Abstract
The modulation by noradrenaline (NA) of synapses among stellate cells was investigated in rat cerebellar slices by using presynaptic loose cell-attached recording and postsynaptic whole-cell recording.
NA increased the frequency of spontaneous IPSCs recorded from stellate cells without changing their mean amplitude.
NA increased the firing rate of stellate cells. This effect persisted after blocking ionotropic glutamate receptors and GABA receptors, indicating that it was independent of synaptic input.
The effects of NA on action potential frequency were mimicked by the β-receptor agonist isoprenaline but not by the α-receptor agonist 6-fluoro noradrenaline, and they were not blocked by the α-receptor antagonist phentolamine, indicating that they were mediated by β-receptors.
In paired recordings of connected stellate cells, NA slightly decreased the success rate of synaptic transmission. A small decrease in mean IPSC amplitude (excluding failures) and a slight increase in latency were also observed in NA.
These results show that, while NA increases the number of action potential-dependent IPSCs by increasing the firing rate of stellate cells, it actually reduces the probability of evoked release. Since previous studies showed that NA increases the rate of miniature IPSCs in this preparation, we conclude that different mechanisms underly the modulation by NA of action potential-dependent and action potential-independent transmitter release.
The cerebellum receives noradrenergic fibres mainly originating from the locus coeruleus and ending both in the granule cell layer, in glomeruli, and in the molecular layer, near Purkinje cell dendrites (Ungerstedt, 1971). Noradrenaline (NA) is a well-known and widely studied modulator in the central nervous system. In the cerebellum it has been shown that NA directly modifies the electrical activity of Purkinje cells (Hoffer, Siggins, Oliver & Bloom, 1972). In addition NA increases the frequency of spontaneous and miniature IPSCs (sIPSCs and mIPSCs, respectively) recorded in stellate or Purkinje cells while mean sIPSC and mIPSC amplitudes are not changed, indicating a presynaptic potentiation (Llano & Gerschenfeld, 1993b). In stellate cells the increase in mIPSC frequency was studied in some detail. This effect was traced to the sequential activation of β-adrenoceptors, of adenylate cyclase and of protein kinase A, and to be targeted at the exocytosis step itself rather than at its regulation by Ca2+ influx (Kondo & Marty, 1997). Since it is often assumed that modifications of the rate of miniature currents are reflected by parallel changes in evoked transmission, it seemed likely that the same mechanism would also account for the enhancement in frequency of sIPSCs described by (Llano & Gerschenfeld, 1993b) in the same preparation. However, the possibility had also to be considered that NA may modify the firing pattern of presynaptic interneurones and thus alter the frequency of sIPSCs. In the hippocampus NA exerts complex effects on the firing rate as well as on the frequency of sEPSCs and sIPSCs in pyramidal cells and in interneurones (Madison & Nicoll, 1986; Chavez-Noriega & Stevens, 1994; Gereau & Conn, 1994; Bergles, Doze, Madison & Smith, 1996). Thus in CA1 interneurones, NA increases the rate of action potentials as well as the frequency of sIPSCs while mIPSCs are not affected, indicating that the increase in sIPSCs frequency is mediated by the frequency increase of presynaptic action potentials (Bergles et al. 1996). In view of these results it was decided to examine whether the effects of NA on stellate cell sIPSCs could be explained by the same mechanism as that underlying the modifications of mIPSCs, or whether effects on presynaptic excitability had to be taken into consideration. Furthermore we wanted to test whether the enhancement in frequency of mIPSCs and of sIPSCs would be paralleled by an increase of mean evoked IPSCs (eIPSCs) upon stimulation of one presynaptic interneurone. To our surprise the results revealed no increase in eIPSCs, but rather a decrease. Analysis of the results suggested that the enhancement in the rate of exocytosis responsible for the increase in the mIPSC frequency played, in fact, no role in the modification of action potential-dependent sIPSCs. Thus the results imply a strong dissociation between the regulation of mIPSCs, sIPSCs, and eIPSCs.
METHODS
Slice preparation and maintenance, recording and data acquisition were performed as described in the accompanying paper (Kondo & Marty, 1998).
Extracellular stimulation
For extracellular stimulation, pipettes suitable for patch-clamp recordings were used. They were filled with extracellular saline and their tips were positioned in the molecular layer at the slice surface. A separate platinum wire was used for earthing the stimulation circuit. Voltage pulses (10-50 V in amplitude, 400 ms in duration) were applied at 1 Hz.
Pharmacological compounds
The glutamate antagonists D(-)-2-amino-5-phosphonopentanoic acid (D-APV) and 6-nitro-7-sulphamoylbenzo(f)quinoxaline-2,3-dione (NBQX), and the GABAA receptor antagonist bicuculline were purchased from Tocris Neuramin (Bristol, UK). Tetrodotoxin (TTX) was purchased from Sigma. Noradrenaline, the α-adrenoceptor agonist 6-fluoronoradrenaline (6FNE), the α-adrenoceptor antagonist phentolamine and the β-adrenoceptor agonist (±)-isoprenaline (Iso) were purchased from RBI (Bethesda, USA). Stock solutions (10 mm) of these adrenergic agonists were prepared in water and were stored at -20°C. Final agonist concentrations were obtained by diluting samples from the stock solutions in saline a few minutes before application to the bath.
Statistics
Statistical results are given as means ±s.d.n is the number of independent experiments. P values indicate the result of Student's paired t test comparisons.
RESULTS
NA increases the frequency of sIPSCs
Application of NA (10 μm) significantly increased the frequency of sIPSCs (mean ratio to the control: 1.81 ± 0.53, n= 11, P < 0.01; Fig. 1; see also Llano & Gerschenfeld, 1993b). There was no significant change in cumulative amplitude distribution upon application of NA (Fig. 1B). The ratio between the means of these distributions in NA over those in the previous control period had a mean value of 0.99 ± 0.25 (n= 10; Fig. 1C). No change of IPSC decay kinetics was detected (data not shown; see also Llano & Gerschenfeld, 1993b). The effect of NA on sIPSCs obtained here is similar to the effect on mIPSCs demonstrated previously (Llano & Gerschenfeld, 1993b; Kondo & Marty, 1997). The mean amplitude did not change significantly in either case, while the frequency increased markedly. Likewise the kinetics of onset and recovery of the effect were also similar. However, the magnitude of the frequency increase was slightly smaller for sIPSCs than for mIPSCs (respective ratios to the control of 1.81 and 2.24, see Kondo & Marty, 1997).
Figure 1. Effect of NA on sIPSCs.
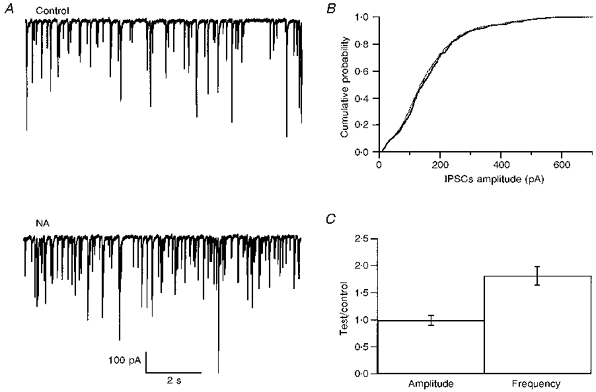
A, spontaneous IPSCs are enhanced in the presence of NA (10 μm). B, the cumulative distribution of sIPSC amplitudes is not modified by NA (continuous line, control; dotted line, NA). C, pooled results from ten experiments as in A, showing that the mean sIPSC amplitude is not altered by NA whereas the mean frequency is significantly increased (P < 0.01). Error bars show ±s.e.m.
NA accelerates action potential firing
The effects of NA on sIPSCs were clearly presynaptic since neither the mean amplitude nor the kinetics of IPSCs were changed. To test whether the generation of presynaptic action potentials was modified, action potentials were recorded from the somata of stellate cells using loose cell-attached patch-clamp recording. This method allowed observation of the resistive and capacitive currents caused by action potentials without perturbing the cell content with dialysis by the pipette solution. Stellate cells had stable mean basal frequencies of spontaneous action potentials in normal saline, which varied from cell to cell between 0.3 and 9 Hz (n= 38). Bath application of NA caused a significant increase in action potential frequency with a mean ratio of 1.6 ± 0.40 (n= 8, P < 0.01) over control values (Fig. 2A and C). The effects of NA were slowly reversible (recovery time > 20 min; Fig. 2C). The magnitude of the increase of firing rate was correlated with the initial action potential frequency: larger increases were obtained from cells which had lower initial firing rates (data not shown).
Figure 2. Effects of NA on interneurone electrical excitability.
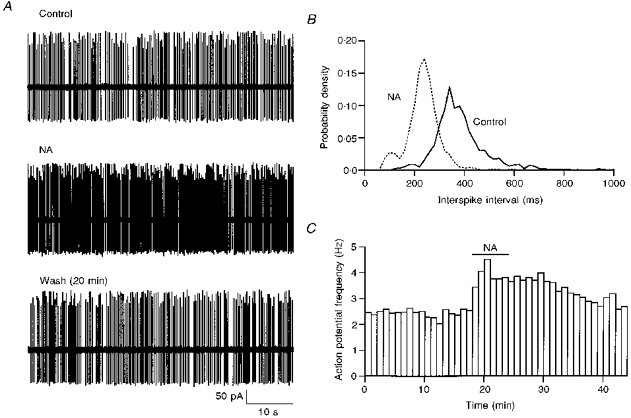
NA application (10 μm) leads to an increase in the frequency of action potentials (A, cell-attached recording) and to a shortening of interspike intervals (B). In NA bursting is more pronounced than in the control. Bursts are manifest as an early component in the interspike interval histograms which becomes more prominent in NA. C, time course of the enhancement of the action potential frequency.
To determine whether the effect of NA on firing frequency resulted from changes of the synaptic input of the recorded cell, we tested the effects of the ionotropic glutamate receptors blockers NBQX (10 μm) and APV (25 μm), as well as of the GABA receptor blocker bicuculline (10 μm). No consistent effect was obtained on the firing rate of stellate cells upon application of a combination of NBQX and APV (n= 5). Upon mixed application of NBQX, APV and bicuculline, variable results were obtained. In some cases the distribution of interspike intervals shifted to a smaller and narrower range, as reported earlier (Midtgaard, 1992; Häusser & Clark, 1997). However, in other cells no effect was observed, or the frequency decreased. On average the ratio of the action potential frequency observed in the presence of the blockers over the control value was 1.01 ± 0.17 (n= 8). These results indicate that the firing pattern of interneurones in slices is minimally affected by excitatory and inhibitory inputs, presumably because the frequency of the corresponding synaptic events is too low to exert a significant effect. In the presence of synaptic transmission blockers the effect of NA on firing frequency persisted (1.44 ± 0.34 of the control, n= 8, P < 0.01). These results suggest that the increase of firing rate by NA is not due to a change in the efficacy of synaptic input but rather to a direct modification of the voltage-dependent channels responsible for the excitability of the cells.
Pharmacology of excitability changes
To examine the nature of the receptors mediating the effects of NA on action potential frequency, we applied different effectors of adrenergic receptors in the presence of NBQX, APV and bicuculline. Application of the selective α-receptor agonist 6-fluoro-noradrenaline (6FNE; 10 μm) did not induce any systematic change of action potential frequency (mean ratio to the control: 1.02 ± 0.17, n= 11). However, in individual experiments either a small increase (6 out of 11 cells) or decrease (5 out of 11 cells) in firing frequency was detected. In contrast, the selective β-receptor agonist isoprenaline (Iso; 10 μm) caused a significant increase in the firing frequency (mean ratio to the control: 1.44 ± 0.30, n= 11, P < 0.01). The increase of firing rate had a dependency on the initial frequency similar to that observed with NA. The effect of Iso had a quick onset and a slow recovery upon washing (> 30 min). Figure 3A illustrates an example where 6FNE reduced the action potential frequency, while Iso caused a marked frequency increase in the same cell. Finally, NA was able to increase the action potential frequency (mean ratio to the control: 1.48 ± 0.32, n= 6, P < 0.02, Fig. 3B) in the presence of the α-receptor antagonist phentolamine. Taken together the results suggest that the NA-induced increase of firing frequency is mainly mediated by β-receptors.
Figure 3. The effects of NA on spike frequency have a β-adrenergic pharmacology.
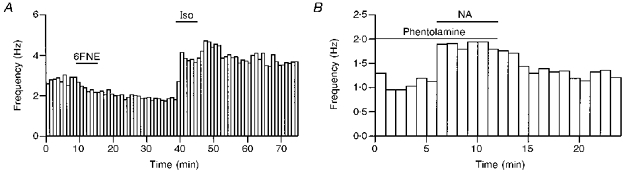
A, in this experiment 6FNE (10 μm), an α-adrenergic agonist, induced a small reduction in spike frequency while isoprenaline (10 μm), a β-adrenergic agonist, induced a strong potentiation. B, here NA (10 μm) is shown to potentiate the spike frequency in the presence of the α-blocker phentolamine (10 μm).
Effects of NA on evoked synaptic transmission
We next examined the effects of NA on evoked synaptic transmission. In a first series of experiments presynaptic interneurones were stimulated with an extracellular pipette located in the molecular layer, typically 100-200 μm from the recorded cell. Using a similar method Mitoma & Konishi (1996) found that NA increases eIPSCs in interneurone- Purkinje cell synapses. An increase in success rate was also observed at synapses among stellate cells, while the mean amplitude of successful events was not modified (Fig. 4). However there was some variability in the results, and for large initial success rates no significant effect was found.
Figure 4. Enhancement of eIPSCs by NA in extracellular stimulation experiments.
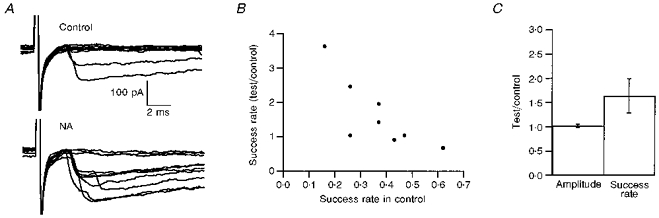
In these experiments eIPSCs were elicited by extracellular stimulation in the molecular layer. A, examples of ten consecutive traces during the control period and in the presence of NA, from an experiment where NA clearly increased the mean IPSC amplitude (including failures) and increased the success rate. B, summary of the results obtained on eight different cells. The effectiveness of NA in increasing the success rate was related to the success rate in the control. C, summary of the effects of NA on mean amplitude (excluding failures) and on success rate, from the same set of eight experiments used for the plot in B. Error bars show ±s.e.m.
When using extracellular stimulation the possibility must be considered that experimental manipulations lead to changes in the number of stimulated release sites. Since NA increased the excitability of interneurones it seemed possible that part or all of the potentiation of eIPSCs could be due to the recruitment of additional presynaptic elements (either new presynaptic cells or, for a single presynaptic cell, new branches of the presynaptic axon). In order to avoid these complications we performed paired recordings between two connected stellate cells. Presynaptic action potentials and postsynaptic currents were respectively monitored by loose cell-attached and by tight-seal whole-cell recording. In the analysis of the results, a control period was defined as just before application of NA and a test period was defined as starting 4 min after the onset of NA application. Among connected pairs the number of analysed postsynaptic responses varied from 300 to 2000. Figures 5 and 6 illustrate the results of such an experiment (from cell pair 1; same numbering as in Table 1 of Kondo & Marty, 1998). Both in the control run and in the presence of NA, amplitude histograms contain one major Gaussian-shaped peak centred near 90 pA, as well as a minor component with amplitudes ranging between 10 and 50 pA (Fig. 5C). The smaller events had slow rise times, and the synapse belonged to the ‘simple + slow’ category defined in the accompanying paper (Kondo & Marty, 1998). Application of NA caused a marked increase in the frequency of presynaptic action potentials (Fig. 5A and 6A). However, eIPSCs were not potentiated. The mean amplitude of eIPSCs excluding failures was reduced (to 83 % of the control; Fig. 5C and Fig. 6B), while the success rate was hardly modified (96 % of the control; Fig. 6A) so that the mean eIPSC including failures was reduced to 80 % of the control. The latency histogram was time shifted by 0.2 ms towards larger values (Fig. 6C). Table 1 summarizes the effect of NA in eight different paired recordings. In none of these experiments was any potentiation of the evoked response registered. Instead, small decreases in success rate and mean amplitudes excluding failures were obtained, with a slight increase in mean latency. Yet in seven out of eight cells, clear effects of NA on the rate of presynaptic action potentials and/or on that of sIPSCs were observed, suggesting that the application of NA was normally effective. These results show that while there is a variable potentiation of eIPSCs by NA with extracellular stimulation, there is none in paired recordings.
Figure 5.
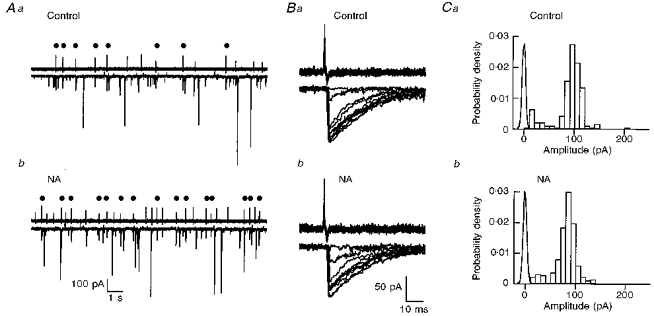
Effect of NA on a paired recording of connected interneuronesA, pre- and postsynaptic traces on a slow time scale, both in the control (Aa, Ba and Ca) and in NA (10 μm; Ab, Bb and Cb). The presynaptic interneurone was recorded in the cell-attached configuration. Dots indicate presynaptic spikes that elicited an IPSC in the recorded postsynaptic neurone. Some of the presynaptic spikes appear smaller than others, but this is an artefact due to insufficient sampling rate. B, successive superimposed presynaptic action potentials (upper traces) and corresponding IPSCs (lower traces). For each panel only one example of failure and of slow event is shown. This does not reflect the actual proportion of these events. C, amplitude histograms of eIPSCs in the control and in the presence of NA. Noise histograms are also displayed. Mean amplitudes excluding failures were 98.1 ± 32.3 and 81.1 ± 23.5 pA (mean ±s.d.) in control and in NA, respectively.
Figure 6. NA does not increase the success rate of eIPSCs.
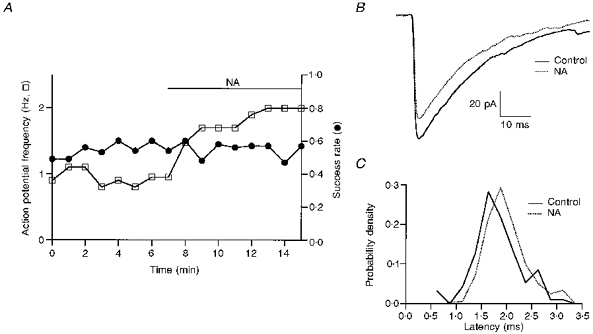
Same experiment as in Fig. 5. A, NA increases the frequency of presynaptic action potentials but does not modify appreciably the success rate (mean values: 0.55 in the control and 0.53 in NA). B, superimposed means of evoked IPSCs, failures excluded. The mean amplitude of successful eIPSCs is less in NA than in the control. C, the latency histogram in NA is time shifted by 0.2 ms compared with the control histogram.
Table 1.
Summary of the effects of noradrenergic agonists in paired recordings
| Treatment | sIPSC frequency | AP frequency | eIPSC frequency | Success rate | Latency | Amp. excl. failures | Amp. incl. failures |
|---|---|---|---|---|---|---|---|
| NA (n= 8) | 1.70 ± 0.39 | 1.59 ± 0.49 | 1.48 ± 0.52 | 0.93 ± 0.09 | 1.1 ± 0.1 | 0.80 ± 0.15 | 0.75 ± 0.17 |
| PT + NA, Iso (n= 3) | 1.57 ± 0.40 | 1.43 ± 0.24 | 1.34 ± 0.13 | 0.95 ± 0.12 | 1.1 ± 0.1 | 0.86 ± 0.12 | 0.84 ± 0.24 |
| Overall mean | 1.66 ± 0.38 | 1.55 ± 0.43 | 1.44 ± 0.44 | 0.93 ± 0.12 | 1.1 ± 0.1 | 0.82 ± 0.14 | 0.77 ± 0.20 |
| t test (P value) | < 0.001 | < 0.01 | < 0.01 | < 0.1 | < 0.01 | < 0.01 | < 0.001 |
NA, noradrenaline (10 μm); PT, phentolamine (10 μm); Iso, isoprenaline (10 μm); Amp. excl. failures, amplitude excluding failures; Amp. incl. failures, amplitude including failures; and AP, action potential. For columns 2-7 the value of the indicated parameter was determined in each experiment in the presence of a noradrenergic agonist and in the control. Entered values are means ±s.d. of the ratios between these measurements. The last line gives the results of t test comparisons between control results and results in the presence of a noradrenergic agonist.
Since the results of Fig. 4 suggest a correlation between the initial success rate and the effectiveness of NA in extracellular stimulation experiments, we examined whether for some reason the initial success rates were systematically lower in paired recordings than in extracellular stimulation experiments. This was not the case. In extracellular experiments NA seemed effective for success rates below 0.4, and ineffective for success rates above 0.4. Seven out of eleven paired recordings had an initial success rate below 0.4, and none of these experiments showed any potentiation following stimulation of noradrenergic receptors. Overall the most likely explanation for the discrepancy between paired recordings and extracellular stimulation experiments is that NA application leads to stimulation of new presynaptic terminals with the latter technique.
Inhibitory effects on Ca2+ current through α-adrenoceptor activation were reported in several preparations including sympathetic neurones (Lipscombe, Kongsamut & Tsien, 1989). To explain that NA treatment did not decrease the failure rate, it was envisaged that an enhancement of transmitter release was compensated by an inhibitory effect of NA on Ca2+ currents. To test this possibility, we applied NA in the presence of the α-receptor-specific antagonist phentolamine (n= 2), and we applied Iso in normal saline solution (n= 1). All three cell pairs tested showed an increase in the frequency of presynaptic action potentials but again, no potentiation of eIPSCs was found. In all respects, the results obtained in these experiments were similar to the effect of NA alone (Table 1). We conclude from these experiments that the lack of enhancement of eIPSCs in NA is not due to the cancellation of a β-receptor-induced potentiation by an inhibitory action of α-receptors.
Dissociated effects of NA on mIPSC frequency and on eIPSC success rate
In paired recordings, the proportion of sIPSCs that were elicited by the recorded presynaptic neurone was rather small, indicating that on average 4.25 interneurones contributed to the sIPSCs (Kondo & Marty, 1998). But in one case (cell pair 15) the proportion of sIPSCs that were associated with presynaptic action potentials was as high as 0.79. Shortly after starting a whole-cell recording in the presynaptic cell, we observed in this experiment the gradual disappearance not only of eIPSCs (presynaptic ‘washout’, see Kondo & Marty, 1998), but also of sIPSCs that were not linked to presynaptic action potentials, indicating that all sIPSCs observed before washout originated from the recorded presynaptic cell. We took advantage of the special situation of this recording to obtain the rate of mIPSCs simply by subtracting eIPSCs from all recorded events. The results indicated that NA increased the frequency of mIPSCs, with a ratio to the control value that was similar to that previously observed in the presence of TTX (Kondo & Marty, 1997). Meanwhile, the success rate of eIPSCs was not modified (Fig. 7B). This result shows that NA increases the frequency of action potential-independent IPSCs even in the absence of TTX while in the same synapse, it fails to increase evoked transmitter release.
Figure 7. NA increases action potential-independent IPSCs recorded without TTX.
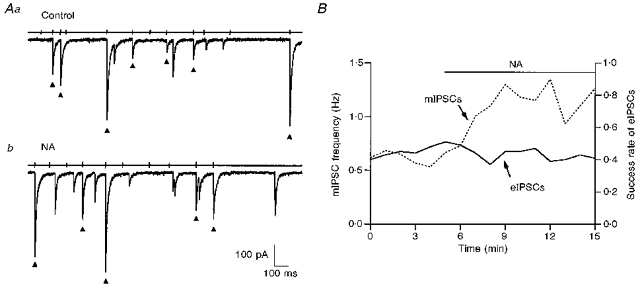
In this paired recording it appeared that all IPSCs, whether they were evoked by a presynaptic action potential or not, originated from a synapse made by the recorded presynaptic interneurone. A, pre- and postsynaptic traces on a slow time scale, both in the control (Aa) and in NA (10 μm; Ab). Spike-evoked IPSCs are marked (▴). B, NA did not increase the proportion of successful IPSCs but it increased significantly the rate of action potential-independent IPSCs (labelled ‘mIPSCs’).
DISCUSSION
The principal result that emerges from the present work is that at synapses among stellate cells sIPSCs, eIPSCs and mIPSCs are regulated separately from each other following NA application. Thus while the frequencies of mIPSCs and sIPSCs are both increased, the underlying mechanisms are different, and the mean amplitude of eIPSCs is actually slightly decreased.
Excitatory action of NA on action potential generation
NA markedly increased the action potential frequency in cerebellar stellate cells. Our results show that this increase arises from a direct effect of NA on action potential generation rather than from a change in the efficacy of excitatory or inhibitory inputs. The NA-induced excitation was mimicked by a specific β-adrenoceptor agonist and persisted in the presence of an α-adrenoceptor antagonist, suggesting that β-adrenoceptors are mainly responsible for this action. The slow recovery of the effects of NA or Iso on spike frequency upon washing are also consistent with the involvement of β-receptors. β-Receptor-mediated excitatory effects on action potentials have been reported in several central nervous system preparations, including hippocampal pyramidal cells and thalamic neurones. In hippocampal pyramidal cells, blockade of slow afterhyperpolarization (AHP) seems to be the major cause for excitation (Madison & Nicoll, 1986) whereas in thalamic neurones, a slow depolarization resulting from a decrease in hyperpolarization-activated current (Ih) predominates (McCormick & Pape, 1990).
Effects of NA on eIPSCs
In paired recordings, NA applications did not lead to the expected increase in mean eIPSC, but to a significant decrease (Table 1). The reduction in mean eIPSC amplitude resulted from decreases in both the success rate and mean amplitude excluding failures, and was accompanied by a small but significant increase in mean latency (Table 1). This pattern of changes is strikingly similar to that observed in control saline during periods of higher presynaptic firing frequency (Kondo & Marty, 1998; Fig. 6B), raising the possibility that the changes in eIPSCs observed in NA are a mere consequence of the increase in presynaptic firing rate.
The increased latency and failure rate indicate that the release probability is reduced in NA. This could happen by either of two mechanisms. First, the increased rate of GABA release associated with the increase in the frequency of sIPSCs could activate presynaptic GABAB receptors and thus reduce the probability of evoked release. Alternatively, the high exocytosis rate could induce a depletion of the pool of vesicles available for evoked release.
The amplitude reduction of eIPSCs excluding failures in NA is observed both for ‘multiple’ and for ‘simple’ synapses (see Fig. 5 and Fig. 6 for the latter). Due to the combination of multivesicular release and of a high degree of receptor occupancy, a reduction in release probability at one release site results in a reduction in the quantal size (C. Auger, S. Kondo & A. Marty, unpublished observations). The reduced probability of evoked release could therefore be the cause of the observed reduction in mean IPSC amplitude. Alternatively, the amplitude decrease could result from cumulative desensitization. Previous results on α-latrotoxin-induced bursts indicate that at single-site synapses, high release rates induce a gradual decrease in peak mIPSC amplitudes, presumably reflecting cumulative desensitization (Auger & Marty, 1997). However, this effect is negligible for mIPSC frequencies below 3 Hz; since the maximum eIPSC frequency observed in the present experiments was 3.3 Hz, cumulative desensitization is unlikely to have played an important part in the reduction in mean eIPSC amplitude.
In summary, the most parsimonious explanation for the NA-induced changes in eIPSCs is that the increased rate of presynaptic firing leads to a decrease in the probability of evoked transmitter release, which then leads to both an increase in latency and to a decrease in mean amplitude of eIPSCs.
Action potential-dependent and -independent transmitter release are differentially modulated by NA
We conclude from this work and from previous publications (Llano & Gerschenfeld, 1993b; Kondo & Marty, 1997) that stimulation of β-adrenergic receptors increases the frequency of both mIPSCs and sIPSCs to a similar extent while eIPSCs are slightly reduced. The effects on mIPSCs and sIPSCs are superficially similar but the underlying mechanisms are different. The increase in mIPSC frequency is not linked to any membrane permeability change and is due to the direct enhancement of the vesicular release probability (Llano & Gerschenfeld, 1993b; Kondo & Marty, 1997). Here we show on the other hand that the effects on sIPSCs are mostly accounted for by an increase in the rate of firing of presynaptic interneurones, apparently due to a change in presynaptic membrane conductance. Thus the location of the NA action is different in the two cases: the enhancement of mIPSC frequency takes place at the nerve terminals whereas the acceleration of sIPSC discharge is elicited in the somato-dendritic domain or near the site of generation of the spikes, at the axon hillock.
The experiment of Fig. 7 suggests that action potential-independent IPSCs occurring in normal saline solution are regulated by NA in the same manner as mIPSCs in TTX. If this is so it should be possible to predict the change in the rate of sIPSCs produced by NA on the basis of the other measurements made in this study and in our preceding work (Kondo & Marty, 1997). To this end we first note that: frequency of sIPSCs = frequency of mIPSCs + frequency of eIPSCs.
To estimate the frequency of mIPSCs, we assume that this frequency remains the same with and without TTX. Therefore, we measured the proportion of action potential-independent IPSCs among the IPSCs occurring in control conditions simply by adding TTX (200 nm) to block the action potentials. The mean proportion of TTX-insensitive IPSCs estimated with this method was 19 % (n= 11; range 10 to 29 %). This number is the same as that previously reported by Llano & Gerschenfeld (1993a).
The above equation is valid both in control saline solution and in the presence of NA:
| (1) |
| (2) |
where sctl, mctl and ectl are the frequencies of sIPSCs, mIPSCs and eIPSCs, respectively, in control conditions, and the same symbols with NA indices are the corresponding quantities in NA. We are assuming here that the frequency increase of action potential-independent release in normal saline solution is the same as in TTX. According to our previous measurements (Kondo & Marty, 1997) the mIPSCs frequency was 2.24 times higher in NA than in the control:
| (3) |
The eIPSC frequency was 1.48 larger in NA than in the control (see Table 1):
| (4) |
Finally, the proportion of mIPSCs in total IPSCs was 0.19:
| (5) |
Equations (1)–(5) are a set of five equations with six unknowns. Therefore these unknowns are linked to each other. One obtains:
This calculation predicts a frequency increase of sIPSCs by NA of 1.62 over the control. This number is quite close to the experimental value (1.70, see Table 1). This match provides evidence for the above hypothesis that sIPSCs are the sum of two components, mIPSCs and eIPSCs, which are regulated by NA independently of each other.
Other examples of differential regulation of evoked and miniature synaptic currents
There are many examples where neuromodulatory substances modify in parallel evoked and miniature synaptic currents, as if a regulatory step downstream of Ca2+ entry was common to spontaneous and triggered exocytosis (see the review by Thompson, Capogna & Scanziani, 1993, for examples in the hippocampus). However, recent measurements of intraterminal Ca2+ concentration suggest that at least in two of these cases (inhibition by adenosine and by GABAB receptor activators of the CA3-CA1 synapse), the regulation of evoked synaptic currents is actually due to modulation of voltage-dependent Ca2+ currents (Wu &, Saggau, 1994, 1995). Such an effect is unlikely to modify miniature synaptic currents (Thompson et al. 1993). Therefore, even though adenosine and GABA similarly affect miniature and evoked release, they appear to do so by separate mechanisms.
Many examples have been previously reported where a neuromodulatory agent changes evoked transmitter release without altering the rate of miniature currents. This is the case for the inhibitory effects of GABAB stimulation at inhibitory synapses in hippocampal CA1 neurones (Scanziani, Capogna, Gähwiler & Thompson, 1992), for those of α-adrenergic stimulation at excitatory synapses in hippocampal CA3 pyramidal cells (Scanziani, Gähwiler & Thompson, 1993), or for those of the metabotropic glutamate agonist trans-ACPD in cerebellar interneurone- interneurone synapses (Llano & Marty, 1995). Such cases can easily be explained by a reduction of Ca2+ influx following the regulation of presynaptic excitability. Thus in all the above examples an inhibition of Ca2+ channels is likely, even though this possibility has not been tested directly.
To our knowledge there has been no report until the present study of a neuromodulatory action where miniature events are modified without an accompanying change in evoked currents. In this case the discrepancy cannot be explained easily on the basis of an alteration in membrane conductances of the presynaptic terminals.
It is finally worth mentioning that in Drosophila, mutations of the synaptotagmin gene can lead to a situation where the frequency of miniature synaptic currents is enhanced while the amplitude of evoked synaptic currents is reduced (DiAntonio & Schwartz, 1994). One possible interpretation of this finding is that the primary defect in mutated animals is an enhancement in the rate of fusion of synaptic vesicles, leading to a decrease in the number of vesicles available for evoked release.
Functional consequences of enhanced mIPSCs and sIPSCs and reduced eIPSCs in the presence of NA
Since in this preparation there is little difference between the mean amplitudes of mIPSCs and of sIPSCs (Llano & Gerschenfeld, 1993a), action potential-dependent and -independent currents contribute in proportion to their relative frequencies (80 and 20 %, respectively) to the inhibition exerted by stellate cells. In slices, excitatory mossy-fibre and climbing-fibre inputs are not active, so that interneurone IPSCs reflect mIPSCs and the random spontaneous firing of presynaptic interneurones. It is this background inhibition which is enhanced by NA. Because of the powerful inhibition resulting from one IPSC it may seem paradoxical that NA manages to enhance significantly the rate of firing of interneurones. Recent results suggest that one IPSC prevents spiking for a period of about 7 ms (Häusser & Clark, 1997). Since the mean frequency of sIPSCs across experiments was 4.7 Hz in the control and 8.7 Hz in NA, the deficit of spiking due to interneurone- interneurone synapses can be estimated at 3 % in the former and at 6 % in the latter case. Thus, due to the low frequency of sIPSCs in interneurones, their rate of firing is determined by their intrinsic action potential generating conductances and not by the rate of synaptic currents (see, however, Häusser & Clark, 1997 for a different point of view). This is confirmed by the lack of net effects of bicuculline on the rate of action potentials in our experiments, and by the finding that the potentiation of the action potential frequency by NA was as strong in control saline solution as in bicuculline.
In vivo, phasic stimulation of the excitatory afferent fibres generates a powerful synchronized inhibition among interneurones and in postsynaptic Purkinje cells (Eccles, Llinas & Sasaki, 1966a,b). The present results indicate that, at interneurone-interneurone synapses, this phasic response is not enhanced, but is somewhat reduced by NA. Even though only interneurone-interneurone synapses have been investigated in the present study it can be speculated that the same situation holds at interneurone-Purkinje cell synapses. If such is the case, NA increases desynchronized inhibitory background activity but decreases the inhibition produced by synchronized parallel fibre stimulation. In Purkinje cells, the increased background inhibition would reduce the spontaneous activity in the absence of stimulation, while the inhibition of eIPSCs would enhance the response to parallel fibre stimulation. Thus noradrenergic modulation appears to tune the contrast between the rate of discharge observed under low excitatory input conditions and the phasic responses to excitatory input stimulation.
Acknowledgments
We thank C. Pouzat for sharing his analysis routines, and C. Auger for comments on the manuscript. This work was supported by the Human Frontier Science Program (fellowship to S. K.) and by the Deutsche Forschungsgemeinschaft (program S. F. B. 406).
References
- Auger C, Marty A. Heterogeneity of functional synaptic parameters among single release site. Neuron. 1997;19:139–150. doi: 10.1016/s0896-6273(00)80354-2. 10.1016/S0896-6273(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Doze VA, Madison DV, Smith SJ. Excitatory actions of norepinephrine on multiple classes of hippocampal CA1 interneurones. Journal of Neuroscience. 1996;16:572–585. doi: 10.1523/JNEUROSCI.16-02-00572.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Stevens CF. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. Journal of Neuroscience. 1994;14:310–317. doi: 10.1523/JNEUROSCI.14-01-00310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A, Schwartz TL. The effect on synaptic physiology of synaptotagmin mutations in Drosophila. Neuron. 1994;12:909–920. doi: 10.1016/0896-6273(94)90342-5. 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K. The inhibitory interneurones within the cerebellar cortex. Experimental Brain Research. 1966a;1:1–16. doi: 10.1007/BF00235206. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Experimental Brain Research. 1966b;1:17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Gereau RW, IV, Conn J. Presynaptic enhancement of excitatory synaptic transmission by β-adrenergic receptor activation. Journal of Neurophysiology. 1994;72:1438–1442. doi: 10.1152/jn.1994.72.3.1438. [DOI] [PubMed] [Google Scholar]
- Häusser M, Clark B. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. 10.1016/S0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Hoffer BJ, Siggins GR, Oliver AP, Bloom FE. Cyclic AMP-mediated adrenergic synapses to cerebellar Purkinje cells. Advances in Cyclic Nucleotides Research. 1972;1:411–423. [PubMed] [Google Scholar]
- Kondo S, Marty A. Protein kinase A-mediated enhancement of miniature IPSC frequency by noradrenaline in rat cerebellar stellate cells. Journal of Physiology. 1997;498:165–176. doi: 10.1113/jphysiol.1997.sp021849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Marty A. Synaptic currents at individual connections among stellate cells in rat cerebellar slices. Journal of Physiology. 1998;509:221–232. doi: 10.1111/j.1469-7793.1998.221bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D, Kongsamut S, Tsien RW. α-Adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature. 1989;340:639–642. doi: 10.1038/340639a0. 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- Llano I, Gerschenfeld HM. Inhibitory synaptic currents in stellate cells of rat cerebellar slices. Journal of Physiology. 1993a;468:177–200. doi: 10.1113/jphysiol.1993.sp019766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Gerschenfeld HM. β-Adrenergic enhancement of inhibitory synaptic activity in rat cerebellar stellate and Purkinje cells. Journal of Physiology. 1993b;468:201–224. doi: 10.1113/jphysiol.1993.sp019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Marty A. Presynaptic metabotropic glutamatergic regulation of inhibitory synapses in rat cerebellar slices. Journal of Physiology. 1995;486:163–176. doi: 10.1113/jphysiol.1995.sp020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape H-C. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. Journal of Physiology. 1990;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Actions of noradrenaline recorded intracellulary in rat hippocampal CA1 pyramidal neurones, in vitro. Journal of Physiology. 1986;372:221–244. doi: 10.1113/jphysiol.1986.sp016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midtgaard J. Membrane properties and synaptic responses of Golgi cells and stellate cells in the turtle cerebellum. Journal of Physiology. 1992;457:329–354. doi: 10.1113/jphysiol.1992.sp019381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitoma H, Konishi S. Long-lasting facilitation of inhibitory transmission by monoaminergic and cAMP-dependent mechanisms in rat cerebellar GABAergic synapses. Neuroscience Letters. 1996;217:141–144. [PubMed] [Google Scholar]
- Scanziani M, Capogna M, Gähwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Gähwiler BH, Thompson SM. Presynaptic inhibition of excitatory synaptic transmission mediated by alpha adrenergic receptors in area CA3 of the rat hippocampus in vitro. Journal of Neuroscience. 1993;13:5393–5401. doi: 10.1523/JNEUROSCI.13-12-05393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Capogna M, Scanziani M. Presynaptic inhibition in the hippocampus. Trends in Neurosciences. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. 10.1016/0166-2236(93)90160-N. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiologica Scandinavica. 1971;(suppl. 367):1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing calcium influx in area CA1 of hippocampus. Neuron. 1994;12:1139–1148. doi: 10.1016/0896-6273(94)90321-2. 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. GABAB receptor-mediated presynaptic inhibition in guinea-pig hippocampus is caused by reduction of presynaptic Ca2+ influx. Journal of Physiology. 1995;485:649–657. doi: 10.1113/jphysiol.1995.sp020759. [DOI] [PMC free article] [PubMed] [Google Scholar]


