Abstract
The aim was to examine whether long-term potentiation (LTP) had effects on short-term synaptic plasticity outside those predicted from its effect on single volley-induced responses. Field recordings from the CA1 region of guinea-pig hippocampal slices were used, and short- term plasticity was evoked by five-impulse trains of 20 and 50 Hz.
The five-impulse trains were evoked in the presence of D(-)-2-amino-5-phosphonopentanoic acid (D-AP5; 20-50 μm), picrotoxin (100 μm), and 2-OH-saclofen (200 μm), and care was taken to avoid initiation of postsynaptic spike activation. Field responses were thus considered to reflect non-NMDA receptor-mediated activity only, and demonstrated a net facilitation during the trains.
The facilitation was found, on average, to be unaffected by LTP, evoked by strong afferent tetanization. This was true also when release probability had been altered either by the adenosine agonist N-cyclohexyladenosine (CHA; 100 nM) or the antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 200 nM). When examined for individual experiments, cases with increases, or decreases, of facilitation following LTP were observed. These deviations showed no relation to initial release probability or to LTP magnitude, and they were also observed in control inputs not subjected to LTP.
Impairment of non-NMDA receptor desensitization by cyclothiazide (30 μm) increased facilitation observed during a 50 Hz, but not a 20 Hz, train. LTP had no effect on facilitation, in the presence of this drug, either during 20 or 50 Hz trains.
The results suggest that the effect of LTP in the hippocampal CA1 region on non-NMDA receptor-mediated synaptic responses to a brief afferent tetanus does not differ from that on a low-frequency, single volley-induced response. They do not support the notion that LTP is based on changes in release probability of previously active synapses. If LTP is based on recruitment of previously, pre- or postsynaptically, silent synapses, these synapses must have, on average, release characteristics similar to the previously active ones.
When evaluating changes in excitatory synaptic transmission in the hippocampal CA1 region occurring in association with long-term potentiation (LTP), responses to single volley activations of presynaptic afferents are generally used. However, the modifications induced by LTP may in principle be such that the synaptic response to a single stimulus is not representative of that resulting from other types of afferent activation (Magleby, 1987). This may be due to a long-term modulation of short-term synaptic plasticity that is inherent in the modification of the single volley response, or one that represents a separate modification process. Evidence for such non-representativeness of the single volley response has recently been found in a neocortical synapse in that the response to a brief train of stimuli was much less enhanced than that to the single volley stimulation (Markram & Tsodyks, 1996). For the hippocampal CA1 region, where LTP mechanisms have been most intensively studied, it is still uncertain whether short-term plasticity following even a paired-pulse stimulation is affected, or not, by LTP (see below). Since the Schaffer collateral-commissural afferents to the pyramidal neurones often discharge in brief high-frequency bursts rather than with single spikes (see Lisman, 1997), the presence of such long-term modulation of short-term plasticity may well be relevant in understanding the functional consequences of LTP in the CA1 region. In addition, modulation of short-term plasticity per se may have consequences for learning and memory (Silva et al. 1996).
To the extent that a modulation of short-term plasticity is an integral part of the LTP of the single volley response, the manner in which short-term plasticity is altered by LTP may also give insights into the still unresolved question of the mechanism(s) of LTP expression. A number of studies have examined how the facilitation resulting from a paired-pulse stimulation is affected. Whereas some of these studies (Christie & Abraham, 1994; Kuhnt & Voronin, 1994; Wang & Kelly, 1996; Kleschevnikov, Sokolov, Kuhnt, Dawe, Stephenson & Voronin, 1997) have indicated a decreased paired-pulse facilitation (PPF) following LTP, suggesting a presynaptic expression mechanism, most others (e.g. McNaughton, 1982; Muller & Lynch, 1989; Manabe, Wyllie, Perkel & Nicoll, 1993; Asztely, Xiao & Gustafsson, 1996) have found that LTP has little effect on this facilitation. However, the CA1 synapses show a great heterogeneity with respect to release properties (Hessler, Shirke & Malinow, 1993; Rosenmund, Clements & Westbrook, 1993) and the CA1 synapses may change their release after LTP in a manner such that PPF measured over a population of synapses is unaltered and the lack of effect, on average, is thus only coincidental (Schultz, Cook & Johnston, 1994, 1995). Alternatively, if LTP selectively affects synapses with initial low release, PPF may not be altered (Kullman & Siegelbaum, 1995). An additional factor is that short-term synaptic facilitation in the hippocampal CA1 region may be determined not only presynaptically but also by desensitization of the postsynaptic receptor (Wang & Kelly, 1996).
It may be considered that to the extent that LTP is due to processes affecting short-term synaptic plasticity, then whatever the nature of these processes, the synaptic response to a brief high-frequency train of afferent activation may be a more sensitive indicator than a paired-pulse test. The present experiments were thus designed to examine the interaction between the synaptic response to a brief afferent tetanus and LTP, and in circumstances in which initial release conditions and desensitization were varied.
METHODS
Preparation and solutions
Experiments were performed on hippocampal slices prepared from forty-four male guinea-pigs, 3-5 weeks old. The animals were anaesthetized by inhalation of isoflurane, the concentration of which was varied to maintain complete anaesthesia, as judged by the absence of a withdrawal reflex when pinching the hindpaw. They were then decapitated and their brains removed. The slices (400 μm thick) were maintained at 30-32°C, either at the interface, or wholly submerged, in a constant flow chamber. Unless otherwise stated, the perfusion fluid contained (mM): NaCl, 124; KCl, 4; CaCl2, 4; MgCl2, 4; NaHCO3, 26; NaH2PO4, 1.25; glucose, 10; and was gassed with 95% O2-5% CO2. Picrotoxin (100 μm) was always present to block GABAA receptor-mediated activity. A surgical cut was always made between the CA1 and CA3 regions. In addition to picrotoxin, the NMDA receptor antagonist D(-)-2-amino-5-phosphonopentanoic acid (D-AP5) and the GABAB receptor antagonist 2-OH-saclofen (200 μm) were always present during application of the test trains (see below). In eleven experiments, using a submerged chamber, tetrodotoxin at low concentration (10 nM) was used to reduce excitability.
In early experiments (13/44), using an interface chamber, D-AP5 was present throughout the experiments at a concentration of 20 μm, which blocks LTP, and NMDA receptor activation, following weak synaptic activation, but allows for the induction of NMDA receptor-dependent LTP following strong tetanization (five twenty-impulse trains at 200 Hz at twice test strength) (Hanse & Gustafsson, 1995). In the majority of the experiments (31/44) a submerged chamber was used, and D-AP5 (50 μm) (and for practical reasons 2-OH-saclofen) were washed out before application of the LTP-inducing tetanization (Fig. 1A). The wash-out generally led to a small increase in the field EPSP (Fig. 1A), as previously reported following wash-out of 2-OH-saclofen (Schultz et al. 1994). Results obtained with these two approaches were similar and have been pooled in the Results section.
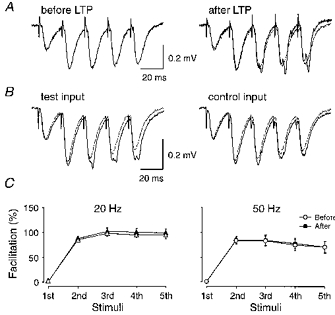
Figure 1. LTP does not interact with synaptic facilitation.
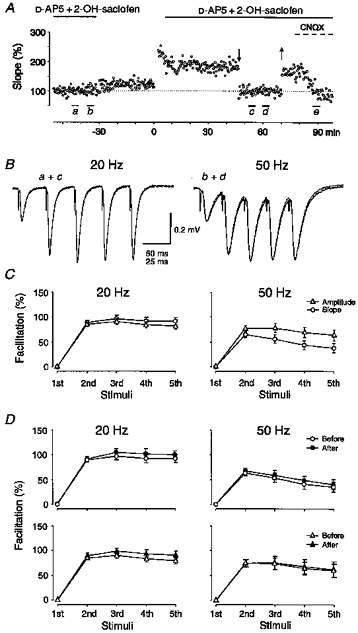
A, measurements of field EPSP initial slope are shown from one experiment for a series of responses evoked at 0.05 Hz. Ten five-impulse trains at 20 and 50 Hz (ten of each) were given at 0.05 Hz before and after induction of LTP, at times indicated (a, c: 20 Hz; b, d: 50 Hz). After the first series of trains D-AP5 and 2-OH-saclofen were washed out and LTP was induced by five twenty-impulse, 200 Hz, trains at twice test strengh. During the second series of test trains the field EPSP was temporarily reduced (downward and upward arrows) to its pre-tetanus value by lowering the stimulation intensity. After the stimulation intensity had been re-established, the non-NMDA antagonist CNQX (1 μm) was applied (dashed line), and a third series of five-impulse 20 Hz trains were given (e). B, averaged field responses (n = 10) induced by 20 and 50 Hz trains before and after LTP are shown superimposed, as indicated in the figure. It can be noted that the field response taken at d is somewhat more negative than that taken at b. In this figure, as in the following ones, stimulation artefacts are retouched. C, average facilitation, expressed as the percentage change in initial slope (○) or peak amplitude (▵) of the 2nd to 5th responses with respect to those of the 1st response, during 20 and 50 Hz trains is shown. Facilitations observed before LTP, estimated by initial slope or peak amplitude, are compared for 20 Hz (n = 19) and 50 Hz (n = 19) trains. D, the upper graphs show facilitations, estimated from initial slope measurements, during 20 Hz (n = 19) and 50 Hz (n = 13) trains obtained before (open symbols) and after (filled symbols) LTP. The lower graphs show the same but using peak amplitude measurements, 20 Hz trains (n = 17), and 50 Hz trains (n = 8). The lower number of experiments involved in the average facilitations obtained using peak amplitude and/or 50 Hz trains depends on the exclusion of field responses displaying evident signs of postsynaptic spike activity.
Recording and analysis
Electrical stimulation of afferent fibres and recordings of extracellular synaptic potentials were carried out in the CA1 hippocampal region. Stimuli consisted of 0.1 ms negative constant current pulses delivered through electrolytically sharpened tungsten wires (bipolar stimulation). Stimulation electrodes were positioned in the stratum radiatum on either side of the recording electrodes to provide two independent afferent inputs projecting to the same dendritic region. Both inputs received a test stimulus every 20 s, but 10 s apart. Recordings were made by means of glass micropipettes (filled with 3 M NaCl), one electrode in the stratum radiatum and the other in the cell body layer. Field potentials were amplified with an Axoclamp-2A (Axon Instruments) and filtered at 1-3 kHz. Data were collected (5 kHz sampling rate) using a 486 PC computer. Facilitation was evaluated as the increase in initial slope (linear regression over 1.2-1.6 ms following presynaptic volley) and peak amplitude (averaged over ± 2 ms of peak value) of the successive dendritic field responses given by five-impulse trains of 20 and 50 Hz. These trains were given before LTP (ten trains of each frequency at 0.05 Hz) and 20-70 min after LTP. Before beginning the data aquisition, test trains (50 Hz) were given to find a stimulus intensity at which no spike activity appeared during the train. Test intensity was then set to an intensity level somewhat below this level. This procedure was adopted to avoid initiation of spike activity during these trains that could interfere with the measurements. For the 20 Hz trains the facilitation observed before induction of LTP differed little whether it was estimated from initial slope or amplitude (Fig. 1C), and for these trains peak magnitude measurements, which were less influenced by noise, were preferentially used. On the other hand, due to the temporal overlap of the successive responses in the 50 Hz trains (Fig. 1B and C), slope measurements were generally used in this case. Statistical deviation from mean values, in the graphs and text, are ± standard error of the mean (s.e.m.).
Since field potentials after LTP resulted in postsynaptic spike activity during the train, comparison of facilitation before and after LTP was made after reduction in stimulus strength to return the field potential to pre-tetanus values. This procedure had the advantage of removing most impact of postsynaptic spike activity on the measurements, and also allowed the comparison of facilitation using equal-sized synaptic responses. On the other hand, it had the obvious drawback that facilitation after LTP would only be tested on a subpopulation of those synapses that were tested before LTP. The assumption would then be that in both cases a large population of synapses are sampled in which synapses with different release probabilities, and thus facilitation characteristics, are present in roughly equal proportions. In some experiments (n = 6), the non-NMDA receptor antagonist CNQX was also applied to reduce the field potential to the pre-tetanus size (Fig. 1A). This resulted in a facilitation that, on average, was superimposable with that obtained when the field potential was reduced by a change in stimulus strength (Fig. 2B). Moreover, when the average facilitation in the test inputs (Fig. 1C, ▵ (20 Hz)) was compared with that of the control inputs (Fig. 2A, ▵ (20 Hz)) in the same slices, these were also equal. Thus, at least when averaged over a number of slices, the above assumption appears valid.
Figure 2. Miscellaneous control procedures.
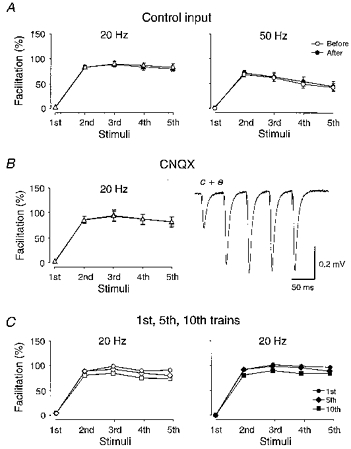
A, average facilitations (n = 16) obtained from control pathways activated alternately to the test pathways. The 20 and 50 Hz trains were given at similar times before (open symbols) and after (filled symbols) LTP of the test pathway, as those given to the test pathway. Facilitation was obtained using amplitude (triangles) and slope (circles) for the 20 and 50 Hz trains, respectively. B, average facilitations (n = 6) obtained when field response was reduced after LTP, by lowering of stimulation strength (open triangles) or by CNQX application (filled triangles). Superimposed field potentials were taken at times c and e as indicated in Fig. 1A. C, average facilitation obtained using the 1st (circles), 5th (diamonds) and 10th trains (squares) in each series of ten trains from some of the experiments (n = 13) used for the graph in Fig. 1D. Data obtained before and after LTP are shown as open and filled symbols, respectively.
Drugs
D(-)-2-amino-5-phosphonopentanoic acid (D-AP5), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and 2-OH-saclofen were obtained from Tocris Neuramin, picrotoxin and N-cyclohexyladenosine (CHA) from Sigma, and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) and cyclothiazide from Research Biochemicals Inc. Drugs were applied to the perfusion line.
RESULTS
Facilitation and LTP
To examine the interaction between LTP and the synaptic response to brief trains of afferent activation, five-impulse tetani at 20 or 50 Hz (ten of each, at 0.05 Hz) were given before and 20-70 min after induction of LTP. Figure 1B shows, from one experiment, dendritic field responses to such five-impulse afferent trains evoked before and after LTP. The trains were evoked in the presence of picrotoxin (100 μm), 2-OH-saclofen (200 μm) and D-AP5 (50 μm) and the field responses were thus considered to represent non-NMDA receptor-mediated events only. The low stimulation intensities used generally prevented the initiation of spike activity in the postsynaptic cells during the trains as evidenced by a recording electrode in the cell body layer. Following the establishment of LTP, stimulus strength was temporarily lowered (arrows in Fig. 1A) to allow for a comparison of synaptic responses of comparable magnitudes before and after LTP. As shown in Fig. 1B, the synaptic responses to both 20 and 50 Hz activations obtained after LTP were superimposable with those obtained before. The synaptic facilitation during these tetani was estimated as the percentage change of either the initial slope, or peak amplitude, of the successive responses with respect to that of the first one. On average, facilitation, both of initial slope (Fig. 1D, upper graphs) and peak amplitude (Fig. 1D, lower graphs), was found to be largely unaffected by LTP. Note that the number of experiments in each graph is not identical (see text and Fig. 1). In these experiments LTP, when measured just prior to the lowering of stimulus strength, varied between 70-190% with a mean value of 114% (n = 19).
The control input, activated alternately with the test one, was also subjected to the same five-impulse trains at about the same times as the test input. Figure 2A shows that the facilitation in these control inputs was the same when examined before (open symbols) and after (filled symbols) LTP of the test input, indicating that the facilitations themselves were, on average, stable throughout the recording period.
As noted above, following the establishment of a stable LTP, the stimulus strength was reduced to return the field response to the pre-tetanus value, and the facilitations observed before and after LTP were thus not sampled from the same population of synapses. Therefore, as illustrated in Fig. 1A, in some of the experiments (n = 6), stimulus strength was returned to the original value, and the non-NMDA receptor antagonist CNQX (1 μm) was applied to reduce the field response to the pre-tetanus value. Figure 2B shows that facilitations observed after LTP, using field responses diminished by reduction in stimulus strength (cf. Fig. 1Ac), or by CNQX (cf. Fig. 1Ae), did not differ from each other (see also Methods).
The facilitation curves were obtained from averaged synaptic responses using ten successive trains evoked at a frequency of 0.05 Hz. To examine for the interaction between these successive activations, the facilitations obtained during the first, fifth and tenth trains from each experiment were averaged. Figure 2C shows that the facilitation became successively smaller with the later trains, indicating that the facilitation curves shown above were influenced by a more long-lasting (> 20 s) component. However, the effect was quite small and its influence did not appear to differ before (Fig. 2C, left graph) and after (Fig. 2C, right graph) LTP.
Dependence on initial release probability
As indicated by studies on the interaction between LTP and PPF, the facilitation may change after LTP in a manner dependent on its initial, pre-tetanus, value. Thus, in experiments with small initial PPF values LTP causes an enhancement of PPF whereas large initial PPF values are associated with an LTP-induced reduction of these values (Schultz et al. 1994, 1995). To vary initial PPF, release probability was altered by application of either the adenosine agonist CHA (100 nM) or the antagonist DPCPX (200 nM). This resulted in appreciable changes in facilitation during the five-impulse trains. In the presence of CHA, which reduced the field EPSP to 59% of its initial value (n = 9), facilitation became significantly larger (Fig. 3A) whereas DPCPX led to a larger field EPSP (199% of initial value, n = 10) and to less facilitation (Fig. 3B). Comparison of changes in EPSP and in the percentage of facilitation induced by DPCPX or CHA observed in these graphs indicates a non-linearity, which can be expected from procedures that alter release probability (see e.g. Debanne, Guérineau, Gähwiler & Thompson, 1996).
Figure 3. Facilitation after alteration of release probability by an adenosine agonist and antagonist.
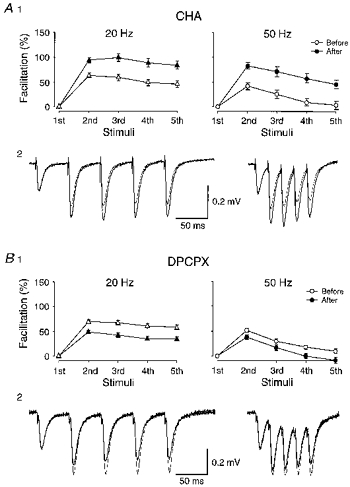
A, effect of the agonist CHA (100 nM). A1 shows facilitation obtained during 20 Hz (n = 7) and 50 Hz (n = 9) trains, before (open symbols) and after (filled symbols) CHA. Facilitation was estimated from peak amplitude (20 Hz) and initial slope (50 Hz) measurements. In A2, field responses obtained from one experiment are shown superimposed. In each experiment, stimulus strength was adjusted to give similarly-sized single volley responses before and after drug application. In A2, the initial slope of the first response was, in addition, scaled to give a perfect match. B, same as in A, but after application of the antagonist DPCPX (200 nm; n = 10). In both A and B, the facilitation was taken from both test and control inputs from each experiment.
Facilitation was subsequently examined before and after induction of LTP, in the presence of either CHA or DPCPX. Figure 4A shows that, in the presence of CHA, facilitation tended, as in absence of this drug (Fig. 1D), to be somewhat greater after LTP, but not significantly so (5th response (20 Hz) paired difference in percentage of facilitation, 12.9 ± 10%, n = 7). Also in the presence of DPCPX, no difference was observed (Fig. 4B). LTP induced under these conditions was quantitatively similar to that in the absence of these drugs, averaging 103% (n = 10) in CHA and 119% (n = 6) in DPCPX.
Figure 4. LTP and facilitation in the presence of an adenosine agonist and antagonist.
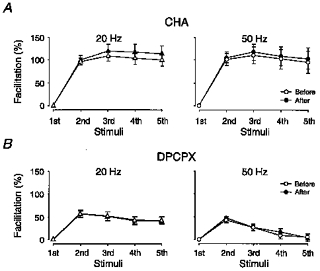
A, average facilitation obtained with 20 Hz (n = 7) and 50 Hz trains (n = 10) before (open symbols) and after (filled symbols) LTP, in the presence of CHA (100 nM). Facilitation was estimated from peak amplitude (20 Hz) and initial slope (50 Hz) measurements. B, same as in A, but in the presence of DPCPX (200 nM) (20 Hz, n = 5; 50 Hz, n = 6). The greater number of values for 50 Hz trains was due to some experiments in which only 50 Hz trains were given.
In Fig. 5A is plotted from the individual experiments (20 Hz trains, both with and without adenosine agonists/antagonists), the change in the percentage of facilitation associated with LTP (post-LTP - pre-LTP) against the pre-LTP value of the facilitation of the 2nd response (initial PPF). It can be noted that the change in facilitation, both with respect to the second and fifth synaptic responses, is scattered on both sides of the line (dashed) denoting zero modification. However, there is no relation to initial PPF. Figure 5B illustrates that the facilitation of the control inputs, when compared at times corresponding to those of the test inputs before and after LTP, displays a similar scatter around the zero modification line. This result would suggest that the changes in facilitation observed in the test input following LTP, may, at least partly, be unrelated to LTP. If these changes in facilitation of test and control inputs were due to some common factor related to the specific conditions during an experiment, a covariation would be expected. A tendency for such covariation was also indicated by the regression line (Fig. 5C) but it was not statistically significant.
Figure 5. Relation between LTP-induced change in facilitation and initial release probability.
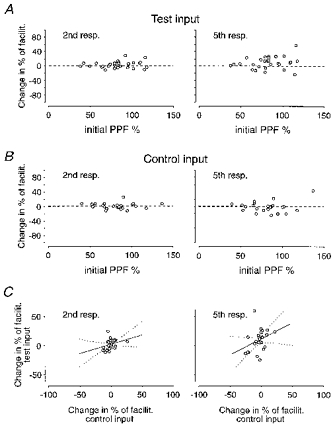
A, the change in percentage of facilitation after LTP (post-LTP - pre-LTP) is plotted against PPF (50 ms interval) for each individual experiment (n = 29). Facilitation values are taken from the 2nd (left graph) and 5th (right graph) responses of 20 Hz trains. B, same as in A but for non-tetanized inputs (n = 26). C, the change in the percentage of facilitation (after LTP of the test input) for test and control inputs, respectively, from each experiment (n = 26) are plotted against each other. A linear regression line (continuous line) and confidence interval (dotted lines) are added to the plots. Regression lines in the left and right graphs are described by the equations: y = 0.31x+ 2.3 (P > 0.05) and y = 0.53x+ 11 (P > 0.05), respectively.
Dependence on LTP parameters
It has also been indicated that the critical parameter in deciding how facilitation is affected by LTP is the magnitude of LTP (Kleschevnikov et al. 1997). Thus, a decrease in PPF has mainly been observed in association with large LTPs (> 100%). This behaviour was not found within the material presented above (Fig. 6A). In experiments in which LTP magnitude exceeded 100% (mean value, 144%; n = 16) the percentage of facilitation (20 Hz trains) was, on average, 3 and 11% greater after than before LTP for the 2nd (PPF) and the 5th response, respectively. The corresponding values in experiments with LTP less than 100% (mean value, 84%; n = 13) were 3 and 7%, respectively. Both groups also showed, on average, the same initial PPF, 82 and 84% for LTPs above and below 100%, respectively (see also Fig. 6B).
Figure 6. Relations between LTP magnitude and facilitation parameters.
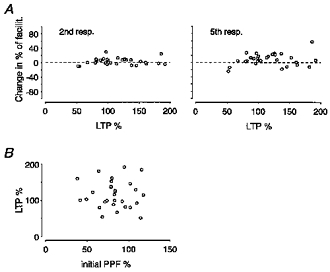
A, the change in the percentage of facilitation after LTP is plotted against LTP magnitude for the 2nd and 5th responses of 20 Hz trains (n = 29 experiments). B, the magnitude of LTP is plotted against the initial value of PPF, demonstrating that equal magnitudes of LTP were evoked under the different conditions of initial release probability.
In the present study, the time at which facilitation was evaluated varied in the different experiments from 20 to 70 min after LTP induction. To examine whether this variation was of consequence, the above material was subdivided into two groups in which facilitation was examined 20-45 min (n = 18) and 45-70 min (n = 11) after LTP induction, respectively. The LTPs (121 vs. 109%) and initial PPFs (81 vs. 86%) were similar for both groups. The facilitation (20 Hz trains) for the 2nd (PPF) and the 5th responses were also affected to a similar extent in the two groups, being, respectively, on average, 4 and 9% greater after LTP in the 20-45 min group compared with 2 and 9% in the 45-70 min group.
Effect of cyclothiazide
The successive field responses during a five-impulse train may be affected not only by presynaptic factors but also by postsynaptic ones, e.g. a progressive desensitization of the postsynaptic receptors (Wang & Kelly, 1996). Application of cyclothiazide (30 μm), a drug that prevents such desensitization, was found to prolong the field EPSP time course (Fig. 7A 2), and to enhance facilitation (measured using initial slope measurements). This enhancement was, however, only observed using 50 Hz trains (5th response paired difference in percentage of facilitation, 31 ± 7%, P < 0.01), whereas that appearing during 20 Hz trains was not significantly affected (Fig. 7A1). Figure 7B shows that facilitation, also in the presence of cyclothiazide (30 μm), was, on average, unaffected by LTP (that averaged 118%, n = 9).
Figure 7. LTP and facilitation after blockade of desensitization.
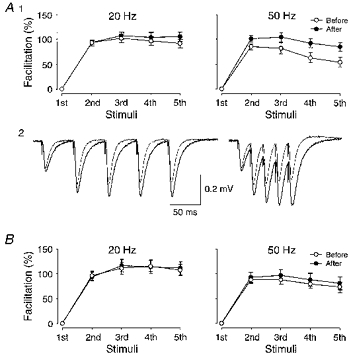
A, facilitation evaluated before and after application of cyclothiazide (30 μm), a blocker of non-NMDA receptor channel desensitization. Due to the prolonged nature of the synaptic response after cyclothiazide (A2) facilitation was evaluated using slope measurements for both 20 Hz (n = 12) and 50 Hz trains (n = 13). A1, facilitations obtained before and after cyclothiazide are shown as open and filled circles, respectively. A2, average (n = 10) field responses during 20 and 50 Hz trains taken before and after cyclothiazide are shown superimposed. B, facilitation obtained before (○) and after (•) LTP in the presence of cyclothiazide (30 μm) for 20 Hz (n = 9) and 50 Hz (n = 9) trains.
EPSP-spike potentiation
Tetanization did not only result in an increased synaptic potential but also in an EPSP-spike (E-S) potentiation (Bliss & Lomo, 1973; Andersen, Sundberg, Sveen, Swann & Wigström, 1980). Thus, in five of the forty-four experiments a 50 Hz train that did not produce postsynaptic firing before LTP did so after return of the field potential to pre-tetanus values, in the absence of any apparent increase of the synaptic field response (Fig. 8A). This result agrees with previous studies indicating that E-S potentiation can also appear in the presence of GABA receptor antagonists (Hess & Gustafsson, 1990; Asztely & Gustafsson, 1994). Data from those experiments, in which this E-S potentiation produced spike activity that was deemed to interfere with the slope measurements, were excluded, which explains the lower number of facilitations with 50 Hz trains than with 20 Hz trains (Fig. 1C and D). It should be noted that, since in our experiments stimulus intensity was deliberately kept low to avoid of E-S potentiation (see Methods), our results do not exclude the possibility that E-S potentiation was present in the remaining thirty-nine experiments as well.
Figure 8. E-S potentiation and field EPSP shape change.
A, averaged (n = 10) field responses during a 50 Hz train taken before and after LTP. The response taken before LTP is shown twice, to the left, and to the right superimposed on the response obtained after LTP. Note that in this experiment equal-sized field EPSPs before and after LTP, as judged from the slope and amplitude of the successive responses, results in spike activity after LTP. B, averaged (n = 10) field responses during 50 Hz trains taken before and after LTP are shown superimposed for test and control inputs. Note the change in field response after LTP, particularly in the late part of each field EPSP, observed in both test and control inputs. C, average facilitation obtained before (○) and after (•) LTP with 20 Hz (n = 27) and 50 Hz trains (n = 25) after excluding experiments performed in the absence of D-AP5 or tetrodotoxin.
In five of the experiments, tetanization also led to a change in the shape of the synaptic response that mainly affected its late part, and which was also seen heterosynaptically (Fig. 8B). Such an effect has previously been reported to occur frequently after repeated strong tetanization in the CA1 region in the presence of GABA receptor antagonists (Hess & Gustafsson, 1990). The less frequent occurrence in the present study is probably due to the fact that most LTPs were evoked either in the presence of D-AP5 (20 μm) or tetrodotoxin (10 nM), since only one clear case of such shape change was observed under those conditions. Since the process underlying this field EPSP modification might have affected the facilitation measurements, facilitation curves were also constructed that excluded all data obtained in the absence of D-AP5 and tetrodotoxin. Figure 8C shows that after this exclusion facilitation during 20 Hz (n = 27) and 50 Hz (n = 25) trains was still, on average, unaffected by LTP.
DISCUSSION
The principal finding is that LTP produced, under various release conditions, little change in the short-term plasticity of the synaptic population response to a brief afferent tetanus (20 and 50 Hz), i.e. LTP altered the non-NMDA receptor-mediated synaptic response to such tetani in proportion to its effect on the single volley-mediated response. Thus our results do not support those studies in the hippocampal CA1 region in which PPF was altered following LTP (Kuhnt & Voronin, 1994; Wang & Kelly, 1996; Kleschevnikov et al. 1997), and offer no support for the notion that LTP is based on, or associated with, an altered probability of release, unless this occurs at previously non-releasing synapses (during five-impulse tetani) which acquire, on average, the same release characteristics after LTP as previously responsive synapses. On the other hand, our results are compatible with an increase in non-NMDA receptor function, either at previously responsive, or silent, postsynaptic sites, if this occurs in a manner unrelated to the release (and thus facilitation) characteristics of the opposing terminals. The notion that such non-NMDA receptor modification should involve an increased desensitization of these receptor channels (Wang & Kelly, 1996) is, however, not in line with the present findings.
It has long been recognized that LTP is not simply expressed as a change in the weight, by a single number, of the non-NMDA receptor-mediated synaptic response. For instance, LTP can be associated with an increase in postsynaptic firing exceeding that of the synaptic potential itself (E-S potentiation) (Bliss & Lomo, 1973; Andersen et al. 1980), and in changes that lead to altered subsequent induction conditions for LTP. This latter ‘metaplasticity’ (Abraham & Bear, 1996) can be due, for example, to concomitant long-term changes in NMDA or GABA receptor-mediated synaptic transmission. Such ‘metaplasticity’ could also relate to long-term modulation of the short-term plasticity of the non-NMDA receptor-mediated synaptic response itself, since such modulation of short-term plasticity, either as a direct consequence of the expression mechanism of ‘conventional’ LTP, or as a separate process, could facilitate, or impair, further induction of LTP. In addition, such long-term modulation of short-term plasticity would also be of importance as it would change the computational properties of the synapse by alteration of the temporal characteristics of the response. Such long-term modulation of short-term plasticity has now been observed in association with LTP in a neocortical synapse, in which LTP was accompanied by an apparent increase in short-term depression leading to a ‘redistribution’, rather than a potentiation, of synaptic efficacy when measured for the response to a stimulus train rather than to a single volley activation (Markram & Tsodyks, 1996). Although the present study cannot exclude such behaviour in some synapses in the hippocampal CA1 region, it shows that these synapses, on average, behave as if LTP changes the synaptic weight by a single number independently of whether they are activated by a single or a brief tetanic activation. Some previous studies have indicated less facilitation (PPF) after LTP if initial release probability is low, and/or LTP magnitude is large (Schultz et al. 1994, 1995; Kleschevnikov et al. 1997). However, even under these conditions we found no evidence that facilitation during a brief tetanus becomes modulated by LTP. The use of whole cell recording and induction of LTP via pairing of presynaptic activity with postsynaptic depolarization in the study of Markram & Tsodyks (1996) may be considered to have created experimental conditions that explain the discrepancy in results. However, use of similar techniques in the CA1 region (Manabe et al. 1993) did not demonstrate changes in PPF after LTP, indicating that such experimental differences are not the critical ones. Our results would then suggest a genuine difference between the LTP evoked in the hippocampal CA1 region and that examined in the neocortex by Markram & Tsodyks (1996).
In a recent paper describing changes in PPF after LTP, several explanations were offered for why a decrease in facilitation has not been found in most previous studies (Kleschevnikov et al. 1997). These explanations include contamination by polysynaptic components in the measurements, small LTP magnitudes, low initial PPF ratios, and measurements of PPF during a phase of LTP not associated with change in PPF. According to the results of Kleschevnikov et al. (1997), tetanization of an input with initial PPF ratios above 1.7, leading to a stable LTP increase of 50-100%, would lead to a large decrease in PPF within the first hour of LTP if the synaptic response was measured early enough to include only the non-NMDA receptor-mediated component. Similarly, the study by Shultz et al. (1995) has indicated that PPF decreases when LTP is large (> 100%) and initial PPF is high. In the present study, no decrease in facilitation was observed even under these conditions. Thus, most initial PPF ratios were larger than 1.7, i.e. within a range in which facilitation was found to be quite sensitive to changes in release probability (Fig. 3A; see also Asztely et al. 1996). Moreover, LTP was, on average, somewhat more than 100%, and facilitation was measured within the first hour of LTP. GABA and NMDA receptor antagonists were used to isolate the non-NMDA receptor-mediated synaptic component, and care was taken to avoid postsynaptic spike activation. A negative aspect of the present study was that facilitation before and after LTP was compared after a reduction in stimulation strength to return the synaptic potential to pre-LTP values. However, we believe that this procedure was valid, since, when tested, the same facilitation was observed independently of whether the reduction of field EPSP size to pre-tetanus values was achieved by CNQX application or by a decrease in stimulus strength. Moreover, when sampled in the same slice, facilitation in synapses belonging to the test or control input also, on average, agreed. A positive aspect of our procedure is that non-linearities due to changes in driving force are removed, and that the influence of postsynaptic spike activity is minimized. There is no doubt that postsynaptic spike activity, by coming early on the synaptic potential, in particular on the second one in a PPF test, can affect even early initial slope measurements after LTP (see Fig. 1A in Kleschevnikov et al. 1997).
In agreement with previous studies, we also noted that facilitation in individual experiments could be larger, or smaller, following LTP. These results have been taken to indicate that LTP is associated with changes in presynaptic function, since these deviations in PPF were found to be correlated with initial release probability, and/or LTP magnitude (Schultz et al. 1994, 1995; Kleschevnikov et al. 1997). As discussed in these previous reports, these deviations in both directions could be explained, for example, if LTP is associated with enhanced release at old release sites in combination with release probabilities less than the pre-LTP ones at new release sites (they could of course equally well be explained if LTP, under various conditions, is associated with increased non-NMDA receptor function preferentially in either low or high releasing synapses; cf. below). We cannot exclude the possibility that our procedure for comparing facilitations before and after LTP after reduction in stimulus strength might have introduced variability that masked a correlation with initial PPF. Nevertheless, in agreement with another recent study (Asztely et al. 1996), we found no indication that these individual deviations correlated with initial PPF. Neither did we find any correlation with LTP magnitude. This difference in results could be due to the way in which initial release probability was modified, by adenosine agonists or antagonists, or by changes in extracellular calcium. If so, the initial release probability per se is not likely to be the decisive factor. A notable observation in the present study was that such deviations, although generally smaller, were also observed in the non-tetanized synapse populations during the 1-2 h period between the facilitation tests. This may indicate that part of the deviation in the test inputs could be unrelated to LTP.
Postsynaptic factors in facilitation
Previous studies in the CA1 region have produced somewhat conflicting results on the question of whether facilitation is also affected by desensitization of the non-NMDA receptor. Thus, whereas some studies have indicated no such effect (Kullman, 1994; Stevens & Wang, 1995), others reported a significant increase in PPF following application of the desensitization blocker cyclothiazide (Wang & Kelly, 1996). Such a discrepancy may relate to experimental factors, such as interstimulus interval, release probability of the synapses, or temperature, affecting desensitization and release kinetics. In the present study, desensitization clearly affected facilitation during a 50 Hz train but not during a 20 Hz train, as indicated by the effect of cyclothiazide. That would mean that if desensitization is substantially increased following LTP (Wang & Kelly, 1996), facilitation during a 50 Hz, but not a 20 Hz, train would be differentially affected by LTP in the presence or absence of cyclothiazide. No such discrepancy was observed in the present study, and thus our results do not support the suggestion that desensitization is altered after LTP.
An additional possible postsynaptic contribution to facilitation is that the synaptic current becomes amplified by local activation of dendritic voltage-gated sodium and/or calcium channels (Magee & Johnston, 1995) in a manner that increases with each successive response. A modulation of such activation by LTP could then emerge as an increase or decrease in facilitation. The ability to detect such effects would, at present, rely on this local activation causing alterations of the field response such that the peak amplitudes and slopes of the successive responses in the train were differentially affected. If this were the case, the present results would appear to suggest that no such difference in local activation occurs after LTP, since no such differential effect was observed generally. However, this suggestion should be tempered by the finding in a few of our experiments that LTP was associated with a change in the field response shape compatible with such local activation. However, other explanations, such as a change in the synaptic non-NMDA receptor-mediated response itself, could equally well apply.
Brief tetanic activation vs. paired-pulse test
The facilitation we observed during the five-impulse 20 and 50 Hz trains appeared to be largely saturated, to about the same level, after the 2nd stimulus, and thereafter stayed much the same with the successive responses. The decrease in facilitation during the 50 Hz trains, not apparent in the 20 Hz ones, was partly found to be related to desensitization, and was also likely to result from non-linear summation. These results would indicate a facilitation that is already nearly maximal with a paired-pulse test (50 ms interval). The question then arises of the extent to which any additional information with respect to the detection of possible alterations of presynaptic release was obtained by examining facilitation during these brief tetani rather than PPF. A consideration is that the observed facilitation probably reflects a balance between facilitatory and depressive effects on the release that may develop during the successive stimuli. A five-impulse tetanus may then test aspects of short-term plasticity other than a paired pulse. In fact, a second question arises of which synapses actually contribute to the successive responses during a five-impulse train. Studies of low-frequency, single volley-induced responses have demonstrated a large heterogeneity among the Schaffer collateral-CA1 pyramidal cell synapses with respect to release probability, with values ranging from 0.6 down to 0.05, the majority belonging to a low release (< 0.1) group (Hessler et al. 1993; Rosenmund et al. 1993). Whereas high-release synapses may also release with high probability in response to the 2nd stimulus (at < 50 Hz) (Stevens & Wang, 1995), it is to our knowledge uncertain to what extent they continue doing so in response to a 3rd, 4th and 5th stimulus. Low-release synapses may release with higher probability in response to the 2nd stimulus, but paired-pulse stimulation still leaves a great number of synapses in a low-release condition (Manabe & Nicoll, 1994). On the other hand, during a five-impulse tetanus these synapses may release with higher probability, and synapses that release with high or low probability to single volley activation may then contribute differentially to PPF and to the facilitation appearing later during the train.
It has been suggested that PPF may not be altered despite LTP being based on an increased release probability, if LTP selectively enhances release at synapses with a very low initial probability of release (Kullman & Siegelbaum, 1995). However, as discussed above, such synapses may release with a much higher probability during a five-impulse tetanus, and, if this is so, LTP would then be associated with a reduced facilitation for the later responses in the train, an effect we did not observe.
Functional implications
As noted above, the synapses in our study display a considerable heterogeneity with respect to release characteristics, and many of them release transmitter in response to single volley activation with a very low probability. However, these low-release synapses may become functional with a brief tetanic activation, allowing most of the afferents to participate, perhaps in a roughly even manner, to the postsynaptic response. Such a switch from unreliable to reliable release of transmitter would suggest that a brief tetanic (burst) rather than single volley activation is the information-carrying unit (Lisman, 1997). The present results would then suggest that these low-release synapses remain in this low-release state, even after LTP, since short-term plasticity is unaltered. It does appear that maintenance of burst pattern as the information-carrying unit, even after LTP, is of greater significance than using long-term modulation of short-term plasticity to expand the computational range of the synapses.
Acknowledgments
This project was supported by the Swedish Medical Research Council (project number 05180). M. P. was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), and H.-X. C. and M. P. by the Barbro Hansson Foundation. We also thank A. Letterfors for helpful contribution in some of the experiments.
References
- Abraham W C, Bear M F. Metaplasticity: the plasticity of synaptic plasticity. Trends in Neurosciences. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. 10.1016/S0166-2236(96)80018-X. [DOI] [PubMed] [Google Scholar]
- Andersen P, Sundberg S H, Sveen O, Swann J W, Wigström H. Possible mechanisms for long-lasting potentiation of synaptic transmission in hippocampal slices from guinea-pigs. Journal of Physiology. 1980;302:463–482. doi: 10.1113/jphysiol.1980.sp013256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztely F, Gustafsson B. Dissociation between long-term potentiation and associated changes in field EPSP waveform in the hippocampal CA1 region: An in vitro study in guinea pig brain slices. Hippocampus. 1994;4:148–156. doi: 10.1002/hipo.450040205. [DOI] [PubMed] [Google Scholar]
- Asztely F, Xiao M-Y, Gustafsson B. Long-term potentiation and paired-pulse facilitation in the hippocampal CA1 region. NeuroReport. 1996;7:1609–1612. doi: 10.1097/00001756-199607080-00016. [DOI] [PubMed] [Google Scholar]
- Bliss T V P, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. Journal of Physiology. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie B R, Abraham W C. Differential regulation of paired-pulse plasticity following LTP in the dentate gyrus. NeuroReport. 1994;5:385–388. doi: 10.1097/00001756-199401120-00003. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guérineau N C, Gähwiler B H, Thompson S M. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. Journal of Physiology. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanse E, Gustafsson B. Long-term potentiation in the hippocampal CA1 region in the presence of N-methyl-D-aspartate receptor antagonists. Neuroscience. 1995;67:531–539. doi: 10.1016/0306-4522(95)00090-6. 10.1016/0306-4522(95)00090-6. [DOI] [PubMed] [Google Scholar]
- Hess G, Gustafsson B. Changes in field excitatory postsynaptic potential shape induced by tetanization in the CA1 region of the guinea-pig hippocampal slice. Neuroscience. 1990;37:61–69. doi: 10.1016/0306-4522(90)90192-7. 10.1016/0306-4522(90)90192-7. [DOI] [PubMed] [Google Scholar]
- Hessler N A, Shirke A M, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Kleschevnikov A M, Sokolov M V, Kuhnt U, Dawe G S, Stephenson J D, Voronin L L. Changes in paired-pulse facilitation correlate with induction of long-term potentiation in area CA1 of rat hippocampal slices. Neuroscience. 1997;76:829–843. doi: 10.1016/s0306-4522(96)00342-9. 10.1016/S0306-4522(96)00342-9. [DOI] [PubMed] [Google Scholar]
- Kuhnt U, Voronin L L. Interaction between paired-pulse facilitation and long-term potentiation in area CA1 of guinea-pig hippocampal slices: application of quantal analysis. Neuroscience. 1994;62:391–397. doi: 10.1016/0306-4522(94)90374-3. 10.1016/0306-4522(94)90374-3. [DOI] [PubMed] [Google Scholar]
- Kullman D M. Role of AMPA receptor desensitization in paired-pulse facilitation of EPSCs recorded in guinea-pig hippocampal CA1 cells. Journal of Physiology. 1994;475.P:152P. [Google Scholar]
- Kullman D M, Siegelbaum S A. The site of expression of NMDA receptor-dependent LTP: New fuel for an old fire. Neuron. 1995;15:997–1002. doi: 10.1016/0896-6273(95)90089-6. 10.1016/0896-6273(95)90089-6. [DOI] [PubMed] [Google Scholar]
- Lisman J E. Burst as a unit of neural information: making unreliable synapses reliable. Trends in Neurosciences. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- McNaughton B L. Long-term synaptic enhancement and short-term potentiation in rat fascia dentata act through different mechanisms. Journal of Physiology. 1982;324:249–262. doi: 10.1113/jphysiol.1982.sp014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J C, Johnston D. Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. Journal of Physiology. 1995;487:67–90. doi: 10.1113/jphysiol.1995.sp020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K L. Short-term changes in synaptic efficacy. In: Edelman G M, Gall V E, Cowan K M, editors. Synaptic Function. New York: John Wiley and Sons; 1987. pp. 21–56. [Google Scholar]
- Manabe T, Nicoll R A. Long-term potentiation: evidence against an increase in transmitter release probability in CA1 region of the hippocampus. Science. 1994;265:1888–1892. doi: 10.1126/science.7916483. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie D J A, Perkel D J, Nicoll R A. Modulation of synaptic transmission and long-term potentiation: effect on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. Journal of Neurophysiology. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Markram H, Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996;382:807–810. doi: 10.1038/382807a0. 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- Muller D, Lynch G. Evidence that changes in presynaptic calcium currents are not responsible for long-term potentiation in hippocampus. Brain Research. 1989;479:290–299. doi: 10.1016/0006-8993(89)91631-4. 10.1016/0006-8993(89)91631-4. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Clements J D, Westbrook G L. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Schultz P E, Cook E P, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. Journal of Neuroscience. 1994;14:5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz P E, Cook E P, Johnston D. Using paired-pulse facilitation to probe the mechanisms for long-term potentiation (LTP) Journal of Physiology (Paris) 1995;89:3–9. doi: 10.1016/0928-4257(96)80546-8. [DOI] [PubMed] [Google Scholar]
- Silva A J, Rosahl T W, Chapman P F, Marowitz Z, Friedman E, Frankland P W, Cestari V, Cioffi D, Sudhof T S, Bourtchuladze R. Impaired learning in mice with abnormal short-lived plasticity. Current Biology. 1996;6:1509–1518. doi: 10.1016/s0960-9822(96)00756-7. 10.1016/S0960-9822(96)00756-7. [DOI] [PubMed] [Google Scholar]
- Stevens C F, Wang Y. Facilitation and depression at single central synapses. Neuron. 1995;14:795–802. doi: 10.1016/0896-6273(95)90223-6. 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]
- Wang J-H, Kelly P T. Regulation of synaptic facilitation by postsynaptic Ca2+/CaM pathways in hippocampal CA1 neurons. Journal of Neurophysiology. 1996;76:276–286. doi: 10.1152/jn.1996.76.1.276. [DOI] [PubMed] [Google Scholar]


