Abstract
The relationship between sympathetic and somatic motor outflows from thoraco-lumbar spinal cord was investigated in a novel arterially perfused trunk-hindquarters preparation of adult mouse.
Ongoing activity was present in both somatic motor (obturator, sciatic or femoral nerves) and sympathetic outflows (either renal nerve or abdominal sympathetic chain). Sympathetic activity was rhythmic with bursts frequencies of 0.6–2.2 Hz. No obvious rhythmic activity was found in the somatic motor outflow. There were periods during which sympathetic and somatic motor activity were correlated.
Addition of NMDA (20–80 μM) to the perfusate elicited repetitive burst discharges in the somatic motor outflow which were sometimes rhythmic. The frequency of these burst discharges/rhythmic activity varied between preparations but in all cases increased with increasing NMDA concentration.
NMDA induced burst discharges in the sympathetic outflow. This bursting activity was of the same frequency as the somatic motor outflow and the two were coupled as revealed by correlation analysis. Periods of coupling persisted for up to 3 min.
Administration of hexamethonium (300 μM), to block sympathetic ganglionic transmission, had no effect on the somatic motor activity but severely attenuated sympathetic nerve discharge.
The thoraco-sacral cord therefore has the neuronal machinery necessary for generating and coupling activity in somatic motor and sympathetic outflows. Our findings indicate a dynamic control over the degree of coupling. We discuss that the synchronization of these neural outflows reflects either coupling between two independent mechanisms or the presence of a common synaptic driver impinging on both somatic motor and sympathetic neurones.
Different behavioural states such as exercise or withdrawal reflexes from noxious stimulation require appropriate co-ordination of sympathetic and somatic motor activity. The role of intrinsic spinal mechanisms in such co-ordination is unclear. There is to our knowledge no data comparing ongoing sympathetic and somatic motor activities simultaneously in adult mammals after spinal section. Independent studies, however, reveal that the spinal cord is capable of generating both sympathetic and somatic motor activity even when isolated from supraspinal structures. In spinal sectioned adult rats, sympathetic postganglionic activity was present (Osborn, Livingstone & Schramm, 1987; Taylor & Schramm, 1987; Osborn, Taylor & Schramm, 1989; Weaver & Stein, 1989); moreover it persisted after dorsal rhizotomy (Taylor & Weaver, 1993) suggesting an intraspinal generator. Spontaneous oscillations in sympathetic preganglionic neurones have been recorded in slices of thoraco-lumbar cord of immature rats at frequencies between 0.1 and 0.5 Hz (Spanswick & Logan, 1990; Logan, Pickering, Gibson, Nolan & Spanswick, 1996). In addition to this sympathetic activity, spinally generated fictive locomotion (0.3–1 Hz) can be induced by additions of serotonin and/or NMDA to isolated spinal cord-hindlimb preparations of neonatal rat (Cazalets, Sqalli-Houssaini & Clarac, 1992; Kjaerulff, Barajon & Kiehn, 1994). All told, the intrinsic circuitry of the spinal cord is capable of generating rhythmic activity destined for somatic motor and sympathetic outflows. The question addressed here was whether sympathetic and somatic motor activity were controlled independently or might, under certain circumstances, become synchronized.
In order to overcome the complexities of supraspinal loops and depressant effects of anaesthesia, we have used a recently developed arterially perfused trunk and hindquarters preparation of adult mouse (Chizh, Headley & Paton, 1997a). Here, we report that somatic motor and sympathetic discharges can show periods of synchronization.
Some of the data presented here have been communicated to the Physiological Society (Chizh et al. 1997b).
METHODS
The perfused trunk-hindquarters preparation of adult mouse has recently been described in detail (Chizh et al. 1997a) and brief details only are given here.
Surgical procedures
Mature mice (MF1; 4–8 weeks) of either sex were anaesthetized deeply with urethane (1.5 g kg−1, intra-peritoneally). Through a midline abdominal incision, the mesenteric root was ligated, the intestines, spleen and liver were removed and the animal bisected at the upper thoracic level. The lower body was placed in ice-chilled Ringer solution gassed continuously with 95 % O2-5 % CO2. The heart, lungs, diaphragm and the skin down as far as the pelvis were removed before transferring to a recording chamber. The descending aorta was cannulated and perfused (see below) at a pressure of 50–70 mmHg (flow rate 14–16 ml min−1) using a roller pump (Watson-Marlow 502S). The perfusate was warmed (33°C), filtered (40 μm pore size) and bubble traps used to remove bubbles and dampen pulsations. Perfusate was recycled after re-gassing.
Recording of motor and sympathetic nerves
An abdominal sympathetic chain or a renal sympathetic nerve was identified anatomically, cut distally and the central end recorded using a glass suction electrode (tip diameter 150–250 μm). Similarly, a somatic motor nerve (femoral, sciatic or obturator) was isolated, sectioned and the cut central end recorded with a second suction electrode. Whole nerve activity was amplified and filtered (Neurolog modules 104 and 125; 8 Hz to 3 kHz), displayed on an oscilloscope (Gould DSO 400), connected to both an audio amplifier (Grass AM8) and thermal chart recorder (Gould TA 11), and digitized and stored on magnetic tape (Instrutech VR100; sampling rate 26 kHz).
Solutions and drugs
The constituents of the Ringer solution were (mM): 10 dextrose, 125 NaCl, 24 NaHCO3, 5 KCl, 2.5 CaCl2, 1.25 MgSO4, 1.25 KH2PO4. The perfusate was Ringer solution plus 22–25 g l−1 dextran (mean MW 260 000; Sigma), an antibiotic cocktail (see Chizh et al. 1997a) and the muscle relaxant vecuronium bromide (40 μg l−1; Norcuron, Organon Teknika, Cambridge, UK). Perfusate pH was 7.35 ± 0.05 on gassing with 95 % O2-5 % CO2. NMDA (20–80 μM; Tocris Cookson) was added to the perfusate to induce rhythmic somatic motor activity. Hexamethonium dichloride (300 μM; Sigma) was used as a ganglion blocker.
Analysis
Multi-unit nerve activity was analysed off-line using a CED 1401 and Spike2 software (Cambridge Electronic Design). Raw signals were digitized at 5 kHz. A window discriminator was set typically at +5 to +15 μV from baseline and spikes were transformed into events. The frequencies of these events were counted and displayed in moving average frequency plots using a time constant of 0.2–0.5 s. We employed two methods of auto- and cross-correlation analysis; digital correlation (i.e. peak to event) and analog correlation.
Peak-to-event correlation
Frequency changes above a certain amplitude were used to define peaks in the moving average frequency plots (representing bursts of activity). The rising phase of these peaks served as triggers for auto- and cross-correlation analysis which was performed on 20–120 s samples of event data. The length of these data sets varied because, in the presence of NMDA, periods of rhythmic activity alternated spontaneously with non-rhythmic discharges. Thus, the results were plotted as histograms of the events discriminated from the original neurogram (bin width 0.2–0.5 s; peak triggering points defined as time 0). Correlated activity was deemed statistically significant if the amplitude of a peak in the histogram was greater than the mean + 2 s.d. (i.e. P < 0.05) or mean + 3 s.d. (P < 0.01) of the activity over the period analysed. n refers to the number of preparations. This analysis relies on the correct definition of the peaks of activity used to produce the triggering signals, but has the advantage of indicating the relationship between peaks in one nerve and events in the other. Interburst frequencies were estimated from the same data sets used for correlation analysis by analysing the intervals between peaks of activity in the moving average frequency plots; these are indicated as means ±s.d.
Analog correlation
Using commercially available software (MATLAB, Cambridge Control Ltd, Cambridge, UK) analog cross-correlation analysis was performed on moving average frequency plots recorded from some experiments.
RESULTS
This report is based on eight preparations of which a full quantitative analysis (i.e. auto- and cross-correlations) of sympathetic and somatic motor activity was performed on five.
Ongoing activity in somatic motor and sympathetic outflows
Following the onset of arterial perfusion by 10–15 min, ongoing activity was present in both somatic nerves and in sympathetic nerves or chain. Recordings from somatic motor nerves revealed ongoing tonic activity with occasional burst discharges (n = 8; Figs 1A and 2A). Auto-correlation and interval analysis of bursts revealed that there was no regular rhythmic activity in somatic outflows as indicated by the large standard error in the mean interburst interval (i.e. 3.0 ± 1.3 s; n = 8). In comparison, analysis of either renal nerve (n = 2) or sympathetic chain activity (n = 6) revealed rhythmic activity with periods in the range of 0.6–2.2 Hz in which the mean interburst interval was 1.4 ± 0.5 s (n = 5). There was no obvious difference in the rhythmic frequencies of renal (0.8–2.1 Hz) and sympathetic chain activity (0.6–2.2 Hz).
Figure 1. Ongoing activity recorded simultaneously in somatic motor and sympathetic outflows of an arterially perfused trunk and hindquarters preparation of adult mouse.

A, traces of multi-unit activity in the whole left femoral nerve (Motor) and abdominal sympathetic chain (Sympathetic). B, activity shown in A presented as moving average frequency plots and expressed in events s−1 (i.e. rate meter; thresholds for spike detection +12 μV for the motor outflow and +8 μV for the sympathetic) smoothed with a time constant of 0.2 s. The ‘peaks/triggers’ indicated above the smoothed frequency rate plots show activity increasing above 25 spikes s−1. Time scale for A and B are the same. C, cross-correlation analysis of sympathetic activity (i.e. events) triggered from the peaks in the motor activity (see Methods). Bin width is 0.2 s throughout. Dashed line indicates mean level of activity, dotted line shows the means ± 2 s.d.; significant histogram peaks at time 0: **P < 0.01. D, cross-correlogram of the same data set using the analog signals (i.e. both from the moving average frequency plots) also showing a highly significant correlation. In this correlogram the two horizontal lines represent the ±95 % confidence limits. Data set analysed was 50 s in duration.
Figure 2. Coupling between spontaneous activity in somatic motor and sympathetic nerves.

A, peaks/triggers points (above) obtained from the discharges of the left femoral nerve (Motor) and abdominal sympathetic chain (Sympathetic) recorded simultaneously (see Methods). The threshold for spike detection was +6 μV for the motor outflow and +4 μV for the sympathetic. After smoothing frequency rate plots (time constant 0.2 s) changes of activity greater than 20 spikes−1 were detected and used to produce the peaks/triggers. B, correlogram using trigger signals derived from the peaks in the somatic motor activity to sympathetic events. C shows the lack of correlation between somatic motor events triggered from the sympathetic peaks of activity. There was, as expected, a ‘mirror image’ correlation using the smoothed frequency plot data (not shown) whether ‘triggering’ from motor (D) or sympathetic activity (E; see Discussion for explanation). In B and C, bin width is 0.2 s; dashed lines indicate mean levels of activity; dotted lines show the mean ± 2 s.d.D and E, analog cross-correlation analysis showing a similar correlation to that in B. The horizontal black lines in the analog cross-correlograms of D and E indicate the extent of the 95 % confidence limit. Significance mark, *P < 0.05 (see Methods). Data set analysed was 40 s in duration.
To assess the possible presence of synchronized activity in motor and sympathetic discharges, cross-correlation analysis was performed on the events in one outflow related to the peaks of activity in the other outflow (see Methods). Figure 1C shows that events in the sympathetic chain were correlated with peaks of activity in the ipsilateral femoral nerve. The same result was obtained in all five preparations analysed. Moreover, there was a correlation between waveforms produced from the plots of the moving average of frequencies of events in somatic and sympathetic outflows (see Fig. 1D for example).
Low levels of activity in the motor output of most preparations precluded a more detailed analysis, but this was possible in three preparations showing higher levels of ongoing somatic motor activity (e.g. Fig. 2A). Figure 2B shows a significant correlation of a proportion of sympathetic chain events with the peaks of activity recorded in a femoral nerve. In contrast, there was no significant cross-correlation in the reverse direction (Fig. 2C), i.e. of the motor events when related to peaks in the sympathetic discharge (Fig. 2A), despite the burst discharges present in the sympathetic outflow (mean interburst interval 0.9 ± 0.3 s in Fig. 2C). A similar pattern was seen in the other two cases analysed. This result implies that some of the inputs affecting somatic motoneurones also influence sympathetic preganglionic neurones but that the sympathetic network receives additional inputs not received, at least to any substantial extent, by somatic motoneurones. Somatic and sympathetic moving average waveforms were correlated significantly (Fig. 2D).
To investigate the relationship between sympathetic and somatic motor outflows further, it was desirable to increase the activity level in one outflow selectively and examine the effect on the other. There is no absolute way of achieving this. Segmental sensory inputs would be expected to feed into both outflows, so that activating afferents was not a suitable strategy. We profited from the nature of the preparation by perfusing NMDA, which we hypothesized should activate somatic motor circuits in a manner analogous to fictive locomotion seen in reduced neonatal preparations (see Introduction). In unparalysed preparations, intra-arterial perfusion of NMDA resulted in rhythmic paddling of hindlimbs, the regularity and frequency of which was variable but dependent on the concentration of NMDA perfused (20–80 μM). When NMDA was added to the perfusate in the present preparations, it increased neural activity in both somatic motor and sympathetic outflows.
NMDA effect on somatic motor and sympathetic activity
NMDA (20 μM; n = 5) increased ongoing tonic activity and induced repetitive burst discharges in somatic motor nerves which showed intermittent periods of rhythmicity (Fig. 3A and B). The frequency of this rhythm was variable between, but relatively constant within, preparations (interburst interval range 18–27 s; n = 5). The analysis below was performed after muscle paralysis, both to eliminate proprioceptive feedback and to reduce movement and enhance mechanical stability.
Figure 3. Rhythmic activity and coupling between somatic motor and sympathetic outflows of the same preparation as in Fig. 1 but in the presence of 20 μM NMDA.
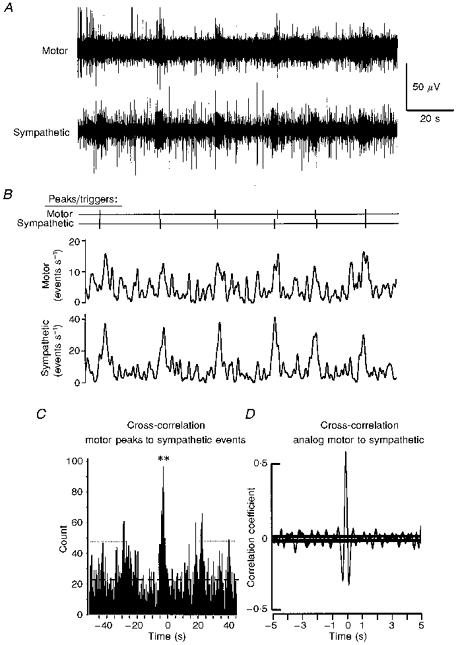
Layout identical to Fig. 1. A, raw data; B, peaks/triggers and moving average frequency plots of the raw data. Time constant for smoothing was 0.5 s; detection was set at changes in the smoothed activity above 10 spikes s−1 for the somatic motor and 20 spikes s−1 for the sympathetic outflow. C, cross-correlogram using the motor peaks as the trigger to sympathetic events (bin width is 0.5 s). Dashed line indicates mean level of activity; dotted line shows the mean ± 2 s.d.; significant histogram peaks at time 0: **P < 0.01. A significant correlation between somatic motor and sympathetic discharges was also observed using the analog moving average frequency plots from the same data (D). For other settings and significance marks see Fig. 1. Data set analysed was 170 s in duration.
NMDA (20 μM; n = 5) also affected sympathetic outflows. It increased the level of ongoing activity and in addition elicited periods during which there were bursts of activity with rhythmic episodes (Fig. 3A and B). Auto-correlation and interburst interval analysis revealed that these discharges had a predominant frequency very similar to that in the somatic motor outflow. For the data shown in Fig. 3 the mean interburst intervals for sympathetic and somatic motor discharges were 22.4 ± 2.9 and 22.6 ± 4.0 s, respectively. Cross-correlation analysis triggered from peaks of motoneurone activity revealed that there was significant synchronization with sympathetic events (Fig. 3C). Under these conditions correlation of sympathetic peaks to motor events demonstrated synchronization of the two outflows (data not shown). Analog cross-correlation analysis also revealed a significant coupling of sympathetic and somatic motor discharges (Fig. 3D).
While 20 μM NMDA clearly increased the frequency of burst discharges, it was evident from the neurograms (and from the auto-correlations) that this was not continuously rhythmic. Episodes of rhythmicity alternated with periods when there was no obvious rhythm. Nevertheless, whether arrhythmic or rhythmic, coupling of burst discharges persisted throughout the application of 20 μM NMDA. Figure 4A shows original traces and Fig. 4B and C the moving averages and cross-correlation, respectively, of somatic motor and sympathetic activity in the presence of 20 μM NMDA during a period when no obvious rhythmicity was seen in either neural outflow. Under these conditions, irregular bursts (mean interburst interval 2.0 ± 1.3 s) of motor activity were superimposed on tonic firing, whereas in the sympathetic outflow bursts occurred at 1.1 ± 0.5 s intervals. However, similar to the situation described above for spontaneous activity there was a significant degree of synchronization (Fig. 4D and E). When peaks of discharge in the somatic motor outflow were used to trigger the cross-correlation analysis of sympathetic events, the correlation was significant (Fig. 4D) whereas when sympathetic peaks were used to trigger the cross-correlation there was not significant correlation (i.e. of motor activity related to sympathetic discharge; data not shown). Cross-correlation of the moving averages of the frequencies of sympathetic and somatic motor events also demonstrated significant coupling (Fig. 4E).
Figure 4. Synchronization of sympathetic and somatic motor burst discharges in the presence of 20 μM NMDA but in the absence of motor rhythm.
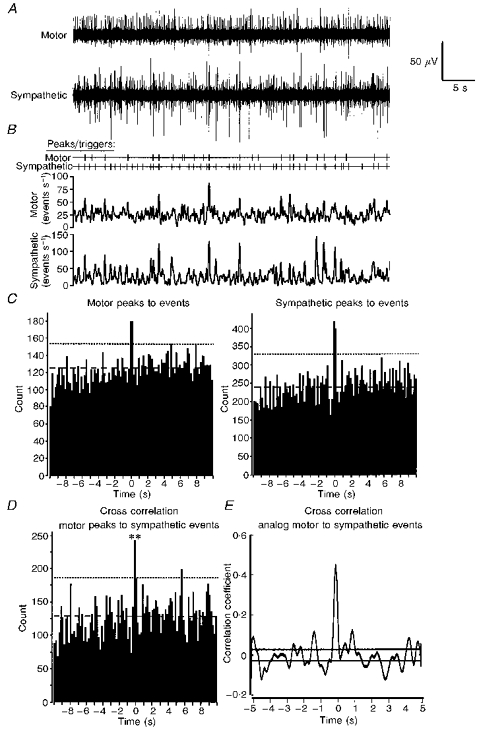
Data are from the preparation shown in Figs 1 and 3 in the presence of 20 μM NMDA but selected during a period when the motor rhythm was less evident. Layout, settings and significance marks are as in Fig. 1. A, raw signals; B, peaks/triggers and moving average frequency plots of raw data; C, auto-correlations of motor (left panel) and sympathetic activity (both peak to event) showing no rhythmic discharge. Bin width is 0.2 s. D, cross-correlation analysis of sympathetic events triggered from the peaks in the motor activity. Bin width is 0.2 s. Dashed lines indicate mean level of activity; dotted lines shows the mean ± 2 s.d.; significant histogram peaks at time 0: **P < 0.01. E, analog cross-correlogram of the same data set using the signals from the moving average frequency plots also shows a highly significant correlation (horizontal lines represent the ±95 % confidence limits). Data set analysed was 50 s in duration.
These results again show that somatic and sympathetic burst discharges can be synchronized independently of rhythm generation. These data with NMDA do not, however, indicate whether the synchronization is of two independent but occasionally synchronized neuronal mechanisms, or reflects coupling of one burst generator to the other output. To assess this more fully, we increased the concentration of NMDA in the perfusate, with the effect of increasing the frequency of somatic motor burst discharges.
In the preparation shown in Figs 1, 3 and 4, increasing NMDA from 20 to 40 μM induced rhythmic activity in both somatic motor and sympathetic outflows as revealed in cross-correlograms. Compared with 20 μM NMDA, the interburst intervals in the somatic motor outflow were reduced from 22.6 ± 4.0 s (Fig. 3) to 3.4 ± 0.4 s (see Fig. 5). Auto-correlation and interburst interval analysis of the sympathetic activity (Fig. 5) indicated that the interburst intervals of rhythmic discharges were reduced to a very similar level (3.0 ± 0.5 s). The decrease in interburst interval in both sympathetic and motor outflows following 40 μM NMDA was seen in all preparations analysed (n = 5). Cross-correlation analysis indicated that the synchronization of sympathetic events to somatic activity peaks was highly significant (Fig. 5C;P < 0.01; n = 5). The episodes of rhythmic motor activity were still, however, intermittent, again lasting up to 3 min at a time.
Figure 5. Rhythmic activity and coupling between somatic motor and sympathetic outflows of the same preparation as in Fig. 1 in the presence of 40 μM NMDA.

Layout as in Fig. 1. Peaks/triggers were detected at event levels above 100 spikes s−1 for both the somatic motor and sympathetic outflow. Moving average frequency plots obtained using a time constant of 0.2 s. Peak-to-event correlations are shown for sympathetic to sympathetic, somatic motor to somatic motor and somatic motor to sympathetic (C). Dashed lines indicate mean level of activity; dotted lines show the mean ± 2 s.d.; significant histogram peaks at time 0: **P < 0.01. Preparation, settings and significance marks are as in Fig. 1. Data set analysed was 32 s in duration.
In three preparations the ganglion blocker hexamethonium (300 μM) was tested to ascertain pharmacologically that the anatomically identified nerves were indeed the sympathetic chain and a somatic motor nerve. In all cases sympathetic chain discharge was substantially reduced after hexamethonium (Fig. 6). The residual low level of activity was likely to be of preganglionic sympathetic origin. In contrast, the periods of rhythmic somatic motor activity were not affected by hexamethonium (mean interburst intervals were 1.1 ± 0.5 s in control and 1.1 ± 0.3 s in the presence of hexamethonium in the example of Fig. 6).
Figure 6. Reduction by hexamethonium of the NMDA-evoked sympathetic, but not of the somatic motor, rhythmic activity.

A, effect of addition of 80 μM NMDA to the preparation shown in Fig. 2. B, activity of the same preparation 3 min after addition of 300 μM hexamethonium. In A and B: a, original traces of somatic motor and sympathetic activity; b, discriminated peaks/triggers and moving average frequency plots; c, correlograms of somatic motor peak to events; d, correlograms of sympathetic peak to events; e, correlograms of sympathetic events triggered from the somatic motor peaks. Dashed lines indicate mean level of activity; dotted lines show the mean ± 2 s.d.; significant histogram peaks at time 0: **P < 0.01. Other experimental details as in Fig. 2. Data sets analysed were both 30 s in duration.
DISCUSSION
The present study is the first to record non-reflexly evoked sympathetic and somatic motor activity simultaneously from a spinally sectioned preparation of an adult mammal. The data reveal that there are neuronal mechanisms within the spinal cord for both the generation and the synchronization of sympathetic and somatic motor outflows. We provide the first evidence for a spinal mechanism that can couple the activity in these separate outflows.
Justification of techniques employed
Since the aim was to assess directly whether the spinal cord could produce synchronized activity in somatic motor and sympathetic outflows, we required a preparation devoid of the complexities of supraspinal loops, descending modulatory influences and depressant effects of anaesthesia (see Weaver & Stein, 1989). Second, we needed to minimize ‘spinal shock’ (see Jänig, 1985, for review) and believe we have succeeded, since both the ongoing activity in sympathetic nerves (this study) and somatic-somatic and visceral-somatic reflexes (Chizh et al. 1997a) were present within minutes of starting arterial perfusion. Third, it was advantageous to avoid (by constant pressure perfusion) the complication due to secondary cardiovascular effects resulting from changes of sympathetic activity that are likely to occur during experimental manipulations in vivo. Fourth, the absence of white blood cells will have reduced the inflammatory response to surgical intervention, a response that can cause progressive changes both of peripheral nociceptors and of spinal function. Fifth, we required the ability to manipulate motor discharge and this could readily be achieved by additions of NMDA to the perfusate. The above, taken together with the robust nature of the ongoing sympathetic activity, validate this novel perfused trunk-hindquarters preparation of adult mouse.
It was advantageous for the purposes of our study to record gross activity in the sympathetic and motor nerves. This follows because the functional heterogeneity within both outflows, and difficulties with functional identification of sympathetic preganglionic neurones, might result in lack of coupling between many pairs of individual neurones even under conditions where this was present within the overall population.
Our hexamethonium experiments rule out the possibility that the rhythmic activity recorded in somatic motor outflows resulted from the sympathetic fibres contained in these nerves, or vice versa. This is based on the finding that rhythmic activity remained the same in somatic nerves at a time when hexamethonium reduced both the rhythmic and tonic activity in the sympathetic chain (Fig. 6). Further, hexamethonium also blocked both the noxious stimulus-evoked increases in sympathetic discharge and NMDA-induced rhythmic bladder contractions (B. A. Chizh., P. M. Headley & J. F. R. Paton, unpublished observations).
We have used two forms of cross-correlation analysis to investigate the degree of coupling between sympathetic and somatic motor activity (see Methods). The two methods yielded closely equivalent results. Triggering from peaks in motor activity revealed significantly correlated sympathetic events whereas using trigger points obtained from the peaks of sympathetic discharge did not reveal a significant correlation to somatic motor events (Fig. 2). The explanation for this is that discriminated sympathetic peaks were not necessarily associated with a high probability of events occurring in the motor outflow, whereas as it can be seen from Fig. 2 there were relatively few discriminated peaks of somatic motor discharge that were not correlated with discriminated sympathetic peaks (i.e. compare peaks derived from the two neurograms in Fig. 2). Whether these activity peaks in the somatic motor outflow reach significance will depend on the length of data set analysed; this was limited in the present experiments by the duration of periods of coupled and/or rhythmic activity. A second factor influencing the accuracy of this analysis is the level of the window discriminator used in defining the trigger points. That this is a valid method is shown by the similar conclusions that can be drawn from peak to event and analog correlation analyses. We suggest that peak-to-event correlation analysis may be advantageous in revealing that peaks of activity in the motor outflow are likely to be associated with events in sympathetic outflows whereas peaks in sympathetic outflows are not necessarily associated with events in the somatic motor outflow. (This insight into the direction of the coupling cannot be realised by the more conventional analog correlation method employed.) Thus, a conclusion that may be drawn from our observations is that the sympathetic outflow receives some robust inputs that are not shared with the somatic motor system whereas, especially in cases of enhanced locomotor drive, much of the somatic output is expressed in the sympathetic outflow.
Mechanism of burst generation and coupling in somatic motor and sympathetic nerves
There is already evidence for spinal rhythm generation within somatic motor and sympathetic circuits, but the data for the two circuits have been obtained independently and only in much reduced preparations of immature animals. Rhythmic activity in flexor and extensor nerves (fictive locomotion; 0.3–1 Hz) can be induced by serotonin and NMDA in neonatal spinal cord preparations with or without the hindlimbs attached (Cazalets et al. 1992; Kjaerulff et al. 1994). This somatic rhythm originates either throughout the lumbar segments (Kjaerulff et al. 1994; Kjaerulff & Kiehn, 1996) or in L1 and L2 (Cazalets, Borde & Clarac, 1995). Sympathetic preganglionic neurones can also show spontaneous oscillations (0.1–0.5 Hz) when recorded in transverse slices of immature thoraco-lumbar spinal cord (Spanswick & Logan, 1990; Logan et al. 1996). The neuronal mechanisms of these sympathetic oscillations remain to be elucidated but their presence in slices indicates a mechanism quite separate from the distributed network responsible for fictive locomotion.
The present experiments have revealed synchronization of some components of somatic motor and sympathetic outflows and this coupling is mediated by a spinal mechanism. Control over the degree of coupling will presumably depend on a complex interaction between the networks generating burst discharges (whether rhythmic or not) and the mechanisms underlying the coupling.
In the absence of NMDA, coupling of somatic motor and sympathetic outflows was significant but not universally strong (see Fig. 1). Some theoretical explanations for the low level of intrinsic coupling merit discussion. First, the coupling depends on a sufficient level and synchrony of somatic motor outflow; second, and given the diversity of functions within somatic motor and especially sympathetic outflows, the coupling is limited to some (presumably functionally related) groups of cells, so that the overall effects in whole nerve recordings depends on the relative degree of activation of these cell groups; third, there is some dynamic control over the degree of coupling depending on other factors such as segmental inputs (see Chizh et al. 1997a) or descending control mechanism; or fourth, the coupling arises from divergent inputs from a common oscillator that is responsible for only the coupled components of activity. At present it is not possible to distinguish between these alternatives.
The most likely source of the activity in somatic motor nerves in the arterially perfused mature mouse preparation is the same as that defined in studies of fictive locomotion in the isolated spinal cord preparations of neonatal rats. This is supported by our findings of rhythmic locomotor activity (i.e. alternating rhythmic paddling movements of the hindlimbs) in the unparalysed preparation during infusions of NMDA (see Results). There is good evidence that NMDA acts on the oscillator that generates fictive locomotion and that this is located in interneurone pools associated with somatic motor co-ordination (e.g. Cazalets et al. 1992) rather than in areas normally associated with sympathetic activity. On the assumption that these fictive locomotion circuits apply in the adult mouse the simplest explanation for our observation of coupled burst discharges/rhythms is that somatic locomotor drive can feed into spinal sympathetic circuits to influence sympathetic preganglionic activity. Whether there is any dynamic control over the degree to which this locomotor activity feeds into sympathetic outflows cannot yet be determined.
The above considerations are consistent with several of our observations. First, much of the activity in both motor and sympathetic outflows was not coupled, so there are neural mechanisms that generate independent activities. Second, the sympathetic outflow showed burst discharges in the absence of coupling to motor outflows. This suggests that the mechanism for generating sympathetic burst activity is not the one responsible for coupling. (Note that non-coupled sympathetic activity could be generated by mechanisms described by others, for example, Logan et al. 1996.) Third, and in contrast, a high proportion of somatic motor burst discharges, and the rhythmic activity seen with NMDA, was strongly correlated with sympathetic activity, indicating that the generating mechanism responsible for somatic locomotor activity is also responsible for inducing coupled sympathetic activity. Fourth, NMDA never generated independent, non-coupled, burst discharges/rhythms in somatic and sympathetic outflows, even when NMDA concentrations were elevated and despite the fact that the perfused NMDA will increase activity/excitability in other spinal neuronal groups including those that affect the two outflows. It cannot be excluded that an independent driver, such as a somato-sympathetic oscillator, drives both somatic and sympathetic circuits, but this seems less likely since it implies duplication of circuitry that is known to generate NMDA-mediated somatic motor rhythms.
Control of coupling
Comparisons of the moving average frequency plots in Figs 1 and 5 demonstrate that the degree of coupling varies markedly under different conditions. It would appear that from our recordings the proportion of synchronized activity (as well as rhythmicity) in sympatho-somatic discharges is under dynamic control, since the strength of coupling between the two rhythmic outflows varied over time. It seems likely that control over the degree of this synchrony can be caused by descending pathways and by segmental sensory inputs including peripheral afferent feedback, which was absent under the conditions of these experiments. Indeed, we have preliminary data suggesting that periods of entrainment can be triggered by synchronized segmental sensory inputs (Chizh, Headley & Paton); such aspects warrant further investigation.
Physiological significance of coupled sympathetic and somatic outflows
Rhythmic activity in sympathetic outflows may be important for increasing the efficacy of neuro-effector transmission (Brock & Cunnane, 1992). Whilst we cannot predict the target of the sympathetic activity recorded here, one possibility is that coupling of sympathetic with somatic motor outflows during exercise allows integration of skeletal muscle with vascular and sudomotor responses. For example, this mechanism could facilitate redistribution of blood flow to exercising muscle from non-exercising muscle within a limb. It may also provide an intrinsic, but exercise-dependent, spinal mechanism for limiting the powerful vasodilatation in exercising muscles that results from the release of local metabolic vasodilator substances and circulating adrenaline. Thus, this spinal mechanism may participate in the ‘sympathetic restraint’ of blood flow to working skeletal muscle that has recently been described in both exercising conscious dogs (Buckwalter, Mueller & Clifford, 1997) and humans (Puvi-Rajasingham, Smith, Akinola & Mathias, 1997). Indeed, coupling of somatic motor to sympathetic outflows could be a potential mechanism in preventing the exercise-induced hypotension observed in patients with sympathetic denervation caused by primary autonomic failure (Puvi-Rajasingham et al. 1997). Thus, we suggest that the synchronized sympatho-somatic discharges support the notion of a central command phenomenon from spinal circuitry in controlling, for example, blood flow and/or sweat production during exercise. In this context, the finding of synchronized muscle sympathetic nerve activity with motor activity during intense intermittent isometric exercise in humans (Victor, Secher, Lyson & Mitchell, 1995) is analogous to the present data. Indeed, based on the finding that partial neuromuscular blockade did not affect this synchronization (or sympathetic burst discharges), Victor et al. (1995) suggested that central command, rather than peripheral afferent feedback, caused the synchronization of muscle sympathetic and somatic motor activity. In addition, identified sympathetic postganglionic sudomotor fibres were seen to discharge synchronously with rhythmic somatic motor activity in chronically spinalized cats (W. Jänig, personal communication).
Conclusions
Our data strongly suggest that the sympathetic network and the somatic motor system can receive a common drive from a spinal locomotor rhythm generator. Thus, the oscillator that has previously been defined as that responsible for the generation of rhythmic somatic locomotor can also drive some (but not all) of the sympathetic outflow; that is, it is a coupled ‘somato-sympathetic generator’. Such a conclusion is consistent with functional considerations: generation of somatic motor activity from sympathetic circuits would be unrealistic, whereas there are clear functional requirements for some sympathetic activity to be correlated with the level of somatic motor outflow whether for regulation of sweat production, redistribution of blood flow to exercising muscles (Victor et al. 1995) or in the prevention of exercise-induced hypotension (Puvi-Rajasingham et al. 1997).
Acknowledgments
We would like to thank Dr H.-S. Chang for his help with the analog signal-correlation analysis and Mr M. V. Holley for his expert technical assistance. The financial support of the British Heart Foundation (BS/93003), Royal Society (14349) and NIH (GM 35523) is fully appreciated.
References
- Brock JA, Cunnane TC. Electrophysiology of neuroeffector transmission in smooth muscle. In: Burnstock G, Hoyle CHV, editors. Autonomic Neuroeffector Mechanisms. Reading UK: Harwood Academic Publishers; 1992. pp. 121–211. [Google Scholar]
- Buckwalter JB, Mueller PJ, Clifford PS. Sympathetic vasoconstriction in active skeletal muscles during dynamic exercise. Journal of Applied Physiology. 1997;83:1575–1580. doi: 10.1152/jappl.1997.83.5.1575. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. The Journal of Physiology. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. Journal of Neuroscience. 1995;15:4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizh BA, Headley PM, Paton JFR. An arterially-perfused spinal cord-hindquarters preparation of adult mouse in vitro. Journal of Neuroscience Methods. 1997a;76:177–182. doi: 10.1016/s0165-0270(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Headley PM, Paton JFR. Coordinated activity in sympathetic and somatic motor outflows from the spinal cord in a novel trunk-hindquarters preparation of adult mouse. The Journal of Physiology. 1997b;504.P:199. P. [Google Scholar]
- Jänig W. Organization of the lumbar sympathetic outflow to skeletal muscle and skin of the cat hindlimb and tail. Reviews in Physiology, Biochemistry and Pharmacology. 1985;102:119–213. doi: 10.1007/BFb0034086. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Gilbey MP. On the dominant rhythm in the discharges of single postganlionic sympathetic neurones innervating the rat tail artery. The Journal of Physiology. 1996;497:241–259. doi: 10.1113/jphysiol.1996.sp021764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Barajon I, Kiehn O. Sulphorhodamine-labelled cells in the neonatal rat spinal cord following chemically induced locomotor activity in vitro. The Journal of Physiology. 1994;478:265–273. doi: 10.1113/jphysiol.1994.sp020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. Journal of Neuroscience. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SD, Pickering AE, Gibson IC, Nolan MF, Spanswick D. Electrotonic coupling between rat sympathetic preganglionic neurones in vitro. The Journal of Physiology. 1996;495:491–502. doi: 10.1113/jphysiol.1996.sp021609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JW, Livingstone RH, Schramm LP. Elevated renal nerve activity after spinal transection: effects on renal function. American Journal of Physiology. 1987;253:R619–625. doi: 10.1152/ajpregu.1987.253.4.R619. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Taylor RF, Schramm LP. Determinants of arterial pressure after chronic spinal transection in rats. American Journal of Physiology. 1989;256:R666–673. doi: 10.1152/ajpregu.1989.256.3.R666. [DOI] [PubMed] [Google Scholar]
- Puvi-Rajasingham S, Smith GDP, Akinola A, Mathias CJ. Abnormal regional blood flow responses during and after exercise in human sympathetic denervation. The Journal of Physiology. 1997;505:841–849. doi: 10.1111/j.1469-7793.1997.841ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick D, Logan SD. Spontaneous rhythmic activity in the intermediolateral cell nucleus of the neonate rat thoracolumbar spinal cord in vitro. Neuroscience. 1990;39:395–403. doi: 10.1016/0306-4522(90)90276-a. 10.1016/0306-4522(90)90276-A. [DOI] [PubMed] [Google Scholar]
- Taylor RB, Weaver LC. Dorsal root influences on tonic firing of renal and mesenteric sympathetic nerves in rats. American Journal of Physiology. 1993;264:R1193–1199. doi: 10.1152/ajpregu.1993.264.6.R1193. [DOI] [PubMed] [Google Scholar]
- Taylor RF, Schramm LP. Differential effects of spinal transection on sympathetic nerve activities in rats. American Journal of Physiology. 1987;253:R611–618. doi: 10.1152/ajpregu.1987.253.4.R611. [DOI] [PubMed] [Google Scholar]
- Victor RG, Secher NS, Lyson T, Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circulation Research. 1995;76:127–131. doi: 10.1161/01.res.76.1.127. [DOI] [PubMed] [Google Scholar]
- Weaver LC, Stein RD. Effects of spinal cord transection on sympathetic discharge in decerebrate-unanesthetized cats. American Journal of Physiology. 1989;257:R1506–1511. doi: 10.1152/ajpregu.1989.257.6.R1506. [DOI] [PubMed] [Google Scholar]


