Abstract
Differential expression of myosin heavy chain (MHC) isoforms dramatically affects mechanical and energetic properties of skeletal muscle fibre types. As many as five different fibre types, each with different mechanical properties, have been reported in frog hindlimb muscles. However, only two frog MHC isoforms have previously been detected by SDS-PAGE and only one adult hindlimb MHC isoform has been cloned.
In the present study, four different fibre types (type 1, type 2, type 3 and tonic) were initially identified in adult Ranapipiens anterior tibialis muscle based on myosin ATPase histochemistry, size and location. Each fibre type exhibited unique reactivity to a panel of MHC monoclonal antibodies. Single fibre analysis using SDS-PAGE revealed that MHCs from immunohistochemically defined type 1, type 2 and type 3 fibres ran as three distinct isoform bands, while MHC of tonic fibres co-migrated with type 1 MHC. The combined data from immunohistochemistry and SDS-PAGE suggests that Rana fibre types are composed of four different MHCs.
Four novel MHC cDNAs were cloned and expression of the corresponding transcripts was measured in single immuno-identified fibres using specific polymerase chain reaction (PCR) primer pairs. Each of the four transcripts was found to be primarily expressed in a different one of the four fibre types.
Coexpression of MHC isoforms was observed only between types 1/2 and types 2/3 at both the protein and mRNA level.
These data provide a molecular basis for differentiation between frog fibre types and permit future molecular studies of MHC structure/function and gene regulation in this classic physiological system.
Comparison of sequence homology among amphibian, avian and mammalian MHC families supports the concept of independent evolution of fast MHC genes within vertebrate classes subsequent to the amphibian/avian/mammalian radiation.
Studies of frog skeletal muscle have provided the mechanical and energetic information that forms much of the basis for current theories of muscle contraction (Huxley, 1974). The ability to perform functional studies on living isolated single frog muscle fibres distinguishes the frog from other species and has allowed for high resolution mechanical and physiological studies. One reason to study muscle at the single fibre level is that it is a stable and relatively homogeneous mechanical system, more easily defined and controlled at the level of the sarcomere than whole muscle. Since fibres of a given muscle are heterogeneous with regard to speed of contraction and metabolism, single fibre studies permit more precise correlations to be made between structural, functional and biochemical properties. Studies of mammalian and avian single skinned fibres have established a detailed correlation between myofibrillar protein isoform composition and a fibre's mechanical properties and calcium sensitivity (Moss, Giulian & Greaser, 1985; Sweeney, Kushmerick, Mabuchi, Gergely & Sreter, 1986; Bottinelli, Betto, Schiaffino & Reggiani, 1994). Studies of skinned single fibres have also provided critical advances in our understanding of the molecular mechanisms of force generation and cross-bridge state transitions (Hibberd, Dantzig, Trentham & Goldman, 1985; Irving et al. 1995). However, there are serious limitations to the study of mammalian and avian single fibres. With the exception of mouse flexor brevis muscle (Westerblad & Allen, 1993), single fibre studies of mammalian and avian muscle are restricted to skinned preparations, in which the fibre membrane is removed or chemically disrupted. Skinning alters the fibre's mechanical properties, uncouples membrane-associated signalling, alters the structural integrity of some cytoskeletal elements, and permits the loss of soluble intracellular components. In the frog, the use of single living fibres permits study of muscle function in an intact cellular environment.
Many contractile characteristics of muscle are defined by the myosin isoforms expressed within the fibres. The myosin molecule exists in skeletal muscle as a hexamer of two myosin heavy chains (MHCs) and four myosin light chains (MLCs), all of which combine to provide a muscle fibre with specific force- and velocity-dependent properties, mechanical power production capabilities, and energetic cost of force production (Curtin & Davies, 1973; Sweeney et al. 1986; Bottinelli et al. 1994). Thus, MHC composition is an important factor in the overall design of a muscle for producing a specific mechanical output. For example, recent studies of locomotion in frogs suggest that MHC composition may be matched to fibre mechanical gearing and joint moments such that, during locomotion, fibres operate at a velocity where near optimal power is generated (Lutz & Rome, 1994, 1996a, b).
The close association between MHC isoform expression and muscle function is also demonstrated by studies of muscle plasticity. For instance, MHC expression changes during development (Miller & Stockdale, 1986) and exhibits plasticity even in fully differentiated adult muscle in response to conditions such as the state of innervation (Engel, Brooke & Nelson, 1966), injury by eccentric contraction (Lieber, Schmitz, Mishra & Fridén, 1994), immobilization (Booth & Kelso, 1973), and various disease states (Karpati & Engel, 1968). It is believed that this adaptation represents the muscle's attempt to match its structural composition to the new functional demands (Pette, 1990).
Despite the advantages of the frog single fibre preparation for studies of muscle function, relatively little is known about the MHC isoform composition of amphibian skeletal muscle. Since the pioneering work of Smith & Ovalle (1973) as many as three different twitch fibre types (types 1, 2 and 3) and two slow or tonic fibre types (types 4 and 5) have been recognized in amphibian skeletal muscle. This classification scheme was based on a combination of metabolic, morphological, ultrastructural and physiological properties. The different muscle fibre types possess a wide range of mechanical properties. For instance, in single Xenopus laevis hindlimb muscle fibres, maximum shortening velocity is 10-fold higher in the fastest twitch fibres than in the slowest tonic fibres (Lännergren, 1992). The different mechanical properties have been attributed to different myosin isoforms, based on a combination of isomyosin banding patterns on native-PAGE gels and MLC content on SDS-PAGE gels (Lännergren & Hoh, 1984; Lännergren, 1987). However, MHC isoform content cannot be unambiguously determined with this methodology.
A detailed and systematic study of fibre types in ranid frog and Xenopus limb muscle using myosin isoform-based criteria has been performed (Rowlerson & Spurway, 1988). In Rana, the genus most commonly used for functional studies, three twitch fibre types (types 1, 2 and 3) and a tonic type were defined based on myosin-ATPase reactivity and differential reactivity to a panel of MHC monoclonal antibodies. In Xenopus, three twitch fibre types, a tonic type and an additional slow fibre type (Lännergren, 1979) were distinguished using the same criteria (Rowlerson & Spurway, 1988). These data provided strong evidence that the different fibre types in Rana and Xenopus limb muscle contained different MHC isoforms.
Despite the indirect evidence that up to five MHC isoforms are present in frog muscle, only two different MHC isoforms have been detected by SDS-PAGE (Lännergren, 1987). In addition, only one adult frog hindlimb MHC has been cloned and its identity was not correlated with a particular fibre type (Radice & Malacinski, 1989). Therefore, the purpose of the present study was to characterize the MHC composition of the various fibre types in ranid hindlimb muscle at the mRNA and protein level. As the physiology of amphibian fibre types has been previously characterized, a link is established between differential MHC gene expression and the mechanical function of Rana fibre types. A brief version of this work was presented in abstract form (Lutz, Ryan & Lieber 1997).
METHODS
Experimental preparation
All experiments were performed on adult male Ranapipiens that were kept in tanks at room temperature and maintained on a diet of live crickets provided twice weekly. Procedures were performed with the approval of the Committee on the Use of Animal Subjects at the University of California and Veterans Affairs Medical Center, San Diego. Frogs were killed by double pithing and anterior tibialis (AT) muscles were removed immediately, snap frozen in liquid nitrogen-cooled isopentane and stored at -80°C. Time from pithing of the frog to tissue freezing was about 5 min. A total of eight muscles from seven different animals were used to generate data on a total of 116 single muscle cells.
Identification of frog fibre types with immunohistochemistry and histochemistry
Serial transverse sections (10 μm) of frozen AT muscle were cut at -20°C and processed for myofibrillar ATPase activity (mATPase) after acid and alkaline preincubations and for immunoreactivity to a panel of monoclonal MHC antibodies. The mATPase protocol was a modification of the methods of Rowlerson & Spurway (1988). For acid inhibition of mATPase activity, sections were preincubated for 3 min in 0.2 M sodium acetate (pH 4.75–4.85). Alkaline inhibition consisted of a 15 min preincubation in 0.075 M sodium barbitone and 0.1 M CaCl2 (pH 10.05–10.15). After preincubation, sections were incubated in a solution containing 2.8 mM ATP, 0.075 M sodium barbitone and 0.1 M CaCl2 (pH 9.4) for either 45 min (acid) or 15 min (alkaline). Slides were washed in three changes of 1 % CaCl2 (5 min each) and transferred to fresh 2 % CoCl2 for 3 min, followed by six rinses of 0.2 % sodium barbitone (30 s each) and four rinses in water. Slides were then placed in freshly prepared 0.1 % ammonium sulphide for 30 s, washed in water (15 min), and fixed in a 1:3 mixture of acetic acid and absolute ethanol (15 min) prior to mounting.
A series of MHC monoclonal antibodies raised against mammalian and avian skeletal muscle were screened for their ability to differentiate between frog fibre types. Fibre type specificity and sources of the various antibodies are detailed in Table 1. Antibody reactivity was visualized using the indirect immunoperoxidase technique (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA, USA).
Table 1.
Histochemical and immunohistochemical reactivity of frog muscle fibres
| Treatment | Type 1 | Type 2 | Type 3 | Tonic |
|---|---|---|---|---|
| mATPase at pH 4.8 | + | 0 | 0 | + |
| mATPase at pH 10.1 | 0 | + | 0 | 0 |
| F8 antibody | + | 0 | + | 0 |
| MHC-S antibody | 0 | 0 | 0 | + |
| NA8 antibody | 0 | + | + | + |
| D5 antibody | 0 | 0 | + | 0 |
The table shows differential reactivity of frog fibre types for mATPase and MHC monoclonal antibodies in the AT muscle of R. pipiens. The staining for each reaction is scored as either positive (+) or negative (0). Dilutions for the antibodies were as follows: F8, 1:30 000; NA8, 1:20000; MHC-S, 1:500; and D5, 1:15, supernatant. The antibodies were all anti-rat except NA8, which was anti-chicken. F8 and D5 were a gift of Professor S. Schiaffino (University of Padua, Italy) and NA8 was a gift of Dr E. Bandman (University of California, Davis, CA, USA). MHC-S was obtained commercially (Vector Laboratories).
SDS-PAGE analysis of MHC
MHC isoforms from whole AT muscle and individual freeze-dried fibre segments (see below) were separated by SDS-PAGE. A myofibril-rich fraction of whole AT muscle was prepared based on previous methods (Talmadge & Roy, 1993) but with the addition of pepstatin (10 μg ml−1), leupeptin (10 μg ml−1) and phenylmethylsulphonyl fluoride (PMSF; 100 μM) to the isolation solutions. The final pellet was resuspended to give 0.125 μg protein μl−1 (BCA protein assay, Pierce, Rockford, IL, USA) in sample buffer consisting of dithiothreitol (DTT; 100 mM), SDS (2 %), Tris base (80 mM) pH 6.8, glycerol (10 %) and Bromphenol Blue (1.2 % w/v), and was boiled (2 min) and stored at -80°C for up to 1 month prior to loading on the gel. Single freeze-dried fibres that had been stored in 10 μl sample buffer for up to 1 week were simply thawed and boiled for 2 min prior to loading the entire volume.
Gel components were identical to those used previously for the separation of rat MHCs (Talmadge & Roy, 1993). Total acrylamide concentration was 4 % and 8 % in the stacking and resolving gels, respectively (bis-acrylamide, 1:50). Gels (16 cm × 22 cm, 0.75 mm thick) were run at a constant current of 10 mA until voltage rose to 275 V and thereafter at constant voltage for 21 h at 4–6°C. Silver staining (BioRad, Hercules, CA, USA) was performed according to the manufacturer's protocol except for an additional 40 min of fixation to further remove glycerol and reduce background staining.
Cloning of novel myosin heavy chains
A polymerase chain reaction (PCR)-based strategy was used to isolate novel MHC clones by amplifying MHC mRNA near the 3′ end of the gene. This region corresponds to exons 36–41 within the rod portion of light meromyosin (Strehler, Strehler-Page, Perriard, Periasamy & Nadal-Ginard, 1986). Total RNA was isolated from the AT muscle using the Trizol method (BRL, Grand Island, NY, USA). Total mRNA was reverse transcribed (Superscript II, BRL) to cDNA with oligo-dT as the primer. PCR was performed using one of three upstream primers: (1) FRG-1; a degenerate 18-mer oligo (5′-TACCAGWCWGARGAAGAC-3′) created based on MHC sequence homology across a variety of species at a site ∼250 bp upstream from the 3′ UTR; (2) EB, a degenerate 19-mer oligo (5′- -AGAAGGCCAARAARGCCAT-3′) based on sequence homology across various mammalian MHCs ∼600 bp upstream from the 3′ UTR; or (3) FC1, a 21-mer oligo (5′-CTGAAGAAGGAGCAGGACACC-3′) based on sequence conservation across multiple species at ∼500 bp upstream from the 3′ UTR. The downstream PCR primer in all three cases was TW2, a non- specific 24-mer poly-T oligo (5′-GCGGATCCTTTT TTTTTTTTTTTT-3′), containing a 5′BamHI site included to match the annealing temperature to the upstream primers. PCR products were subcloned and sequenced using standard methods. From the seventeen MHC clones investigated, three distinct MHC clones were obtained using the FRG1-TW2 primer pair and a fourth clone was obtained using the EB-TW2 primer pair. The sequences of the three clones originally isolated using the FRG1-TW2 primer set were subsequently extended to obtain longer sequence information by reverse transcription (RT) and PCR using the primer pairs FC1-TW2 or EB-TW2.
Correlation between MHC and fibre type
To correlate the novel MHCs with the fibre types in which they were expressed, a strategy was used whereby immunohistochemically identified fibre segments were microdissected from freeze-dried transverse sections (60 μm) and tested for the presence of each of the four MHC clones by RT-PCR (Staron & Pette, 1986). The protocol involved cutting four serial transverse sections (10 μm) for immuno-identification of fibre types using a panel of MHC monoclonal antibodies (Table 1) and then cutting a 60 μm section that was freeze-dried for RT-PCR of isolated single fibres (see below). In other muscles, two consecutive 60 μm sections were cut and freeze-dried and MHC protein isoform composition of immuno-identified single fibres was determined in the first 60 μm section by SDS-PAGE and mRNA expression in the same fibre of the next section by RT-PCR.
Microdissection of single freeze-dried fibres
The 60 μm transverse sections directly serial to those obtained for immunohistochemistry were freeze dried before storing at -80°C. Prior to use, sections were thawed under vacuum for 1 h and individual immuno-identified single fibre segments were isolated by microdissection with insect pins at room temperature (Staron & Pette, 1986). The fibre segments were picked up on the tip of a pin and placed in the bottom of a 200 μl tube within 1 h of exposure to room air. Fibre segments were suspended in either 12.5 μl of RNAse-free water containing RNAsin (1 U μl−1) for RT-PCR or 10 μl of SDS-PAGE sample buffer and frozen immediately in liquid N2. Due to the small size of the type 3 and tonic fibres, in 50 % of the cases, a pair of fibres of a given immunohistochemical type were placed together into one tube.
Clone-specific RT-PCR
Identification of the presence of the various MHC transcripts in fibre segments was accomplished by creating highly specific PCR primer pairs to amplify each novel MHC transcript without cross-amplification. Primer pairs were constructed from regions of low sequence homology between the four clones and were chosen to provide different PCR product lengths for each pair (Table 2). The four primer pairs were matched in optimal PCR annealing temperature so that amplification of all four MHCs within a given sample could be performed during the same PCR run. The PCR solution (100 μl) contained: PCR buffer (50 mM KCl, 10 mM Tris-HCl, pH 8.3; Perkin Elmer, Foster City, CA, USA), 2.5 mM MgCl2, 1.0 μM dNTPs, 0.5 μM each of the upstream and downstream primers and 1.25 units of thermostable DNA polymerase (AmpliTaq gold, Perkin Elmer). To demonstrate primer pair specificity and determine optimal amplification conditions, PCR was performed on plasmid cDNA of each of the four clones (Fig. 2). Amplification conditions (Perkin Elmer Model 2400 Thermal Cycler) consisted of preactivation of the Taq by heating at 94°C for 10 min. Amplification cycling conditions were: denaturation at 94°C for 30 s followed by primer annealing at 52°C for 1 min and DNA extension at 72°C for 30 s, repeated for 35 cycles followed by a 7 min final extension at 72°C. RT of single freeze-dried fibre segments was performed without prior extraction of total RNA. Due to the small size of type 3 fibres, we performed PCR at both 35 and 45 cycles to test for adequate amplification.
Table 2.
Clone-specific primer pairs and corresponding PCR product length
| PCR primer | Nucleotide sequence | 5′ start position | PCR product length (bp) |
|---|---|---|---|
| RPM1 (S) | 5′-gaatctgaggaacaagccaatgc-3′ | 346 | 133 |
| RPM1 (AS) | 5′-tgatatcccggctcttgactctc-3′ | 478 | |
| RPM2 (S) | 5′-agtggaggaacaggctac-3′ | 348 | 251 |
| RPM2 (AS) | 5′-tatttgcacctagagctacac-3′ | 598 | |
| RPM3 (S) | 5′-ttgaggagcaatccaatgtta-3′ | 397 | 178 |
| RPM3 (AS) | 5′-caccttgaggctcagtcact-3′ | 574 | |
| RPMT (S) | 5′-acgaatgcaggacttgatag-3′ | 329 | 290 |
| RPMT (AS) | 5′-agggattgtgaagtctgtctc-3′ | 618 |
The PCR primer pairs (sense (S) and antisense (AS)) used for clone-specific amplification of four MHC transcripts. The 5′ position of each primer (as numbered in Fig. 1) is indicated along with the expected PCR product length for each primer pair.
Figure 2. Clone-specfic PCR amplification of four MHC transcripts.
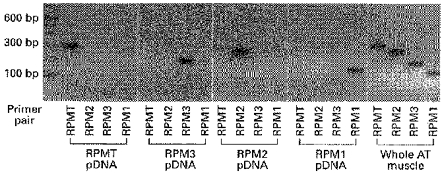
The four clone-specific RT-PCR primer pairs each specifically amplify only one of the MHCs. Amplification of each of the four MHCs is demonstrated on the plasmid DNAs (pDNA; 1 ng) from which the four MHCs were cloned and on cDNA obtained from whole AT muscle.
RESULTS
Four fibre types were identified
Fibres were initially classified as one of four different types (types 1, 2, 3 or tonic) based on their relative mATPase reactivity, size, and location within the muscle cross-section (Table 1 and Fig. 3) according to previous nomenclature for amphibian skeletal muscle (based on similar criteria; Rowlerson & Spurway, 1988). Under acid preincubation conditions, only the large type 1 fibres and much smaller tonic fibres had significant mATPase activity, whereas after alkaline preincubation only type 2 fibres showed significant activity. Type 3 fibres had mATPase activity that was inhibited under both acid and alkaline conditions. In agreement with previous findings, these small type 3 fibres were always found closely associated with the acid stable tonic fibres within the ‘slow’ region of the muscle cross-section (Rowlerson & Spurway, 1988). However, very few pure type 3 fibres were observed, as the expression of type 3 MHC was usually accompanied by expression of type 2 MHC (see below).
Figure 3. Frog fibre types are identified by mATPase histochemistry and MHC immunohistochemistry.
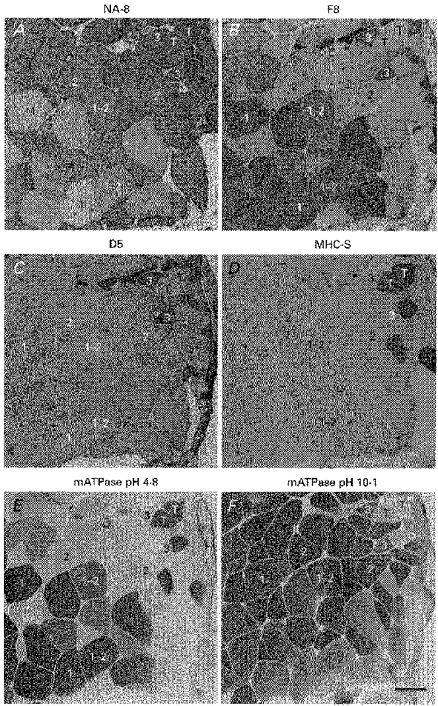
Serial transverse sections (10 μm) of the anterior tibialis muscle are stained for reactivity to the MHC monoclonal antibodies NA8 (A), F8 (B), D5 (C) and MHC-S (D) and mATPase activity with acid (pH 4.8; E) and alkaline (pH 10.1; F) preincubations. Selected examples of type 1, type 2, type 1–2, type 3 and tonic (T) fibre types are labelled in accordance with the scheme outlined in Table 1. Note the occurrence of many intermediate type 1–2 fibres (positive reactivity to both F8 and NA8). The small type 3 and tonic (T) fibres are found closely associated within the slow tonus region of the muscle (upper right-hand portion of micrographs). All type 3 fibres in view are type 2/3 hybrids with no pure type 3 fibres present. Scale bar, 100 μm.
Each of the four fibre types, defined by their mATPase reactivity, was shown in serial sections to posses a distinct immunohistochemical reactivity to a panel of MHC monoclonal antibodies (Table 1 and Fig. 3). Type 1 fibres were identified by positive reactivity to F8 antibody and negative reactivity to NA8 and D5 antibodies. Type 2 fibres were identified as positive for NA8 and negative for F8 and MHC-S antibodies. Type 3 and tonic fibres were characterized by their highly specific positive reactivity to D5 and MHC-S antibodies, respectively. These data support the previous finding using a different set of antibodies that each fibre type identified by mATPase activity has a distinct immunohistochemical reactivity (using different antibodies; Rowlerson & Spurway, 1988) and suggest, as confirmed below, that the four fibre types each contained a distinct MHC isoform. Further, fibres with positive reactivity to both F8 and NA8 and negative reactivity to D5 were identified as type 1–2 fibres, indicating coexpression of MHCs corresponding to type 1 and type 2 fibres. Also, most fibres identified as type 3 based on their positive reactivity to D5 antibody demonstrated significant mATPase activity under alkaline conditions (a property found only in type 2 fibres), suggesting coexpression of MHCs corresponding to type 2 and type 3 fibres. In contrast to immunohistochemical demonstration of type 1–2 fibres, it was not possible to demonstrate coexpression of type 2 and type 3 MHCs by immunohistochemistry alone due to the lack of an antibody that was positive for type 2 and negative for type 3 MHC (Table 1). Nevertheless, the antibodies used in this study provided suitable specificity to establish a distinct immunohistochemical identity for each fibre type. Thus, immunohistochemistry was used to identify fibre types in all subsequent experiments where MHC protein isoform or transcript expression was correlated with fibre type.
The presence of different MHC isoforms in the four immunohistochemically defined fibre types was shown directly using SDS-PAGE (Figs 4 and 5, and Table 3). To characterize the MHC identity on the gel, the banding pattern of whole AT muscle was compared with the pattern obtained from individual 60 μm segments of immuno-typed fibres (Figs 4D and 5D). Three distinct bands were found from whole AT muscle. Single fibre analysis (Table 3) revealed that type 1 fibres contained MHC that ran as the middle band, type 2 fibres had MHC that ran as the upper band and tonic fibres contained MHC that also migrated as the middle band. This indicates that type 1 and tonic MHCs co-migrate as a single band in whole muscle, explaining the presence of only three bands from whole muscle. Type 3 fibres contained two MHC bands, a band which ran at the level of the bottom band and an upper band corresponding to type 2 MHC. There were virtually no type 3 fibres that contained only type 3 MHC. This is in agreement with the coexpression of two MHC transcripts (RPM2 and RPM3) observed in the majority of type 3 fibres (see below). These data demonstrate that the four immuno-identified fibre types are composed of four different MHCs, two of which comigrate on SDS-PAGE gels.
Figure 4. MHC mRNA transcripts and protein isoforms in single fibres obtained from the fast region of the anterior tibialis muscle.
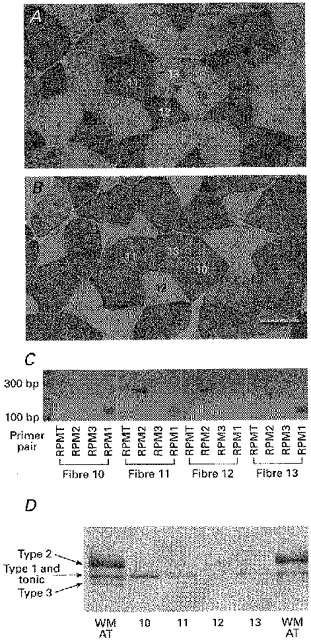
Expression of MHC mRNA transcripts and protein isoforms in immunohistochemically identified frog fibre types obtained from the fast region of the anterior tibialis muscle. A and B, serial sections of anterior tibialis muscle stained for reactivity to the MHC monoclonal antibodies NA8 (A) and F8 (B). Scale bar, 100 μm. C, agarose gels showing expression pattern of the four MHC transcripts in 60 μm segments of immuno-identified fibres (fibre numbers correspond to those shown in A and B), measured by RT-PCR using the four clone-specific PCR primer pairs. D, MHC isoforms measured by SDS-PAGE of 60 μm segments of the same fibres shown in A and B are compared with the banding pattern from whole AT muscle (WM AT). The immuno-classified type 1 fibre (no. 10) expressed only RPM1 mRNA (C) and a single MHC protein band that migrated at the position of the middle band of the triplet seen in whole AT muscle (D). The type 2 fibre (no. 12) expressed only RPM2 mRNA and a single protein band corresponding to the upper band of the triplet. The immuno-classified type 1–2 fibres (nos 11 and 13) coexpressed both RPM1 and RPM2 transcripts and contained 2 MHC protein bands corresponding to the upper and middle bands of the triplet. The upper band (type 2) is barely visible in fibre 11 and was barely detectable in the original gel for fibre 13 and is not apparent in the scanned figure. This banding pattern matches the faint immunoreactivity to NA8 antibody in fibre 13.
Figure 5. MHC mRNA transcripts and protein isoforms in single fibres obtained from the slow region of the anterior tibialis muscle.
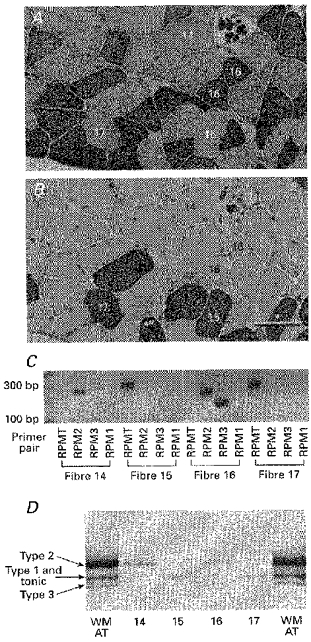
Expression of MHC mRNA transcripts and protein isoforms in immunohistochemically identified frog fibre types obtained from the slow region of the anterior tibialis muscle (labelling scheme as in Fig. 4). A and B, serial sections of anterior tibialis muscle stained for reactivity to the MHC monoclonal antibodies D5 (A) and MHC-S (B). Scale bar, 100 μm. Immuno-classified tonic fibres (no. 15 and no. 17) expressed only the RPMT mRNA (C) and contained a single MHC protein band migrating at the position of the middle band of the triplet seen in whole AT muscle. The type 3 fibre (no. 16) coexpressed RPM3 and RPM2 transcripts and contained 2 protein bands migrating at the position of the upper and lower bands. The type 2 fibre (no. 14) expressed RPM2 and a single protein isoform at the position of the upper band.
Table 3.
SDS-PAGE analysis of MHC protein isoforms in immuno-identified frog single muscle fibres
| SDS-PAGE band | ||||||
|---|---|---|---|---|---|---|
| Immuno-identified fibre type | No. of fibres | Middle only | Upper and middle | Upper only | Upper and lower | Lower only |
| Type 1 | 14 | 11 | 3 | - | - | - |
| Type 1–2 | 19 | 1 | 18 | - | - | - |
| Type 2 | 19 | - | 2 | 17 | - | - |
| Type 3 | 16 | - | - | 1 | 14 | 1 |
| Tonic | 8 | 8 | - | - | - | - |
Four novel MHCs were cloned from the AT muscle
RT-PCR amplification of whole AT muscle with the primer pair FRG1-TW2 resulted in the isolation of three novel MHC clones and the EB-TW2 primer pair yielded a fourth novel clone (Fig. 1). All four transcripts were obtained from the 3′ end of the cDNA and included a portion of the coding region and all of the 3′ untranslated region. These four clones were identified as MHCs based on their approximately 75 % nucleotide and amino acid homology to skeletal muscle MHCs from other vertebrate species. The clones were named based on their pattern of expression in immuno-typed fibres (see below).
Figure 1. Four novel MHC clones obtained from the anterior tibialis muscle of Ranapipiens.
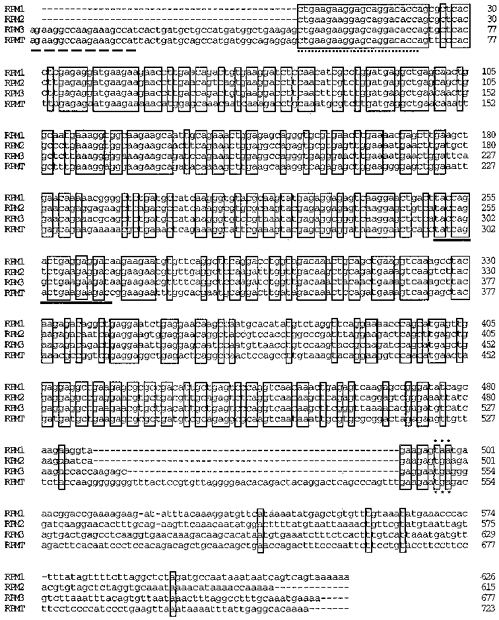
The cDNA sequence of the four MHC clones is shown, with regions of complete sequence homology between all four transcripts indicated by a box and the stop codons indicated by ***. The RPM1, RPM2 and RPMT transcripts were cloned using the primer pair FRG1 (continuous underline)-TW2 and RPM3 was cloned using the primer pair EB (dashed underline)-TW2. Additional sequence information of the clones originally obtained with FRG1-TW2 was obtained by RT-PCR using either EB-TW2 (RPM3) or FC1 (dotted underline)-TW2 (RPM1 and RPM2). These clones have been submitted to GenBank/NCBI with the following accession numbers: RPM1, AF013132; RPM2, AF013133; RPM3, AF013134; and RPMT, AF013135.
Alignment of the four MHC sequences revealed that the RPMT clone contained an additional 54 bp insert immediately upstream from the stop codon (Fig. 1). Excluding this insert, sequence homology of the coding region was less between the RPMT clone and any of the other three clones (67 %) than between any two of the three other clones (81 %). In the UTR, the sequence homology between pairs of clones ranged from a high of 62 % between RPM1 and RPM2 to a low of 47 % between RPM1 and RPMT.
PCR primer pairs (Table 2) were developed to amplify each of the four novel MHC transcripts specifically during a single PCR run. The primer pairs were found to be highly specific since each pair amplified only the plasmid DNA from which it was derived and produced a reaction product of the expected length (Fig. 2 and Table 2). The four MHC transcripts were always readily amplified in cDNA prepared from whole AT muscle (Fig. 2).
Correlation between MHC clones and fibre types
To establish a correlation between the expression of the four cloned MHC transcripts and fibre type, immunohistochemically defined fibre segments were dissected from freeze-dried transverse sections (60 μm) of AT muscle and expression of each of the transcripts was measured using RT-PCR (Table 4 and Figs 4 and 5). Each MHC transcript was found to be predominantly expressed in one of the four fibre types and the clones were named based on this association. Thus, the MHC transcripts expressed primarily in type 1, type 2, type 3 and tonic fibres were named RPM1 (i.e. Ranapipiens myosin 1), RPM2, RPM3 and RPMT, respectively (Table 4). In addition, many fibres coexpressed two different MHC transcripts. Consistent with the immunohistochemical pattern, both RPM1 and RPM2 transcripts were detected in eighteen of twenty-two type 1–2 fibres. Also, expression of both RPM2 and RPM3 transcripts was detected in fifteen of twenty-one type 3 fibres. A qualitatively similar result was obtained for type 3 fibres using thirty-five or forty-five amplification cycles except that more blank results and slightly less coexpression was observed with thirty-five cycles. Thus, results from forty-five cycles of amplification are reported exclusively. There was virtually no coexpression of RPMT with any other MHC transcript with the exception that RPMT was detected in 3 of 18 type 3 fibres that also expressed RPM2 and RPM3 (not shown). These results suggest that the specific expression of the four MHC transcripts gives rise to the four immunohistochemically distinct fibre types and that pure type 3 fibres are rarely observed in the AT muscle.
Table 4.
MHC transcript expression in immuno-identified frog single muscle fibres
| MHC mRNA transcript expression | |||||||
|---|---|---|---|---|---|---|---|
| Immuno-identified fibre type | No. of fibres | RPM1 | RPM1 and RPM2 | RPM2 | RPM2 and RPM3 | RPM3 | RPMT |
| Type 1 | 15 | 10 | 5 | - | - | - | - |
| Type 1–2 | 22 | 2 | 18 | 2 | - | - | - |
| Type 2 | 21 | - | - | 20 | 1 | - | - |
| Type 3 | 21 | - | - | - | 15 | 6 | - |
| Tonic | 21 | - | - | - | - | - | 21 |
A direct correlation between MHC protein isoforms and the four MHC transcripts was obtained by comparing transcript expression with MHC isoform content in consecutive 60 μm segments of immuno-identified fibres (Table 5). All fibres that expressed the RPMT transcript contained only the middle MHC isoform band that corresponds to tonic MHC. Fibres that expressed only the RPM2 transcript contained primarily the upper MHC band corresponding to type 2 MHC. Fibres expressing only the RPM1 transcript were found to contain primarily the middle MHC band corresponding to type 1 MHC. Finally, nearly all fibres expressing RPM2 and RPM3 mRNA were found to contain both the lower and upper bands corresponding to type 3 and type 2 MHCs, respectively. Thus, the MHC protein content of single fibre segments was highly correlated with the pattern of MHC transcript expression.
Table 5.
Correlation between MHC protein isoforms and mRNA transcripts in single frog fibres
| SDS-PAGE band | ||||||
|---|---|---|---|---|---|---|
| mRNA transcripts | No. of fibres | Middle only | Upper and middle | Upper only | Upper and lower | Lower only |
| RPM1 | 12 | 10 | 2 | - | - | - |
| RPM1 and RPM2 | 17 | 2 | 15 | - | - | - |
| RPM2 | 17 | - | 2 | 14 | 1 | - |
| RPM2 and RPM3 | 19 | - | - | 1 | 17 | 1 |
| RPM3 | 3 | - | - | - | 3 | - |
| RPMT | 6 | 6 | - | - | - | - |
DISCUSSION
MHC protein and transcript expression patterns provide a molecular basis for Ranapipiens muscle fibre type identity
In this study, four fibre types (type 1, type 2, type 3 and tonic), initially identified based on their mATPase reactivity, morphology and location within the AT muscle, were each shown to possess unique reactivity to a panel of monoclonal MHC antibodies (Fig. 3). Using SDS-PAGE, the four immunohistochemically distinct fibre types were shown to consist of two MHC isoforms that run as unique bands (types 2 and 3) and two that comigrate (types 1 and tonic; Figs 4 and 5, and Table 3). The combined immunohistochemistry and SDS-PAGE analysis suggests that R.pipiens AT muscle fibre types are composed of four different MHCs. To characterize further the MHC isoforms in these fibre types, four novel MHC mRNA transcripts were cloned from the AT muscle and their expression pattern within single immuno-identified fibres was determined by RT-PCR using specific primer pairs (Figs 2, 4 and 5, and Table 4). Each MHC transcript was predominantly expressed in one of the four fibre types and was named based on this expression pattern. The four transcripts, RPM1, RPM2, RPM3 and RPMT, were expressed predominantly in type 1, type 2, type 3 and tonic fibre types, respectively. Thus, it appears that the four MHC transcripts encode four distinct MHC isoforms that define the immunohistochemically distinct fibre types, demonstrating a molecular basis for Rana fibre types.
Our results do not preclude the existence of additional MHC isoforms within the hindlimb muscles of adult R.pipiens. At present, only the AT muscle has been studied and it is possible that other muscles may express different MHCs. Other MHCs could also exist within the AT itself. Despite the use of consensus sequence PCR primers, based on sequence homology between known MHCs, our cloning strategy was not designed to be exhaustive. Thus, a more complex pattern of MHC isoform expression and coexpression may exist than was elucidated by our study. An exhaustive search for all possible MHCs may require the use of a protein expression library and a myosin antibody that recognizes all myosins such as was performed recently in chicken muscle (Moore, Arrizubieta, Tidyman, Herman & Bandman, 1992). However, it should be noted that the same immunohistochemically defined fibre types were found in many other R.pipiens hindlimb muscles (G. J. Lutz, S. N. Bremner, N. Lajevardi, R. L. Lieber & L. C. Rome, unpublished observations) indicating that the expression of these same isoforms occurs within other muscles.
Correlation of MHC transcript with fibre type required detection of each transcript without cross-reactivity of the other transcripts. For this purpose, the experimental approach of single cell RT-PCR offers many advantages over the commonly used in situ hybridization technique (DeNardi et al. 1993). First, to increase signal strength, in situ hybridization probes are necessarily longer than PCR primers. The extra length makes the in situ probes less specific as they cannot be restricted to the short segments of divergent mRNA sequences (Fig. 1). Second, it is difficult to distinguish between cross-reactivity of in situ probes and coexpression of multiple MHC transcripts. In contrast, the clone-specific PCR primer pairs used at present are short enough to be highly specific and their cross-reactivity was tested directly on high copy plasmid cDNA from which the clones were obtained (Fig. 2).
Single fibres coexpressed multiple MHCs
Most immuno-identified type 1–2 fibres expressed both RPM1 and RPM2 mRNA and contained both type 1 and type 2 MHC protein isoforms as shown by SDS-PAGE (Figs 4 and 5, and Tables 3 and 4). This coexpression was reliably predicted using a combination of the two MHC antibodies, F8 and NA8, which both selectively differentiated between type 1 and type 2 MHCs. In addition, most immuno-identified type 3 fibres expressed both RPM2 and RPM3 transcripts and contained both type 2 and type 3 MHC protein isoforms (Figs 4 and 5, and Tables 3 and 4).
The functional significance of MHC coexpression within normal adult single fibres has not been determined. Previous reports have documented transcript coexpression within single fibres (DeNardi et al. 1993; Peuker & Pette, 1997), within neighbouring myofibrils of a sarcomere (Gauthier, 1990) or even within a single thick filament (Saad, Pardee & Fischman, 1986). For example, in adult rat muscle, coexpression in single muscle fibres of type 2A and 2X and type 2B and 2X MHCs was reported in 10 % and 20 % of the fibres studied, respectively, using in situ hybridization (DeNardi et al. 1993). Coexpression of type 2A and 2B transcripts within a single cell was not observed. Similarly, we observed coexpression of specific MHC transcript pairs within single fibres: type 1/2 and type 2/3 coexpressed, but type 1/3 coexpression was not observed. Further, we have recently found in R.pipiens that fibres coexpressing type 1 and type 2 MHCs account for over 25 % of the muscle volume in four different hindlimb muscles, including the AT (G. J. Lutz, S. N. Bremner, N. Lajevardi, R. L. Lieber & L. C. Rome, unpublished observations). This relatively common occurrence of coexpression, as well as the stereotypic combination of transcripts, implies that coexpression within single cells reflects the normal state of adult Rana muscle.
Despite the reports of widespread occurrence of MHC coexpression, there are at present relatively few data documenting the mechanical consequences of coexpression. The question that arises is whether fibres that coexpress two different isoforms of MHC have intermediate mechanical properties in direct proportion to the ratio of the two isoforms. Alternatively, in a given fibre, the slower cross-bridges could limit mechanical function by a dominant-negative mechanism. The limited data from two studies using skinned mammalian fibres suggest that Vmax of a fibre that coexpresses two MHC isoforms is intermediate between the Vmax values of fibres expressing only the faster or slower isoform (Bottinelli et al. 1994; Galler, Schmitt & Pette, 1994).
If coexpression does in fact result in intermediate properties rather than dominant-negative properties, it may have a functional role in providing a muscle with a continuum of mechanical properties. Many studies have documented that mechanical properties of different fibre types vary smoothly between types, rather than falling into discrete units (Edman, Reggiani & Te Kronnie, 1985; Staron & Pette, 1987; Elzinga, Howarth, Rall, Wilson & Woledge, 1989; Bottinelli, Schiaffino & Reggiani, 1991). However, a clear understanding of the degree to which coexpression of MHC isoforms contributes to a continuum of mechanical properties may be complicated by variability in MLC isoform expression. Interestingly, Edman et al. (1985) demonstrated using immunohistochemistry in Rana temporaria that MHC composition varied along the length of single isolated fibres and was correlated with variations inVmax. The variability in Vmax between adjacent segments of a given fibre was as large as the variability between individual fibres. The functional role of length-dependent variation in MHC isoform content along a single fibre is not currently understood.
Amphibian fibre type nomenclature and mechanical properties
Previously, Rowlerson & Spurway (1988) were able to define three twitch fibre types (type 1, type 2 and type 3) and a tonic type in the hindlimb muscle of ranid frogs using a combination of mATPase reactivity and differential reactivity to a panel of MHC monoclonal antibodies. Using the same techniques, these authors found that Xenopus muscle could also be classified into three twitch types and two slow types. The relative mATPase activity of fibre types under acid preincubation conditions in the present study is identical to previous findings for ranid muscle (Rowlerson & Spurway, 1988). However, these authors found type 1 and type 2 fibres to have similar levels of alkaline stability, in contrast to our finding that type 2 fibres were considerably more alkaline stable than type 1 fibres. This difference may represent species differences, as the previous study was performed on a variety of ranid species without specific attention to variation between individual species. Alternatively, differences in methodology could explain the discrepancy, as the previous authors stated that they had difficulties obtaining a consistent alkaline ATPase pattern (Rowlerson & Spurway, 1988).
Based on the similarity between our results and those of Rowlerson & Spurway (1988) for mATPase reactivity, size and location of the various fibre types, we are likely to be characterizing the same fibre types. Although we used a different antibody set from that used previously, the fact that the four fibre types each had unique reactivity to MHC antibodies in both studies further supports the consistency in fibre type identification.
The five different fibre types of Xenopus have been shown to have differences in maximum shortening velocity in the order, type 1 > type 2 > type 3 > type 4 > tonic (Lännergren, 1987, 1992) but the MHC isoform content of individual fibres has not been measured directly. Post-mechanics analysis of the isomyosin banding patterns on native-PAGE gels and MLC content on SDS-PAGE gels (Lännergren & Hoh, 1984; Lännergren, 1987) suggests that the five fibre types are composed of five different MHCs. However, MHC isoform content could not be unambiguously determined with this methodology. The mechanical properties of the four fibre types within R.pipiens have not yet been determined but, based on the similarity to Xenopus in mATPase and actomyosin reactivity (Rowlerson & Spurway, 1988), we expect a similar pattern of functional properties. However, while a clear correlation between MHC content and fibre type has been made in the present study, it remains to be determined whether the mechanical properties correlate with fibre type in the precise order that is intimated by the nomenclature.
Our categorization of muscle fibres into four immunohistochemically distinct types ignores the variable expression of other proteins involved in contractile function, such as troponin, MLCs, metabolic proteins and the Ca2+-ATPase within the sarcoplasmic reticulum, many of which exist as isoforms within certain fibre types. Intact muscle fibres contain a complex complement of protein isoforms that probably results in muscle fibres with a continuum of functional properties. Because our study was not designed to be quantitative, fibres were arbitrarily grouped into types, such that discrete divisions were favoured over a continuum of MHC expression. These results do not, therefore, provide a measure of the relative amount of MHC transcript or protein isoform expression or coexpression within a given fibre. This is particularly true for the small type 3 and tonic fibres since the detection of bands was near the limits of sensitivity of the silver stain. However, despite the qualitative nature of the methods, we have no reason to doubt the validity of the correlation between the presence of mRNA transcripts and MHC isoforms of the various fibre types.
Sequence homology and evolutionary relatedness of MHCs
Among the four R. pipiens MHC transcripts, we noted regions of complete sequence identity interspersed with regions that were highly divergent (Fig. 1). The functional significance of such sequence diversity along the rod portion of MHC is not currently understood (Moore, Tidyman, Arrizubieta & Bandman, 1993). The rod portion is involved in myofibrillogenesis (McNally, Kraft, Bravo-Zehnder, Taylor & Leinwand, 1989) and myosin filament assembly (Leinwand, Sohn, Frankel, Goodwin & McNally, 1989; Sinard, Stafford & Pollard, 1989). In this regard it is interesting to speculate that a possible role of the extra insert in the RPMT clone may be to prevent improper filament assembly with other MHCs or simply to minimize the occurrence of heterogeneous filaments during exchange of molecules with a heterogeneous MHC monomer pool (Saad et al. 1986; Saad, Dennis, Tan & Fischman, 1991). This notion is supported by the lack of coexpression between tonic MHC and any other MHC.
The sequence homology within the MHC rod has been previously used to evaluate evolutionary relatedness of avian and mammalian isoforms of MHC. The level of homology between the four R. pipiens clones and those of other species was evaluated by aligning a 488 bp segment within the coding region of each clone (located immediately upstream of the 3′ UTR) using clustal analysis (Fig. 6; GeneWorks, Oxford Molecular Group, Campbell, CA, USA). The pattern of homology supports the idea that slow MHC isoforms (e.g. frog tonic, avian slow and avian cardiac) diverged from other MHCs in an ancient split, prior to the amphibian/avian/mammalian radiation. In contrast, the development of fast MHC isoforms occurred after the amphibian/avian/mammalian radiation, since the fast isoforms within a vertebrate class are always more similar to each other than to the fast MHCs of other classes. The evolution of fast MHC genes within the various vertebrate classes thus appears to provide a classic example of parallel evolution, with fish, amphibian, avian and mammalian species each forming their own branch containing a complement of fast isoforms, although different numbers of these isoforms have arisen within different species (Moore et al. 1993).
Figure 6. Evolutionary relatedness of MHCs.
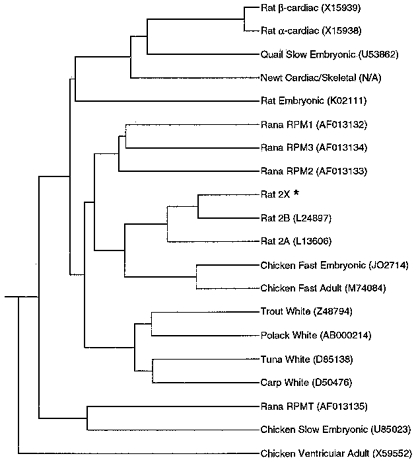
Evolutionary tree comparing the homology between the four novel MHCs from R. pipiens skeletal muscle with MHCs of other species (see Discussion for details). Alignment was performed using clustal analysis (Geneworks, Oxford Molecular Group). GenBank/NCBI accession numbers are indicated in parenthesis. *Sequence from S. D. Shoemaker, A. F. Ryan & R. L. Lieber (unpublished observations).
Acknowledgments
This work was supported by NIH grants AR35192 and AR07484, DC00137, and the Veterans Affairs Medical Research and Rehabilitation R & D Services. We thank Professor Everett Bandman (University of California Davis) and Professor Stefano Schiaffino (University of Padua) for the use of their antibodies. We also thank Dr Vince Caiozzo (University of California Irvine) for providing antibodies and helpful discussion. We also thank Shannon Bremner and Christina Taylor for their excellent technical assistance.
References
- Booth FW, Kelso JR. Effect of hind-limb immobilization on contractile and histochemical properties of skeletal muscle. Pflügers Archiv. 1973;342:123–238. doi: 10.1007/BF00591371. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Betto R, Schiaffino S, Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. The Journal of Physiology. 1994;478:341–349. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. The Journal of Physiology. 1991;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin NA, Davies RE. Chemical and mechanical changes during stretching of activated frog skeletal muscle. Cold Spring Harbor Symposia on Quantitative Biology. 1973;37:619–626. [Google Scholar]
- DeNardi C, Ausoni S, Moretti P, Gorza L, Velleca M, Buckingham M, Schiaffino S. Type 2X-myosin heavy chain is coded by a muscle fiber type-specific and developmentally regulated gene. Journal of Cell Biology. 1993;123:823–835. doi: 10.1083/jcb.123.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Reggiani C, Te Kronnie G. Differences in maximum velocity of shortening along single muscle fibres of the frog. The Journal of Physiology. 1985;365:147–163. doi: 10.1113/jphysiol.1985.sp015764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga G, Howarth JV, Rall JA, Wilson MGA, Woledge RC. Variation in the normalized tetanic force of single frog muscle fibres. The Journal of Physiology. 1989;410:157–170. doi: 10.1113/jphysiol.1989.sp017526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel WK, Brooke MH, Nelson PG. Histochemical studies of denervated or tenotomized cat muscle. Annals of the New York Academy of Sciences. 1966;138:160–185. doi: 10.1111/j.1749-6632.1966.tb41164.x. [DOI] [PubMed] [Google Scholar]
- Galler S, Schmitt TL, Pette D. Stretch activation, unloaded shortening velocity, and myosin heavy chain isoforms of rat skeletal muscle fibres. The Journal of Physiology. 1994;478:513–521. doi: 10.1113/jphysiol.1994.sp020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier GF. Differential distribution of myosin isoforms among the myofibrils of individual developing muscle fibers. Journal of Cell Biology. 1990;110:693–701. doi: 10.1083/jcb.110.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd MG, Dantzig JA, Trentham DR, Goldman YE. Phosphate release and force generation in skeletal muscle fibers. Science. 1985;228:1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Huxley AF. Muscular contraction. The Journal of Physiology. 1974;243:1–43. [PMC free article] [PubMed] [Google Scholar]
- Irving M, St Claire Allen T, Sabido-David C, Craik JS, Brandmeier B, Kendrick-Jones J, Corrie JE, Trentham DR, Goldman YE. Tilting of the light-chain region of myosin during step length changes and active force generation in skeletal muscle. Nature. 1995;375:688–691. doi: 10.1038/375688a0. [DOI] [PubMed] [Google Scholar]
- Karpati G, Engel WK. Correlative histochemical study of skeletal muscle after suprasegmental denervation, peripheral nerve section, and skeletal fixation. Neurology. 1968;18:681–692. doi: 10.1212/wnl.18.7.681. [DOI] [PubMed] [Google Scholar]
- Lännergren J. An intermediate type of muscle fibre in Xenopus laevis. Nature. 1979;279:254–256. doi: 10.1038/279254a0. [DOI] [PubMed] [Google Scholar]
- Lännergren J. Contractile properties and myosin isoenzymes of various kinds of Xenopus twitch muscle fibres. Journal of Muscle Research and Cell Motility. 1987;8:260–273. doi: 10.1007/BF01574594. [DOI] [PubMed] [Google Scholar]
- Lännergren J. Fibre types in Xenopus muscle and their functional properties. In: Simmons RM, editor. Muscular Contraction. Cambridge University Press; 1992. pp. 181–188. [Google Scholar]
- Lännergren J, Hoh JFY. Myosin isozymes in single muscle fibers of Xenopus laevis: analysis of five different functional types. Proceedings of the Royal Society. 1984;B 222:401–408. doi: 10.1098/rspb.1984.0072. [DOI] [PubMed] [Google Scholar]
- Leinwand LA, Sohn R, Frankel SA, Goodwin EB, McNally EM. Bacterial expression of eukaryotic contractile proteins. Cell Motility and the Cytoskeleton. 1989;14:3–11. doi: 10.1002/cm.970140104. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Schmitz MC, Mishra DK, Fridén J. Contractile and cellular remodeling in rabbit skeletal muscle after cyclic eccentric contractions. Journal of Applied Physiology. 1994;77:1926–1934. doi: 10.1152/jappl.1994.77.4.1926. [DOI] [PubMed] [Google Scholar]
- Lutz GJ, Rome LC. Built for jumping: the design of the frog muscular system. Science. 1994;263:370–372. doi: 10.1126/science.8278808. [DOI] [PubMed] [Google Scholar]
- Lutz GJ, Rome LC. Muscle function during jumping in frogs. I. Sarcomere length change, EMG pattern, and jumping performance. American Journal of Physiology. 1996a;271:C563–570. doi: 10.1152/ajpcell.1996.271.2.C563. [DOI] [PubMed] [Google Scholar]
- Lutz GJ, Rome LC. Muscle function during jumping in frogs. II. Mechanical properties of muscle: implications for system design. American Journal of Physiology. 1996b;271:C571–578. doi: 10.1152/ajpcell.1996.271.2.C571. [DOI] [PubMed] [Google Scholar]
- Lutz GJ, Ryan AF, Lieber RL. Novel myosin heavy chain clones correlated with fiber type from skeletal muscle of Rana pipiens. FASEB Journal. 1997;11:A61. [Google Scholar]
- McNally EM, Kraft R, Bravo-Zehnder M, Taylor DA, Leinwand LA. Full-length rat alpha and beta cardiac myosin heavy chain sequences. Comparisons suggest a molecular basis for functional differences. Journal of Molecular Biology. 1989;210:665–671. doi: 10.1016/0022-2836(89)90141-1. [DOI] [PubMed] [Google Scholar]
- Miller JB, Stockdale FE. Developmental origins of skeletal muscle fibers; clonal analysis of myogenic cell lineages based on expression of fast and slow myosin heavy chains. Proceedings of the National Academy of Sciences of the USA. 1986;83:3860–3864. doi: 10.1073/pnas.83.11.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LA, Arrizubieta MJ, Tidyman WE, Herman LA, Bandman E. Analysis of the chicken fast myosin heavy chain family localization of isoform-specific antibody epitopes and regions of divergence. Journal of Molecular Biology. 1992;225:1143–1151. doi: 10.1016/0022-2836(92)90114-y. [DOI] [PubMed] [Google Scholar]
- Moore LA, Tidyman WE, Arrizubieta MJ, Bandman E. The evolutionary relationship of avian and mammalian myosin heavy-chain genes. Journal of Molecular Evolution. 1993;36:21–30. doi: 10.1007/BF02407303. [DOI] [PubMed] [Google Scholar]
- Moss RL, Giulian GG, Greaser ML. The effects of partial extraction of TnC upon the tension-pCa relationship in rabbit skinned skeletal muscle fibers. Journal of General Physiology. 1985;86:585–600. doi: 10.1085/jgp.86.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D. The Dynamic State of Muscle Fibers. Berlin: Walter de Gruyter & Company; 1990. [Google Scholar]
- Peuker H, Pette D. Quantitative analysis of myosin heavy-chain mRNA and protein isoforms in single fibers reveal a pronounced fiber heterogeneity in normal rabbit muscles. European Journal of Biochemistry. 1997;247:30–36. doi: 10.1111/j.1432-1033.1997.00030.x. [DOI] [PubMed] [Google Scholar]
- Radice GP, Malacinski GM. Expression of myosin heavy chain transcripts during Xenopus laevis development. Developmental Biology. 1989;133:562–568. doi: 10.1016/0012-1606(89)90058-4. [DOI] [PubMed] [Google Scholar]
- Rowlerson AM, Spurway NC. Histochemical and immunohistochemical properties of skeletal muscle fibers from Rana and Xenopus. Histochemical Journal. 1988;20:657–673. doi: 10.1007/BF01845796. [DOI] [PubMed] [Google Scholar]
- Saad AD, Dennis JE, Tan IP, Fischman DA. Visualization of myosin exchange between synthetic thick filaments. Journal of Muscle Research and Cell Motility. 1991;12:225–234. doi: 10.1007/BF01745111. [DOI] [PubMed] [Google Scholar]
- Saad AD, Pardee JD, Fischman DA. Dynamic exchange of myosin molecules between thick filaments. Proceedings of the National Academy of Sciences of the USA. 1986;83:9483–9487. doi: 10.1073/pnas.83.24.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinard JH, Stafford WF, Pollard TD. The mechanism of assembly of Acanthamoeba myosin-II minifilaments: minifilaments assemble by three successive dimerization steps. Journal of Cell Biology. 1989;109:1537–1547. doi: 10.1083/jcb.109.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Ovalle WK., Jr Varieties of fast and slow extrafusal muscle fibers in amphibian hind limb muscles. Journal of Anatomy. 1973;116:1–24. [PMC free article] [PubMed] [Google Scholar]
- Staron RS, Pette D. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in rabbit muscle fibers. Histochemistry. 1986;86:19–23. doi: 10.1007/BF00492341. [DOI] [PubMed] [Google Scholar]
- Staron RS, Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit soleus muscle. Biochemical Journal. 1987;243:687–693. doi: 10.1042/bj2430687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler EE, Strehler-Page MA, Perriard JC, Periasamy M, Nadal-Ginard B. Complete nucleotide and encoded amino acid sequence of a mammalian myosin heavy chain gene. Journal of Molecular Biology. 1986;190:291–317. doi: 10.1016/0022-2836(86)90003-3. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Kushmerick MJ, Mabuchi K, Gergely J, Sreter FA. Velocity of shortening and myosin isozymes in two types of rabbit fast-twitch muscle fibers. American Journal of Physiology. 1986;251:C431–434. doi: 10.1152/ajpcell.1986.251.3.C431. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR. Elecrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. Journal of Applied Physiology. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. The Journal of Physiology. 1993;466:611–628. [PMC free article] [PubMed] [Google Scholar]


