Abstract
This review focuses on recent advances in our understanding of temporal pattern coding in the motor systems of animals and man. Examples of millisecond time scale rhythmic synchronization in the visual system are considered. Results of experiments that demonstrate similar phenomena in the motor system are discussed. Finally problems concerning the nature of the correlation between neurophysiological signals and the relationship of correlation to motor behaviour are explored.
In this paper I shall examine the hypothesis that rhythmic synchronization of neurophysiological activity provides a mechanism for integration of the distributed motor and sensory systems involved in co-ordinated movement and posture. First, I shall discuss cortical oscillations in the mammalian visual system. Second, I shall present experimental data from studies of oscillatory behaviour in the motor systems of primates and man. Finally, I shall discuss specific problems concerning the functional interpretation of oscillatory activity in the motor system.
The visual system is confronted with the problem of identification of objects or groups of objects that may be embedded in a complex background. Visual information comprises local borders of luminance and wavelength distributed across the field of vision. Although the possible feature combinations are potentially limitless, when confronted with any novel visual scene the system rapidly and accurately segregates the components (for reviews, see Engel, Konig, Kreiter, Schillen & Singer, 1992; Singer, 1993; Gray, 1994; Singer & Gray, 1995). In doing this the system needs to avoid the ‘superposition catastrophe’ and a potential ‘combinatorial explosion’ of neural representations. In addition, our knowledge of the architecture of the visual system indicates that it is organized in a parallel fashion with different processing areas sharing information. How are relationships established and broken between spatially distributed neuronal populations? At some point the information must be gathered together as a single percept. Whilst to an extent there exists a hierarchy of processing systems, there is no single neurone onto which streams of information converge for every single object or potential object. How is activity in different visual areas bound together? Finally, in the cerebral cortex processing systems spatially overlap. A visual task may, therefore, require segregation of neuronal assemblies at one point in time and binding together of assemblies at another point in time. In recent years many sensory physiologists have championed the idea that in part these problems, referred to in general terms as the binding problem, may be solved through transient temporal synchronization of the discharges of populations of neurones (see Von der Malsburg, 1995).
Does the binding problem arise in motor system physiology and if so are there theoretical and experimental reasons for supposing that millisecond time scale synchronization between neurones of the sensorimotor cortex may provide a solution?
What can be learnt from studies of visual perception?
The population coding approach assumes that individual neurones can participate at different times in the representation of different sensory patterns. The code is assumed to be relational, by which it is meant that the significance of an individual cell's response depends on the context set by other members of the neuronal assembly. Active cells participating in a representation must be unambiguously identified as belonging together. This may be achieved through rate coding. However, there is a problem with simple rate coding; for in order to encode a particular visual feature and separate it from background, a neurone or group of neurones in an assembly must become segregated and as a consequence alter firing rate(s). In doing this the system loses flexibility, since any necessary interactions or sharing of information between the segregated assembly and other assemblies will affect the firing rate and, therefore, introduce ambiguity. The synaptic influence of multiple neurones converging onto others is enhanced if they fire in coincidence. Rather than simply increasing or decreasing neuronal discharge rate, it has been proposed that response selection and binding may occur through the increased saliency achieved through transient millisecond time scale synchronization of neuronal discharges across a distributed neural network. Thus the relative timing of neuronal discharges, their temporal pattern, assumes importance.
The above considerations have led to the following predictions concerning multiunit recordings from the mammalian cerebral cortex (Singer & Gray, 1995). Correlated firing should occur between cells recorded in (a) the same cortical column of a given area to enable the coding of local features, (b) different columns within an area to enable the linking of local features, (c) different cortical areas to provide for the binding of information across different feature categories and different locations in space, (d) the two cerebral hemispheres to link information present in the two visual fields, and (e) different sensory and motor modalities to contribute to the processes of sensorimotor integration. The work of the visual system neurophysiologists has produced empirical support for predictions (a)-(d) in mammals and primates. As will be discussed, there is emerging evidence for conjecture (e) in primates and man.
Visual system experiments have established several important facts. First, populations of neurones in the primary visual cortex of cat display regular rhythmic firing at approximately 40 Hz. Second, when presented with visual stimuli organized in a certain way the ∼40 Hz rhythmic firing of separate neurones may become synchronized. This has been established through cross-correlation analysis of discharges from different neurones within striate cortex and between striate and extra-striate visual areas (Eckhorn et al. 1988; Gray, Konig, Engel & Singer, 1989; Gray & Singer, 1989; Engel, Kreiter, Konig & Singer, 1991); the resulting correlogram contains a peak at time zero with side bands indicating that the neuronal discharges are both oscillatory and synchronized. The perceptual conditions which favour this type of synchronous discharge behaviour are those in which ‘Gestalt’ criteria are met. For example, two separate bars moving across the visual field in the same direction share a common fate, whereas bars moving in opposite directions do not. The important result being that in the former experiment the two groups of neurones synchronize their discharges, while in the latter situation the same two groups of neurones fire asynchronously (see Fig. 1). In addition, the presence of synchronization correlates with the animal's perception as determined by its behavioural response (Fries, Roelfsema, Engel, Konig & Singer, 1997). Another important point to arise from these studies is that distant neurones, for example in homologous left and right visual cortical areas, may, given the right perceptual conditions, synchronize their discharges at zero phase delay despite the significant conduction times involved in interneuronal communication.
Figure 1. The sensitivity of synchronization between primary and accessory visual areas to global visual stimulus features.

A, position of the cortical recording electrodes. A17, area 17; PMLS, posteromedial lateral suprasylvian; LAT, lateral sulcus; SUPS, suprasylvian sulcus; P, posterior; L, lateral. B1-B3, plots of the receptive fields of the PMLS and area 17 cell. The circle represents the visual centre. The bars represent the stimulus conditions used. B1, the stimulus is a single moving bar. B2, the stimuli are two bars moving at the same rate in the same direction. B3, the stimuli are two bars moving at the same rate in opposite directions. C1-C3, post-stimulus time histograms (PSTHs) for multiunit PMLS and area 17 recordings for each of the three stimulus conditions. The vertical lines indicate the 1 s time window from which the autocorrelograms (D) and cross-correlograms (E) were constructed. D1-D3, comparison of the autocorrelograms for the two visual areas in each of the three conditions. E1-E3, cross-correlograms constructed between the area 17 and PMLS discharges for each of the three stimulus conditions. The mean level of the auto- and cross-correlograms changes little as a result of change in stimulus condition, indicating that there is little alteration in firing rate of the neurones. The peak at time zero indicates that the responses are synchronized when the stimuli are moving in the same direction (E1 and E2). The synchronization between area 17 and PMLS is absent when the stimuli are moving in opposite directions (E3). The presence of side bands in E1 and E2 indicates that the synchronization is oscillatory, with a period of ∼25 ms (∼40 Hz). (Reproduced with permission from Engel et al. 1991.)
Is there evidence for synchronous oscillations in the motor system?
Oscillatory neural activity has been detected in the sensorimotor cortex of the cat and the awake behaving monkey (Bouyer, Montaron, Vahnee, Albert & Rougeul, 1987; Murthy & Fetz, 1992, 1996a, b; Sanes & Donoghue, 1993; Baker, Olivier & Lemon, 1997). It is best appreciated through the recording of local field potentials (LFPs) which may oscillate in the frequency range ∼20-30 Hz. These reflect the average activity of large groups of neurones in the vicinity of a recording electrode and are also influenced by subthreshold fluctuations in the membrane potential (see Fig. 2). Single corticomotoneurones (CMs) may discharge in a Poisson fashion. However, it can be demonstrated that during periods of LFP oscillation individual CM discharges may become temporally synchronous with deflections in the LFP, and the CM firing may become oscillatory at a frequency ∼20-30 Hz (Murthy & Fetz, 1996b).
Figure 2. Sensorimotor cortex local field potentials in an awake behaving primate.

Upper traces: LFPs recorded simultaneously in five anterior-posterior tracks each separated by 2 mm. Lower left: electrode sites, marked on a sketch of the cortical surface, straddled the central sulcus. Lower right: averages of LFPs aligned on triggers from oscillatory cycles in LFP 1. Note that LFPs 1-5 are rhythmic and occur synchronously. The monkey was reaching for a raisin offered to the side of its head by the experimenter. Vertical calibration bars are in μV, horizontal calibration bars are in ms. (Reproduced with permission from Murthy & Fetz, 1992.)
Single motor unit recordings in humans performing steady isometric contraction reveal short-term motor unit synchronization between pairs of discharges, indicative of common last-order branched stem presynaptic drive to motoneurones (Sears & Stagg, 1976; Kirkwood & Sears, 1978; Datta & Stephens, 1990). Short-term synchronization is likely to result from activity in common presynaptic inputs of corticospinal origin (Farmer, Ingram & Stephens, 1990b; Datta, Farmer & Stephens, 1991; Farmer, Swash, Ingram & Stephens, 1993b). It has been further demonstrated during steady isometric contraction in man that the motoneurones are commonly modulated by rhythmic inputs at frequencies in excess of the mean motor unit firing rate of ∼10 Hz. Coherence analysis of the single motor unit records shows the presence of temporal correlation between pairs of motor units in the frequency ranges 1-12 and 16-32 Hz (Farmer, Farmer, Halliday, Rosenberg & Stephens, 1990a; Farmer, Bremner, Halliday, Rosenberg & Stephens, 1993a; Mills & Schubert, 1995). Indirect evidence from patients with peripheral and central nervous lesions suggests that the oscillatory common drive to motoneurones is transmitted via the corticospinal tract (see Fig. 3). This common oscillatory modulation, although well focused, may be shared between different muscles, e.g. first and second dorsal interosseous (1DI and 2DI) activated during co-abduction of the index and middle fingers (Farmer et al. 1993a). Thus it has been proposed that human motor unit firing may during weak isometric contraction carry an echo of oscillatory synchronization between neurones of the primary motor cortex.
Figure 3. Comparison of synchronization and coherence between single motor unit discharges in healthy subjects and patients with central motor pathway lesions.
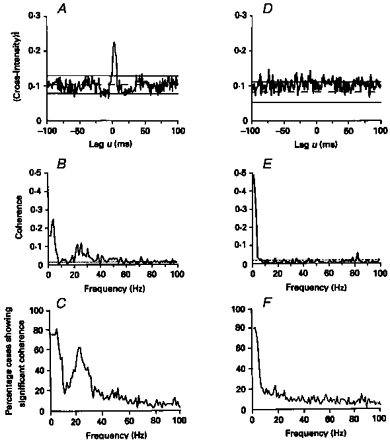
A, cross-correlogram constructed between the discharges of two single motor units from first dorsal interosseus muscle (1DI) in a healthy subject. B, coherence spectra for the data used to construct the cross-correlogram in A. D, cross-correlogram constructed between the discharges of two motor units recorded from within 1DI of the affected (left) hand in a patient who had suffered an infarct of the right internal capsule 4 months previously. E, coherence spectra for the data used to construct the cross-correlogram in D. The central cross-correlogram peak is absent in the data recorded from the stroke patient; the corresponding coherence is significant only at low frequencies. Bin width in A and D, 1 ms; frequency resolution in B and E, 1 Hz. C and F, the percentage of 1DI motor unit pairs that showed significant coherence between 1 and 100 Hz. C, data from 1DI of sixteen healthy subjects (49 motor unit pairs); F, data from 1DI of eleven stroke patients (91 motor unit pairs). (Reproduced from Farmer et al. 1993a.)
The recognition that motor unit synchronization in man might reflect synchronization between pyramidal neurones of the primary motor cortex prompted studies of the temporal relationship between magneto- (MEG) and electroencephalographic (EEG) signals and the electromyogram (EMG). The human EEG may be thought of as spatially averaged local field potentials (LFPs) and as such, represents the summated activity of subthreshold soma and dendritic membrane potential fluctuations as well as action potentials (see Nunez, 1995). The EEG best represents activity in gyri, while because of perpendicular magnetic flux, the MEG better represents activity in the sulci. The detection of both signals is strongly enhanced by temporal synchronization of the underlying neural activity.
The human EEG and MEG may undergo changes in frequency structure before and during movement. Beta activity (13-35 Hz) may be initially suppressed during movement (Penfield, 1954; Pfurtscheller, 1981; Salmelin & Hari, 1994) and re-established after movement and during maintained tonic contraction (Penfield, 1954). During isometric contraction of muscles acting on the finger, frequency components in the human MEG recorded from the sensorimotor cortex are found in the ranges ∼10 Hz, ∼16-26 Hz and ∼40-50 Hz (Conway et al. 1995). The ∼16-26 Hz component of the MEG is correlated with rectified surface EMG recorded from the contralateral hand muscles. This correlation is manifest as coherence in the frequency range ∼16-26 Hz at zero phase delay (see Fig. 4). These results were subsequently confirmed using a wider array of MEG recordings, and using source mapping, it has been established that the correlation is maximal between EMG and MEG recorded from the primary motor cortex (Salenius, Portin, Kajola, Salmelin & Hari, 1997). Furthermore, motor cortex somatotopy can be demonstrated in that coherence between hand muscle EMG and the cortex is found in a lateral position, whereas that between leg muscle EMG and the cortex occurs in a more medial position (Salenius et al. 1997). Recently, the MEG results have been replicated using the EEG. Halliday, Conway, Farmer & Rosenberg 1998 have shown ∼16-26 Hz EEG/EMG coherence during steady isometric contraction in man.
Figure 4. The relation between MEG and EMG during simultaneous contraction of left and right 1DI muscles.

A, estimated autospectrum of MEG recorded from above the left sensorimotor cortex during simultaneous isometric abduction of left and right index fingers. B, estimated autospectrum of surface EMG recorded from left 1DI. C, estimated autospectrum of surface EMG recorded from right 1DI. D, coherence between left cortex MEG and left 1DI EMG. E, coherence between left cortex MEG and right 1DI EMG. F, cumulant density (time domain) function between left cortex MEG and left 1DI EMG. G, cumulant density function between left cortex MEG and right 1DI EMG. The vertical bars next to the autospectra represent 95 % confidence intervals. The horizontal dotted lines represent 95 % confidence intervals for the coherence. The dotted line and parallel continuous lines represent the mean and ±95 % confidence intervals for the cumulants. Coherence in the frequency range 18-25 Hz is present between the MEG and contralateral EMG only. The corresponding time domain measure demonstrates rhythmic synchronization between MEG and EMG. (Reproduced from Conway et al. 1995.)
Primate experiments have established ∼20 Hz coherence between oscillatory LFPs and contralateral EMG in awake behaving monkeys (Baker et al. 1997). As predicted from human experiments, in primates the EMGs from different muscles co-activated during performance of a task are also coherent at ∼20 Hz (see Fig. 5). Thus, in humans, individual motor unit discharges within and between muscles and surface EMGs are commonly modulated by ∼16-32 Hz drive. This rhythmic, synchronizing modulation of EMG is correlated with the MEG/EEG in humans and with LFP oscillations in primates.
Figure 5. Coherence between EMGs and between EMGs and primary motor cortex local field potentials in a primate performing a steady pincer grip task.

A-C relate to first dorsal interosseous muscle (1DI); D-F relate to adductor pollici muscle (AdP). The bars at the top of the two columns indicate the range over which the amplifiers had a flat frequency response. A and D, EMG power spectra. A small ∼20 Hz peak is present in D. B and E, coherence between cortical slow wave and simultaneously recorded EMG from 1DI and AdP, respectively. C and F, coherence between cortical slow wave recording from a different cortical site and simultaneously recorded 1DI and AdP EMGs. Large values of coherence at 0 Hz (DC) have been removed. The insets in B-F represent cross-correlations between the slow wave and the EMG; the arrow indicates time zero. Scale bars are 20 ms, r= 0.01. G, coherence between simultaneously recorded EMGs from 1DI and AdP during a steady pincer grip. Coherence at ∼20 Hz is present between the cortical slow wave and EMGs from 1DI and AdP. Due to electrical cross-talk coherence is present between 1DI and AdP EMGs at all frequencies. However, a large peak is present at ∼20 Hz, the same frequency at which coherence is observed between the cortical slow wave and EMG. Compare, in this paper, Fig. 5G with Fig. 3B-C and Fig. 5B-F with Fig. 4E and G. (Reproduced from Baker et al. 1997.)
Does the experimental data from the motor system in primates and man allow for certain problems of motor control to be construed in terms of binding and combination with a solution in terms of temporal correlation? The architecture of the primary and pre-motor areas consists of multiple and spatially overlapping representations within an area (Kwan, MacKay, Murphy & Wong, 1978; Sanes, Donoghue, Thangaraj, Edelman & Warach, 1995) and through branching of corticospinal axons which are distributed to functionally related muscle groups, the existence of muscle fields (Shinoda, Zarzecki & Asanuma, 1979; Cheney & Fetz, 1985; for review, see also Porter & Lemon, 1993). As in the sensory system, the motor system must solve the problem of segregation and co-operation between neuronal assemblies whose relation to one another and their target motoneurones is spatially complex. The binding problem may be defined for the motor system as the formation of associations between the distributed motor systems necessary for the spatiotemporal co-ordination of the activity of different muscles involved in the same motor task. Avoidance of the ‘combinatorial explosion’ might involve the establishment of separate neural assemblies within the motor system in order to accomplish separate yet contemporaneous tasks, for example, walking and chewing gum, to quote a famous aphorism! For the reasons so clearly put forward by the sensory physiologists, the solutions to these problems offered by millisecond time scale temporal correlations would seem highly attractive and an essential addition to simple rate coding solutions. In the following sections I shall consider the problems and controversies that have arisen during the initial attempts to construe motor and sensorimotor integration in terms of millisecond time scale synchronization within and between rhythmically firing upper and lower motoneurones.
The question of phase
The studies of visual cortex demonstrate that the ∼40 Hz oscillations are synchronized, and thus in the time domain there is a peak at time zero despite significant conduction delays (see Fig. 1). In the frequency domain the phase would be flat at zero radians across the frequencies at which the signals are coherent. Singer (1993) has argued that it is the very rhythmicity of the neuronal firing that allows zero phase lag synchronization to develop within a few cycles. Because, during rhythmic firing, the time of occurrence of one spike predicts with accuracy the time of occurrence of the next spike, two groups of neurones firing rhythmically at similar frequencies may, within a few cycles, synchronize their discharges. Recently a mechanism of doublet firing has been proposed to explain zero time lag synchronization across large cortical distances (Traub, Whittington, Stanford & Jefferys, 1996). Data from the primate motor system suggest that at the level of the cortex synchronization is maintained across considerable distances and even between homologous left and right cortical areas (Murthy & Fetz, 1996b). However, phase reversal may occur as a function of cortical depth (Murthy & Fetz, 1996a). Recordings of human MEG and EMG suggest that the signals are synchronized (see Fig. 4G). Although there is no pure delay, the phase between MEG and EMG may vary between 0 and 180 deg. Thus Conway et al. (1995) failed to demonstrate a consistent phase lag corresponding to the known 20-25 ms conduction delay between sensorimotor cortex and muscles activated during isometric contraction (for discussion, see also Halliday et al. 1998). Volkmann et al. (1996) investigated MEG-EMG correlations using magnetic field tomography to define the spatiotemporal distribution of cortical currents during Parkinsonian resting tremor. They found that in relation to the onset of flexor muscle EMG a magnetic source appeared in the sensorimotor cortex at -2 ms, i.e. practically synchronous with the EMG burst. In contrast, the study of Salenius et al. (1997), whilst confirming the presence of ∼20 Hz coherence between MEG and EMG during steady muscle contraction, claimed also to show a phase lag corresponding to the conduction time between motor cortex and muscle. This study used spike-triggered averaging to calculate the lag between MEG and EMG. It can be seen from their published records that the spike-triggered averaged MEG may have undergone phase reversal, possibly reflecting differing dipole orientation in leg compared with hand areas of the motor cortex. Possibly this, rather than a pure phase delay, explains the apparent shift which, it is claimed, mirrors the known increase in cortex to muscle conduction time when comparing arm with leg muscles.
The available monkey data derived from cycle triggered averages and cross-correlations demonstrates co-modulation of LFP oscillations and EMG. The co-modulation appears out of phase but there is no consistent phase delay (Murthy & Fetz, 1992, 1996a; Baker et al. 1997). The timing of CM discharge with respect to rhythmic fluctuations in the LFP is still being studied. Murthy & Fetz (1996b) noted a close temporal relationship, with the CM discharging 2.7 ms before the peak LFP negativity.
Studies of hippocampus suggest an interesting way of considering the phase relationship between unitary and slow wave activity. It has been shown that a systematic change in the phase relationship between complex spike discharges of single CA1 and CA3 units and the hippocampal theta rhythm oscillations accompanies alterations in an animal's spatial position (O'Keefe & Reece, 1993). In the motor system the overall correlation between MEG/EEG/LFP and EMG may be at zero delay; it is possible, however, that the discharges of single corticomotoneurones may vary their timing systematically with respect to the ∼20 Hz cortical rhythm. Whether such a delay can be demonstrated to reflect systematically either differing limb to cortex conduction times or changes in limb position it is not yet known. The issue of phase, phase reversal and phase delay clearly will remain contentious. The studies discussed above apply only to maintained posture. We do not know if correlation between cortical activity and the EMG occurs during fast ballistic movements and whether during such movements, which are less dependent on sensory feedback, if correlation is present, a different phase relationship obtains.
The question of frequency
There are numerous rhythms represented in the animal and human brain. Studies of visual processing have focused on ∼40 Hz oscillations in the so-called gamma range. The primate motor system studies suggest that the important oscillations are somewhat slower at ∼20-30 Hz (the beta range of frequencies), although in cat 30-100 Hz oscillations in the cerebellothalamic pathway have been found to be synchronous with motor cortex potentials (Timofeev & Steriade, 1997). The ∼20-30 Hz motor cortex oscillations are most prominent during preparation to move and during maintained posture. During actual movement they reduce (Sanes & Donoghue, 1993). In man, slow finger movements can be shown to be discontinuous with a frequency of ∼10 Hz (Valbo & Wessberg, 1993). Synchronization at ∼10 Hz has been detected between different muscles during movement (Conway, Biswas, Halliday, Farmer & Rosenberg, 1997) but as yet ∼10 Hz synchronization has not been found in MEG-EMG coherence (B. A. Conway, D. M. Halliday, S. F. Farmer & J. R. Rosenberg, unpublished observations). It is possible that during slow movement ∼10 Hz drive may not be expressed at the level of the motor cortex, and one might speculate at this point about the possible role of the somatosensory cortex and cerebellum in producing such rhythms (see Salmelin & Hari, 1994; for discussion of 13-18 Hz cerebellar LFP oscillations, see Welsh & Llinas, 1997; Pellerin & Lamarre, 1997).
Do the various different frequencies expressed at the levels of cortex and muscle have differing significance? The study of Roelfsema, Engel, Konig & Singer (1997) has demonstrated zero time lag synchronization between different cortical areas involved with visual processing and behavioural response. In this study, correlations between LFPs occured in the range ∼20-25 Hz. The correlations were maximal between functionally related areas, for example between areas 7 and 17 (visual cortex and posterior parietal association area) and between areas 5 and 4 (anterior parietal association area and motor cortex). Correlations were absent between the visual (area 17) and motor (area 4) cortices. The earlier studies suggested that during feature detection visual cortical synchronization occurs at ∼40 Hz. The sensorimotor cortex synchronization occurs at ∼20-30 Hz. Do different frequencies modulate one another? Do these areas interact through an interim frequency? Can one frequency act as a carrier? Are the frequencies task dependent? Also it should be noted that within a functional system, frequencies can reduce with time from the gamma to the beta range (Whittington, Traub, Faulkner, Stanford & Jefferys, 1997; Roelfsema et al. 1997). We do not yet know at the level of the cortex how different frequencies interact; the necessary mathematical analysis required in order to demonstrate such interaction is complex and non-linear (Bullock & Achimowicz, 1994; Halliday, Rosenberg, Amjad, Breeze, Conway & Farmer, 1995; Friston et al. 1996). Nevertheless the relationship between different frequency ranges and its functional significance needs to be addressed.
The behavioural significance of oscillations
The components of movement may be summarized as follows: (a) preparation to move; (b) movement (with varying types and amount of sensory feedback); (c) maintenance of posture (again with differing sensory feedback). Components (a)-(c) may involve motor synergies as well as isolated movement.
In insects it has been demonstrated that chemical blocking of ∼30 Hz LFP olfactory oscillations results in impairment of odour discrimination and degradation of a conditioned behavioural task (Stopfer, Bhagavan, Smith & Laurent, 1997). At the level of the primate motor cortex, synchronous ∼20 Hz oscillations may be proposed to have a functional role in segregating and integrating the activities of different muscle representations engaged in a motor task. Furthermore, task-dependent coherence may be detected between cortical activity and the EMG and between EMGs of different muscles. Such a model predicts that the different cortical representations of muscles and the motoneurones of different muscles, engaged in the same motor task, will be commonly modulated by high frequency ∼20 Hz oscillations and that this common modulation will be lost when the muscles are acting independently. Farmer et al. (1993a) found that during co-abduction of the index and middle fingers, the motor unit discharges of first and second dorsal interosseous muscles were coherent at zero phase lag in the frequency range 16-32 Hz. In monkey and man it has been shown that ∼20 Hz LFP/MEG/EEG oscillations are present during motor preparation, decrease during limb movement and are re-established during maintained contraction (Penfield, 1954; Pfurtscheller, 1981; Pfurtscheller & Arinbar, 1989; Sanes & Donoghue, 1993; Feige, Kristeva-Feige, Rossi, Pizzella & Rossini, 1996; Baker et al. 1997; Pfurtscheller, Stancak & Edlinger, 1997). During a ramp and hold pincer grip task in monkey task related changes in ∼20 Hz coherence have been demonstrated (Baker et al. 1997). During the movement phase of the task there is little ∼20 Hz coherence between LFPs from different cortical sites, between the LFPs and the EMG or between EMGs of functionally related muscles (1DI and AdP; see Fig. 5). However, synchronous ∼20 Hz activity develops between cortical sites, between the LFP and EMG and between the EMGs themselves during the maintained (hold) phase of the task. There is some evidence that this also occurs in humans. Conway et al. (1997) demonstrated that coherence between the EMGs of different muscles during slow movements occurs at ∼10 Hz. In contrast, during maintained co-contraction of the same muscle pair, ∼20 Hz coherence is observed between the EMGs. Coherence between the MEG and EMG in man is not present during movement but develops in the frequency range ∼16-26 Hz during maintained contraction (B. A. Conway, D. M. Halliday, S. F. Farmer & J. R. Rosenberg, unpublished observations).
The difficult question of how rhythmic synchronization may help to unite distributed neuronal assemblies in the monkey motor cortex has been further addressed in a detailed study by Murthy & Fetz (1996a, b). In this study, motor cortex LFPs along with single and multiple CM unit activity and EMGs were recorded from awake behaving monkeys performing a variety of tasks. Murthy & Fetz (1996b) defined task-sensitive CM cells as those that modulated their discharge rates in response to exploratory or manipulative hand movements. The degree of synchronization between neurones that were sensitive to the same task was compared with that seen when one or both of the neurones were not task sensitive. Some tasks were well rehearsed and required little sensory feedback for successful completion; other tasks, for example use of a Kluver board, or retrieval of a reward from a position away from the monkey's line of vision, were complex and judged to require sustained attention and high levels of sensorimotor integration. The temporal binding hypothesis suggests that the more demanding tasks will be associated with greater prevalence and more widespread synchronization between disparate cell groups in the motor cortex. In addition, neurones with similar task sensitivity may show greater synchronization than neurones with differing task sensitivity. Furthermore, tasks requiring bilateral hand movements should show greater inter-hemispheric synchronization than tasks requiring unilateral hand movements. Finally, it was predicted that, to a degree, the presence or absence of synchronization would be independent of CM firing rate.
In the event, Murthy & Fetz (1996a, b) failed to establish a simple relationship between task, the performance of complex movements and the need for greater levels of intra- and inter-cortical synchronization. They have interpreted their findings as supporting a role for ∼20-30 Hz oscillations in tasks requiring sustained attention. However, two points need to be made. First, the study only used time domain analysis. Comparisons across tasks were to some extent made difficult because only data where visible oscillations were present were used in the analysis. Frequency domain analysis may, through emphasizing the data differently, have given different results. Secondly, although the tasks differed in complexity all required fine finger movements during which period, according to the findings of Sanes & Donoghue (1993) and Baker et al. (1997), a reduction in ∼20-30 Hz oscillations would have been expected. The experiments did not examine different maintained ‘hold’ tasks of varying type and complexity when oscillations might be expected to be more prevalent and important.
The relationship between motor task, cortical oscillations and EMG modulation is therefore complex. I believe human and primate data may be summarized as follows. In monkey, Sanes & Donoghue (1993) and Baker et al. (1997) have shown a decrease in ∼20 Hz oscillations with movement, with an increase during maintained contraction. Murthy & Fetz (1992, 1996a, b) have used more complex natural tasks and observe a positive relationship between the prevalence of ∼20 Hz oscillations and task complexity yet do not demonstrate a simple relationship between time domain measures of synchronization and task-related neurones. Baker et al. (1997) further demonstrated increased coherence during maintained co-contraction. In man, the primate findings are mirrored by ∼20 Hz EEG/MEG power decreases before and during movement (Pfurtscheller, 1981; Pfurtscheller & Arinbar, 1989; Feige et al. 1996), yet interestingly, and in contrast to the findings of Baker et al. (1997), coupling between different motor areas as expressed by ∼20 Hz EEG coherence has recently been shown to actually increase during movement (Leocani, Toro, Manganotti, Zhuang & Hallett, 1997). This raises the interesting possibility of a task-related dissociation between the ∼20 Hz coherence observed to involve different motor cortex areas (e.g. primary motor cortex and supplementary motor area) and that observed within primary motor cortex or between primary motor cortex and muscle.
Some of the apparent differences between the above may yet be resolved through the more uniform application of signal analysis techniques. Ultimately, however, the difficulty may be that the very nature of synchronization, both at the level of the cortex, between cortex and EMG and between EMGs, is extremely dynamic and flexible and thus difficult to associate closely with motor task. This may explain why, so far, close relationships have been demonstrated only for relatively simple ramp and hold pincer grip tasks. It is of great interest, therefore, that the use of a dynamic correlation measure in which temporally nearly coincident (within 5 ms) CM discharges were logged, has shown task- and context-dependent synchronization (Riehle, Grun, Diesmann & Aertsen, 1997). Tightly synchronized CM discharges may occur in temporal relation to different phases of a voluntary movement. They were shown to occur in temporal relation to external events, i.e. stimuli and movements, but also during periods of stimulus expectancy. During the latter paradigm, spike synchronization occurred independently of firing rate modulations. Interestingly, as far as could be ascertained using time domain measures, the synchronization was not conspicuously periodic. Thus in primates brief time varying temporal associations may provide a flexible code suitable for solving the motor system binding problem.
Conclusions
Whilst there is much more work to be done, the evidence in sensory and motor systems of animals and man indicates that synchronous oscillatory activity is ubiquitous, spreads across large anatomical distances and is behaviourally relevant. Temporal pattern coding is likely to prove an important addition to rate coding and may well provide a solution to the binding problem for the motor system. Finally, in man many pathological states such as Parkinson's disease, essential tremor, dystonia and myoclonus may involve abnormal synchronization between motoneurones themselves and between EMG activity and the motor cortex (Volkmann et al. 1995; Halliday et al. 1997; Farmer et al. 1998; P. Brown, S. F. Farmer, D. M. Halliday & J. R. Rosenberg, unpublished observations). Time and frequency domain analysis of simultaneous MEG/EEG and EMG recordings affords a fascinating opportunity to uncover more of the pathophysiology of these conditions.
Acknowledgments
My great thanks to Dr Bernie Conway, Dr David Halliday and Professor Jay Rosenberg for our friendship, collaboration and discussions over many years.
References
- Baker SN, Olivier E, Lemon RN. Coherent oscillations in the monkey motor cortex and hand muscle EMG show task-dependent modulation. Journal of Physiology. 1997;501:225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer JJ, Montaron MF, Vahnee JM, Albert MP, Rougeul A. Anatomical localization of cortical beta rhythms in cat. Neuroscience. 1987;22:863–869. doi: 10.1016/0306-4522(87)92965-4. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Achimowicz JZ. A comparative study of event-related brain oscillations. In: Pantev C, Elbert T, Lutkenhoner B, editors. Oscillatory Event-related Brain Dynamics. New York: Plenum Press; 1994. pp. 11–26. [Google Scholar]
- Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimulation in primate. Evidence for functional groups of CM cells. Journal of Neurophysiology. 1985;53:786–804. doi: 10.1152/jn.1985.53.3.786. [DOI] [PubMed] [Google Scholar]
- Conway BA, Biswas P, Halliday DM, Farmer SF, Rosenberg JR. Task-dependent changes in rhythmic motor output during voluntary elbow movement in man. Journal of Physiology. 1997;501.P:48–49P. [Google Scholar]
- Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, Rosenberg JR. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. Journal of Physiology. 1995;489:917–924. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta AK, Farmer SF, Stephens JR. Central nervous pathways underlying synchronization of human motor unit firing studied during voluntary contractions. Journal of Physiology. 1991;428:561–577. doi: 10.1113/jphysiol.1991.sp018391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta AK, Stephens JA. Short-term synchronization of motor unit activity during voluntary contraction in man. Journal of Physiology. 1990;422:397–419. doi: 10.1113/jphysiol.1990.sp017991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitbeock HJ. Coherent oscillations: a mechanism for feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biological Cybernetics. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Engel AK, Konig P, Kreiter AK, Schillen TB, Singer W. Temporal coding in the visual cortex: new vistas on integration in the nervous system. Trends in Neurosciences. 1992;15:218–226. doi: 10.1016/0166-2236(92)90039-b. 10.1016/0166-2236(92)90039-B. [DOI] [PubMed] [Google Scholar]
- Engel AK, Kreiter AK, Konig P, Singer W. Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proceedings of the National Academy of Sciences of the USA. 1991;88:6048–6052. doi: 10.1073/pnas.88.14.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer C, Farmer SF, Halliday DM, Rosenberg JR, Stephens JA. Coherence analysis of motor unit firing recorded during voluntary contraction in man. Journal of Physiology. 1990a;420:22P. [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. Journal of Physiology. 1993a;470:127–155. doi: 10.1113/jphysiol.1993.sp019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Ingram DA, Stephens JA. Mirror movements studied in a patient with Klippel-Feil syndrome. Journal of Physiology. 1990b;428:467–484. doi: 10.1113/jphysiol.1990.sp018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Sheean GL, Mayston MJ, Rothwell JC, Marsden CD, Conway BA, Halliday DM, Rosenberg JR, Stephens JA. Abnormal motor unit synchronization between antagonist muscles underlies pathological co-contraction in upper limb dystonia. Brain. 1998;121:799–812. doi: 10.1093/brain/121.5.801. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous lesions in man. Journal of Physiology. 1993b;463:83–105. doi: 10.1113/jphysiol.1993.sp019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige B, Kristeva-Feige R, Rossi S, Pizzella V, Rossini P-M. Neuromagnetic study of movement-related changes in rhythmic brain activity. Brain Research. 1996;734:252–260. [PubMed] [Google Scholar]
- Fries P, Roelfsema PR, Engel AK, Konig P, Singer W. Synchronization of oscillatory responses in visual cortex correlates with perception in interocular rivalry. Proccedings of the National Academy of Sciences of the USA. 1997;94:12699–12704. doi: 10.1073/pnas.94.23.12699. 10.1073/pnas.94.23.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Stephan KM, Heather JD, Frith CD, Ioannides AA, Liu LC, Rugg MD, Vieth J, Keber H, Hunter K, Frackowiack RSJ. A multivariate analysis of evoked responses in EEG and MEG data. Neuroimage. 1996;3:167–174. doi: 10.1006/nimg.1996.0018. 10.1006/nimg.1996.0018. [DOI] [PubMed] [Google Scholar]
- Gray CM. Synchronous oscillations in nervous systems: Mechanisms and functions. Journal of Computational Neuroscience. 1994;1:11–38. doi: 10.1007/BF00962716. [DOI] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proceedings of the National Academy of Sciences of the USA. 1989;86:1678–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. On the use of the EEG to explore functional aspects of correlations between cortical recordings and muscle EMG during voluntary contractions in human. Neuroscience Letters. 1998;241:5–8. doi: 10.1016/s0304-3940(97)00964-6. 10.1016/S0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Shahani U, Russell A, Farmer SF, Weir AI, Rosenberg JR. Coherence estimates between cortical activity and motor output in subjects with essential tremor. Journal of Physiology. 1997;501.P:38P. [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data-theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Progress in Biophysics and Molecular Biology. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. 10.1016/S0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Synaptic connections to intercostal motoneurones as revealed by the common excitation potential. Journal of Physiology. 1978;275:102–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan HC, Mackay WA, Murphy JT, Wong YC. Spatial organization of precentral cortex in awake primates. II Motor outputs. Journal of Neurophysiology. 1978;41:1120–1131. doi: 10.1152/jn.1978.41.5.1120. [DOI] [PubMed] [Google Scholar]
- Leocani L, Toro C, Manganotti P, Zhuang P, Hallett M. Event-related coherence and event-related desynchronization/synchronization in the 10 Hz and 20 Hz EEG during self-paced movements. Electroencephalography and Clinical Neurophysiology. 1997;104:199–206. doi: 10.1016/s0168-5597(96)96051-7. 10.1016/S0168-5597(96)96051-7. [DOI] [PubMed] [Google Scholar]
- Mills KR, Schubert M. Short term synchronization of human motor units and their responses to transcranial magnetic stimulation. Journal of Physiology. 1995;483:511–523. doi: 10.1113/jphysiol.1995.sp020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proceedings of the National Academy of Sciences of the USA. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: Synchronization of local field potentials and relation to behaviour. Journal of Neurophysiology. 1996a;76:3949–3967. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Synchronizaton of neurones during local field potential oscillations in sensorimotor cortex of awake monkeys. Journal of Neurophysiology. 1996b;76:3968–3982. doi: 10.1152/jn.1996.76.6.3968. [DOI] [PubMed] [Google Scholar]
- Nunez PL. Neocortical Dynamics and Human EEG Rhythms. New York: Oxford University Press; 1995. [Google Scholar]
- O'Keefe J, Reece ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Pellerin J-P, Lamarre Y. Local field potential oscillations in primate cerebellar cortex during voluntary movement. Journal of Neurophysiology. 1997;78:3502–3507. doi: 10.1152/jn.1997.78.6.3502. [DOI] [PubMed] [Google Scholar]
- Penfield W. Mechanisms of voluntary movement. Brain. 1954;77:1–17. doi: 10.1093/brain/77.1.1. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Central beta rhythm during sensorimotor activities in man. Electroencephalography and Clinical Neurophysiology. 1981;51:253–264. doi: 10.1016/0013-4694(81)90139-5. 10.1016/0013-4694(81)90139-5. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Event-related desynchronization detected by power measurements of scalp EEG. Electroencephalography and Clinical Neurophysiology. 1989;72:250–258. doi: 10.1016/0013-4694(77)90235-8. 10.1016/0013-4694(89)90250-2. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Edlinger G. On the existence of different types of central beta rhythms below 30 Hz. Electroencephalography and Clinical Neurophysiology. 1997;102:316–325. doi: 10.1016/s0013-4694(96)96612-2. 10.1016/S0013-4694(96)96612-2. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon R. Monographs of the Physiological Society, No. 45. New York: Oxford University Press; 1993. Corticospinal Function and Voluntary Movement. [Google Scholar]
- Riehle A, Grun S, Diesmann M, Aertsen A. Spike synchronization and rate modulation differentially involved in motor cortical function. Science. 1997;278:1950–1953. doi: 10.1126/science.278.5345.1950. 10.1126/science.278.5345.1950. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Engel AK, Konig P, Singer W. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature. 1997;385:157–161. doi: 10.1038/385157a0. 10.1038/385157a0. [DOI] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. Journal of Neurophysiology. 1997;77:3401–3405. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience. 1994;60:537–550. doi: 10.1016/0306-4522(94)90263-1. 10.1016/0306-4522(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex. Proceedings of the National Academy of Sciences of the USA. 1993;90:4470–4474. doi: 10.1073/pnas.90.10.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP, Thangaraj V, Edelman RR, Warach S. Shared neural correlates controlling hand movements in human motor cortex. Science. 1995;268:1775–1777. doi: 10.1126/science.7792606. [DOI] [PubMed] [Google Scholar]
- Sears TA, Stagg D. Short-term synchronization of intercostal motoneurone activity. Journal of Physiology. 1976;263:357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Zarzecki P, Asanuma H. Spinal branching of pyramidal tract neurones in the monkey. Experimental Brain Research. 1979;34:59–72. doi: 10.1007/BF00238341. [DOI] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annual Review of Physiology. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annual review of Neuroscience. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Fast (mainly 30-100 Hz) oscillations in the cat cerebellothalamic pathway and their synchronization with cortical potentials. Journal of Physiology. 1997;504:153–168. doi: 10.1111/j.1469-7793.1997.153bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Stanford IM, Jefferys JGR. A mechanism for the generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- Valbo AB, Wessberg J. Organization of motor output in slow finger movements in man. Journal of Physiology. 1993;469:673–691. doi: 10.1113/jphysiol.1993.sp019837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann J, Joliot M, Mogilner A, Ionnides AA, Lado F, Fazzini E, Ribary U, Llinas R. Central motor loop oscillations in Parkinsonian resting tremor revealed by magnetoenephalography. Neurology. 1996;46:1359–1370. doi: 10.1212/wnl.46.5.1359. [DOI] [PubMed] [Google Scholar]
- Von der Malsburg C. Binding in models of perception and brain function. Current Opinion in Neurobiology. 1995;5:520–526. doi: 10.1016/0959-4388(95)80014-x. 10.1016/0959-4388(95)80014-X. [DOI] [PubMed] [Google Scholar]
- Welsh J, Llinas R. Some organizing principals for the control of movement based on olivocerebellar physiology. In: De Zeeuw CI, Strata P, Voogd J, editors. The Cerebellum from Structure to Control. Vol. 114. New York: Elsevier; 1997. pp. 449–461. Progress in Brain Research. [Google Scholar]
- Whittington MA, Traub RD, Faulkner HJ, Stanford IM, Jefferys JGR. Recurrent excitatory post synaptic potentials induced by synchronized fast oscillations. Proceedings of the National Academy of Sciences of the USA. 1997;94:12198–12203. doi: 10.1073/pnas.94.22.12198. 10.1073/pnas.94.22.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]


