Abstract
Ionic currents were simultaneously recorded at macroscopic and unitary level using the whole-cell and cell-attached patch-clamp procedures together on the same portion of isolated mouse skeletal muscle fibres.
In the presence of Tyrode solution in the patch pipette and Tyrode-TTX solution in the bath, macroscopic and unitary currents through delayed rectifier K+ channels were simultaneously recorded in response to depolarizing pulses of 1 s duration.
In five fibres, successive long-lasting incremental depolarizing levels induced, at -40 mV or -30 mV, the opening of a high conductance channel carrying an outward current superimposed on delayed rectifier K+ channel activity. Opening of this high conductance channel was not observed when the depolarization steps were applied in the patch pipette.
Using the same depolarizing protocol, activation of a high conductance channel was also observed in two fibres in the presence of a K+-rich solution in the pipette (145 mm K+).
With either Tyrode or K+-rich solution in the pipette, unitary current amplitudes of the high conductance channel matched well with the values obtained for Ca2+-activated K+ (KCa) channels in inside-out patches under similar ionic conditions.
Indo-1 fluorescence measurements showed that the stimulation protocol that led to KCa channel opening induced stepwise increases in intracellular [Ca2+] in the submicromolar range.
Our results provide evidence that activation of sarcolemmal KCa channels can be induced by a rise in intracellular [Ca2+] following voltage-activated sarcoplasmic reticulum Ca2+ release.
High conductance Ca2+-activated K+ (KCa) channels are found in a large variety of both electrically excitable and non-excitable cells. The main characteristic of these KCa channels is that their activity is enhanced by an increase in intracellular Ca2+ concentration ([Ca2+]i) as well as membrane depolarization. In smooth muscle, these channels may be involved in the regulation of vascular tone while they are thought to regulate firing in neurons (for review, see Blatz & Magleby, 1987; Latorre, Oberhauser, Labarca & Alvarez, 1989; Marty, 1989; McManus, 1991; Kaczorowski, Knaus, Leonard, McManus & Garcia, 1996). In skeletal muscle, the biophysical properties as well as the calcium and voltage dependences of the KCa channels have been extensively studied using the excised configurations of the patch-clamp technique (Barrett, Magleby & Pallota, 1982; Latorre, Vergara & Hidalgo, 1982). However, up to now, the conditions which may lead to activation of these channels in their cellular environment have not been clearly defined. Allard, Bernengo, Rougier & Jacquemond (1996) suggested that these channels are involved in the control of muscle cell excitability during synaptic transmission. They showed that, in depolarized mouse skeletal muscle cells, Ca2+ entering through nicotinic receptors induced an increase in intracellular Ca2+ concentration that could activate KCa channels. Besides, during physiological activation, skeletal muscle undergoes a large increase in [Ca2+]i associated with membrane depolarization, both of which should favour KCa channels opening. The aim of this study was to determine whether or not it is possible to detect KCa channel opening at the unitary level when intracellular Ca2+ is released by the sarcoplasmic reticulum in response to membrane depolarization in mouse skeletal muscle. Using a whole-cell clamp technique together with single channel recording, we demonstrated that during prolonged membrane depolarization, the subsarcolemmal [Ca2+] may reach a high enough level to trigger KCa opening.
METHODS
Isolation and preparation of the muscle fibres
Male mice 2-4 months old were killed by cervical dislocation. The flexor digitorum brevis and interosseal muscles were removed and treated with collagenase (Sigma, Type 1) for 60-90 min at 37°C as previously described (Allard et al. 1996). Single skeletal muscle fibres were then obtained by gently triturating the muscles within the experimental chamber through the cut disposable tip of a Pipetman (Gilson). Most of the experiments described in this paper required whole-cell voltage clamping of a short portion of a single fibre extremity while unitary channel activity was simultaneously measured on that same portion of fibre with a patch-clamp pipette in cell-attached configuration. Electrical insulation of the major part of a fibre was performed as previously described (Jacquemond, 1997). In brief, prior to trituration of the enzyme-treated muscle, the bottom of the experimental chamber was covered with a thin layer of silicone grease and filled with culture medium containing 10 % bovine fetal serum (MI199, Eurobio, France). Single fibres were then left to settle on the silicone layer. After ∼10 min, the culture medium was replaced by a tetrodotoxin (TTX)-containing Tyrode solution. A single fibre was then covered with some more silicone so that only a short portion (∼50 μm long) of the fibre extremity was left free. All experiments were performed at room temperature (20-22°C).
Electrophysiology
The experimental arrangement used for measuring the unitary ionic channel activity from a voltage clamped portion of muscle fibre is schematized in Fig. 1. Voltage clamping of the silicone-free end of single fibres was performed using an RK400 patch-clamp amplifier in whole-cell configuration (Bio-Logic, Claix, France) with a single microelectrode filled with 3 M potassium acetate and 0.02 M KCl (resistance 1-3 MΩ). As already described (Jacquemond, 1997) the tip of the microelectrode was inserted across the silicone within the insulated part of the fibre. The holding command potential was always set to -80 mV with the patch-clamp amplifier. Analog compensation was used to decrease the effective series resistance and speed up the charging of the membrane capacitance. Thereafter, the tip of a patch pipette was sealed on the silicone-free portion of the fibre in order to record single channel activity in the cell-attached configuration with an additional RK400 patch-clamp amplifier. Currents flowing into the patch pipette were considered to be positive. Patch pipettes were filled with Tyrode solution or with a K+-rich (containing 140 mm KCl) solution (see below in ‘Solutions’ for details).
Figure 1. Experimental arrangement for measurement of single channel activity on a voltage-clamped portion of skeletal muscle fibre.
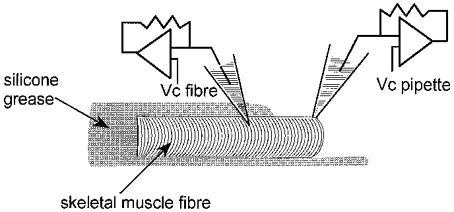
The fibre is shown partially embedded within silicone grease leaving only a short portion of the fibre end free of silicone. Whole-cell voltage clamp was achieved with a patch-clamp amplifier using a single microelectrode, the tip of which was inserted within the silicone-embedded part of the fibre (command voltage, Vc fibre). Simultaneous single channel recording was performed by sealing a patch pipette on the voltage-clamped silicone-free portion of fibre (command voltage, Vc pipette).
In some experiments, only the cell-attached configuration of the patch-clamp technique was used to record single channel activity on resting fibres (results illustrated in the right panel of Fig. 2B).
Figure 2. Current through delayed rectifier K+ channels in response to depolarizing pulses.
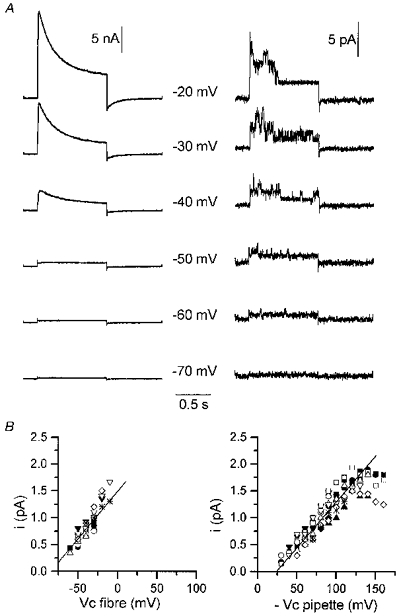
A, simultaneous records of delayed rectifier K+ channel currents at the macroscopic level (left panel) and at the unitary level (right panel) in response to 1 s depolarizing pulses of increasing amplitude applied to the portion of fibre under study. Experimental arrangement was as described in Fig. 1. The value of the command potential is indicated in between each corresponding pair of traces. The holding command potential was -80 mV. B, single channel current-voltage relationships for delayed rectifier K+ channels obtained in cell-attached patches by applying depolarizing pulses to the fibre (Vc fibre; left panel) and to the patch membrane (-Vc pipette) through the patch pipette on fibres that were not simultaneously voltage clamped (right panel). Different symbols correspond to 11 distinct fibres (left panel) and 11 distinct membrane patches (right panel). On both graphs, the continuous line corresponds to the mean conductance calculated from the individual series of measurements. Bath and patch pipette contained Tyrode-TTX solution and Tyrode solution, respectively.
Command voltage pulse generation and data acquisition were done using commercial software (Biopatch Acquire, Bio-Logic) driving an A/D, D/A converter (Lab Master DMA board, Scientific Solutions Inc., Solon, OH, USA). The current from the patch pipette was also digitized with a PCM converter (701 Sony) and stored on videotape for further analysis.
In some experiments, short step changes in the potential of the patch pipette were applied while long-lasting successive depolarizing levels of the fibre command potential were generated with the D/A converter; in this case, the voltage pulses through the patch pipette were delivered with a stimulator (Tektronix 2601, Beaverton, OR, USA).
Currents were analysed using Bio-Patch software (Bio-Logic). Single channel current amplitudes were determined using amplitudes histograms.
Intracellular calcium measurements
Intracellular free calcium concentration ([Ca2+]i) was calculated from the fluorescence of isolated fibres that were partly embedded within silicone as described above (‘Isolation and preparation of the muscle fibres’) and microinjected with indo-1 (pentapotassium salt, Molecular Probes). Details concerning the procedure for intracellular dye loading, the optical apparatus, the [Ca2+] calculation and the calibration of indo-1 fluorescence have been published elsewhere (Allard et al. 1996; Jacquemond, 1997). In brief, a modified Nikon Diaphot microscope was used in diafluorescence mode: the UV light for dye excitation was provided by a high pressure mercury bulb set on the top of the microscope. The beam of light was passed through a 335 nm interference filter and focused on the preparation. The emitted fluorescent light collected by the objective was split and filtered allowing simultaneous detection at 405 and 470 nm by two photomultipliers. Fluorescence signals were digitized and stored on disk using the same hardware and software as for the electrophysiological measurements. The fluorescence measurement field was 40 μm in diameter and the silicone-free extremity of each tested fibre was placed in the middle of the field. The standard calibration parameters Rmin, Rmax, KD and β (Grynkiewicz, Poenie & Tsien, 1985) obtained from in vivo calibration of the indo-1 fluorescence ratio at 405 and 470 nm (F405/F470) were used to calculate the myoplasmic [Ca2+] from the indo-1-microinjected fibres (Jacquemond, 1997).
Solutions
The external Tyrode solution contained (mm): 140 NaCl, 5 KCl, 2.5 CaCl2, 1 MgCl2, 10 Hepes; adjusted to pH 7.4 with NaOH. The external K+-rich solution contained (mm): 140 KCl, 2.5 CaCl2, 1 MgCl2, 10 Hepes, adjusted to pH 7.4 with KOH. TTX (Sigma) was used at a concentration of 1 μm. Some experiments were performed in the absence of extracellular calcium. For this, CaCl2 was replaced equimolarly by MgCl2 in the TTX-containing Tyrode solution, and EGTA was added at a concentration of 0.5 mm. That same solution was used in the patch pipette except that EGTA was used at 0.05 mm. In these experiments, fibres were bathed in regular TTX-containing Tyrode solution and the external solution was superfused on each tested fibre using a thin polyethylene capillary system operating by gravity.
Statistics
Least-squares fits were performed using a Marquardt-Levenberg algorithm routine included in Microcal Origin (Microcal software Inc., Northampton, MA, USA). Data values are presented as means ±s.e.m.
RESULTS
A first series of experiments was performed to determine what kind of ionic channel activity could be detected under our experimental conditions in response to depolarizing command pulses. Figure 2A shows ionic currents simultaneously recorded at macroscopic (left panel) and unitary (right panel) level using whole-cell and cell-attached patch-clamp procedures together on the same portion of a muscle fibre (see Methods). The potential of the cell-attached patch pipette was held at 0 mV. Depolarizing command pulses of 1 s duration were applied with the microelectrode from a holding command potential of -80 mV. At the unitary as well as at the macroscopic level, membrane depolarization elicited outward currents, the amplitude of which increased with the magnitude of voltage steps. During the course of the voltage steps, unitary channel activity displayed a time-dependent decay, while similarly, macroscopic currents inactivated with time. Similar results were observed in twelve distinct fibres on which depolarizing pulses up to -20 mV or -10 mV were tested. In these twelve cell-attached patches, the single channel current amplitude was plotted versus the command voltage (Fig. 2B, left panel). The mean slope conductance was 17.7 ± 1.6 pS (n= 11) and the mean current-voltage relationship displayed a reversal potential at -85 mV, the presumed K+ equilibrium potential under asymmetrical K+ concentrations (5 mm K+ in the pipette and in vivo intracellular [K+] assumed to be 140 mm). These data strongly suggest that the channel activated by membrane depolarization is the delayed rectifier K+ channel already described in mammalian skeletal muscle cells (Duval & Leoty, 1978; Beam & Donaldson, 1983; Brinkmeier, Zachar & Rüdel, 1991; Hocherman & Bezanilla, 1996).
The current-voltage relationship shown on the right panel of Fig. 2B was obtained from cell-attached patches performed on resting fibres that were not simultaneously handled with the silicone-whole-cell-voltage-clamp procedure. Data points correspond to the amplitude of unitary currents measured when depolarizing pulses were applied with the patch pipette. Saturation of unitary current amplitude was apparent at high potentials. The mean slope conductance calculated in the linear region was 17.1 ± 0.6 pS (n= 11), a value not significantly different from the one previously obtained for the delayed rectifier K+ channel when depolarizing the cell (Fig. 2B, left panel). These results indicate that our experimental silicone-voltage-clamp method allowed satisfactory voltage control of the internal surface membrane potential.
In all of the cell-attached patches tested under these conditions, the only channel activity we detected was rare brief openings of channels carrying inward current; the characteristics of these openings were not investigated here. When recording single channel activity with the patch pipette, membrane potentials more depolarized than -10 mV could rarely be tested because under these conditions, contraction of the fibre was so vigorous that the contact between the tip of the pipette and the membrane was generally lost. Considering the well-known voltage dependency of maxi-KCa channels activity, we thought that a higher amplitude of depolarizations, not easily achievable under our conditions, may be needed to activate these channels. We thus attempted to explore the phenomenon during long-lasting successively increasing depolarizing levels for which the depolarization-induced SR calcium release process should be partially inactivated (Hodgkin & Horowicz, 1960). In such conditions we expected the amplitude of the contractile output to be less and thus to be able to test higher values of membrane depolarization. Figure 3 shows simultaneous records of unitary ionic currents (A) and macroscopic currents (B) in response to successive long-lasting step changes in membrane potential (indicated in the figure as Vc fibre) to -50, -40 and -30 mV while the patch membrane potential (Vc pipette) was held at 0 mV (C). It shows that each depolarizing step led to the opening of channels that could be easily identified, on the basis of their conductance properties and pattern of activity, as delayed rectifier K+ channels. Brief openings of channels carrying inward currents were also observed at all the potentials. Interestingly, 1.3 s after the beginning of the pulse at -30 mV, superimposed on the delayed rectifier channel activity, brief openings of a channel carrying an outward current of much higher amplitude was observed. A short segment of the main trace on an expanded scale indicates that the unitary current amplitude through this channel was 3 pA (giving a conductance of 54 pS at -30 mV for a K+ channel, assuming a K+ equilibrium potential at -85 mV). After returning the command potential to -80 mV, the same stimulation protocol was applied to the patch pipette. Figure 4 shows that, under these conditions, at -50, -40 as well as -30 mV, only the activity of the delayed rectifier K+ channel could be detected.
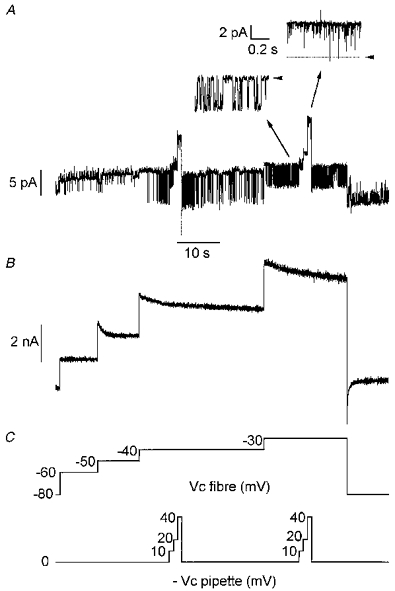
Figure 3. Simultaneous recording of ionic currents at the unitary (A) and macroscopic level (B) in response to successive long-lasting depolarizing levels of increasing amplitude.
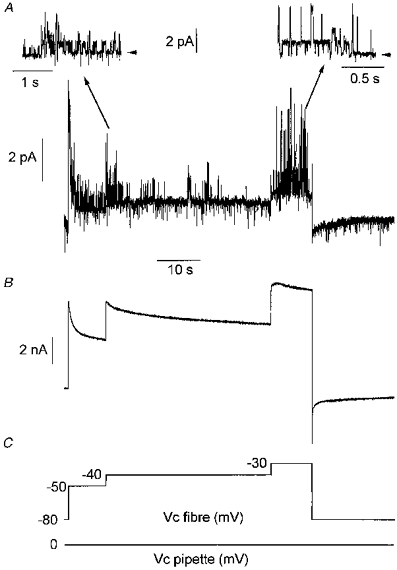
Experimental procedure was as described in Fig. 1. Voltage protocols applied in the fibre and in the pipette are shown in C. In A, insets show short segments of the main trace on an expanded scale at -40 (left trace) and -30 mV (right trace). Arrowheads indicate the zero current level. Note that at -40 mV only one type of channel, identified as the delayed rectifier K+ channel (see text), was active while an additional channel type of higher conductance (54 pS, see text) opened at -30 mV. Bath and patch pipette contained Tyrode-TTX solution and Tyrode solution, respectively.
Figure 4. Single channel recording performed in the same cell-attached patch as in Fig. 3 in response to successive long-lasting depolarizing levels of increasing amplitude applied to the patch membrane through the patch pipette.
The current trace is shown in A. Only the activity of the delayed rectifier K+ channel was observed. Voltage protocols applied in the fibre and in the pipette are indicated in B.
In Fig 3 and Fig 4, the potential reached by the membrane patch was the same under each of the two experimental procedures. However, the fibre depolarization at -30 mV was likely to induce an increase in [Ca2+]i, whereas the same depolarization applied with the pipette should not. Thus, one possibility is that the activation of the channel carrying a high amplitude current was induced by an increase in [Ca2+]i. As the channel displayed an apparent high conductance, these results strongly suggest that the channel activated by fibre depolarization is the Ca2+-activated maxi-K channel which is known to be present in the sarcolemma of adult muscles. Opening of this channel was observed in four other fibres at -40 mV. The amplitude of unitary current through this channel was plotted against membrane potential in the five fibres where channel activation occurred; this is shown in Fig. 5A. It can be seen that data points match well with the current-voltage relationship of the KCa channel recorded in inside-out patches under asymmetrical K+ concentrations in a preceding study (Allard et al. 1996, Fig. 4B).
Figure 5. Single channel current-voltage relationships for the large conductance channel activated during successive long-lasting depolarizing levels applied to the fibre in the presence of Tyrode solution (A) and K+-rich solution (B) in the pipette.
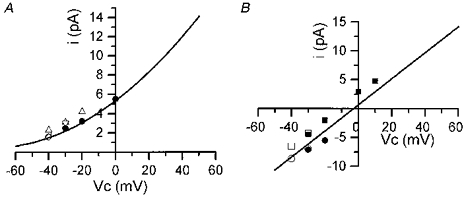
Data points in A and B were obtained from experiments as described in Figs 3 and 6, respectively. In A, open symbols correspond to unitary current amplitudes through the large conductance channel measured at different depolarizing levels applied to the fibre. Symbols of different shapes correspond to 4 distinct fibres. Filled circles correspond to data points that were obtained by depolarizing the patch membrane through the pipette during a burst of activity observed while the fibre internal potential was held at -40 mV. In B, squares and circles correspond to two different fibres. Open symbols correspond to the amplitudes of the currents through the large conductance channel measured at different depolarizing levels applied to the fibre. Filled symbols correspond to data points that were obtained by depolarizing the patch membrane through the patch pipette during a burst of activity in a fibre depolarized at -40 and -30 mV (squares) and in another fibre depolarized at -40 mV (circles). Continuous lines correspond to the mean current-voltage relationship of the KCa channel in inside-out patches from a previous study (see text), in the presence of Tyrode solution (A) and of a K+-rich solution (B) in the pipette.
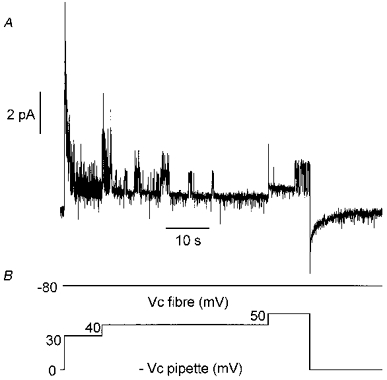
The effects of a similar stimulation protocol on single channel activity was tested in the presence of an external K+-rich solution in the patch pipette. Figure 6 illustrates the results from such an experiment. In this fibre, a sustained activity of channels carrying an inward current was observed throughout the experiment. On the basis of their conductance properties (25 pS), these channels were characterized as inward rectifier K+ channels (Burton & Hutter, 1990). It can also be seen that the voltage step at -50 mV induced a transient opening of channels which superimposed on inward rectifier channel activity and probably corresponded to the opening of delayed rectifier K+ channels. Subsequent depolarization at -40 mV induced, after a delay of 2 s, activation of a channel of much higher conductance (-6.5 pA at -40 mV) carrying an inward current. Activity of this channel progressively increased, reaching a maximum ∼3 s after the onset of activation. Channel activity remained high during 8 s then decreased to lower values. Within the same delay of 2 s, cell depolarization at -30 mV increased channel activity again, and this activity remained stable throughout the voltage step. Activity of this channel of high conductance stopped upon returning the command potential to -80 mV. Then only inward rectifier K+ channel opening could be observed. At both potentials (-40 mV and -30 mV), three depolarization steps of 10, 20 and 40 mV amplitude were applied in the pipette during the bursts. It could be observed that the more depolarized the potential the longer the time the channel of high conductance spent in the open state. In this fibre and in another in which activation of this high conductance channel occurred, amplitudes of unitary currents were plotted against membrane potential (Fig. 5B). It can be seen that data points are also in agreement with the current-voltage relationship of the KCa channel recorded in inside-out patches under symmetrical K+ concentrations in a preceding study (Allard et al. 1996, Fig. 4B).
Figure 6. Simultaneous recording of ionic currents at the unitary (A) and macroscopic level (B) in response to successive long-lasting depolarizing levels of increasing amplitude in the presence of a K+-rich solution in the patch pipette.
Voltage protocols applied in the fibre and in the pipette are shown in C. In A, note that at -40 mV and -30 mV the activity of a large conductance channel carrying an inward current was superimposed on the opening of the inward rectifier K+ channel (25 pS, see text). During the bursts of activity of the large conductance channel, depolarizing pulses were applied to the patch membrane through the pipette as indicated in C. Insets show short segments of the main trace on an expanded scale when the fibre command potential was -30 mV (left trace) and in response to a 40 mV amplitude depolarizing pulse applied to the patch membrane through the patch pipette from this fibre command potential (right trace). Arrowheads indicate the zero current level. Bath contained Tyrode-TTX solution.
In order to check whether calcium entry from the external medium may contribute to the increase in [Ca2+]i responsible for the observed high conductance channel activation, a series of experiments was performed in zero-calcium EGTA-containing Tyrode solution as external medium as well as patch pipette solution. Under these conditions, using a similar stimulation protocol to that shown in Fig 3 and Fig 6, activation of the high conductance channels was observed in three fibres at -40, -30 and -20 mV, respectively (not illustrated).
Indo-1 fluorescence measurements were performed in a series of fibres in order to determine the maximal average [Ca2+]i reached under the conditions that led to the opening of the KCa channels. Figure 7A shows the membrane current (top trace) and [Ca2+]i (bottom trace) simultaneously measured from a fibre depolarized by long-lasting incremental voltage steps up to -20 mV. The command voltage protocol is shown in Fig. 7C. [Ca2+]i increased in a stepwise manner in response to the successive voltage jumps, reaching a maximum of ∼0.3 μm at -20 mV. It is worth noticing that with this protocol, [Ca2+]i remained elevated and displayed only a very slow decay during the depolarizations. The mean ±s.e.m. [Ca2+]i recorded from twelve fibres on which that same protocol was applied is presented in Fig. 7B. The s.e.m. is shown as vertical bars superimposed on the [Ca2+]i trace. The mean maximal [Ca2+]i reached at the potentials of -50, -40, -30 and -20 mV was 40 ± 5, 80 ± 15, 95 ± 15 and 165 ± 40 nm, respectively, from a mean resting level of 25 ± 5 nm.
Figure 7. Intracellular [Ca2+] changes elicited by successive long-lasting depolarizing levels of increasing amplitude.
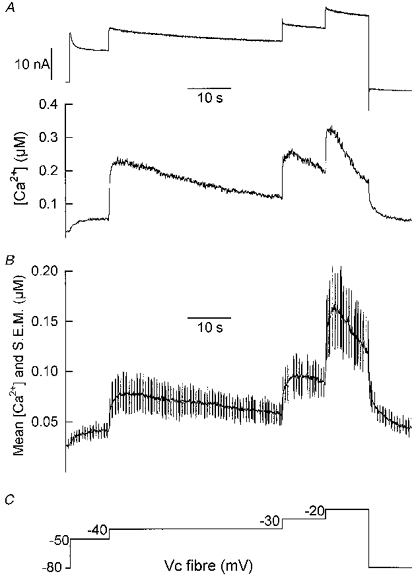
A, changes in macroscopic ionic current (top trace) and [Ca2+]i (bottom trace) measured from one fibre in response to the depolarizing protocol shown in C. B, mean [Ca2+]i (continuous line) and s.e.m. (superimposed vertical bars) measured from twelve fibres on which the depolarizing protocol shown in C was applied.
DISCUSSION
In mammalian skeletal muscle, no clear demonstration was previously made at the single channel level as to whether or not the high conductance Ca2+-activated K+ channels could be activated in response to an intracellular [Ca2+] rise induced by membrane depolarization. In the present study, we found that these channels can be activated when the membrane potential of an isolated portion of mouse skeletal muscle fibre is held at depolarized levels for several seconds. We used a technical procedure that allows, for the first time in adult skeletal muscle, recording of the sarcolemmal ion channel activity at the unitary level while having the internal potential of the fibre portion under study simultaneously clamped. In response to 1 s duration single step depolarizations of the fibre up to -10 mV, we were able to record the unitary activity of delayed rectifying K+ channels. However, under these conditions, the unitary activity of channels featuring the properties of KCa channels was never detected. Unexpectedly, when successive long-lasting depolarizations of incremental amplitude were applied, a unitary current of large amplitude could be observed for quite low levels of membrane depolarization (-40, -30 mV). Analysis of the voltage dependence and conductance properties of that channel activity with the patch pipette containing either Tyrode or a K+-rich solution led to unequivocal identification of the KCa channels.
On the other hand, results from indo-1 fluorescence measurements showed that during such long depolarizations, the cytoplasmic [Ca2+] concentration was only slightly increased from the resting level, and in any case was much smaller than that expected to activate the KCa channels. Indeed, in a preceding study (Allard et al. 1996), analysis of [Ca2+] and voltage dependences of KCa channels in inside-out membrane patches indicated that a [Ca2+] higher than 3 μm had to be reached at the cytoplasmic face to elicit KCa channel opening at membrane potentials of -40 or -30 mV. This is thus more than ten times higher than the [Ca2+]i changes induced by the long-lasting fibre depolarizations at -40 or -30 mV under the present conditions. A similar dissociation between the presumed subsarcolemmal [Ca2+] estimated from the KCa channel activity and the global cytosolic [Ca2+] was already suggested in a previous study; Allard et al. (1996) showed that, in response to acetylcholine application at the muscle endplate, KCa channels activated, indicating that subsarcolemmal Ca2+ may exceed micromolar concentrations while cytoplasmic [Ca2+] reached a maximum of about 0.2 μm. However, in this case, calcium was likely to originate exclusively from outside (via the nicotinic receptors) which would favour the development of a [Ca2+] gradient from the cytoplasmic face of the membrane into the cell. In the present study, since KCa channels activation could occur in the absence of extracellular calcium, the increase in subsarcolemmal [Ca2+]i responsible for channel opening is likely to result exclusively from voltage-activated sarcoplasmic reticulum calcium release. Therefore, one way of allowing the released calcium to become more highly concentrated underneath the surface membrane than in the bulk would require the existence of a highly restricted space between the site of calcium release and the membrane cytoplasmic face. This could indeed be the case since peripheral couplings between junctional sarcoplasmic reticulum and surface sarcolemma have been reported in adult vertebrate skeletal muscle (Spray, Waugh & Sommer, 1974). Another possibility is that the [Ca2+] sensitivity of KCa channels is different in situ from the excised patch conditions (McManus, 1991). However, only considering a [Ca2+]i-dependent activation process, it remains hard to understand why the channels would not be activated in response to 1 s single voltage steps up to -10 mV, when they could open during long-lasting depolarizations at lower values. Though we have no straightforward clue to explain this, it may be that the observed small prolonged rise in [Ca2+]i induced by the first depolarizing level(s) is important for activation of the KCa channels. Under these conditions, it may be speculated that changes in the activity of intracellular factors modulating the [Ca2+] sensitivity of KCa channels or changes in the properties of the channel protein itself occur. Otherwise it would indicate that, at low depolarizing levels, the subsarcolemmal [Ca2+] reaches a higher level when long-lasting rather than when 1 s depolarizing pulses are delivered. Whatever the mechanism, our results suggest that the opening of KCa channels in skeletal muscle may be physiologically relevant during sustained muscle activity or in exhausted fibres that have been shown to display an increased resting [Ca2+]i (Westerblad & Allen, 1991). In such conditions, increase in K+ outflow through these channels may have a protective effect by forcing the membrane potential towards hyperpolarized levels.
Acknowledgments
This study was supported by the Centre National de la Recherche Scientifique (CNRS) the Université Claude Bernard and by the Association Française contre les Myopathies (AFM). We wish to thank Carlos Ojeda, Oger Rougier and Yves Tourneur for helpful discussion while preparing the manuscript.
References
- Allard B, Bernengo J-C, Rougier O, Jacquemond V. Intracellular Ca2+ changes and Ca2+-activated K+ channel activation induced by acetylcholine at the endplate of mouse skeletal muscle fibres. Journal of Physiology. 1996;494:337–349. doi: 10.1113/jphysiol.1996.sp021496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JN, Magleby KL, Pallotta BS. Properties of single calcium-activated potassium channels in cultured rat muscle. Journal of Physiology. 1982;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam KG, Donaldson PL. A quantitative study of potassium channel kinetics in rat skeletal muscle from 1 to 37°C. Journal of General Physiology. 1983;81:485–512. doi: 10.1085/jgp.81.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz AL, Magleby KL. Calcium-activated potassium channels. Trends in Neurosciences. 1987;10:463–467. [Google Scholar]
- Brinkmeier H, Zachar E, Rüdel R. Voltage-dependent K+ channels in the sarcolemma of mouse skeletal muscle. Pflügers Archiv. 1991;419:486–491. doi: 10.1007/BF00370793. [DOI] [PubMed] [Google Scholar]
- Burton FL, Hutter OF. Sensitivity to flow of intrinsic gating in inwardly rectifying potassium channel from mammalian skeletal muscle. Journal of Physiology. 1990;424:253–261. doi: 10.1113/jphysiol.1990.sp018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval A, Leoty C. Ionic currents in mammalian fast skeletal muscle. Journal of Physiology. 1978;278:403–423. doi: 10.1113/jphysiol.1978.sp012312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien R. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hocherman SD, Bezanilla F. A patch-clamp study of delayed rectifier currents in skeletal muscle of control and mdx mice. Journal of Physiology. 1996;493:113–128. doi: 10.1113/jphysiol.1996.sp021368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Horowicz P. Potassium contractures in single muscle fibres. Journal of Physiology. 1960;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemond V. Indo-1 fluorescence signals elicited by membrane depolarization in enzymatically isolated mouse skeletal muscle fibers. Biophysical Journal. 1997;73:920–928. doi: 10.1016/S0006-3495(97)78124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski GJ, Knaus H-G, Leonard RJ, McManus OB, Garcia ML. High-conductance calcium-activated potassium channels; structure, pharmacology and function. Journal of Bioenergetics and Biomembranes. 1996;28:255–267. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- Latorre R, Oberhauser A, Labarca P, Alvarez O. Varieties of calcium-activated potassium channels. Annual Review of Physiology. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Latorre R, Vergara C, Hidalgo C. Reconstitution in planar lipid bilayers of a Ca2+-activated K+ channel from transverse tubule membranes isolated from rabbit skeletal muscle. Proceedings of the National Academy of Sciences of the USA. 1982;77:7484–7486. doi: 10.1073/pnas.79.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus OB. Calcium-activated potassium channels: regulation by calcium. Journal of Bioenergetics and Biomembranes. 1991;23:537–559. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- Marty A. The physiological role of calcium dependent channels. Trends in Neurosciences. 1989;12:420–424. doi: 10.1016/0166-2236(89)90090-8. [DOI] [PubMed] [Google Scholar]
- Spray TL, Waugh RA, Sommer JR. Peripheral couplings in adult vertebrate skeletal muscle. Anatomical observations and functional implications. Journal of Cell Biology. 1974;62:223–227. doi: 10.1083/jcb.62.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. Journal of General Physiology. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]


