Abstract
Electrophysiological experiments on single myocytes obtained from Purkinje fibres and ventricular tissue of adult rabbit hearts were done to compare the contributions of three potassium (K+) currents to the action potentials in these two tissues.
In Purkinje cells reductions in extracellular potassium, [K+]o, from normal (5.4 mM) to 2.0 mM resulted in a large hyperpolarization and marked lengthening of the action potential. In ventricular myocytes, these changes were much less pronounced. Voltage clamp measurements demonstrated that these differences were mainly due to a much smaller inward rectifier K+ current, IK1, in Purkinje cells than in ventricular myocytes.
Application of 4-aminopyridine (4-AP, 2 mM) showed that all Purkinje cells exhibited a very substantial Ca2+-independent transient K+ outward current, It. 4-AP significantly broadened the early, rapid repolarization phase of the action potential.
Selective inhibitors of the fast component, IK,r (MK-499, 200 nM) and the slow component IK,s (L-735821 (propenamide), 20 nM) of the delayed rectifier K+ currents both significantly lengthened the action potential, suggesting that these conductances are present, but very small (< 20 pA) in Purkinje cells. Attempts to identify time- and voltage-dependent delayed rectifier K+ current(s) in Purkinje cells failed, although a slow delayed rectifier was observed in ventricular myocytes.
These results demonstrate significant differences in action potential waveform, and underlying K+ currents in rabbit Purkinje and ventricular myocytes. Purkinje cells express a much smaller IK1, and a larger It than ventricular myocytes. These differences in current densities can explain some of the most important electrophysiological properties of these two tissues.
Marked differences in the shape and duration of the cardiac action potential have been described in different regions of the rabbit heart (Giles & Imaizumi, 1988) and even within the same tissue, e.g. the epicardium versus the endocardium of rabbit ventricle (Fedida & Giles, 1991; for review see Antzelevitch, Sicouri, Lukas, Nesterenko, Liu & Di Diego, 1995; Giles, Clark & Braun, 1996). In mammalian hearts (including human), the Purkinje fibre action potential is much longer in duration than the ventricular action potential (reviewed by Dangman, 1991; Surawicz, 1992). Although this suggests that the underlying ionic currents are different in these two tissues, the ionic basis for many of these differences is not understood. Repolarization of the cardiac action potential is initiated and controlled by activation of a number of time- and voltage-dependent K+ currents. In rabbit heart, at least three types of K+ currents (Lindblad, Murphey, Clark & Giles, 1996) play important roles in regulating the cardiac action potential duration. These include: (i) an inwardly rectifying K+ current, IK1; (ii) a Ca2+-independent transient outward K+ current, It; and (iii) delayed rectifier K+ current(s), IK.
In Purkinje fibre tissue isolated from dog, cow, sheep and rabbit the action potential exhibits a very rapid phase 1 repolarization (Callewaert, Carmeliet & Vereecke, 1984), suggesting the presence of a large Ca2+-independent transient outward K+ current, It. This type of K+ current has been identified in sheep Purkinje fibres (Coraboeuf & Carmeliet, 1982), and its properties have been studied in detail in this, and many other, cardiac tissues and cell types (Campbell, Rasmusson, Comer, Strauss, 1995; Giles et al. 1996). A Ca2+-dependent chloride current has also been identified in rabbit Purkinje cells (Sipido, Callewaert & Carmeliet, 1993; Papp, Sipido, Callewaert & Carmeliet, 1995). Detailed analysis of this current has revealed two components: a rapid component which plays a role in repolarization and excitation-contraction coupling on a beat-to-beat basis, and a slower component which appears when cytosolic Ca2+ levels are elevated (Papp et al. 1995).
The inwardly rectifying K+ current (IK1) is much larger in rabbit ventricles than in atria (Giles & Imaizumi, 1988) and a similar pattern has been demonstrated in guinea-pig hearts (Hume & Uehara, 1985). In rabbit pacemaker tissue, IK1 appears undetectable (Irisawa, Brown & Giles, 1993), and it is also very small in rabbit crista terminalis (Giles & van Ginneken, 1985). In canine cardiac Purkinje cells, IK1 appears to be relatively small, although no direct comparison with canine ventricle or atrium has been made (Oliva, Cohen & Pennefather, 1990). IK1 is the main conductance underlying the resting potential in most cardiac tissues (Hutter & Noble, 1960; Carmeliet, 1982; Harvey & Ten Eick, 1988). In rabbit ventricle, the outward component of IK1 is responsible for the terminal phase of repolarization of the action potential (Shimoni, Clark & Giles, 1992), and somewhat similar results have been published from guinea-pig ventricle (Nichols, Makhina, Pearson, Sha & Lopatin, 1996).
Hutter & Noble (1960) and Noble & Tsien (1968) published the first detailed studies of the time- and voltage-dependent outward K+ currents in sheep Purkinje fibres. The electrophysiological properties of rabbit Purkinje strands have also been investigated quite extensively (Colatsky, 1980; Carmeliet, 1982; Carmeliet & Mubagwa, 1986; Adamantidis, Lacroix, Caron & Dupuis, 1995) but relatively few studies on single rabbit Purkinje cells have been published (Scamps & Carmeliet, 1989; Glitsch & Tappe, 1995). However, the first description of the delayed rectifier K+ currents in rabbit Purkinje cells reported that reduction of [K+]o resulted in a marked decrease in its magnitude (Scamps & Carmeliet, 1989).
The main goal of the present study was to compare the electrophysiological properties of K+ currents in rabbit Purkinje cells with those of myocytes from rabbit ventricle. Enzymatically isolated single Purkinje cells were used to: (i) describe differences in the action potential waveforms in Purkinje and ventricular cells, (ii) identify the types of K+ currents which are expressed, and (iii) determine which K+ current(s) initiate and control repolarization. Preliminary results have been presented as an abstract (Cordeiro, Spitzer & Giles, 1997).
METHODS
Isolation of Purkinje cells
Purkinje cells were prepared from rabbit hearts using techniques described previously (Scamps & Carmeliet, 1989) with some modifications. Male New Zealand White rabbits (Ellis & Pritchard, America Fork, UT, USA) were housed according to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85–23, revised 1985). Animals (2.0–3.0 kg) were killed with an overdose of sodium pentobarbitone. The hearts were removed and perfused retrogradely through the aorta with nominally Ca2+-free Tyrode solution of the following composition (mM): NaCl, 126; KCl, 5.4; MgCl2, 5.0; NaH2PO4, 1.0; glucose, 22; Hepes, 24; taurine, 20; creatine, 5; sodium pyruvate, 5. This solution was equilibrated with 100 % O2 and its pH was adjusted to 7.4 with NaOH. After 6–8 min, the heart was perfused for approximately 18 min with the solution described above supplemented with collagenase (273 U ml−1, Worthington type II), protease (5.2 U ml−1, Sigma Type XIV), and 0.1 mM CaCl2. Following this enzyme digestion, the heart was perfused for about 5 min with enzyme-free Tyrode solution containing 0.1 mM Ca2+. The heart was then placed in a dissecting dish containing 0.1 mM Ca2+ Tyrode solution. Purkinje fibres from both ventricles were dissected out and placed in a small dish containing fresh enzyme solution (described above). Dissociation of individual cells from the isolated fibres was aided by agitation of the enzyme solution with a stream of 100 % O2. The temperature was maintained at 37°C throughout. Periodically, aliquots containing Purkinje cells in suspension were removed and added to about 5 ml of 0.1 mM Ca2+ Tyrode solution. Fresh enzyme solution was then added to the undigested Purkinje fibres to maintain a volume of approximately 2 ml. Digestion of the Purkinje fibres into populations of myocytes required between 15–60 min under these conditions.
Isolation of ventricular cells
Ventricular cells were also isolated using the method described above. After the Purkinje strands had been dissected from both ventricles, the left ventricle was removed, minced and gently swirled in 0.1 mM Ca2+ Tyrode solution. The resulting suspension containing ventricular cells was then diluted with Tyrode solution and stored in a beaker at room temperature (21–22°C) until use.
Populations of Purkinje cells or ventricular cells were placed in a perfusion chamber mounted on the stage of an inverted microscope (Nikon, Diaphot), allowed to settle and adhere to the bottom of the chamber for about 10 min, and then superfused with oxygenated Hepes Tyrode solution. Recordings were made from myocytes which were fully relaxed and had smooth surfaces. All experiments were performed at 36°C.
Solutions
All solutions were made with Milli-Q grade water. Cells were superfused with oxygenated Hepes Tyrode solution of the following composition (mM): NaCl, 126; KCl, 5.4; MgCl2, 1.0; CaCl2, 1.0; Hepes, 24; glucose, 11. pH was adjusted to 7.4 with NaOH. In some experiments, the extracellular K+ concentration [K+]o was changed to either 2 or 10 mM by adding/removing the appropriate amount of KCl. The internal pipette solution consisted of (mM): potassium aspartate, 90; KCl, 30; K2ATP, 5.0; Hepes, 5.0; EGTA, 10; MgCl2, 1.0; NaCl, 15. pH was adjusted to 7.2 with KOH.
Experimental methods and recording techniques
Myocytes were visualized with a video camera (Pulnix model TM540) and television monitor (Panasonic model WVBM1700). Patch pipettes were fabricated from borosilicate glass capillaries (o.d. 1.5 mm; World Precision Instruments, Sarasota, FL, USA). They were pulled using a gravity puller (BHI, model 7005) and the tips were fire polished. The pipette resistance ranged from 1–4 MΩ when filled with the internal solution. The inclusion of potassium aspartate caused a liquid junction potential of about -10 mV, which was compensated electronically at the start of each recording.
Transmembrane voltages and ionic currents were recorded with the Axoclamp-2B console (Axon Instruments). The series resistance was compensated by approximately 70 % in voltage clamp mode. In ‘Bridge mode’, action potentials were initiated by 3 ms current pulses (0.5–2.0 nA) delivered through the microelectrode at a cycle length of 2000 ms.
Data acquisition and analysis
Membrane potential and current were monitored on a storage oscilloscope, and recorded and analysed on a microcomputer using pCLAMP software (Axon Instruments). Results from pooled data are presented as means ±s.e.m.
Drugs
Tetrodotoxin, nicardipine and 4-aminopyridine were purchased from Sigma. L-735821 (propenamide) and MK-499 were generous gifts from Merck Research Labs (West Point, PA, USA).
RESULTS
Cell morphology
The shape and dimensions of rabbit Purkinje cells differ significantly from ventricular cells obtained from the same heart. The photomicrograph (Fig. 1) shows that rabbit Purkinje cells are longer (approximately 130–140 μm vs. 90–100 μm) but somewhat smaller in width than ventricular cells (10–15 μm vs. 15–20 μm). The input resistance (measured by applying small hyperpolarizing currents from the resting potential) of the Purkinje cell averaged 179 ± 11 MΩ (n = 25) and the mean capacitance was 66.5 ± 2.5 pF (n = 65). In comparison, rabbit ventricular cells had an input resistance of 38 ± 12.6 MΩ and a capacitance of 86.7 ± 8.9 pF.
Figure 1.

Photomicrograph of an enzymatically isolated rabbit Purkinje cell (left) and a ventricular myocyte (right).
Effects of changes in external K+ on action potentials
As an initial basis for comparison of electrophysiological differences, the action potential waveforms of these two types of rabbit myocytes were recorded at 0.5 Hz (36°C). Figure 2A shows three representative action potentials from a rabbit Purkinje cell when the external potassium concentration ([K+]o) was varied from normal (5.4 mM) to 2 and then to 10 mM. Figure 2B illustrates three action potentials from a rabbit ventricular cell following the same changes in [K+]o. Under control conditions, Purkinje cells exhibited a much more prominent phase 1 repolarization, and their action potential duration (APD) was longer than the ventricular myocyte. Most Purkinje cells had a stable resting potential of approximately -85 mV; however, a few showed spontaneous pacemaker activity. In normal [K+]o some Purkinje cells exhibited one or two early after-depolarizations before fully repolarizing.
Figure 2. Representative examples of action potentials from rabbit Purkinje and ventricular myocytes recorded in Tyrode solution containing three different extracellular K+ concentrations ([K+]o).

The horizontal line in each panel denotes 0 mV. A, action potential records from a Purkinje cell exposed to 2.0, 5.4 or 10 mM [K+]o. B, action potentials from a ventricular cell exposed to the same three [K+]o levels. Elevating [K+]o resulted in a marked hyperpolarization in both cell types. In 10 mM [K+]o much less shortening of action potential duration (APD) was observed in the ventricle than in the Purkinje cell. Temperature in this and all subsequent figures, 36 °C.
As shown in Fig. 2A, following exposure to 10 mM [K+]o, the APD of the Purkinje cell shortened markedly. In comparison, exposure of a ventricular cell to 10 mM [K+]o resulted in a less pronounced shortening of APD. When Purkinje cells were exposed to 2.0 mM [K+]o, repolarization slowed and the APD prolonged significantly. In addition, early after-depolarizations were often observed and, less frequently, delayed after-depolarizations occurred (data not shown). In contrast, when ventricular cells were exposed to 2.0 mM [K+]o, the APD was only slightly prolonged and after-potential activity was never observed. Somewhat similar results have been obtained in multicellular preparations from these two tissues excised from human hearts (Christe, 1982).
Comparison of IK1 in Purkinje and ventricular cells
Figures 3A and B show a family of membrane currents recorded from a rabbit Purkinje cell and ventricular myocyte, respectively. In these experiments, each myocyte was held at -80 mV and stepped for 1 s to membrane potentials between -120 and +60 mV in 10 mV increments. Hyperpolarization activated an inwardly rectifying background K+ current (IK1) in both types of cells. IK1 was always much smaller in Purkinje cells (Fig. 3A) than ventricular myocytes (Fig. 3B), being approximately 1 and 5 nA at -120 mV, respectively. No hyperpolarization-activated pacemaker current (If) was observed under our recording conditions.
Figure 3. Inward rectifier K+ current (IK1) in rabbit Purkinje cells and ventricular myoctyes.

A and B, comparison of membrane currents recorded from a rabbit Purkinje (A) and ventricular (B) cells in normal (5.4 mM) [K+]o Tyrode solution. In each experiment, the myocyte was held at -80 mV and membrane currents were elicited by stepping the voltage to potentials between -130 and +50 mV. Na+ and Ca2+ currents were blocked with TTX (30 μM) and nicardipine (5 μM), respectively. Note that the inward rectifier K+ currents, IK1, are much larger in ventricular myocytes than Purkinje cells. Panels C and D compare I–V relations for IK1, in Purkinje (C) and ventricular (D) cells recorded in three different [K+]o Tyrode solutions. Lowering the [K+]o from the normal level of 5.4 mM (•) to 2.0 mM (▴) resulted in a decrease in IK1; raising [K+]o to 10 mM (▪) increased it. All ventricular myocytes expressed a much larger IK1 than the Purkinje cells. Note that between approximately -80 and −40 mV ventricular cells exhibit a more prominent outward component of IK1 than Purkinje cells.
Previous studies in ventricular myocytes have demonstrated that increases in [K+]o cause an anomalous increase in conductance of IK1 at membrane voltages positive to the reversal potential for K+, EK (Harvey & Ten Eick, 1988; Shimoni et al. 1992; for review see Nichols et al. 1996). Figure 3C shows an I–V relationship for IK1 from a rabbit Purkinje cell following selected changes in [K+]o. Each myocyte was held at -80 mV and stepped for 1 s to potentials between -130 and +50 mV in 10 mV increments. K+ currents were measured at the end of the 1 s voltage clamp step. Under control conditions ([K+]o = 5.4 mM, •), the inward component of IK1 is prominent in both ventricular and Purkinje cells. However, in Purkinje cells very little outward current and no negative slope conductance was observed in the voltage range -80 to −40 mV. Increasing the [K+]o to 10 mM produced a substantial increase in inward current (IK1) at hyperpolarized potentials (Fig. 3C), and a small increase in the outward component of IK1. Exposure of the Purkinje cell to 2.0 mM [K+]o produced a marked reduction in the inward component of IK1 with little apparent change in the outward component. The magnitude of IK1 was also studied in ventricular cells isolated from rabbit heart (Fig. 3D). Alterations in [K+]o from control conditions ([K+]o = 5.4 mM, •) showed that at hyperpolarized membrane potentials the density of IK1 is 3–4 times larger in ventricular cells than Purkinje cells. Furthermore, there is a relatively large outward component of IK1 (0.4 nA at the peak) and a prominent region of negative slope conductance between the voltages of -60 and -20 mV (Fig. 3D). Exposure of the same ventricular cell to 2.0 mM [K+]o (▴) resulted in a decrease in the magnitude of both the inward and outward K+ currents, and the peak of the outward current was shifted to more negative potentials (Fig. 3D). Exposure to 10 mM [K+]o (▪) produced an increase in the magnitude of IK1 at hyperpolarized potentials. In addition, the peak outward current shifted to more depolarized potentials and the outward component became larger than in the control recordings. These characteristics of IK1 in rabbit ventricular cells are very similar to those reported previously (Giles & Imaizumi, 1988; Shimoni et al. 1992).
Since IK1 is quite small in Purkinje cells, it seemed possible that leakage current through the electrode/sarcolemma seal resistance may limit accurate measurement of the outward current. IK1 was therefore re-evaluated as the difference current under (i) control conditions ([K+]o = 5.4 mM) and (ii) after addition of 0.1 mM BaCl2. The left panel of Fig. 4A consists of a family of IK1 currents measured in a Purkinje cell under control conditions ([K+]o = 5.4 mM) from a holding potential of -80 mV. Addition of 0.1 mM BaCl2 (right side of figure) strongly inhibited the inward K+ current but did not change the large transient outward current, It.
Figure 4. K+ currents recorded from rabbit Purkinje and ventricular cells.

A, current changes were elicited by stepping the voltage from the holding potential (-80 mV) to membrane potentials between -130 and +50 mV. B, I–V relation for IK1 measured in normal conditions ([K+]o, 5.4 mM) as the Ba2+-sensitive (0.1 mM) difference current (mean ±s.e.m., n = 8). The IK1 current density in Purkinje cells (•) is approximately half that in ventricular myocytes (▾). C, effects of changes in [K+]o on the inwardly rectifying background K+ current in rabbit Purkinje cells (mean ±s.e.m., n = 8). Reduction of [K+]o to 2.0 mM (▿) caused a marked decrease in the magnitude of inward K+ current at hyperpolarized potentials. Raising [K+]o to 10 mM resulted in an increase in inward current (▪).
The mean current-voltage relation for IK1 from eight Purkinje cells and ventricular myocytes in 5.4 mM [K+]o, measured as the Ba2+-sensitive difference current is shown in Fig. 4B. The slope conductance of the inward component of IK1 in ventricular cells is 2–3 times larger than that of Purkinje cells; and the outward component of IK1 is also much larger in ventricular cells. Note also that IK1 in Purkinje cells (recorded with this improved resolution) has a negative slope conductance, although this feature is much less prominent than in ventricular cells. Figure 4C shows the mean I–V relation for IK1 in Purkinje cells in the presence of 2.0 and 10 mM [K+]o, again measured as the Ba2+-sensitive difference current. In 2.0 mM [K+]o, the magnitude of current at hyperpolarized potentials decreased with respect to control (▿). Exposure to [K+]o of 10 mM resulted in a large increase in the inward current, and a smaller but significant increase in the outward current. Together, these changes result in a ‘cross-over’ phenomenon, which is characteristic of inwardly rectifying K+ current in mammalian heart (Hutter & Noble, 1960; Shimoni et al. 1992; Nichols et al. 1996).
Previous studies have demonstrated that IK1 is important for maintaining the resting potential and have also shown that its outward component is mainly responsible for the terminal phase of repolarization in rabbit ventricular cells (Shimoni et al. 1992). Figure 5A consists of superimposed current traces from rabbit Purkinje and ventricular cells recorded using a voltage clamp protocol which consisted of an initial step from the holding potential of -80 mV to -120 mV, followed by a 1 s ramp to a potential of +20 mV. In this figure, the horizontal line represents the 0 current level. In the ventricular cell, as the voltage is ramped between -120 and +20 mV, a large outward hump is observed. The Purkinje cell exhibited a much smaller outward component in response to the same voltage ramp (Fig. 5A). To confirm that these current changes in the Purkinje cell were due to IK1, the ramp protocol was repeated in the presence of 0.1 mM Ba2+ (Fig. 5B); both the small outward hump and the large inward component of IK1 were blocked. In the I–V curve for the Ba2+-sensitive difference current (Fig. 5C) the outward component of IK1 peaks at about -70 mV and negative slope conductance is observed between -60 and -30 mV.
Figure 5. Rectifier properties of IK1 in rabbit Purkinje and ventricular cells studied using a ramp voltage clamp protocol.

As shown at the top, the holding potential was -80 mV, and the potential was ramped from -120 to +20 mV in a 1 s period. The recording from the ventricular cell (A) shows a prominent outward ‘hump’ and negative slope conductance. In contrast, the current recorded from the Purkinje cell shows a much smaller outward current. B shows currents recorded from a different Purkinje cell in the absence and presence of 0.1 mM Ba2+. C shows the I–V relation for the Ba2+-sensitive difference in a Purkinje cell. The reversal potential is near -80 mV, and the small outward hump of IK1 peaks at approximately -70 mV. A small negative slope conductance is present between -70 and -30 mV.
Transient outward K+ current in Purkinje cells and ventricular cells
In rabbit Purkinje cells, the prominent phase 1 repolarization suggests the presence of a large transient outward K+ current (It), as has been demonstrated in Purkinje fibres from other species (Coraboeuf & Carmeliet, 1982; Dangman, 1991). Three different types of transient outward currents have been identified in rabbit heart: (i) a Ca2+-independent K+ current (reviewed by Campbell et al. 1995; Giles et al. 1996); (ii) a Ca2+-dependent K+ current (Hiraoka & Kawano, 1987); and (iii) a Ca2+-activated chloride current (Zygmunt & Gibbons, 1991, 1992; Sipido et al. 1993). The Ca2+-independent K+ current can be inhibited by millimolar concentrations of 4-aminopyridine (4-AP, 1–5 mM). Figure 6A shows a rabbit Purkinje cell action potential under control conditions ([K+]o = 5.4 mM) and after addition of 2 mM 4-AP at a basic cycle length of 0.5 Hz. In the absence of 4-AP, the Purkinje action potential exhibited a rapid phase 1 repolarization and the plateau of the action potential was at approximately -20 mV. After addition of 2 mM 4-AP, the prominent phase 1 of repolarization was reduced substantially, the plateau of the action potential depolarized to approximately 0 mV, and the duration of the action potential lengthened dramatically. Figure 6B shows a ventricular action potential under control conditions and after addition of 2 mM 4-AP at the same stimulus frequency. Under control conditions, the ventricular action potential has a much less prominent phase one repolarization than the Purkinje action potential. Addition of 2 mM 4-AP caused the small notch in the ventricular action potential to disappear; however, this concentration of 4-AP did not alter the plateau of the ventricular action potential, nor did it change the duration.
Figure 6. Effect of 4-aminopyridine (4-AP) on action potentials recorded from a Purkinje cell and ventricular myocyte.

Horizontal line denotes 0 mV. Addition of 2 mM 4-AP to the Purkinje cell (A) resulted in slowing of phase 1 repolarization, a substantial increase in the height of the plateau and action potential prolongation. Addition of 2 mM 4-AP to the ventricular cell (B) caused slowing of the early repolarization phase; however, neither the plateau height nor the duration of the action potential were altered significantly. The frequency of stimulation was 0.5 Hz.
Voltage clamp measurements of the I–V curve and reactivation kinetics of It in rabbit Purkinje cells are shown in Figs 7 and 8. In these experiments the Ca2+-activated K+ and Cl− currents were strongly suppressed by inclusion of EGTA (10 mM) in the recording pipette, and the L-type Ca2+ current was blocked by nicardipine (5 μM). The holding potential was -80 mV and the membrane voltage was stepped for 1 s to potentials between -60 and +50 mV. Figure 7A shows a family of currents recorded from a Purkinje cell under control conditions. The rapid, transient component of outward K+ current increased progressively with more positive voltage clamp steps. Addition of 2 mM 4-AP resulted in a marked decrease in the initial transient component of this current (Fig. 7B). It was measured as the difference between the peak current and the steady-state current at the end of the 1 s voltage clamp pulse. The current-voltage relationship for It in Fig. 7C (n = 8) shows that It is activated at approximately -20 mV.
Figure 7. 4-AP-sensitive membrane currents recorded from rabbit Purkinje cells.

Membrane currents were elicited by stepping the voltage from the holding potential, -80 mV, to membrane potentials between -50 and +60 mV. Addition of 2 mM 4-AP (B) caused inhibition of the transient component of outward current. C shows mean I–V relation for It under control conditions (•) and after 2 mM 4-AP application (▴). Values are means ±s.e.m. recorded from 8 Purkinje cells.
Figure 8. Reactivation of It in rabbit Purkinje cells.

A shows representative transient outward currents measured by applying two identical voltage clamp steps to +40 mV from a holding potential of -80 mV separated by selected time intervals. Nicardipine (5 μM) was present to block the L-type Ca2+ current, ICa,L. The recovery time course of It recorded from 4 Purkinje cells is shown in B. The half-time of reactivation at -80 mV was approximately 275 ms.
In myocytes from rabbit crista terminalis, ventricle and atrium, It modulates the early repolarization of the action potential due to the frequency dependence of its reactivation (cf. Campbell et al. 1995; Giles et al. 1996). The experimental results in Fig. 8A demonstrate somewhat similar frequency-dependent changes in It in rabbit Purkinje cells. The reactivation time course of It recorded from four Purkinje cells is illustrated in Fig. 8B. Reactivation of It at -80 mV followed a monotonic time course, with a half-time of reactivation of approximately 275 ms. These results are consistent with It being responsible for the phase 1 repolarization, and can explain the observed changes in phase 1 repolarization in response to altered stimulation frequency.
Delayed rectifier K+ current(s) in rabbit Purkinje cells
Our previous work shows that the delayed rectifier K+ current (IK) is very small in both atrial and ventricular cells from rabbit heart (Giles & Imaizumi, 1988; Muraki, Imaizumi, Watanabe, Habuchi & Giles, 1995; but see Salata et al. 1997). In most mammalian hearts, this K+ conductance is composed of two components: a slowly activating one, denoted IK,s, and a rapidly activating, inwardly rectifying component, IK,r (Sanguinetti & Jurkiewicz, 1990, 1992; Salata et al. 1997). In the present experiments the role of the delayed rectifier(s) in repolarization of the Purkinje action potential was investigated using recently described, selective pharmacological blockers for IK,r and IK,s. Figure 9A shows action potentials and corresponding dV/dt measurements from a Purkinje cell in the presence of the IK,r blocker MK-499 (Sanguinetti & Jurkiewicz, 1990; Lynch et al. 1994). Each trace is an average of three action potentials recorded at 0.5 Hz. In the absence of the drug, the action potential duration was about 450 ms. Addition of 200 nM MK-499 produced a marked lengthening of the action potential within 8–10 min. Longer exposure to MK-499 at this concentration resulted in the appearance of early after-depolarizations and finally the cell failed to repolarize. In corresponding voltage clamp experiments, the voltage was stepped for 500 ms from the holding potential (-45 mV) to +65 mV in 10 mV increments. Figure 10 shows the family of currents recorded from a rabbit ventricular and a Purkinje cell. In the Purkinje cell, there was very little development of outward current in response to depolarization (Fig. 10B), while in the ventricular cell under the same recording conditions a measurable outward current was observed (Fig. 10A) Addition of MK-499 caused no measurable change (< 20 pA) in the magnitude of the outward current in this Purkinje cell (data not shown), or in three others.
Figure 9. Attempts to identify delayed rectifier K+ currents in rabbit Purkinje cells.

A, representative action potentials and corresponding dV/dt measurements from a Purkinje cell under control conditions and in the presence of a blocker (MK-499) of the fast component of delayed rectification, IK,r. Under control conditions (at 0.5 Hz), the action potential duration was about 450 ms. Exposure to 200 nM MK-499 caused the action potential to lengthen significantly. B, action potentials recorded from a Purkinje cell under control conditions and in the presence of 20 nM L-735821 (propenamide), a blocker of the slow component of delayed rectification, IK,s. Under control conditions (at 0.5 Hz), the action potential duration was about 425 ms. L-735821 (20 nM) caused the action potential to lengthen significantly. In both panels the large positive deflection of the dV/dt (corresponding to the upstroke of the action potential) is truncated. The -dV/dt records show that although both drugs prolong the action potential, the rate of final repolarization (due to IK1) is not changed significantly.
Figure 10. Comparison of outward K+ currents in rabbit ventricular (A) and Purkinje (B) cells in 5.4 mM K+ Tyrode solution.

Cells were depolarized from the holding potential (-45 mV) to +45 mV for 500 ms in 10 mV increments. Nicardipine (5 μM) and 4-AP (2 mM) were present. Note that an outward current was observed in the ventricular cell (A); but not in the Purkinje cell (B). C shows current traces recorded from a Purkinje cell immediately after a brief hyperpolarizing pulse. In this protocol, a 500 ms pulse (P1) to +20 mV from a holding potential of -45 mV was applied; and then a second identical pulse preceded by a brief (5 ms) hyperpolarizing pulse to -110 mV was applied. The hyperpolarizing pulse was applied to remove inactivation of the rapid delayed rectifier component, IK,r (see text). The current traces in C illustrate the last 100 ms of P1, the brief hyperpolarizing pulse, and the first 75 ms of the second pulse. Following the brief hyperpolarizing pulse, a small outward current was observed during the second depolarization. Addition of MK-499 resulted in a reduction of this current, suggesting that it could be generated by IK,r.
We reasoned that our inability to detect IK,r may be able to be overcome by enhancing this current using voltage clamp steps which remove its steady-state inactivation (Spector, Curran, Zou, Keating & Sanguinetti, 1996; Yang, Snyders & Roden, 1997). Figure 10C shows K+ current traces recorded immediately following a brief hyperpolarizing voltage clamp pulse (which removes inactivation of IK,r). In these experiments, Purkinje cells were held at -45 mV and stepped to +20 mV for 500 ms; thereafter a second 500 ms pulse to +20 mV was applied following a brief (5 ms) hyperpolarizing pulse to -110 mV. The current record trace in Fig. 10C illustrates the current changes during: (i) the last 100 ms of the first voltage clamp pulse, (ii) the brief hyperpolarizing pulse, and (iii) the first 75 ms of the second voltage clamp depolarization. Note that a small outward current was observed following the hyperpolarizing prepulse. Addition of 200 nM MK-499 strongly inhibited this current, suggesting that it could be generated by IK,r.
Very recently, a selective blocker of the slow component of the cardiac delayed rectifier K+ current has been described (Salata et al. 1997). This agent, L-735821 or propenamide, was applied to Purkinje cells at 20 nM, using the same stimulation protocol as in the experiment shown in Fig. 9A. Within approximately 10 min, L-735821 resulted in marked prolongation of the action potential (Fig. 9B). This agent was applied to three other Purkinje cells and very similar changes in the action potential were observed. In corresponding voltage clamp experiments, to elicit IK,s the voltage was stepped for 3 s from the holding potential (-45 mV) to +65 mV in 10 mV increments. Nicardipine (5 μM) and 4-AP (2 mM) were present in these experiments. Figure 11A and B shows the family of currents recorded from a rabbit ventricular and a Purkinje cell under these conditions. In the Purkinje cell, a very small outward current was observed at +55 mV (Fig. 11B). A much larger outward current was recorded in the ventricular cell in response to the same voltage clamp protocol (Fig. 11A). Figure 11C shows the mean isochronal current-voltage relation for the six Purkinje cells. Note that this outward current is very small (about 25 pA) even at +60 mV. To determine whether this current was generated by IK,s, L-735821 (20 nM) was applied to the Purkinje cells; it resulted in an approximately 50 % reduction in the magnitude of current measured at potentials more positive than 0 mV.
Figure 11. Comparison of delayed rectifier currents recorded from a rabbit ventricular myocyte (A) and Purkinje cell (B).
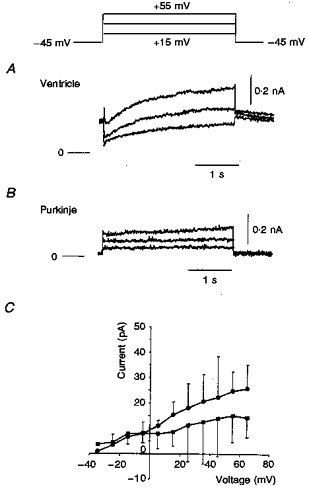
In these experiments cells were depolarized for 3 s in 10 mV increments to +45 mV from the holding potential (-45 mV). Nicardipine (5 μM) and 4-AP (2 mM) were present throughout. Large and long depolarizations elicited a measurable outward current in the ventricular cell (A). In comparison, in the Purkinje cell, only a very small outward current developed (B). C shows a mean isochronal I–V relation (5 s) recorded from 6 Purkinje cells. The maximum current change was very small, about 25 pA at +60 mV. The apparent decrease after L-735821 (20 nM) suggests that this current change was due to the slow delayed rectifier, IK,s. •, control (5.4 mM K+); ▪, with 20 nM L-735821.
DISCUSSION
Summary of main findings
Our results, and previous work (Callewaert et al. 1984) demonstrate that viable single Purkinje cells can be isolated consistently from adult rabbit hearts using a straightforward enzymatic isolation procedure. Rabbit Purkinje cells are longer and somewhat narrower than ventricular cells (Fig. 1). They have a smaller capacitance (approximately 66 vs. 88 pF) and a much higher input resistance (approximately 180 vs. 38 MΩ). The action potential duration (APD) of Purkinje cells is much longer and it has more prominent phase 1 repolarization and a lower plateau than the ventricular waveform. Voltage clamp measurements show that Purkinje cells express a significantly smaller inward rectifier, IK1, and a larger Ca2+-independent transient outward K+ current, It, than ventricular myocytes from the same rabbit heart. Our results also suggest that the delayed rectifier K+ currents are considerably smaller in Purkinje cells than in ventricular myocytes.
Action potential waveforms and responses to changes in [K+]o
The resting potential (approximately -85 mV) and the action potential configuration of rabbit single Purkinje cells are both very similar to those which have been recorded in vitro from intact Purkinje fibres (Callewaert et al. 1984; Adamantidis et al. 1995; cf. Dangman, 1991). In Purkinje cells, reducing [K+]o (from 5.4 to 2.0 mM) resulted in a pronounced prolongation of the action potential and elevating [K+]o (from 5.4 to 10.0 mM) caused a marked abbreviation of it. Both of these responses are opposite to those predicted from the changes in electrochemical driving force for K+. This ‘anomalous’ dependence of APD and resting potential in Purkinje fibres was first described more than 35 years ago by Hutter & Noble (1960) who showed that this could be attributed to the non-linearities due to inwardly rectifying K+ conductances.
The rabbit Purkinje cell action potential waveform was also changed markedly by the addition of 4-AP (2 mM); phase 1 repolarization was nearly abolished and the APD increased significantly (Fig. 5). Since 4-AP inhibits the Ca2+-independent transient outward K+ current, It (cf. Giles, Clark & Braun, 1996), this finding strongly suggests that It is responsible for phase 1 repolarization. The reasons for the observed increase in APD following 4-AP treatment were not addressed in this study. It is plausible, however, that the increased (more depolarized) plateau level resulted in a reduced contribution of IK1 to repolarization of the action potential.
Previous studies have shown that there are significant differences in the ventricular action potential waveforms from epicardial and endocardial tissue in dog (Antzelevitch et al. 1995), rat (Clark, Bouchard, Salinas-Stefanon, Sanchez-Chapula & Giles, 1993) and rabbit (Fedida & Giles, 1991). All of these studies demonstrated that the 4-AP-sensitive current, It, is more prominent in epicardial than endocardial tissue. It is therefore of interest that Purkinje cells (located on the endocardium) express a substantial It (Figs 6-8). In fact, It in rabbit Purkinje cells is slightly larger (approximately 10.5 pA pF−1) than the corresponding K+ current of ventricular cells from the epicardium (approximately 8 pA pF−1) of rabbit ventricular cells (Fedida & Giles, 1991).
K+ currents in Purkinje and ventricular cells
Our findings demonstrate that the two largest K+ currents in rabbit Purkinje cells are a Ca2+-independent transient outward current, It, and an inward rectifier, IK1. The size of IK1 varies markedly when different regions of the rabbit heart are compared: it is large in the ventricles, much smaller in atria (Hume & Uehara, 1985; Giles & Imaizumi, 1988; Muraki et al. 1994) and crista terminalis (Giles & van Ginneken, 1985) and virtually absent from pacemaker tissue (Irisawa et al. 1993). The magnitude of IK1 in Purkinje cells, measured as a slope conductance between -120 and -80 mV, was one-third to one-half of that of ventricular cells. Whole-cell voltage clamp measurements of IK1 have demonstrated that changing [K+]o results in an anomalous change in the magnitude of IK1 and a ‘cross-over’ effect (Carmeliet, 1982; Shimoni et al. 1992; Nichols et al. 1996). This ‘cross-over’ of the I–V curve is the reason that increases in [K+]o result in a marked shortening of the action potential duration in Purkinje cells; and that reductions in [K+]o result in a lengthening of APD (Hutter & Noble, 1960; Shimoni et al. 1992; Nichols et al. 1996). Corresponding changes in [K+]o caused a similar, but less pronounced, pattern of changes in the action potential of ventricular cells (Fig. 2 and Shimoni et al. 1992).
Previous studies have shown that the delayed rectifier K+ current is very small in rabbit atrial (Muraki et al. 1995) and ventricular cells (Giles & Imaizumi, 1988; but see Salata et al. 1997). In Purkinje cells (Fig. 9), addition of MK-499, an IK,r inhibitor (Lynch et al. 1994) caused a significant prolongation of the action potential, and prolonged exposure to MK-499 often resulted in the appearance of early after-depolarizations. These findings suggest that IK,r is important in initiating repolarization. Bath application of L-735821 (20 nM), a recently described (Salata et al. 1996) potent blocker of IK,s, also significantly lengthened the APD of Purkinje cells. However, prolonged exposure to this agent did not cause the appearance of early after-depolarizations (n = 4). Attempts to measure IK,r and IK,s in Purkinje cells yielded equivocal results (Figs 10 and 11) due to the very small size (apparently < 20 pA) of these delayed rectifier K+ currents. It is unlikely, however, that our failure to record larger K+ currents was due to myocyte damage at the time of isolation since (i) action potential waveforms were comparable to those recorded in rabbit Purkinje fibres, (ii) resting potential was stable and (iii) larger delayed rectifier currents were recorded in ventricular myocytes prepared from the same hearts (Fig. 11).
Contributions of K+ currents to repolarization in Purkinje cells
To understand the functional roles of the three main K+ currents in rabbit Purkinje cells (IK1, IK, It) it is useful to first ask the question: how much net current is present during the final phase of repolarization? An approximate answer, in a space clamped preparation, can be obtained from the product of the first derivative of the action potential during repolarization (-dV/dt) and the cell capacitance, Cm. In rabbit Purkinje cells, paced at 0.5 Hz, -dV/dt is between -0.5 and -1 V s−1 at 36°C and Cm is approximately 80 pF. Thus, approximately 40–80 pA of net outward current must be present within the voltage range of the final repolarization phase of the action potential (approximately -30 to -80 mV). Of this, the outward ‘hump’ of IK1 contributes a large fraction, approximately 50 pA (Figs 4 and 5). An electrogenic Na+-K+ pump current, Ip, has been identified in single Purkinje cells. From published information on rabbit Purkinje myocytes (Glitsch & Tappe, 1995) Ip would be expected to contribute 10–30 pA. The data of Scamps & Carmeliet (1989) suggest that IK could contribute approximately an additional 30 pA. Thus, final repolarization from the plateau in a single Purkinje cell is regulated by IK1, Ip and the delayed rectifier(s). The pharmacological results in Figs 9-11 suggest that the delayed rectifier in rabbit Purkinje fibres could be generated by both IK,r and IK,s. At present we know of no way of artificially augmenting the size of either of these K+ currents. Detailed studies of their biophysical properties in rabbit Purkinje cells is therefore not feasible.
Our results provide several new insights into the mechanism(s) of repolarization and the underlying ionic currents in rabbit Purkinje cells, and demonstrate the major differences in K+ currents between Purkinje and ventricular cells in adult rabbit hearts.
It is of interest that the complement of K+ currents which we have recorded in Purkinje fibres can account for the strong and anomalous dependence upon changes in the electrochemical driving force for [K+]. In Purkinje fibres inward rectification, due to IK1 and its negative slope conductance and cross-over of the I–V curves have been identified and their functional significance understood for approximately 30 years (Hutter & Noble, 1960; cf. Shimoni et al. 1992; Nichols et al. 1996). It has recently been shown that the fast component of delayed rectification in mammalian heart also exhibits substantial inward rectification and strong dependence upon [K+]o (Spector et al. 1996; Yang et al. 1997). It is likely therefore that the marked changes in action potential wave shape which we have identified depend on changes in both IK,r and IK1. Unfortunately, the size of IK,r in our rabbit Purkinje cells is too small to allow detailed study of any of the properties of this current. However, Scamps & Carmeliet (1989) have described an anomalous dependence of the size of the delayed rectifier in Purkinje cells on [K+]o.
The strong inward rectification of IK1 which we have identified, and which has been described for IK,r in other preparations, makes it likely that the contribution of either of these currents to the early repolarization or the plateau phase of the action potential is minimal. Perhaps this is one reason why Purkinje cells exhibit after-potential activity (early and delayed after-depolarizations) more frequently than ventricular cells. Although isolated Purkinje cells apparently have a lower safety factor for repolarization than ventricular cells (Christe, 1982; Dangman, 1991), in vivo the electrotonic coupling of Purkinje cells to ventricular cells would, of course, tend to hyperpolarize their resting membrane potential and shorten their action potential.
Acknowledgments
This study was supported by operating grants to Dr W. Giles from the Canadian Medical Research Council and the Heart and Stroke Foundation of Canada; and to Dr K. W. Spitzer from the National Institutes of Health, the Nora Eccles Treadwell Foundation and the Richard A. and Nora Eccles Harrison Fund for Cardiovascular Research. J. M. Cordeiro is a recipient of a Heart and Stroke Foundation of Canada Fellowship. W. Giles holds a Medical Scientist Award from the Alberta Heritage Foundation for Medical Research. The authors thank Gary Webster for excellent technical assistance, Karen Burrell for secretarial assistance, and Dr Robert B. Clark for helpful discussions. L-735821 and MK-499 were generous gifts from Merck Research Laboratories.
References
- Adamantidis MM, Lacroix DL, Caron JF, Dupuis BA. Electrophysiological and arrhythmogenic effects of the histamine type-1 receptor antagonist astemizole on rabbit Purkinje fibres: clinical relevance. Journal of Cardiovascular Pharmacology. 1995;26:319–327. doi: 10.1097/00005344-199508000-00019. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Sicouri S, Lukas A, Nesterenko VV, Liu DW, Di Diego JM. Regional differences in the electrophysiology of ventricular cells: Physiological and clinical implications. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: from Cell to Bedside. 2. Philadelphia: Saunders Co. Ltd; 1995. pp. 228–245. [Google Scholar]
- Callewaert G, Carmeliet E, Vereecke J. Single cardiac Purkinje cells: general electrophysiology and voltage-clamp analysis of the pacemaker current. The Journal of Physiology. 1984;349:643–661. doi: 10.1113/jphysiol.1984.sp015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DL, Rasmusson RL, Comer MB, Strauss HC. The cardiac calcium-independent transient outward potassium current: kinetics, molecular properties and role in ventricular repolarization. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: from Cell to Bedside. 2. Philadelphia: Saunders Co. Ltd; 1995. pp. 83–96. [Google Scholar]
- Carmeliet E. Induction and removal of inward-going rectification in sheep cardiac Purkinje fibers. The Journal of Physiology. 1982;327:285–308. doi: 10.1113/jphysiol.1982.sp014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E, Mubagwa K. Characterization of the acetylcholine-induced potassium current in rabbit cardiac Purkinje fibres. The Journal of Physiology. 1986;371:219–237. doi: 10.1113/jphysiol.1986.sp015970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christe G. Effects of low [K+]o on the electrical activity of human cardiac ventricular and Purkinje cells. Cardiovascular Research. 1982;17:243–250. doi: 10.1093/cvr/17.4.243. [DOI] [PubMed] [Google Scholar]
- Clark RB, Bouchard RA, Salinas-Stefanon E, Sanchez-Chapula J, Giles WR. Heterogeneity of action potential waveforms and potassium currents in rat ventricle. Cardiovascular Research. 1993;27:1795–1799. doi: 10.1093/cvr/27.10.1795. [DOI] [PubMed] [Google Scholar]
- Colatsky TJ. Voltage clamp measurements of sodium channel properties in rabbit cardiac Purkinje fibers. The Journal of Physiology. 1980;305:215–234. doi: 10.1113/jphysiol.1980.sp013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E, Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflügers Archiv. 1982;392:352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Cordeiro JM, Spitzer KW, Giles WR. A comparison of repolarizing K+ currents in rabbit Purkinje cells and ventricular myocytes. Biophysical Journal. 1997;72:A49. [Google Scholar]
- Dangman KH. Electrophysiology of the Purkinje fiber. In: Dangman KH, Miura DS, editors. Electrophysiology and Pharmacology of the Heart. A Clinical Guide. New York: Marcel Dekker, Inc.; 1991. pp. 161–199. [Google Scholar]
- Fedida D, Giles WR. Regional variations in action potentials and transient outward current in myocytes isolated from rabbit left ventricle. The Journal of Physiology. 1991;442:191–209. doi: 10.1113/jphysiol.1991.sp018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles WR, Clark RB, Braun A. Ca2+-independent transient outward current in mammalian heart. In: Morad M, Kurachi Y, Noma A, Hosada M, editors. Molecular Physiology and Pharmacology of Cardiac Ion Channels and Transporters. Amsterdam: Kluwer Press Ltd; 1996. pp. 141–168. [Google Scholar]
- Giles WR, Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. The Journal of Physiology. 1988;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles WR, van Ginneken ACG. A transient outward current in isolated cells from the crista terminalis of rabbit heart. The Journal of Physiology. 1985;368:243–264. doi: 10.1113/jphysiol.1985.sp015856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch HG, Tappe A. Change of Na+ pump current reversal potential in sheep cardiac Purkinje cells with varying free energy of ATP hydrolysis. The Journal of Physiology. 1995;484:605–616. doi: 10.1113/jphysiol.1995.sp020690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RD, Ten Eick RE. Characterization of the inward-rectifying potassium current in cat ventricular myocytes. Journal of General Physiology. 1988;91:593–615. doi: 10.1085/jgp.91.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M, Kawano S. Mechanism of increased amplitude and duration of the plateau with sudden shortening of diastolic intervals in rabbit ventricular myocytes. Circulation Research. 1987;60:14–26. doi: 10.1161/01.res.60.1.14. [DOI] [PubMed] [Google Scholar]
- Hume JR, Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. The Journal of Physiology. 1985;368:525–544. doi: 10.1113/jphysiol.1985.sp015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter OF, Noble D. Rectifying properties of heart muscle. Nature. 1960;188:495. doi: 10.1038/188495a0. [DOI] [PubMed] [Google Scholar]
- Irisawa H, Brown HF, Giles WR. Cardiac pacemaking in the sinoatrial node. Physiological Reviews. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Lindblad DS, Murphey CR, Clark JW, Giles WR. A model of the action potential and underlying membrane currents in a rabbit atrial cell. American Journal of Physiology. 1996;271:H1666–1696. doi: 10.1152/ajpheart.1996.271.4.H1666. [DOI] [PubMed] [Google Scholar]
- Lynch JJ, Wallace AA, Stupienski RF, Baskin EP, Beare CM, Appleby SD, Salata JJ, Jurkiewicz NK, Sanguinetti MC, Stein RB, Gehret JR, Kothstein T, Claremon DA, Elliot JM, Bitcher JW, Remy DC, Baldwin JJ. Cardiac electrophysiologic and antiarrhythmic actions of two long-acting spirobenzopyran piperidine class III agents, L-702,958 and L-706,000 [MK-499] Journal of Pharmacology and Experimental Therapeutics. 1994;269:541–554. [PubMed] [Google Scholar]
- Muraki K, Imaizumi Y, Watanabe M, Habuchi Y, Giles WR. Delayed rectifier K+ current in rabbit atrial cells. American Journal of Physiology. 1995;269:H524–532. doi: 10.1152/ajpheart.1995.269.2.H524. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Makhina WL, Pearson WL, Sha Q, Lopatin AN. Inward rectification and implications for cardiac excitability. Circulation Research. 1996;78:1–7. doi: 10.1161/01.res.78.1.1. [DOI] [PubMed] [Google Scholar]
- Noble D, Tsien RW. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. The Journal of Physiology. 1968;195:185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva C, Cohen IS, Pennefather P. The mechanism of rectification of IK1 in canine Purkinje myocytes. Journal of General Physiology. 1990;96:299–319. doi: 10.1085/jgp.96.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp Z, Sipido KR, Callewaert G, Carmeliet E. Two components of [Ca2+]i-activated Cl− current during large [Ca2+]i transients in single rabbit heart Purkinje cells. The Journal of Physiology. 1995;483:319–330. doi: 10.1113/jphysiol.1995.sp020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata JJ, Jurkiewicz NK, Jow B, Folander K, Guinosso PJ, Raynor B, Swanson R, Fermini B. IK of rabbit ventricle is composed of two currents: evidence for IK,s. American Journal of Physiology. 1997;271:H2477–2489. doi: 10.1152/ajpheart.1996.271.6.H2477. [DOI] [PubMed] [Google Scholar]
- Salata JJ, Jurkiewicz NK, Sanguinetti MC, Siegl PK, Clareman DA, Remy DC, Elliot JM, Libby BE. The novel class III antiarrhythmic agent, L-735–821 is a potent and selective blocker of IK,s in guinea pig ventricular myocytes. Circulation. 1996;94:I–529. [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current: differential sensitivity to block by Class III antiarrhythmic agents. Journal of General Physiology. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Role of external Ca2+ and K+ in gating of cardiac delayed rectifier K+ currents. Pflügers Archiv. 1992;420:180–186. doi: 10.1007/BF00374988. [DOI] [PubMed] [Google Scholar]
- Scamps F, Carmeliet E. Delayed K+ current and external K+ in single cardiac Purkinje cells. American Journal of Physiology. 1989;257:C1086–1092. doi: 10.1152/ajpcell.1989.257.6.C1086. [DOI] [PubMed] [Google Scholar]
- Sheets MF, January CT, Fozzard HA. Isolation and characterization of single canine Purkinje cells. Circulation Research. 1983;53:544–548. doi: 10.1161/01.res.53.4.544. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Clark RB, Giles WR. Role of an inwardly rectifying potassium current in rabbit ventricular action potential. The Journal of Physiology. 1992;448:709–727. doi: 10.1113/jphysiol.1992.sp019066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido KR, Callewaert G, Carmeliet E. [Ca2+]i-dependent chloride current in single Purkinje cells from rabbit heart. The Journal of Physiology. 1993;468:641–667. doi: 10.1113/jphysiol.1993.sp019793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector PS, Curran ME, Zou A, Keating MY, Sanguinetti MC. Fast inactivation causes rectification of the IK,r channel. Journal of General Physiology. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surawicz B. Role of potassium channels in cycle length dependent regulation of action potential duration in mammalian cardiac Purkinje and ventricular muscle fibers. Cardiovascular Research. 1992;26:1021–1029. doi: 10.1093/cvr/26.11.1021. [DOI] [PubMed] [Google Scholar]
- Yang T, Snyders DJ, Roden DM. Rapid inactivation determines the rectification and [K+]o dependence of the rapid component of the delayed rectifier K+ current in cardiac cells. Circulation Research. 1997;80:782–789. doi: 10.1161/01.res.80.6.782. [DOI] [PubMed] [Google Scholar]
- Zygmunt AC, Gibbons WR. Calcium-activated chloride current in rabbit ventricular myocytes. Circulation Research. 1991;68:424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]
- Zygmunt AC, Gibbons WR. Properties of the calcium-activated chloride current in the heart. Journal of General Physiology. 1992;99:391–414. doi: 10.1085/jgp.99.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]


