Abstract
The role of synaptic inhibition in respiratory rhythm generation was analysed by microinjections of GABAA and glycine receptor antagonists into the bilateral pre-Bötzinger complex (PBC) of anaesthetized cats. Central respiratory activity was monitored by phrenic nerve recordings.
Bilateral injections of bicuculline (50 or 100 μm) irreversibly slowed respiratory frequency and induced apneustic patterns.
Bilateral injections of strychnine (50 or 100 μm) greatly reduced phrenic burst amplitudes leading to increased burst frequency or irreversibly blocked rhythmic phrenic discharges. After unilateral tetrodotoxin (TTX) blockade in the PBC, strychnine injection into the contralateral PBC blocked rhythmic phrenic discharges.
Bilateral blockade of both GABAergic and glycinergic inhibition abolished rhythmic burst discharges and only tonic phrenic activity remained. Such tonic activity was blocked only by TTX (1 μm).
Potentiation of synaptic inhibition by the serotonin 1A receptor agonist 8-hydroxydipropylaminotetralin (8-OH-DPAT; 50 μm) restored rhythmic activity only when given shortly after strychnine and bicuculline applications. It was, however, ineffective after blockade of synaptic inhibition was complete.
The study demonstrates the significance of synaptic inhibition in the process of respiratory generation in the adult cat in vivo.
Respiratory activity generated within the brainstem of adult mammals consists of rhythmically oscillating burst discharges of antagonistically coupled neurons. In a specific type of respiratory neuron this is reflected by an alternation of excitatory synaptic drive mediated by glutamate and synaptic inhibition mediated by GABA and glycine (Schmid, Foutz & Denavit-Saubie, 1996). Synaptic inhibition results in reversible and irreversible switching off of phasic activities and shaping of neuronal discharge patterns (Richter, Camerer, Meesmann & Röhrig, 1979; Richter, 1982; Ballantyne & Richter, 1986; Remmers, Richter, Ballantyne, Bainton & Klein, 1986; Anders, Ballantyne, Bischoff, Lalley & Richter, 1991; Haji, Takeda & Remmers, 1992; Klages, Bellingham & Richter, 1993; Schmid et al. 1996).
Blockade of GABAergic and glycinergic mechanisms by systemic or local administration of receptor antagonists greatly disturbs rhythmic respiratory functions when studied under in vivo conditions (Hayashi & Lipsky, 1992) or under in vitro conditions in slice preparations that contain more rostral medullary and pontine structures (Paton, Ramirez & Richter, 1994; Paton & Richter, 1995). These findings, however, are not consistent with reports on experiments performed in en bloc brainstem-spinal cord (Feldman & Smith, 1989; Onimaru, Arata & Homma, 1990) or medullary slice preparations lacking the pons (Ramirez, Quellmalz & Richter, 1996) showing that synaptic inhibition is not essential for the generation and maintenance of the respiratory activity. Such diverse findings resulted in contradictory discussions about the principal mechanisms of rhythm generation. The suggestion was made that respiratory activity originates from pacemaker cells within the medullary respiratory network (Onimaru, Arata &, Homma, 1988, 1989; Feldman & Smith, 1989; Feldman et al. 1990; Smith, Ellenberger, Ballanyi, Richter & Feldman, 1991), while an opposing proposal was that synaptic inhibition is essential for rhythm generation because it conditions specific neuronal properties favouring burst discharges and organizes phase switching under in vivo conditions (Richter, 1982; Richter, Ballanyi & Schwarzacher, 1992; Ogilvie, Gottschalk, Anders, Richter & Pack, 1992; Richter, Champagnat, Jacquin & Benacka, 1993; Ramirez & Richter, 1996; Rybak, Paton & Schwaber, 1997).
Such discrepancies between experimental data concerning the role of synaptic inhibition in the respiratory network led us to perform in vivo experiments in which synaptic inhibitory mechanisms within the pre-Bötzinger complex (PBC) were pharmacologically modified in the intact anaesthetized cat. The PBC has been recently described as the core region involved in primary rhythm generation under in vitro conditions (Smith et al. 1991) which has been confirmed for the in vivo rat and cat (Connelly, Dobbins & Feldman, 1992; Schwarzacher, Smith & Richter, 1995; Koshia & Guyenet, 1996; Ramirez, Schwarzacher, Pierrefiche, Olivera & Richter, 1998).
We found that respiratory rhythmicity was greatly disturbed if not completely abolished when synaptic inhibition mediated through GABAergic and glycinergic synapses was blocked.
METHODS
Surgical procedures and phrenic nerve recording
Experiments were performed on thirteen adult cats of either sex. Animals were anaesthetized with sodium pentobarbitone (Nembutal, Sanofi, CEVA, Garbsen, Germany) at an initial dose of 40 mg kg−1, i.p. Supplementary anaesthetic doses were given i.v. (1.3-2.5 mg kg−1) whenever spontaneous increases in heart rate or arterial blood pressure (above 130 mmHg) occurred or if phrenic activity increased in frequency. Additional anaesthetic was also administered in case of increases in central respiratory activity or in arterial blood pressure when a slight nociceptive stimulus was applied to the paw. Atropine sulphate (B. Braun AG, Melsungen, Germany; 0.1-0.2 mg kg−1, i.v.) and dexamethasone (Fortecortin Mono, Merck, Darmstadt, Germany; 0.2 mg kg−1i.m.) were administered to block mucus secretion and to prevent brain oedema, respectively. Catheters were inserted into one femoral artery for monitoring arterial blood pressure and into both femoral veins for drug administration. If necessary, arterial blood pressure was maintained above 100 mmHg by i.v. infusion of a Ringer solution containing adrenaline (Suprarenin, Hoechst AG, Frankfurt, Germany, 40 μg ml−1) and glucose (27 mg ml−1). Body temperature was maintained between 36 and 38°C by means of external heating. Artificial ventilation was performed with a positive pressure pump using oxygen-enriched air (40-50 % O2) connected to a cannula inserted into the trachea caudal to the larynx. Inspiratory and end-expiratory pressures were controlled by continuous tracheal pressure monitoring. Animals were paralysed by gallamine triethiodide (Flaxedil, RhTMne-Poulenc Rorer, Paris; initial dose 10 mg kg−1i.v., followed by 5 mg kg−1 h−1). A pneumothorax was performed bilaterally to reduce respiratory-related movements of the thorax and to increase stability of the brainstem. Atelectasis of the lungs was prevented by applying positive pressure of 1-2 cmH2O to the expiratory flow resistance. End-tidal CO2 was monitored (DATEX normocap, Hoyer AG, Bremen, Germany) and maintained at 30-40 Torr by adjusting the ventilatory rate. Asphyxia tests were performed by arresting artificial ventilation for variable time.
Both phrenic nerves were prepared by a dorsal approach and both vagal nerves were severed. The head of the animal was fixed in a ventroflexed position and an occipital craniotomy exposed the dorsal surface of the brainstem. The dura was opened and the caudal part of the cerebellum was carefully dissected free from connective tissue under microscopic observation. The cerebellum was then slightly lifted and pushed rostrally with a loop of lead wire in order to give access to the region of the pre-Bötzinger complex (PBC) on both sides. The arachnoidal membrane was removed over the medulla and the pial membrane was opened at the location of micropipette insertion. Phrenic nerves were cut peripherally, desheathed and placed on bipolar silver hook electrodes. Their discharges were amplified (10000 times), filtered (band pass, 0.1-3 kHz), and displayed on an oscilloscope and a chart recorder in original and integrated forms (time constant τ= 10-100 ms). Unitary respiratory activity was recorded in current clamp with fine-tipped glass microlectrodes filled with 3 M NaCl to localize the pool of rostral expiratory neurons within the Bötzinger complex and the region directly caudal to it containing a heterogeneous population of respiratory neurons (for details see Schwarzacher et al. 1995).
Phrenic activity was stored throughout the course of an experiment by an Apple Macintosh computer with the use of MacLab software (AD Instruments Ltd, Hastings, UK).
Micropressure injections
Micropipettes for microinjections were made of borosilicate glass (tip diameter, 10-25 μm) and were filled with Ringer solution with no additions (control) or containing 50-100 μm bicuculline, the fluorescent dye Bodipy-Fl-strychnine (50-100 μm, Molecular Probes), 50-100 μm strychnine, 1 μm tetrodotoxin (TTX) or 50 μm 8-hydroxydipropylaminotetralin (8-OH-DPAT, a serotonin 1A receptor agonist (5-HT1A). The test substances were injected with the use of a Picospritzer (NPI, Tamm, Germany) connected to compressed air (1-3 × 105 Pa). The injected volumes (10-20 nl) were calculated by measuring the displacement of the meniscus of the micropipette solutions with a microscope fitted with an ocular micrometer (accuracy, ±0.1 mm). For each injection, the position of the electrode was measured in relation to the obex and to the dorsal surface of the brainstem. Injections of test substances which elicited a change in the arterial blood pressure were discarded in this study. Control injections using Ringer solution had no effect on phrenic activity. Changes in phrenic activity were determined by comparing twenty control cycles with twenty cycles during steady state of drug effects. All changes described as significant had a P φ; 0.0001.
Histology
The rostral medulla was explored at co-ordinates of 2.6-4.2 mm rostral to the obex, 3.0-4.5 mm lateral to mid-line and 3.5-5.5 mm ventral to the dorsal surface for sites that were reactive to injection of small amounts (10 nl) of neuromodulators or blockers of ligand-controlled inhibitory receptors. These sites included the PBC (Schwarzacher et al. 1995), which is thought to be the region of primary respiratory rhythm generation (Smith et al. 1991). A single Bodipy-strychnine injection was made bilaterally into the sites that proved to be most reactive. This induced a similar change of phrenic activity to that seen with injections of unlabelled strychnine.
At the end of experiments animals were killed during deep anaesthesia (100 mg kg−1 pentobarbitone, i.v.) with an intravenous injection of a 3 M KCl solution. After a 3 min in situ prefixation with 4 % paraformaldehyde, the brainstem was removed and transferred into a fixative of 4 % paraformaldehyde in phosphate buffer for 12-24 h and then transferred into a 30 % sucrose solution. The fixed brainstem was mounted in the Horsely-Clarke coronal plane and cut into serial 100 μm sections with a frigocut (Reichert-Jung, Nußloch, Germany). Sections were embedded in Histogel without counterstaining, examined from the obex level to the level of the facial nucleus with a fluorescence microscope, and anatomically reconstructed with a camera-lucida system.
Localization of injection sites
Based on the distribution of respiratory neuronal activity and anatomical reconstructions, the precise localization of the pre-Bötzinger complex (PBC) of the cat has been described in a previous paper (Schwarzacher et al. 1995). It is a region of the ventrolateral reticular formation located directly caudal to the retrofacial nucleus, which in the cat is 3.0-3.5 mm rostral to the obex. In the transverse plane the PBC coexists with the rostral compact and semicompact part of the nucleus ambiguus with precise distances to characteristic cytoarchitectural landmarks, such as the lateral reticular nucleus and the borders of the subnuclei of the inferior olive, and extends ventrally from the nucleus ambiguus until approximately 0.9 mm dorsal to the ventral surface of the medulla. In the present experiments, the localization of injection sites relative to the position of the PBC was also defined on the basis of the distances to cytoarchitectural landmarks of the obex, the caudal end of the facial nucleus, the lateral reticular nucleus, the borders of the subnuclei of the inferior olive, spinal trigeminal nucleus and the configuration of the ambigual complex, which were measured and normalized for an averaged sized medulla (Fig. 1).
Figure 1. Injection sites.
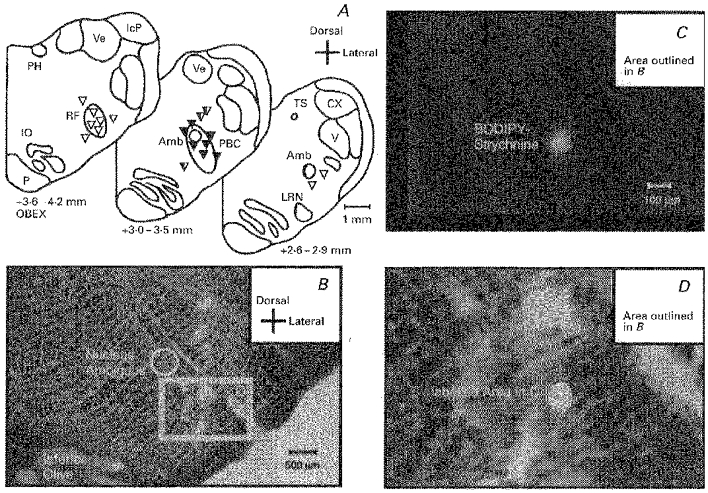
A, the injection sites as shown at levels 2.6-2.9, 3.0-3.5 and 3.6-4.2 mm rostral to the obex. The edged area corresponds with the localization of the pre-Bötzinger complex (PBC) as defined by Schwarzacher et al. (1995). Black triangles, effective injection sites; black and white trangles, injection sites with delayed effects; white triangles, ineffective injection sites. B, overview of the ventrolateral medulla with the visible electrode track used for injection. The area labelled D is shown at higher magnification in D to indicate better the deposition of Bodipy-strychnine (circular white spot) at the area of maximal response as verified in fluorescence microscopy in C. Amb, ambigual nucleus; CX, external cuneate nucleus; IcP, inferior peduncle of the cerebellum; IO, inferior olive; LRN, lateral reticular nucleus; P, pyramidal tract; PH, nucleus prepositus hypoglossi; RF, retrofacial nucleus; TS, solitary tract; V, spinal trigeminal nucleus; Ve, vestibular nuclei; X, dorsal nucleus of the vagal nerve; XII, hypoglossal nucleus.
RESULTS
Histology
We searched with microelectrodes for single neuronal discharges in the rostral medulla at co-ordinates of 3.5-4.5 mm rostral to the obex and 3.5-4.0 mm lateral to mid-line to determine the caudal border of the rostral group of expiratory neurons within the Bötzinger complex. Slightly caudal to this border, we tested various positions for their reactivity to injections of small amounts (10 nl) of 8-OH-DPAT (50 μm), a 5-HT1A receptor agonist, or strychnine (50 μm), a blocker of glycine-controlled inhibitory receptors. Once such a reactive site was localized, we injected 10 nl Bodipy-Fl-strychnine (50-100 μm), which induced a similar response of phrenic activity as seen after injections of unlabelled strychnine (see below).
Histological examination of the ventrolateral region of medullary brainstem tissue labelled with Bodipy injections revealed circular fluorescent spots with diameters of 100-500 μm (Fig. 1).
Based on histological verification (n= 19 bilateral single injections in 10 animals), the injection sites were separated into three groups. (i) Bodipy labelled regions proved that effective injection sites with an accuracy of ± 200 μm were localized within the PBC, i.e. directly around and ventrolateral to the nucleus ambiguus (NA) at a rostro-caudal position of 3.0-3.5 mm (n= 7). (ii) Some positions (n= 3) were found more distant from the PBC, i.e. more than 600 μm from the centre of the PBC (see Schwarzacher et al. 1995). Injection into such neighbouring regions corresponded with delayed functional effects which became obvious after a latency of 20-30 min, indicating that diffusion of drugs to the PBC was necessary. (iii) Injections (n= 9) at sites that were clearly outside the PBC were ineffective in changing the respiratory rhythm even after additional doses of blocking agents were applied. The sites of injections are shown in Fig. 1.
Effects of strychnine injections
Micropressure injections of 10-20 nl strychnine (50 or 100 μm) into the unilateral PBC depressed burst amplitudes of phrenic discharges to 68 ± 16.7 % and reduced inspiratory burst duration to 71.5 ± 14.0 % (from 1.37 to 0.98 s), while the frequency of bursts increased to 174 ± 43.3 % (from 18.8 to 33.5 cycles min−1; n= 6 in 6 animals).
The effects were more pronounced when strychnine was bilaterally injected into the PBC. Amplitudes were reduced to 55 ± 12.1 %, burst durations were reduced to 52 ± 21.3 % (from 1.47 to 0.67 s) and frequencies were increased to 217 ± 75.2 % (from 19.3 to 38.3 cycles min−1; n= 3/6). In the three other preparations, bilateral strychnine injections were sufficient to abolish completely and irreversibly all rhythmic burst activities in phrenic nerves (Fig. 2Aa and Ba). In such cases, there remained weak tonic dischargeswith irregular fluctuations in amplitude that did not summate to a clear burst-like phrenic discharge. Tonic discharges were finally abolished by injection of TTX (1 μm) into the same area (not illustrated).
Figure 2. Two typical effects of bilateral injections of strychnine into the pre-Bötzinger complex.
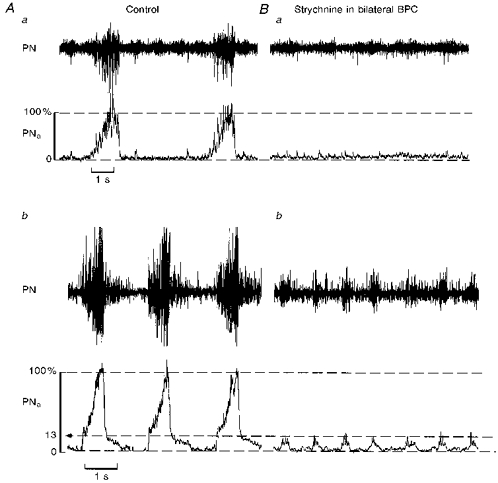
Aa and Ba, strychnine (100 μm) injections into bilateral PBCs totally abolished rhythmic phrenic activity. Note that under control there were fluctuations in tonic PN discharges that remained after strychnine injection. Ab and Bb, in a different experiment, injection of strychnine (100 μm) greatly reduced the amplitude of phrenic burst activity to approximately 13 % of control. This reduction of burst amplitudes was followed by a significant increase in burst frequency clearly indicating a direct effect on the central mechanisms responsible for rhythm generation. PN, raw phrenic activity; PNa, integrated phrenic activity.
A similar effect was observed in two additional experiments (n= 2/8) in which we first blocked synaptic interaction within the PBC on one side with microinjections of TTX and then injected 50 or 100 μm strychnine into the PBC of the contralateral side (Fig. 3A-C). Blockade of one PBC induced a significant decrease in phrenic burst amplitude (to 51.5 ± 1.5 %) and an increase in burst frequency to 148 ± 6.0 % (from 16 to 23.5 cycles min−1; Fig. 3B). Subsequent strychnine injections into the PBC on the contralateral side produced disappearance of rhythmic phrenic discharge. The complete blockade of rhythmic phrenic activity was irreversible even during prolonged asphyxia (n= 4/8), which increased tonic phrenic activity but never restored rhythmic burst discharges (Fig. 3D).
Figure 3. Ipsilateral injection of strychnine into PBC blocks rhythmic phrenic activity after prior TTX blockade of the contralateral PBC.
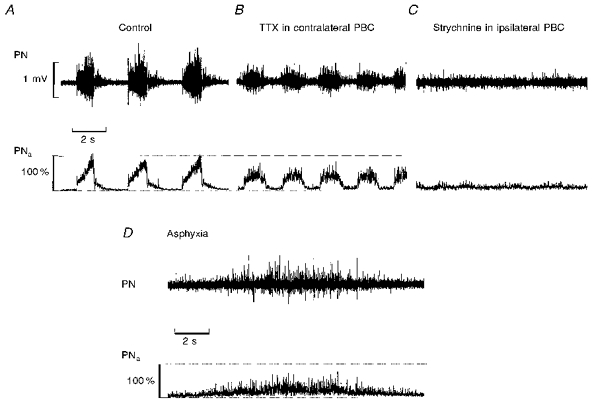
A, phrenic activity (PN) and its moving average (PNa) as recorded under control conditions. B, injection of TTX (1 μm) into the PBC of the contralateral side of phrenic nerve recording reduced the amplitudes and increased inspiratory burst frequencies. C, subsequent strychnine injection (100 μm) into the ipsilateral PBC blocked the remaining respiratory activity. D, phrenic activity did not resume rhythmic bursting although prolonged asphyxia tests induced activation of the network as seen in the tonic output activity in phrenic motoneurons.
Effects of bicuculline injections
Uni- and bilateral injections of bicuculline (50 or 100 μm) normally evoked a pronounced slowing of central respiratory frequency (n= 5/5). Inspiratory burst discharges were significantly shortened to 60.6 ± 17.2 % (from 1.05 to 0.63 s) and occurred at low frequencies of 39.4 ± 19.0 % of control (from 21.6 to 8.9 cycles min−1). During the long interburst intervals phrenic motoneurons discharged tonically. Inspiratory bursting was terminated by transient suppression of tonic phrenic discharges (Fig. 4B). All steady state effects of bicuculline were irreversible.
Figure 4. Blockade of GABAergic and glycinergic synaptic inhibition within PBC blocks rhythmic respiratory output activity.
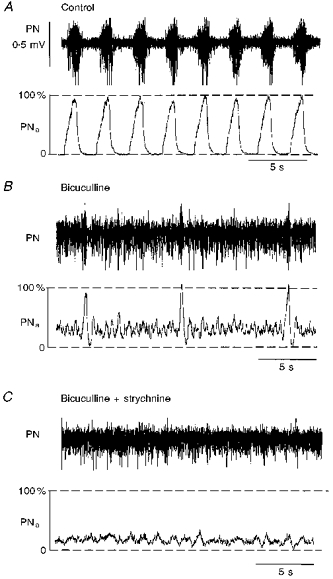
A, control activity of phrenic nerve (PN) with its moving average (PNa). B, bilateral injection of bicuculline (100 μm) into PBC induced steady tonic phrenic output activity that was interrupted by regular short-lasting bursts of phrenic activity. Each burst was terminated by an inhibitory process (presumably glycinergic) as seen by a transient complete suppression of tonic phrenic activity. C, subsequent bilateral injections of strychnine (100 μm) abolished phasic burst activities in phrenic nerve leading to tonic discharges of low amplitude.
Cumulative effects of strychnine and bicuculline injections
Bilateral PBC injections of 100 μm bicuculline followed by 100 μm strychnine injections, or bilateral injections of 100 μm strychnine followed by 100 μm bicuculline irreversibly blocked all rhythmic burst discharges in phrenic nerves (n= 5/6; Fig. 4C, 5Ad, and Fig. 6A and B). Only tonic discharges remained, which sometimes varied in amplitude, but never generated clear burst activity. Such tonic discharges were blocked by TTX injections (1 μm) into the same regions (not illustrated). In two experiments (n= 2/7), rhythmic respiratory activity persisted after bilateral strychnine (100 μm) and bicuculline (100 μm) injections, but the amplitudes of rhythmic activity were greatly reduced to 9.2 or 10.3 % of control. Histological verification of the injection sites of the latter two experiments revealed that injections were into the PBC in one case and more than 500 μm outside the PBC in the other case.
Figure 5. Activation of 5-HT1A receptors does not restore rhythmic phrenic activity after complete blockade of synaptic inhibition.
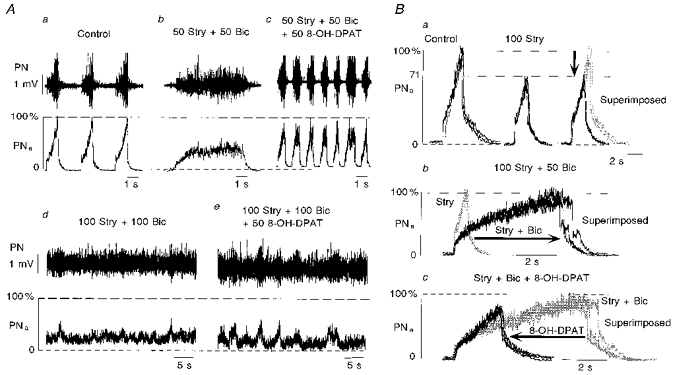
Aa-e and Ba-c illustrate sequences of drug applications in two different experiments. Aa, control recordings before injection of drugs. Ab, successive application of strychnine (Stry, 50 μm) and bicuculline (Bic, 50 μm) induced a regular apneustic breathing pattern characterized by a prolongation of the inspiratory burst duration. Ac, following development of such apneustic activity pattern, an injection of 8-OH-DPAT (50 μm; a 5-HT1A agonist) restored rhythmic phrenic activity, its bursting occurring at higher frequencies. This effect was only transient and vanished after 1-2 min. Ad, additional injections of strychnine (100 μm) and bicuculline (100 μm) completely abolished rhythmic bursting of phrenic activity. Ae, subsequent injections of 8-OH-DPAT (50 μm) led to increased fluctuations of tonic phrenic activity, which for short periods, but not persistently, could appear rhythmic. Ba, ipsilateral strychnine injection (100 μm) reduced the amplitude and the duration of phrenic burst activity without changing the early inspiratory burst pattern as can be seen in the superimposed traces on the right. Bb, inspiratory burst duration was dramatically increased (arrow) and the slope of phrenic burst discharge slowed after additional injection of bicuculline (50 μm). This led to an apneustic activity pattern. Bicuculline also reduced the amplitude at onset of phrenic burst activity. Bc, within the following few minutes, subsequent activation of 5-HT1A receptors on PBC cells by 8-OH-DPAT (50 μm) reversed the apneustic activity pattern. Bursts again showed a steady augmentation of inspiratory activity and their durations were reduced to almost normal (arrow). When rhythmic phrenic activity was blocked after a longer latency, 8-OH-DPAT injections were ineffective (see Ae). PN, original phrenic activity; PNa, moving average of phrenic activity.
Figure 6. Proposal of the organization of inhibitory synaptic pathways to the respiratory network in PBC.
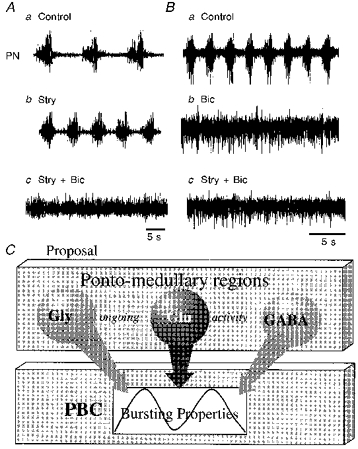
Aa-c, illustration of the abolition of rhythmic phrenic activity after bilateral sequential injections of strychnine (100 μm) and bicuculline (100 μm). Ba-c, rhythmic phrenic activity is abolished by the same drugs (same concentration used) also when applied in opposite sequences. C, schematic diagram illustrating our proposal for the organization of inhibitory pathways from ponto-medullary regions to the PBC. Much of this proposal is based on the experiments described by Lumsden (1923). For details see the Discussion.
In cases (n= 6/6) in which strychnine and bicuculline were injected in lower concentrations (bicuculline, 50 μm; and strychnine, 50 or 100 μm), the amplitude of inspiratory bursts was depressed to 64.7 ± 11.3 %, burst duration was greatly increased to 431.0 ± 236.6 % (from 1.0 to 4.0 s), while burst frequency was reduced to 52.3 ± 24.4 % (from 24.7 to 12.8 cycles min−1; Fig. 5Ab and Bb). This activity pattern resembled neuronal apneusis (Fig. 5Ab).
Restoration of the respiratory rhythm during incomplete block of inhibition
In order to compensate for blockade of synaptic inhibition by strychnine and/or bicuculline by potentiating the remaining inhibitory currents and by activating persistent potassium currents (Lalley, Bishoff & Richter, 1994a), we injected 8-OH-DPAT (50 μm), an agonist of 5-HT1A receptors, into the PBC (n= 6). Figure 5 illustrates the effects of 8-OH-DPAT injections. After injections of strychnine at 50 or 100 μm and bicuculline at 50 μm concentrations that induced apneustic activity patterns (Fig. 5Ab), activation of 5-HT1A receptors with 8-OH-DPAT led to shortening of the apneustic inspiratory bursts (Fig. 5Ac and 5Bc) and restored a regular inspiratory bursting for the next 1-2 min (n= 4/6; Fig. 5Ac). After diffusion of blockers for a few more minutes, however, rhythmic phrenic discharges vanished again. The same effects were seen after additional injections of strychnine and bicuculline (Fig. 5Ad). Under such conditions, rhythmic phrenic activity could no longer be recovered by successive 8-OH-DPAT injections (Fig. 5Ae).
DISCUSSION
This study describes the effects of antagonists of glycinergic and GABAergic synaptic receptors and a 5-HT1A receptor agonist injected bilaterally into the PBC of adult cats. The data reveal the essential role of inhibitory synaptic transmission in the process of respiratory rhythm generation in the intact central nervous system.
Specificity of drug application
Substances used in the present study are known to cross the blood-brain barrier and previous reports have described their effects on respiratory activity when administered systemically or into the fourth ventricle. Such applications produce a large variety of effects including increase of inspiratory activity, reduction of respiratory frequency and apneusis (Schmidt, Boehmer & Gebauer, 1991; Steele, Siddiqi & Sica, 1994). We concluded that such measurements are not specific because the effects are probably not restricted to the structures involved in respiratory rhythm generation. Therefore, we employed microinjection into the PBC, a region that has been shown to play a specific role in respiratory rhythm generation both in vitro (Smith et al. 1991) and in vivo (Koshia & Guyenet 1996; Ramirez et al. 1998). Our data confirm such a specific role of the PBC since microinjections of bicuculline and glycine outside this area did not persistently affect phrenic activity.
Blockade of inhibitory synaptic transmission
Blocking inhibitory neurotransmission within the bilateral PBC revealed two types of effects which can be attributed to two inhibitory pathways. (1) Blockade of glycinergic transmission was capable of abolishing rhythmic discharges. In many cases, however, it greatly reduced the amplitude of the phrenic burst discharges while the respiratory frequency was increased. (2) Blockade of GABAergic transmission with bicuculline slowed respiratory frequency. A combined application of both antagonists into the PBC always elicited a complete arrest of rhythmic activity leading to tonic phrenic discharges at low amplitudes. The finding that respiratory burst frequency increased whenever burst amplitudes decreased reveals that the drugs acted specifically on the primary mechanisms of respiratory rhythm generation (see Euler, 1986 for review). This provides evidence that inhibitory GABAergic and glycinergic synaptic inputs target neurones in the PBC and control in vivo generation of rythmic respiratory activity.
Source of the inhibitory pathways
Our data contradict those from in vitro experiments on isolated brainstem-spinal cord and transverse medullary slice preparations (Feldman et al. 1990; Ramirez et al. 1996). Only some in vitro studies on mice describe a similar sensitivity of central respiratory activity to strychnine and bicuculline (Paton et al. 1994; Paton & Richter, 1995). In the latter studies, bicuculline triggered an apneustic discharge in respiratory output nerves regardless of the age of the mice, and strychnine greatly disturbed the rhythm in an age-dependent manner, the sensitivity being higher in mature preparations. Such discrepancies between various in vitro studies may arise from differences in the preparations. One major difference is that the tilted sagittal slice of mice (Paton et al. 1994; Paton & Richter, 1995) retains rostral medullary and pontine respiratory areas that are absent in most other in vitro preparations in which there was an apparent absence of significant inhibitory mechanisms. Therefore, we conclude that inputs from more rostral structures including the pons are a major source of inhibition (see Fournier, Richter & Feldman, 1987). This view is consistent with in vitro studies on en bloc brainstem showing complete blockade of rhythmic discharges in phrenic nerves when the pons was kept intact (Hilaire, Bou & Monteau, 1997). Our suggestion follows the original in vivo observation of Lumsden (1923) that lesions of the pons in vagotomized animals lead to apneustic breathing, which he interpreted as a result of deleting pontine inhibitory mechanisms. Thus, the effects of strychnine and bicuculline injections into the PBC could be related to local blockade of inhibitory inputs from medullary and/or pontine interneurons leading to dominance of synaptic activation of respiratory neurons within the PBC. Pontine influences seem to be complex (Euler, 1986) and besides inhibitory pathways also involve excitatory pathways projecting directly to the PBC (Ellenberger & Feldman, 1994). Hence excitatory pontine drive (see apneustic drive in the model of Lumsden, 1923) might enhance the activity level of inspiratory PBC neurons which makes synaptic inhibition even more essential for rhythm generation in intact preparations.
Such a connectivity pattern is schematically illustrated in Fig. 6. We propose that inhibitory neurons, possibly localized within the PBC, have to be active under normal conditions to ensure termination of inspiratory bursting to allow rhythmic oscillations of respiratory activity. If such inhibition is disturbed, tonic activation and apneusis may result (Foutz, Champagnat & Denavit-Saubie, 1989; Feldman, Windhorst, Anders & Richter, 1992; Lalley, Bischoff, Richter, 1994b) leading to systemic hypoxia and further life-threatening disruption of synaptic interaction within the respiratory centre (Wilken et al. 1997).
Inhibition of pacemaker neurons
Several laboratories have reported the existence of potential pacemaker neurons within the medullary respiratory network inside and outside the PBC (Onimaru et al. 1988, 1989,1990; Smith et al. 1991; Johnson, Smith, Funk & Feldman, 1994). A crucial question therefore is whether such pacemaker-like neurons are involved in respiratory rhythm generation in vivo. Experimental (Smith et al. 1991) studies of such pacemaker neurons indicate that they oscillate rhythmically in a voltage-dependent manner due to activation/inactivation of specific membrane currents. Moderate membrane depolarization, therefore, could result in an increase of oscillatory frequency, while further depolarization of neurons would arrest pacemaker oscillations. The increase in respiratory frequency following blockade of glycinergic inhibition, therefore, could indeed result from moderate membrane depolarization of pacemaker cells. A different mechanism, however, would be necessary to account for the observed decrease in respiratory frequency following initial bicuculline injections. In the present study, we also tried to counteract disinhibition-induced membrane depolarization of PBC neurons with 8-OH-DPAT injections. Such stimulation of 5-HT1A receptors would activate persistent potassium currents and compensate for disinhibitory membrane depolarization after blockade of hyperpolarizing glycinergic and GABAergic currents (Lalley et al. 1994a). Indeed, we saw recovery from apneustic disturbances after blockade of GABAergic inhibition and there was also recovery of rhythmic respiratory activity after incomplete blockade of glycinergic inhibition. However, we did not see any recovery of rhythmic respiratory activity once the blockade of inhibitory synaptic transmission was complete.
We conclude that (1) inhibitory synaptic transmission within the pre-Bötzinger complex is essential for the genesis of respiratory rhythm in intact in vivo mammals. (2) Release from such synaptic inhibition is obviously not sufficient to allow putative pacemaker cells to develop functional pacing, which indicates that (3) additional decrease of excitatory synaptic inflow is necessary for potential endogenous bursting of PBC-neurons. (4) This might indicate that under in vivo conditions synaptic conductances are permanently activated also in respiratory pacemaker neurons and dominate over endogenous/voltage-dependent conductance changes.
Acknowledgments
The work was supported by the Deutsche Forschungsgemeinschaft.
References
- Anders K, Ballantyne D, Bischoff AM, Lalley PM, Richter DW. Inhibition of caudal medullary expiratory neurones by retrofacial inspiratory neurones in the cat. Journal of Physiology. 1991;437:1–25. doi: 10.1113/jphysiol.1991.sp018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D, Richter DW. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones in the cat. Journal of Physiology. 1986;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CA, Dobbins EG, Feldman JL. Pre-Boetzinger complex in cats: respiratory neuronal discharge patterns. Brain Research. 1992;390:337–340. doi: 10.1016/0006-8993(92)91118-x. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Origins of excitatory drive within the respiratory network: anatomical localization. NeuroReport. 1994;5:1933–1936. doi: 10.1097/00001756-199410000-00023. [DOI] [PubMed] [Google Scholar]
- Euler C Von. Brain stem mechanisms for generation and control of breathing pattern. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, section 3, The Respiratory System, Control of Breathing. II. Bethesda, MD, USA: American Physiological Society; 1986. pp. 1–67. chap. 1. [Google Scholar]
- Feldman JL, Smith JC. Cellular mechanisms underlying modulation of breathing pattern in mammals. Annals of the New York Academy of Sciences. 1989;563:114–130. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Smith JC, Ellenberger HH, Connelly CA, Liu GS, Greer JJ, Lindsay AD, Otto MR. Neurogenesis of respiratory rhythm and pattern: emerging concepts. American Journal of Physiology. 1990;259:R879–886. doi: 10.1152/ajpregu.1990.259.5.R879. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Windhorst U, Anders K, Richter DW. Synaptic interaction between medullary respiratory neurones during apneusis induced by NMDA-receptor blockade in cat. Journal of Physiology. 1992;450:303–323. doi: 10.1113/jphysiol.1992.sp019128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M, Richter DW, Feldman JL. Effects of rostral pontine stimulation on membrane potential of expiratory medullary neurones. Society of Neuroscience Abstracts. 1987;13:1587. [Google Scholar]
- Foutz AS, Champagnat J, Denavit-Saubie M. Involvement of N-methyl-D-aspartate (NMDA) receptors in respiratory rhythmogenesis. Brain Research. 1989;500:199–208. doi: 10.1016/0006-8993(89)90314-4. 10.1016/0006-8993(89)90314-4. [DOI] [PubMed] [Google Scholar]
- Haji A, Takeda R, Remmers JE. Evidence that glycine and GABA mediate postsynaptic inhibition of bulbar respiratory neurons in the cat. Journal of Applied Physiology. 1992;73:2333–2342. doi: 10.1152/jappl.1992.73.6.2333. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Lipsky J. The role of inhibitory amino acids in control of respiratory motor output in an arterially perfused rat. Respiratory Physiology. 1992;89:47–63. doi: 10.1016/0034-5687(92)90070-d. 10.1016/0034-5687(92)90070-D. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Bou C, Monteau R. Rostral ventrolateral medulla and respiratory rhythmogenesis in mice. Neuroscience Letters. 1997;224:13–16. doi: 10.1016/s0304-3940(97)13458-9. 10.1016/S0304-3940(97)13458-9. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Funk GD, Feldman JL. Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. Journal of Neurophysiology. 1994;72:2598–2608. doi: 10.1152/jn.1994.72.6.2598. [DOI] [PubMed] [Google Scholar]
- Klages S, Bellingham MC, Richter DW. Late expiratory inhibition of stage 2 expiratory neurons in the cat - a correlate of expiratory termination. Journal of Neurophysiology. 1993;70:1307–1315. doi: 10.1152/jn.1993.70.4.1307. [DOI] [PubMed] [Google Scholar]
- Koshia N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. Journal of Physiology. 1996;491:859–869. doi: 10.1113/jphysiol.1996.sp021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, Bischoff A-M, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. Journal of Physiology. 1994a;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, Bischoff A-M, Richter DW. Serotonin 1A-receptor activation suppresses respiratory apneusis. Neuroscience Letters. 1994b;172:59–62. doi: 10.1016/0304-3940(94)90662-9. 10.1016/0304-3940(94)90662-9. [DOI] [PubMed] [Google Scholar]
- Lumsden T. Observations on the respiratory centers in the cat. Journal of Physiology. 1923;57:153–160. doi: 10.1113/jphysiol.1923.sp002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie MD, Gottschalk A, Anders K, Richter DW, Pack AI. A network model of respiratory rhythmogenesis. American Journal of Physiology. 1992;263:R962–975. doi: 10.1152/ajpregu.1992.263.4.R962. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Primary respiratory rhythm generator in the medulla of brainstem-spinal cord preparation from newborn rat. Brain Research. 1988;445:314–324. doi: 10.1016/0006-8993(88)91194-8. 10.1016/0006-8993(88)91194-8. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Firing properties of respiratory rhythm generating neurons in the absence of synaptic transmission in rat medulla in vitro. Experimental Brain Research. 1989;76:530–536. doi: 10.1007/BF00248909. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Inhibitory synaptic inputs to the respiratory rhythm generator in the medulla isolated from newborn rats. Pflügers Archiv. 1990;417:425–432. doi: 10.1007/BF00370663. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Ramirez J-M, Richter DW. Mechanisms of respiratory rhythm generation change profoundly during early life in mice and rats. Neuroscience Letters. 1994;170:167–170. doi: 10.1016/0304-3940(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Richter DW. Role of fast inhibitory synaptic mechanisms in respiratory rhythm generation in the maturing mouse. Journal of Physiology. 1995;484:505–521. doi: 10.1113/jphysiol.1995.sp020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J-M, Quellmalz UJA, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. Journal of Physiology. 1996;491:799–812. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J-M, Richter DW. The neuronal mechanisms of respiratory rhythm generation. Current Opinion in Neurobiology. 1996;6:817–825. doi: 10.1016/s0959-4388(96)80033-x. [DOI] [PubMed] [Google Scholar]
- Ramirez J-M, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Bötzinger complex in vivo eliminates breathing but not gasping. Journal of Physiology. 1998;507:895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, Richter DW, Ballantyne D, Bainton CR, Klein JP. Reflex prolongation of stage I of expiration. Pflügers Archiv. 1986;407:190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- Richter DW. Generation and maintenance of the respiratory rhythm. Journal of Experimental Biology. 1982;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Richter DW, Ballanyi K, Schwarzacher S. Mechanisms of respiratory rhythm generation. Current Opinion in Neurobiology. 1992;281:788–793. doi: 10.1016/0959-4388(92)90135-8. [DOI] [PubMed] [Google Scholar]
- Richter DW, Camerer H, Meesmann M, Röhrig N. Studies on the synaptic interconnection between bulbar respiratory neurones of cats. Pflügers Archiv. 1979;380:245–257. doi: 10.1007/BF00582903. [DOI] [PubMed] [Google Scholar]
- Richter DW, Champagnat J, Jacquin T, Benacka R. Calcium currents and calcium-dependent potassium currents in mammalian medullary respiratory neurones. Journal of Physiology. 1993;470:23–33. doi: 10.1113/jphysiol.1993.sp019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak IA, Paton JFR, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern II. Network models of the central respiratory pattern generator. Journal of Neurophysiology. 1997;77:2007–2026. doi: 10.1152/jn.1997.77.4.2007. [DOI] [PubMed] [Google Scholar]
- Schmid K, Boehmer G, Gebauer K. Glycine-receptor mediated fast synpatic inhibition in the brainstem respiratory system. Respiratory Physiology. 1991;84:351–361. doi: 10.1016/0034-5687(91)90129-7. [DOI] [PubMed] [Google Scholar]
- Schmid K, Foutz AS, Denavit-Saubie M. Inhibitions mediated by glycine and GABAA receptors shape the discharge pattern of bulbar respiratory neurons. Brain Research. 1996;710:150–160. doi: 10.1016/0006-8993(95)01380-6. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Boetzinger complex in the cat. Journal of Neurophysiology. 1995;73:1452–1461. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Boetzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AM, Siddiqi ZA, Sica AL. Effects of bicuculline on inspiratory activities in piglets. Brain Research. 1994;650:166–169. doi: 10.1016/0006-8993(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Wilken B, Lalley P, Bischoff AM, Christen HJ, Behnke J, Hanefeld F, Richter DW. Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. Journal of Pediatrics. 1997;130:89–94. doi: 10.1016/s0022-3476(97)70315-9. [DOI] [PubMed] [Google Scholar]


