Physiology. 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hicks R G, Stephen J P H. Corticospinal volleys evoked by anodal and cathodal stimulation of the human motor cortex. Journal of Physiology. 1990;425:283–299. doi: 10.1113/jphysiol.1990.sp018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B L, Dressler D, Maertens De Noordhout A, Marsden C D, Nakashima K, Rothwell J C, Thompson P D. Electric magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. Journal of Physiology. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B L, Rothwell J C, Thompson P D, Dick J P R, Kachi T, Cowan J M A, Marsden C D. Motor cortex stimulation in intact man. II. Multiple descending volleys. Brain. 1987;110:1191–1120. doi: 10.1093/brain/110.5.1191. [DOI] [PubMed] [Google Scholar]
- Edgley S A, Eyre J A, Lemon R N, Miller S. Excitation of the corticospinal tract by electromagnetic and electric stimulation of the scalp in the macaque monkey. Journal of Physiology. 1990;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S A, Eyre J A, Lemon R N, Miller S. Comparison of activation of corticospinal neurons and spinal motor neurons by magnetic and electrical transcranial stimulation investigated in the lumbosacral cord of the anaesthetised monkey. Brain. 1997;120:839–854. doi: 10.1093/brain/120.5.839. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Shiraishi G, Ito T. Spinal cord potentials after transcranial magnetic stimulation during muscle contraction. Muscle and Nerve. 1996a;19:659–661. doi: 10.1002/(SICI)1097-4598(199605)19:5<659::AID-MUS17>3.0.CO;2-I. 10.1002/(SICI)1097-4598(199605)19:5<659::AID-MUS17>3.3.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Shirsaishi G, Ito T. Effect of stimulus intensity and voluntary contraction on corticospinal potentials following transcranial magnetic stimulation. Journal of the Neurological Sciences. 1996b;139:131–136. [PubMed] [Google Scholar]
- Maertens De Noordhout A, Pepin L, Gerard P, Delwaide P J. Facilitation of responses to motor cortex stimulation: effect of isometric voluntary contraction. Annals of Neurology. 1994;32:365–370. doi: 10.1002/ana.410320310. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R, Rothwell J C, Day B L, Thompson P D. Effect of tonic voluntary activity on the excitability of human motor cortex. Journal of Physiology. 1994;474:261–267. doi: 10.1113/jphysiol.1994.sp020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton P A, Morton H B. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227. doi: 10.1038/285227a0. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Direct and indirect activation of human corticospinal neurones by transcranial magnetic and electric stimulation. Neuroscience Letters. 1996;210:45–48. doi: 10.1016/0304-3940(96)12659-8. 10.1016/0304-3940(96)12659-8. [DOI] [PubMed] [Google Scholar]
- Patton H D, Amassian V E. Single- and multiple-unit analysis of cortical stage of pyramidal tract activation. Journal of Neurophysiology. 1954;17:345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical premotoneurones. Progress in Neurobiology. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Priori A, Bertolasi L, Dressler D, Rothwell J C, Day B L, Thompson P D, Marsden C D. Transcranial electric and magnetic stimulation of the leg area of the human motor cortex: single motor unit and surface EMG responses in the tibialis anterior muscle. Electroencephalography and Clinical Neurophysiology. 1993;89:131–137. doi: 10.1016/0168-5597(93)90095-7. 10.1016/0168-5597(93)90095-7. [DOI] [PubMed] [Google Scholar]


