Abstract
This study was performed to test the hypothesis that inflammatory cytokines are produced in skeletal muscle in response to prolonged intense exercise. Muscle biopsies and blood samples were collected from runners before, immediately after, and 2 h after a marathon race.
The concentration of interleukin (IL)-6 protein in plasma increased from 1.5 ± 0.7 to 94.4 ± 12.6 pg ml−1 immediately post-exercise and to 22.1 ± 3.8 pg ml−1 2 h post-exercise. IL-1 receptor antagonist (IL-1ra) protein in plasma increased from 123 ± 23 to 2795 ± 551 pg ml−1, and increased further to 4119 ± 527 pg ml−1 2 h post-exercise.
The comparative polymerase chain reaction technique was used to evaluate mRNA for IL-6, IL-1ra, IL-1β and tumour necrosis factor (TNF)-α in skeletal muscle and blood mononuclear cells (BMNC) (n = 8). Before exercise, mRNA for IL-6 could not be detected either in muscle or in BMNC, and was only detectable in muscle biopsies (5 out of 8) after exercise. Increased amounts of mRNA for IL-1ra were found in two muscle biopsies and five BMNC samples, and increased amounts of IL-1β mRNA were found in one muscle and four BMNC samples after exercise. TNF-α mRNA was not detected in any samples.
This study suggests that exercise-induced destruction of muscle fibres in skeletal muscles may trigger local production of IL-6, which stimulates the production of IL-1ra from circulating BMNC.
Tissue injury induces a complex cascade of non-specific events known as the inflammatory response, which provides early recognition by restricting tissue damage to the site of injury. The local response involves the production of cytokines that are released at the site of inflammation. In the acute phase response, interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α have been most extensively studied. IL-1 receptor antagonist (IL-1ra) exerts its effect by blocking the biological activities of IL-1α and IL-1β. IL-6 has been shown to up-regulate IL-1ra (Seckinger, Lowenthal, Williamson, Dayer & Macdonald, 1987; Eisenberg et al. 1990; Hannum et al. 1990; Mazzei, Seckinger, Dayer & Shaw, 1990). Several studies suggest that eccentric exercise induces muscle damage and an acute phase response.
Recently Bagby, Crouch & Shepherd (1996) suggested that although IL-1 was believed to be the cytokine responsible for the exercise-induced plasma activities described by Cannon & Kluger (1983), Cannon, Evans, Hughes, Meredith & Dinarello (1986) and Evans et al. (1986), the possibility exists that other cytokines were measured. The latter studies were conducted prior to the availability of recombinant IL-1 proteins. Furthermore, the thymocyte proliferation bioassay also detects IL-6. Therefore, the possibility exists that the cytokine responsible for the activity measured in the thymocyte bioassay, or for the fever-inducing properties of plasma, was IL-6 and not IL-1. As pointed out by Bagby et al. (1996), there have been a number of studies that failed to detect significantly elevated levels of IL-1 in plasma (Cannon et al. 1991; Northoff & Berg, 1991; Sprenger et al. 1992; Ullum, Haahr, Diamant, Palmo, Halkjaer & Pedersen, 1994). However, IL-6 was enhanced in studies by Northoff & Berg (1991), Ullum et al. (1994), Sprenger et al. (1992), Castell, Poortmans, Leclercq, Brasseur, Duchateau & Newsholme (1997) and Bruunsgaard, Galbo, Halkjaer, Johansen, Maclean & Pedersen (1997). In the latter study concentric versus eccentric exercise was compared to test the hypothesis that exercise-induced increase in cytokine levels is associated with muscle damage. The creatine kinase (CK) level increased almost 50-fold 4 days after eccentric exercise, whereas no changes were found in the CK level in relation to concentric exercise. The IL-6 level increased 5-fold in relation to eccentric exercise and was significantly correlated to CK in the following days, whereas no changes were found in relation to concentric exercise. An increased cytokine level after exercise has primarily been found after eccentric muscle contractions, and the study by Bruunsgaard et al. (1997) supported the hypothesis that the post-exercise cytokine production was related to skeletal muscle damage. The critical question is whether a causal relationship exists, i.e. does muscle damage cause cytokine production or vice versa? The present study was performed to test the hypothesis that the cytokine response is locally produced in response to mechanically damaged myofibres or disrupted connective tissue in the muscle, and that a local cytokine response initiates the systemic inflammatory response.
METHODS
Subjects
Sixteen male subjects participated in the study (age, 30.5 ± 1.9 years; weight, 74.1 ± 2.3 kg; height, 182.3 ± 1.5 cm; maximal oxygen consumption (VO2,max), 4.36 ± 0.2 l min−1). The experimental protocol was approved by the ethics committee and all subjects were informed of the risks and purposes of the study before their written consent was obtained. The subjects took part in the Copenhagen Marathon (19 May, 1996); running time was 03:17:03 ± 00:07:39 (h:min:s). Blood samples (n = 16) were drawn from the antecubital vein 1 week before, immediately after (the delay from stoppage of running to the taking of the first blood sample did not exceed 10 min) and 2 h after the marathon. Muscle biopsies (n = 8) from vastus lateralis of quadriceps femoris were taken 1 week before, immediately after and 2 h after the race, and were frozen in liquid nitrogen immediately after sampling. The subjects prepared for the pre-exercise sample in a similar way as they would prepare for the marathon, and they refrained from exercise for 2 days before the pre-exercise samples were taken. The subjects were allowed to consume fluid and carbohydrate before, during and after running.
Isolation of blood mononuclear cells
Blood was drawn into 10 ml glass tubes containing 250 i.u. of heparin. Blood mononuclear cells (BMNC) were isolated by density gradient centrifugation (Lymphoprep Nyegaard, Oslo, Norway) in LeucoSep tubes (Greiner, Frickenhausen, Germany) and washed once in glutamine-free RPMI 1640 (R5632, Sigma). Cells were lysed by addition of 1 ml GIT-solution (GIT-buffer: 4 M guanidinium isocyanate; Sigma), 25 mM sodium citrate (Merck, Darmstadt, Germany) (pH 7) immediately before freezing and storage in liquid nitrogen.
Preparation of RNA from blood and tissue
Total RNA was isolated from BMNC using the RNeasy kit (Qiagen, Santa Clarita, CA, USA). In the case of muscle biopsies, stored in liquid nitrogen, the biopsy was poured into a denaturing solution containing GIT-buffer (described above), 25 mM sodium citrate (pH 7 in millipore water) and immediately thawed and homogenized using a polytron (Ultra-Turrax T8, Ika Labortechnik, Staufen, Germany). The weight of the biopsies was in the range 20–30 mg. Following homogenization, RNA was prepared from the sample using established techniques; the following were added to the homogenized solution: 14 μl β-mercaptoethanol (Sigma), 17 μl sarcosyl (30 %) (Sigma), 100 μl 2 M sodium acetate (pH 4.0) (Sigma), 200 μl chloroform/isoamyl alcohol (24:1) (Sigma) and 1 ml water-saturated phenol (Merck). After centrifugation, RNA was extracted in the upper aqueous phase, precipitated by adding 1 volume of isopropanol (Merck) and incubated at -20°C for 1 h. Following centrifugation the pellet was redissolved in 600 μl of the above-described GIT-buffer plus 8 μl β-mercaptoethanol, precipitated in isopropanol, and incubated at -20°C for 1 h. After centrifugation the pellet was washed in 80 % ethanol, and finally the RNA pellet was dissolved in sterile water. The concentration and purity of the RNA was determined by absorbance at 260 and 280 nm.
Preparation of cDNA
Immediately after RNA preparation, cDNA was synthesized using approximately 0.5 μg of RNA, 2.5 μM oligo-dT primer (Pharmacia Biotech, Uppsala, Sweden), 1 mM dNTP (Pharmacia Biotech), 20 U RNase inhibitor (rRNasin, Promega, Madison, WI, USA) and 50 U Superscript, Reverse Transcriptase (Life Technologies, Gaithersburg, MD, USA) incubated at 42°C for 1 h, followed by 5 min at 96°C and soaking at 4°C. Prepared cDNA was stored at -80°C.
Semiquantitative polymerase chain reaction
The sequence of interest was co-amplified with a sequence from glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), used as an internal control to correct for unequal efficiencies in RNA isolation, reverse transcription and the polymerase chain reaction (PCR). The PCR mixture contained 1.25 μl of the above cDNA mixture, 250 μM of each dNTP, 6 mM MgCl2, 1 × PCR buffer, 2 U DNA polymerase (AmpliTaq Gold, Perkin Elmer (Roche, Branchburg, NJ, USA)) and 0.4 μM of each of four primers. Total volume was 25 μl. The reaction mixture was first heated to 94°C for 10 min then amplified for thirty cycles by 45 s at 94°C, 1 min at 60°C and 1.5 min at 72°C. The amplification was carried out in a PTC-100 thermal cycler (MJ Research, Inc., Watertown, MA, USA). PCR products were separated by electrophoresis in a 2 % agarose gel, and visualized on a UV-transilluminator after staining with ethidium bromide. The gel was photographed (Polaroid, Birkerød, Denmark), scanned (Arcus II, Agfa), and analysed using image analysis software (Cream 1-D for Windows, KEM-EN-TEC, Copenhagen, Denmark). Relative cytokine expression was determined in different samples, by comparing in-sample ratios (R) of the level of cytokine relative to the level of GAPDH.
Determination of cytokine proteins in plasma
Three millilitres of blood were drawn in glass tubes containing 11.7 μmol dipotassium-EDTA and 500 kallikrein inactivator units (KIU) Trasylol (Bayer, Leverkusen, Germany). Tubes were kept on ice until centrifugation at 3000 r.p.m. for 5 min, and the plasma was stored at -80°C. The concentrations of cytokines in plasma were measured using commercially available ELISA kits: IL-1βHS, TNF-αHS, IL-6HS and IL-1ra (R&D Systems, Minneapolis, MN, USA). All measurements were performed in duplicate. The coefficient of (intra-assay) variation was 7.8, 6.6, 7.0 and 4.9 % for the assays of IL-1β, TNFα, IL-6 and IL-1ra, respectively.
Measurement of creatine kinase
Creatine kinase (CK) was determined by the Central Laboratory at the University Hospital of Copenhagen using standard laboratory procedures. CK was measured in lithium heparinized plasma using automated enzyme reactions (automated analysis for Hitachi System 717, Boehringer Mannheim Diagnostica, Germany).
Statistics for protein data
For each parameter the data were tested for a normal distribution by plotting the values against the corresponding expected value. If the probability plot indicated that the data did not follow a normal distribution the data were log transformed and tested again. This was the case for IL-6, IL-1ra, IL-1β and TNF-α protein data. To analyse changes over time a repeated measures analysis of variance was used. If significance was indicated, a Tukey's post hoc test was used to determine where the significance occurred. Results are expressed as means ± standard error of the mean (s.e.m.).
RESULTS
Detection of plasma cytokine proteins
Plasma IL-6 increased from 1.5 ± 0.7 pg ml−1 before to 94.4 ± 12.6 pg ml−1 immediately after the race, and declined to 22.1 ± 3.8 pg ml−1 2 h after exercise (Fig. 1). The plasma IL-1ra concentration was 123 ± 23 pg ml−1 before, increased to 2795 ± 551 pg ml−1 immediately after the exercise, and further increased to 4119 ± 527 pg ml−1 2 h after (Fig. 2). IL-1β and TNF-α increased only 1.5- and 2-fold in response to exercise (Table 1).
Figure 1. The plasma concentration of interleukin-6 (IL-6) before (Pre), immediately after (Post) and 2 h after (2 h post) a marathon race.
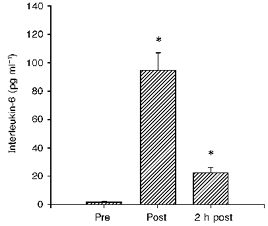
* Significant difference from pre-exercise value, P < 0.0001.
Figure 2. The plasma concentration of interleukin-1 receptor antagonist (IL-1ra) before (Pre), immediately after (Post) and 2 h after (2 h post) a marathon race.
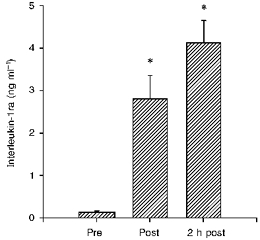
* Significant difference from pre-exercise value, P < 0.0001.
Table 1.
Plasma concentration of interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α), before (Pre), immediately after (Post) and 2 h after (2 h post) the marathon race
| Pre | Post | 2 h post | |
|---|---|---|---|
| IL-1β (pg ml−1) | 0.61 ± 0.24 | 0.92 ± 0.26 * | 0.71 ± 0.24 |
| TNF-α (pg ml−1) | 0.96 ± 0.09 | 2.43 ± 0.27 † | 1.82 ± 0.25 * |
Significant difference from pre-exercise value, P < 0.005.
Significant difference from pre-exercise value, P < 0.0001.
Creatine kinase
The CK levels increased from 225 U l−1 before, to 2360 U l−1 and 1193 U l−1 on days 1 and 2, respectively. Messenger RNA for IL-6, IL-1ra, IL-1β and TNF-α was undetectable in muscle biopsies before exercise (Table 2). Messenger RNA for IL-6 was detected in four muscle biopsies immediately after exercise and in two biopsies 2 h after exercise. In a total of five out of eight samples mRNA for IL-6 was elevated in skeletal muscle in response to exercise. Messenger RNA for IL-6 was not detected in BMNC.
Table 2.
Cytokine mRNA in BMNC and muscle as determined by comparative PCR
| Pre | Post | 2 h post | Increase | ||
|---|---|---|---|---|---|
| IL-6 | Muscle tissue | 0 | 4 | 2 | 5 |
| BMNC | 0 | 0 | 0 | 0 | |
| IL-1ra | Muscle tissue | 0 | 1 | 2 | 2 |
| BMNC | 5 | 4 | 5 | 5 | |
| IL-1β | Muscle tissue | 0 | 0 | 1 | 1 |
| BMNC | 2 | 3 | 3 | 4 | |
| TNF-α | Muscle tissue | 0 | 0 | 0 | 0 |
| BMNC | 1 | 0 | 0 | 0 |
The first three columns show the number of detectable PCR product bands in an agarose gel at rest (Pre), immediately after (Post), and 2 h after (2 h post) the marathon race. ‘Increase’ denotes the number of samples with an increase in the ratio R (level of cytokine/level of GAPDH) of the sample at Post or 2 h post compared with the R from the pre-exercise sample.
Increased amounts of mRNA for IL-1ra were found in a total of two muscle biopsies and five BMNC samples in response to exercise. Increased amounts of mRNA for IL-1β were found in a total of one muscle and four BMNC samples after exercise.
Messenger RNA for TNF-α was detected in no muscle biopsies, and only in one BMNC sample obtained before exercise.
DISCUSSION
The major finding of this study is that mRNA for IL-6 was detectable in skeletal muscle after intense prolonged exercise (a marathon). Since mRNA for IL-6 was not detectable in circulating BMNC, the finding of mRNA for IL-6 in skeletal muscle is not likely to be due to contamination of the muscles by blood, but indicates that IL-6 is locally produced in the skeletal muscle. The source of this local IL-6 mRNA production has yet to be determined, but in inflammation one of the first events is adherence of neutrophils and macrophages to the vascular endothelium in the damaged tissue. It is possible that during the adherence, or later during the infiltration of the damaged tissue, the neutrophil or macrophage is activated, and thus becomes the local source of IL-6 mRNA.
In this study plasma IL-6 and IL-1ra concentrations were markedly increased after the race. This increase is more pronounced than, but still in accordance with, previous studies (Cannon et al. 1991; Northoff & Berg, 1991; Sprenger et al. 1992; Ullum et al. 1994; Castell et al. 1997).
The finding of increased amounts of mRNA for IL-1ra and mRNA for IL-1β in BMNC after the marathon race indicates that IL-1ra and IL-1β are produced systemically by circulating BMNC.
The plasma IL-6 level was increased immediately after exercise and declined thereafter, whereas the level of IL-1ra in plasma continued to increase 2 h post-exercise. Although more detailed studies are required in order to estimate the time course of these cytokines in relation to exercise and in relation to each other, the present results indicate that the local production of IL-6 in muscle stimulates the production of IL-1ra by BMNC and the release of this cytokine into the circulation. IL-6 has previously been shown to stimulate the production of IL-1ra (Eisenberg et al. 1990; Hannum et al. 1990).
In this study we also found increased amounts of mRNA for IL-1β in BMNC samples, but only a very modest increase in the plasma IL-1β. This could indicate that IL-1β is produced in response to exercise and rapidly removed from the circulation, which is in accordance with the study by Sprenger et al. (1992), where increased levels of cytokines were found in the urine after a 20 km run. Thus exercise may alter the production of several cytokines and these may have important paracrine functions.
In prolonged intense running, e.g. a marathon, there is a large component of eccentric exercise. Increased levels of PGE2 and delayed onset muscle soreness have been found 24 h after a single bout of isolated eccentric exercise. The time course for PGE2 and muscle pain indicates a possible relationship. The source of the prostaglandin production could be the macrophages, which are the predominant invading cell type in muscle at 24 h. However, the stimulus for PGE2 release is not known. It has been shown that endothelial rat cells stimulated with lipopolysaccharide (LPS) responded with an augmented PGE2 synthesis, and that cultures treated with IL-1β or IL-6 showed a further stimulation of PGE2 release (de Vries et al. 1995). Thus in theory, local cytokine production may stimulate the production of prostaglandins. In support of this theory is also the fact that high levels of plasma IL-6 are found immediately after the completion of exhaustive exercise, preceding neutrophil and macrophage accumulation in the muscle. The interactions between cytokine production and prostaglandins are highly complex. A recent study showed inhibitory effects of PGE2 on TNF-α and IL-6 production by LPS-stimulated macrophages (Strassmann, Patil, Finkelman, Fong & Kambayashi, 1994). The authors suggested that the anti-inflammatory effect of PGE2 on mononuclear phagocytes is mediated in part by an autocrine feedback mechanism involving IL-10. In relation to the acute phase response in exercise, this indicates that high levels of PGE2 exert a negative feedback mechanism on the cytokine response and thereby limit the inflammatory response.
The present study suggests that IL-6 is produced locally in the skeletal muscle in response to prolonged exercise with a large eccentric component. With regard to cytokine-producing cells in the skeletal muscle, further studies involving in situ techniques will have to address this problem.
Acknowledgments
The excellent technical assistance of Ruth Rousing, Birgit Jensen, Leila B. Jacobsen, Hanne Willumsen and Lynda Powell is acknowledged. The study was supported by The Danish National Research Foundation (504–14).
References
- Bagby GJ, Crouch LD, Shepherd RE. Exercise and cytokines: Spontaneous and elicited response. In: Hoffman GL, editor. Exercise and Immune Function. New York: CRC Press; 1996. pp. 55–78. [Google Scholar]
- Bruunsgaard H, Galbo H, Halkjaer KJ, Johansen TL, Maclean DA, Pedersen BK. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. The Journal of Physiology. 1997;499:833–841. doi: 10.1113/jphysiol.1997.sp021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JG, Evans WJ, Hughes VA, Meredith CN, Dinarello CA. Physiological mechanisms contributing to increased interleukin-1 secretion. Journal of Applied Physiology. 1986;61:1869–1874. doi: 10.1152/jappl.1986.61.5.1869. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Kluger MJ. Endogenous pyrogen activity in human plasma after exercise. Science. 1983;220:617–619. doi: 10.1126/science.6836306. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Meydani SN, Fielding RA, Fiatarone MA, Meydani M, Farhangmehr M, Orencole SF, Blumberg JB, Evans WJ. Acute phase response in exercise. II. Associations between vitamin E, cytokines, and muscle proteolysis. American Journal of Physiology. 1991;260:R1235–1240. doi: 10.1152/ajpregu.1991.260.6.R1235. [DOI] [PubMed] [Google Scholar]
- Castell LM, Poortmans JR, Leclercq R, Brasseur M, Duchateau J, Newsholme EA. Some aspects of the acute phase response after a marathon race, and the effects of glutamine supplementation. European Journal of Applied Physiology. 1997;75:47–53. doi: 10.1007/s004210050125. [DOI] [PubMed] [Google Scholar]
- De Vries HE, Hoogendoorn KH, Van Dijk J, Zijlstra FJ, Van Dam AM, Breimer DD, Van Berkel TJ, De Boer AG, Kuiper J. Eicosanoid production by rat cerebral endothelial cells: stimulation by lipopolysaccharide, interleukin-1 and interleukin-6. Journal of Neuroimmunology. 1995;59:1–8. doi: 10.1016/0165-5728(95)00009-q. 10.1016/0165-5728(95)00009-Q. [DOI] [PubMed] [Google Scholar]
- Eisenberg SP, Evans RJ, Arend WP, Verderber E, Brewer MT, Hannum CH, Thompson RC. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990;343:341–346. doi: 10.1038/343341a0. 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- Evans WJ, Meredith CN, Cannon JG, Dinarello CA, Frontera WR, Hughes VA, Jones BH, Knuttgen HG. Metabolic changes following eccentric exercise in trained and untrained men. Journal of Applied Physiology. 1986;61:1864–1868. doi: 10.1152/jappl.1986.61.5.1864. [DOI] [PubMed] [Google Scholar]
- Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Mazzei GJ, Seckinger PL, Dayer JM, Shaw AR. Purification and characterization of a 26-kDa competitive inhibitor of interleukin 1. European Journal of Immunology. 1990;20:683–689. doi: 10.1002/eji.1830200332. [DOI] [PubMed] [Google Scholar]
- Northoff H, Berg A. Immunologic mediators as parameters of the reaction to strenuous exercise. International Journal of Sports Medicine. 1991;12(1):S9–15. doi: 10.1055/s-2007-1024743. suppl. [DOI] [PubMed] [Google Scholar]
- Seckinger P, Lowenthal JW, Williamson K, Dayer JM, Macdonald HR. A urine inhibitor of interleukin 1 activity that blocks ligand binding. Journal of Immunology. 1987;139:1546–1549. [PubMed] [Google Scholar]
- Sprenger H, Jacobs C, Nain M, Gressner AM, Prinz H, Wesemann W, Gemsa D. Enhanced release of cytokines, interleukin-2 receptors, and neopterin after long-distance running. Clinical Immunology and Immunopathology. 1992;63:188–195. doi: 10.1016/0090-1229(92)90012-d. 10.1016/0090-1229(92)90012-D. [DOI] [PubMed] [Google Scholar]
- Strassmann G, Patil KV, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. Journal of Experimental Medicine. 1994;180:2365–2370. doi: 10.1084/jem.180.6.2365. 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullum H, Haahr PM, Diamant M, Palmo J, Halkjaer KJ, Pedersen BK. Bicycle exercise enhances plasma IL-6 but does not change IL-1α, IL-1β, IL-6, or TNF-α pre-mRNA in BMNC. Journal of Applied Physiology. 1994;77:93–97. doi: 10.1152/jappl.1994.77.1.93. [DOI] [PubMed] [Google Scholar]


