Abstract
The effects of unaccustomed eccentric exercise on exercise metabolism during a subsequent bout of graded concentric exercise were investigated in seven healthy male subjects. Arterial and bilateral femoral venous catheters were inserted 2 days after eccentric exercise of one thigh (eccentric thigh) and blood samples were taken before and during graded two-legged concentric knee-extensor exercise. Muscle biopsies were obtained from the eccentric and control vastus lateralis before (rest) and after (post) the concentric exercise bout.
Maximal knee-extensor concentric exercise capacity was decreased by an average of 23 % (P < 0.05) in the eccentric compared with the control thigh.
The resting muscle glycogen content was lower in the eccentric thigh than in the control thigh (402 ± 30 mmol (kg dry wt)−1vs. 515 ± 26 mmol (kg dry wt)−1, means ± s.e.m., P < 0.05), and following the two-legged concentric exercise this difference substantially increased (190 ± 46 mmol (kg dry wt)−1vs. 379 ± 58 mmol (kg dry wt)−1, P < 0.05) despite identical power and duration of exercise with the two thighs.
There was no measurable difference in glucose uptake between the eccentric and control thigh before or during the graded two-legged concentric exercise. Lactate release was higher from the eccentric thigh at rest and, just before termination of the exercise bout, release of lactate decreased from this thigh (suggesting decreased glycogenolysis), whereas no decrease was found from the contralateral control thigh. Lower glycerol release from the eccentric thigh during the first, lighter part of the exercise (P < 0.05) suggested impaired triacylglycerol breakdown.
At rest, sarcolemmal GLUT4 glucose transporter content and glucose transport were similar in the two thighs, and concentric exercise increased sarcolemmal GLUT4 content and glucose transport capacity similarly in the two thighs.
It is concluded that in muscle exposed to prior eccentric contractions, exercise at a given power output requires a higher relative workload than in undamaged muscle. This increases utilization of the decreased muscle glycogen stores, contributing to decreased endurance.
It is recognized that strenuous unaccustomed exercise is followed by muscle soreness and the sensation of muscle weakness, as well as by decreased work capacity during a subsequent bout of dynamic exercise (Sargeant & Dolan, 1987). The mechanism(s) behind the latter feature remain(s) obscure, but could involve altered muscle metabolism. Eccentric exercise involves forced lengthening of active muscle, and a strenuous unaccustomed bout of this exercise type causes temporary ultrastructural muscle damage (Armstrong, Ogilvie & Schwane, 1983; Newham, McPhail, Milles & Edwards, 1983; Friden, Sjøstrøm & Ekblom, 1983), delayed-onset muscle soreness (Schwane, Scarlet, Vandenakker & Armstrong, 1983), muscle stiffness (Jones, Newham & Clarkson, 1987), reduced muscle strength (Davies & White, 1981; Newham, Jones & Clarkson, 1987; Nosaka, Clarkson, McGuiggin & Byrne, 1991; Golden & Dudley, 1992), and sustained decreased muscle glycogen content (O'Reilly, Warhol, Fielding, Frontera, Meredith & Evans, 1987; Costill, Pascoe, Fink, Robergs, Barr & Pearson, 1990; Widrick, Costill, McConell, Anderson, Pearson & Zachwieja, 1992; Doyle, Sherman & Strauss, 1993; Asp, Daugaard & Richter, 1995a; Asp, Kristiansen & Richter, 1995b), all factors that could have an effect on subsequent concentric exercise. A study using magnetic resonance spectroscopy failed to detect any effect of prior eccentric exercise on phosphate metabolism during subsequent concentric exercise (Rodenburg, De Groot, Echteld, Jongsma & Bar, 1995), but this negative finding might reflect the relatively low exercise intensities employed, and changes in other metabolic pathways are not necessarily excluded. For instance, prior eccentric contractions of rat muscle impairs the increase in muscle glucose transport during subsequent electrical stimulation (Kristiansen, Asp & Richter, 1996a). A previous study from our laboratory (Asp et al. 1995a) revealed that the total content of the exercise-/insulin-regulatable and most abundant glucose transporter in skeletal muscle, GLUT4, is reduced after strenuous unaccustomed eccentric contractions. In addition, since resting muscle glycogen content, as mentioned above, is decreased in muscle for several days after eccentric exercise, it can be speculated that decreased glucose and/or glycogen availability may play a role in the impaired concentric exercise capacity following eccentric exercise. The aim of the present study was to investigate the effect of unaccustomed eccentric exercise on metabolism during subsequent concentric exercise, and to elucidate mechanism(s) behind the decreased work capacity in damaged muscle.
METHODS
Subjects
Seven healthy untrained male students, aged 21-27 years, with no relevant medical history and especially no history of cardiovascular disease, clotting disorders, diabetes or other endocrine diseases, served as subjects. Subjects were recruited by advertisement and were fully informed of any risks and the discomfort associated with the experiment before giving their informed consent to participate. All were paid a small honorarium for the time and discomfort involved. Their mean weight and height were 78 kg (range, 70-87 kg) and 186 cm (range, 182-189 cm), respectively. None participated in competitive sports, but all used bicycles for local transportation. Subjects were initially accustomed to the one-legged knee-extensor apparatus (Andersen, Adams, Sjøgaard, Thorboe & Saltin, 1985) before an incremental concentric knee-extensor test was performed on the non-dominant thigh to determine the maximal work capacity of the knee extensors. The test was performed at least 1 week prior to the one-legged eccentric exercise bout. Maximal work capacity was defined as the highest load at which the subjects could maintain the kicking frequency (60 r.p.m.) for more than 2 min, and the mean value was 63 ± 4 W. Seven additional subjects, aged 20-36 years, 72 kg (range, 61-79 kg) in weight, and 178 cm (range, 172-185 cm) in height, were included to estimate the maximal work capacity in control and eccentric thigh (random dominant and non-dominant thigh), 2 days after the one-legged eccentric exercise. The study was approved by the Copenhagen Ethics Committee and conforms with the code of ethics of the World Medical Association (Declaration of Helsinki). Subjects were covered by state medical insurance and, in addition, by the same insurance as hospitalized patients, in case of complications.
Diet
Two days before the one-legged eccentric exercise, subjects commenced a standard weight-maintaining diet, containing at least 5 g carbohydrate per kilogram of body weight, and maintained a constant activity level where slow or leisurely walking and bicycling were allowed, but the subjects abstained from exercise. The carbohydrate-rich diet was consumed until the end of the experiment, and the subjects were instructed to avoid alcohol, smoking and drugs during this period.
Experimental procedure
On the morning of the one-legged eccentric exercise, subjects had fasted for 10-12 h. A venous blood sample was obtained and thigh muscle soreness was evaluated. Eccentric exercise was then performed as described previously (Asp et al. 1995a). The subjects performed maximal voluntary eccentric contractions with one thigh (randomized dominant/non-dominant) while seated on a chair. This involved resisting the backward motion of the lower leg enforced through a rod by a motor-driven device. The session consisted of four 5 min bouts, with a motor velocity of 30 r.p.m., interrupted by 2 min rest periods. In order to vary the strain on the muscle, the fixed hip angle (which was dependent on the angle of the chair back) and the knee angle around which the lower leg moved equidistantly (dependent on the distance between the chair and motor-driven device) were varied in the different exercise bouts. The hip/knee angles during the different bouts were 90/90 deg, 120/90 deg, 90/120 deg and 120/120 deg. In theory, the contralateral thigh could either have been an unexercised control or the subjects could have performed concentric exercise with similar total work output as during the eccentric exercise (with reverse signs). However, it would have been impossible for the subjects to perform concentric exercise with the same high power output as during the eccentric exercise and exercise time should have been introduced as a new variable to uniform total work output. For these reasons we decided to use the contralateral thigh as unexercised muscle. One and two days after the eccentric exercise bout, following an overnight fast, the subjects returned to the laboratory. Muscle soreness was evaluated and blood samples were obtained from an arm vein. On day 2, after resting 30 min in the supine position in a room specially arranged for invasive procedures, Teflon catheters were placed in both femoral veins and in one femoral artery using aseptic techniques, and the tips were advanced centrally to approximately 2 cm below (venous catheter) and above (arterial catheter) the inguinal ligament. A thermistor probe (Edslab probe 94-030-2.5-F, Baxter), for measuring blood temperature, was inserted through each venous catheter and advanced 8-10 cm proximal to the catheter tip. Catheterization and biopsies have been employed in many previous studies (Andersen & Saltin, 1985; Richter, Mikines, Galbo & Kiens, 1989) and were performed by a medical doctor with more than 4 years’ experience in these procedures. Blood was then obtained from the three femoral catheters simultaneously. Resting femoral venous blood flow was measured by the thermodilution method using bolus injections of 3 ml ice-cold sterile saline (Andersen & Saltin, 1985). Whenever blood was sampled, or flow measured, pneumatic cuffs below the knees were inflated to 230 mmHg to exclude circulation to the lower leg. Three resting muscle samples were obtained from each vastus lateralis under local anaesthesia (Xylocain, 20 mg ml−1; Astra, Sweden) using the Bergstrøm needle technique (Bergstrøm, 1962) with suction. Biopsies were taken alternating between the eccentric and control muscle. A portion of each specimen was immediately frozen in liquid nitrogen and kept at -80°C awaiting assay for metabolites. A second portion was treated with collagenase for subsequent harvest of giant sarcolemmal vesicles, as described previously (Kristiansen, Hargreaves & Richter, 1996b). The rest of the biopsy was embedded in tissue-tek (Miles, Elkhart, IN, USA) and frozen in isopentane, cooled over liquid N2, for histochemical determination of glycogen using the periodic acid-Schiff (PAS) reaction and fibre composition.
The subjects were then placed in a chair with rods connecting their ankles to the pedals of two identical and independent knee-extensor apparatuses constructed locally, based on the Monark Ergomedic 829E bicycle ergometer (Monark, Sweden). Two or three prior practice runs had accustomed the subjects to this apparatus and its operation. Two-legged concentric knee-extensor exercise was performed at two levels, each lasting approximately 25 min, and the thighs performed uniform exercise (load and r.p.m.) throughout the entire exercise period. During step 1, the absolute load for each thigh was equivalent to the load at 42 ± 2 % of the non-dominant maximal work capacity (mean, 26 W; range, 22-32 W). During step 2 of the exercise it was equivalent to 57 ± 2 % (mean, 35 W; range, 30-44 W), with a constant pedal frequency of 60 r.p.m. Blood samples were obtained from all femoral catheters at 10, 20 and 25 min during each exercise step, and flow in each femoral vein was measured immediately before sampling by the thermodilution technique described by Andersen and Saltin (Andersen & Saltin, 1985). Muscle exertion in each thigh was rated subjectively at the same time points, using the Borg scale. Immediately after termination of exercise, three more muscle biopsies (post) were obtained from each vastus lateralis muscle, alternating between eccentric and control thighs.
Analytical procedures
Blood and plasma glucose and blood lactate were measured with glucose and lactate analysers, respectively (Yellow Springs, OH, USA). Plasma long-chain non-esterified fatty acids (NEFA) were determined using an enzymatic kit (Wako, Neuss, Germany) and plasma glycerol by the glycerolkinase method, and both assays were run on a Cobas Fara (Roche, Switzerland). Creatine kinase was measured at 30°C using a commercially available kit (Sigma). Blood haemoglobin and oxygen saturation were measured on an OSM3 analyser (Radiometer, Copenhagen, Denmark). Muscle biopsies were freeze-dried and dissected free of blood and connective tissue before analysis. Glycogen was measured by a hexokinase method after acid hydrolysis (Lowry & Passonneau, 1972). Giant sarcolemmal vesicles were obtained as previously described (Kristiansen et al. 1996b), and the vesicle GLUT4 protein content was quantified by Western blot using a mouse monoclonal primary antibody directed against the thirteen C-terminal amino acids of GLUT4 and a horseradish peroxidase-labelled goat anti-mouse antibody as described previously (Asp et al. 1995a). For histological determination of glycogen and muscle fibre type, serial transverse sections (10 μm) of the embedded frozen muscle samples were cut in a cryotome at -20°C. The sections were mounted on coverslips and stained for myofibrillar actomyosin ATPase at pH 9.4 after both acid (pH 4.3 and 4.6) and alkaline (pH 10.3) preincubations (Brooke & Kaiser, 1970), to identify type I, II A and II B fibres. Serial transverse sections were stained for glycogen using the PAS reaction (Pearse, 1968), and the relative glycogen contents in the individual muscle fibres were estimated from PAS staining intensity (Gollnick, Piehl & Saltin, 1974), which was quantified using a COMFAS image scanner (SbsysCOMFAS, Scan Beam, Hadsund, Denmark). Muscle soreness was subjectively rated by palpation of the proximal, intermediate and distal areas of the vastus lateralis of the quadriceps muscles, using a rating scale from 0 (no soreness) to 4 (extreme soreness). Palpation and recording were performed throughout the study by the same person. Soreness scores were averaged (proximal, intermediate and distal) each day for statistical comparison. Muscle exertion in each thigh was rated subjectively at 10, 20 and 25 min during step 1 and step 2 of the two-legged concentric exercise, using the Borg scale ranging from 6 (lighter than very, very light) to 20 (harder than very, very hard).
Statistics
Plasma and blood values at rest were compared using Student's paired t test. To compare mean values in plasma during each exercise step, a two-way analysis of variance for repeated measures was employed, and the Student-Newman-Keuls test was used for posthoc comparisons. Muscle values before and after the exercise, and differences (control thigh vs. eccentric thigh), were compared by Student's paired t test, using the Bonferroni correction if multiple comparisons were made. Since the creatine kinase and soreness data were not distributed normally, a non-parametric test was used (Wilcoxon) for these data. The level of significance was set at P < 0.05 for all tests. Figures and tables display means ±s.e.m., and n= 7 for all groups.
Calculations
Local exchanges over the two thighs were calculated as the product of arterio-venous concentration difference and perfusion. Blood oxygen content was calculated by multiplying the arterio-venous difference in oxygen content by the thigh blood flow. Mean arterial lactate and glycerol concentrations and total glycerol exchanges over the thighs were calculated on each exercise step, with the 10, 20 and 25 min values contributing 50, 37.5 and 12.5 %, respectively.
RESULTS
Maximal knee-extensor concentric exercise capacity was 23 % lower in the eccentric thigh than in the control thigh (52 ± 4 vs. 67 ± 3 W; P < 0.05) 2 days after the eccentric exercise. Muscle soreness in the eccentrically exercised thigh increased from 0 (range, 0-0), to 1.3 (range, 0.8-2.1) and 1.9 (range, 0.8-2.5) arbitrary units (a.u.) on day 1 and 2, respectively (P < 0.05). Soreness scores in the control thigh showed no tendency towards a change from 0 (data not shown).
Creatine kinase increased from 54 U l−1 (range, 32-86 U l−1) before the eccentric exercise to 620 U l−1 (range, 117- 1571 U l−1) on day 1 and 2211 U l−1 (range, 87-8138 U l−1) on day 2.
During the two-legged concentric exercise bout, the subjective rating of muscle exertion increased in the control and eccentric thigh, and the values were higher in the eccentric thigh throughout the exercise. The mean ratings were 12.3 ± 0.6 vs. 10.3 ± 0.4, 14.0 ± 0.6 vs. 12.2 ± 0.5 and 14.3 ± 0.6 vs. 12.3 ± 0.5 in the eccentric and control thigh, respectively, after 10, 20 and 25 min during step 1 of the exercise, and 17.3 ± 0.6 vs. 14.4 ± 1.0, 18.7 ± 0.6 vs. 15.7 ± 1.3 and 19.7 ± 0.3 vs. 17.6 ± 1.0 in the eccentric and control thigh, respectively, at the same time points during step 2 of the exercise.
Prior to concentric exercise the glycogen content was lower in the previously eccentrically exercised thigh than in the control thigh (402 ± 30 vs. 515 ± 26 mmol (kg dry wt)−1; P < 0.05). Following concentric exercise this difference substantially increased (190 ± 46 vs. 379 ± 58 mmol (kg dry wt)−1; P < 0.05). The glycogen utilization was significantly higher (P < 0.05) in the eccentric thigh (212 ± 32 mmol (kg dry wt)−1) compared with control (137 ± 49 mmol (kg dry wt)−1) (Fig. 1).
Figure 1. Glycogen concentration in eccentric and control muscle before and after two-legged concentric exercise.
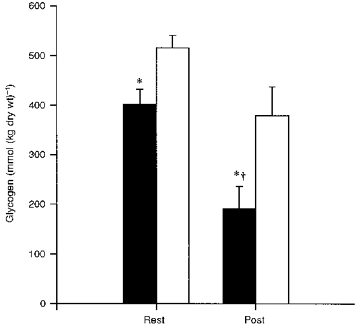
Each bar shows the mean ±s.e.m. of 7 observations, for values from the eccentric thigh that had performed eccentric exercise 2 days prior to the concentric exercise (▪) and values from the contralateral control thigh (□). Rest and Post represent the values before and after the concentric exercise bout, respectively. * Significantly (P < 0.05) different from the control thigh. † Decrease in concentration during concentric exercise is significantly (P < 0.05) different from the decrease in the control thigh.
Prior eccentric exercise had a pronounced effect on the resting glycogen content in the type II fibres, and less so on the type I fibres. The intensity of the PAS staining in the eccentric type II fibres before the concentric exercise resembled the staining intensity in the control type II fibres after the concentric exercise. After the concentric exercise bout, none (0 %) of the fibres in muscle from the eccentric thigh were darkly stained, whereas in the control muscle this occurred in 26, 51 and 36 % of the type I, II A and II B fibres, respectively (Fig. 2).
Figure 2. Glycogen depletion pattern with concentric exercise in the different muscle fibre types in eccentric and control muscle.
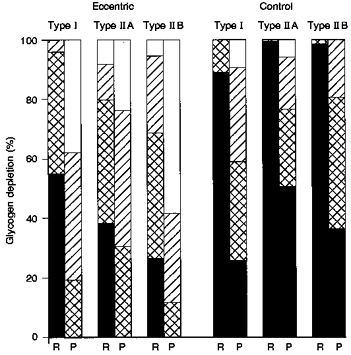
Histochemical estimation of glycogen distribution in the type I, II A and II B fibres in eccentric and control vastus lateralis at rest (R) and after two-legged concentric exercise (P), using a scale of dark (▪), moderate ( ), light (
), light ( ) and negative (□) stained. Dark stained represents fibres which are glycogen filled, and negative stained represents fibres which are glycogen depleted.
) and negative (□) stained. Dark stained represents fibres which are glycogen filled, and negative stained represents fibres which are glycogen depleted.
At rest, giant sarcolemmal vesicle GLUT4 content was not statistically different between eccentric and control muscle (0.23 ± 0.05 and 0.31 ± 0.06 a.u., respectively). The concentric exercise increased the GLUT4 content 2.1 (± 0.6)-fold in vesicles from eccentric muscle and 1.9 (± 0.4)-fold in vesicles from control muscle (Fig. 3). There was no significant difference in vesicle glucose transport between eccentric and control muscle at rest (0.48 ± 0.07 and 0.59 ± 0.10 pmol μg−1 min−1, respectively) (Fig. 3), and the concentric exercise induced an identical 1.7 (± 0.2)-fold increase in glucose transport in vesicles obtained from either thigh (Fig. 3).
Figure 3. Changes with concentric exercise in GLUT4 content and glucose transport in giant sarcolemmal vesicles from eccentric and control muscle.
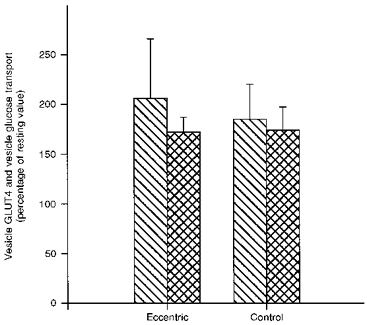
Bars show means ±s.e.m. of 7 observations.  , percentage change from resting to post muscle biopsies in vesicle GLUT4 content;
, percentage change from resting to post muscle biopsies in vesicle GLUT4 content;  , percentage change in vesicle glucose transport.
, percentage change in vesicle glucose transport.
Thigh (venous) blood flow and oxygen uptake increased dramatically from rest to exercise. There were no differences in blood flow or oxygen uptake between the two thighs throughout the experiment (Table 1).
Table 1.
Blood flow, oxygen and glucose uptake, lactate release, NEFA uptake and glycerol release in eccentric and control thigh before (rest) and at each step during the two-legged concentric exercise
| Blood flow (l thigh−1 min−1) | Oxygen uptake (ml thigh−1 min−1) | Glucose uptake (μmol thigh−1 min−1) | Lactate release (μmol thigh−1 min−1) | NEFA uptake (μmol thigh−1 min−1) | Glycerol release (μmol thigh−1 min−1) | ||
|---|---|---|---|---|---|---|---|
| Eccentric thigh | |||||||
| Rest | 0.25 ± 0.04 | 10.7 ± 2.2 | 31.0 ± 8.9 | 37.0 ± 5.8* | −9.1 ± 7.0 | 6.7 ± 3.7 | |
| Step 1 | 10 min | 4.1 ± 0.21 | 510 ± 39 | 306 ± 72 | 846 ± 206 | 62 ± 35 | 1.1 ± 10.6 |
| 20 min | 3.9 ± 0.22 | 493 ± 8 | 391 ± 76 | 611 ± 238 | 69 ± 30 | −11.2 ± 12.9 | |
| 25 min | 4.0 ± 0.17 | 504 ± 30 | 510 ± 62 | 286 ± 138 | 129 ± 46 | −11.4 ± 14.4 | |
| Step 2 | 10 min | 4.5 ± 0.38 | 622 ± 52 | 642 ± 142 | 1496 ± 478 | 60 ± 28 | 11.1 ± 2.0 |
| 20 min | 4.1 ± 0.29 | 565 ± 37 | 742 ± 118 | 603 ± 396 | 80 ± 58 | 29.0 ± 5.4 | |
| 25 min | 4.3 ± 0.40 | 592 ± 61 | 1166 ± 164 | 358 ± 288;dagger; | 174 ± 58 | 1.4 ± 12.2 | |
| Control thigh | |||||||
| Rest | 0.23 ± 0.03 | 11.2 ± 2.7 | 32.5 ± 5.9 | 21.2 ± 6.7 | −9.3 ± 8.0 | 5.3 ± 3.8 | |
| Step 1 | 10 min | 4.3 ± 0.28 | 540 ± 42 | 520 ± 280 | 807 ± 322 | −1.2 ± 55 | 19.8 ± 13.2 |
| 20 min | 4.2 ± 0.32 | 552 ± 49 | 389 ± 74 | 126 ± 204 | 25 ± 15 | 37.9 ± 20.4 | |
| 25 min | 4.0 ± 0.32 | 530 ± 43 | 620 ± 87 | 201 ± 166 | 117 ± 77 | 22.9 ± 13.3 | |
| Step 2 | 10 min | 4.4 ± 0.43 | 606 ± 44 | 498 ± 98 | 604 ± 291 | 69 ± 43 | 6.3 ± 14.8 |
| 20 min | 4.3 ± 0.36 | 570 ± 47 | 557 ± 128 | 990 ± 466 | 51 ± 34 | 17.6 ± 17.8 | |
| 25 min | 4.2 ± 0.44 | 596 ± 60 | 901 ± 222 | 1376 ± 438 | 101 ± 32 | 1.3 ± 16.2 | |
Values are means ±s.e.m. of 7 observations in each group. Eccentric thigh, the thigh which performed the eccentric exercise 2 days before the two-legged concentric exercise; Control thigh, the contralateral thigh that acted as unexercised control thigh.
Significantly different from contralateral control (P < 0.05).
Significantly different from the ipsilateral 10 min value on the same exercise step (P < 0.05).
Arterial glucose concentrations were stable during the exercise bout (Table 2). Glucose uptake was similar in the eccentric and control thigh at rest. During exercise, glucose uptake increased similarly in the two thighs (Table 1).
Table 2.
Arterial concentrations for glucose, lactate, NEFA and glycerol before (rest) and at each step during the two-legged concentric exercise
| Glucose (mmol l−1) | Lactate (mmol l−1) | Mean lactate (mmol l−1) | NEFA (μmol l−1) | Glycerol (μmol l−1) | Mean glycerol (μmol l−1) | ||
|---|---|---|---|---|---|---|---|
| Rest | 4.9 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.0 | 423 ± 85 | 78 ± 12 | 78 ± 12 | |
| Step 1 | 10 min | 5.0 ± 0.2 | 1.6 ± 0.1 | − | 394 ± 66 | 115 ± 14 | — |
| 20 min | 4.9 ± 0.3 | 1.6 ± 0.2 | 1.6 ± 0.2* | 395 ± 87 | 116 ± 13 | 117 ± 12* | |
| 25 min | 4.9 ± 0.3 | 1.4 ± 0.2 | − | 434 ± 89 | 125 ± 12 | − | |
| Step 2 | 10 min | 4.9 ± 0.3 | 2.5 ± 0.2 | − | 439 ± 83 | 129 ± 13 | — |
| 20 min | 4.9 ± 0.3 | 2.8 ± 0.2 | 2.7 ± 0.2* | 464 ± 95 | 137 ± 15 | 136 ± 14* | |
| 25 min | 5.0 ± 0.3 | 2.9 ± 0.2 | − | 510 ± 105 | 160 ± 20 | — |
Values are means ±s.e.m. of 7 observations in each group. Mean lactate and glycerol represent the mean values for each step (rest, step 1 and step 2).
Mean value is significantly different from the mean value on the previous step.
Arterial lactate concentrations increased from rest to step 1, and further from step 1 to step 2 (Table 2). Lactate release was higher from the eccentric thigh compared with control at rest (P < 0.05; Table 1). During step 1 of the exercise, releases were similar from the two thighs, whereas they differed during step 2. During step 2, less lactate was released from the eccentric thigh at 25 min compared with at 10 min (P < 0.05), whereas no significant changes occurred in release from the control thigh.
Arterial concentration of NEFA did not change from rest to exercise (Table 2). At rest, no net exchange of NEFAs was detectable (Table 1), whereas there was a net NEFA uptake in both thighs during the exercise. There were no significant differences between the two thighs.
Arterial concentration of glycerol increased from rest to step 1 and a further slight increase was observed from step 1 to step 2 (Table 2). Total glycerol release was lower from the eccentric thigh (-0.22 ± 0.23 mmol thigh−1) than from the control thigh (0.67 ± 0.29 mmol thigh−1) (P < 0.05) during step 1 of the exercise (Fig. 4). There were no significant differences in total release during step 2 (0.42 ± 0.07 vs. 0.25 ± 0.32 mmol thigh−1) or when summarized over both exercise steps.
Figure 4. Total glycerol release in eccentric and control thigh during step 1 and step 2 of the two-legged concentric exercise.
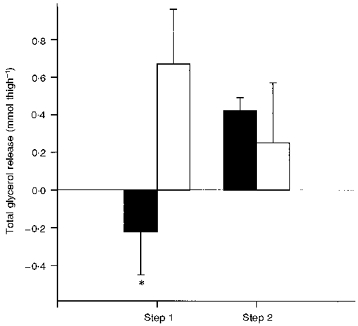
Each bar shows mean ±s.e.m. of 7 observations, for values from the eccentric thigh that had performed eccentric exercise 2 days prior to the concentric exercise (▪) and values from the contralateral control thigh (□). Glycerol release was measured at rest, and at 10, 20 and 25 min during step 1 (lighter exercise) and step 2 (heavier exercise) of the two-legged concentric exercise, and on each step the total release was calculated from these values. * Significantly (P < 0.05) different from the control thigh at the same exercise step.
DISCUSSION
The present study shows that 2 days after unaccustomed eccentric exercise, muscle glycogen content and maximal concentric exercise capacity are decreased. Thus, during concentric exercise at a given power output, eccentrically damaged muscle is working at a higher relative workload, resulting in increased glycogen utilization rate and, in turn, decreased endurance.
Strenuous unaccustomed exercise is followed by reductions in both isometric force (Davies & White, 1981; Newham et al. 1987; Nosaka et al. 1991) and short-term dynamic power output (Sargeant & Dolan, 1987). These functional changes may last several days or weeks, but the mechanisms involved are not completely understood. The reduction of dynamic power output may partly originate from contractile protein destruction (Armstrong et al. 1983; Newham et al. 1983; Friden et al. 1983), muscle soreness (Schwane et al. 1983) or stiffness (Jones et al. 1987), but disturbed metabolism could also play a role. Prior eccentric contractions were found to impair the increase in rat muscle glucose transport during subsequent electrical stimulation (Kristiansen et al. 1996a), and eccentric contractions are also known to be followed by sustained decreased muscle glycogen content (O'Reilly et al. 1987; Costill et al. 1990; Widrick et al. 1992; Doyle et al. 1993; Asp et al. 1995a) and decreased muscle GLUT4 content (Asp et al. 1995a, b Asp, Daugaard, Kristiansen, Kiens & Richter, 1996; Kristiansen et al. 1996a). These changes could have an effect on a subsequent bout of concentric exercise, but this has not yet been thoroughly investigated. Studies in man, in which phosphorous magnetic resonance spectroscopy was used at rest after eccentric exercise, reported changes in the ratio between inorganic phosphate and phosphocreatine, suggesting an increase in resting metabolism (McCully, Shellock, Bank & Posner, 1992; Rodenburg et al. 1995), whereas one study using this technique failed to detect any effect of prior eccentric exercise on phosphate metabolism during subsequent concentric exercise (Rodenburg et al. 1995). However, the latter negative finding might reflect the relatively low intensities of exercise used, and does not necessarily exclude changes in other pathways of exercise metabolism.
In the current study, the mean resting glycogen content was 22 % lower in eccentric compared with control thigh muscle. This magnitude of difference is in accordance with previous findings (Asp et al. 1995a, 1996). The resting glycogen content of the type II fibres was particularly affected (Fig. 2). This predominant effect of eccentric contractions on fast-twitch fibres is in accordance with previous findings in humans (Jones, Newman, Round & Tolfree, 1986) and rats (Lieber, Woodburn & Friden, 1991; Asp et al. 1995b). Increased glycogenolysis might play a role in the resting glycogen pattern and could be the basis for the higher resting lactate release from the eccentric thigh reported here (Table 1) and previously (Asp et al. 1996), which, furthermore, fits the above mentioned change in resting metabolism (McCully et al. 1992; Rodenburg et al. 1995). Interestingly, in the current study, the resting glycogen concentration in the eccentric thigh was very similar to the concentration in the control thigh after the concentric bout, and a similar pattern was found with the PAS staining intensity in the type II fibres, whereas the type I fibres appeared more depleted by the concentric exercise than by prior eccentric contractions (Fig. 2).
Maximal work capacity was 23 % lower with the eccentric than with the control thigh on the two-legged concentric exercise day, and the eccentric thigh therefore performed exercise at a higher relative intensity compared with the control thigh during the exercise bout, since the same power output was generated by each thigh. Increasing the relative exercise intensity is known to increase the relative utilization of carbohydrate at the expense of fat in undamaged muscle, and therefore one might expect a higher carbohydrate oxidation during exercise in the eccentric than in the control thigh. In fact glycogen breakdown was increased by 55 % in the eccentric compared with the control thigh (Fig. 1), whereas glucose uptake was similar in the two thighs (Table 1). Because work efficiency was not different in the two thighs, as judged by the similar oxygen uptake during exercise (Table 1), an increase in glycogen utilization in the eccentric thigh should result in decreased utilization of other substances, in particular fat. Lower glycerol release from the eccentric thigh (-0.22 ± 0.23 mmol thigh−1) than from the control thigh (0.67 ± 0.29 mmol thigh−1) (P < 0.05) during step 1 of the exercise is indirect evidence suggesting impaired muscle triacylglycerol breakdown (Fig. 4), but it can quantitatively account for only approximately 16 % of the difference in glycogen utilization during the entire exercise bout. Net uptake of NEFA was not different in the two thighs (Table 1), but the variability in these data may preclude detection of relatively small differences. Thus, a plausible explanation for the higher glycogen utilization during exercise in the eccentric than in the control thigh, at least in part, is a higher relative workload in the eccentric thigh. As previously discussed, the reason for this reduction in dynamic work capacity after eccentric exercise is not well understood. From the present data, showing severe glycogen depletion in the eccentric thigh at the end of exercise (Fig. 2), it might be suggested that decreased glycogen availability is an important contributing factor. This suggestion is supported by the decreased lactate release from the eccentric thigh towards exhaustion.
In a previous study in rats we showed that prior eccentric contractions decreased the subsequent glucose transport and uptake during isometric contractions in the perfused hindlimb (Kristiansen et al. 1996a). Thus, one might have expected that in the present study glucose uptake during exercise would increase less in the eccentric muscle compared with control. Nevertheless, such a difference was not found (Table 1). While differences between the current study and the perfusion study are not readily reconciled, it should be borne in mind that the degree of muscle damage and decrease in GLUT4 protein content are more marked in rat muscle after electrically induced eccentric in situ contractions (Asp et al. 1995b) than in human muscle after in vivo contractions (Asp et al. 1995a). Therefore, the difference might be ascribed to the more severe effects on rat than on human muscle in our studies. On the other hand, in the present study - in contrast to the previous rat study (Kristiansen et al. 1996a) - muscle glycogen concentrations towards the end of exercise were markedly lower in eccentric compared with control muscle. In a preliminary communication it was reported that part of the intracellular GLUT4 pool may be translocated in response to decreasing glycogen concentrations (Coderre, Vallega & Pilch, 1994). If such a scheme is correct, it might mean that in eccentric muscle two opposing regulators of GLUT4 translocation operate: one could be the decreased total GLUT4 pool (Asp et al. 1995a) causing a smaller translocation of GLUT4 during exercise, while the other might be the low muscle glycogen content in turn increasing GLUT4 translocation. The net result could explain the present finding that GLUT4 translocation in eccentric muscle during exercise is similar to that in control muscle (Fig. 3).
It is concluded that eccentric exercise causes sustained decreased muscle glycogen content and decreased maximal concentric exercise capacity. Thus, at a given absolute workload, muscle subjected to prior eccentric damage is working at a higher relative workload, resulting in increased utilization of the decreased muscle glycogen stores and, in turn, in decreased endurance.
Acknowledgments
Betina Bolmgren and Nina Pfluzek provided skilled technical assistance. Nick Oakes is sincerely thanked for constructive criticism. The study was supported by grants from the Danish Sport Research Council (project no. 55272) and by the Danish National Research Foundation (grant no. 504-14). Søren Kristiansen is supported by the Danish Research Council (grant no. 9600422).
References
- Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as a model for study of isolated exercising muscle in humans. Journal of Applied Physiology. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. Journal of physiology. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. Journal of Applied Physiology. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Asp S, Daugaard JR, Kristiansen S, Kiens B, Richter EA. Eccentric exercise decreases maximal insulin action in humans: muscle and systemic effects. Journal of Physiology. 1996;494:891–898. doi: 10.1113/jphysiol.1996.sp021541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp S, Daugaard JR, Richter EA. Eccentric exercise decreases glucose transporter GLUT4 protein in human skeletal muscle. Journal of Physiology. 1995a;482:705–712. doi: 10.1113/jphysiol.1995.sp020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp S, Kristiansen S, Richter EA. Eccentric muscle damage transiently decreases rat skeletal muscle GLUT4 protein. Journal of Applied Physiology. 1995b;79:1338–1345. doi: 10.1152/jappl.1995.79.4.1338. [DOI] [PubMed] [Google Scholar]
- Bergstrøm J. Muscle electrolytes in man. Scandinavian Journal of Clinical Laboratory Investigation. 1962;14(suppl. 68):11–13. [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind. Archives of Neurology. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Coderra L, Vallega G, Pilch PF. Association of GLUT4 vesicles with glycogen particles in skeletal muscle: identification of a contraction-sensitive pool. Diabetes. 1994;43:159A. [Google Scholar]
- Costill DL, Pascoe DD, Fink J, Robergs RA, Barr SI, Pearson D. Impaired muscle glycogen resynthesis after eccentric exercise. Journal of Applied Physiology. 1990;69:46–50. doi: 10.1152/jappl.1990.69.1.46. [DOI] [PubMed] [Google Scholar]
- Davies CTM, White MJ. Muscle weakness following eccentric work in man. Pflügers Archiv. 1981;392:168–171. doi: 10.1007/BF00581267. [DOI] [PubMed] [Google Scholar]
- Doyle JA, Sherman WM, Strauss RL. Effect of eccentric and concentric exercise on muscle glycogen replenishment. Journal of Applied Physiology. 1993;74:1848–1855. doi: 10.1152/jappl.1993.74.4.1848. [DOI] [PubMed] [Google Scholar]
- Friden J, Sjøstrøm M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. International Journal of Sports Medicine. 1983;4:170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- Golden CL, Dudley GA. Strength after bouts of eccentric or concentric actions. Medicine and Science in Sports and Exercise. 1992;24:926–933. [PubMed] [Google Scholar]
- Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle fibers after exercise of varying intensity and at varying pedalling rates. Journal of Physiology. 1974;241:45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA, Newham DJ, Clarkson PM. Skeletal muscle stiffness and pain following eccentric exercise of the elbow flexors. Pain. 1987;30:233–242. doi: 10.1016/0304-3959(87)91079-7. [DOI] [PubMed] [Google Scholar]
- Jones DA, Newham DJ, Round JM, Tolfree SEJ. Experimental human muscle damage: morphological changes in relation to other indices of damage. Journal of Physiology. 1986;375:435–448. doi: 10.1113/jphysiol.1986.sp016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen S, Asp S, Richter EA. Decreased muscle GLUT-4 and contraction-induced glucose transport after eccentric contractions. American Journal of Physiology. 1996a;271:R477–482. doi: 10.1152/ajpregu.1996.271.2.R477. [DOI] [PubMed] [Google Scholar]
- Kristiansen S, Hargreaves M, Richter EA. Exercise-induced increase in glucose transport, GLUT4 and VAMP-2 in plasma membrane from human muscle. American Journal of Physiology. 1996b;270:E197–201. doi: 10.1152/ajpendo.1996.270.1.E197. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Woodburn TM, Friden J. Muscle damage induced by eccentric contractions of 25 % strain. Journal of Applied Physiology. 1991;70:2498–2507. doi: 10.1152/jappl.1991.70.6.2498. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. [Google Scholar]
- McCully K, Shellock FG, Bank WJ, Posner JD. The use of nuclear magnetic resonance to evaluate muscle injury. Medicine and Science in Sports and Exercise. 1992;24:537–542. [PubMed] [Google Scholar]
- Newham DJ, Jones DA, Clarkson PM. Repeated high-force eccentric exercise: effects on muscle pain and damage. Journal of Applied Physiology. 1987;63:1381–1386. doi: 10.1152/jappl.1987.63.4.1381. [DOI] [PubMed] [Google Scholar]
- Newham DJ, McPhail G, Mills KR, Edwards RHT. Ultrastructural changes after concentric and eccentric contractions of human muscle. Journal of the Neurological Sciences. 1983;61:109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Nosaka K, Clarkson PM, McGuiggin ME, Byrne JM. Time course of muscle adaptation after high force eccentric exercise. European Journal of Applied Physiology. 1991;63:70–76. doi: 10.1007/BF00760804. [DOI] [PubMed] [Google Scholar]
- O'Reilly KP, Warhol MJ, Fielding RA, Frontera WR, Meredith CN, Evans WJ. Eccentric exercise-induced muscle damage impairs muscle glycogen repletion. Journal of Applied Physiology. 1987;63:252–256. doi: 10.1152/jappl.1987.63.1.252. [DOI] [PubMed] [Google Scholar]
- Pearse AGE. Histochemistry - Theoretical and Applied. London: J. & A. Churchill Ltd; 1968. pp. 307–322. [Google Scholar]
- Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. Journal of Applied Physiology. 1989;66:876–885. doi: 10.1152/jappl.1989.66.2.876. [DOI] [PubMed] [Google Scholar]
- Rodenburg JB, de Groot MCH, Echteld CJA, Jongsma HJ, Bar PR. Phosphate metabolism of prior eccentrically loaded vastus medialis muscle during exercise in humans. Acta Physiologica Scandinavica. 1995;153:97–108. doi: 10.1111/j.1748-1716.1995.tb09840.x. [DOI] [PubMed] [Google Scholar]
- Sargeant AJ, Dolan P. Human muscle function following prolonged eccentric exercise. European Journal of Applied Physiology. 1987;56:704–711. doi: 10.1007/BF00424814. [DOI] [PubMed] [Google Scholar]
- Schwane JA, Scarlet RJ, Vandenakker CB, Armstrong RB. Delayed-onset muscular soreness and plasma CPK and LDH activities after downhill running. Medicine and Science in Sports and Exercise. 1983;15:51–56. [PubMed] [Google Scholar]
- Widrick JJ, Costill DL, McConell GK, Anderson DE, Pearson DR, Zachwieja JJ. Time course of glycogen accumulation after eccentric exercise. Journal of Applied Physiology. 1992;72:1999–2004. doi: 10.1152/jappl.1992.72.5.1999. [DOI] [PubMed] [Google Scholar]


