Abstract
We performed an RNase protection assay on cultured C2C12 mouse myotubes, demonstrating that the γ subunit of the fetal muscle acetylcholine receptor (AChR) exists as two splice variants, which differ in the presence of the amino terminal exon 5.
We studied unitary ACh-evoked events in fibres acutely dissociated from the hindlimb flexor digitorum brevis muscle of BALB/C mice aged between embryonic day 16 (E16) and postnatal day 6 (P6).
At all ages, the channel conductance was about 30 pS, typical of the fetal form of the AChR. The mean open time increased significantly from 6 ms at E16 to 9 ms at E19, then decreased to about 5 ms during the first postnatal week. The lengthening of the open time was considerably delayed in hypothyroid mice. Data were recorded at 24-26 °C.
On the basis of previously reported experiments in heterologous expression systems, we suggest that the modulation of channel open time is related to the expression of the AChR incorporating the γs subunit. These events might be coupled to the crucial modifications in muscle innervation that take place during the same developmental period.
The fetal muscle acetylcholine receptor (AChR) is a pentameric protein assembled from α, β, γ and δ subunits, with the stoichiometry α2βγδ. In mature neuromuscular junctions, an ε subunit replaces the γ subunit. In mouse hindlimbs, the mRNA encoding the γ subunit is abundant in late fetal life, and gradually disappears within 2 weeks of postnatal life, whereas ε mRNA is first detected at birth, and is present throughout adulthood (Martinou & Merlie, 1991). In fetal rat muscles, mRNA for the γ subunit is uniformly expressed over the entire fibre (Kues, Sakmann & Witzemann, 1995), in agreement with the distributed sensitivity to ACh (Diamond & Miledi, 1962). After birth, γ subunit mRNA and ACh sensitivity tend to colocalize with the sites of innervation, whereas ε subunit mRNA is invariably associated with subsynaptic nuclei (Kues et al. 1995). A similar sequence of events takes place in other vertebrate species (Hall & Sanes, 1993). The γ to ε switch is under neural and hormonal control, as it is delayed in hypothyroid mice (Martinou & Merlie, 1991).
In mouse muscles, the reverse transcription-polymerase chain reaction (RT-PCR) identified two variants of the γ subunit, one identical to the previously cloned γ subunit (Yu, La Polla & Davidson, 1986) and the other missing the fifty-two amino acids encoded by exon 5 (short γ, γs) (Mileo, Monaco, Palma, Grassi, Miledi & Eusebi, 1995). This domain contains the disulphide-bonded cysteine residues characteristic of the receptor superfamily, one glycosylation site and two residues likely to determine the binding selectivity of competitive antagonists (reviewed by Karlin, 1993). Lacking exactly one exon, γs is likely to be a splice variant of the γ subunit.
Other spliced variants of the muscle AChR subunits have been identified, as both the human α and the rat β subunit have been reported as alternatively spliced (Goldman & Tamai, 1989; Newland, Beeson, Vincent & Newsom-Davis, 1995), although the α variant is non-functional in Xenopus oocytes (Newland et al. 1995) and no expression data are available for the β alternative form. By contrast, both mouse αβγδ AChRs (γ AChR) and αβγsδ AChRs (γs AChR) are functional in Xenopus oocytes and transfected BOSC 23 human cells (Mileo et al. 1995; Fucile, Mileo, Grassi, Salvatore, Alemà & Eusebi, 1996). It is therefore conceivable that γs plays a role in developing mouse muscle fibres, where it is naturally expressed. This paper confirms, by means of RNase protection, the previous hypothesis that the γs subunit is a splice variant of the γ subunit. In an attempt to elucidate its developmental significance, we also examined the single-channel behaviour of fetal AChR- channels in normal and hypothyroid mice. In particular, we expected to find differences in the duration of ACh-evoked unitary events, considering that, in transfected BOSC 23 cells, channel openings are four times longer for the γs AChR than for the γ AChR (Fucile et al. 1996), and the amount of mRNA encoding either variant of the γ subunit appears to be regulated during muscle development in vivo (Mileo et al. 1995).
METHODS
Cell culture
The C2C12 myogenic satellite cell line (kindly provided by Dr S. Alemà, Instituto di Biologia Cellulare, CNR, Roma, Italy) was cultured in Dulbecco's modified Eagle's medium (Biowhittaker, Verviers, Belgium) supplemented with calf serum (20% v/v; Gibco) to stimulate cell mitosis. When 70% confluence was reached (after about 48 h in culture), the serum supplement was switched to horse serum (2% v/v; Gibco), to induce myotube differentiation.
mRNA preparation and RNase protection assay
Poly(A)+ RNA was extracted from differentiated C2C12 myotubes (4-5 days in culture) using Fast Track (Invitrogen Corporation, Carlsbad, CA, USA) according to the manufacturer's instructions. The RNase protection assay was performed using the RPA kit (Ambion Inc., Austin, TX, USA), according to the manufacturer's protocol. A cDNA fragment from the C2C12 γ subunit (532-725 nucleotides) (Mileo et al. 1995) was cloned into pBluescript II KS (Stratagene). Taq1 linearization of the plasmid and in vitro transcription using T7 RNA polymerase (Promega, Madison, WI, USA) generated a 255 bp antisense probe complementary to γ subunit RNA (nucleotides 478-671). The probe was labelled with [α-32P] UTP (800 Ci mmol−1; Amersham) to a specific activity of 7 × 108 c.p.m. μg−1. Yeast RNA (10 μg) or 2 μg of C2C12 poly(A)+ RNA (dissolved in 30 μl of hybridization buffer) was hybridized with a large excess of the probe (8 × 105 c.p.m. reaction−1) at 45°C for 12-16 h. After addition of RNaseA (0.5 units ml−1) and RNaseT1 (20 units ml−1) diluted in 200 μl of RNase digestion buffer, the incubation continued at 37°C for 1 h. The RNase digestion was terminated and the RNA precipitated by addition of 300 μl of RNase inactivation-precipitation mixture. The final product was then resolved on a 6% polyacrylamide-8 M urea sequencing gel, and detected by autoradiography. 32P-labelled riboprobes of known length (217 and 171 bp) were used as markers.
Mice
BALB/C mice were used in all experiments. The day of the vaginal plug was counted as embryonic day 0 (E0) and the day of birth as postnatal day 0 (P0). Newborn mice were made hypothyroid by feeding pregnant females from E14 with low-iodine Remington's diet (Mucedola, Italy) and adding 0.1% propylthiouracil (Sigma) to their drinking water, as described by Martinou & Merlie (1991). Hypothyroid mice were markedly smaller than controls, as expected (Kawa & Obata, 1982).
Muscle fibre isolation
Flexor digitorum brevis (FDB) muscles were dissected from the hindlimbs of fetal or newborn mice, killed by decapitation. Experiments were performed according to national guidelines for animal experiments. To obtain fetuses, pregnant mice were killed by cervical dislocation and the uterus removed. Fetuses were immediately decapitated and hindlimbs maintained in ice-cold saline. Muscles from four to six fetuses or one to two newborn mice of the same litter were incubated with Type I collagenase (2 mg ml−1; Sigma) for 15-20 min at 37°C in minimum essential medium (MEM; Gibco). After equilibrating in Ca2+-free MEM (Gibco) for 30 min, fibres were dissociated in 35-mm Petri dishes using plastic Pasteur pipettes, and the Ca2+ concentration was restored (see below) to allow fibre adhesion, which developed within 20-30 min, at room temperature (24-26°C). Fibres were studied within 3-4 h of isolation. Muscles were obtained from mice of three litters at E19 (n = 18 patches) and P0 (n = 14), one litter at E16 (n = 5), and two litters at all other ages (n = 8-9). Two hypothyroid litters were examined at all ages.
Single-channel recordings
Single-channel currents were recorded (at 24-26°C) in cell-attached mode from fibres bathed in MEM with the following ionic composition (mM): 116 NaCl, 5.4 KCl, 1.8 CaCl2, 0.8 MgSO4, 1 NaH2PO4, 10 glucose, 25 Hepes-NaOH, pH 7.3. Borosilicate glass pipettes (3-5 MΩ resistance), filled with MEM plus ACh (100 nM to 100 μM), were connected to an Axopatch 200 amplifier (Axon Instruments). Data were sampled at 10 kHz and analysed after digital filtering at 2 kHz, using a threshold-crossing method by pCLAMP 6.0 routines (Axon Instruments). For high ACh concentrations (10-100 μM), sampling and filtering frequencies were 20 and 4 kHz, respectively. Only single openings (200-3000 at each potential in every patch) were used to calculate slope conductance and mean channel duration. Cell resting potential was estimated from current-voltage relations, assuming a reversal potential of about 0 mV for ACh-evoked events (Fucile et al. 1996). Kinetic properties were compared at similar transmembrane potential (i.e. estimated resting plus pipette potential) for all patches. Open and closed time histograms were fitted by a non-linear algorithm (pCLAMP 6.0) with the sums of two or three exponential components, respectively. Burst duration (τburst) was calculated as in Fucile et al. (1996) using a critical time between 1 and 2 ms.
Unless indicated otherwise, results are given as means ±s.e.m.; P < 0.05 was considered significant (Student's t test).
RESULTS
RNase protection assay
To investigate whether the mRNA lacking exon 5 is indeed produced by cell processing of the RNA encoding the γ subunit, yielding a splice variant of the subunit, we performed an RNase protection assay in cultured mouse myotubes of the myogenic cell line C2C12 (4-5 days in culture). These cells yield a large amount of poly(A)+ RNA, and RT-PCR reliably identified both γ and γs mRNAs, with a marked preponderance of the γ transcript (Mileo et al. 1995).
Using a riboprobe that contained a region across exons 5 and 6 of the γ subunit, we detected two protected bands, of 193 and 166 bp, corresponding to γ and γs variants, respectively (Fig. 1). In all the four poly(A)+ RNA preparations tested, the mRNA encoding the γ subunit was notably more abundant than that encoding the γs transcript, confirming the results obtained previously using RT-PCR (Mileo et al. 1995).
Figure 1. RNase protection assay in cultured myotubes.
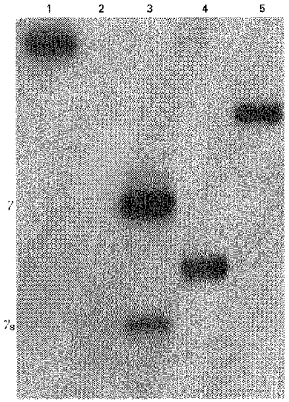
Poly(A)+ RNA from C2C12 myotubes (4 days in culture) was assayed by RNase protection with a γ probe containing parts of exons 5 and 6. Lane 1, 32P-labelled antisense γ probe (255 bp) not digested with RNase. Lane 2, probe hybridized to 10 μg yeast RNA after RNaseA-T1 digestion. Note the absence of signal. Lane 3, probe hybridized to 2 μg of C2C12 poly(A)+ RNA: protected γ (193 bp) and γs (166 bp) fragments after RNaseA-T1 digestion. Note that the γ fragment is markedly more abundant than the γs fragment. Lanes 4 and 5, riboprobes used as RNA markers (171 and 217 bp, respectively).
Single-channel recordings
The confirmation that the short variant of the γ subunit is a naturally occurring messenger prompted us to investigate whether the reported changes in the abundance of γ and γs mRNAs in mouse muscles around birth (Mileo et al. 1995) affect the behaviour of ACh-evoked channel openings during in vivo myogenesis. In fact, the mean open time (τop) is longer for γs AChR-channels than for γ AChR- channels (Fucile et al. 1996). To this aim, we studied ACh-evoked unitary events in acutely dissociated muscle fibres obtained from mice aged between E16 and P6. In all fibres tested, channel openings were observed irrespective of electrode placement, and appeared as squared pulses, with normally distributed amplitudes (Fig. 2A and B). The corresponding slope conductance was about 30 pS, with a maximal value of 33 pS at E16 and a minimum value (28 pS) at E19 (Fig. 2C). Starting from P4, we occasionally observed events of higher amplitude (not shown), corresponding to a slope conductance of about 40-50 pS, and mean τop of 1-2 ms, typical of receptors containing the ε subunit (ε AChRs). With 100 nM ACh in the patch pipette, the frequency of ACh-induced events was above 1 Hz in all patches (occasionally as high as 50 Hz), independent of animal age, and remained stable up to 45 min of recording. The mean τop of the ACh-evoked channels increased significantly from 6 to 9 ms between E16 and E19, then decreased to 5 ms in the first postnatal week (Fig. 3A and B). In fetuses, litter-to-litter variation of τop was small both at E18 and E19 (Table 1). We examined newborn mice from three litters, and found differences in τop at P0, but channel open duration was about 5 ms by P4 (Table 2). Thus, the long mean τop previously observed at P3 (9 ms; Mileo et al. 1995) in fibres acutely dissociated from mice of a different strain (C3H) possibly fell within this litter-to-litter variability. In patches with long τop, events as long as 100 ms were observed. The open time histograms were adequately fitted by two exponential components at all animal ages (Fig. 3A). The fast component was remarkably constant during development, while the slow component closely followed the changes in mean open time, accounting for the existence of very long events at E18-E19 (Fig. 3B). The voltage dependence of channel open duration was characterized by calculating the change in membrane potential yielding an e-fold increase in τop. Comparable values (140-200 mV) were obtained throughout the period considered (e.g. Fig. 3C), and they were consistent with the values reported previously for mouse fetal AChRs (Kopta & Steinbach, 1994). When the same fibre was examined at two different places, τop varied by less than 1 ms (5 cells), while the rate of channel opening occasionally differed by as much as 7-fold (not shown). These data suggest that AChRs with rather homogeneous properties were unevenly distributed on the fibre surface. In all patches, more than one channel was active, as revealed by the presence of multiple openings, and therefore a detailed analysis of channel kinetics was not performed. The closed time histograms were adequately fitted by three components (e.g. Fig. 4A), and mean channel closed time decreased e-fold for hyperpolarizations of about 150 mV, reflecting a voltage-dependent increase in opening frequency, with no age-dependent trend (not shown). τburst was comparable with τop (Fig. 4b; cf. Fig. 3B), indicating that channel bursting behaviour was virtually absent at this ACh concentration.
Figure 2. ACh-evoked unitary events in muscle fibres.

A, cell-attached currents were recorded in acutely dissociated FDB muscle fibres from mice at the ages indicated. Pipette potential was set to 0 (E16 and P4) or 30 mV (E19) to obtain an estimated transmembrane potential of about -80 mV. [ACh], 100 nM. Upward deflections represent inward currents. B, amplitude distribution of the events recorded in the same patches as in A. Histograms were best fitted with single Gaussian functions (superimposed) with mean ±s.d. values of 2.7 ± 0.2 pA (E16), 2.5 ± 0.1 pA (E19) and 2.4 ± 0.1 pA (P4). Slope conductances were 33.3 pS (E16), 31.6 pS (E19) and 29.2 pS (P4). Note that all the histograms are skewed to the left, due to the effects of filtering on the fastest openings. C, time course of slope conductance during mouse development. For each age, the mean slope conductance was calculated from the number of patches reported in Methods. Error bars represent s.e.m. Values at E16 vs. E18 and at E19 vs. P0 are statistically different (Student's t test, P < 0.014). All other points are not significantly different from their next neighbours.
Figure 3. Channel open time changes around birth.

A, open time histograms of the same patches as in Fig. 2, with the indicated mean open times (τop). Superimposed are the best fits with the sum of two exponentials, with the following time constants: E16: τ1= 0.5 ms (18%), τ2= 6.8 ms (82%); E19: τ1= 0.8 ms (16%), τ2= 11.3 ms (84%); P4: τ1= 0.9 ms (32%), τ2= 4.2 ms (68%). Note the different axes. B, time course of mean τop (•) and of the best fitting time constants τ1 (□) and τ2 (○) during mouse development, from values averaged as in Fig. 2C. τop at E19 is statistically different from values at E18 and P0 (P = 0.014 and P = 0.0004, respectively). C, semilogarithmic plot of τopvs. pipette potential for the same patches as in A, to show similar voltage dependence of channel open time at all ages. The hyperpolarization required for an e-fold increase in τop is 170 mV at E16, 148 mV at E19 and 140 mV at P4.
Table 1.
Duration of ACh-evoked channel openings in FDB fibres from fetuses of different ages
| Age | τop (ms) |
|---|---|
| E16 | 6.1 ± 0.3 (5) |
| E18 | 6.5 ± 0.5 (5) |
| 7.3 ± 0.5 (6) | |
| E19 | 9.3 ± 0.7 (5) |
| 8.7 ± 1.1 (6) | |
| 10.0 ± 1.2 (7) |
Mean ±s.e.m. (n cells) duration of ACh-evoked channel openings in fibres obtained from fetuses of six different litters, at the indicated ages. Fibres were isolated from both FDB muscles of four to six fetuses from each litter.
Table 2.
Duration of ACh-evoked channel openings in FDB fibres from mice of different litters at various ages
| τop (ms) | |||
|---|---|---|---|
| Age | Litter 1 | Litter 2 | Litter 3 |
| P0 | 4.2 ± 0.3 (5) | 7.2 ± 0.3 (4) | 7.0 ± 0.4 (5) |
| P2 | 5.0 ± 0.4 (6) | 8.9 ± 0.7 (2) | — |
| P4 | 4.6 ± 0.4 (5) | 3.8 ± 0.3 (3) | — |
| P6 | 5.4 ± 0.5 (3) | — | 5.7 ± 0.3 (5) |
Mean ±s.e.m. (n cells) duration of ACh-evoked channel openings in fibres obtained from mice of three litters. One newborn mouse was killed at each indicated age, except for P2, litter 1 (two mice).
Figure 4. Channel closed time and bursting behaviour.

A, closed time distributions in the same patches as in Fig. 2. Superimposed are the best fits with the sum of three exponentials, with the following time constants: E16: τc1= 0.14 ms (22%), τc2= 2.0 ms (4%), τc3= 209 ms (74%); E19: τc1= 0.2 ms (10%), τc2= 2.6 ms (4%), τc3= 300 ms (87%); P4: τc1= 0.12 ms (48%), τc2= 1.8 ms (3%), τc3= 179 ms (49%). Note the different vertical axes. B, time course of mean burst duration (τburst) during mouse development. Data obtained at an ACh concentration of 100 nM were averaged as in Fig. 2C. Values at E16 vs. E18 and E19 vs. P0 are statistically different (P < 0.01). C, consecutive traces recorded in the same fibre (P0) with 100 nM (top two traces) and 100 μM (bottom traces) ACh in the patch pipettes. Note the longer open time and the bursting behaviour in the patch with the high ACh concentration.
When the ACh concentration was raised to 10-100 μM, the pattern of channel openings changed as expected for desensitizing transmitter doses (Sakmann, Patlak & Neher, 1980; Sine & Steinbach, 1987). Events were grouped in bursts (Fig. 4C, bottom traces), and their frequency markedly decreased during prolonged recordings (not shown). Due to the high density of AChRs, most bursts were made of overlapping openings, even after 15 min of recording. This made the characterization of channel behaviour quite approximate. However, at ACh concentrations of 10-100 μM, mean channel open time was at least 40% longer than the τop measured in the same fibre, either from fetuses or newborn mice, with 100 nM ACh in the patch pipette (n = 5 cells). The open time histograms were fitted with a single exponential distribution (not shown), in agreement with other studies (Sakmann et al. 1980). τburst, measured only for bursts made up by single openings, was comparably longer. For instance, in one fibre at P0, with 100 μM ACh, τop was 20 ms and τburst was 46 ms, whereas at 100 nM the values were 8 and 9 ms, respectively (Fig. 4C). Taken together, these findings indicate that bursting activity appears only at high ACh concentrations throughout muscle development.
In hypothyroid mice, the mean open time of the unitary events evoked by ACh (100 nM) was 4.6 ± 0.2 ms (n = 10 cells) at P0 and significantly increased to 6.2 ± 0.3 ms (n = 9; Student's t test, P = 0.0004) at P3, then decreased to 3.7 ± 0.5 ms (n = 5, P = 0.0005) at P8.
DISCUSSION
In this paper we demonstrate, by RNase protection assay in cultured mouse C2C12 myotubes, that the mRNA encoding the AChR γ subunit exists as two alternatively spliced variants, which differ in the presence of exon 5. The ‘long’ variant (γ) was first identified in the BC3H-1 cell line (Yu et al. 1986), whereas the short variant (γs) has been identified by RT-PCR in C2C12 cultured myotubes and developing mouse muscles (Mileo et al. 1995). The present data rule out the possibility that γs mRNA was actually an artefact of PCR amplification. Having established that γs mRNA exists in native preparations, we studied ACh-evoked unitary events in mouse FDB muscle fibres during the perinatal period, when the mRNAs for the two variants of the γ subunit are detected (Mileo et al. 1995). In acutely dissociated muscle fibres, the open duration of the fetal AChR-channel changed with a well-defined time course around birth, with a peak value at E19. Channel conductance showed a small reduction by the same day, whereas no consistent variation was observed for closed times during this period. These events are concomitant with the establishment of nerve-muscle contacts, and the maturation of muscle fibres. In the rat, innervation of hindlimb muscles begins at E16-E17 (Kues et al. 1995). In the mouse, neuromuscular synapses are formed around E16 and develop to the adult morphology during the first postnatal weeks, with considerably different timing among fibres of a single muscle (Slater, 1982; Balice-Gordon, Chua, Nelson & Lichtman, 1993). A similar variability might explain the differences in τop observed at P0-P2 in different litters. ACh-evoked events were detected all over the fibres, even at P6, and in all patches we observed multiple openings, indicating that AChR density was quite high. At this time, extrasynaptic ACh sensitivity is more limited in the rat (Diamond & Miledi, 1962), and we have also observed a strong reduction of ACh-evoked events in distal parts of muscle fibres from P4 rats (F. Grassi & F. Eusebi, unpublished data). Openings of putative ε AChR-channels appear in the extrajunctional membrane after P4, possibly as the ε subunit begins to replace the γ subunit, since the majority of AChR-channels in adult mouse FDB fibres behave as the junctional type (Brehm & Kullberg, 1987). In hypothyroid mice, the increase in channel duration took place during the first postnatal week. This observation emphasizes the developmental significance of τop lengthening, as in hypothyroid rats the maturation of the neuromuscular junction is comparably retarded (Kawa & Obata, 1982), and the γ subunit is still present in hypothyroid mice at P20 (Martinou & Merlie, 1991).
As the open times of the several channels present in each patch were invariably averaged, the long τop values observed in fetal fibres, particularly at E19, are possibly caused by an enhanced expression of γs AChRs with respect to other ages. In fact, in human BOSC 23 transfected cells, the γs AChR has a 4-fold longer open time than the γ AChR (15 and 4 ms, respectively; Fucile et al. 1996). A weighted average of these two values shows that the τop values observed in dissociated fibres might be obtained if the fraction of γs AChRs increases from 17% at E16 to 50% at E19, then decreases to about 10% in early postnatal life. A day-by-day regulation of the amount of AChRs incorporating either variant of γ subunit is not unlikely, since the turnover rate for the fetal AChR is less than 1 day (reviewed by Hall & Sanes, 1993). Interestingly, in mRNA samples obtained from the hindlimbs or the FDB muscles of littermates of the mice used for electrophysiology, RT-PCR determinations identified mRNAs encoding both γ and γs subunits throughout the period considered (A. M. Mileo, B. Barabino & F. Eusebi, unpublished results), though with no conclusive evidence on the relative expression of the two variants. In fact, the relative abundance of the γ and γs transcripts was highly variable, possibly because the differences in the amount of each transcript variant present in the mRNA preparations tested were emphasized by the variability inherent to the RT-PCR amplification technique. Thus, the detection of only the γs mRNA variant in mouse muscles at P1 (Mileo et al. 1995) possibly stems from this variability.
The α (Newland et al. 1995) and β (Goldman & Tamai, 1989) subunits of the AChR also exist as splice variants, but the γs subunit appears to be the only one capable of assembling into a functional AChR in heterologous systems (Mileo et al. 1995; Newland et al. 1995; Fucile et al. 1996). In addition, we constructed a mutant ε subunit lacking exon 5. Although γ and ε subunit nucleotide sequences are 72% identical and have an equivalent gene organization, with a highly conserved exon-intron structure (Buonanno, Mudd & Merlie, 1989), the mutant ε variant failed to assemble into functional receptors in Xenopus oocytes or transfected human cells (authors’ unpublished observations). All these considerations suggest that the γs subunit subserves a physiological role in developing muscle fibres, the native cell type where the γs subunit was identified. It is likely that the regulation of γ and γs AChR expression contributes to the modulation of channel open time during in vivo myogenesis. A developmentally regulated switch between alternatively spliced receptor forms has been described for a glutamate receptor (Monyer, Seeburg & Wisden, 1991).
The function of extrasynaptic AChRs during myogenesis is still unclear, in spite of the many years that have elapsed since the first report of a diffuse sensitivity to ACh in developing muscle fibres (Diamond & Miledi, 1962). Given this lack of knowledge, we cannot attribute any exact role to the lengthening of channel open time and the putative expression of γs AChR. However, nerve-induced AChR clusters at developing neuromuscular junctions are in part formed by aggregation of extrasynaptic receptors (reviewed by Hall & Sanes, 1993). Since we found AChRs with rather homogeneous properties along the length of developing mouse muscle fibres, we may infer that the formation of end-plates incorporates the same percentage of γ and γs AChRs as the extrasynaptic regions. The presence of AChRs with long open times might be required to initiate any of the activity-mediated events in muscle fibres, for instance because during prolonged openings more Ca2+ ions enter into the cell.
This is the first report showing that the kinetics of the fetal AChR-channel change prior to the switch to the junctional form of the receptor in mammals, as previously described for Xenopus myotomal fibres (Leonard, Nakajima, Nakajima & Takahashi, 1984). In mouse, it is likely that changes in channel open time during the perinatal period are due, at least in part, to a concomitant modulation of the expression of the γs subunit. All these considerations justify a further search for a physiological role for this phenomenon.
Acknowledgments
We would like to thank Dr C. Passananti for helpful suggestions, Drs S. Alemà, C. Limatola and L. Monaco for critical reading of the manuscript, Mr F. Galli for technical assistance, and Mr G. Bertini for animal care. The work was supported by MURST grants (to F. G. and F. E.).
References
- Balice-Gordon R J, Chua C K, Nelson C C, Lichtman J W. Gradual loss of synaptic cartels precedes axon withdrawal at developing neuromuscular junctions. Neuron. 1993;11:801–815. doi: 10.1016/0896-6273(93)90110-d. [DOI] [PubMed] [Google Scholar]
- Brehm P, Kullberg R. Acetylcholine receptor channels on adult mouse skeletal muscle are functionally identical in synaptic and nonsynaptic membrane. Proceedings of the National Academy of Sciences of the USA. 1987;84:2550–2554. doi: 10.1073/pnas.84.8.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Mudd J, Merlie J P. Isolation and characterization of the β and ɛ subunit genes of mouse muscle acetylcholine receptor. Journal of Biological Chemistry. 1989;264:7611–7616. [PubMed] [Google Scholar]
- Diamond J, Miledi R. A study of foetal and new-born rat muscle fibres. Journal of Physiology. 1962;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S, Mileo A M, Grassi F, Salvatore A M, Alemà S, Eusebi F. Identification of a determinant of AChR gating kinetics in the extracellular portion of the γ subunit. European Journal of Neuroscience. 1996;8:2564–2570. doi: 10.1111/j.1460-9568.1996.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Goldman D, Tamai K. Coordinate regulation of RNAs encoding two isoforms of the rat muscle acetylcholine receptor β-subunit. Nucleic Acids Research. 1989;17:3049–3056. doi: 10.1093/nar/17.8.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z W, Sanes J R. Synaptic structure and development: the neuromuscular junction. Neuron. 1993;10(suppl.):99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- Karlin A. Structure of nicotinic acetylcholine receptors. Current Opinion in Neurobiology. 1993;3:299–309. doi: 10.1016/0959-4388(93)90121-e. 10.1016/0959-4388(93)90121-E. [DOI] [PubMed] [Google Scholar]
- Kawa K, Obata K. Altered developmental changes of neuromuscular junction in hypo- and hyperthyroid rats. Journal of Physiology. 1982;329:143–161. doi: 10.1113/jphysiol.1982.sp014295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopta C, Steinbach J H. Comparison of mammalian adult and fetal nicotinic acetylcholine receptors stably expressed in fibroblasts. Journal of Neuroscience. 1994;14:3922–3933. doi: 10.1523/JNEUROSCI.14-06-03922.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kues W A, Sakmann B, Witzemann V. Differential expression patterns of five acetylcholine receptor subunit genes in rat muscle during development. European Journal of Neuroscience. 1995;7:1376–1385. doi: 10.1111/j.1460-9568.1995.tb01129.x. [DOI] [PubMed] [Google Scholar]
- Leonard R J, Nakajima S, Nakajima Y, Takahashi T. Differential development of two classes of acetylcholine receptors in Xenopus muscles in culture. Science. 1984;226:55–57. doi: 10.1126/science.6474189. [DOI] [PubMed] [Google Scholar]
- Martinou J-C, Merlie J P. Nerve-dependent modulation of acetylcholine ɛ-subunit gene expression. Journal of Neuroscience. 1991;11:1291–1299. doi: 10.1523/JNEUROSCI.11-05-01291.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileo A M, Monaco L, Palma E, Grassi F, Miledi R, Eusebi F. Two forms of acetylcholine receptor γ subunit in mouse muscle. Proceedings of the National Academy of Sciences of the USA. 1995;92:2686–2690. doi: 10.1073/pnas.92.7.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Seeburg P H, Wisden W. Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron. 1991;6:799–810. doi: 10.1016/0896-6273(91)90176-z. 10.1016/0896-6273(91)90176-Z. [DOI] [PubMed] [Google Scholar]
- Newland C F, Beeson D, Vincent A, Newsom-Davis J. Functional and non-functional isoforms of the human muscle acetylcholine receptor. Journal of Physiology. 1995;489:767–778. doi: 10.1113/jphysiol.1995.sp021090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B, Patlak J, Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980;286:71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Sine S M, Steinbach J H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. Journal of Physiology. 1987;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater C R. Postnatal maturation of nerve-muscle junctions in hindlimb muscles of the mouse. Developmental Biology. 1982;94:11–22. doi: 10.1016/0012-1606(82)90063-x. [DOI] [PubMed] [Google Scholar]
- Yu L, La Polla J, Davidson N. Mouse muscle acetylcholine receptor γ subunit: cDNA sequence and gene expression. Nucleic Acids Research. 1986;14:3539–3555. doi: 10.1093/nar/14.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]


