Abstract
Protein kinase modulation of glycine-activated currents was examined in acutely dissociated ganglion cells from tiger salamander retina using whole-cell voltage-clamp techniques.
Glycine (100 μM) induced an outward chloride current in cells clamped at 0 mV. Co-application of 50 μM forskolin made the glycine-induced current more transient. The combination of forskolin and glycine reduced the later portion of current response without changing the initial peak amplitude.
3-Isobutyl-1-methylxanthine (IBMX) or 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP) produced effects similar to those of forskolin. H-89, a protein kinase A (PKA) inhibitor, blocked the effect of forskolin.
A protein kinase C (PKC) activator, OAG (1-oleoyl-2-acetyl-sn-glycerol), also made the glycine response more transient. Unlike PKA analogues, OAG enhanced the glycine peak response without changing the glycine late response. OAG effects were blocked by 1 μM GF-109203X, a PKC inhibitor.
Nanomolar concentrations of strychnine selectively blocked the fast phase of the glycine current and reversed the effect of OAG, but not that of forskolin. Conversely, forskolin occluded the effect of 5,7-dichlorokynurenic acid, which selectively suppresses the late phase of the glycine current. The action of OAG was not blocked by 5,7-dichlorokynurenic acid.
Thus, through a differential modulation, both protein kinase A and C shorten the decay time of the glycine current. PKA suppresses the slow component, while PKC potentiates the fast component.
Phosphorylation of ligand-gated ion channels has been proposed as a possible mode of synaptic plasticity. The glycine receptor, a member of a family of ligand-gated ion channels, has been shown to be phosphorylated by intracellular second messenger-triggered protein kinases, including protein kinase A and C (Ruiz-Gomez, Vaello, Valdivieso & Mayor, 1991; Vaello, Ruiz-Gomez, Lerma & Mayor, 1994). Both kinases alter the magnitudes of glycine-evoked currents; however, there are discrepancies regarding their precise effects. Protein kinase A (PKA) has been reported to enhance the glycine response in Xenopus oocytes injected with rat brain mRNA (Vaello et al. 1994). On the other hand, PKA reduced glycine responses in rat substantia nigra neurones (Inomata, Nabekura & Akaike, 1993). Agopyan, Tokutomi & Akaike (1993) reported that PKA activation reduced the open probability of the glycine receptor in the acutely isolated rat ventromedial hypothalamic neurones, while Song & Huang (1990) reported that PKA increased the channel open probability in the trigeminal neurones of the rat spinal cord. Protein kinase C (PKC) has been shown to increase the glycine current in rat sacral dorsal commissural neurones (Xu, Nabekura & Akaike, 1996), rat substantia nigra (Nabekura, Omura, Horimoto Ogawa & Akaike, 1996), and rat hippocampal neurones (Schonrock & Bormann, 1995), but to decrease current of glycine receptor subunits expressed in Xenopus oocytes (Uchiyama, Hirai, Hishinuma & Akagi, 1994; Vaello et al. 1994).
These conflicting results could be due to regional differences in the expression of subunits making up the glycine receptor complex. Molecular biological studies have shown the existence of heterogeneity of glycine receptor subunits in rats, mice and humans, with four different α subunit isoforms and a single β subunit (Grenningloh et al. 1987; Grenningloh, Pribilla, Prior, Multhaup, Beyreuther & Taleb, 1990; Kuhse, Schmieden & Betz, 1990; Akagi, Hirai & Hishinuma, 1991; Handford, Lynch, Baker, Webb, Ford & Sutherland, 1996). The diverse regional and developmental distribution of these subunits has been confirmed by immunohistochemical staining and in situ hybridization in the spinal cord and higher regions of the central nervous system (Triller, Cluzeaud, Pfeiffer, Betz & Korn, 1985; Altschuler, Betz, Parakkal, Reeks & Wenthold, 1986; Malosio, Marqueze-Pouey, Kuhse & Betz, 1991; Kirsch & Betz, 1993). This is relevant to retinal ganglion cells. Greferath, Brandstatter, Wässle, Kirsch, Kuhse & Grunert (1994) have described differential expression of glycine α subunits (α1, α2 and α3) in rat retinal ganglion cells.
Recently, we found that retinal ganglion cells possess two types of glycine responses, which can be distinguished by their kinetics and their pharmacology (Han, Zhang & Slaughter, 1997). One has a fast desensitization rate and is blocked by nanomolar concentrations of strychnine. The other, a more sustained current, is suppressed by 5,7-dichlorokynurenic acid (DCKA). This offers an opportunity to simultaneously compare the effects of protein kinases on two distinct glycine currents in the same neurone. Our experiments indicate that PKA reduces the slow component of the glycine current while PKC enhances the fast component of the current. Since the time course of the overall glycine current is the sum of both of these components, the net effect of either kinase is an acceleration of the decay of the glycine response.
METHODS
Retinal dissociation
Experiments were carried out on acutely isolated retinal ganglion cells from the tiger salamander Ambystoma tigrinum (Kons Scientific, Germantown, WI, USA) by methods previously described (Bader, MacLeish & Schwartz, 1979; Han et al. 1997). All procedures were performed in accordance with the USA Animal Welfare Act, National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 85–23), and approved by the University's Animal Care Committee. The animals were stunned, rapidly decapitated, double-pithed and the eyes enucleated. The retina was isolated and incubated for 30–60 min at room temperature (22°C) in 400 μl of enzyme solution containing 2 U ml−1 papain (Worthington Biochemicals) and 5 mM L-cysteine in amphibian Ringer solution. At the end of the incubation, the retina was rinsed five times with amphibian Ringer solution, transferred to calcium-free Ringer solution, and shaken until the tissue dissociated. The cells were dropped on a lectin-coated coverslip, placed in a culture dish containing Ringer solution, and stored in a 17°C incubator. Experiments were performed on acutely isolated cells, usually within 5 h of dissociation.
Electrophysiological recordings
The whole-cell voltage-clamp technique was used. Data were recorded with an Axopatch-2B amplifier in combination with a Pentium computer and pCLAMP 6 software (Axon Instruments). Origin Software (Microcal Corp.) was used for data analysis, curve fitting and figure illustration. Isolated neurones were superfused at room temperature with amphibian Ringer solution consisting of (mM): 111 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2 and 10 dextrose, buffered with 5 mM Hepes and adjusted to pH 7.8 with NaOH. The recording pipettes were filled with (mM): 5 NaCl, 110 potassium gluconate, 1 MgCl2 and 5 EGTA, buffered with 5 mM Hepes and adjusted to pH 7.4 with KOH. With this internal solution, electrodes had resistances of 5–10 MΩ. The currents shown are raw data, and were not corrected for electrode junctional potentials or access resistance.
A dual superfusion system was used. A gravity-fed system with a 1 min exchange time bathed all the cells on the coverslip, while a computer-controlled, pressurized system with an exchange time of less than 40 ms (DAD-12, ALA Scientific Instruments) was used to locally apply drugs near the recorded neurone. Oxygenated control Ringer solution was bath superfused continuously. Glycine was applied through the local superfusion system. PKA experiments employed the PKA activators forskolin, 8-bromo-cAMP (8-Br-cAMP), the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX), and a specific PKA inhibitor, H-89 dichloride. PKC experiments employed the diacylglycerol analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG) and a specific PKC antagonist, GF-109203X. These drugs and DCKA were obtained from Research Biochemicals International. Sigma Chemical Company was the source of all the other chemicals. All drugs were diluted in amphibian Ringer solution except OAG and GF-109203X, which were dissolved in DMSO as stock solution, then diluted 200-fold and 1000-fold, respectively, into Ringer solution. Initially, cells were pretreated for 1–2 min with kinase regulators by bath perfusion, followed by rapid, local application of glycine plus the regulator. This was required for H-89, OAG, and GF-109203X. However, forskolin, 8-Br-cAMP, and IBMX gave similar results with and without pretreatment. Whether pretreatment was used or not, the effects of glycine were tested in the continued presence of kinase activators or inhibitors.
Ganglion cells were identified based on a combination of morphological and physiological characteristics, particularly large soma size and magnitude of the inward sodium current. Recordings from the retinal slice preparation indicated that the presence of TTX-sensitive sodium currents and lack of inwardly rectifying currents could distinguish amacrine and ganglion cells from other neurones (N. Tian & M. M. Slaughter, unpublished observations). Generally, sodium currents in ganglion cells exceeded those in amacrine cells. These criteria did not positively exclude amacrine cells, but based on the number of cells studied and the consistency of the results it is reasonable to conclude that they are representative of ganglion cell responses.
RESULTS
PKA modulation of glycine-induced currents
PKA has been implicated in the modulation of glycine receptors in several systems (Song & Huang, 1990; Agopyan et al. 1993; Inomata et al. 1993; Vaello et al. 1994). Forskolin (50 μM), a membrane-permeable activator of adenylyl cyclase, was used to explore PKA modulation of glycine receptors on isolated retinal ganglion cells. With neurones voltage clamped at 0 mV, glycine (100 μM) induced an outward current (Fig. 1A). Comparing the glycine response under control conditions with that of neurones pretreated with forskolin revealed that the peak of the glycine current was unchanged, but the late phase of the current was reduced in the presence of forskolin. This selective reduction in the late current resulted in a more rapid decay of the glycine current. Results from eight cells are summarized in Fig. 1B and C. The responses of each neurone were normalized to its peak glycine-induced current under control conditions. There was no significant change in the peak glycine response when cells were pretreated with forskolin (Iforskolin = 1.00 ± 0.04, normalized to Icontrol). The apparent decay time constant was determined by fitting the glycine-induced current with a single exponential function. Forskolin reduced the mean apparent decay time constant (τdecay) by 45 % (from 1650 ± 175 ms in control to 915 ± 101 ms).
Figure 1. Forskolin hastened the decay of the glycine-evoked current.
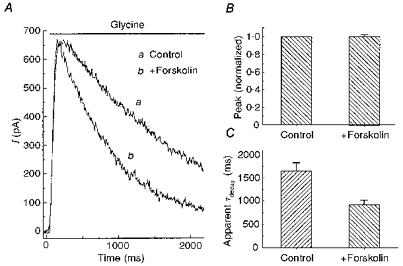
A, in a neurone voltage clamped at 0 mV, rapid application of 100 μM glycine induced an outward current (a). If 50 μM forskolin was applied to the cell, and then glycine (100 μM) and forskolin (50 μM) were rapidly co-applied, the glycine-induced current was more transient, although the peak current was unchanged (b). B and C, summary of results of similar experiments performed on 8 neurones. For each cell, the amplitude of the current produced by glycine plus forskolin was normalized to the current produced by glycine alone. The apparent decay time constant (τdecay) was obtained by fitting the decline of the glycine current to a single exponential function.
When forskolin (50 μM) was co-applied with glycine (100 μM) in the rapid puff system, it produced an effect that was similar to the response of neurones that had been pretreated with forskolin. This indicated that forskolin acted rapidly. To test this, glycine was applied for 2.5 s and then forskolin was applied in the presence of glycine (Fig. 2A). Forskolin reduced the glycine current, with an average time constant of 148 ± 14 ms (n = 8). This indicates that PKA can rapidly be recruited to reduce the late phase of the glycine current. When similar experiments were performed with an analogue of forskolin that does not activate PKA (1,9-dideoxyforskolin), it had little effect on the glycine current (Fig. 2B).
Figure 2. Time course of the action of forskolin.
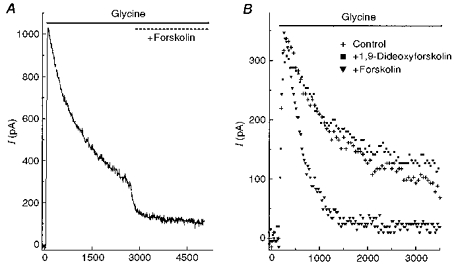
A, glycine (100 μM) was rapidly applied to a neurone and maintained for 5 s. At the 2.5 s mark of glycine application, 50 μM forskolin was co-applied. B, in one neurone, 100 μM glycine was applied alone (+); without pretreatment, glycine and 50 μM forskolin were co-applied (▾); and without pretreatment, glycine was co-applied with 50 μM 1,9-dideoxyforskolin (▪). To distinguish the traces, the control trace was plotted as one point every 25 ms, and for the other two traces one point was plotted every 40 ms. Cells were voltage clamped at 0 mV.
To confirm that forskolin was acting through a PKA pathway, 8-Br-cAMP and IBMX were tested. 8-Br-cAMP is a membrane-permeable cAMP analogue. IBMX is a phosphodiesterase inhibitor. They produced effects on glycine-induced currents that were similar to those elicited by forskolin (Fig. 3). Like forskolin, 8-Br-cyclic AMP and IBMX were applied prior to, or only simultaneously with, glycine. Results were similar, indicating that both the kinase and the phosphodiesterase provide a rapid regulation of the glycine receptor.
Figure 3. Other PKA activators mimicked the effect of forskolin on the glycine current.
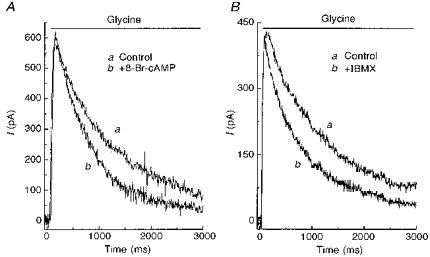
A, outward current was elicited by application of 100 μM glycine alone (a), or by co-application of glycine plus 50 μM 8-Br-cAMP (b). B, outward current produced by application of 100 μM glycine alone (a) or glycine plus 500 μM IBMX. Neurones were voltage clamped at 0 mV.
As a further test, H-89 dichloride, a cAMP-dependent kinase inhibitor, was used to block the forskolin effect (Fig. 4). Co-application of 50 μM forskolin and 100 μM glycine produced a current with a relatively rapid decay (Fig. 4Ab) compared with the response to glycine alone (Fig. 4Aa). However, addition of H-89 (1–2 min pretreatment was required) reversed the effect of forskolin and prolonged the glycine current (Fig. 4Ac). Neither forskolin nor H-89 altered the peak glycine current. H-89 alone had no effect on the glycine current. The mean apparent decay time constant of the glycine current (fitted by a single exponential function) was 1839 ± 236 ms under control conditions. It decreased to 877 ± 75 ms in the presence of forskolin alone, but increased to 1449 ± 165 ms in the presence of forskolin and H-89 (n = 4).
Figure 4. A protein kinase A inhibitor reversed the action of forskolin.
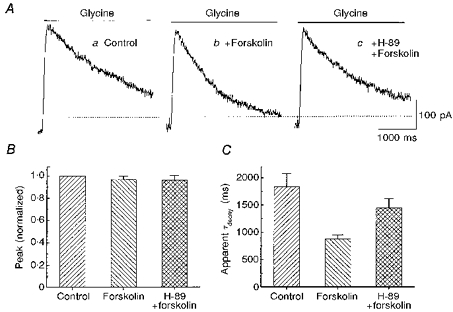
A, responses from one neurone show the outward current produced by 100 μM glycine alone (a), glycine plus 50 μM forskolin (b), and glycine plus forskolin during application of 1 μM H-89, a protein kinase A inhibitor (c). B, the graph compares the peak amplitude of the glycine current under these 3 conditions in 4 cells (mean and standard error). For each cell, the currents were normalized to the peak glycine current in control. C, in the same group of cells and for the same 3 conditions, the decays of the glycine current were compared. For each cell the decay of the current was fitted to a single exponential function. Cells were voltage clamped at 0 mV.
PKC-mediated modulation of glycine-induced currents
Protein kinase C modulation of glycine currents has also been described in other systems (Uchiyama et al. 1994; Vaello et al. 1994; Schonrock & Bormann, 1995; Xu et al. 1996). We used OAG, a diacylglycerol analogue, to investigate PKC regulation of glycine currents. OAG was applied through bath perfusion as well as through the local perfusion system. Unlike forskolin, pretreatment of cells with OAG was required. Figure 5A shows the typical OAG effect on glycine-induced currents. Like PKA, activation of PKC caused the glycine response to become more transient. The apparent decay time constant decreased by 37 %, from 1925 ± 180 to 1225 ± 77 ms (n = 6) (Fig. 5C). But unlike PKA, PKC enhanced the initial, peak amplitude and did not change the late, plateau phase of the glycine current. Normalized peak responses in the presence of OAG were 26 % greater than control currents (mean IOAG = 1.26 ± 0.026, normalized to control; n = 6).
Figure 5. A PKC activator enhanced the peak of the glycine current.
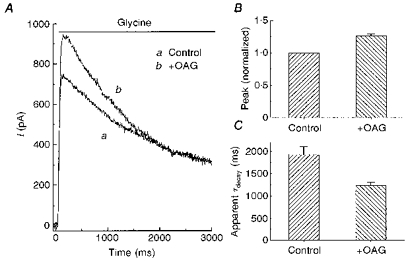
A, 100 μM glycine was applied to a neurone under control conditions (a) or after pretreatment with 50 μM OAG, a PKC activator (b). B and C, a summary of experiments similar to that in A, performed on 5 cells. The experimental design and presentation format are the same as in Fig. 4.
The effect of OAG could be blocked by GF-109203X, a specific PKC inhibitor. Figure 6A shows that the early, but not the late, phase of the glycine current was enhanced by OAG. In the same cell, 1 μM GF-109203X in the bath solution reversed the effect of OAG (Fig. 6B). Note that the response in the presence of OAG and GF-109203X (Fig. 6B, trace c) is very similar to the control glycine current (Fig. 6A, trace a). This experiment was repeated in four other cells with similar results. Thus, while PKA suppressed the late phase of the glycine current, PKC augmented the early phase of the glycine current.
Figure 6. A PKC inhibitor reversed the action of OAG.
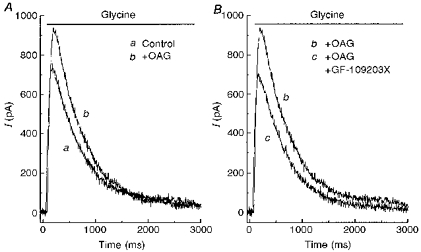
A, 100 μM glycine was applied alone (a) and then after the cell was pretreated with 50 μM OAG (b). B, in the same cell, glycine was applied after the cell was pretreated with OAG plus 1 μM GF-109203X (c). Trace b is from A, replotted for comparison. The neurone was voltage clamped at 0 mV.
Selectivity of protein kinase regulation of the glycine response
Previous studies indicated that glycine currents could be separated into two components: a rapidly activating and desensitizing current, and a slowly developing, less-desensitizing current. These two currents could be deciphered pharmacologically, since the former was more sensitive to strychnine while the latter was suppressed by 5,7-dichlorokynurenic acid (DCKA) (Han et al. 1997). The similarity between the temporal properties of glycine responses in the presence of these two glycine antagonists and the two protein kinases implied a correlation. It suggested that each protein kinase modulated only one of the kinetically distinct glycine currents. To test this hypothesis, strychnine and DCKA were used to separate the fast and slow glycine currents and examine the sensitivity of each component to PKA and PKC. At 500 nM, strychnine can block nearly all of the fast, glycine-evoked current, while leaving a slow, sustained current. On the other hand, 500 μM DCKA can selectively suppress the slow glycine current. (Dose-response curves can be found in Han et al. 1997.)
When the fast component was blocked by 500 nM strychnine, 50 μM forskolin still reduced the slow component of the glycine response (Fig. 7A). Similar results were obtained in four other cells. This indicates that the slow glycine current is forskolin sensitive. In another five cells, glycine was applied in the presence of forskolin alone or both forskolin and 500 μM DCKA. There was no difference in the response to glycine under the two conditions (Fig. 7B, compare b with c); that is, DCKA did not produce an additional inhibition of the glycine current, beyond the effect of forskolin. Under normal conditions, DCKA produces a significant decrease in the late glycine current (Han et al. 1997). This indicates that forskolin occluded the action of DCKA, indicating both drugs affect the same component of the glycine current.
Figure 7. Forskolin affects the strychnine-insensitive glycine current.
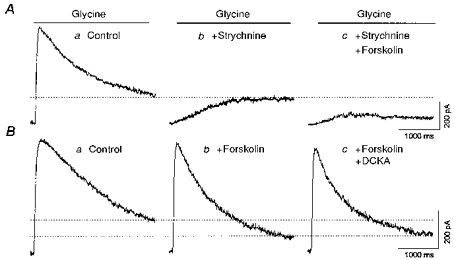
A, in one neurone, 100 μM glycine was applied alone (a), after pretreatment of the neurone with 500 nM strychnine (b), and in the presence of both 500 nM strychnine and 50 μM forskolin (c). B, in another cell, 100 μM glycine was applied alone, in combination with 50 μM forskolin, or with forskolin plus 500 μM DCKA. The neurones were voltage clamped at 0 mV.
In another experiment to determine whether forskolin selectively reduced the slow component, we examined the forskolin effect in five cells while changing the balance between the two current components. If forskolin only modified the slow glycine response, the forskolin response should be the same whether or not the fast response was present. Strychnine (50 nM) was used to partially block the fast glycine component, without affecting the slow one (Han et al. 1997). Initially, glycine was applied alone or in the presence of forskolin (Fig. 8A). As seen previously, the glycine current decayed faster in the presence of forskolin. Subtracting the glycine currents with and without forskolin revealed a slow current (Slow1). This is the current affected by forskolin. In the same neurone, the experiment was repeated in the presence of 50 nM strychnine. Strychnine suppressed the fast glycine current (Fig. 8B, trace c). Then the glycine current was elicited in the presence of both strychnine and forskolin (Fig. 8B, trace d). Subtracting the glycine currents in the presence and absence of forskolin revealed a slow glycine response (Slow2). Comparing the forskolin-sensitive currents in the absence and presence of 50 nM strychnine (Fig. 8, traces Slow1 and Slow2, respectively) showed that the forskolin effect was similar regardless of the amplitude of the fast glycine current. This indicates that forskolin acted selectively on the strychnine-insensitive glycine current, supporting the conclusion that the PKA pathway selectively modulated the slow glycine current component.
Figure 8. Forskolin had a similar effect on glycine current when the fast current was suppressed.
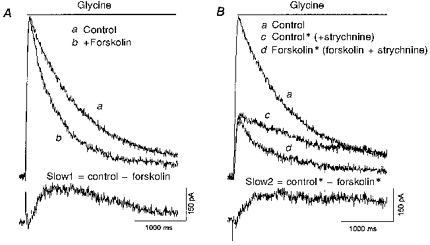
A, glycine (100 μM) was puffed onto a neurone in the presence (b) and the absence (a) of 50 μM forskolin. The difference current, Slow1, represents the glycine current that was sensitive to forskolin. B, the same cell was then pretreated with 50 nM strychnine, then in the continued presence of strychnine glycine was applied (c), or glycine and forskolin were applied (d). The difference current, Slow2, represents the forskolin-sensitive current in the presence of strychnine. Trace a is the same as trace a from A. The cell was voltage clamped at 0 mV.
The complementary experiment was performed to examine the characteristics of the glycine current that was modulated by PKC. In the presence of 500 nM strychnine, which blocked the fast component of the glycine current, OAG had no effect on the remaining, slow glycine current (comparing Fig. 9A traces b and c, n = 6). However, in the presence of 500 μM DCKA, which suppressed the slow component, OAG was still able to enhance the fast component of the glycine current (Fig. 9B, trace c, n = 5). This indicates that PKC acts selectively to enhance the fast component of the glycine current.
Figure 9. Strychnine occludes the response to a PKC activator.
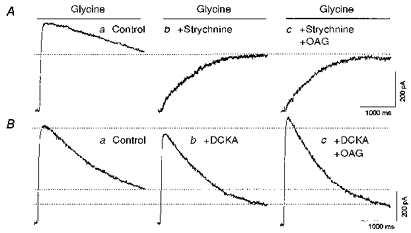
A, glycine (100 μM) was rapidly applied to a neurone under control conditions (a), after pretreatment with, and in the presence of 500 nM strychnine (b), and after pretreatment with, and in the presence of 500 nM strychnine and 50 μM OAG (c). B, in another neurone, glycine was applied alone (a), in the presence of 500 μM DCKA (b), and in the presence of both DCKA and 50 μM OAG. Neurones were clamped at 0 mV.
DISCUSSION
Kinases make the glycine current more transient
These experiments demonstrate that both PKA and PKC shaped the glycine-induced current response. In each case, the overall response waveform became more transient, although the mechanisms of action were different. The glycine current could be separated into two components, with each kinase modulating one component. The PKA pathway selectively reduced the sustained component. The result was a more transient glycine response because it was generated mainly by the rapidly desensitizing glycine response component. In contrast, PKC augmented the transient glycine component, so that it now represented a larger fraction of the total glycine current. Again, this resulted in a more transient current waveform.
Comparing our results with others, we find two shared observations. Firstly, PKA and PKC often have opposite effects on glycine responses. Secondly, these kinases can regulate different temporal components of glycinergic current. In the retinal ganglion cells, we found that PKA selectively down-regulated the glycine slow component while PKC selectively up-regulated the glycine fast component. Vaello et al. (1994) reported that PKA and PKC also had opposing actions on glycine responses in Xenopus oocytes injected with rat brain mRNA. Phorbol esters reduced the glycine current while cAMP enhanced the current. They did not distinguish temporal components of the current. Agopyan et al. (1993) found that PKA selectively suppressed the fast-desensitizing, but not the non-desensitizing, glycine current in dissociated ventromedial hypothalamic neurones. But, in each case, these results were opposite in polarity to our observations. Nevertheless, these studies show that protein kinases can differentially modulate glycine responses, thus providing a mechanism for alteration of inhibitory signals.
PKA modulation of glycine current
The slow glycine current appeared to be tightly regulated by PKA. The application of forskolin or 8-Br-cAMP produced a rapid down-regulation of the slow glycine current. The resolution of our system was approximately 100 ms, so it is likely that forskolin began to activate the entire transduction cascade within this time frame. IBMX can also suppress the slow glycine current, indicating that cAMP phosphodiesterase is constitutively active. The effectiveness of forskolin, 8-Br-cAMP, IBMX and H-89 indicate that a PKA pathway modulates the glycine current. This contrasts with results on GABA receptors in rabbit bipolar cells, where Gillette & Dacheux (1996) found that forskolin had an effect unrelated to the PKA system.
This action of PKA might serve as a desensitization mechanism for the slow glycine current, which, under control conditions, manifests little desensitization. Our experiments do not distinguish between a desensitization mechanism, a kinase-mediated lowering of receptor affinity, or a decrease in the conductance properties of the channel. However, if PKA mediates a desensitization of the slow glycine current, it is likely to be a heterologous desensitization since it was not observed under control conditions. On the other hand, neither PKA nor PKC seemed to be involved in regulating the very prominent desensitization of the fast glycine current. The enhancement of the fast glycine current by PKC is similar to the effect of PKA on the fast GABA current in rat amacrine cells (Feigenspan & Bormann, 1994a).
Comparison of glycine and GABA
Retinal ganglion cells possess both ionotropic GABA and glycine receptors. Since these two transmitter systems provide the preponderance of inhibitory input in the inner plexiform layer, comparison of their regulation reveals the interplay of inhibitory mechanisms that shape the responses of ganglion cells. There have been a few studies of kinase regulation of retinal GABA receptors. One intriguing set of observations, by Feigenspan & Bormann (1994a, b), indicates that there may be a differential regulation of GABA receptors that is symmetrical to the effects on glycine receptors that we have observed. In rat retina, they found that PKA enhanced the GABAA receptor response in amacrine cells by increasing open probability. But in rat retinal bipolar cells, they reported that PKC down-regulated the GABAC receptor. While both the GABAA and GABAC receptors are ionotropic, the former is fast activating and fast desensitizing, while the latter is slow activating and displays little desensitization. Thus, they are functionally similar to the fast and slow components of the glycine current. As with the glycine receptor, PKA and PKC made the GABA response more transient. The former enhanced the fast GABAA response, the latter suppressed the slow GABAC response. It would be interesting to know if both systems functioned in the same retinal preparation. If so, it could provide a mechanism for protein kinase modulation of the balance between these inhibitory transmitters. The speculation would be that PKA suppresses the slow glycine current and enhances the fast GABAA current, while PKC suppresses the slow GABAC current and enhances the fast glycine current.
Retinal physiology
Transient and sustained synaptic responses, both excitatory and inhibitory, are fundamental properties of ganglion cells. Glycine generates both types of synaptic currents (Miller, Frumkes, Slaughter & Dacheux, 1981), although Belgum, Dvorak & McReynolds (1984) found that transient glycine responses predominate. Therefore, kinase regulation of glycinergic currents can play an important role in synaptic integration. It seems likely that glycine does not alter kinase activity directly, since the existence of a metabotropic glycine receptor has not been reported. However, several retinal neurotransmitters, such as dopamine and GABA, have been reported to regulate PKA. Therefore, it is likely that the temporal characteristics of glycinergic inhibition are malleable and may change with the adaptational state of the retina.
Acknowledgments
This work was supported by grant no. EY05725 from the National Eye Institute.
References
- Agopyan N, Tokutomi N, Akaike N. Protein kinase A-mediated phosphorylation reduces only the fast desensitizing glycine current in acutely dissociated ventromedial hypothalamic neurons. Neuroscience. 1993;56:605–615. doi: 10.1016/0306-4522(93)90360-r. 10.1016/0306-4522(93)90360-R. [DOI] [PubMed] [Google Scholar]
- Akagi H, Hirai K, Hishinuma F. Cloning of a glycine receptor subtype expressed in rat brain and spinal cord during a specific period of neuronal development. FEBS Letters. 1991;281:160–166. doi: 10.1016/0014-5793(91)80383-e. 10.1016/0014-5793(91)80383-E. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Betz H, Parakkal MH, Reeks KA, Wenthold RJ. Identification of glycinergic synapses in the cochlear nucleus through immunocytochemical localization of the postsynaptic receptor. Brain Research. 1986;369:316–320. doi: 10.1016/0006-8993(86)90542-1. 10.1016/0006-8993(86)90542-1. [DOI] [PubMed] [Google Scholar]
- Bader CR, MacLeish PR, Schwartz EA. A voltage-clamp study of the light response in solitary rods of the tiger salamander. The Journal of Physiology. 1979;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgum JH, Dvorak DR, McReynolds JS. Strychnine blocks transient but not sustained inhibition in mudpuppy retinal ganglion cells. The Journal of Physiology. 1984;354:273–286. doi: 10.1113/jphysiol.1984.sp015375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Facilitation of GABAergic signaling in the retina by receptors stimulating adenylate cyclase. Proceedings of the National Academy of Sciences of the USA. 1994a;91:10893–10897. doi: 10.1073/pnas.91.23.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Modulation of GABAC receptors in rat retinal biploar cells by protein kinase C. The Journal of Physiology. 1994b;481:325–330. doi: 10.1113/jphysiol.1994.sp020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette MA, Dacheux RF. Protein kinase modulation of GABAA currents in rabbit retinal rod bipolar cells. Journal of Neurophysiology. 1996;76:3070–3086. doi: 10.1152/jn.1996.76.5.3070. [DOI] [PubMed] [Google Scholar]
- Greferath U, Brandstatter JH, Wässle H, Kirsch J, Kuhse J, Grunert U. Differential expression of glycine receptor subunits in the retina of the rat: a study using immunohistochemistry and in situ hybridization. Visual Neuroscience. 1994;11:721–729. doi: 10.1017/s0952523800003023. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Pribilla I, Prior P, Multhaup G, Beyreuther K, Taleb OBH. Cloning and expression of the 58 kd beta subunit of the inhibitory glycine receptor. Neuron. 1990;4:963–970. doi: 10.1016/0896-6273(90)90149-a. 10.1016/0896-6273(90)90149-A. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen M, Beyreuther K, Gundelfinger ED, Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Han Y, Zhang J, Slaughter MM. Partition of transient and sustained inhibitory glycinergic input to retinal ganglion cells. Journal of Neuroscience. 1997;17:3392–3400. doi: 10.1523/JNEUROSCI.17-10-03392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford CA, Lynch JW, Baker E, Webb GC, Ford JH, Sutherland GR, Schofield PR. The human glycine receptor beta subunit: primary structure, functional characterisation and chromosomal localisation of the human and murine genes. Molecular Brain Research. 1996;35:211–219. 10.1016/0169-328X(95)00218-H. [PubMed] [Google Scholar]
- Inomata H, Nabekura J, Akaike N. Suppression of taurine response in acutely dissociated substantia nigra neurons by intracellular cyclic AMP. Brain Research. 1993;615:347–350. doi: 10.1016/0006-8993(93)90048-r. 10.1016/0006-8993(93)90048-R. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Widespread expression of gephyrin, a putative glycine receptor-tubulin linker protein, in rat brain. Brain Research. 1993;621:301–310. doi: 10.1016/0006-8993(93)90120-c. 10.1016/0006-8993(93)90120-C. [DOI] [PubMed] [Google Scholar]
- Kuhse J, Schmieden V, Betz H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. Journal of Biological Chemistry. 1990;265:22317–22320. [PubMed] [Google Scholar]
- Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO Journal. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RF, Frumkes TE, Slaughter M, Dacheux RF. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. II. Amacrine and ganglion cells. Journal of Neurophysiology. 1981;45:764–782. doi: 10.1152/jn.1981.45.4.764. [DOI] [PubMed] [Google Scholar]
- Nabekura J, Omura T, Horimoto N, Ogawa T, Akaike N. Alpha 1 adrenoceptor activation potentiates taurine response mediated by protein kinase C in substantia nigra neurons. Journal of Neurophysiology. 1996;76:2455–2460. doi: 10.1152/jn.1996.76.4.2455. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gomez A, Vaello ML, Valdivieso F, Mayor F., Jr Phosphorylation of the 48-kDa subunit of the glycine receptor by protein kinase C. Journal of Biological Chemistry. 1991;266:559–566. [PubMed] [Google Scholar]
- Schonrock B, Bormann J. Modulation of hippocampal glycine receptor channels by protein kinase C. NeuroReport. 1995;6:301–304. doi: 10.1097/00001756-199501000-00019. [DOI] [PubMed] [Google Scholar]
- Song YM, Huang LY. Modulation of glycine receptor chloride channels by cAMP-dependent protein kinase in spinal trigeminal neurons. Nature. 1990;348:242–245. doi: 10.1038/348242a0. 10.1038/348242a0. [DOI] [PubMed] [Google Scholar]
- Triller A, Cluzeaud F, Pfeiffer F, Betz H, Korn H. Distribution of glycine receptors at central synapses: an immunoelectron microscopy study. Journal of Cell Biology. 1985;101:683–688. doi: 10.1083/jcb.101.2.683. 10.1083/jcb.101.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama M, Hirai K, Hishinuma F, Akagi H. Downregulation of glycine receptor channels by protein kinase C in Xenopus oocytes injected with synthetic RNA. Molecular Brain Research. 1994;24:295–300. doi: 10.1016/0169-328x(94)90142-2. 10.1016/0169-328X(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Vaello ML, Ruiz-Gomez A, Lerma J, Mayor F., Jr Modulation of inhibitory glycine receptors by phosphorylation by protein kinase C and cAMP-dependent protein kinase. Journal of Biological Chemistry. 1994;269:2002–2008. [PubMed] [Google Scholar]
- Xu TL, Nabekura J, Akaike N. Protein kinase C-mediated enhancement of glycine response in rat sacral dorsal commissural neurones by serotonin. The Journal of Physiology. 1996;496:491–501. doi: 10.1113/jphysiol.1996.sp021701. [DOI] [PMC free article] [PubMed] [Google Scholar]


