Abstract
Fast excitatory neurotransmission in the central nervous system is mediated through glutamate acting on ionotropic glutamate receptors. However, glutamate acting on metabotropic glutamate receptors (mGluRs) can also exert an inhibitory action. Here, we report by immunocytochemistry and physiology, to our knowledge, the first glutamate receptor to be found in terminals of photoreceptors in the mammalian retina—the group III metabotropic glutamate receptor mGluR8. Glutamate is the transmitter of photoreceptors, and thus mGluR8 functions as an autoreceptor. Activation of mGluR8 by the group III mGluR agonists l-2-amino-4-phosphonobutyrate and l-serine-O-phosphate, or by glutamate itself, evokes a decrease in the intracellular calcium ion concentration ([Ca2+]i) in isolated photoreceptors. This effect is blocked by the group III mGluR antagonists (RS)-α-methyl-4-phosphonophenylglycine and (RS)-α-methylserine-O-phosphate. Agonists for other classes of glutamate receptors—n-methyl-d-aspartic acid, quisqualic acid, kainic acid, or (RS)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid—have no effect on the [Ca2+]i in isolated photoreceptors. The down-regulation of the [Ca2+]i in photoreceptors by mGluR8 provides evidence for an inhibitory feedback loop at the photoreceptor synapse in the mammalian retina. This negative feedback may be a mechanism for the fine adjustment of the light-regulated release of glutamate from photoreceptors and may serve as a safety device against excitotoxic levels of release at this tonic synapse. Such a mechanism may provide a model for feedback inhibition in other parts of the central nervous system.
Glutamate is the neurotransmitter of the vertical signal pathway through the mammalian retina. Photoreceptors and their postsynaptic partners, the bipolar cells, release glutamate to mediate the fast transfer of light signals onto the ganglion cells, the output neurons of the retina (1, 2). In recent years, different glutamate receptors with specific functional properties have been described and localized to identified synapses in the retina (3–9).
In contrast to ionotropic glutamate receptors, which mediate fast excitatory synaptic transmission (10), metabotropic glutamate receptors (mGluRs) are coupled to G-proteins and act on various intracellular second messenger systems. mGluRs are located pre- and postsynaptically and play important roles in modulating synaptic activity (11, 12). Indeed, glutamate acting via mGluRs can exert not only an excitatory but also an inhibitory action (11, 12). In the mammalian retina, the group III mGluR, mGluR6, is expressed postsynaptically at the synapse between photoreceptor cells and ON-bipolar cells (13). In darkness, when photoreceptors release glutamate, activation of mGluR6 produces a hyperpolarization of the ON-bipolar cells (4, 14–17).
mGluR8 is another member of the group III mGluRs that are activated by agonists such as l-2-amino-4-phosphonobutyrate (l-AP4) and l-serine-O-phosphate (l-SOP) (12, 18). We generated a mGluR8-specific antiserum and report the immunocytochemical localization of mGluR8 in the terminals of photoreceptors. Using fluo-3 acetoxymethylester (fluo-3) as an intracellular calcium indicator dye and video imaging microscopy, we show in isolated photoreceptors that activation of mGluR8 by glutamate, l-AP4, and l-SOP, but not by other glutamate receptor agonists, decreases [Ca2+]i. The calcium concentration in presynaptic nerve terminals and transmitter release are intimately related (19, 20). Our study provides evidence for the possible autoreceptor inhibition of glutamate release at photoreceptor synapses via a mGluR, a mechanism that could modulate and fine tune the glutamatergic synaptic transmission between the photoreceptors and their postsynaptic partners.
MATERIALS AND METHODS
All experiments were performed in compliance with the guidelines for the welfare of experimental animals issued by the Federal Government of Germany, the National Institutes of Health, and the Max Planck Society.
Generation and Characterization of the Antiserum Against mGluR8.
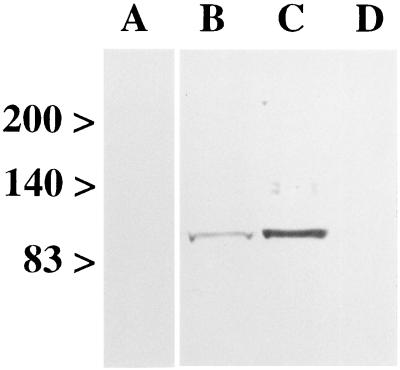
A synthetic peptide corresponding to the carboxyl-terminal amino acid sequence of mouse mGluR8 (residues 890–908: ETNTSSTKTTYISYSDHSI) was coupled to keyhole limpet haemocyanin by using glutaraldehyde. A polyclonal antiserum was raised in New Zealand white rabbits according to standard techniques (21). Antibodies were precipitated with ammonium sulfate and purified by immunoaffinity chromatography with peptide-coupled Affigel 10/15 (Bio-Rad). The specificity of the mGluR8 antiserum was tested by immunoblotting (8) of membrane proteins (80 μg/lane) of rat retina, olfactory bulb, and liver (Fig. 1). The labeling could be specifically blocked by preadsorption of the antiserum with its antigen (Fig. 1, lane A). The antiserum was used at a concentration of 0.1 μg protein/ml.
Figure 1.
Specificity of the antiserum against mGluR8. Eighty μg of protein of membranes from rat retina (lanes A, B), rat olfactory bulb (lane C), and rat liver (lane D) were separated on a 7.5% SDS/PAGE gel. Numbers and arrowheads (Left) indicate the position and relative molecular mass (kDa) of the marker bands. With the antiserum against mGluR8, bands at approximately 100 kDa were detected in retina (lane B) and olfactory bulb (lane C). The labeling could be specifically blocked by preadsorption of the antiserum with its antigenic peptide (lane A; retina), and no band was detected in liver (lane D).
Tissue Preparation and Light and Electron Microscopic Immunocytochemistry of Rat Retina Sections.
Retinae of adult albino rats were investigated. The preparation of retinal tissue for light and electron microscopy, the immunostaining procedure, and the microscopic analysis were performed as described (5).
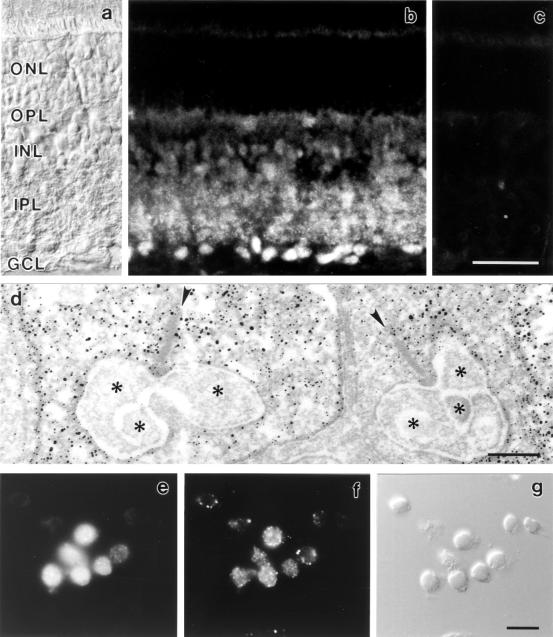
Briefly, the tissue was fixed for light microscopy (LM) in 4% (wt/vol) paraformaldehyde (PA) in phosphate buffer (PB; 0.1 M, pH 7.4) for 15 min, and for electron microscopy (EM) in 4% (wt/vol) PA and 0.05% (vol/vol) glutaraldehyde in PB for 10 min, followed by an additional 40 min in 4% (wt/vol) PA in PB. The mGluR8 antiserum was used at a concentration of 0.1 μg protein/ml. Incubation time in the primary antiserum for LM was overnight at room temperature and for EM, 4 d at 4°C. In controls, the mGluR8 antiserum was preadsorbed with a 10-fold excess of its respective antigenic peptide for 1 hr, resulting in a complete loss of specific staining (Fig. 2c).
Figure 2.
(a–c) Micrographs of a vertical cryostat section through rat retina. (a) Nomarski micrograph showing the retinal layers (ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer). (b) Retina section stained with the antiserum against mGluR8. The antiserum is directed against the carboxyl-terminal end of the receptor, which is located intracellularly. Immunofluorescence is present in both synaptic layers, the IPL and the OPL, and in somata of cells in the INL and GCL. (c) Control; the anti-mGluR8 antiserum was preadsorbed with the antigen, resulting in a complete loss of specific immunoreactivity. (d) Electron micrograph showing the presynaptic localization of mGluR8 immunoreactivity in rod spherules. The presynaptic ribbon in the rod spherule is marked by an arrowhead. Postsynaptic processes are marked by stars. (e–g) Double labeling of isolated photoreceptors with an antibody against arrestin (e) and the antiserum against mGluR8 (f). (g) Nomarski micrograph showing the isolated cells. (Bars = 30 μm in c for a–c; 0.2 μm in d; and 10 μm in g for e–g).
Freshly Isolated Photoreceptors and Immunocytochemistry.
Retinae were mechanically and enzymatically dissociated as described (22). Cells were plated on poly-l-lysine-coated coverslips and allowed to settle for 30 min for tight attachment to the surface. For immunocytochemistry, the cells were fixed for 5 min in 4% (wt/vol) PA in PB and processed for immunocytochemistry as described for retinal sections but with reduced incubation times—1 to 2 hr each for preincubation and primary and secondary antibody incubations. The mGluR8 antiserum was used at a concentration of 0.1 μg protein/ml; the antibody against the splice variant of arrestin (S85–63) (23) was diluted 1:10 (the anti-arrestin antibody was kindly provided by P. A. Hargrave, Department of Ophthalmology, College of Medicine, University of Florida, Gainesville, FL).
Calcium Indicator Dye Labeling.
After dissociation and during the experiments, the cells were maintained in extracellular solution (ECS, in mM: NaCl, 137; KCl, 5; CaCl2, 2; Na2HPO4, 1; MgSO4, 1; Hepes, 10; glucose, 22; pH 7.4). Before the optical recording, cells were incubated in ECS containing 1 μM cell permeant fluo-3 (fluo-3 acetoxymethyl ester; Molecular Probes) with 0.045% DMSO and 0.005% pluronic acid (Molecular Probes) for 15–30 min. Kept in ECS and at room temperature, cells showed reproducible responses to pharmacological stimuli for up to 5 hr after dissociation. Only these experimental data were incorporated in Results. A live/dead staining of dissociated cells was performed according to the manufacturer‘s instructions (LIVE/DEAD Viability/Cytotoxicity Kit for Animal Cells; Molecular Probes) before and after the experiments to ensure the viability of the cells; less than 1% of the cells were not viable during the time period the experiments were conducted.
Calcium Optical Recording.
The fluo-3 fluorescence present in loaded cells was measured with a Merlin system for low-light-level real-time ratiometric fluorescence imaging (Life Science Resources, Cambridge, U.K.), consisting of a SpectraMASTER high-speed monochromator, an 8-bit analog CCD camera with a single-stage intensifier, merlin 1.87 software, computer workstation for image acquisition and analysis, and a Zeiss Axioskop equipped with an appropriate Zeiss fluorescence filter set (FITC: 450–490, FT 510, LP 520) and an Achroplan ×63/0.90 W objective. Images were acquired every 0.5–1.5 sec as an average of four frames.
Drug Application and Drugs Used.
During the optical recording experiments, cells were kept in a chamber on the microscope stage at room temperature and were superfused constantly with ECS at a flow rate of 1 ml/min. Drugs were bath-applied directly into the chamber, which had a volume of 0.5 ml.
l-2-amino-4-phosphonobutyrate (l-AP4), nifedipine, N-methyl-d-aspartic acid (NMDA), (S)-3-carboxy-4-hydroxyphenylglycine (S-CHPG), (±) Bay K 8644, l-quisqualic acid, l-serine-O-phosphate (l-SOP), kainic acid, (2S, 1′S, 2′S)-2-(carboxycyclopropyl) glycine (L-CCG-I), and l-glutamic acid (Glu) were obtained from Alexis (Grünberg, Germany); (RS)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (CAMPA), (RS)-α-methyl-4-phosphonophenylglycine (MPPG), (RS)-α-methylserine-O-phosphate (MSOP), and trans-azetidine-2, 4-dicarboxylic acid (t-ADA) were obtained from Tocris Cookson (Bristol, U.K.); N6, 2′-O-dibutyryladenosine 3′:5′-cyclic monophosphate (DB-cAMP) and 8-bromoadenosine 3′:5′-cyclic monophosphate (8-Br-cAMP) were obtained from Sigma; and 1-(5-isoquinolinesulfonyl)-2-methylpiperazine⋅2 HCl (H-7) was purchased from Biomol (Plymouth Meeting, PA). For the NMDA experiments, the standard ECS was modified in that it contained 1 μM glycine (Sigma) and no magnesium. When drugs had to be dissolved in DMSO or ethanol, the appropriate controls were performed and showed no effects on the intracellular calcium ion concentration ([Ca2+]i). To keep the osmolarity constant in high-potassium medium, the NaCl concentration was adjusted. The calcium-free ECS consisted of (in mM): NaCl, 137; KCl, 5.4; MgCl2, 2.5; Hepes, 5; EGTA, 5). The cobalt containing ECS consisted of (in mM): NaCl, 132; KCl, 1; MgCl2, 1; Hepes, 10; glucose, 22; CoCl2, 4).
Analysis of the Calcium Transients.
Changes in fluorescence intensity of fluo-3-labeled cells were determined for individual photoreceptor cells by using the Merlin system. Fluo-3, on binding of calcium ions, undergoes a 40- to 200-fold increase in fluorescence (24, 25) measured at 488 nm. Changes in fluorescence intensity were calculated by dividing the measured fluorescence intensity during drug application (F) by the measured average baseline fluorescence intensity (F/F0).
Random spontaneously occurring changes in fluorescence intensity, as well as changes after the application of control substances, were in the range of 1–3% of F/F0. Because there is no linear correlation between the F/F0 values and changes in the [Ca2+]i, the present data provide a qualitative estimate of the drug-evoked calcium responses in photoreceptor cells.
“Outer Retina” Experiments: Determination of cAMP Concentrations.
Eyes of adult rats were dissected as described (5). The “outer retina” preparation, retina tissue without the inner cell layers (inner nuclear layer, ganglion cell layer), was carried out under daylight illumination and at room temperature after a modified procedure as described for Xenopus laevis retina (26). One eyecup was superfused with ECS but otherwise remained untreated and served as a control. The other eyecup was filled with 0.5% Triton X-100 in deionized water for 2 min, flushed with deionized water twice, and superfused with ECS for 1 hr. After the incubation, the retinae of the experimental and the control eye were removed from the eyecups. The inner cell layers of the Triton X-100-treated retina were taken off with forceps and a paintbrush. To check whether the destruction of the inner cell layers was successful and whether the outer nuclear layer (ONL) and the outer plexiform layer (OPL) were still intact, a live/dead staining of a tissue sample was performed according to the manufacturer’s instructions before the experiments (LIVE/DEAD Viability/Cytotoxicity Kit for Animal Cells; Molecular Probes). Typically, only the ONL, the OPL, and very few cells of the inner nuclear layer close to the OPL (putative horizontal cells) were found to be intact. The intact retina and the “outer retina” were cut into small pieces of 5–10 mg wet weight and were incubated in ECS that contained glutamate or l-AP4 (concentrations as used for the calcium imaging experiments) to test the effects of group III mGluR agonists on the cAMP concentration. To determine the control cAMP levels, pieces of the intact retina and of the “outer retina” were incubated in buffer alone. The total cAMP content of retinal tissue was determined as pmol cAMP/g (wet weight) of retinal tissue by using the cAMP-EIA kit from Cayman Chemicals (Ann Arbor, MI).
RESULTS
mGluR8 Is Expressed in the Rat Retina.
An antiserum was raised against a peptide corresponding to the carboxyl-terminal amino acid sequence of mGluR8 and was purified by immunoaffinity chromatography. The specificity of the purified antiserum was analyzed by immunoblotting rat retina membranes after SDS/PAGE. The immunoblot incubated with the antiserum directed against mGluR8 showed one labeled band of protein in rat retina (Fig. 1, lane B) and rat olfactory bulb (Fig. 1, lane C) membrane preparations. The labeled protein had an apparent molecular mass of 100 kDa, which is in agreement with the molecular mass deduced from the cDNA sequence of mGluR8 (18). The labeling could be specifically blocked by preadsorption of the antiserum with its antigen (Fig. 1, lane A), and no band was detected in rat liver (Fig. 1, lane D).
mGluR8 immunoreactivity was present in both plexiform layers of the retina (Fig. 2b). Somata in the inner nuclear layer and the ganglion cell layer were labeled with varying intensities. Preadsorption of the anti-mGluR8 antiserum with the antigenic peptide before application to retinal sections resulted in a complete absence of specific staining (Fig. 2c). Because the antiserum was generated against the mouse carboxyl-terminal peptide of mGluR8 [one amino acid difference between the two species (27)], we also checked the specificity of the antiserum in mouse retina. The mGluR8 staining pattern in mouse retina was identical to that of rat retina and could be blocked specifically by preadsorption of the antiserum with the peptide.
mGluR8 Is a Presynaptic Autoreceptor in Photoreceptor Terminals.
We used a highly sensitive immunocytochemical method (see Materials and Methods) to examine by electron microscopy the subcellular distribution of mGluR8.
The most striking finding was the presence of mGluR8 immunoreactivity presynaptically in the terminals of photoreceptors, in the rod spherules (Fig. 2d), and in the cone pedicles; the distribution of the immunoreactivity in the terminals was diffuse (Fig. 2d). The preembedding immunoelectron microscopic technique as used in this study is a very sensitive technique. This advantage, however, is compromised by a low spatial resolution caused by the diffusion of the diaminobenzidine reaction product, sometimes over a few micrometers, causing the diffuse appearance of the staining. Occasionally, mGluR8 immunoreactivity was also found in postsynaptic processes at photoreceptor synapses (data not shown).
Immunoreactivity in the inner nuclear layer, the inner plexiform layer, and the ganglion cell layer was clearly associated with amacrine cells and ganglion cells and was found postsynaptically at bipolar cell ribbon synapses on processes of ganglion cells and amacrine cells.
Immunocytochemical Characterization of Isolated Photoreceptors.
In dissociates of rat retina, two types of cell can be identified easily by using morphological criteria: rod bipolar cells (22, 28, 29) and Müller cells (22). Most of the other retinal cell types, including photoreceptors, tend to retract their processes or have already lost their processes leading to spherically shaped cells. To study mGluR8 function in isolated photoreceptors, we first had to establish criteria by which photoreceptors could be identified even in unstained material. Staining of dissociates with an antibody against a splice variant of arrestin (23), which specifically stains photoreceptors in retinal sections, produced a high number of labeled putative photoreceptors. In fact, most cells with a round shape and a diameter of 4–5 μm, usually the smallest cells in the dissociates, were arrestin positive (Fig. 2e). In double-labeling experiments, the arrestin-positive photoreceptors clearly expressed mGluR8 (Fig. 2 e and f). Typically, when focusing through the whole depth of the isolated photoreceptor cell, the anti-mGluR8 staining seemed to be associated with the plasma membrane of the cell distributed in several small patches (Fig. 2f), whereas arrestin staining seemed to be present intracellularly throughout the cell’s cytoplasm (Fig. 2e). The small size and round shape were the main criteria used to select photoreceptor cells for functional analysis in the calcium imaging experiments.
Activation of mGluR8 Reduces [Ca2+]i in Isolated Photoreceptors.
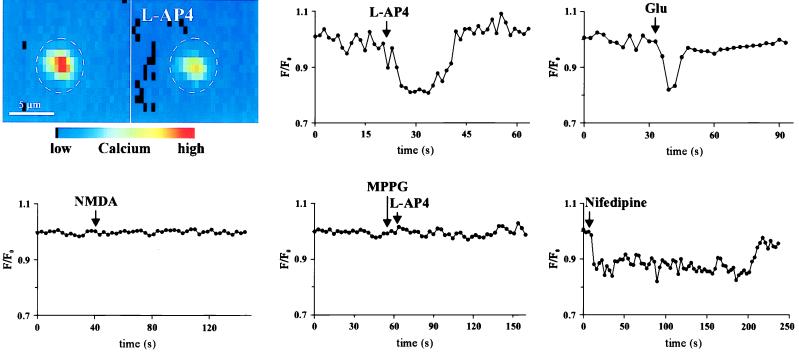
The [Ca2+]i of freshly isolated photoreceptors was monitored optically by loading the cells with the calcium indicator fluo-3. To test the effects of mGluR8 activity, different glutamate receptor agonists and antagonists were applied and changes in the [Ca2+]i, as the change of fluorescence intensity over the basal level of the fluorescence intensity (F/F0), were optically recorded. l-AP4, l-SOP and l-glutamate, each applied into the bath at a concentration of 10 to 200 μM, produced a reversible decrease in the [Ca2+]i (Fig. 3; Table 1). Characteristically, this effect lasted several seconds. Because of the bath application of the drugs, no precise temporal analysis of the calcium response was possible. The effect was reversible with washout of the agonists (Fig. 3), and the application of the drugs could be repeated several times; however, with increasing numbers of applications, only partial recoveries could be achieved after washout. Bath application of other agonists of mGluRs, trans-azetidine-2, 4-dicarboxylic acid, quisqualic acid, (S)-3-carboxy-4-hydroxyphenylglycine, and (2S,1′S,2′S)-2-(carboxycyclopropyl)glycine, and of agonists of ionotropic glutamate receptors, NMDA, kainic acid, and (RS)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, were ineffective in changing the [Ca2+]i in isolated photoreceptors over a range of 10 μM-10 mM (Fig. 3; Table 1). Furthermore, the decrease in the [Ca2+]i on activation of mGluR8 in photoreceptors could be specifically blocked by the group III mGluR antagonists MPPG (10–300 μM) and MSOP (10–300 μM), with MPPG showing greater potency (Fig. 3; Table 1).
Figure 3.
The color figure shows a typical calcium imaging experiment performed on a freshly isolated photoreceptor loaded with the calcium indicator fluo-3. The high [Ca2+]i in the photoreceptor before the application of l-AP4 (Left) significantly drops after the application of l-AP4 (Right). As shown in the graphs representing single experiments, l-AP4 and glutamate lead to a decrease in the [Ca2+]i in photoreceptors; NMDA does not. The decrease of the [Ca2+]i by l-AP4 can be specifically blocked by the group III mGluR antagonist MPPG. Application of the l-type calcium channel blocker nifedipine leads to a decrease of the [Ca2+]i in a range comparable to that elicited by l-AP4 or glutamate.
Table 1.
Calcium imaging experiments
| Drug | Cells nr. | Mean % | SD % | S.L. |
|---|---|---|---|---|
| l-AP4 | 429 | 88 | 7 | *** |
| l-SOP | 253 | 91 | 6 | *** |
| l-glutamic acid | 213 | 89 | 8 | *** |
| MPPG/L-AP4 | 30 | 100 | 1 | |
| MSOP-L-AP4 | 16 | 96 | 5 | |
| MPPG/L-SOP | 12 | 100 | 1 | |
| MSOP/L-SOP | 11 | 98 | 5 | |
| MPPG/Glu | 34 | 99 | 1 | |
| MSOP/Glu | 23 | 99 | 1 | |
| MPPG | 19 | 104 | 1 | |
| MSOP | 21 | 107 | 3 | * |
| 8-Br-cAMP/l-AP4 | 38 | 93 | 3 | ** |
| 8-Br-cAMP/Glu | 21 | 92 | 2 | * |
| 8-Br-cAMP/l-SOP | 10 | 93 | 1 | * |
| 8-Br-cAMP | 38 | 100 | 3 | |
| DB-cAMP/l-AP4 | 14 | 92 | 1 | ** |
| DB-cAMP | 14 | 100 | 2 | |
| H-7 | 4 | 100 | 4 | |
| NMDA | 33 | 100 | 4 | |
| l-CCG-I | 49 | 98 | 4 | |
| Kainic acid | 71 | 100 | 4 | |
| l-Quisqualic acid | 38 | 100 | 4 | |
| AMPA | 19 | 100 | 4 | |
| S-CHPG | 15 | 100 | 4 | |
| t-ADA | 25 | 100 | 4 | |
| ECS | 21 | 100 | 1 | |
| ECS ([K+], 40 mM) | 3 | 100 | 1 | |
| ECS ([Co2+], 4 mM) | 48 | 57 | 13 | *** |
| ECS ([Ca2+], 0 mM) | 59 | 82 | 8 | ** |
| Nifedipine | 16 | 85 | 6 | ** |
| Bay K 8644 | 22 | 108 | 1 | * |
Significance levels (S.L.): ∗∗∗, 0.1%; ∗∗, 1%; ∗, 5%. No symbol, not significantly different from control.
mGluR8, Calcium Channels, and the cAMP Pathway.
The [Ca2+]i of photoreceptors could not be significantly increased by the application of high concentrations of potassium (20–40 mM) in the ECS. Control application of standard ECS, to check for possible artefacts caused by the bath application procedure, revealed no changes in [Ca2+]i. However, if calcium was removed from the extracellular medium or if Co2+ was present in the extracellular medium, intracellular calcium levels dropped significantly and recovered only partially (Table 1).
Nifedipine, a l-type calcium channel blocker, also produced a significant decrease in the [Ca2+]i in a range comparable to the effects caused by l-AP4, l-SOP, or glutamate (Fig. 3; Table 1). The decrease was smaller than the Co2+-induced decrease and showed a faster and complete return of the [Ca2+]i to basal levels after washout. The application of (±) Bay-K 8644 (100–500 μM), a known activator of high-threshold voltage-activated l-type calcium channels (30), resulted in a small but significant increase in the [Ca2+]i (Table 1).
To study the proposed involvement of the cAMP pathway in mGluR8 function (11, 12) and the down-regulation of the [Ca2+]i, we compared cAMP levels in pieces of intact retina tissue with tissue with the inner cell layers removed (“outer retina” preparation; see Materials and Methods). On the basis of localization studies of retinal mGluRs (9), “outer retina” preparations should contain mGluR8 as the only mGluR potentially using cAMP as messenger. The effects of drugs on the cAMP concentration in the tissue were calculated as percentages of values derived from buffer control applications. When “outer retina” preparations were treated with 10–200 μM glutamate (108 ± 13%; n = 4) or 10–200 μM l-AP4 (99 ± 22%; n = 12), no significant differences in the cAMP concentrations were observed compared with controls. However, when we measured the cAMP levels in the intact retina, which contains mGluR2 and all known group III mGluRs (9), a significant decrease in the cAMP content was found when treated with 10–200 μM glutamate (78 ± 18%; n = 4) or 10–200 μM l-AP4 (66 ± 15%; n = 12) compared with buffer control applications.
To examine further possible mechanisms involved in the down-regulation of [Ca2+]i by mGluR8, we manipulated intracellular signaling pathways postulated for use by group III mGluRs (11, 12). Preincubation with or coapplication of 8-Br-cAMP and DB-cAMP, hydrolysis-resistant analogs of cAMP or of H-7 (10–100 μM), a protein kinase A, protein kinase C, and protein kinase G, together with l-AP4, l-SOP or glutamate, had no significant effect on the mGluR8-mediated decrease of the [Ca2+]i (Table 1); the drugs alone had no effect (Table 1).
DISCUSSION
The cellular and subcellular distribution of mGluRs in the mammalian retina have been studied extensively in recent years (9), and so far only mGluR7 has been localized presynaptically (5). With mGluR8, a member of the same group of mGluRs as mGluR7 (12), the present study reports the second presynaptic mGluR in the retina, but the first to be present in photoreceptor terminals. It is intriguing to study the autoreceptor function of mGluR8, because it has been a long-standing issue of how photoreceptors regulate their glutamate release (31, 32). The experiments on mGluR8 in isolated rat photoreceptors by using calcium imaging techniques suggest a presynaptic role of mGluR8 in modulating the [Ca2+]i in photoreceptor terminals.
Stimulation with high potassium (40 mM) did not lead to an increase in the [Ca2+]i, compared with resting levels, suggesting that the mechanically and enzymatically isolated photoreceptors are in a depolarized state, probably because of the loss of the light-perceiving apparatus that, on light activation, hyperpolarizes the cell (33). Nevertheless, the recorded photoreceptors were alive over several hours, as determined by a viability stain assay (see Materials and Methods).
Of all the different drugs applied, only l-AP4, l-SOP, and glutamate caused a small (10–15%) but highly significant and reversible decrease in the [Ca2+]i. Moreover, the decrease could be specifically blocked with the group III mGluR antagonists MPPG and MSOP. Thus, the measured effects show the specificity of group III mGluRs and because mGluR8 is the only mGluR to be found in photoreceptors, of mGluR8. In intact photoreceptors, the calcium channels are localized in the immediate vicinity of the release site at the ribbon synapse (32, 34). In isolated photoreceptors, a redistribution of the calcium channels occurs and, as the fluo-3 assay reflects the average bulk cytosolic changes in the [Ca2+]i, the observed effect on the decrease in [Ca2+]i underestimates the true local calcium changes that occur directly at the release site.
Group III mGluRs are involved in mediating presynaptic autoreceptor inhibition of neurotransmitter release at glutamatergic synapses (12, 35–38). Possible mechanisms are the down-regulation of the presynaptic voltage-gated calcium channels through G-proteins, an increase of a potassium conductance, or a direct presynaptic receptor action on the exocytotic machinery downstream of calcium (39). Many studies have shown that presynaptic inhibition of elicited neurotransmitter release is largely because of the inhibition of voltage-gated calcium channels (39–41).
In the photoreceptor terminals, the main voltage-gated calcium channels are the slowly inactivating l-type channels (26, 42–44). The maximal decrease in the [Ca2+]i after the application of the l-type calcium channel blocker nifedipine was in a range comparable to the maximal decrease caused by l-AP4 or glutamate. This observation suggests that l-type calcium channels are the main target for the modulatory presynaptic action of mGluR8 at photoreceptor synapses. The result of the experiments with Co2+ in the ECS, leading to a greater and longer-lasting decrease in the [Ca2+]i, is possibly caused by the presence of additional calcium channels of the N and/or P/Q-type in photoreceptor terminals. Whether mGluR8 modulates only l-type channel activity or whether other calcium channels are also affected remains to be shown. We favor l-type channels, because they have been shown to be present in photoreceptor terminals directly at the site of transmitter release (32), and in the experiments applying Bay K 8644, which promotes the open state of l-type calcium channels, a significant increase in the [Ca2+]i was observed. Furthermore, in Xenopus photoreceptors, it has been shown that glutamate release is controlled mainly by the dihydropyridine-sensitive l-type channels (26, 44).
The observed decrease in the [Ca2+]i in the photoreceptors caused by l-AP4, l-SOP, and glutamate could be (i) because of a direct membrane-delimited G-protein-coupled interaction between mGluR8 and calcium channels, or (ii) because of a negative coupling between mGluR8 and adenylyl cyclase activity, causing a decrease in cAMP levels and indirectly an attenuation of voltage-dependent calcium entry into photoreceptor terminals. It was not possible to examine the first point, namely the involvement of pertussis toxin (PTX)-sensitive G-proteins in the inhibition of high-threshold calcium channels, as shown for a presynaptic l-AP4 receptor on cultured olfactory-bulb neurons (36), because the isolated photoreceptors did not survive the long pretreatment required with PTX. The examination of the second point, however, was possible.
The observations that 8-Br-cAMP and DB-cAMP had no significant effect on the mGluR8-induced decrease in the [Ca2+]i in isolated photoreceptors and the fact that cAMP levels in the “outer retina” preparation did not differ from buffer-treated retina preparation after drug treatment imply that the effect does not rely on a cAMP-dependent mechanism. Furthermore, the results of the experiments with the kinase inhibitor H-7 suggest that protein phosphorylation is also not likely to be involved in the observed changes in the [Ca2+]i.
Our study suggests that glutamate released from photoreceptors may activate in a feedback fashion the presynaptically localized mGluR8. mGluR8, most likely negatively coupled to the activity of l-type calcium channels, would then down-regulate the amount of glutamate released from photoreceptors. In conclusion, this negative feedback through mGluR8 at photoreceptor terminals could play a significant role in modulating and fine tuning the light-regulated release of glutamate and could serve as a safety device against excitotoxic levels of glutamate release at this tonic synapse. Finally, such a mechanism may provide a model for feedback inhibition in other parts of the central nervous system.
Acknowledgments
We thank A. Hildebrand, W. Hofer, G. S. Nam, and D. Benzaid for excellent technical assistance and A. Hirano for reading and improving the manuscript. This study was supported by a BASF Fellowship to P.K., a grant from the Deutsche Forschungsgemeinschaft (SFB 269/B4), and a Heisenberg Fellowship to J.H.B.
ABBREVIATIONS
- l-AP4
l-2-amino-4-phosphonobutyrate
- l-SOP
l-serine-O-phosphate
- mGluR
metabotropic glutamate receptor
- MPPG
(RS)-α-methyl-4-phosphonophenylglycine
- MSOP
(RS)-α-methylserine-O-phosphate
- NMDA
N-methyl-d-aspartic acid
- ECS
extracellular solution
- 8-Br-cAMP
8-bromoadenosine 3′:5′-cyclic monophosphate
- DB-cAMP
N6,2′-O-dibutyryladenosine 3′:5′-cyclic monophosphate
- H-7
1-(5-isoquinolinesulfonyl)-2-methylpiperazine⋅2 HCl
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Marc R E, Liu W-L S, Kallionatis M, Raiguel S F, van Haesendonck E. J Neurosci. 1990;10:4006–4034. doi: 10.1523/JNEUROSCI.10-12-04006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massey S M. In: Progress in Retinal Research. Osborne N, Chader J, editors. Oxford: Pergamon; 1990. pp. 399–425. [Google Scholar]
- 3.Hartveit E, Brandstätter J H, Sassòe-Pognetto M, Laurie D J, Seeburg P H, Wässle H. J Comp Neurol. 1994;348:570–582. doi: 10.1002/cne.903480407. [DOI] [PubMed] [Google Scholar]
- 4.Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, et al. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- 5.Brandstätter J H, Koulen P, Kuhn R, van der Putten H, Wässle H. J Neurosci. 1996;16:4749–4756. doi: 10.1523/JNEUROSCI.16-15-04749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koulen P, Malitschek B, Kuhn R, Wässle H, Brandstätter J H. Eur J Neurosci. 1996;8:2177–2187. doi: 10.1111/j.1460-9568.1996.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 7.Brandstätter J H, Koulen P, Wässle H. J Neurosci. 1997;17:9298–9307. doi: 10.1523/JNEUROSCI.17-23-09298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koulen P, Kuhn R, Wässle H, Brandstätter J H. J Neurosci. 1997;17:2200–2211. doi: 10.1523/JNEUROSCI.17-06-02200.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandstätter J H, Koulen P, Wässle H. Vision Res. 1998;38:1385–1397. doi: 10.1016/s0042-6989(97)00176-4. [DOI] [PubMed] [Google Scholar]
- 10.Hollmann M, Heinemann S. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 11.Pin J-P, Duvoisin R. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 12.Conn P J, Pin J-P. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 13.Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 14.Slaughter M M, Miller R F. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- 15.Nawy S, Jahr C E. Nature (London) 1990;346:269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- 16.Shiells R A, Falk G. Proc R Soc Lond Ser B. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita M, Wässle H. J Neurosci. 1991;11:2372–2382. doi: 10.1523/JNEUROSCI.11-08-02372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duvoisin R M, Zhang C, Ramonell K. J Neurosci. 1995;15:3075–3083. doi: 10.1523/JNEUROSCI.15-04-03075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz B, Miledi R. J Physiol (London) 1967;192:407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith S J, Augustine G J. Trends Neurosci. 1988;11:458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- 21.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, New York: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 22.Hartveit E, Brandstätter J H, Enz R, Wässle H. Eur J Neurosci. 1995;7:1472–1483. doi: 10.1111/j.1460-9568.1995.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith W C, Milam A H, Dugger D, Arendt A, Hargrave P A, Palczewski K. J Biol Chem. 1994;269:15407–15410. [PubMed] [Google Scholar]
- 24.Minta A, Kao J P Y, Tsien R Y. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- 25.Harkins A B, Kurebayashi N, Baylor S M. Biophys J. 1993;65:865–881. doi: 10.1016/S0006-3495(93)81112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz Y, Witkovsky P. Neurocience. 1997;78:1209–1216. doi: 10.1016/s0306-4522(96)00678-1. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita A, Ohishi H, Neki A, Nomura S, Shigemoto R, Takada M, Nakanishi S, Mizuno N. Neurosci Lett. 1996;207:61–64. doi: 10.1016/0304-3940(96)12489-7. [DOI] [PubMed] [Google Scholar]
- 28.Greferath U, Grünert U, Wässle H. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- 29.Karschin A, Wässle H. J Neurophysiol. 1990;63:860–876. doi: 10.1152/jn.1990.63.4.860. [DOI] [PubMed] [Google Scholar]
- 30.Hille B. Ionic Channels of Excitable Membranes. 2nd Ed. Sunderland, MA: Sinauer; 1992. pp. 83–114. [Google Scholar]
- 31.Picaud S, Larsson H P, Wellis D P, Lecar H, Werblin F. Proc Natl Acad Sci USA. 1995;92:9417–9421. doi: 10.1073/pnas.92.20.9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgans C W, El Far O, Berntson A, Wässle H, Taylor W R. J Neurosci. 1998;18:2467–2474. doi: 10.1523/JNEUROSCI.18-07-02467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen W G. Annu Rev Physiol. 1987;49:743–764. doi: 10.1146/annurev.ph.49.030187.003523. [DOI] [PubMed] [Google Scholar]
- 34.Taylor W R, Morgans C. Visual Neurosci. 1998;15:541–552. doi: 10.1017/s0952523898153142. [DOI] [PubMed] [Google Scholar]
- 35.Forsythe I D, Clements J D. J Physiol (London) 1990;429:1–16. doi: 10.1113/jphysiol.1990.sp018240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trombley P Q, Westbrook G L. J Neurosci. 1992;12:2043–2050. doi: 10.1523/JNEUROSCI.12-06-02043.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakanishi S. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi T, Forsythe I D, Tsujimoto T, Barnes-Davies M, Onodera K. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- 39.Wu L-G, Saggau P. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 40.Hille B. Neuron. 1992;9:187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- 41.Dolphin A C. J Physiol (London) 1998;506:1. doi: 10.1111/j.1469-7793.1998.003bx.x. , 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bader C R, Bertrand D, Schwartz E A. J Physiol (London) 1982;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rieke F, Schwartz E A. J Physiol (London) 1996;493:1. doi: 10.1113/jphysiol.1996.sp021360. , 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witkovsky P, Schmitz Y, Akopian A, Krizaj D, Tranchina D. J Neurosci. 1997;17:7297–7306. doi: 10.1523/JNEUROSCI.17-19-07297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]