Abstract
A soluble form of the tumour necrosis factor (TNF) type 1 receptor (referred to as TNF binding protein, TNF-bp) at a dose of 1 mg per animal, or an equivalent volume of solvent, was injected together with 10 μg kg−1 lipopolysaccharide (LPS) or 50 μg kg−1 muramyl-dipeptide (MDP) directly into the arterial circulation of guinea-pigs and the effects on circulating TNF or interleukin-6 (IL-6) and on abdominal temperature were studied.
At 15 or 60 min after injection, LPS-induced and MDP-induced circulating TNF was below the detection limit of the assay and thus completely neutralized in animals treated with TNF-bp. In the control group, TNF was still below the limit of detection in most animals 15 min after LPS was injected; in some animals small traces of TNF could already be detected at that time. However, 60 min after administration of LPS, large amounts of TNF (19508 ± 4682 pg ml−1) were measured in the control group. MDP-induced TNF in plasma was below the limit of detection 15 min after MDP was injected, and rose to 10862 ± 3029 pg ml−1 60 min after injection.
Low levels of circulating IL-6 (20-40 international units (IU) ml−1) were measured in all groups of animals 15 min after injection of LPS or MDP. This value corresponds to the baseline activity of IL-6 in plasma of guinea-pigs. One hour after administration of LPS, IL-6 rose to 5442 ± 1662 IU ml−1 in the control group and to a significantly lower value of 1485 ± 179 IU ml−1 in guinea-pigs treated with TNF-bp. One hour after injection of MDP, circulating IL-6 was 2614 ± 506 IU ml−1 in the control group, while the corresponding value in animals treated with TNF-bp again was significantly lower (873 ± 312 IU ml−1).
The second phase of the characteristic biphasic LPS fever in guinea-pigs was significantly attenuated in animals treated with TNF-bp. The shorter first phase of the febrile response to LPS was identical in both groups of animals.
The late phase of MDP-induced fever (7-22 h after injection) was depressed by treatment with TNF-bp, while the first phase of MDP-induced fever (0-7 h after injection) was significantly enhanced by the neutralization of TNF by TNF-bp.
Tumour necrosis factor (TNF) is a cytokine that is detected in the circulation of man or experimental animals after administration of a fever-inducing dose of lipopolysaccharide (LPS; Kluger, 1991). Muramyl-dipeptide (MDP) from Gram-positive bacterial cell walls is capable of evoking a febrile response in guinea-pigs that is preceded by high circulating levels of TNF (Roth et al. 1997). In this context the question arose whether TNF released endogenously under the influence of LPS or MDP represents one of the mediators of the febrile responses, and is thus one of the endogenous pyrogens. Studies with the aim of investigating this question led, in part, to conflicting results and indicated some species-specific differences in the reactivity to TNF. While there is experimental evidence suggesting that TNF is, per se, a pyrogenic molecule in man (Michie et al. 1988), rabbits (Dinarello et al. 1986; Kapas et al. 1992) and guinea-pigs (Goldbach, Roth, Störr & Zeisberger, 1997a; Roth, McClellan, Kluger & Zeisberger, 1994b), in several studies in rats (Long, Kunkel, Vander & Kluger, 1990; Klir, McClellan, Kozak, Szelenyi, Wong & Kluger, 1995) or mice (Kozak, Conn, Klir, Wong & Kluger, 1995; Leon, Kozak, Peschon & Kluger, 1997), neutralization of circulating TNF enhances fever. In order to obtain precise information concerning the quantitative participation of a given cytokine in the response of an organism to exogenous pyrogens, e.g. LPS or MDP, substances that are able to neutralize the biological activity of the cytokine are necessary. Because cytokine proteins are species specific, antibodies against the cytokine of a different species of experimental animal might fail to neutralize the cytokine. For TNF, soluble neutralizing forms of both receptors exist and it has been demonstrated that soluble 55 kDa but not soluble 75 kDa TNF receptor has protective effects in an experimental model of Gram-negative sepsis (Evans et al. 1994). This discrepancy between the two soluble TNF receptors is due to the fact that TNF is released again after binding to the 75 kDa receptor, but not from the complex of TNF with the soluble 55 kDa receptor.
In the present study we used a novel dimeric polyethylene glycol-linked form of the 55 kDa TNF receptor, TNF-bp (Martin, Near, Bendele & Russell, 1995), to neutralize endogenously released TNF in response to a fever-inducing dose of LPS or MDP in guinea-pigs. The pyrogen and TNF-bp were injected into the same compartment, the arterial circulation. The febrile response and circulating levels of TNF and interleukin-6 (IL-6) were used as parameters to study the effects of TNF-bp on the LPS- or MDP-induced responses.
METHODS
Animals
The present study was performed in male guinea-pigs (Cavia aperea porcellus) with a body weight of 380 ± 10 g at the beginning of the experiments. The animals were housed in individual cages at 22°C with a 12 h light-12 h dark cycle (lights off at 7.00 p.m.). The animals had access to food and water ad libitum. Twice a week they received fresh pellet food and water, and at least once a week their cages were changed. Within the week before the experiment was performed, the animals were prepared surgically (see below) and habituated once or twice to the blood sampling procedure by withdrawal of a small volume of blood and flushing back the blood plus approximately 0.1 ml sterile heparinized saline. The national guidelines for experiments with vertebrate animals were followed, and approval by the local ethics commitee was obtained for the experimental protocols. One day after the experiment was performed a lethal dose of urethane was injected into the general circulation via the intra-arterial catheter.
Surgery
At least 1 week before the start of the experimental procedure, the animals were implanted with intra-arterial catheters as described previously (Roth, Conn, Kluger & Zeisberger, 1993; Roth, McClellan, Kluger & Zeisberger, 1994a). Briefly, the guinea-pigs were anaesthetized with 100 mg kg−1 ketamine hydrochloride and 4 mg kg−1 xylazine. Polyethylene catheters were inserted through the left carotid artery and extended up to the aortic arch. The distal ends of the catheters were tunnelled subcutaneously to the interscapular region of the back where they emerged through the skin. After implantation, the catheters were flushed with sterile heparinized saline and sealed by heating.
Substances
Bacterial LPS (derived from Escherichia coli, O111:B4; Sigma) was suspended in sterile pyrogen-free 0.9 % saline at a concentration of 100 μg ml−1. A dose of 10 μg kg−1 was injected into the intra-arterial catheter.
MDP (N-acetylmuramyl-L-Ala-D-isoglutamine; ICN Biomedicals, Aurora, OH, USA) was dissolved in sterile pyrogen-free 0.9 % saline at a concentration of 500 μg ml−1. A dose of 50 μg kg−1 was injected into the intra-arterial catheter.
The potent TNF inhibitor, a dimeric polyethylene glycol-linked form of the type 1 soluble receptor of TNF (PEG-(rsTNF-RI)2), was obtained from Amgen Inc., Boulder CO, USA; this substance is referred to as TNF-binding protein (TNF-bp) throughout this paper. TNF-bp was dissolved in sterile pyrogen-free saline at a concentration of 5 mg ml−1. A dose of 1 mg per animal was used for intra-arterial injections.
Measurement of body temperature
Abdominal temperature was measured by use of battery-operated biotelemetry transmitters (VM-FH discs, Mini-Mitter Co., Sunriver, OR, USA) implanted intraperitoneally after placement of the intra-arterial catheter. Output (frequency in Hz) was monitored by an antenna placed under each cage (RA 1000 radioreceivers, Mini-Mitter Co.) and multiplexed by means of a BCM 100 consolidation matrix to an IBM personal computer system. A Dataquest IV data acquisition system (Data Sciences Inc., St Paul, MN, USA) was used for automatic control of data collection and analysis. Body temperature was monitored and recorded at 5 min intervals. For the analysis and graphical documentation, temperature data at adequate time intervals between 15 and 60 min were used.
Bioassays for TNF and IL-6
Determination of TNF was performed by a bioassay based on the cytotoxic effect of TNF on the mouse fibrosarcoma cell line WEHI 164 subclone 13 (Espevic & Nissen-Meyer, 1986). The assay was performed in sterile, 96-well microtiter plates. Serial dilutions of biological samples or different concentrations of TNF standard (code 88/532, National Institute for Biological Standards and Control, South Mimms, UK) were incubated for 24 h in wells that had been seeded with 50 000 actinomycin D-treated WEHI cells. The number of surviving cells after 24 h was measured with the dimethylthiazol-diphenyl tetrazolium bromide (MTT) colorimetric assay (Holt, Cooper & Hopkins, 1991).
Determination of IL-6 was performed by a bioassay based on the dose-dependent growth stimulation of IL-6 on the B9 hybridoma cell line (Aarden, DeGroot, Schaap & Landsdorp, 1987). The assay was performed in sterile, 96-well microtiter plates. In each well, 5000 B9 cells were incubated for 72 h with serial dilutions of biological samples or with different concentrations of IL-6 standard (code 89/548, National Institute for Biological Standards and Control, South Mimms, UK). The number of cells in each well was measured by use of the MTT assay (see above).
Experimental protocols
To determine its TNF-neutralizing capacity, different amounts of TNF-bp in the range of 12.5-500 ng were incubated together with 20 ng murine TNF in the presence of 50 000 WEHI subclone 13 cells in wells of microtiter plates. The amount of bioactive TNF in each well was measured with the TNF bioassay described above.
To determine the effects of TNF-bp on LPS-induced fever and on circulating bioactive IL-6 in response to LPS, 1 mg TNF-bp dissolved in 0.2 ml of 0.9 % sterile pyrogen-free saline (n= 8) or 0.2 ml of solvent alone (n= 9) were injected directly into the intra-arterial catheters together with 10 μg kg−1 LPS.
To determine the effects of TNF-bp on MDP-induced fever and on circulating bioactive IL-6 in response to MDP, 1 mg TNF-bp dissolved in 0.2 ml of 0.9 % sterile pyrogen-free saline (n= 7) or 0.2 ml of solvent alone (n= 7) were injected directly into the intra-arterial catheters together with 50 μg kg−1 LPS.
After the injection of substances the catheter was flushed with saline. In control animals TNF-bp or solvent were intra-arterially injected without LPS or MDP, but together with an equivalent volume of 0.9 % sterile saline (n= 4 in each group). During the experimental period, single blood samples (0.6 ml) were slowly (within 1 min) withdrawn into a sterile syringe, transferred into a polypropylene tube, and immediately centrifuged. Sterile 0.9 % saline (0.2-0.3 ml) was slowly injected into the catheter after each blood sampling procedure; heparinized saline was used after the last blood sampling of each experiment. The blood plasma was stored at -70°C for later determination of cytokines. The samples were collected 15 and 60 min after the intra-arterial injections.
Evaluation and statistics
In graphs of the thermal responses to LPS or MDP injections, the mean abdominal temperatures were plotted against time. At each time point abdominal temperatures were expressed as means ± standard error of the mean (s.e.m.). An analysis of variance (ANOVA) for repeated measurements, followed by Scheffe's post hoc test, was used to compare thermal responses. The calculations were carried out on an Apple Macintosh computer using the software package StatView (Abacus Concepts, Berkeley, CA, USA).
Circulating levels of TNFα or IL-6 in response to injection of LPS or MDP were compared by Student's t tests. Because the values for cytokine concentrations are not normally distributed, a log-transformation of the cytokine values was performed for the t tests.
RESULTS
Data proving the capacity of TNF-bp to neutralize TNF are shown in Table 1. The upper part of Table 1 shows the ability of different amounts of TNF-bp to block TNF bioactivity of 20 ng murine TNF. According to these data, 25 ng TNF-bp is able to neutralize 20 ng TNF. The lower part of Table 1 shows that TNF is recovered almost completely by the assay in the absence of TNF-bp.
Table 1.
Neutralization of TNF by TNF-bp in vitro
| TNF per well | TNF-bp per well | Detectable TNF per well |
|---|---|---|
| 20 ng | 500 ng | n.d. |
| 20 ng | 250 ng | n.d. |
| 20 ng | 125 ng | n.d. |
| 20 ng | 62.5 ng | n.d. |
| 20 ng | 50 ng | n.d. |
| 20 ng | 25 ng | n.d. |
| 60 pg | — | 65 pg |
| 20 pg | — | 16 pg |
Amounts of bioactive TNF recovered after incubation for 24 h with different concentrations of TNF-bp. Each sample was analysed in triplicate. n.d., not detectable.
In vivo, circulating levels of bioactive TNF in response to intra-arterial injections of 10 μg kg−1 LPS along with 1 mg TNF-bp or solvent are shown in Fig. 1.
Figure 1. Neutralization of LPS-induced TNF by TNF-bp in vivo.
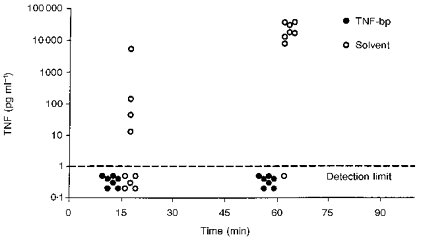
Individual data from TNF measurements in plasma samples collected 15 and 60 min after intra-arterial injections of 10 μg kg−1 LPS together with 1 mg per animal of TNF-bp (n= 8) or solvent (n= 9). In one animal treated with TNF-bp, the substance could be administered but blood could not be withdrawn; the value of one 60 min sample from an animal treated with LPS and solvent is missing because of an erroneous sample pre-dilution.
Fifteen minutes after LPS was injected into the arterial circulation, no bioactive TNF was detected in the plasma of guinea-pigs treated with LPS and TNF-bp. In four out of nine guinea-pigs treated with LPS and solvent, low levels of TNF were detected at this time point. One hour after injection of LPS together with solvent, high circulating levels of TNF were measured in all investigated guinea-pigs, with only one exception. Again, no bioactive TNF could be detected in the animals treated with LPS and TNF-bp.
The influence of TNF-bp on the LPS-induced levels of circulating IL-6 is shown in Fig. 2. Figure 2A shows the mean values of the individual TNF data from Fig. 1. In contrast to the remarkable amount of 19509 ± 4682 pg ml−1 in guinea-pigs injected with LPS and solvent, no bioactive TNF was present in arterial plasma of animals injected with LPS and TNF-bp, 60 min after injection, a time when circulating TNF reaches peak values in guinea-pigs (Roth et al. 1993). The mean amounts of bioactive IL-6 in the same plasma samples are shown in Fig. 2B. The level of IL-6 in plasma was 48 ± 23 IU ml−1 in animals injected with LPS and solvent versus 28 ± 10 IU ml−1 in guinea-pigs injected with LPS and TNF-bp, 15 min after injection. One hour after injection, IL-6 in plasma rose to 1485 ± 179 IU ml−1 in guinea-pigs injected with LPS and TNF-bp and to a significantly higher value of 5442 ± 1661 IU ml−1 in animals injected with LPS and solvent (t= 2.482; P= 0.0264). In animals injected with 0.9 % saline instead of LPS, no bioactive TNF was detected in any sample, while a baseline level of IL-6 in the range of 8-29 IU ml−1 was measured.
Figure 2. LPS-induced IL-6 under the influence of TNF-bp.
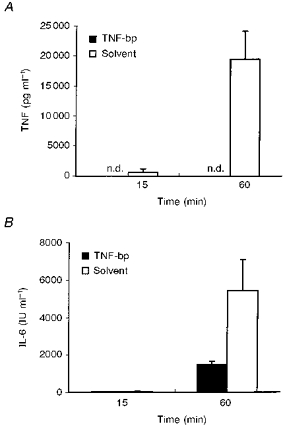
A, mean TNF values (+s.e.m.) in plasma of guinea-pigs collected 15 and 60 min after intra-arterial injections of 10 μg kg−1 LPS together with 1 mg per animal of TNF-bp or solvent. B, mean IL-6 values (+s.e.m.) in the same samples.
The influence of TNF-bp on LPS-induced fever is shown in Fig. 3. The febrile response of guinea-pigs to intra-arterial injections of 10 μg kg−1 LPS together with TNF-bp and solvent is shown in Fig. 3A. It is obvious that the second phase of the biphasic febrile response from 120 to 270 min after injection is significantly depressed by the treatment with TNF-bp (F= 30.65; P= 0.0001; ANOVA), while the first phase from 0 to 90 min after injection was not altered by this treatment (F= 3.36; P= 0.087, ANOVA). Figure 3B shows that TNF-bp alone had no significant influence on the abdominal temperature in guinea-pigs.
Figure 3. LPS-induced fever under the influence of TNF-bp.

A, febrile response of guinea-pigs to intra-arterial injections of 10 μg kg−1 LPS together with 1 mg per animal of TNF-bp (n= 8) or solvent (n= 9). B, abdominal temperature of guinea-pigs (n= 4 in each group) after intra-arterial injection of 1 mg per animal of TNF-bp or 0.2 ml solvent (0.9 % NaCl).
Individual circulating levels of bioactive TNF in response to intra-arterial injections of 50 μg kg−1 MDP together with 1 mg TNF-bp or solvent are shown in Fig. 4.
Figure 4. Neutralization of MDP-induced TNF by TNF-bp in vivo.
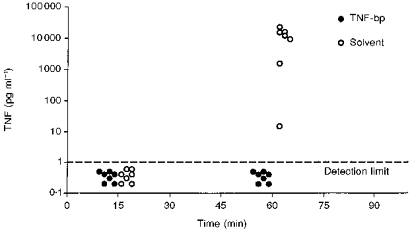
Individual data from TNF measurements in plasma samples collected 15 and 60 min after intra-arterial injections of 50 μg kg−1 MDP together with 1 mg per animal of TNF-bp or solvent (n= 7 in each group).
Fifteen minutes after MDP was injected into the arterial circulation, no bioactive TNF was detected in plasma of guinea-pigs treated with MDP and TNF-bp or with MDP and solvent. One hour after injection of MDP together with solvent, high circulating levels of TNF were measured in all animals, while no bioactive TNF could be detected in guinea-pigs treated with MDP and TNF-bp. The influence of TNF-bp on MDP-induced levels of circulating IL-6 is shown in Fig. 5.
Figure 5. MDP-induced IL-6 under the influence of TNF-bp.
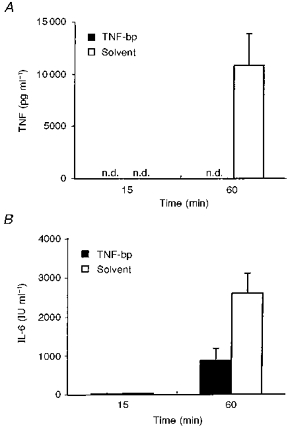
A, mean TNF values (+s.e.m.) in plasma of guinea-pigs collected 15 and 60 min after intra-arterial injections of 50 μg kg−1 MDP together with 1 mg per animal of TNF-bp or solvent. B, mean IL-6 values (+s.e.m.) in the same samples.
Figure 5A shows the mean values of the individual TNF data from Fig. 4. MDP-induced circulating TNF was completely neutralized by TNF-bp 1 h after injection of MDP, the time when MDP-induced circulating TNF reaches its peak in guinea-pigs (Roth et al. 1997). At this time, the level of TNF in plasma of animals injected with MDP and solvent was 10 862 ± 3029 pg ml−1. The mean amounts of bioactive IL-6 in the same plasma samples are shown in Fig. 5B. The level of IL-6 in plasma was 31 ± 5 IU ml−1 in animals injected with MDP and solvent versus 24 ± 6 IU ml−1 in guinea-pigs injected with MDP and TNF-bp, 15 min after injection. One hour after injection, IL-6 in plasma rose to 873 ± 312 IU ml−1 in guinea-pigs injected with MDP and TNF-bp and to a significantly higher value of 2613 ± 507 IU ml−1 in animals injected with MDP and solvent (t= 3.486; P= 0.0051). The influence of TNF-bp on MDP-induced fever is shown in Fig. 6.
Figure 6. MDP-induced fever under the influence of TNF-bp.
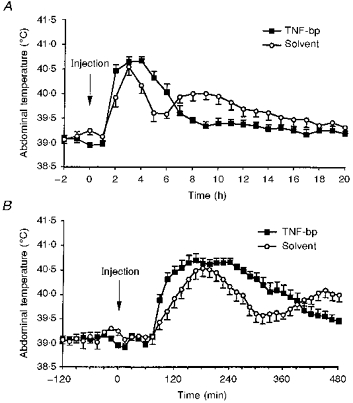
A, complete febrile response of guinea-pigs to intra-arterial injections of 50 μg kg−1 MDP together with 1 mg per animal of TNF-bp (n= 7 in each group). B, detailed graph of the first phase of MDP-induced fevers shown in A.
The complete febrile response of guinea-pigs to intra-arterial injections of 50 μg kg−1 MDP together with TNF-bp or solvent is shown in Fig. 6A. The first phase of the febrile response to MDP lasting 6-7 h was enhanced by treatment with TNF-bp. This phase of fever is documented in detail in Fig. 6B. From 90 to 360 min after injection of MDP, abdominal temperature of guinea-pigs treated with TNF-bp was significantly higher than the corresponding values of control animals (F= 5.32; P= 0.0438; ANOVA) due to a faster rate of rise and a slower fall in body temperature. The second phase from 420 to 1020 min after injection was, however, depressed in guinea-pigs treated with TNF-bp (F= 6.46; P= 0.0292; ANOVA).
Taken together, the present results indicate that a complete neutralization of LPS- or MDP-induced circulating TNF results in a significant attenuation of endogenous formation of IL-6 and an attenuation of the second phase of fever in guinea-pigs.
DISCUSSION
Within the last few years it has been demonstrated that TNF-soluble receptors circulate during experimental-induced and clinical inflammation and can protect against excessive levels of TNF (Van Zee, Kohno, Fischer, Rock, Moldawer & Lowry, 1992). The soluble TNF receptor type 1, corresponding to TNF-bp used in our study, is even present in the blood of septic patients in the absence of circulating TNF (Rogy et al. 1994). Studies on the role of endogenous TNF in the febrile response to bacterial pyrogens (see Introduction) and other inflammatory stimuli (Cooper et al. 1994; Leon et al. 1997) indicate distinct reactions depending on the investigated species of experimental animal, and on the exogenous stimulus for the induction of the acute-phase response.
In contrast to a number of studies in rats and mice (Long et al. 1990; Klir et al. 1995; Kozak et al. 1995; Takahashi, Brouckaert & Fiers, 1995), our previous experiments support the view that TNF is a pyrogenic substance in guinea-pigs. This view is based on the following observations. (1) After injection of the bacterial pyrogens LPS (Roth et al. 1993; Jansky et al. 1995) or MDP (Roth et al. 1997), bioactive TNF is detectable in the circulation of guinea-pigs briefly after pyrogen administration. (2) There was a good correlation between circulating TNF and the extent of fever (Roth et al. 1994a). (3) Fever of a similar time course as LPS-induced fever followed intra-arterial infusions of TNF in doses comparable to the amounts released during the febrile response to LPS (Roth et al. 1994b). (4) Blockade of endogenous formation of TNF by pretreatment with pentoxifylline resulted in an attenuation of the second phase of LPS fever (Goldbach et al. 1997a). The problem with the use of pentoxifylline as an anti-TNF tool in fever studies is that this drug per se can influence body core temperature (Goldbach et al. 1997a; LeMay, Vander & Kluger, 1990), and does not depress production of TNF exclusively.
In order to obtain better information on the role of LPS-induced and MDP-induced formation of circulating TNF in our experimental animal model, we injected TNF-bp together with either bacterial pyrogens directly into the arterial circulation of guinea-pigs, controlled the neutralization of TNF by TNF-bp and documented the effects on fever and circulating IL-6. Although the pattern of release of TNF and IL-6 are rather similar after injection of LPS or MDP in guinea-pigs (Roth et al. 1993, 1997), time course, duration and shape of the fever curves are different in response to each of both bacterial pyrogens in guinea-pigs. In addition, endogenous TNF seems to play distinct roles in LPS- and MDP-induced fever in guinea-pigs (see Figs 3 and 6). The second phase of the febrile response to LPS and MDP is significantly attenuated by treatment with TNF-bp. This observation corresponds to the reduced formation of IL-6 in response to both pyrogens in guinea-pigs injected with TNF-bp. However, even in guinea-pigs treated with TNF-bp and showing no detectable levels of TNF, there is still an increase in circulating IL-6 and development of fever. This means that TNF is not the only inducer of IL-6 after injection of LPS or MDP in guinea-pigs. The most likely candidate as a second inducer of IL-6 (Luheshi, Miller, Brouwer, Dascombe, Rothwell & Hopkins, 1996), and the most important endogenous mediator of fever (Dinarello, 1994), is IL-1β. Administration of an IL-1 receptor antagonist blocks a significant component of LPS-induced formation of IL-6 and attenuates LPS-induced fever in rats (Luheshi et al. 1996). The effects of IL-1 appear to be mediated by local production of this cytokine because IL-1 does not spill over into the circulation in large amounts after LPS injection, and therefore it frequently escapes detection (see Kluger, 1991, for review).
The first phase of LPS-induced fever is unimpaired by neutralization of TNF and seems thus to develop independently of the presence or absence of circulating cytokines (at least TNF and IL-6). Currently, there is an ongoing debate whether the appearance of cytokines in the blood is really necessary to induce fever, or rather an activation of neuronal pathways by bacterial pyrogens themselves or locally produced cytokines account for the generation of a febrile response (Dantzer, 1994; Watkins, Maier & Goehler, 1995; Sehic & Blatteis, 1996; Blatteis & Sehic, 1997; Goldbach, Roth & Zeisberger, 1997b; Rothwell, 1997). In this context it has been suggested that the quick onset of LPS-induced fever after intravenous injection of this pyrogen may be triggered by a very rapidly evoked neuroactive substance, the signal of which may be transmitted to the brain via peripheral sensory nerves (Blatteis & Sehic, 1997). Afferents of the vagus nerve especially seem to be part of the rapid communication pathway for pyrogenic signals between the periphery and the brain, because subdiaphragmatic vagotomy blocks LPS-fever and abrogates the concomitant rise of hypothalamic levels of prostaglandin E2 in guinea-pigs, which is regarded as the final neuronal mediator of the febrile response to pyrogens (Sehic & Blatteis, 1996).
According to the results presented in this paper, circulating cytokines participate at least in the maintenance of the second phase of fever induced by LPS or MDP in guinea-pigs, since a complete neutralization of TNF and an accompanied reduction of IL-6 result in a suppression of the late phase of both febrile responses. Interestingly, the first 6 h of MDP-induced fever are significantly enhanced by a complete neutralization of TNF. A similar observation was made in transgenic mice lacking both TNF receptors. These mice showed an exacerbated ‘high-dose LPS fever’ during its first phase, while the second phase was unimpaired and no modulation of ‘low-dose LPS fever’ and turpentine-induced fever were observed (Leon et al. 1997). Thus our results add conflicting data on the role of TNF in fever (see Kluger, 1991, for review). A pro-pyretic or, under certain circumstances, an anti-pyretic role of TNF in fevers induced by a variety of pyrogenic or inflammatory agents may depend on the stimulus (Long et al. 1990; Cooper et al. 1994; Leon et al. 1997), the dose of the stimulus (Leon et al. 1997) and the investigated species of experimental animal. In addition, the use of species-specific TNF may evoke responses different from those seen after administration of human-recombinant TNF due to distinct receptor activation (Stefferl, Hopkins, Rothwell & Luheshi, 1996).
For our experiments in guinea-pigs it can be stated that TNF plays a role in the induction of substances responsible for maintenance of the second phase of fever, while this cytokine has no influence on the first phase of LPS-induced fever and seems to be responsible for a limitation of the first phase of MDP-induced fever.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft and Amgen Inc. (Boulder, CO, USA). We thank Dr Stephen Hopkins (University of Manchester, UK) for providing us with the WEHI 164 subclone 13 and the B9 cell lines and informing us about important details of the cytokine assays. We also thank Dr Robert L. Snipes for linguistic revision of the manuscript.
References
- Aarden LA, DeGroot ER, Schaap OL, Landsdorp PM. Production of hybridoma growth factors by human monocytes. European Journal of Immunology. 1987;17:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Sehic E. Fever: How may circulating pyrogens signal the brain. News in Physiological Sciences. 1997;12:1–9. [Google Scholar]
- Cooper AL, Brouwner S, Turnbull AV, Luheshi GN, Hopkins SJ, Kunkel SL, Rothwell NJ. Tumor necrosis factor-α and fever after peripheral inflammation in the rat. American Journal of Physiology. 1994;267:R1431–1436. doi: 10.1152/ajpregu.1994.267.6.R1431. [DOI] [PubMed] [Google Scholar]
- Dantzer R. How do cytokines say hello to the brain? Neural versus humoral mediation. European Cytokine Network. 1994;5:271–273. [PubMed] [Google Scholar]
- Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB Journal. 1994;8:1314–1325. [PubMed] [Google Scholar]
- Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, Figari JS, Palladino MA, O'Connor JV. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. Journal of Experimental Medicine. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espevic T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. Journal of Immunological Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Evans TJ, Moyes D, Carpenter A, Martin R, Loetscher H, Lesslauer W, Cohen J. Protective effect of 55- but not 75 kD soluble tumor necrosis factor receptor-immunoglobulin G fusion proteins in an animal model of gram-negative sepsis. Journal of Experimental Medicine. 1994;180:2173–2179. doi: 10.1084/jem.180.6.2173. 10.1084/jem.180.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach JM, Roth J, Störr B, Zeisberger E. Influence of pentoxifylline on fevers induced by bacterial lipopolysaccharide and tumor necrosis factor-α in guinea-pigs. European Journal of Pharmacology. 1997a;319:273–278. doi: 10.1016/s0014-2999(96)00845-x. 10.1016/S0014-2999(96)00845-X. [DOI] [PubMed] [Google Scholar]
- Goldbach JM, Roth J, Zeisberger E. Fever suppression by subdiaphragmatic vagotomy in guinea-pigs depends on the route of pyrogen administration. American Journal of Physiology. 1997b;272:R675–681. doi: 10.1152/ajpregu.1997.272.2.R675. [DOI] [PubMed] [Google Scholar]
- Holt I, Cooper RG, Hopkins SJ. Relationship between local inflammation, interleukin-6 concentration and the acute phase response in arthritic patients. European Journal of Clinical Investigation. 1991;21:479–484. doi: 10.1111/j.1365-2362.1991.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Jansky L, Vybiral S, Pospisilova D, Roth J, Dornand J, Zeisberger E, Kaminkova J. Production of systemic and hypothalamic cytokines during the early phase of endotoxin fever. Neuroendocrinology. 1995;62:55–61. doi: 10.1159/000126988. [DOI] [PubMed] [Google Scholar]
- Kapas L, Hong L, Cady AB, Opp MR, Postlethwaite AE, Seyer J, Krueger JM. Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factor-α and TNF-α fragments. American Journal of Physiology. 1992;263:R708–715. doi: 10.1152/ajpregu.1992.263.3.R708. [DOI] [PubMed] [Google Scholar]
- Klir JJ, McClellan JL, Kozak W, Szelenyi Z, Wong GHW, Kluger MJ. Systemic but not central administration of tumor necrosis factor-α attenuates LPS-induced fever in rats. American Journal of Physiology. 1995;268:R480–486. doi: 10.1152/ajpregu.1995.268.2.R480. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiological Reviews. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak W, Conn CA, Klir JJ, Wong GHC, Kluger MJ. TNF soluble receptor and antiserum against TNF enhance lipopolysaccharide fever in mice. American Journal of Physiology. 1995;269:R23–29. doi: 10.1152/ajpregu.1995.269.1.R23. [DOI] [PubMed] [Google Scholar]
- LeMay LG, Vander AJ, Kluger MJ. The effects of pentoxifylline on lipopolysaccharide (LPS) fever, plasma interleukin 6 (IL 6), and tumor necrosis factor (TNF) in the rat. Cytokine. 1990;2:300–306. doi: 10.1016/1043-4666(90)90032-o. [DOI] [PubMed] [Google Scholar]
- Leon LR, Kozak W, Peschon J, Kluger MJ. Exacerbated febrile responses to LPS, but not turpentine, in TNF double receptor knockout mice. American Journal of Physiology. 1997;272:R563–569. doi: 10.1152/ajpregu.1997.272.2.R563. [DOI] [PubMed] [Google Scholar]
- Long NJ, Kunkel SL, Vander AJ, Kluger MJ. Antiserum against tumor necrosis factor enhances lipopoly-saccharide fever in rats. American Journal of Physiology. 1990;258:R332–337. doi: 10.1152/ajpregu.1990.258.2.R332. [DOI] [PubMed] [Google Scholar]
- Luheshi G, Miller AJ, Brouwer S, Dascombe MJ, Rothwell NJ, Hopkins SJ. Interleukin-1 receptor antagonist inhibits endotoxin fever and systemic interleukin-6 induction in rats. American Journal of Physiology. 1996;270:E91–95. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- Martin D, Near SL, Bendele A, Russell DA. Inhibition of tumor necrosis factor is protective against neurologic dysfunction after active immunization of Lewis rats with myelin basic protein. Experimental Neurology. 1995;131:221–228. doi: 10.1016/0014-4886(95)90044-6. 10.1016/0014-4886(95)90044-6. [DOI] [PubMed] [Google Scholar]
- Michie HR, Spriggs DR, Kanogue KR, Sherman ML, Revhaug A, O'Dwyer S, Arthur K, Dinarello CA, Cerami A, Wolff SM, Kufed DW, Wilmore W. Tumor necrosis factor and endotoxin induce similar metabolic responses in human beings. Surgery. 1988;104:280–286. [PubMed] [Google Scholar]
- Rogy MA, Coyle SM, Oldenburg HSA, Rock CS, Barie PS, van Zee KJ, Smith CG, Moldawer LL, Lowry SF. Persistently elevated soluble tumor necrosis factor receptor and interleukin-1 receptor antagonist levels in critically ill patients. Journal of the American College of Surgery. 1994;178:132–138. [PubMed] [Google Scholar]
- Roth J, Conn CA, Kluger MJ, Zeisberger E. Kinetics of systemic and intrahypothalamic IL-6 and tumor necrosis factor during endotoxin fever in guinea-pigs. American Journal of Physiology. 1993;265:R653–658. doi: 10.1152/ajpregu.1993.265.3.R653. [DOI] [PubMed] [Google Scholar]
- Roth J, Hopkins SJ, Hoadley ME, Tripp A, Aslan T, Störr B, Luheshi GN, Zeisberger E. Fever and production of cytokines in response to repeated injections of muramyl-dipeptide in guinea-pigs. Pflügers Archiv. 1997;434:525–533. doi: 10.1007/s004240050432. [DOI] [PubMed] [Google Scholar]
- Roth J, McClellan JL, Kluger MJ, Zeisberger E. Attenuation of fever and release of cytokines after repeated injections of lipopolysaccharide in guinea-pigs. Journal of Physiology. 1994a;477:177–185. doi: 10.1113/jphysiol.1994.sp020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J, McClellan JL, Kluger MJ, Zeisberger E. Changes in body temperature and circulating levels of interleukin-6 after intra-arterial injections or infusions of tumor necrosis factor α in guinea-pigs. Experientia. 1994b;50:815–820. doi: 10.1007/BF01956462. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. Neuroimmune interactions: the role of cytokines. British Journal of Pharmacology. 1997;121:841–847. doi: 10.1038/sj.bjp.0701248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehic E, Blatteis CM. Blockade of lipopolysaccharide-induced fever by subdiaphragmatic vagotomy in guinea-pigs. Brain Research. 1996;726:160–166. [PubMed] [Google Scholar]
- Stefferl A, Hopkins SJ, Rothwell NJ, Luheshi GN. The role of TNF-α in fever: opposing actions of human and murine TNF-α and interactions with IL-1β in the rat. British Journal of Pharmacology. 1996;118:1919–1924. doi: 10.1111/j.1476-5381.1996.tb15625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Brouckaert R, Fiers W. Mechanism of tolerance to tumor necrosis factor: receptor specific pathway and selectivity. American Journal of Physiology. 1995;269:R398–405. doi: 10.1152/ajpregu.1995.269.2.R398. [DOI] [PubMed] [Google Scholar]
- Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor αin vitro and in vivo. Proceedings of the National Academy of Sciences of the USA. 1992;88:10535–10539. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review and analysis of alternative mechanisms. Life Sciences. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]


