Abstract
Human hand vein endothelial cells were isolated from blood obtained by traumatic venepuncture. Cells were identified as endothelial by staining with endothelium-specific antibodies. The subject groups studied were (i) non-pregnant, (ii) pregnant (mean, 35 weeks gestation) and (iii) pre-eclamptic women (mean, 36 weeks gestation).
Fura-2 was used to measure agonist-induced responses in intracellular Ca2+ in single endothelial cells isolated and maintained in vitro. All of the cells examined responded to adenosine triphosphate (ATP) with a large transient increase in Ca2+ followed by a sustained plateau.
The responses to ATP were significantly larger in the cells from pregnant women than in those from non-pregnant and pre-eclamptic women, but no other differences were observed. The amplitudes of the responses to ATP were (means ± s.e.m.) 0.56 ± 0.04, 1.42 ± 0.24 and 0.65 ± 0.09 fura-2 ratio units for cells from non-pregnant, pregnant and pre-eclamptic subjects, respectively.
In cells isolated from non-pregnant subjects, the amplitude of the responses to carbachol, histamine and bradykinin were all smaller than those activated by ATP: 5.1, 13.9 and 4.4%, respectively. Not all cells responded to these agonists: 25% responded to carbachol, 70.5% responded to histamine and 12.5% responded to bradykinin. Sixty-five per cent of the cells from normotensive pregnant subjects responded to bradykinin compared with 25% in the non-pregnant and 13.9% in the pre-eclamptic subjects.
These data suggest that there may be differences in the responsiveness of venous endothelial cells in pregnancy and that pre-eclamptic cells behave differently.
During human pregnancy both blood volume and cardiac output increase (Robson, Hunter, Boys & Dunlop, 1989). These changes are accompanied by a fall in blood pressure indicative of a profound fall in peripheral resistance. Although there have been many attempts to identify the processes responsible for this fall in peripheral resistance the precise nature of the underlying mechanisms remain elusive (see Poston, McCarthy & Ritter, 1995). It is now widely accepted that the endothelium is likely to play a pivotal role in the normal vascular changes in pregnancy (Roberts, Taylor, Musci, Rodgers, Hubel & McLaughlin, 1989; Tsukimori, Maeda, Shingu, Koyanagi, Nobunaga & Nakano, 1992; Roberts & Redman, 1993). The physiological changes are consistent with the concept that the endothelium is ‘upregulated’ in pregnancy, thus producing an increased vasodilatation either as a result of an increase in the release of vasodilators or a decrease in vasoconstrictor output. An increase in endothelial cell activity in pregnancy has yet to be demonstrated directly. Consequently, the nature of the induced alterations and the processes which trigger such changes remain poorly understood (Poston et al. 1995).
Approximately 5% of primigravidae develop pre-eclampsia, a condition characterized by an elevated blood pressure and proteinuria and associated with substantial maternal mortality and fetal morbidity (Chesley, 1978; Campbell & MacGillivray, 1985; Davey & MacGillivray, 1988; Roberts & Redman, 1993). Despite the prevalence and clinical importance of pre-eclampsia, its aetiology remains poorly understood. If the endothelium plays a part in the normal cardiovascular changes in pregnancy it is a simple extension of this idea to propose that endothelial dysfunction might be one of the underlying causes of pre-eclampsia (Roberts et al. 1989). Morphological changes have been described in the endothelium in the kidney (Fisher, Luger, Spargo & Lindheimer, 1981), uterine spiral arteries (Robertson, Brosens & Dixon, 1967; Robertson & Khong, 1987) and umbilical vein (Cester et al. 1995), pointing to endothelial damage and dysfunction in pre-eclampsia. Endothelial dysfunction has been suggested from work on isolated blood vessels from normal pregnant women and those with pre-eclampsia when agonists induced relaxations to bradykinin (Knock & Poston, 1996) and to acetylcholine and histamine (Oguogho, Aloamaka & Ebeigbe, 1996). However, it is not known how any of these changes in endothelial morphology and function are brought about. There is some evidence, using animal endothelial cell models, for the presence of substances in the plasma of pre-eclamptic women which affect the endothelium. These experiments suggest that serum from pre-eclamptic women can activate endothelial cells (Davidge, Signorella, Lykins, Gilmour & Roberts, 1996). Experiments have also been done using fetal endothelial cells, human umbilical vein endothelial (HUVE) cells, and sera from non-pregnant, normal pregnant and pre-eclamptic women (Rodgers, Taylor & Roberts, 1988; Tsukimori et al. 1992). These studies, like those using animal cell models, tend to support the concept that pre-eclamptic serum contains substances which are toxic and reduce endothelial cell function. Recently, plasma levels of vascular endothelial growth factor (VEGF) have been reported to be elevated in pre-eclamptic women compared with normal pregnant controls. Consequently, it has been suggested that VEGF may be involved in endothelial dysfunction in pre-eclampsia (Sharkey et al. 1996).
Given the contradictory experimental evidence from animal experiments and fetal endothelium, there is a need to study directly endothelial cells from women undergoing a normal pregnancy and from women with pre-eclampsia. We have developed an approach which allows us to isolate endothelial cells from blood taken following a traumatic venepuncture. Using hand veins, endothelial cells can be isolated and maintained in vitro for physiological and pharmacological study. Samples can, in principle, be taken serially so that changes in the responsiveness of the endothelium can be studied in the same subject. In this paper we present data from endothelial cells isolated from non-pregnant women and from pregnant women who were assessed as normotensive or pre-eclamptic. These experiments have focused specifically on the ability of different agonists to induce a rise in intracellular Ca2+ which can be used as an indicator of cell activation. These data suggest that endothelial cells from women in late pregnancy, uncomplicated by pre-eclampsia, are more responsive to specific agonists than those of non-pregnant women. Furthermore, cells from pre-eclamptic women fail to show this pregnancy-induced alteration.
METHODS
Subject identification
Human hand vein endothelial (HHVE) cells were obtained from eight healthy non-pregnant volunteers (mean age, 30.9 ± 1.6 years). They were of parity 0 to 2 (median 0) and were on day 10 ± 1 of their menstrual cycles when the samples were taken. Their mean systolic and diastolic blood pressures were 106 ± 4 and 68 ± 3 mmHg, respectively. HHVE cells were obtained from twenty-three normal pregnant primigravidae (mean age, 27.6 ± 1.3 years) at 34.7 ± 0.8 weeks gestation. The mean systolic and diastolic blood pressures of this group, at the time of sampling, were 111 ± 2 and 66 ± 1 mmHg, respectively. Delivery took place at a median of 40.0 weeks gestation (range, 38.0-41.7), and the birth weight of their babies corresponded to 33 ± 6 centile. HHVE cells were also obtained from nine pre-eclamptic primigravid women (mean age, 25.9 ± 1.4 years) at a mean gestation of 36.2 ± 1.3 weeks. At the time of sampling, the mean systolic and diastolic blood pressures of this group were 147 ± 2 and 94 ± 1 mmHg, respectively, and mean proteinuria was 1.10 ± 0.24 g (24 h)−1. These pre-eclamptic subjects delivered at a median of 38.6 weeks gestation (range, 30.4-41.1 weeks) and the mean birth weight of their babies corresponded to 21 ± 7 centile.
Venepuncture technique and the isolation of endothelial cells
A tourniquet was placed on the left or right arm or forearm of the subject. A vein of sufficient bore (3-5 mm) to comfortably fit a 14G cannula (Venflon 2; Ohmeda, Helsingborg, Sweden) was identified on the dorsal surface of the hand. The skin was cleansed with 70% (v/v) isopropyl alcohol and 1% lignocaine was injected intradermally at the intended site of venepuncture and along the exterior of the vein. The inner metal cannula was removed from its plastic sheath and attached to a 20 ml syringe (Omnifix; B. Braun Melsungen AG, D-34209 Melsungen, Germany). The tip of the cannula was pushed through the anaesthetized skin into the vein and advanced for 4-6 cm. The tip of the cannula was then repeatedly brought into contact with the venous wall at various sites along the length of the vein by gently massaging the overlying skin while simultaneously withdrawing blood. The blood sample was then aliquoted into tubes containing 3.8% sodium citrate as anticoagulant. Ethical approval for this procedure was obtained from Newcastle Area Health Authority and all subjects gave written informed consent.
Endothelial cell isolation from blood using isopycnic centrifugation
The method was modified from that of Sbarbati, de Boer, Marzilli, Scarlattini, Rossi & van Mourik (1991) using the principles outlined by Roos & de Boer (1986). A solution of 1.54 M sodium chloride (NaCl; BDH Laboratory Supplies) with 1 M sodium dihydrogen phosphate (NaH2PO4·H2O; Sigma) in deionized water was made up and filter sterilized with a Flowpore 0.22 μm sterile filter (ICN Pharmaceuticals Inc., Costa Mesa, CA, USA). Percoll (density, 1.130 ± 0.005 g ml−1; viscosity, 10 ± 5 centipoise at 20°C; osmolality, < 20 mosmol (kg H2O)−1; pH 8.9 ± 0.3 at 20°C; from Sigma) was used to prepare a Percoll stock suspension by adding 7 ml of the above solution to 93 ml of sterile Percoll suspension. One hundred millilitres of Percoll suspension of specific gravity 1.060 g ml−1 was made up using 46 ml Percoll stock suspension, 10 ml human albumin (5 mg ml−1 human plasma albumin, fraction V; Calbiochem-Novabiochem Corporation, La Jolla, CA, USA), 34 ml phosphate buffered saline (PBS; ICN Pharmaceuticals Inc.) and 10 ml of 3.8% sodium citrate (prepared from trisodium citrate in deionized water; BDH Laboratory Supplies). Preparation was carried out under aseptic conditions in a Class II Cabinet and all solutions (except Percoll) were filter sterilized. The prepared Percoll suspension of specific gravity 1.060 g ml−1 was stored at 4°C until use.
A 10 ml plastic centrifuge tube (Bibby Sterilin Ltd, Stone, Staffordshire, UK) was half-filled with Percoll suspension. The blood sample was layered over the Percoll to fill the rest of the tube. This was then centrifuged at room temperature at 1000 g for a further 10 min. The top layer was collected and centrifuged at 400 g for 10 min. The supernatant from this second centrifugation was discarded and the cell pellet resuspended in culture medium and seeded on to appropriate culture surfaces.
Endothelial cell culture
The culture medium used was Medium 199 (modified, with Earle's salts, without L-glutamine) (ICN Pharmaceuticals) containing 20% (v/v) fetal bovine serum, penicillin (100 i.u. ml−1), streptomycin (100 μg ml−1), L-glutamine (4 μmol ml−1), sodium heparin (17.92 USP units ml−1) and endothelial cell growth supplement (all from Sigma). Cells were seeded on to 10 mm glass coverslips in 35 mm Petri dishes with 4 × 11 mm wells (Cell-Cult) and incubated at 37°C in a mixture of 95% air and 5% carbon dioxide. The culture medium was changed every 2-3 days. Cells were used from day 14-28 in culture. No systematic analysis was made of the effects of time in culture on the responsiveness of the cells.
Immunocytochemical identification of the endothelium
This was done using anti von Willebrand factor antibody or anti PECAM-1 conjugated to fluorescein isothiocyanate (FITC). Between day 7 and day 21 of culture, a 5 μl aliquot of antibody was added to 1 ml culture medium in a Petri dish. After 30 min incubation, the medium containing antibody was washed off and replaced with PBS and the cells viewed using an inverted fluorescence microscope (490 nm excitation, > 520 nm emission).
Intracellular Ca2+ determination
Cells were loaded with fura-2 (Molecular Probes Ltd) by incubation for 30 min at 37°C in 1 ml of culture medium containing 3 μg ml−1 of the acetoxymethylester form of the dye. Coverslips were then mounted in a superfusion chamber on the stage of a microspectrofluorimeter based on a Nikon Diaphot EPI fluorescence microscope fitted with an excitation filter changer and multi-point imaging system (Newcastle Photometric Systems Ltd, Newcastle upon Tyne, UK). Up to sixteen single cells per experiment were monitored. The excitation wavelengths used were 350 nm and 380 nm and the emitted fluorescence was measured at wavelengths above 520 nm. Fluorescence measurements were taken every 500 ms and the ratios of emission intensities at each excitation wavelength were calculated for each cell and displayed approximately every 1.6 s. For the purposes of the experiments presented here, which relate to time-dependent changes in Ca2+ concentration, no attempt was made to calibrate the fluorescence signals in terms of absolute Ca2+ concentrations. However, only cells whose initial resting ratios were in the range 0.9-1.1 were used for analysis. Solutions were perfused through the experimental chamber (volume, 100 μl) at a rate of 2-3 ml min−1 at a temperature of 32-35°C. The bathing solution contained (mM): 140 NaCl, 4.5 KCl, 2.5 CaCl2, 1 MgCl2, 1 NaH2PO4, 10 D-glucose, 10 mM Hepes, pH 7.4. Ca2+-free solution contained 0.2 mM EGTA to buffer free Ca2+. Bovine serum albumin (1 mg ml−1; BDH Laboratory Supplies) was present in all solutions.
Definitions of response and amplitude of response
A response to any agonist was defined as a positive deflection in the fluorescence ratio of at least 3% of the amplitude of response of the same cell to ATP, this elevation persisting over more than two consecutive fluorescence ratio recordings before returning towards baseline. This had to follow the application of the agonist concerned within 100 s and be accompanied by a corresponding divergent change in the single wavelength recordings of the response. The amplitude of response was defined as the difference between the peak of a response and the baseline fluorescence ratio just before the onset of the response.
Statistical analysis
Most of the numerical results in this study were presented as the arithmetic mean ± standard error of the mean (s.e.m.).
RESULTS
Human hand vein endothelial (HHVE) cells were obtained from 91% of the subjects subjected to traumatic venepuncture. Figure 1A shows a phase contrast photomicrograph of cells immediately (∼1 h) after isolation. The cells were small, approximately 5 μm in diameter, and often were found in small ‘raspberry like’ clusters. Confirmation that they were endothelial cells was obtained using the endothelium-specific antibodies anti PECAM-1 (Fig. 1B) and anti von Willebrand factor (not shown). Figure 1C illustrates a clump of endothelial cells which had been maintained in culture for 28 days. In vitro the rate of growth of the HHVE cells was variable but patches of cells growing in contact with each other with a ‘flat’ appearance could be seen after 7 days in culture. These cultures were routinely confirmed to contain endothelial cells by staining with FITC-conjugated anti PECAM-1 and anti von Willebrand factor antibodies. Ninety per cent of these round cells in culture expressed the von Willebrand factor antigen and 87% stained positively with anti PECAM-1 antibody. Cells, 7-21 days in culture, in areas demonstrating this ‘flat’ morphology were used for all physiological measurements. Elongated and spindle-shaped cells were also seen. These were presumably contaminating fibroblasts or smooth muscle cells and did not stain with anti von Willebrand factor antibody.
Figure 1. Photomicrographs of human hand vein endothelial (HHVE) cells.

A shows a phase contrast image of cells 2 h after isolation. B is the same field of view as in A but using fluorescence (FITC). Cells were stained with an endothelial cell-specific marker anti PECAM-1. Positive cells can be seen to cluster together or can be detected as single spherical cells. C is a phase contrast image of cells maintained for 11 days in tissue culture. At this time the cells have attained a flat ‘cobblestone’ appearance. Scale bars in A and C represent 20 μm.
A preliminary study of the responsiveness of isolated human hand vein endothelial (HHVE) cells showed that 100% of the cells responded to the agonists adenosine triphosphate (ATP; 0.1-10 μM) and uridine triphosphate (UTP; 0.1-10 μM) with an elevation in intracellular Ca2+. For this reason, responses to ATP (or UTP) were used in this study, as the ‘standard response’ to confirm that every cell was viable and sensitive to agonists. Figure 2A demonstrates the typical response obtained to ATP (1 μM) in five single adjacent HHVE cells in vitro isolated from a non-pregnant individual. On application of ATP there was an initial rapid transient increase in Ca2+ which was followed by a sustained elevation in Ca2+, the plateau phase. In many cells the plateau phase showed signs of a slow gradual increase in Ca2+ throughout the exposure to agonist. Occasionally, small fluctuations were noted within responses. Figure 2B illustrates a typical response of HHVE cells to ATP in the absence of extracellular Ca2+. There is a rapid but transient rise in Ca2+ with no indication of a sustained response. This type of response has been seen in many cell types and is indicative of an intracellular release process almost certainly dependent on IP3. It is noteworthy that on returning to a solution containing 2 mM Ca2+ there is a large transient increase in Ca2+. This phenomenon has been described in many cells and appears, in part, to be related to store-regulated Ca2+ entry (Putney & Bird, 1993) but may also be related to changes in intracellular Na+ as a result of the exposure to Ca2+-free solutions (Gillespie, Johnson, Nicholls, Lynch & Greenwell, 1992).
Figure 2. Intracellular Ca2+ recordings from HHVE cells.
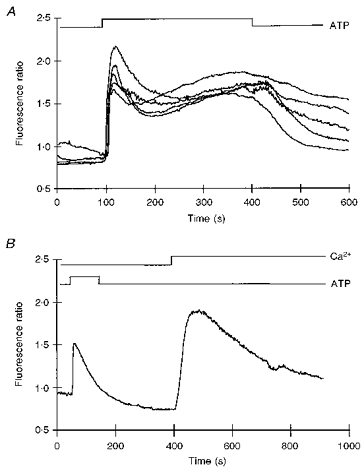
A shows the simultaneous recording of intracellular Ca2+ from 5 cells exposed to 1 μM ATP on a confluent region of human hand vein endothelial cells maintained in vitro for 14 days. Temperature, 33 °C. B illustrates the mobilization of intracellular Ca2+ by ATP (1 μM). The cells were washed in medium which was nominally Ca2+ free before application of ATP (1 μm). Temperature, 33 °C.
The responsiveness of HHVE cells from non-pregnant, normal pregnant and pre-eclamptic subjects to ATP was measured and compared. Ca2+ signals, similar to those in Fig. 2A were broken down into (a) the amplitude of the initial peak response (Fig. 3A) and (b) the maximum amplitude of the sustained response (Fig. 3B). The amplitudes of the ATP-induced Ca2+ transients, initial and sustained phases, from pregnant cells were significantly larger than those derived from the non-pregnant cells (P < 0.01; n = 50; Student's t test). The responses of cells from pre-eclamptic subjects were significantly different from those from pregnant subjects but not from those from non-pregnant subjects (P < 0.01, n = 48).
Figure 3. The responsiveness of HHVE cells to ATP.
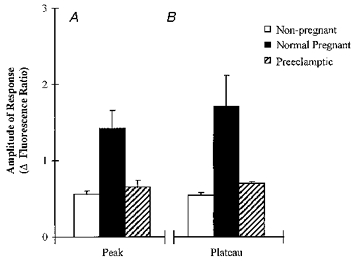
Histograms of the amplitude of the Ca2+ transients of peak (A) and plateau (B) responses to ATP (1 μm). □, data from ‘non-pregnant’ cells; ▪, data from ‘pregnant’ cells;  , data from ‘pre-eclamptic’ cells. Details of numbers of cells in each group are given in the text.
, data from ‘pre-eclamptic’ cells. Details of numbers of cells in each group are given in the text.
Responses to carbachol, bradykinin and histamine
Endothelial cells from different origins express several re ceptor types on their surface membrane. Therefore, we examined the effects of a number of other agonists; carbachol (20 μm), bradykinin (1 μm) and histamine (30 μm). Figure 4A-C illustrates typical responses seen. Invariably, these responses were smaller than those seen with purinergic agonists. In addition the responses were relatively slow in onset and transient, with no indication of a plateau phase of activation. Magnified sections of the records are also illustrated in Fig. 4A-C. In fact, not all cells responded with a definite rise in Ca2+. In order to be certain that each cell was viable we adopted the protocol of following the exposure to a test agonist with a standard application of a maximal dose of ATP (10 μm). This allowed us to determine the fraction of cells sensitive to any particular agonist and to calculate the amplitude of the response relative to the response to ATP.
Figure 4. The effects of carbachol (A; 1 μm), bradykinin (B; 0.1 μm) and histamine (C; 1 μm) on intracellular Ca2+ in isolated HHVE cells.
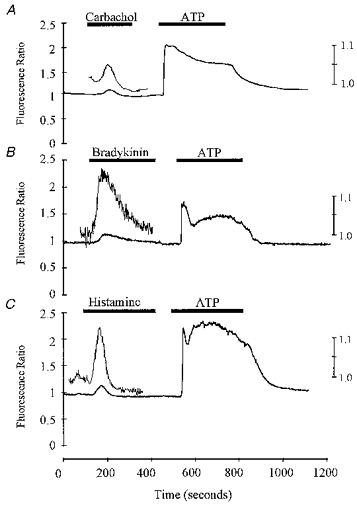
In each experiment the exposure to the test agonist was followed by exposure to ATP (10 μm). Ordinates show the fura-2 fluorescence ratio. The left ordinate is the low gain and the right the high gain. All experiments were carried out at a temperature of 32-35 °C.
Figure 5 shows data illustrating the percentages of cells from non-pregnant, normal pregnant and pre-eclamptic individuals responding to the different agonists (ATP, histamine, carbachol and bradykinin). For the cells isolated from non-pregnant subjects, 70.5% responded to histamine, 25% to carbachol and 12.5% to bradykinin. No significant differences in the numbers of cells responding to histamine and carbachol were noted between non-pregnant and pregnant individuals. However, the proportion of cells responding to bradykinin was significantly greater in the cells from pregnant subjects (65%). No significant differences in the numbers of cells responding were detected when cells from non-pregnant individuals were compared with those from pre-eclamptic women. Thus the major difference noted in this analysis was that a higher proportion of cells responded to bradykinin in preparations from normal pregnant subjects compared with non-pregnant or pre-eclamptic subjects.
Figure 5. Responses to histamine, carbachol and bradykinin in HHVE cells isolated from non-pregnant, pregnant (normotensive) and pre-eclamptic subjects.
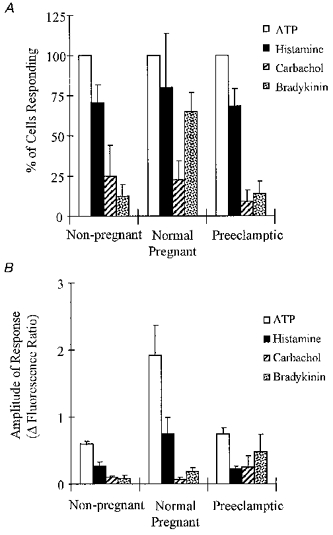
A, the numbers of cells responding; B, relative amplitudes of the intracellular Ca2+ transients activated; □, ATP; ▪, histamine;  , carbachol;
, carbachol;  , bradykinin. In A, ATP was used as an internal control and the proportion of cells responding to the test agonist is calculated using only those cells which also responded to ATP. Data show mean values ±s.e.m. All experiments were carried out at a temperature of 32-35 °C.
, bradykinin. In A, ATP was used as an internal control and the proportion of cells responding to the test agonist is calculated using only those cells which also responded to ATP. Data show mean values ±s.e.m. All experiments were carried out at a temperature of 32-35 °C.
Figure 5B shows an analysis of the amplitudes of the responses measured with the agonists, ATP, histamine, carbachol and bradykinin. Firstly, comparing non-pregnant and pregnant samples, the amplitudes of the Ca2+ transients seen with ATP and histamine were significantly larger in the cells from pregnant women (P < 0.01, see also Fig. 3). The responses to carbachol and bradykinin did not differ statistically between the groups. When the responses to ATP, histamine and carbachol from cells isolated from pre-eclamptic women were compared with those from the cells of non-pregnant women, no significant differences were seen.
DISCUSSION
The principal new observations from the present study are that HHVE cells, isolated from pregnant women, are more responsive to purinergic activation and to bradykinin than those isolated from non-pregnant controls. Furthermore, HHVE cells from pre-eclamptic women do not show the upregulation seen in the normal pregnant cells, supporting the idea that one factor contributing to pre-eclampsia may be a defect in the ability of the endothelium to adapt in pregnancy.
Many aspects of the cardiovascular complications seen in pre-eclampsia could be a result of endothelial dysfunction involving either an increase in vasoconstrictor output or a reduction in vasodilator output. Such alterations could arise by at least two basic mechanisms: the endothelial cells may be different in pre-eclamptics in that they fail to adapt to normal pregnancy; alternatively, there may be factors in the blood or tissues of pre-eclamptic women which damage the endothelium and subsequently impair its function.
The possibility that the serum of pre-eclamptic women contains agents which reduce endothelial cell function has been studied extensively. Early work on endothelial dysfunction in pre-eclampsia indicated that human umbilical vein endothelial (HUVE) cells preloaded with 51Cr released more of this isotope when exposed to sera obtained from pre-eclamptic women than after exposure to sera from normal pregnant women (Rodgers et al. 1988; Tsukimori et al. 1992). This was interpreted as reflecting the presence of a lethal cytotoxic effect of pre-eclamptic sera on endothelial cells (Rodgers et al. 1988; Tsukimori et al. 1992). More recently, it has been suggested that the nature of the toxic agents involves an alteration in the ratio of protective toxicity preventing activity (TxPA) to ‘toxic’ very low density lipoproteins (Arbogast, Leeper, Merrick, Olive & Taylor, 1996). However, a contrary view has emerged. A morphological analysis of HUVE cells obtained from cases of pre-eclampsia showed evidence of cellular activation rather than injury when compared with cells obtained from normal pregnancies (Roberts, Edep, Goldfein & Taylor, 1992; Endresen, Tøsti, Lorentzen & Henriksen, 1995; Cester et al. 1995). Subsequent experiments also showed an increase in nitric oxide synthase (NOS) expression (Davidge, Baker & Roberts, 1995), NO production (Baker, Davidge & Roberts, 1995) and PGI2 synthesis (de Groot, Davidge, Friedman, McLaughlin, Roberts & Taylor, 1995) by HUVE cells exposed to plasma from pre-eclamptic women. In a further study using bovine coronary endothelial cells, there are data suggesting that serum from pregnant women stimulates the production of vasodilator agents (Davidge et al. 1995). It is not clear how a stimulation of endothelial cell function could result in the cardiovascular change underlying pre-eclampsia. In an attempt to address this apparent contradiction, it has recently been shown that mechanical stimulation of bovine coronary endothelium reduced the stimulatory effects of pre-eclamptic serum (Baker, Stranko, Davidge, Davies & Roberts, 1996). These data suggest that the endothelium may not be defective in pre-eclampsia and that its activation may represent a secondary response to overcome a different basic defect. Thus, in pre-eclampsia the endothelium may eventually reach such a state of over-stimulation that it malfunctions. Caution must be exercised in the interpretation of these results since the majority of the data are derived from studies on the effects of maternal serum on endothelium derived from animal tissues or on human fetal endothelial cells. These models may not represent the situation in vivo, and therefore the precise role of the endothelium in the aetiology of pre-eclampsia must still remain controversial.
In the present study we have attempted to circumvent some of the experimental problems in the study of pre-eclampsia by developing an approach which uses endothelial cells from pregnant and non-pregnant subjects in each of whom the blood pressure had been recorded. The use of hand vein endothelium appears to provide a simple cellular model with which to approach the study of endothelial changes in pregnancy and pre-eclampsia. However, the model may still not be ideal. For example, it is possible that the characteristics of endothelium in veins differ from those of arterial endothelium. Furthermore, in this study, we have used cells which were maintained in vitro in order to allow them to attach to an appropriate substrate, but this time in vitro may alter with the physiological properties of the cells. Nevertheless, despite these potential limitations, the HHVE model does provide data which may give an insight into adult human endothelial cell function and dysfunction in pregnancy.
The larger Ca2+ transients observed in normal pregnant compared with non-pregnant women in response to ATP and histamine are potentially interesting observations, implying that the HHVE cells may be more sensitive during pregnancy to purinergic activation. Since the production of both NO and PGI2 are activated by Ca2+, the output of vasoactive compounds by HHVE cells might thus be increased during pregnancy. Enhanced local production of these vasodilators might contribute to the generalized vasodilatation of normal pregnancy. HHVE cells from pre-eclamptic women did not show this altered responsiveness to purinergic activation, producing results which were similar to those obtained from the cells of non-pregnant women. One interpretation of these data would be that the HHVE cells from pre-eclamptic subjects failed to upregulate their responsiveness to purinergic and histamine receptor activation and were consequently less able to contribute to a vasodilatation.
The Ca2+ transients induced by carbachol, bradykinin and histamine were different from those activated by ATP: they had a slower rate of rise and were smaller in amplitude. Since the Ca2+ transients were small this might imply that the ability of these agonists to stimulate the production of vasodilators was limited. The reduced responsiveness, particularly to carbachol may, however, be misleading since it has been reported that there is a downregulation of M1 and M3 but not of M2 muscarinic receptor subtypes in certain cultured endothelial cell types (Peach, Singer & Loeb, 1985; Loeb, Johns, Milner & Peach, 1987; Lueckhoff, Busse, Winter & Bassenge, 1987; Tracey & Peach, 1992; Wang, Lau, Li, Yoshikawa & van Breemen, 1995). The M3 muscarinic receptor stimulates IP3 production whereas the M2 is a weak activator of IP3 production (Clapham & Sneyd, 1995) and this may account for the weak mobilization of Ca2+ by carbachol in HHVE cells in vitro.
A significantly higher percentage of cells from normal pregnant women responded to bradykinin than that from non-pregnant and pre-eclamptic subjects, although the amplitudes of the Ca2+ transients were not significantly different. This observation raises the possibility that the HHVE cells are not homogeneous. Furthermore, it suggests that an increased vasodilator response might be achieved in vivo by simply increasing the number of cells that respond to a particular agonist. Therefore, the data presented here point to two mechanisms whereby the endothelium, as a whole, might be upregulated in pregnancy: (i) by an increase in the Ca2+ transients activated by purinergic activation and histamine, and (ii) by an increase in the number of cells responding to bradykinin. These observations fit with the concept that in a normal pregnancy there is a generalized reduction in arterial vascular tone, affecting blood pressure, and venous tone, resulting in an increased venous compliance (McCauseland, Hyman, Winsor & Trotter, 1961; Barwin & Roddie, 1976; Robson et al. 1989).
It has been reported that bradykinin is capable of inducing relaxations in maternal resistance arteries isolated from women with normal pregnancies and with pre-eclampsia. However, the bradykinin-induced relaxations were significantly blunted in vessels from the pre-eclamptics compared with those from controls (Knock & Poston, 1996). These data point to an alteration in the mechanisms of peptidergic activation of the endothelium. The results presented here are in keeping with this idea. There appears to be an ‘upregulation’ in the responsiveness of cells from normotensive women, and a failure for this to occur in pre- eclamptics. A contrary result has been reported in resistance arteries where no change in bradykinin sensitivity was noted between the non-pregnant and pregnant states (Ashworth, Warren, Baker & Johnson, 1996).
The proposition that in women with pre-eclampsia the endothelium may be activated (Davidge et al. 1995; Baker et al. 1995; de Groot et al. 1995) differs from the conclusion arrived at in the present study. In order to account for this paradox it might be suggested that in the endothelium of normal pregnancy both the Ca2+ signalling system elements of cell activation and the enzymes needed to produce vasodilator agents are upregulated. However, in the endothelium from pre-eclamptics the Ca2+ signalling system may be deficient although the upregulation of the vasodilator production is retained. If this were the case, the overall effect would be a defect in the vasodilator capacity of the endothelium in pre-eclampsia. Further studies into the nature of the processes which activate the endothelium in normal pregnancy and the failure of these process to develop in pre- eclamptics may therefore afford new insights into the pathophysiology of this clinical condition.
Acknowledgments
We gratefully acknowledge the support of the Medical Research Council and Well Being for funding this work.
References
- Arbogast B W, Leeper S C, Merrick R D, Olive K E, Taylor R N. Plasma factors that determine endothelial cell lipid toxicity in vitro correctly identify women with preeclampsia in early and late pregnancy. Hypertension in Pregnancy. 1996;15:263–279. [Google Scholar]
- Ashworth J R, Warren A Y, Baker P N, Johnson I R. A comparison of endothelium-dependent relaxation in omental and myometrial resistance arteries in pregnant and nonpregnant women. American Journal of Obstetrics and Gynecology. 1996;175:1307–1312. doi: 10.1016/s0002-9378(96)70046-7. [DOI] [PubMed] [Google Scholar]
- Baker P N, Davidge S T, Roberts J M. Plasma from women with preeclampsia increases endothelial cell nitric oxide production. Hypertension. 1995;26:244–248. doi: 10.1161/01.hyp.26.2.244. [DOI] [PubMed] [Google Scholar]
- Baker P N, Stranko C P, Davidge S T, Davies P S, Roberts J M. Mechanical stress eliminates the effects of plasma from patients with preeclampsia on endothelial cells. American Journal of Obstetrics and Gynecology. 1996;174:730–736. doi: 10.1016/s0002-9378(96)70457-x. [DOI] [PubMed] [Google Scholar]
- Barwin B N, Roddie I C. Venous distensibility determined by graded venous congestion. American Journal of Obstetrics and Gynecology. 1976;125:921–923. doi: 10.1016/0002-9378(76)90489-0. [DOI] [PubMed] [Google Scholar]
- Born G V R, Kratzer M A A. Source and concentration of extracellular adenosine triphosphate during homeostasis in rats, rabbits and man. Journal of Physiology. 1984;354:419–429. doi: 10.1113/jphysiol.1984.sp015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D M, MacGillivray I. Pre-eclampsia in second pregnancy. British Journal of Obstetrics and Gynaecology. 1985;92:131–140. doi: 10.1111/j.1471-0528.1985.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Cester N, Romanini C, Pugnaloni A, Biagini G, Rizzoli C, Salvolini E, Staffolani R, Mazzanti L, Rabini R A. Pregnancy-induced hypertension is associated with an activation of endothelial cells. Hypertension in Pregnancy. 1995;14:227–234. [Google Scholar]
- Chesley L C. Hypertensive Disorders in Pregnancy. New York: Appleton-Century-Crofts; 1978. pp. 225–228. [Google Scholar]
- Clapham D E, Sneyd J. Intracellular calcium waves. Advances in Second Messenger and Phosphoprotein Research. 1995;30:1–24. doi: 10.1016/s1040-7952(05)80003-5. [DOI] [PubMed] [Google Scholar]
- Davey D A, MacGillivray I. Severe hypertension, preeclampsia and eclampsia. American Journal of Obstetrics and Gynecology. 1988;158:982–988. doi: 10.1016/0002-9378(88)90090-7. [DOI] [PubMed] [Google Scholar]
- Davidge S T, Baker P N, Roberts J M. NOS expression is increased in endothelial cells exposed to plasma from women with preeclampsia. American Journal of Physiology. 1995;269:H1106–1112. doi: 10.1152/ajpheart.1995.269.3.H1106. [DOI] [PubMed] [Google Scholar]
- Davidge S T, Signorella A P, Lykins D L, Gilmour C H, Roberts J M. Evidence of endothelial activation and endothelial activators in cord blood of infants of preeclamptic women. American Journal of Obstetrics and Gynecology. 1996;175:1301–1306. doi: 10.1016/s0002-9378(96)70045-5. [DOI] [PubMed] [Google Scholar]
- de Groot C J, Davidge S T, Friedman S A, McLaughlin M K, Roberts J M, Taylor R N. Plasma from pre-eclamptic women increases human endothelial cell prostacyclin production without changes in cellular enzyme activity or mass. American Journal of Obstetrics and Gynecology. 1995;172:976–985. doi: 10.1016/0002-9378(95)90030-6. 10.1016/0002-9378(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Endresen M J, Tøsti E, Lorentzen B, Henriksen T. Sera of pre-eclamptic women are not cytotoxic to endothelial cells in culture. American Journal of Obstetrics and Gynecology. 1995;172:196–201. doi: 10.1016/0002-9378(95)90112-4. 10.1016/0002-9378(95)90112-4. [DOI] [PubMed] [Google Scholar]
- Fisher K A, Luger A, Spargo B H, Lindheimer M D. Hypertension in pregnancy. Clinical and pathological correlations and remote prognosis. Medicine. 1981;60:267–276. [PubMed] [Google Scholar]
- Gillespie J I, Johnson C, Nicholls J, Lynch M, Greenwell J R. Is there a ‘calcium paradox’ in isolated bovine aortic endothelial cells? Experimental Physiology. 1992;77:393–396. doi: 10.1113/expphysiol.1992.sp003601. [DOI] [PubMed] [Google Scholar]
- Jaffe E A, Nachman R L, Becker C G, Minick C R. Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunological criteria. Journal of Clinical Investigation. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knock G A, Poston L. Bradykinin-mediated relaxation of isolated maternal resistance arteries in normal pregnancy and preeclampsia. American Journal of Obstetrics and Gynecology. 1996;175:1668–1674. doi: 10.1016/s0002-9378(96)70123-0. [DOI] [PubMed] [Google Scholar]
- Loeb A L, Johns R A, Milner P, Peach M J. Endothelium-derived relaxing factor in cultured cells. Hypertension. 1987;9:III-186–III-192. doi: 10.1161/01.hyp.9.6_pt_2.iii186. [DOI] [PubMed] [Google Scholar]
- Lueckhoff A, Busse R, Winter I, Bassenge E. Characterization of vascular relaxant factor released from cultured endothelial cells. Hypertension. 1987;9:295–303. doi: 10.1161/01.hyp.9.3.295. [DOI] [PubMed] [Google Scholar]
- McCauseland A M, Hyman C, Winsor T, Trotter A D. Venous distensibility during pregnancy. American Journal of Obstetrics and Gynecology. 1961;81:472–478. doi: 10.1016/s0002-9378(15)33663-2. [DOI] [PubMed] [Google Scholar]
- Oguogho A, Aloamaka C P, Ebeigbe A B. Depression of endothelium dependent relaxation responses to acetylcholine and histamine in isolated human epigastric arteries from pre-eclamptic women. Clinical Autonomic Research. 1996;6:153–155. doi: 10.1007/BF02281902. [DOI] [PubMed] [Google Scholar]
- Peach M J, Singer H A, Loeb A L. Mechanisms of endothelium-dependent vascular smooth muscle relaxation. Biochemical Pharmacology. 1985;34:1867–1874. doi: 10.1016/0006-2952(85)90300-4. 10.1016/0006-2952(85)90300-4. [DOI] [PubMed] [Google Scholar]
- Pearson J D, Carter T D. Effects of extracellular ATP on the release of vasoactive mediators from endothelium. Annals of the New York Academy of Sciences. 1990;603:267–274. doi: 10.1111/j.1749-6632.1990.tb37678.x. [DOI] [PubMed] [Google Scholar]
- Poston L, McCarthy A L, Ritter J M. Control of vascular resistance in the maternal and feto-placental arterial beds. Pharmacological Therapeutics. 1995;65:215–239. doi: 10.1016/0163-7258(94)00064-a. 10.1016/0163-7258(94)00064-A. [DOI] [PubMed] [Google Scholar]
- Putney J W, Jr, Bird G St J. The signal for capacitative calcium entry. Cell. 1993;75:199–201. doi: 10.1016/0092-8674(93)80061-i. [DOI] [PubMed] [Google Scholar]
- Roberts J M, Edep M E, Goldfien A, Taylor R N. Sera from pre-eclamptic women specifically activate human umbilical vein endothelial cells in vitro: morphological and biochemical evidence. American Journal of Reproductive Immunology. 1992;27:101–108. doi: 10.1111/j.1600-0897.1992.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Roberts J M, Redman C W G. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447–1450. doi: 10.1016/0140-6736(93)90889-o. 10.1016/0140-6736(93)90889-O. [DOI] [PubMed] [Google Scholar]
- Roberts J M, Taylor R N, Musci T J, Rodgers G M, Hubel C A, McLaughlin M K. Preeclampsia: An endothelial cell disorder. American Journal of Obstetrics and Gynecology. 1989;161:1200–1205. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- Robertson W B, Brosens I, Dixon H G. The pathological response of the vessels of the placental bed to hypertensive pregnancy. Journal of Pathology and Bacteriology. 1967;93:581–592. doi: 10.1002/path.1700930219. [DOI] [PubMed] [Google Scholar]
- Robertson W B, Khong T Y. Pathology of the uteroplacental bed. In: Sharp F, Symonds E M, editors. Hypertension in Pregnancy. Ithaca, NY, USA: Perinatology Press; 1987. pp. 101–113. [Google Scholar]
- Robson S C, Hunter S, Boys R J, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. American Journal of Physiology. 1989;256:H1060–1065. doi: 10.1152/ajpheart.1989.256.4.H1060. [DOI] [PubMed] [Google Scholar]
- Rodgers G M, Taylor R N, Roberts J M. Preeclampsia is associated with a serum factor cytotoxic to human endothelial cells. American Journal of Obstetrics and Gynecology. 1988;159:908–914. doi: 10.1016/s0002-9378(88)80169-8. [DOI] [PubMed] [Google Scholar]
- Roos D, de Boer M. Purification and cryopreservation of phagocytes from human blood. Methods in Enzymology. 1986;132:225–243. doi: 10.1016/s0076-6879(86)32010-x. [DOI] [PubMed] [Google Scholar]
- Sbarbati R, de Boer M, Marzilli M, Scarlattini M, Rossi G, van Mourik J A. Immunologic detection of endothelial cells in human whole blood. Blood. 1991;77:764–769. [PubMed] [Google Scholar]
- Sharkey A M, Cooper J C, Balmforth J R, McLaren J, Clark D E, Charnock-Jones D S, Morris N H, Smith S K. Maternal plasma levels of vascular endothelial growth factor in normotensive pregnancies and in pregnancies complicated by pre-eclampsia. European Journal of Clinical Investigation. 1996;26:1182–1185. doi: 10.1046/j.1365-2362.1996.830605.x. 10.1046/j.1365-2362.1996.830605.x. [DOI] [PubMed] [Google Scholar]
- Tracey W R, Peach M J. Differential muscarinic receptor mRNA expression by freshly isolated and cultured bovine aortic endothelial cells. Circulation Research. 1992;70:234–240. doi: 10.1161/01.res.70.2.234. [DOI] [PubMed] [Google Scholar]
- Tsukimori K, Maeda H, Shingu M, Koyanagi T, Nobunaga M, Nakano H. The possible role of endothelial cells in hypertensive disorders during pregnancy. Obstetrics and Gynaecology. 1992;80:229–233. [PubMed] [Google Scholar]
- Wang X, Lau F, Li L, Yoshikawa A, van Breemen C. Acetylcholine-sensitive intracellular Ca2+ store in fresh endothelial cells and evidence for ryanodine receptors. Circulation Research. 1995;77:37–42. doi: 10.1161/01.res.77.1.37. [DOI] [PubMed] [Google Scholar]


