Abstract
A supervised learning procedure was applied to individual cat area 17 neurons to test the possible role of neuronal co-activity in controlling the plasticity of the spatial ‘on-off’ organization of visual cortical receptive fields (RFs).
Differential pairing between visual input evoked in a fixed position of the RF and preset levels of postsynaptic firing (imposed iontophoretically) were used alternately to boost the ‘on’ (or ‘off’) response to a ‘high’ level of firing (S+ pairing), and to reduce the opponent response (respectively ‘off’ or ‘on’) in the same position to a ‘low’ level (S− pairing). This associative procedure was repeated 50-100 times at a low temporal frequency (0.1-0.15 s−1).
Long-lasting modifications of the ratio of ‘on-off’ responses, measured in the paired position or integrated across the whole RF, were found in 44% of the conditioned neurons (17/39), and in most cases this favoured the S+ paired characteristic. The amplitude change was on average half of that imposed during pairing. Comparable proportions of modified cells were obtained in ‘simple’ (13/27) and ‘complex’ (4/12) RFs, both in adult cats (4/11) and in kittens within the critical period (13/28).
The spatial selectivity of the pairing effects was studied by pseudorandomly stimulating both paired and spatially distinct unpaired positions within the RF. Most modifications were observed in the paired position (for 88% of successful pairings).
In some cells (n = 13), a fixed delay pairing procedure was applied, in which the temporal phase of the onset of the current pulse was shifted by a few hundred milliseconds from the presentation or offset of the visual stimulus. Consecutive effects were observed in 4/13 cells, which retained the temporal pattern of activity imposed during pairing for 5-40 min. They were expressed in the paired region only.
The demonstration of long-lasting adaptive changes in the ratio of ‘on’ and ‘off’ responses, expressed in localized subregions of the RF, leads us to suggest that simple and complex RF organizations might be two stable functional states derived from a common connectivity scheme.
The pioneering findings of Hubel & Wiesel (1962) led to the qualitative classification of cortical receptive fields (RFs) in areas 17 and 18 of the cat into two major categories, namely ‘simple’ and ‘complex’. One of the four criteria used by these authors to define RF type was the topographic organization of responses to the presentation (‘on’) and extinction (‘off’) of an optimally oriented slit of light (Hubel & Wiesel, 1962). Quantitative assessment of the degree of spatial overlap of the ‘on’ and ‘off’ fields shows a bimodal distribution in area 17. Less than 50% overlap is found in simple RFs, whereas a very high degree of overlap (70%) is observed for complex RFs (Heggelund, 1986). This classification led to the functional identification of two populations of visual cortical RFs, differing not only in the spatial segregation of ‘on’ and ‘off’ responses but also in their excitability, the selectivity of their visual properties (e.g. orientation and velocity tuning), and their location in the cortical laminae.
Initial models of RF organization proposed that simple and complex types resulted from significant differences in the pattern of convergence of afferent inputs. Hubel & Wiesel (1962) assumed that simple RFs were formed by the convergence of a row of geniculate RFs, each with a centre of the same type and all aligned in the visual field. Complex cells would be fed by simple neurons of similar orientation preference, analysing overlapping regions in the visual field. In spite of the success of this hierarchical model, three observations are clearly at variance with its predictions: (i) certain classes of visual stimuli which do not trigger postsynaptic activity in simple cells selectively activate complex neurons (Hammond & McKay, 1977); (ii) a significant proportion of complex neurons in area 17 were found to be monosynaptically activated by thalamic neurons (Tanaka, 1983); and (iii) the unmasking of excitatory ‘on’ and ‘off’ responses across the whole extent of simple RFs during pharmacological blockade of intracortical inhibition suggests that at the subthreshold level, complex and simple RFs could share a similar contingent of excitatory input (Sillito, 1975; Shulz, Bringuier & Frégnac, 1993).
Studies of development of the spatial organization of visual cortical RFs and of their activity-dependent plasticity also provide data that are difficult to reconcile with the Hubel & Wiesel (1962) hierarchical model. In the young kitten before natural eye opening (6-7 days), the first visual RFs are complex-like (Albus & Wolf, 1984) and found in layer IV, which at the adult stage contains mostly simple cells (Hubel & Wiesel, 1962). Furthermore, manipulation of environmental constraints during a critical postnatal period shows that the spatial distribution of ‘on’ and ‘off’ responses cannot be considered as a property inherited from a fixed connectivity. Visual deprivation (Singer & Tretter, 1976), exposure to environments deprived of edges (Pettigrew & Freeman, 1973) or restricted to patterns of fixed orientation and spatial frequency (Singer, Tretter & Cynader, 1975) all induce a degradation or reorganization of the topography of ‘on’ and ‘off’ zones. Although these data suggest that visual experience plays an important role in shaping the ‘on-off’ response profile in cortical RFs, they do not provide a causal relationship between the activity pattern that has been imposed in the cortical network by visual experience and the final structure of the RF.
To address the possibility of a causal relationship more explicitly, we have attempted to demonstrate that imposed changes in the relative weight of ‘on’ and ‘off’ responses during the time of recording of a single visual cortical neuron can induce long-term changes in the spatial organization of the RF in a predictable way. The cellular conditioning protocol we applied to change the balance between ‘on’ and ‘off’ responses is based on the hypothesis that temporal covariance between afferent presynaptic and postsynaptic activity controls the gain of activated synapses (Hebb, 1949; reviewed in Frégnac & Shulz, 1994). This theoretical framework predicts that periods of increased or reduced temporal correlation will result in a strengthening or weakening of the active synaptic connections. A simple way for the experimenter to control the level of correlation between pre- and postsynaptic activity is to artificially manipulate the postsynaptic state of the neuron during the time of presentation of the visual stimulus (Frégnac, Shulz, Thorpe & Bienenstock, 1988). In order to clamp the respective weight of ‘on’ and ‘off’ responses to preset values in a controlled way, ‘high’ and ‘low’ levels of postsynaptic activity were imposed alternately by extracellular iontophoresis with KCl-filled electrodes, and paired respectively with the presentation (‘on’) and extinction (‘off’) of the same stimulus shown in a fixed position of the RF. The spatial spread of the effects of the conditioning was studied by measuring responses in different test positions in the RF before and after pairing. This ‘generalization’ protocol made it possible to determine whether the effects were confined to the conditioned region, or extended to the remainder of the RF.
Similar conditioning procedures have been used successfully to modify functional properties of visual cortical cells assessed with moving stimuli such as orientation selectivity, ocular dominance and interocular orientation disparity (Frégnac et al. 1988; Frégnac, Shulz, Thorpe & Bienenstock, 1992; Shulz & Frégnac, 1992). In the present series of experiments, stationary flashed ‘on’ and ‘off’ stimuli were used as conditioned inputs under the assumption that the two different stimuli effectively activate separate sets of synaptic afferents. This hypothesis that the sets of stimulated inputs are indeed separate is supported by the fact that ‘on’ and ‘off’ channels, originating from two specialized and segregated networks in the retina, can only be activated asynchronously. At the geniculate level, the two input pathways remain largely separated anatomically and functionally (Bowling & Caverhill, 1989). The simplified view we have adopted here is that the ‘on’ and ‘off’ centre inputs converge at the level of the cortical cells through distinct retinogeniculocortical pathways. This assumption of separate input pathways, which seems to hold at least at the level of first-order cortical cells (Reid & Alonso, 1995), may apply to a large contingent of the afferents which reach most simple RFs in the cat visual cortex (which represent 70% of the population of cells in area 17) and, to a lesser extent, to complex cells. The chosen opposing pairing protocols are designed to promote competition between positively and negatively reinforced inputs to the same cell. This situation may be considered as similar to the effect of alternate strabismus, where the RFs from each eye will never be activated synchronously. According to the theories of Hebb (1949) and Stent (1973), activity-dependent competition will occur at the level of the first-order cells where the two inputs converge, resulting in the functional disconnection of afferents which were silent when the postsynaptic cell was forced to fire. Afferents whose activity was most correlated in time with the output of the postsynaptic cell will win control of the visual response.
METHODS
Surgery and animal preparation
The basic surgical methods used in these experiments have been described in detail elsewhere (Frégnac et al. 1992). Neurons were recorded in area 17 of the striate cortex of normally reared kittens (aged from 4 weeks to 1 year) and adult cats (> 1 year old). Animals were anaesthetized by an intramuscular injection of Althesin (Glaxo; 1.2 ml kg−1, equivalent to 10.8 mg kg−1 alfaxalone and 3.6 mg kg−1 alfadolone acetate). All skin incisions were infiltrated at regular time intervals with 1% lidocaine (lignocaine). After cannulation of the femoral vein and tracheotomy, the animal was paralysed by an intravenous infusion of Flaxedil (gallamine triethiodide, 15 mg kg−1 h−1). Anaesthesia and paralysis were maintained by continuous intravenous infusion of Althesin and Flaxedil (3 mg kg−1 h−1 and 15 mg kg−1 h−1, respectively). End-tidal CO2 concentration was regulated to between 3.8 and 4.2%, ECG was monitored throughout the experiment and body temperature was kept at 38°C. Nictitating membranes were retracted with neosynephrine (0.5%). Neutral contact lenses were placed on the cornea immediately after paralysis in order to protect the eye from drying. The head was fixed in a stereotaxic Horsley-Clarke frame, and the skull was cemented to an additional metal support which ensured optimal recording stability. Contact points of the head with the stereotaxic frame (ear bars) were coated with lidocaine gel (0.5%). Two small holes (2 mm diameter) were made bilaterally in the skull in order to expose the region of area 17 corresponding to the representation of area centralis (stereotaxic co-ordinates, L1-L2, P1-P3).
All surgical procedures were performed in conformity with national (JO 87-848) and European legislation (86/609/CEE) on animal experimentation, and strictly adhered to the recommendations of The Physiological Society. The stability of the ECG and the absence of pupillary reaction during surgery, and during an initial 2 h observation period prior to the use of the muscle relaxant, were taken as indicators that an adequate level of anaesthesia had been reached. The concentration of the anaesthetic in the intravenous infusion was increased by steps of 10% if spontaneous changes in heart rate were observed or if the heart rate changed by more than 10% while pinching the paw of the animal.
Pupil dilatation and accommodation blockade were induced by eye drops containing atropine (1%). Optical correction was measured by ophthalmoscopy and retinoscopy (skiascopy) in kittens younger than 8 weeks old, and by reflection of direct tapetal illumination on a stimulation screen in older animals.
Electrophysiological techniques
Glass micropipettes (impedance, 6-22 MΩ; filled with a solution of 3 M KCl and Pontamine Sky Blue 6BX Gurr, 1.6%) were used for extracellular recordings. Electrodes which appeared to leak electrolyte under microscopic examination of their tip, or for which an increase in spontaneous activity was observed during preliminary phases of recording, were discarded. For each cell, the shape of the action potential (amplitude ranging from 2-40 mV) was continuously monitored on a digital oscilloscope and compared with its initial shape in order to ensure that the same neuron was recorded throughout the experiment. The recording electrode was also used to control postsynaptic activity levels iontophoretically (see Methods in Frégnac et al. 1992). The intensity of the current pulses was limited to between -11 and +11 nA, which still allowed recording in balanced bridge mode. Dye labelling of the recording site after completion of the recording was achieved by passing a current of -10 μA for 10-20 min.
Visual stimulation
RF properties such as orientation and velocity selectivity, ocular dominance and end-stopping were first assessed using an overhead projector. The stimulus configuration (orientation, velocity and length) which elicited the stronger response through the dominant eye was used to plot the minimal discharge field on a drawing table. Principal ‘on’ and ‘off’ discharge fields were determined by moving light and dark moving edges over short distances and by marking where firing started and stopped. In most cases this criterion allowed a better distinction of the number of ‘on’ and ‘off’ regions and their overlap than using stationary stimuli. The spatial map of the RF was then established by flashing the same test light bar in contiguous positions across the RF (stationary stimulation mode). The width of the bar ranged between 0.3 and 0.5 deg of the visual field, and its optimal length was chosen to maximize firing according to the length summation characteristic of the recorded cell.
After the qualitative characterization of the static RF, ‘on’ and ‘off’ responses were recorded during computer-controlled sequences of stimulation. Two automated experimental set-ups for visual stimulation were used in this study. No differences in the functional properties of the recorded neurons were observed between these two systems. In the first series of experiments (n = 57), a slit of light, whose luminance and size matched that shown with the overhead projector, was presented on the frontal stimulation screen using an optical bench (mean luminance, 50-150 cd m−2; contrast, 0.7-0.9). The open ‘on’ and closed ‘off’ states of an electronic shutter were controlled by a timer device. Different preset positions in the RF (charted on the drawing table) were stimulated sequentially. The second mode of stimulation (n = 120) was fully automated and relied on image synthesis (Picasso; Innisfree, Cambridge, MS, USA) on a cathode ray tube (CRT) screen (Tektronix 608, P31). The upwardly oriented CRT was positioned using a mobile carriage sliding on two orthogonal horizontal rails. Its optical distance (through an intermediate reflecting 45 deg mirror) from the retinal planes of the cat was the same as that of the frontal stimulation screen on which the back-projection of the fundus of the eyes was made. The use of a common co-ordinate system for the drawing table, the frontal stimulation screen and the displacement reference for the horizontal rails allowed us to align the centre of the CRT screen with the centre of the RF (see Fig. 1 in Frégnac et al. 1992). Light and dark bars were generated on the screen by the Picasso stimulator and were controlled by customized software provided by Cambridge Electronic Design (Cambridge, UK). The luminance of the light bars ranged between 15 and 50 cd m−2, and the contrast of the test stimulus (0.4-0.8) was adjusted to give a stable baseline of ‘on’ and ‘off’ responses. Automated sequences of exploration of the RF in two to eight positions were made in an interleaved fashion and repeated 10-50 times.
Figure 1. Experimental protocol.
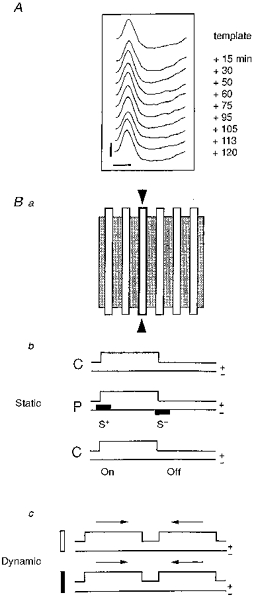
A, for each cell, the shape of the extracellular action potential (AP) was monitored on a digital oscilloscope and continuously compared with an initial template in order to make sure that the same neuron was recorded throughout the experiment (calibration bars: 1 mV; 1 ms). Ba, the spatial distribution of ‘on’ and ‘off’ responses in the RF (shaded area) was explored (‘static’ mode) with an optimally oriented stationary bar flashed sequentially in several positions (open bars). b, during control conditions (C) the optimal stimulus was presented 10-50 times without iontophoretic current (upper line: ‘on-off’ state of the stimulus with ‘on’ duration, 3000 ms and ‘off’ duration, 3000 ms; lower line: no iontophoretic current). During pairing (P), the visual stimulation was restricted to a given position in the RF (outlined contour indicated by 2 arrowheads in a). Iontophoretic pulses of opposite polarity (filled rectangles on the lower line; upward for positive, downward for negative current) were applied through the KCl recording electrode concomitantly with the presentation (‘on’) and the extinction (‘off’) of the visual stimulus. Intensity and polarity (+/−) of the current was adjusted in such a way as to impose a significant increase (S+) or decrease (S−) of the visual response. Following pairing the RF was explored in the paired and unpaired positions under the same conditions as the initial control (C, without current). c, in a few cells, responses to moving stimuli (dynamic mode) were also determined using optimally oriented light (open) and dark (filled) bars moving back and forth (arrows) through the RF.
Functional properties of visual cortical RFs
The visual response was assessed in the centre of ‘on’ and ‘off’ subfields in the case of multimodal simple RFs, or in two positions at least half the RF width apart in unimodal simple (S1) and complex RFs. Depending on the cell, the ‘on’ duration varied from 3-5 s, and the presentation of the test stimulus was repeated every 6-10 s. The temporal frequency was low enough to avoid short-term adaptation processes which occur in vivo with a stimulus presentation rate above 2 Hz (Marlin, Douglas & Cynaner, 1991), and did not induce significant trends with time in ‘on’ and ‘off’ response levels. In most neurons stimulated with the Picasso/CRT system, direction selectivity was also assessed using light and dark bars moving in both directions. A shift in the time of the peak response to any of the moving stimuli (detected in the PSTHs during control recordings) was taken as indicative of a drift in eye position, and the cell was discarded.
Protocols
Two types of activity control were imposed when presenting a stimulus (conditioned input) in a fixed position in the RF. The S+ pairing procedure was used to enhance the cell's firing and was applied to favour the weakest of the two control ‘on’ and ‘off’ responses or even, in extreme cases, replace an initially absent or subthreshold response. The S− pairing procedure was used in a symmetrical way to reduce or suppress the dominant response. Alternation of ‘on’ and ‘off’ activation with opposite changes in postsynaptic activity levels maintained the average activity of the cell constant throughout the control and conditioning periods, and allowed us to dissociate the associative effects from non-associative or global changes in excitability levels. The simultaneous comparison of visually evoked responses to two test stimuli yields more reliable results than the direct observation of absolute changes in the response level to a single stimulus (Frégnac et al. 1992).
In order to achieve this ‘differential’ pairing protocol (P, Fig. 1), the S+ paired visual stimulation (e.g. an ‘on’ transition) was associated with application of a positive current through the recording electrode, leading to ejection of K+ into the extracellular medium and a ‘high’ level of discharge. The opposite S− paired visual stimulus (in this case, an ‘off’ transition) was associated with the application of negative current, imposing a ‘low’ level of discharge (generally corresponding to a significant reduction or total blockade of the visually evoked discharge). This latter effect was probably due to a field effect, more effective when recordings were juxtacellular (Frégnac et al. 1988; Andrew & Fagan, 1990), which depended on the relative geometry of the cell and the tip of the electrode. In the few cases where this type of action was ineffective, no current was applied and this condition is referred to in the text as a neutral S0 condition (see Fig. 6). In the case of unimodal simple RFs (S1 type), we tried to induce a de novo response or potentiate possible subthreshold events which were not initially apparent in the extracellular recording. When the RFs were of the complex type, or when a simple RF had overlapping ‘off’ and ‘on’ fields (S2 type) the conditioning stimulus was shown in a position which had already elicited both ‘on’ and ‘off’ responses before pairing. Spatial generalization effects of the conditioning procedure were studied before and after pairing by flashing an optimally oriented bar in different positions chosen to span the RF width (see Fig. 1).
Figure 6. Modification of a pure ‘off’ simple cell into a complex cell recorded in a 6-week-old kitten.
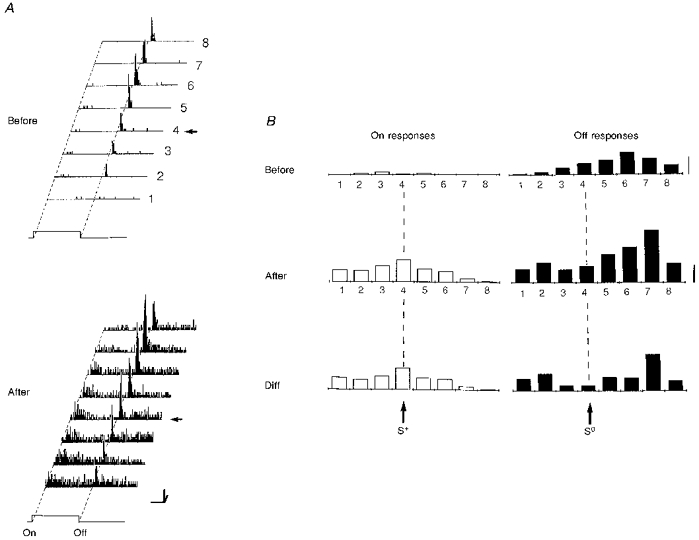
A, the RF of this neuron was characterized before and after conditioning in 8 positions. The pairing procedure (64 associations; not shown) was performed on the fourth position (arrow) by applying a positive current pulse (S+, +8 nA) through the recording pipette while the stimulus was turned ‘on’. No current was passed at the extinction of the stimulus (S0). Fifteen minutes after pairing, an ‘on’ response was unmasked in the RF (LCR, P < 0.0001), and the global complexity ratio (GCR) increased significantly from 0.06 to 0.33 (P < 0.0003). Calibration bars: horizontal, 1 s; vertical, 20 AP s−1; oblique, 1 deg. B, the spatial distribution of ‘on’ and ‘off’ responses given by the histograms on the right were computed after subtracting the corresponding levels of spontaneous activity. Calibration bar: 5 AP trial−1. General conventions for Figs 6–7 are as follows. Left panel (A): RF response planes. PSTHs are represented for 8 contiguous positions, numbered 1 to 8. The position used during pairing is indicated by a horizontal arrow. Right panel (B): from top to bottom, spatial distribution of ‘on’ responses (open bars, left column) and ‘off’ responses (filled bars, right column), before (upper row) and after (middle row) pairing. The lower histograms (Diff) represent the spatial distribution of absolute gain and losses in visual responses across the RF, given by the difference between the positional tuning curves measured after and before pairing.
In order to modulate the neuronal activity as soon as the visual stimulus was presented, iontophoresis was initiated usually a few tens of milliseconds before the onset and offset of the visual stimulus. Currents delivered by iontophoretic units ranged between 3 and 11 nA for both polarities, and were applied for 1.5-3 s in order to manipulate the eventual tonic component of the visual response. In a few cases, the onset of the positive current was delayed so that it followed the visual stimulus by 500-1000 ms, imposing a significant increase in the activity level which preceded or lagged the ‘on’ or ‘off’ transition of the stimulus by a few hundred milliseconds. The aim of this fixed delay pairing (FDP) protocol was to study possible temporal reorganization of sub- or suprathreshold long-latency components of the visually evoked responses.
The specificity of the effects of the pairing procedure, repeated 50-100 times, was assessed by measuring ‘on’ and ‘off’ responses in both paired and unpaired positions of the RF in the absence of any current applied through the recording electrode (C in Fig. 1B).
Data analysis
A ‘local complexity ratio’ (LCR), given by ‘on’/(‘on’+‘off’), was computed on the basis of ‘on’ and ‘off’ responses in each position of the RF, before and after the pairing procedure. This ratio was calculated using a moving average established by pooling responses for each 2-4 consecutive sequences (see also Frégnac et al. 1992). This allowed us to test the stationarity of the visual responses before the conditioning procedure and to study the kinetics of eventual effects appearing after the pairing procedure. The counting of spikes was limited to the period during which S+ and S− iontophoretic actions were applied, since the precise timing of current application was adjusted to affect the early phasic component and most of the tonic component of both ‘on’ and ‘off’ responses (with the exception of FDP protocols). For S2 cells where ‘on’ and ‘off’ fields initially presented no overlap, a ‘global complexity ratio’ (GCR) was computed by summing all the ‘on’ and all the ‘off’ responses over the RF width, after subtracting spontaneous activity from visual activity measured for the same epoch duration during the same trial.
Criteria of modification
Two criteria, based respectively on spatial and temporal modifications of the firing rate, were used to assess the significance of the effects induced by the pairing procedure. The first examined changes in LCR and GCR and was applied when the control of activity imposed during pairing had a differential effect on the ‘on’ and ‘off’ responses (see examples of S+S− protocols in Figs 3–6). In the case of contiguous (zero-delay correlation) pairing, LCR and GCR were compared before and after conditioning using a Mann-Whitney non-parametric test with a level of significance of 0.005. The relative weight (balance) between ‘on’ and ‘off’ responses was considered to be modified when the pairing resulted in a significant change (i) of the LCR in the paired and/or unpaired positions, or (ii) of the GCR in the case of S2 simple RFs.
Figure 3. Potentiation of the ‘off’ response of a simple cell recorded in a 5-week-old kitten.
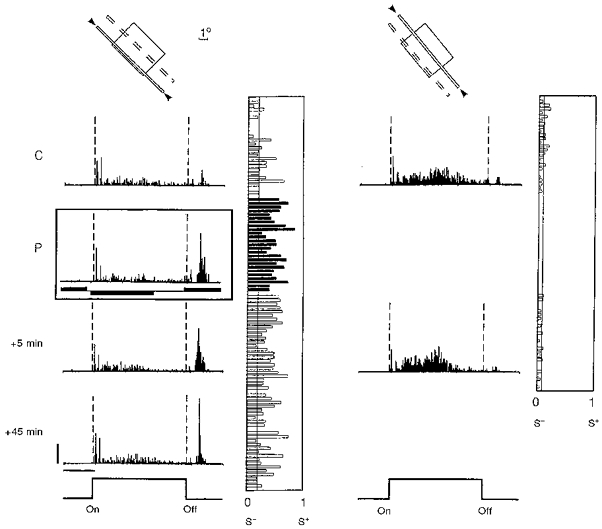
Response histograms for the paired and control (unpaired) positions are shown in the left and right panels, respectively. Before pairing, this cell exhibited a strong dominant ‘on’ response in both positions and was classified as simple. The pairing procedure consisted of 50 associations of a negative current pulse (-4 nA, 2000 ms duration) with the presentation of the stimulus, and of a positive current (+4 nA, 2000 ms) with the extinction of the same stimulus. The onset of the current pulses preceded the ‘on’ and ‘off’ transitions of the visual stimulus by 100 ms. During pairing (P, inset) a significant modification of the ‘on/(on + off)’ ratio was imposed. After pairing (+5 min), the ‘off’ response in the paired position was significantly potentiated (P < 0.0001). This effect was selective for the paired position (P < 0.005vs. P > 0.10 for the unpaired position) and lasted for 45 min until the cell was lost. Calibration bars: 1 s; 10 AP s−1. In this figure and the following ones, each RF is schematized by a rectangle at the top of the panels whose relative size and orientation corresponds to that charted on a drawing table (open zone, ‘on’ field; filled zone, ‘off’ field; chequerboard pattern, complex RF). The positions within the RF used for automated recordings are represented by thin, oriented open bars. The continuous outline and the arrowheads indicate the test stimulus which was flashed ‘on’ and ‘off’ to build the PSTHs placed in the same column; the other test positions, which were presented alternately, are indicated by a dotted outline. PSTHs are shown from top to bottom sequentially during an initial control period (C), during pairing (P, inset, with filled rectangles to indicate the polarity and timing of the iontophoretic pulses), and at various times following pairing (in minutes). The temporal evolution of the local complexity ratio (LCR), given by S+/(S++ S−) and calculated by a moving average technique over 2-4 epochs of visual stimulation (see Methods), is represented to the right of each corresponding histogram. The narrow vertical line indicates the mean value of the complexity ratio in the control period.
A different criterion, the modification of the temporal pattern of discharge for the paired stimulus, was chosen when the pairing was exerted out of phase with the ‘on-off’ transitions of the visual stimulation (see FDP protocols in Figs 10 and 11). Such modifications were usually not apparent when comparing LCRs, since significant changes in the discharge profile were found to occur outside the temporal windows defined by the initial ‘on’ and ‘off’ responses, and then appeared to be in phase with the onset of the applied iontophoretic pulse (see Figs 10 and 11). Consequently the responses used to calculate the complexity ratios for FDP protocols were taken as the number of spikes which occurred during the time window starting from the onset of the iontophoretic pulse and ending at the next ‘on’ or ‘off’ transition of the visual stimulus (if this preceded the end of the iontophoretic pulse).
Figure 10. Effects of a fixed delay protocol in a complex cell recorded in a 6-week-old kitten.
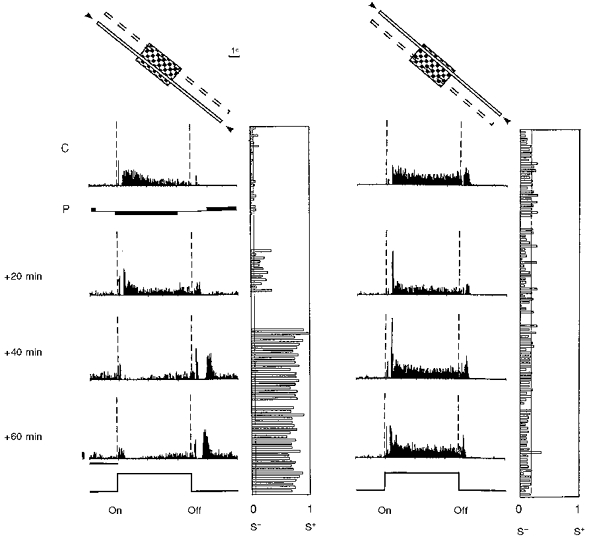
Conventions are as in Fig. 3. PSTHs are shown in the left column for the paired position, and in the right column for the unpaired position. Before pairing, a tonic ‘on’ response and a more transient ‘off’ response were observed uniformly across the RF. The pairing procedure (P, data not shown) consisted of 50 associations of a negative current (-3 nA, 2120 ms duration) with the onset of the light bar and a positive current (+3.2 nA, 2150 ms) following the offset of the same stimulus with a constant delay of 500 ms. A progressive change developed over 40 min after pairing, resulting in a significant depression of the ‘on’ response (P < 0.0005), whereas a late ‘off’ response appeared de novo in the paired position. The latency of the new response precisely matched the onset delay of the iontophoretic pulse used during pairing. The ‘on-off’ ratio was unchanged in the unpaired position. The modification in the paired position was still present more than 1 h after the end of the pairing procedure, at which time the neuron was lost. Calibration bars: 1 s; 20 AP s−1.
Figure 11. Effects of fixed delay pairing.
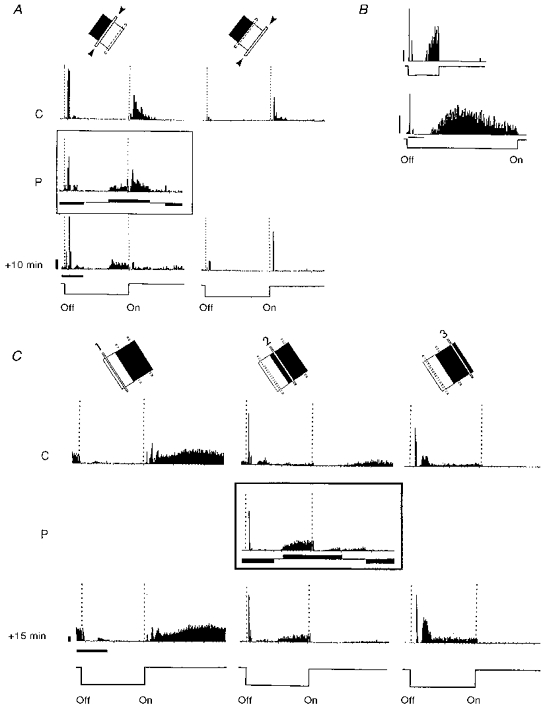
Conventions are as in Fig. 3, except for the fact that the visual stimulation has been synchronized with the ‘off’ transition of the stimulus. A, de novo appearance of a late ‘off’ response in a simple cell recorded in a 15-week-old kitten. The paired position (left column), located at the border between ‘on’ and ‘off’ fields, initially exhibited a mixed ‘on-off’ response. During pairing, a positive current pulse (S+, +5 nA) was applied systematically 1 s before the onset of the stimulus, and resulted in a significant imposed tonic level of activity during the late part of the ‘off’ period. The early ‘off’ response was paired with a pulse of negative current (S−, -5 nA) applied 1 s before the extinction of the stimulus, and its amplitude was reduced significantly (as shown in P). When recording continued without any current (+10 min), the neuron fired exactly 1 s before the effective onset of the light bar in the paired position, reproducing the exact temporal pattern of response that had been imposed during pairing. No significant changes in ‘on-off’ responses were observed in the unpaired position (right column), suggesting that the effect of the pairing was associative and restricted to the inputs during conditioning. Calibration bars: 1 s; 10 AP s−1. B, lagged ‘off’ responses (latency > 1 s) were occasionally observed during control recordings in young kittens (here 6 weeks old). The latency of this form of late response is not the result of a global synchronization effect of neuronal activity with the rate of stimulation, since it was not affected when the duration of the ‘off’ period was increased from 2 to 7 s. A strong inhibition of this response was induced when the stimulus was again turned ‘on’, an observation which is similar to that also observed in A and C. Calibration bars: 1 s; 10 AP s−1. C, simple cell recorded in a 17-week-old kitten. Two positions, chosen in the centre of the ‘on’ field (left column) and ‘off’ fields (right column) were unpaired, whereas a more intermediate position between the 2 fields was selected as the position to be stimulated during pairing (P). A +4 nA current pulse (S+) was applied 1 s before the ‘on’ transition of the stimulus, which resulted in a tonic activation of the cell (similar to that shown in A). A negative -5 nA pulse (S−) was also used to block the late ‘on’ activity of the cell and reduced the amplitude of the early ‘off’ response of the cell. This differential FDP was repeated 50 times. Fifteen minutes after pairing, the temporal activity pattern observed in the paired position in the absence of iontophoretic current still mimicked that imposed during pairing, whereas the ‘on’ and ‘off’ responses in the unpaired regions had similar time courses to those observed before pairing. Calibration bars: 1 s; 20 AP s−1.
Averaged normalized changes
In order to compare the spatial profile of ‘on’ and ‘off’ responses across the width of the RF we applied a method of normalization similar to that used for a previous study on orientation selectivity (Frégnac et al. 1992): the areas of the spatial ‘on’ and ‘off’ tuning curves observed before and after pairing were normalized to 100%, and the difference between the normalized responses of the same type recorded after and before pairing were computed for each test position. The resulting histogram (Diff in Figs 6 and 7) expresses the relative gain or loss in visual responsiveness as a function of the position in the RF.
Figure 7. Spatial generalization of the effect of conditioning in a simple cell recorded in a 12-week-old kitten.
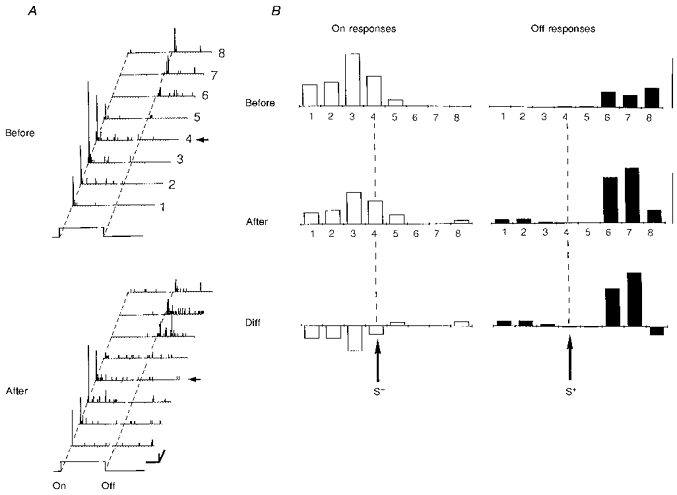
Conventions are as in Fig. 6. The RF was characterized before and after pairing in 8 positions and showed a simple structure with non-overlapping ‘on’ and ‘off’ fields. The fourth position (arrow) was submitted to 40 differential pairing trials (data not shown) during which the ‘on’ response was associated with a negative pulse of current (S−, -7 nA) and the ‘off’ response paired with a positive current (S+, +11 nA). After conditioning, the balance of ‘on’ and ‘off’ responses was modified across the whole extent of the RF: ‘on’ responses in the ‘on’ field were significantly decreased, while ‘off’ responses evoked in the ‘off’ field were potentiated. The GCR changed accordingly from 0.27 to 0.57 (P < 0.0011). Calibration bars: A, 1 s, 10 AP s−1, 0.5 deg; B, 5 AP trial−1.
Changes in the temporal pattern of discharge for a given characteristic of the stimulus were quantified in the following way: for each bin of the PSTH, the difference between the absolute count of spikes elicited during pairing (Imposed, Fig. 12A), or evoked after pairing (Induced, Fig. 12B) and that observed in the control period, was divided by the mean number of spikes. This latter measure was obtained by averaging spike activity over the whole duration of the appropriate ‘on’ or ‘off’ period of the visual stimulation for both the control and pairing (imposed or induced) conditions. This avoided the use of a null reference value for bins where no activity was present initially. Such normalization avoided giving too much weight to changes in marginal responses (border effect), at the start and end of the visual response, and allowed comparison of response patterns between cells independently of their mean level of firing.
Figure 12. Imposed and induced modifications of the temporal patterns of ‘on’ and ‘off’ responses associated with S+ and S−.
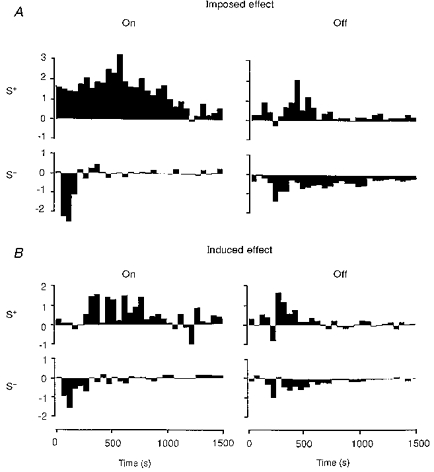
This analysis was performed only for cells whose responses were significantly modified following the application of contiguous ‘on’ or ‘off’ pairings (thus excluding FDP protocols). A, imposed effects of contiguous S+ and S− pairings on ‘on’ and ‘off’ responses. The histograms of response changes (‘during pairing’ minus ‘before pairing’), synchronized with the ‘on’ transition (left column) or the ‘off’ transition (right column), have been normalized relative to the mean count of each bin estimated by averaging responses before and during pairing (see Methods). A facilitation of ‘on’ and ‘off’ responses (upward histograms) was observed as expected during S+ pairings, whereas a depression was imposed during S− pairings (downward histograms). B, induced effects of contiguous S+ and S− pairings on ‘on’ and ‘off’ responses. The histograms of response changes (‘after pairing’ minus ‘before pairing’), synchronized with the ‘on’ or ‘off’ transition, have been normalized relative to the average count of each bin estimated by averaging responses before and after pairing (see Methods). Gains in visual responsiveness (upward histograms) were induced for both ‘on’ and ‘off’ transitions of the stimulus following their respective pairing with a ‘high’ level of activity (S+). Losses of responsiveness (downward histograms) were induced for ‘on’ and ‘off’ characteristics associated with a reduced level of activity (S−). Both the early and late components of the response (up to 800-1000 ms after an ‘on’-S+ pairing) could be modified by the pairing procedure.
Histology
The laminar locations of recording sites were determined by the ejection of Pontamine Blue. For most electrode tracks, two points at least 500 μm apart were labelled. The positions of the recorded cells were interpolated from the stereotaxic co-ordinates of the two marker injection sites. At the end of the experiment, after a lethal dose of anaesthetic, an intracardiac perfusion was performed with 0.9% NaCl followed by an isotonic solution of 2% paraformaldehyde and 2% glutaraldehyde in phosphate buffer. The visual cortex was removed, kept overnight in a 30% sucrose, 10% formaldehyde solution and 80 μm thick sections were cut in the frontal plane on a freezing microtome. Slices were stained with Cresyl Violet and electrode tracks reconstructed with a camera lucida.
RESULTS
The spatial organization of the RFs of 177 neurons was studied quantitatively in kitten and cat area 17, from age 4 weeks to adulthood. Most RFs could be classified into simple (116 cells) and complex (55 cells) types, except for six neurons which responded only to moving stimuli. One hundred and ten cells (64.3%) were recorded in 4- to 15-week-old kittens, and sixty-one cells (35.7%) in juvenile (from 16-52 weeks) and adult cats (> 1 year). Forty-two cells were recorded for a period long enough (> 1 h) to complete the pairing protocols (including subsequent controls), in addition to the quantitative maps of spatial organization of the RF. The distributions of ocular dominance, orientation preference and visual latency, and the laminar distribution of RF types (simple vs. complex) for the whole population of recorded cells were similar to those reported in the literature for the same eccentricity, i.e. within an area of 5 deg around area centralis. This suggests that the particular electrode characteristics (electrolyte and impedance) used in the present study did not introduce a sampling bias as to the type of neurons recorded. The general properties of the paired cells were also similar to those established for the rest of the sample.
Intrinsic stability of the spatial organization of ‘on-off’ zones
The stability of the spatial structure of simple and complex RFs was studied as a function of time (Fig. 2A); the mapping of the response plane in fifteen RFs was repeated systematically at 20-40 min intervals. Fourteen of these (93%) showed no change in the topography of the spatial profile of ‘on-off’ responses, as demonstrated by the stationarity of the GCR computed across the whole extent of the RF, and by that of the LCR relative to each test position. Only one simple RF showed a spontaneous modification of the LCR measured in one position at 20 min intervals.
Figure 2. Intrinsic variability of the spatial RF organization.
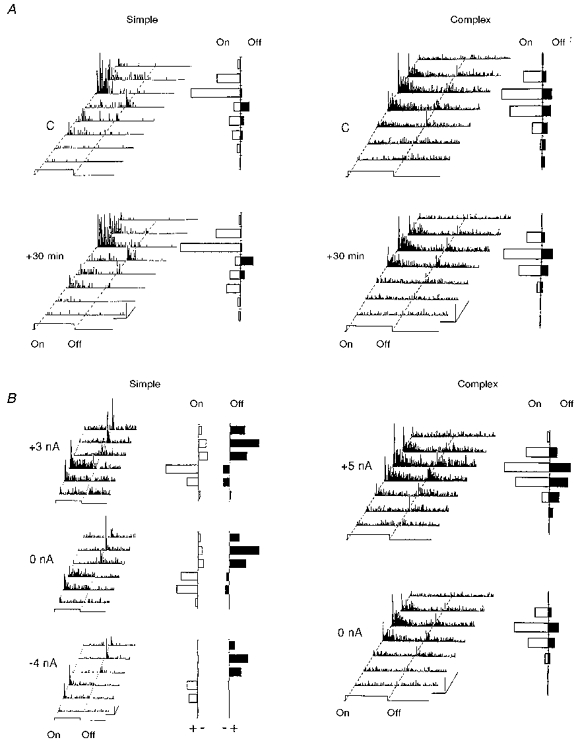
A, the temporal stability of the spatial profile of ‘on’ and ‘off’ responses was assessed in simple (left panel) and complex (right panel) cells by repetitively exploring the whole extent of the RF with a light bar flashed ‘on’ and ‘off’ in 8 positions, 30 min apart. The cumulative ‘on’ and ‘off’ responses shown for each position (to the right of the corresponding PSTH) remained unchanged. Calibration bars: horizontal, 1 s; vertical, 10 AP s−1; oblique, 1 deg (RF width axis). B, left panel: in order to study the degree of invariance of the spatial RF organization as a function of the level of excitability artificially imposed by a constant iontophoretic current, three RF maps of the same simple cell recorded in an 8-week-old kitten were constructed during the constant application of either positive current (+3 nA), no current (0 nA), or negative current (-4 nA) through the recording pipette. Cumulative excitatory (+) and inhibitory (-) responses were quantified for the ‘on’ (open bars) or ‘off’ periods (filled bars) by subtracting the spontaneous activity (measured for the same temporal window duration between trials) from the visual activity. An increase in early inhibition triggered by the characteristic antagonistic to that of the field under test was apparent when the neuron was held at the more depolarized level. In spite of the large changes in mean level of activity imposed by iontophoresis, the general spatial organization of the RF and the degree of overlap between the ‘on’ and ‘off’ zones remained unchanged whatever the value and polarity of the applied current. Right panel: a complex RF map recorded in a 24-week-old kitten is shown in the control situation (0 nA), and when an iontophoretic current is applied (+5 nA). No inhibitory responses were revealed even under sustained depolarization (+5 nA), in contrast to that observed for simple cells. Calibration bars: horizontal, 1 s; vertical, 10 AP s−1; oblique, 1 deg.
In order to determine the possible dependency of the RF structure on the state of excitability of the neuron, which may vary during long-duration recording of the same cell in the anaesthetized animal, the ‘on-off’ organization of five simple and complex neurons was quantitatively tested whilst artificially changing the mean discharge level from 20 to 500% of the control value. This change in discharge level was obtained by injection of a constant current of fixed polarity (positive or negative) unrelated to the timing of the ‘on-off’ transition of the visual stimulus. No significant changes in the RF structure and local (LCR) and global (GCR) complexity ratios were found during the imposed increase or decrease in visual responsiveness (Fig. 2B). We conclude from this non-associative conditioning protocol that ‘on’ and ‘off’ responses covary with the level of applied current in a similar way. As a result, the balance between the ‘on’ and ‘off’ responses is unchanged. We conclude that the spatial organization of simple and complex RFs remains invariant independent of the change in excitability imposed by iontophoretic means.
Pairing-induced modifications of ‘on’ and ‘off’ responses
General effects of the pairing procedure
Seventy-four pairing procedures were performed on forty-two neurons. Four initial pairing procedures (3 cells), following which a shift in the position of the RF was apparent (see Methods) were discarded from the final analysis. Significant modifications (P < 0.005) in the response to a stationary flashed stimulus (Static mode in Fig. 1Bb) were found in 44% of the conditioned neurons (17/39). The responses of these neurons were modified in different ways. The majority (n = 10) showed a non-uniform reorganization of the spatial distribution of ‘on’ and ‘off’ responses across their RF. This effect is shown by differential modifications of the LCRs measured in the ‘paired’ position (see Fig. 1B) and outside (‘unpaired’ positions) the conditioning site. Three S2 simple cells, with initially no spatial overlap between the antagonistic subfields, kept a clear segregation of ‘on’ and ‘off’ zones, but the balance (given by the GCR) of the ‘on’ and ‘off’ responses, when summated irrespective of the position of the stimulus in the RF, appeared significantly modified. The four remaining modified cells had been submitted to a fixed delay pairing procedure, which resulted in a drastic modification of long-latency responses. Apart from one case, the pairing procedure did not induce subsequent changes in the level of excitability of the conditioned neurons. No change in the shape of the extracellularly recorded AP was induced following pairing.
In the vast majority of cases, modifications were largest in the position that had been used during conditioning (15/17, 88% of modified neurons). In ten cells (62% of cases), the effect of pairing was restricted to that sole position. Changes in the unpaired position were observed in one-third of the cases. Among these neurons, two showed a modification in the unpaired position only. One modified cell was tested only in the paired position.
Simple (Figs 3, 5–8 and 11) and complex (Figs 4 and 10) RFs were modified in a similar proportion (simple: 48.1%, 13/27; complex: 33.3%, 4/12; χ2 test, P > 0.389), and no significant difference in the probability of inducing a change was observed between young kittens (46%, 13/28) and older animals (36%, 4/11; χ2 test, P > 0.57). Recording sites could be localized with precision in thirty-eight out of thirty-nine cases (16 out of the 17 modified cells). The percentage of modified cells did not significantly differ across layers (χ2 test, 3 degrees of freedom, P > 0.32; see Table 1).
Figure 5. De novo induction of an ‘on’ response in a pure ‘off’ simple cell recorded in a 27-week-old cat.
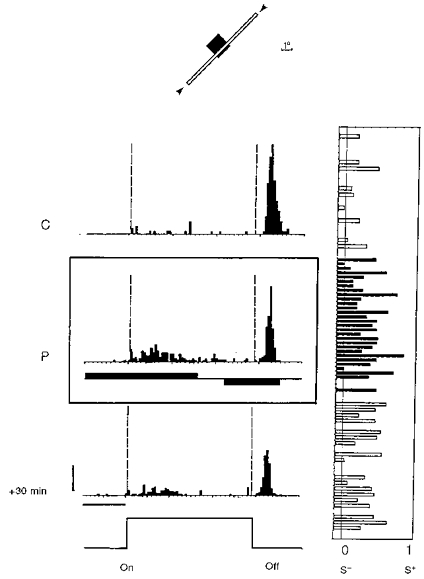
Conventions are as in Fig. 3. This neuron initially responded only to the extinction of the stimulus within its RF (C). Occasionally, one AP occurred during the ‘on’ period without a fixed latency. However, most of the presentations of the test stimulus failed to evoke any responses, as indicated by the LCR plot on the right. An ‘on’ response was imposed during the conditioning (P; 110 associations) by applying a positive current pulse (+3 nA), while the ‘off’ response was reduced when passing negative current (-3 nA). In spite of the fact that the current pulse started almost 1 s before the onset of the stimulus, its action was effective in triggering postsynaptic activity only during the ‘on’ period. After pairing, the cell fired in a consistent way during the presentation of the stimulus (119 APs for the ‘on’ period vs. 208 APs for the ‘off’ stimulation), although the response remained more transient and synchronized for the offset of the stimulus. The mean LCRs shifted from 0.094 to 0.207 following pairing (P < 0.005). This effect was still present 45 min after pairing (data not shown; P < 0.002). Calibration bars: 1 s; 10 AP s−1.
Figure 8. Modification of directional selectivity in a simple cell recorded in a 5-week-old kitten.
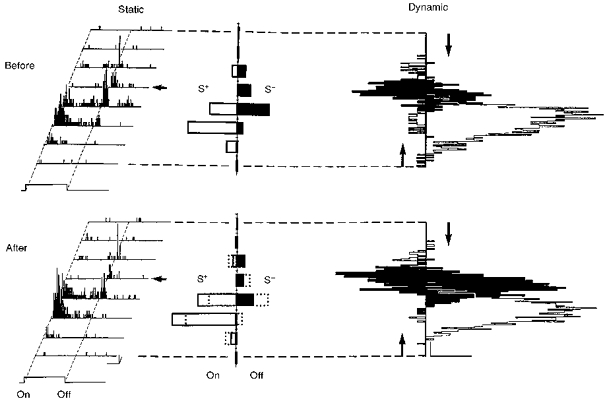
This receptive field was studied using stationary (left panel) and moving (right panel) stimuli. The fifth position (arrow) was submitted to 50 pairing trials (data not shown) during which the ‘on’ response was associated with a positive current (S+, +4 nA) and the ‘off’ response with a negative current (S−, -10 nA). After pairing, static ‘on’ responses were increased while ‘off’ responses were depressed (see histograms of cumulative ‘on’ and ‘off’ responses after pairing where the superimposed dotted lines represent the initial control responses). The GCRs shifted from 0.61 to 0.76 (P < 0.023) and the LCRs were significantly modified (P < 0.001) in 2 positions. Calibration bars: 1 s; 10 AP s−1; 0.5 deg. In the right panel (Dynamic) light and dark bars were moved in opposite directions across the RF. The empty and filled downward and upward histograms represent the visual responses to the bright and dark stimuli, respectively. The responses in the opposite direction are shown in a mirror-like fashion and aligned on the same positional axis in the RF. This simple cell showed a directional preference before pairing which reversed when the contrast of the stimulus was inverted. A significant change in the response to the dark bar was observed after pairing, whereas the response to the light stimulus remained the same. Calibration bars: 1 s; 20 AP s−1.
Figure 4. Differential potentiation and depression of ‘on’ and ‘off’ responses of a complex cell recorded in a 6-week-old kitten.
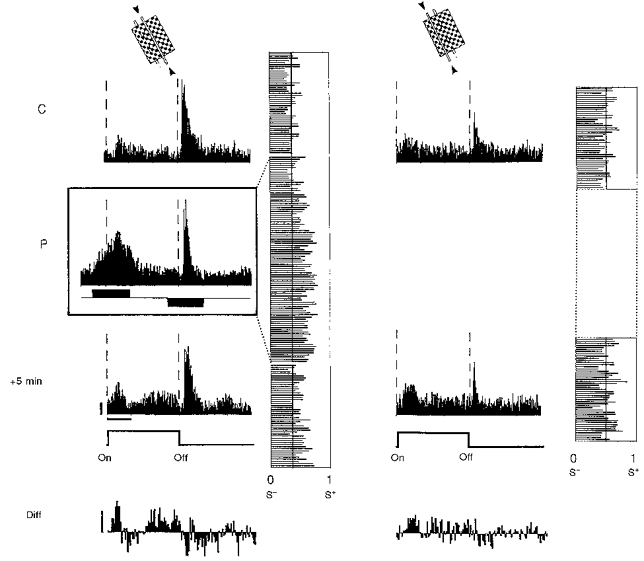
Conventions are as in Fig. 3. PSTHs are shown in the left panel for the paired position, and in the right panel for the unpaired position. Before pairing, a small ‘on’ response and a dominant transient ‘off’ response were observed in the two test positions. During pairing the ‘on’ response was increased to a high level using a +3 nA current pulse for 1500 ms (S+), whereas the dominant ‘off’ response was paired with a -4 nA current pulse of the same duration (S−). A significant change in the LCR was found in the paired position following pairing (P < 0.002), indicating a potentiation of ‘on’ responses of short and medium latency (100-300 ms) and a depression of ‘off’ responses. This finding is illustrated in the lower histogram (Diff) given by the difference, bin by bin, of the PSTHs after and before pairing (gain upward, loss downward). Calibration bars: horizontal, 1 s; vertical, 10 AP s−1.
Table 1.
Laminar distribution of modified RFs
| Layer | II–III | IV | V | VI | Total |
|---|---|---|---|---|---|
| Paired cells | 6 | 7 | 14 | 11 | 38* |
| Modified cells | 2 | 2 | 5 | 7 | 16* |
| Percentage modified | 33 | 29 | 36 | 64 | 42.1 |
Recording sites could be identified for 38 out of the 39 cells submitted to a pairing procedure, and for 16 out of the 17 modified cells.
‘Homopositional’ changes
By analogy to homosynaptic plasticity, which refers to a synaptic change induced primarily by the activity of the corresponding presynaptic axon, ‘homopositional’ modifications here refer to changes in responses induced in the paired position. In 87% (n = 13) of such cases (n = 15 out of 17 paired cells), the LCR moved significantly in favour of the characteristic which had been associated with a ‘high’ level of activity during pairing. This finding is in agreement with the prediction made on the basis of Hebb's postulate and the assumption of adequate input separability. Only three cells (13%) did not comply with this theory of plasticity: one neuron behaved in an inconclusive way, in that two identical pairing procedures induced opposite changes, and was counted twice in the statistics; and two other cells underwent a change favouring the stimulus characteristic which had been paired with a ‘low’ level of activity.
Ten out of the thirteen modified c ells showing modification as predicted by the Hebbian theory of plasticity had been submitted to a differential S+S− pairing procedure. A general observation was that pairing-induced modifications of the ‘on/(on + off)’ ratio resulted from an increase in the absolute value of the response (which was ‘reinforced’ during the S+ pairing for 7/10 cells) as well as from a decrease in the response that had been associated with a negative current (S−; 7/10 cells). Two other neurons (2/13) which showed a significant effect had been forced to fire with a ‘high’ level of activity (S+) for one characteristic of the visual stimulus, the other characteristic remaining unpaired (S0) (see S+S0 protocol in Methods). In both cases a potentiation of the response to the positively reinforced characteristic was induced. Lastly, for another neuron submitted to an S−S0 protocol (for which one stimulus characteristic only was paired, in this case with a ‘low’ level of activity), a potentiation of the late component of the ‘on’ response was observed, which corresponded temporally to the rebound of spiking activity following the end of the pulse of negative current (S−). In terms of activity control, this procedure might in fact be considered as an early ‘on’-S−, late ‘on’-S+ pairing.
Two major types of potentiation of visual response could be distinguished for the stimulus that had been paired with a ‘high’ level of activity: (i) an increase in a pre-existing extracellular response during the initial control (7/12 cells; see Figs 3 and 4), and (ii) the emergence of a de novo response or the unmasking of a subliminal event which was initially undetectable extracellularly (5/12 cells; see Figs 5, 7, 10 and 11). On the other hand, the depressions of the S− paired visual response, found in seven out of ten neurons modified with an S+S− protocol, could not be explained by a general depression of activity since, as mentioned earlier, the antagonistic S+ characteristic was often potentiated and no change in spontaneous activity was observed.
Spatial selectivity of RF modifications
The spatial selectivity of the effects of conditioning was tested in all but one cell by studying at least one other position in addition to the one stimulated during conditioning. One neuron with a small (1 deg) pure ‘off’ RF was studied for only one position, since the stimulus width needed to trigger spike activity (and used during pairing) already extended over two-thirds of the RF. Ten of the sixteen cells tested for at least two RF positions showed a modification restricted to the paired position (62% of the modified cells).
In three modified neurons (S2 simple cells) where no overlap zone could be found between the ‘on’ and ‘off’ fields during the control phase of recording, the RF profile was determined by exploring eight positions and comparing them before and after pairing. The difference established between the two spatial sensitivity profiles can be considered, using the terminology of behavioural learning, as equivalent to a generalization gradient. This gradient - which shows how pairing-induced changes in the unpaired positions depend on their relative proximity to the paired position - might be informative regarding input separability at the synaptic level. In one of these cells, which was submitted to a S+S0 procedure, the largest change in the ‘on’ response to the paired stimulus was found in the paired position (see middle panel of Fig. 6B) which was also the location at which the smallest increase in the S0 response was apparent. This functional modification corresponded to the unmasking of, or the de novo induction of, a response for the characteristic (‘on’) that had been associated with a ‘high’ level of activity. The ‘off’ response, although unpaired, also appeared to be increased in a position that was not stimulated during pairing. Consequently, the homogeneous ‘off’ field before pairing had become complex-like following conditioning with two dominant ‘on’ and ‘off’ regions. This profound restructuring of the RF could not be explained by a non-associative sensitization resulting in a global change in the excitability of the cell (an ‘iceberg’ effect): such opposite changes between ‘on’ and ‘off’ responses were never observed spontaneously or during application of an iontophoretic current maintained constant irrespective of visual stimulation (compare Figs 6 and 2). In the two other cells submitted to an S+S− procedure, the spatial structure of the RF was unchanged, and the local complexity ratio measured in the paired position was unaffected. However, a reorganization of the balance between ‘on’ and ‘off’ responses becomes apparent when separately summating the ‘on’ and ‘off’ responses across the whole width of the RF. The induced change in the GCR shows precisely that the amplitudes of the ‘on’ and ‘off’ responses change in opposite directions irrespective of the position of the stimulus (see Methods, and Figs 7 and 8). The data show that ‘on’ and ‘off’ responses were also altered outside the paired position, and that the sign of the change in the ‘on-off’ balance is the same as that imposed by the differential pairing procedure.
These two examples demonstrate the existence of similar generalization gradients, even when the RF remains simple after pairing. Both examples share common points: (i) the RF was initially of the S2 type; (ii) the stimulation of the position chosen for pairing initially elicited only one type of response (‘on’ in Fig. 7, ‘off’ in Fig. 8); and (iii) there was no overlap between the antagonistic fields as judged from the discharge pattern. The characteristic of the visual stimulation associated with a ‘high’ level of activity was always selected as antagonistic to that expressed dominantly in the paired position (i.e. ‘off’ in Fig. 7, and ‘on’ in Fig. 8). Following conditioning, the response, evoked in the unpaired antagonistic region of the RF (‘off’ field in Fig. 7 and ‘on’ field in Fig. 8) that was potentiated, shared the same triggering characteristic (respectively ‘off’ or ‘on’) as that which had been associated with a ‘high’ level of activity in the paired region (S+). Similarly, the dominant response (respectively ‘on’ or ‘off’) which had been paired with a reduced level of activity (S−) was depressed over the whole extent of the corresponding subfield (respectively ‘on’ or ‘off’).
Correlation between the spatial spread of functional modification across the RF width and the initial direction preference of the conditioned cell
Non-linear excitatory and inhibitory interactions within a given response zone, or even between ‘on’ and ‘off’ zones in simple cells, are thought to be responsible for the directional asymmetry in the rate of firing often shown by cortical cells in response to bars moving in opposite directions (Hubel & Wiesel, 1962). In view of the spatial non-homogeneity of changes found within the RF in our pairing experiments, we looked for a possible correlation between the spatial generalization gradient of modifications and the initial direction preference or selectivity of the conditioned cells. In addition, we tried to test whether some predictions could be made about consecutive changes in directional selectivity, based solely on the knowledge of distribution of changes in ‘on’ and ‘off’ responses to stationary flashed stimuli across the width of the RF.
Since the pairing procedure was done in different RF positions depending on the cell, we observed that the peak of the response changes was generally centred within 1 deg around the position used during conditioning, regardless of its exact location (border or central part of complex RFs, border or central parts of ‘on’ or ‘off’ fields in simple RFs). In order to facilitate data analysis and comparison of RF layouts which differed in size, orientation and directional preference, the following transformations were applied to the raw data. (1) The width of each RF was normalized to a constant spatial spread reference value. (2) RFs were artificially rotated and aligned so that the transformed RFs all shared the same orientation and direction preference. The spatial profile of ‘on’ and ‘off’ responses over the width of the RF was then subjected to a linear interpolation, making data taken from each cell comparable at any point along the width of the ‘normalized’ RF. These conventions are similar to those introduced by Marlin and collaborators (Marlin et al. 1991). The result of the data manipulation is that RF positions at positive distances from the conditioning position will be stimulated by a bar of light moving from the paired position in the cell's preferred direction.
The results shown in Fig. 9 indicate that there was an asymmetric spread of the adaptation effects across the RF, correlated with the direction preference of the cells. In a given position of the RF, the changes observed for the S− paired characteristic of the stimulus tend to be opposite in sign to those observed for the S+ paired characteristic. Changes in visual responsiveness opposite to those induced in the paired position were observed both for the positively reinforced (Fig. 9A) and negatively reinforced characteristics (Fig. 9B). They were most apparent in the unpaired positions of the RF, which would be stimulated just ahead of the paired position by a light bar entering the RF and moving in the preferred direction. The magnitude of the induced changes in responsiveness was greatest for the ‘off’ responses both after S+ and S− pairings (data not shown).
Figure 9. Spatial gradient of generalization of the functional changes and directional preference of visual cortical cells.
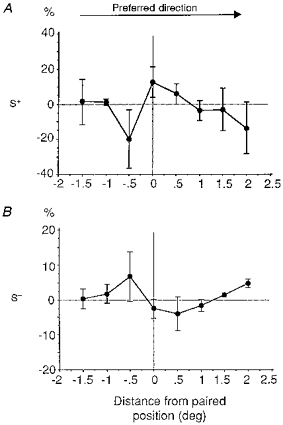
Distribution of the mean relative change in responses (%, ±s.e.m.) induced by pairing as a function of the distance across the RF width from the paired position (in degrees). The RF response profiles were aligned as a function of the preferred direction of each cell and linearly interpolated in order to be able to compare the behaviour of different cells at similar eccentricities from the paired position. The normalized changes in responses were established separately for the S+ (A) and the S− characteristic (B) and averaged across cells. The two graphs appear to be organized in mirror fashion and show an asymmetric spread of the long-term adaptation effect across the RF width which is correlated with the directional preference of the cell.
In order to further test for possible relationships between the properties of visual cortical RFs established using stationary and dynamic stimuli, we tried to assess the consequences of a spatial reorganization of the balance of ‘on’ and ‘off’ responses on the directional properties of the cells. In four cells in which pairing using stationary stimuli resulted in a significant change in the relative weight of ‘on’ and ‘off’ responses, we tested dynamic responses to light and dark bars moving in both directions, before and after the pairing procedure. A significant modification in the index of direction selectivity was observed in only one case, illustrated in Fig. 8. A conditioning protocol, performed at the borderline of the ‘on’ and ‘off’ zones, induced a potentiation of the ‘on’ responses evoked in the ‘on’ field and a depression of those elicited in the ‘off’ field (as described in the previous section). Before pairing, the preferred directions measured for light and dark bars were opposite, and could be predicted from the map of the stationary responses. After pairing, the preferred direction was the same, whatever the contrast. This change in dynamic property was due predominantly to a potentiation of the response to the dark bar in the previously non-preferred direction. It is difficult to predict this increase in responsiveness selective to the contrast and direction of a moving stimulus from the changes in response to a stationary stimulus (decrease of the ‘off’ response in the ‘off’ field in the present case). Nevertheless one cannot exclude the fact that alterations in the ‘on-off’ balance across the RF result in profound modifications in the dynamic interaction between antagonistic fields when the stimulus moves in the null direction.
Temporal conditioning and effects of the FDP
For thirteen cells, pairing was achieved by delaying or advancing the iontophoretic pulses by a few hundred milliseconds from the ‘on’ or ‘off’ transition of the visual stimulus. Significant changes in the temporal modulation of the patterns of discharge were observed in four cells that had been subjected to pairings where the time onset of the current pulse preceded the beginning of the effective visual stimulation by 500-1000 ms. In three out of these four neurons, the de novo appearance of a late ‘off’ response, which was absent in the initial control period, could be induced (see Figs 10 and 11).
In the example shown in Fig. 10, the S+ current pulse started 500 ms after extinction of the light bar. During the first 40 min following pairing, a new ‘off’ response developed progressively over time, and exhibited a latency precisely matching that of the onset of the positive current pulse of the pairing. Two other examples illustrated in Fig. 11 similarly show the development of a delayed rise in tonic activity synchronized with the ‘off’ response as a consequence of the FDP (Fig. 11A and C). In both cells, the depolarizing current injection for the S+ procedure was applied 1000 ms before the ‘on’ transition of the visual stimulation and resulted in an immediate increase in activity starting with the onset of the pulse of positive current. When the visual response was tested without current following pairing, a significant build-up of activity preceded the presentation of the previously paired stimulus. Its timing corresponded exactly to the period of iontophoresis of positive current which had preceded the ‘on’ transition during pairing. Such recall of the pattern of activity that had been imposed during pairing is very similar to previous observations using similar conditioning protocols, but with moving stimuli (Fig. 5 in Frégnac et al. 1992 and Fig. 4 in Shulz & Frégnac, 1992). This effect was retained for 5-40 min following pairing. Although the new firing pattern of the cell seemed to predict the ‘on’ transition of the stimulation by a fixed delay, this behaviour cannot be explained by a global phase-locking to the stimulation cycle used in our protocols. The modification of the response pattern was shown to be linked in phase with the ‘off’ transition of the stimulus (and not its ‘on’ transition), since changing the period of stimulation by increasing the duration between two successive presentations did not affect the latency of the ‘off’ evoked discharge (see Fig. 11B). Furthermore, these changes were clearly associative, as recall of the memorized activity pattern was observed only when stimulating the region of the RF activated during pairing, and was absent when alternately stimulating the unpaired region of the RF. These modifications of the visual response could be attributed to the potentiation of late or lagged ‘off’ responses, which are sometimes observed in control recordings in kitten visual cortex at the beginning and peak of the critical period (Fig. 11B), and might have been present at a subthreshold level in our conditioned neurons.
Activity dependence of the induction process
An important aim was to determine how these functional changes were related to the control of activity imposed during pairing. The rate of success in inducing plastic changes was higher when the two modes of control of activity (S+ and S−) were applied in parallel to antagonistic ‘on’ and ‘off’ responses (16/47 S+S− protocols) than when only one type of transition of the visual stimulus was paired (3/23 S+S0 or S0S− protocols). Most changes were observed when a differential control of activity had been achieved during pairing, i.e. when the visual response of the conditioned cell was alternately significantly increased for S+ and significantly decreased for S−. For example, repetitive increased firing for a given characteristic (S+) appeared less effective when applied alone (1/19) than when interleaved with periods of reduced activity imposed during S− pairings (16/47; χ2, P < 0.015). These observations suggest that, in addition to Hebbian synaptic potentiation, some forms of heterosynaptic competition and/or homosynaptic depression might participate in the observed effects. No changes in response patterns were found in the five control cases when the conditioning current was not effective in influencing the activity of the recorded cell.
We conclude from these observations that both up- and down-modulations of postsynaptic activity were necessary during pairing in order to induce long-lasting changes in ‘on’ and ‘off’ responses. A more quantified assessment of how these effects depend on the control of activity is obtained by comparing the amplitude and time course of the discharge pattern induced after pairing with the template of activity imposed by the current pulses during the pairing procedure (Fig. 12). On average, the induced change had the same sign and half the amplitude of that achieved during iontophoresis. This finding parallels our previous conclusions on orientation selectivity using very similar pairing protocols (Frégnac et al. 1992). A more precise analysis of the latency distribution of modified responses was not possible as, depending on the cell, the pairing resulted in alteration of the firing pattern during current injection at different delays following the stimulus onset or offset. Nevertheless we observed on average that both early and late components of the visual response were modified following pairing. In addition, in the case of the ‘on’ S+ protocol (upper left histogram, Fig. 12B) the induced increase in visual responsiveness could even extend for several hundreds of milliseconds following onset of the stimulus presentation.
Time constant of the extinction process
Induced modifications lasted longer than 10 min. The full time course of extinction of the effect was followed in nine cases and reached its end within 10-75 min following pairing (mean, 19 min). In the ten other cases the effect was still present up to the point where the neuron was lost, i.e. 10-75 min after pairing (mean, 28 min). Although the distribution of extinction time constants forms a continuum, the functional modifications can be qualified as long-term potentiation and/or depression of visual responses since, in most cases, they extend beyond several tens of minutes. The low rate of stimulation used during pairing (below 0.2 Hz) and the associative characteristic of the induction process clearly distinguish these changes from those produced by the continuous or periodic stimulation of the RF, which are classic examples of short-term adaptation (Marlin et al. 1991).
DISCUSSION
The present results demonstrate that imposed changes in temporal correlation between pre- and postsynaptic activity can lead to long-lasting changes (> 10 min) in the spatial distribution of ‘on’ and ‘off’ responses within visual cortical RFs. These changes appear to result from the strengthening and weakening of short- and long-latency visually evoked responses, mainly apparent in the region of the RF which has been stimulated during pairing. The direction of the changes in firing rate (potentiation or depression) was in most cases predicted by the sign of the change in covariance between presynaptic and postsynaptic activity imposed during pairing: 87% of significant modifications of LCRs and GCRs were correlated with the imposed changes in ‘on’ and ‘off’ responses. Plastic changes reflected, both in their amplitude and their time course, the altered pattern of discharge imposed during the pairing procedure. These findings generalize conclusions reached in previous experiments (Frégnac et al. 1988; Frégnac et al. 1992; Shulz & Frégnac, 1992) where functional selectivity to moving stimuli of different orientations and/or ocularity was shown to be modifiable in a predictable way using Hebbian-like conditioning procedures.
Two hypotheses can be forwarded concerning the cells whose RFs were not modified by the pairing procedure. Firstly, we cannot exclude the possibility that a certain proportion of cells could be under the control of strong endogenous constraints and that their RF organization is insensitive to activity-dependent processes. For example, in the orientation domain, the presence of specific monocular vertical and horizontal orientation detectors, forming input-resistant ‘seeds’ in the cortical network, has been detected at an early stage of development (Frégnac & Imbert, 1978). Secondly, the proportion of modified cells might depend on the quality of control of the postsynaptic activity during the pairing procedure and on the presence of extraretinal gating factors. The comparison of intracellular pairing studies in the motor cortex of the cat (Baranyi & Szente, 1987), using a supervised learning scheme comparable to our S+ protocol, shows that the proportion of modifiable cells in awake cats was twice that observed in anaesthetized animals. Furthermore, the proportion of modifiable cells was higher when using single-electrode voltage clamp than when using current clamp to impose changes in membrane potential during the pairing procedure. These observations are compatible with the present state of knowledge on the role of postsynaptic membrane potential at the synaptic site in the induction of synaptic potentiation. Accordingly, one may reasonably assume that localized extracellular K+ iontophoresis (the present case) is less effective in producing synaptic potentiation than direct intracellular depolarization using single-electrode voltage clamp, and this might in fact lead to an underestimation of the proportion of modifiable cells in our study.
We confirm that visual cortical cells retain a potential for plasticity throughout postnatal life, which can be revealed even in adulthood using associative supervised learning protocols. Although the probability of observing a significant change after conditioning was similar in young kittens (4-15 weeks) and in adult or juvenile animals outside the critical period (from 16 weeks to adulthood), the amplitude of the effect in the youngest kittens (4-6 weeks) was more pronounced than in older animals (compare Figs 5 and 6). Similar observations have already been reported in our previous study concerning the plasticity of orientation tuning and ocular dominance (Frégnac et al. 1992; Shulz & Frégnac, 1992).
Before discussing the implication of our findings in terms of cellular mechanisms involved during functional epigenesis of visual cortical RFs, and presenting an alternative model of synaptic organization which could provide the adequate substrate to account for such plasticity, we will briefly review the arguments favouring the associative nature of the functional changes. We will compare the present evidence for a synaptic basis of the plasticity of ‘on’ and ‘off’ visual responses with previous data in the literature.
Associative features of the effects of conditioning
Spatial input specificity
The effects induced by our pairing procedures were restricted to the position that was stimulated during conditioning in almost two-thirds of cases (62%). This spatial limitation favours the conclusion that pairing-induced response modulations proceed from regulation of the efficacy of transmission of the synapses activated during conditioning (homosynaptic plasticity). In most other cases, the modification affected the paired and unpaired positions equally. It can thus be hypothesized that spatial selectivity of the induced modifications, where it exists, reflects the spatial separation of the inputs activated within or across ‘on’ and ‘off’ fields. The spatial selectivity probably depends mainly on the relative location and overlap of the RFs of the ‘on’ and ‘off’ centre afferent cells projecting to the recorded cortical cell, which were activated by visual stimulation in the paired and the other unpaired positions. However, the number of parallel independent feedforward input lines activated when exploring adjacent positions in an RF is not known in our experimental conditions. The mean number of geniculate RFs converging to form an ‘on’ or ‘off’ subfield in first-order cortical cells has been estimated as being close to ten (Tanaka, 1983; Reid & Alonso, 1995), but the spatial distribution of the RF centres of all input cells (geniculate and cortical) which participate in the response of second-order cortical cells, and their dominant type of activation (‘on’, ‘off’, complex ‘on-off’), remains largely undocumented.
Changes in the spatial distribution of ‘on’ and ‘off’ responses observed over the width of simple S2 receptive fields (see Figs 6 and 7) suggest that the spatial spread of the modified zone may in certain cases be larger than the ‘on’ or ‘off’ subfields assessed extracellularly, and may extend beyond the paired position region itself. In these conditioned cells, the positively reinforced characteristic (S+) did not evoke any response either before or after pairing in the position used for the conditioning. Despite the absence of modification of the LCR measured in the paired region, a push-pull behaviour of ‘on’ and ‘off’ responses was found across the subfields of the RF. Although not specific to the paired position, these changes are still thought to be associative in nature, since (i) modifications in GCR were never observed spontaneously during long-duration control recordings (Fig. 2A), and (ii) they differed from the covariation of ‘on’ and ‘off’ responses when postsynaptic firing was manipulated in a non-associative way, by passing constant current through the recording electrode irrespective of the visual stimulation (Fig. 2B).
These effects may at first glance appear paradoxical since on the one hand the spatial separation of the RF in ‘on’ and ‘off’ zones is maintained whereas on the other hand, a significant change in the balance between ‘on’ and ‘off’ responses is observed. The absence of position specificity in the induced modifications can however be explained by assuming the existence of subthreshold excitatory input covering both ‘on’ and ‘off’ zones, whose functional expression is normally masked by local intracortical inhibition. A simple hypothesis would be that, for a given ‘on’ or ‘off’ transition of the stimulus, various locations in the RF (even situated in antagonistic subfields) feed a common excitatory interneuron in a graded manner, and this interneuron then synapses with the recorded cell. According to the theory of generalized covariance plasticity, the pairing procedure in the conditioned position would potentiate only the synapses made by this hypothetical summating interneuron. The functional modification would be apparent only in the regions of the RF where the opponent inhibition for the tested ‘on’ or ‘off’ characteristic is absent, or too weak, to mask the excitation coming from the interneuron.
Temporal associativity
Several observations suggest that there is temporal associativity underlying the plasticity processes. Most changes in responsiveness after conditioning were found in the precise temporal window (of the stimulation cycle) during which the conditioning current had been applied. Furthermore, modifications in late components of ‘on’ (1 cell) and ‘off’ responses (3 cells) were induced when the iontophoretic action was delayed by 500-1000 ms following the onset or offset of the stimulus. In particular, lagged ‘off’ responses were revealed following FDP protocols for latencies corresponding precisely to the onset of imposed firing during the pairing itself (Figs 11 and 12).
In order to explain the very precise time locking of the potentiated response, a first interpretation could be that the modified temporal response profile corresponds to the potentiation of a late subthreshold ‘on’ or ‘off’ response. This would involve polysynaptic reverberating activity, recruiting synaptic circuits different to those activated during the early component of the visual response. Such long-latency delayed activation has been observed by Shinkman and colleagues, who showed that the presentation of a spot of light for a period as short as 25 ms was sufficient to influence cortical activity in a phase-locked manner for several hundreds of milliseconds (Shinkman, Bruce & Pfingst, 1974). The plasticity of long-latency responses revealed by our FDP protocol, in addition shares some similarity with the effects of the global intracranial reinforcement schedule engineered by these authors. Using an operant conditioning procedure in the paralysed but unanaesthetized cat, they demonstrated that long-latency responses (300-800 ms) of visual cortical neurons could be selectively potentiated after repeated reinforcement (by electrical stimulation of the lateral hypothalamus) of all activity peaks arising at the same latency that had reached a preset level of firing.
A simpler but related hypothesis could be that the potentiation of late responses demonstrated in Figs 11 and 12 corresponds to a non-specific temporal synchronization of the response with the cycle of visual stimulation. Indeed the temporal conditioning with full-field stimuli periodically flashed ‘on’ and ‘off’ at low temporal frequency (0.1-0.25 Hz) induces the progressive build-up of rebound synchronized long-latency activity in kitten visual cortex (Frégnac & Imbert, 1984). However, in this latter case changes in visual activity appeared unrelated to the spatial structure of the RF. These effects differ from the present situation, where the delayed activation patterns we observed were apparent only in the paired position of the RF, and were absent during interleaved stimulation of unpaired regions of the RF. In addition the latency of the late ‘off’ responses in the present experiments was not affected by the rate of stimulation. This demonstrates that the increase in activity was phase-locked with the ‘off’ characteristic of the visual stimulus and unrelated to the later onset of the subsequent stimulation.
Another possibility arises from the atypical visual latency of geniculate ‘lagged’ afferents originally described by Mastronarde (1987), which present delayed spike activation due to the presence of early intrageniculate inhibition. FDP protocols could potentiate synapses between lagged lateral geniculate nucleus (LGN) cells and cortical neurons, whose functional influence is occasionally recorded in young kitten cortex independently of any pairing procedures (see Fig. 11B).
A final scenario assumes that the build-up of delayed activity induced by FDP is the result of adaptive changes regulating the expression of intrinsic conductances. Conductance-based models have been proposed to account for the persistent firing described mainly in frontal and inferotemporal neurons but occasionally observed in cells recorded in monkey primary visual cortex V1 cells during memory tasks (J. M. Fuster, personal communication). Recent simulations show that activity-dependent kinetics of the slowly inactivating potassium IKs conductance and the persistent non-inactivating sodium INap conductance may be used to generate a sustained activity as a response to a transient input (Delord, Klaasen, Burnod, Costalat & Guigon, 1998). The latency of the onset of activity is controlled by the relative balance of the two conductances. This theoretical view is supported by the demonstration that repeated intracellular depolarizations induce a gradual reduction in the delayed activation of prefrontal neurons and can favour spontaneous plateau depolarizations lasting for several seconds (Hammond & Crépel, 1992). Interestingly, these authors showed that a brief hyperpolarizing pulse was sufficient to reset the conductances in their original states. In our case, this resetting could be produced by the synaptic inhibitory input elicited by the next (antagonizing) stimulus transition, which may explain the silencing (or suppression) of the potentiated response occurring abruptly at the following ‘on’ transition of the stimulus (examples in Fig. 11A and B). Adding a spatial restriction to the localization of dendritic conductance changes (Lytton & Sejnowski, 1991), thus making it input-specific, could account for the complex functional modifications induced by FDP pairings.
Synaptic events underlying functional plasticity
Potentiation of visual response
Several studies of synaptic plasticity are based on high-frequency tetanus of white matter or intracortical afferents in visual cortex, or on low-frequency pairing of synaptic activation with postsynaptic depolarization (Frégnac, Burke, Smith & Friedlander, 1994). They demonstrate the role of positive covariance between pre- and postsynaptic activity in the induction of long-term potentiation or transient facilitation of excitatory neocortical synapses. The depolarization of the postsynaptic neuron during our S+ pairings most likely causes a large postsynaptic calcium influx through the ionophore channel associated with the NMDA receptor and/or through voltage-dependent calcium channels, leading to potentiation of the active synapses. A large calcium influx could also be induced in our protocols during the activity rebound produced by withdrawal of the hyperpolarizing current step used during S− pairing. Low-threshold calcium spikes (LTS) are known to favour the induction of synaptic potentiation (Komatsu & Iwakiri, 1992).
Depression of visual response
While potentiation of the S+ paired responses agrees with classical schemes of Hebbian plasticity, depression in response to S− paired stimuli may at first glance seem paradoxical in view of the strong dogma that inhibition freezes the induction of synaptic plasticity. However, similar reductions in sensory responses have been observed in vivo in cortical neurons, following pairing of the sensory input with a blockade of firing imposed by a field effect (Frégnac et al. 1988; Cruikshank & Weinberger, 1996). Depression of composite synaptic potentials has been demonstrated in vitro following repeated association of low-frequency stimulation of a test synaptic pathway with hyperpolarizing pulses of current injected in the postsynaptic neuron (Frégnac et al. 1994; see also Fig. 3 in Stanton & Sejnowski, 1989) or with the concomitant activation of an inhibitory pathway (Thiels, Barrionuevo & Berger, 1994).
Our experiments may also involve another form of associative synaptic depression specifically linked to the asynchronous nature of our protocol: the two stimuli, associated respectively with ‘high’ and ‘low’ levels of response, are applied sequentially at fixed intervals. It has been shown in the CA1 field of hippocampal organotypic cultures (Debanne, Gähwiler & Thompson, 1994) that the delayed activation of a set of synapses following intracellular application of strong depolarizing current pulses in the postsynaptic cell (by up to 1600 ms depending on the duration of the unconditioned postsynaptic activation) resulted in a long-lasting decrease in the synaptic efficacy of the test pathway. This situation may share some similarities with our protocol, since in some of our conditioned cells, one visual characteristic was positively covaried with the postsynaptic activity of a cortical neuron while a few seconds later, the other visual characteristic was unpaired or negatively covaried with the postsynaptic activity (S+/S−). However the degree of involvement of such an adaptive process in the functional changes reported here remains a matter of debate for the following reasons: (i) associative depression appears to be less apparent in the in vitro or in vivo preparation than in organotypic cultures which may amplify the expression of competitive growth processes; (ii) the temporal delay between S+ and the presentation of the second stimulus used in our experiments was significantly longer (1-5 s) than that reported to be optimal in the hippocampal organotypic culture; (iii) if S+-induced heterosynaptic plasticity was a major source of synaptic changes produced during differential pairing, one would have expected a similar rate of change following S+S− or S+S0 protocols, which was not the case. Furthermore no depression per se of the response to the unpaired stimulus (S0) was observed following the latter type of pairing.
Our data in fact confirm our previous claim that the additional constraint of a reduced postsynaptic activity (S−) for one of the two stimuli appears to be a more favourable protocol for inducing changes (Frégnac et al. 1988). The most probable mode of action of negative current applied extracellularly during S− pairing is the hyperpolarization of the recorded cell by field effect (Andrew & Fagan, 1990), which in certain cases effectively blocks all discharge in response to the preferred stimulus. Since it has been shown that the calcium rise evoked by concomitant synaptic activation is reduced when the cell is kept hyperpolarized (Miyakawa et al. 1992; see also Fig. 2B in Magee & Johnston, 1997), S− pairing may lead to homosynaptic depression by reducing the amplitude of the intracellular calcium concentration, which is normally evoked by visual stimulation, below a critical threshold that has yet to be defined.
In a small number of cells, the functional modification favoured the response to the S− paired stimulus. This kind of anti-Hebbian plasticity has been reported mostly in inhibitory output cells in other cortical structures (e.g. cerebellum and electrosensory lobe of the electric fish, see review in Frégnac, 1995) and its existence in a particular class of visual cortical cells cannot be excluded. It is also possible that if the iontophoretic action of the S+ pairing were less effective in raising the level of visual discharge than initially planned, activity might remain below the critical threshold for the calcium surge necessary to induce long-term potentiation (LTP) and thus depression of the response, rather than strengthening, might be observed (Artola & Singer, 1993).
A possible role of inhibitory plasticity
Up to now we have tried to explain the functional modifications on the sole basis of the plasticity of excitatory synapses. However, since inhibitory intracortical circuits are also recruited following geniculate afferent activation, in particular when stimulating simple RF subfields, one cannot exclude a depression of the co-activated inhibitory component as a substrate for potentiation of the visual response. Theoreticians, ahead of experimenters, proposed symmetrical plasticity rules for inhibitory synapses: forced periods of co-activation would induce the reinforcement of functional links between neurons by the combined interplay of increase in excitatory synaptic gain and decrease in inhibitory synaptic gain (Stent, 1973). Recent data (see below) obtained in visual cortex and hippocampal slices support such complementary rules of plasticity.
Let us consider first the S+ pairing effect. When synaptic input is paired with depolarization of the postsynaptic cell, the evoked depolarizing responses may be large enough to activate NMDA receptor channels and lead to depression of inhibition (Komatsu, 1994). Such a process could help to potentiate responses to the S+ stimulus in our in vivo protocols. An illustration can be seen in the ‘on’ response in Fig. 4 where the transient reduction of discharge following the onset of the stimulus is no longer apparent after an S+ pairing, suggesting a depression of short-latency inhibition.
The S− situation may potentially put into play different types of GABA receptors. When synaptic input is paired with hyperpolarization, visual stimulation may be unable to activate NMDA receptor channels, but can still potentiate concomitantly elicited GABAB IPSPs. The interpretation given by Komatsu (1994) of our own experimental paradigm (in the context of our previous orientation preference conditioning study) is based on the fact that the LTP of inhibitory synapses he describes is input-specific and independent of the postsynaptic membrane potential (Komatsu, 1994). As a result, since the voltage condition imposed during our S− pairing protocol blocks the induction of LTP of excitatory synapses, LTP of IPSPs will predominate: consequently, the composite response to the paired stimulus - combining excitatory and inhibitory inputs - becomes depressed. In the cell illustrated in Fig. 10, the tonic ‘on’ response after pairing is antagonized by a powerful inhibition that was virtually absent before the S− pairing (see a similar effect of an S− protocol applied in vitro in Fig. 4 in Frégnac et al. 1994).
In the case of GABAA inhibition, a more graded framework can be put forward. In visual cortex, the efficacy of GABAA inhibitory synapses has been shown to be increased or decreased depending on whether the postsynaptic calcium influx is mediated respectively by the mobilization of intracellular stores (Komatsu, 1996) or by the activation of NMDA receptors (Komatsu, 1994). More recently, a similar dependence on the origination of free calcium has been described in neonate slices of hippocampus in order to induce long-term depression (LTD) or LTP of inhibitory transmission (McLean, Caillard, Ben-Ari & Gaiarsa, 1996).
On the basis of these data, we propose a more general plasticity algorithm that would apply for most cortical neurons: patterns of weak afferent stimulation - or, in our case, pairing of strong input with hyperpolarization (S−) - both of which would result in a moderate increase in the postsynaptic concentration of free calcium during synaptic activation, would promote parallel LTD at the excitatory input and LTP of inhibitory synapses. The combined effect would be a net weakening of the apparent efficacy of the conditioned pathway. In contrast, patterns of stimulation - or, in our case, S+ pairing - which may be intense enough to activate NMDA receptors and induce a high intracellular concentration of free calcium, would favour both potentiation of excitatory synapses and depression of inhibitory ones. The combined effect would be a net strengthening of the apparent efficacy of the conditioned pathway. Although at each level of intracellular surge of free calcium the changes in the synaptic gain of excitatory and inhibitory pathways are of the opposite sign, the two effects add up at the postsynaptic level in such a way that the induced functional modification of the apparent coupling of the conditioned pathway benefits synergistically from both types of plasticity. Such mechanisms could play an important role in controlling the balance between excitatory and inhibitory events, thus affecting the size of the composite test postsynaptic potential in a reversible manner.
‘Simplex’ model
The modulation of ‘on-off’ responses in the RF is a rather unusual concept compared to classical models of RF organization (Hubel & Wiesel, 1962; Heggelund, 1981; Palmer & Davis, 1981; Heggelund, 1986; Ferster, 1988; Reid & Alonso, 1995). The cited studies support the view that ‘on’ or ‘off’ subfields in simple RFs are generated by excitatory inputs from geniculate neurons of the same centre type and receive spatially opponent inhibition from other cortical simple cells. They predict an absence of effect for our pairing procedures, since there is no position corresponding to a mixing of excitatory ‘on’ and ‘off’ inputs.
Our plasticity data suggest a somewhat revised model of cortical RF (the ‘simplex’ model, Fig. 13A) in which we have tried to incorporate the ‘canonical’ circuit organization proposed by Douglas & Martin (1991). For the simplicity of the diagram, only the connectivity outline for granular and supragranular cells is shown, but the proposed connectivity could be extended to infragranular cells as well. Intracolumnar connections between supra- and infragranular cells are not included in this model, since they are not required in our hypothetical schema. The basic framework of geniculocortical connections is assumed to be of a complex type and each position is fed by direct parallel ‘on’ and ‘off’ inputs: LGN ‘on’ centre and ‘off’ centre cells make contact, with variable efficacy, with three types of cortical targets (smooth interneurons, spiny stellate and spiny pyramidal cells). Layer IV spiny stellate cells and layer II-III spiny pyramidal cells are grouped together for simplicity in the block diagram of Figure 13A illustrates the possible final simple state emerging from a mixed ‘on-off’ connectivity scheme with unequal synaptic weights (symbolized by the different diameters of the presynaptic terminals), thus imposing in each position a bias towards ‘on’ or ‘off’ activation. Reciprocal excitatory connections between cortical cells also convey mixed ‘on’ and ‘off’ information (open triangles, Fig. 13A).
Figure 13. The ‘simplex’ model.
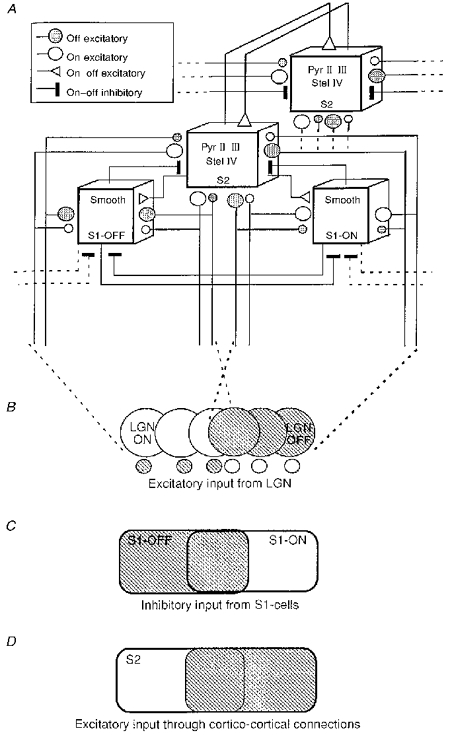
A, connectivity scheme: the model assumes that two types of thalamic inputs (‘on’ centre represented by ○, and ‘off’ centre by  ) converge monosynaptically or disynaptically (through inhibitory smooth interneurons of the S1 class) on layer IV spiny stellate cells and layer II-III pyramidal cells of the S2 class. The size of each symbol represents the relative efficacy of synaptic transmission. For the sake of clarity only a subset of the global envelope of geniculate inputs is represented. Excitatory intracortical connections which convey mixed ‘on’ and ‘off’ information are represented by open triangles. The two S2 cells linked by reciprocal connections belong to the same isofunctional column and share common inputs of both geniculate and cortical origin. The RFs depicted in B-D represent the spatial distribution of excitatory and inhibitory inputs to the S2 cell. B, the distribution of RF centres of a small proportion of the geniculate inputs to the S2 cell are shown as a cross-section of the cortical RF width. The RFs of strong and weak excitatory LGN afferents, the size of which indicates their relative synaptic efficacy, were displaced vertically for the sake of clarity. Each position of the RF of the cortical cell is fed by both ‘on’ and ‘off’ inputs. C, S1 cells also receive mixed ‘on’ and ‘off’ inputs from the LGN. However, one of these inputs predominates over the entire RF, the ‘off’ for the S1 ‘off’ cell type and the ‘on’ for the S1 ‘on’ cell type. Reciprocal inhibitory connections (thick terminal segments) between these cells are responsible for their antagonist response. In addition, these cells receive excitatory intracortical input from spiny S2 cells, and through a local recurrent loop send an inhibitory feedback onto the same group of cells. D, supragranular and granular cells of the S2 type make reciprocal connections with each other. The S2 RF schematized here has separate ‘on’ and ‘off’ fields and a central overlap zone of variable extent, characteristic of simple cells. Since the two S2 cells share common inputs, their RFs are identical.
) converge monosynaptically or disynaptically (through inhibitory smooth interneurons of the S1 class) on layer IV spiny stellate cells and layer II-III pyramidal cells of the S2 class. The size of each symbol represents the relative efficacy of synaptic transmission. For the sake of clarity only a subset of the global envelope of geniculate inputs is represented. Excitatory intracortical connections which convey mixed ‘on’ and ‘off’ information are represented by open triangles. The two S2 cells linked by reciprocal connections belong to the same isofunctional column and share common inputs of both geniculate and cortical origin. The RFs depicted in B-D represent the spatial distribution of excitatory and inhibitory inputs to the S2 cell. B, the distribution of RF centres of a small proportion of the geniculate inputs to the S2 cell are shown as a cross-section of the cortical RF width. The RFs of strong and weak excitatory LGN afferents, the size of which indicates their relative synaptic efficacy, were displaced vertically for the sake of clarity. Each position of the RF of the cortical cell is fed by both ‘on’ and ‘off’ inputs. C, S1 cells also receive mixed ‘on’ and ‘off’ inputs from the LGN. However, one of these inputs predominates over the entire RF, the ‘off’ for the S1 ‘off’ cell type and the ‘on’ for the S1 ‘on’ cell type. Reciprocal inhibitory connections (thick terminal segments) between these cells are responsible for their antagonist response. In addition, these cells receive excitatory intracortical input from spiny S2 cells, and through a local recurrent loop send an inhibitory feedback onto the same group of cells. D, supragranular and granular cells of the S2 type make reciprocal connections with each other. The S2 RF schematized here has separate ‘on’ and ‘off’ fields and a central overlap zone of variable extent, characteristic of simple cells. Since the two S2 cells share common inputs, their RFs are identical.
While the simplex model incorporates the connectivity pattern of Hubel & Wiesel's simple cells, it should be noted that it predicts a distinct outcome in the case of a blockade of intracortical inhibition which, according to our model, should unmask ‘on’ and ‘off’ responses in all subfields. The modification of simple RFs into complex ones and the loss of antagonist responses observed under bicuculline application with extracellular (Sillito, 1975; Ramoa, Paradiso & Freeman, 1988) and intracellular (Shulz et al. 1993) recording techniques, as well as during intracellular blockade of inhibition (Nelson, Toth, Sheth & Sur, 1994), strongly support this view.
The two major assumptions made here link the number of zones expressed by cortical simple RFs to their ordinal position in the canonical Douglas & Martin microcircuit. The first assumption states that unimodal first-order S1 RFs would be predominantly of the smooth cell type. The second is that RFs of stellate and pyramidal cells are of the S2 type. As shown in Fig. 13C, inhibitory antagonist responses in S1 cells are obtained through the reciprocal inhibitory connections between S1 ‘on’ and S1 ‘off’ cells with partially overlapping RFs. Depending on the degree of overlap and the presence of lateral inhibitory connections with other S1 cells (see dotted lines in Fig. 13A) the model predicts that the minimal discharge field of these cells would be flanked by inhibitory zones responding only to the antagonistic transition of the stimulus. Such RFs were described by Palmer & Davis (1981; their Figs 3 and 4). The antagonistic inhibitory responses of layer IV spiny stellate cells and layers II-III pyramidal cells are obtained through separate connections with smooth inhibitory cells of the S1 type (Fig. 13C). All the simplex cells of the smooth, spiny stellate and pyramidal type included in the model receive mixed ‘on’ and ‘off’ excitatory inputs from every position in the RF, which could drive the cell in the absence of intracortical inhibition.
In order to account for their adaptive properties, as described in the previous section, the rules of plasticity at excitatory and inhibitory synapses are assumed to obey a generalized covariance scheme: an increase in covariance between pre- and postsynaptic activity leads to the strengthening of excitatory synapses (rule 1) and weakening of inhibitory ones (rule 2), whereas converse effects are induced by negative changes in covariance levels (rule 3, depression of excitatory synapses and rule 4, potentiation of inhibitory synapses). This set of rules allows us to compare qualitatively the predictions of the model with the spatial spread of the changes in the ‘on-off’ balance induced by our pairing procedures.
Prediction 1: input separability in S1 RF
Because of the spatial separation of ‘on’ and ‘off’ input assumed at the level of the smooth interneurons, an initially ‘on’ S1 RF (e.g. the smooth S1 ‘on’ cell in the microcircuit drawn in Fig. 13A) can be changed into a complex RF by an S−-‘on’,S+-‘off’ pairing procedure, due to selective potentiation (rule 1) and depression (rule 3) of excitatory inputs. Such a response modulation is shown in Fig. 3, where the effect of the pairing is restricted to the paired position of the RF, strongly suggesting that the unpaired position is fed by separate afferents whose synaptic influence remained unchanged due to the fact that these were silent during pairing. Similarly, an initially S1 ‘off’ RF (e.g. the smooth S1 ‘off’ cell of Fig. 13A) can be modified to become a complex-like RF by selectively potentiating the initially subthreshold ‘on’ input and depressing the initially dominant ‘off’ response. Two such examples are illustrated in Figs 5 and 6.
Prediction 2: spatial gradient of the pairing effects in S2 subfields
In most cases of simple RFs composed of several antagonistic subfields, as seen in the discharge patterns (see extracellular examples in Figs 7 and 8), the effect of the pairing did spread over to unpaired positions of the RF. In the example presented in Fig. 7, the S− pairing induces a reduction of the ‘on’ response which is generalized to the whole ‘on’ field of this S2 cell (corresponding to the group of spiny stellate and pyramidal cells in the model). This behaviour can be simulated by the depression (rule 3) of the efficacy of reciprocal connections between supragranular layer cells in the model, in addition to the specific depression (rule 3) of the direct LGN pathway activated during negative covariance pairing. The hypothesis proposed here is equivalent to the general ‘excitatory interneuron’ hypothesis on spatial input specificity discussed earlier. A second observation, made for the same cell, is that the effect of the ‘off’-S+ pairing applied in the same position of the RF is shown to generalize only to the pre-existing ‘off’ field. No de novo ‘off’ response appears in the ‘on’ field (Fig. 7). This effect can be simulated by the model if one assumes that the inhibitory link between the S1 ‘off’ smooth cell and the conditioned cell is depressed (rule 2) because of the concomitant activation of these connections and the iontophoretic depolarization of the recorded cell during pairing. Inhibition fed by the smooth cell is assumed to be still strong enough to counteract the weak excitatory LGN ‘off’ input in the paired position, but should be much less effective in counteracting the strong LGN ‘off’ input provided by the adjacent ‘off’ field. The delayed activation of this inhibitory loop with respect to the direct excitatory afferents also predicts an increase in the late or tonic component of the ‘off’ response as seen in Fig. 7 (PSTHs of positions 6-8). A complementary explanation of these heteropositional effects is that both the paired region and the unpaired ‘off’ field provide a common subthreshold excitatory input. Its potentiation following the S+ pairing (rule 1) would be masked by antagonistic inhibition in the paired position and be expressed only in the ‘off’ subfield where the inhibition is not present. A similar reasoning could be applied to explain depression effects generalized to the ‘on’ field following the S−-‘on’ pairing (rule 3).
In conclusion, the results presented in this study support the hypothesis that simple and complex RFs, classically considered as two exclusive classes at the adult stage, could arise from a common omnipotent simplex connectivity scheme. After a developmental stage during which activity-dependent processes progressively shape the spatial structure of the cortical RFs, only a restricted part of the connections would be functionally expressed. The connections not recruited by simple RFs might still be anatomically present, but would be depressed or masked by inhibitory processes. Nevertheless, it remains plausible that later coherent changes in co-activity levels, induced by anomalous visual experiences or imposed through learning, might reveal local adaptation in the complexity ratio expressed in subregions of the RF, even at the adult age.
Acknowledgments
This work was funded by the Human Frontier Science Program (RG 69-93) and CNRS Cognisciences grants to Y. F. D. D. was supported by the French Ministry of Research and Technology, and by the Singer Polignac Foundation during his PhD studies. We thank Nicolas Gazères, Lyle Borg-Graham and Paul Salin for helpful comments on the manuscript and Kirsty Grant for help with the English.
References
- Albus K, Wolf W. Early postnatal development of neuronal function in the kitten visual cortex: a laminar analysis. Journal of Physiology. 1984;349:153–185. doi: 10.1113/jphysiol.1984.sp015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R D, Fagan M. A technique for controlling the membrane potential of neurons during unit recording. Journal of Neuroscience Methods. 1990;33:55–60. doi: 10.1016/0165-0270(90)90082-q. 10.1016/0165-0270(90)90082-Q. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends in Neurosciences. 1993;16:480–487. doi: 10.1016/0166-2236(93)90081-v. 10.1016/0166-2236(93)90081-V. [DOI] [PubMed] [Google Scholar]
- Baranyi A, Szente M B. Long-lasting potentiation of synaptic transmission requires postsynaptic modifications in the neocortex. Brain Research. 1987;423:378–384. doi: 10.1016/0006-8993(87)90867-5. [DOI] [PubMed] [Google Scholar]
- Bowling D B, Caverhill J I. ON/OFF organization in the cat lateral geniculate nucleus: sublaminae versus columns. Journal of Comparative Neurology. 1989;283:161–168. doi: 10.1002/cne.902830114. [DOI] [PubMed] [Google Scholar]
- Cruikshank S J, Weinberger N M. Receptive field plasticity in adult auditory cortex induced by Hebbian covariance. Journal of Neuroscience. 1996;16:861–875. doi: 10.1523/JNEUROSCI.16-02-00861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Gähwiler B H, Thompson S M. Asynchronous presynaptic and postsynaptic activity induces associative long-term depression in area CA1 of the rat hippocampus in vitro. Proceedings of the National Academy of Sciences of the USA. 1994;91:1148–1152. doi: 10.1073/pnas.91.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delord B, Klaassen A J, Burnod Y, Costalat R, Guigon E. Bistable behavior in a neocortical neuron model. NeuroReport. 1998;8:1019–1023. doi: 10.1097/00001756-199703030-00040. [DOI] [PubMed] [Google Scholar]
- Douglas R J, Martin K A C. A functional microcircuit for cat visual cortex. Journal of Physiology. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. Spatially opponent excitation and inhibition in simple cells of the cat visual cortex. Journal of Neuroscience. 1988;8:1172–1180. doi: 10.1523/JNEUROSCI.08-04-01172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frégnac Y. Comparative and developmental aspects of Hebbian synaptic plasticity. In: Arbib M, editor. Handbook of Brain Theory and Neural Networks. USA: MIT Press; 1995. pp. 459–464. [Google Scholar]
- Frégnac Y, Burke J, Smith D, Friedlander M J. Temporal covariance of pre- and postsynaptic activity regulates functional connectivity in the visual cortex. Journal of Neurophysiology. 1994;71:1403–1421. doi: 10.1152/jn.1994.71.4.1403. [DOI] [PubMed] [Google Scholar]
- Frégnac Y, Imbert M. Early development of visual cells in normal and dark reared kittens: relationship between orientation selectivity and ocular dominance. Journal of Physiology. 1978;278:27–44. doi: 10.1113/jphysiol.1978.sp012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frégnac Y, Imbert M. Development of neuronal selectivity in the primary visual cortex of the cat. Physiological Reviews. 1984;64:325–434. doi: 10.1152/physrev.1984.64.1.325. [DOI] [PubMed] [Google Scholar]
- Frégnac Y, Shulz D. Models of synaptic plasticity and cellular analogs of learning in the developing and adult vertebrate visual cortex. In: Casagrande V, Shinkman P, editors. Advances in Neural and Behavioral Development. New Jersey, USA: Neural Ablex Publishers; 1994. pp. 149–235. [Google Scholar]
- Frégnac Y, Shulz D, Thorpe S, Bienenstock E. A cellular analogue of visual cortical plasticity. Nature. 1988;333:367–370. doi: 10.1038/333367a0. 10.1038/333367a0. [DOI] [PubMed] [Google Scholar]
- Frégnac Y, Shulz D, Thorpe S, Bienenstock E. Cellular analogs of visual cortical epigenesis: I. Plasticity of orientation selectivity. Journal of Neuroscience. 1992;12:1280–1300. doi: 10.1523/JNEUROSCI.12-04-01280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Crépel F. Evidence for a slowly inactivating K+ current in prefrontal cortical cells. European Journal of Neuroscience. 1992;4:1087–1092. doi: 10.1111/j.1460-9568.1992.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Hammond P, McKay D M. Differential responsiveness of simple and complex cells in cat striate cortex to visual texture. Experimental Brain Research. 1977;30:275–296. doi: 10.1007/BF00237256. [DOI] [PubMed] [Google Scholar]
- Hebb D O. The Organization of Behavior. New York: J. Wiley and Sons; 1949. [Google Scholar]
- Heggelund P. Receptive field organization of simple cells in cat striate cortex. Experimental Brain Research. 1981;42:89–98. doi: 10.1007/BF00235733. [DOI] [PubMed] [Google Scholar]
- Heggelund P. Quantitative studies of the discharge fields of single cells in cat's striate cortex. Journal of Physiology. 1986;373:277–292. doi: 10.1113/jphysiol.1986.sp016047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D H, Wiesel T N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. Journal of Physiology. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y. Age-dependent long-term potentiation of inhibitory synaptic transmission in rat visual cortex. Journal of Neuroscience. 1994;14:6488–6499. doi: 10.1523/JNEUROSCI.14-11-06488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y. GABAb receptors, monoamine receptors and postsynaptic inositol trisphosphate-induced Ca2+ release are involved in the induction of long-term potentiation at visual cortical inhibitory synapses. Journal of Neuroscience. 1996;16:6342–6352. doi: 10.1523/JNEUROSCI.16-20-06342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Iwakiri M. Low-threshold Ca2+ channels mediate induction of long-term potentiation in visual kitten cortex. Journal of Neurophysiology. 1992;67:401–410. doi: 10.1152/jn.1992.67.2.401. [DOI] [PubMed] [Google Scholar]
- Lytton W W, Sejnowski T J. Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. Journal of Neurophysiology. 1991;66:1059–1079. doi: 10.1152/jn.1991.66.3.1059. [DOI] [PubMed] [Google Scholar]
- McLean H A, Caillard O, Ben-Ari Y, Gaiarsa J L. Bidirectional plasticity expressed by GABAergic synapses in the neonatal rat hippocampus. Journal of Physiology. 1996;496:471–477. doi: 10.1113/jphysiol.1996.sp021699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J C, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Marlin S G, Douglas R M, Cynaner M S. Position-specific adaptation in simple cell receptive fields of the striate cortex. Journal of Neurophysiology. 1991;66:1769–1784. doi: 10.1152/jn.1991.66.5.1769. [DOI] [PubMed] [Google Scholar]
- Mastronarde D N. Two classes of single-input X-cells in cat lateral geniculate nucleus. I. Receptive-field properties and classification of cells. Journal of Neurophysiology. 1987;57:357–380. doi: 10.1152/jn.1987.57.2.357. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Ross W N, Jaffe D, Callaway J C, Lasser-Ross N, Lisman J E, Johnston D. Synaptically activated increases in Ca2+ concentration in hippocampal CA1 pyramidal cells are primarily due to voltage-gated Ca2+ channels. Neuron. 1992;9:1163–1173. doi: 10.1016/0896-6273(92)90074-n. [DOI] [PubMed] [Google Scholar]
- Nelson S, Toth L, Sheth B, Sur M. Orientation selectivity of cortical neurons during intracellular blockade of inhibition. Science. 1994;265:774–777. doi: 10.1126/science.8047882. [DOI] [PubMed] [Google Scholar]
- Palmer L A, Davis T A. Receptive field structure in cat striate cortex. Journal of Neurophysiology. 1981;46:260–295. doi: 10.1152/jn.1981.46.2.260. [DOI] [PubMed] [Google Scholar]
- Pettigrew J D, Freeman R D. Visual experience without lines: effect on developing cortical neurons. Science. 1973;182:599–601. doi: 10.1126/science.182.4112.599. [DOI] [PubMed] [Google Scholar]
- Ramoa A S, Paradiso M A, Freeman R D. Blockade of intracortical inhibition in kitten striate cortex: effects on receptive field properties and associated loss of ocular dominance plasticity. Experimental Brain Research. 1988;73:285–296. doi: 10.1007/BF00248220. [DOI] [PubMed] [Google Scholar]
- Reid R C, Alonso J-M. Specificity of monosynaptic connections from thalamus to visual cortex. Nature. 1995;378:281–284. doi: 10.1038/378281a0. 10.1038/378281a0. [DOI] [PubMed] [Google Scholar]
- Shinkman P G, Bruce C J, Pfingst B E. Operant conditioning of single-unit response patterns in visual cortex. Science. 1974;184:1194–1196. doi: 10.1126/science.184.4142.1194. [DOI] [PubMed] [Google Scholar]
- Shulz D, Bringuier V, Frégnac Y. Complex-like structure of simple visual cortical receptive fields is masked by GABAA intracortical inhibition. Society for Neuroscience Abstracts. 1993;19:628. [Google Scholar]
- Shulz D, Frégnac Y. Cellular analogs of visual cortical epigenesis: II. Plasticity of binocular integration. Journal of Neuroscience. 1992;12:1301–1318. doi: 10.1523/JNEUROSCI.12-04-01301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in striate cortex of the cat. Journal of Physiology. 1975;250:305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Tretter F. Unusually large receptive fields in cats with restricted visual experience. Experimental Brain Research. 1976;26:171–184. doi: 10.1007/BF00238281. [DOI] [PubMed] [Google Scholar]
- Singer W, Tretter F, Cynader M. Organization of cat striate cortex: a correlation of receptive field properties with afferent and efferent connections. Journal of Neurophysiology. 1975;38:1080–1098. doi: 10.1152/jn.1975.38.5.1080. [DOI] [PubMed] [Google Scholar]
- Stanton P K, Sejnowski T J. Associative long-term depression in the hippocampus induced by Hebbian covariance. Nature. 1989;339:215–218. doi: 10.1038/339215a0. 10.1038/339215a0. [DOI] [PubMed] [Google Scholar]
- Stent G. A physiological mechanism for Hebb's postulate of learning. Proceedings of the National Academy of Sciences of the USA. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. Cross-correlation analysis of geniculo-striate neuronal relationships in cats. Journal of Neurophysiology. 1983;49:1303–1318. doi: 10.1152/jn.1983.49.6.1303. [DOI] [PubMed] [Google Scholar]
- Thiels E, Barrionuevo G, Berger T W. Excitatory stimulation during postsynaptic inhibition induces long-term depression in hippocampus in vivo. Journal of Neurophysiology. 1994;71:3009–3016. doi: 10.1152/jn.1994.72.6.3009. [DOI] [PubMed] [Google Scholar]


