Abstract
Acetylcholine (ACh) is important as the transmitter responsible for neuromuscular transmission. Here we report the non-quantal release of ACh from embryonic myocytes.
Co-cultures of spinal neurons and myotomal muscle cells were prepared from 1-day-old Xenopus embryos. Single channel currents were recorded in the non-innervated myocytes. When the patch pipette was filled with Ringer solution alone, spontaneous single channel currents occurred, which were inhibited by d-tubocurarine (d-Tc).
The channel conductance appearing in Ringer solution (37.3 pS) was similar to that of an embryonic-type ACh channel (36.9 pS), indicating that ACh is probably released from myocytes in normal Ringer solution.
When the patch pipette was filled with anticholinesterase alone to prevent hydrolysis of ACh released from myocytes, both physostigmine and neostigmine in a concentration-dependent manner increased channel open probability; it was reduced by d-Tc or α-bungarotoxin.
Vesamicol and quinacrine, vesicular transporter inhibitors, reduced the channel open probability caused by ACh released from myocytes in the presence of neostigmine or physostigmine.
Intracellular alkalinization with NH4Cl inhibited the ACh release from myocytes, whereas, extracellular alkalinization, brought about by replacing normal Ringer solution, with pH 8.6 Ringer solution enhanced ACh release.
The immunocytochemistry of choline acetyltransferase (ChAT) showed that ChAT exists in both myocytes and neuronal cells but not in fibroblasts.
These results suggest that embryonic myocytes are capable of synthesizing and releasing ACh in a non-quantal manner. Extracellular alkalinization enhanced and intracellular alkalinization inhibited ACh release from myocytes.
It is widely accepted that the evoked release of ACh from the motor nerve terminals occurs in discrete quanta, with each quantum probably representing the content of one synaptic vesicle (Katz, 1969; Ceccarelli & Hurlbut, 1980). Electrophysiologically, a single quantum of ACh brings about the appearance of a single miniature end-plate potential. Several types of experiments have led to the suggestion, however, that the liberation of ACh from neuromuscular preparations which occurs under resting condition, is mainly non-quantal. Non-quantal ACh release also occurs spontaneously (Katz & Miledi, 1977), but is not enhanced by nerve impulses (Katz & Miledi, 1981). The function of non-quantal ACh release is not yet well understood. It is probable that non-quantally released ACh may act on the nerve terminal itself. Evidence has been accumulated that non-quantal ACh release may also contribute in part to the neurotrophic regulation of muscle properties (Katz & Miledi, 1977; Drachman, Stanley, Pestronk, Griffin & Price, 1982), including acetylcholinesterase induction or regulation (Katz & Miledi, 1977), ACh receptor distribution (Mathers & Thesleff, 1978; Drachman et al. 1982), and resting membrane potential (Bray, Forrest & Hubbard, 1982; Drachman et al. 1982). However, not all ACh released from nerve-muscle preparations during incubation in vitro originates from the nerve fibres. It has been known for a long time that ACh is also released from denervated muscles and from aneural parts of innervated muscles (Miledi, Molenaar, Polak, Tas & van der Laaken, 1982; Dolezal & Tucek, 1983). At present, very little is known about the function of the ACh released from myocytes. To learn more about the mechanism and the factors controlling the non-quantal release of ACh from myocytes, the effects of various drugs or pH changes on the non-quantal ACh release have been investigated in cultured embryonic Xenopus myocytes in the present work. The release of ACh was quantified by the apparent open probability of single ACh channels. It was found that embryonic myocytes are capable of synthesizing and releasing ACh in a non-quantal manner. Extracellular and intracellular alkalinization enhanced and inhibited the release of ACh from myocytes, respectively.
METHODS
Cell culture
Xenopus nerve-muscle cultures were prepared as reported previously (Tabti & Poo, 1991). Briefly, the neural tube and the associated myotomal tissues of 1-day-old Xenopus embryos (stages 20-22; Nieuwkoop & Faber, 1967) were dissociated in Ca2+- and Mg2+-free Ringer solution supplemented with EDTA. The cells were plated on clean glass coverslips and were used for experiments after 24 h at room temperature (20-22°C). The culture medium consisted of 50 % (v/v) Ringer solution (115 mM NaCl, 2 mM CaCl2, 1.5 mM KCl, 10 mM Hepes, pH 7.6), 49 % L-15 Leibovitz medium (Sigma), 1 % fetal bovine serum (Gibco) and antibiotics (100 units ml−1 penicillin and 100 μg ml−1 streptomycin).
Electrophysiology
The cell-attached patch-clamp recording method was similar to that described previously by Hamill, Marty, Neher, Sakmann & Sigworth (1981). Single ACh channel currents were measured with a patch-clamp amplifier (Axopatch-200A, Axon Instruments) in 1-day-old Xenopus cultured myocytes at an applied pipette potential of +60 mV by using fire-polished and Sylgard-coated glass electrodes (1-5 MΩ). Recordings were made at room temperature in Ringer solution. The pipette was filled with Ringer solution with or without 1 nM ACh. The current signals were filtered at 1 kHz and digitized by a digitizing unit (Neuro-Corder DR390, New York) and stored on a videotape recorder for later playback. The data were digitized at 100 μs intervals and the kinetics and the apparent open propability (NPo) of ACh channels were analysed by using the pCLAMP program (Axon Instruments). The acquisition time of the single channel recording per file was on average 1-3 min. Events corresponding to the opening of more than one channel were excluded from the open time analysis. The open time histograms were fitted with a single exponential curve and the amplitude histograms with a Gaussian distribution, using the least-squares method in both cases. The apparent NPo was expressed as the mean of the fraction of the total channel opening time per 10 s (including both high- and low-conductance ACh channels). In some conditions, more than two channels opened simultaneously and only the opening of the level 1 channel was counted. The drugs were either bath applied or applied in the patch pipette as indicated in Results and the ACh channel recordings were compared in the same or different patches of the same myocyte before and after drug application. Synaptic currents were recorded from innervated myocytes by whole-cell recording in the voltage-clamp mode. For whole-cell recordings, the solution inside the recording pipette contained 150 mM KCl, 1 mM NaCl, 1 mM MgCl2 and 10 mM Hepes (pH 7.2). The results were expressed as the means ±s.e.m. Statistical significance was evaluated by Student's t test.
Measurement of pHi
Measurement of pHi has been described in detail elsewhere (Wu, Tsai & Tseng, 1994). In brief, cultures were loaded with the acetoxymethyl ester of 5 μM 2,7-bis(carboxyethyl)-5,6-carboxyfluorescein (BCECF AM; Molecular Probes) for 5-10 min at room temperature in Ringer solution and then washed with the same solution three times. A single soma was excited by alternate flashes of 490 and 440 nm wavelength light, using a filter wheel (Cairn Research, Kent, UK) rotating at 32 Hz. The excitation light was transmitted to the cells of interest using a 510 nm dichroic mirror under the microscope nosepiece, and the resulting fluorescence was collected via a × 40 oil-immersion lens. The overall sampling rate was 0.5 Hz. The 490 nm/440 nm emission ratio from the intracellular BCECF was calculated and converted to a linear pH scale using in situ calibration data obtained at the end of the experiment by the nigericin technique as described elsewhere (Rink, Tsien & Pozzan, 1982). A calibration curve similar to that used by Wu et al. (1994) was established by measuring the fluorescence ratio values between pHi 4.5 and 9.5. Between pHi 5.5 and 9.0, the response is linear and fits the equation:
where R is the ratio of 530 nm fluorescence at 490 nm excitation to 530 nm fluorescence at 440 nm excitation. Rmax and Rmin are the maximum and minimum ratio values from the data curve and K is the dissociation constant for the dye, taken as 55 nM.
Immunocytochemical procedures
Cultures were washed briefly in serum-free culture medium and fixed by 4 % formaldehyde in 0.1 % phosphate buffered saline (PBS) at room temperature for 10 min. After washing with PBS twice, cultures were permeabilized with 0.15 % Triton X-100 in PBS for another 10 min. Non-specific labelling was blocked by incubation in 10 % normal goat serum. The cultures were washed with PBS and cooled to 4°C during this wash, and all subsequent incubations were performed at this temperature. Mouse anti-choline acetyltransferase (anti-ChAT) antibody (Chemicon International, Temecula, CA, USA) was diluted to 1:100 and applied to the cultures for 20 h. The cultures were then washed with two changes of PBS. The immunoperoxidase procedure was then applied by using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) based on the properties introduced by Hsu, Raine & Fanger (1981).
Chemicals
α-Bungarotoxin, carbonyl cyanide m-chlorophenylhydrazone (CCCP) d-tubocurarine, neostigmine methyl sulphate, physostigmine (eserine), quinacrine, NH4Cl and sodium acetate were obtained from Sigma; vesamicol ((±)-2-(4-phenylpiperidino)cyclohexanol, AH 5183) was obtained from RBI.
RESULTS
Release of ACh from embryonic myocytes
Single channel recordings were made on isolated myocytes with micropipettes containing Ringer solution alone. Figure 1A shows the typical patterns of single channel events observed when the myocyte was hyperpolarized +60 mV from rest. The open probability was very low (NPo= 0.011 ± 0.005, n= 9). After addition of 0.1 μM physostigmine into the patch pipette to prevent hydrolytic activity of acetylcholinesterase, the opening events were enhanced and the open probability was increased to 0.085 ± 0.034 (n= 7) (Fig. 1B), which was inhibited by the simultaneous addition of 4 μM d-tubocurarine in the patch pipette or bath pretreatment with 0.1 μM α-bungarotoxin.
Figure 1. Spontaneous ACh release from embryonic myocytes.
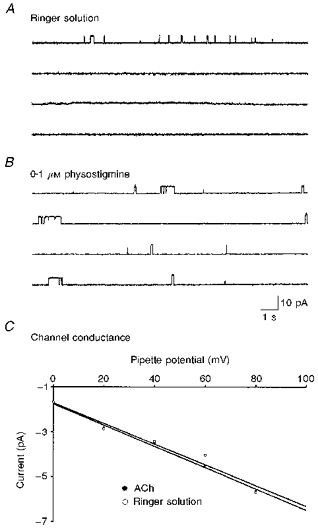
Samples of the recordings of single channel currents before (A) and after (B) application of 0.1 μM physostigmine in the patch pipette were obtained from different cell-attached patches of the same isolated 1-day-old cultured muscle cell. The pipette was filled with Ringer solution and the patch was hyperpolarized +60 mV from rest. C, current-voltage relations for channel activated in Ringer solution alone (○) or Ringer solution containing 1 nM ACh (•).
When the patch pipette was filled with another anticholinesterase, neostigmine, the channel open probability increased in a dose-dependent manner (Fig. 2A). In order to show the anticholinesterase activity of neostigmine, the spontaneous synaptic currents (SSCs) were recorded from innervated myocyte by the whole-cell voltage clamp method. As shown in Fig. 2B, application of 0.1 μM neostigmine prolonged the decay time of SSCs. To characterize further the channel opening in Ringer solution, the single-channel conductance was determined from the slope of current-voltage relations. Since the opening events of adult-type channels were rare in 1-day-old cultures in response to Ringer solution, only the conductance of embryonic-type channels was measured. The channel conductance was about 37.3 pS, which was similar to that of embryonic-type ACh channels activated by the addition of 1 nM ACh in the patch pipette (36.9 pS) (Fig. 1C). These results suggest that ACh may be released in a non-quantal manner from the myocyte, which is detectable by single channel recordings. To further strengthen the ‘non-quantal’ ACh releasing ability of myocytes, the opening rates of single ACh channels at the same membrane patch (using Ringer solution alone as intrapipette solution of the cell-attached patch) before and after intracellular loading of ACh were compared using another whole-cell recording pipette (intrapipette ACh concentration, 5 mM; holding potential, -60 mV). It was found that there was an immediate marked increase of the open probability of single ACh channels following intracellular loading of ACh (NPo was 2450 ± 988 % of control, n= 4).
Figure 2. Potentiating effect of anticholinesterase agents.
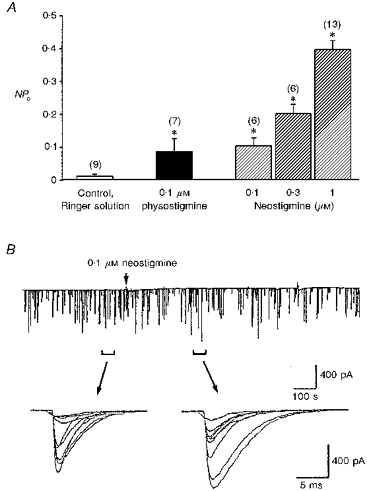
A, single channel currents were recorded by cell-attached patch clamp in 1-day-old Xenopus myocytes. The myocyte was hyperpolarized +60 mV from rest. Note that the open probability caused by ACh release from the myocyte was enhanced by anticholinesterase agents. Data are presented as means ± s.e.m (n). *P < 0.05 compared with control (Student's t test). B, the continuous trace depicts the spontaneous synaptic currents recorded from an innervated muscle cell (holding potential, -60 mV, filtered at 150 Hz). Samples of synaptic currents were superimposed and shown below at higher time resolution.
Regulation of ACh release from embryonic myocytes
It was proposed that the ACh transport system of synaptic vesicles may play a role in non-quantal release of ACh from nerve terminals (Edwards, Dolezal, Tucek, Zemkova & Vyskocil, 1985; Vyskocil, Zemkova & Edwards, 1989). To investigate whether this transport system is also responsible for the non-quantal ACh release from myocyte, the effects of ACh transporter inhibitors (Anderson, King & Parsons, 1983a,b) were examined. The most potent inhibitors of ACh transport in Torpedo synaptic vesicles among the eighty compounds investigated by Anderson et al. (1983b) were vesamicol and quinacrine. The channel open probability caused by ACh released from myocyte in the presence of neostigmine or physostigmine was reduced by 75-92 % at low concentrations of vesamicol and quinacrine (10 nM of each) (Fig. 3). Vesamicol and quinacrine at this concentration did not affect the open duration and the current amplitude of the single ACh channel (embryonic-type, low-conductance ACh channel) when the patch pipette was filled with 1 nM ACh (control: 3.7 ± 0.4 ms, 4.3 ± 0.2 pA, n= 18; vesamicol: 4.7 ± 0.8 ms, 4.1 ± 0.4 pA, n= 7; quinacrine: 4.2 ± 0.8 ms, 4.7 ± 0.2 pA, n= 6). These results indicate that the ACh transporter in the myocyte membrane is also vesamicol and quinacrine sensitive.
Figure 3. Inhibitory effect of transporter blockers on the ACh release from myocytes.
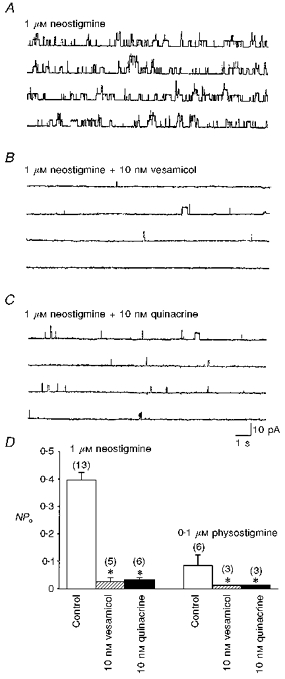
Single channel recordings were made by cell-attached patch clamp in 1-day-old Xenopus myocytes. The patch was hyperpolarized +60 mV from rest. Note that the single channel currents were markedly enhanced when the patch pipette was filled with Ringer solution containing 1 μM neostigmine (A). Application of ACh transporter blockers vesamicol (B) or quinacrine (C) in the patch pipettes inhibited these currents. The current recordings were obtained from different patches of the same myocyte. Summary of the inhibitory effects of vesamicol and quinacrine is shown in D. Data are presented as means ± s.e.m (n). *P < 0.05 compared with respective control.
Previous work has shown the existence of Ca2+-dependent quantal release of ACh from these myocytes after loading of ACh into the cytoplasm (Dan & Poo, 1992). The spontaneous ‘non-quantal’ release of ACh from the myocyte appears Ca2+ independent, since bath application of the mitochondrial uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP; 10 μM), to increase intracellular Ca2+, did not significantly affect the NPo when the intrapipette was filled with 0.3 μM neostigmine (112.5 ± 12.5 % of control, n= 4). The transport of ACh into the synaptic vesicle of nerve terminals has been reported to be coupled to a proton concentration gradient, in which the vesicle interior is more acidic (Anderson, King & Parsons, 1982). If the non-quantal ACh leakage of the myocyte similarly required a proton gradient, an increase in the bath pH should somehow inhibit the release by setting up a proton gradient in the direction opposite to that required for H+-ACh exchange. As shown in Fig. 4, extracellular alkalinization by replacing normal Ringer solution with pH 8.6 Ringer solution increased the channel open probability, which resulted from the enhanced ACh release from the myocyte. Vesamicol and quinacrine antagonized this potentiating effect (Fig. 4C). In contrast, intracellular alkalinization with NH4Cl inhibited ACh release from myocytes in the presence of 1 μM neostigmine in a concentration-dependent manner (Fig. 5). The NPo was inhibited by more than 90 % in the presence of 15 mM NH4Cl. In addition, the enhancement of NPo in pHo 8.6 Ringer solution was antagonized by the cytosolic alkalinization (Fig. 4C). The change of cytosolic pH in the myocyte was monitored by the BCECF fluorescence method and Fig. 6A shows the pHi alteration by NH4Cl application. The baseline pHi value was 7.09 ± 0.02 (n= 10). Exposure of the myocyte to NH4Cl resulted in a rapid increase in pHi and the peak pHi value was 7.18 ± 0.01 (n= 5), 7.39 ± 0.11 (n= 5) and 7.44 ± 0.01 (n= 6) for 1.5, 5 and 15 mM NH4Cl, respectively (Fig. 6C). The initial alkalinization seen during the first moments of exposure to NH4+ is presumably caused by the rapid, passive entry of NH3 and its subsequent hydration to form NH4+ and OH− (Roos & Boron, 1981). On the other hand, cytosolic acidification was induced by the substitution of all extracellular NaCl with weak organic acid. As shown in Fig. 6B and C, exposure of myocytes to acetate resulted in an acute acid load to the cytoplasm. The pHi reached a peak value of 6.70 ± 0.01 (n= 5). Intracellular acidification did not significantly affect the release of ACh from myocytes in the presence of 0.1 μM neostigmine (NPo values were 0.104 ± 0.028, n= 6 and 0.041 ± 0.011, n= 4 for control and intracellular acidification, respectively; P > 0.05).
Figure 4. Enhancement of ACh release from myocytes by extracellular alkalinization.
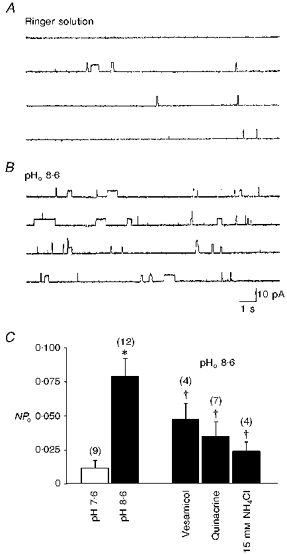
Single channel recordings were made by cell-attached patch clamp in 1-day-old Xenopus cultured myocytes. The patch was hyperpolarized +60 mV from rest. Compared with control in normal Ringer solution (pH 7.6, A), a patch pipette filled with pH 8.6 Ringer solution enhanced the opening of ACh channels (B and C). Application of vesamicol or quinacrine in the patch pipette or bath application of NH4Cl to cause intracellular alkalinization inhibited the potentiating effect (C). Data are presented as means ± s.e.m (n). *P < 0.05 compared with pH 7.6 normal Ringer solution. †P < 0.05 compared with pH 8.6 Ringer solution.
Figure 5. Inhibition of ACh release from myocyte by intracellular alkalinization.
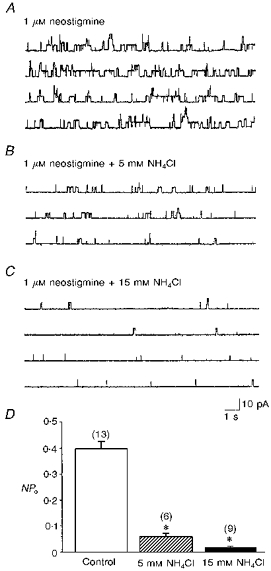
Single channel recordings were made by cell-attached patch clamp in 1-day-old Xenopus cultured myocytes. The patch was hyperpolarized +60 mV from rest. The patch pipette was filled with Ringer solution containing 1 μM neostigmine to induce ACh-activated single channel current (A). Intracellular alkalinization by bath application of NH4Cl in a dose-dependent manner inhibited channel opening (B-D). Data are presented as means ± s.e.m (n). *P < 0.05 compared with control.
Figure 6. Intracellular pH changes in cultured Xenopus myocytes.
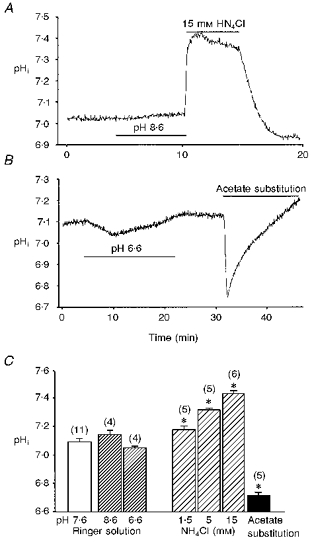
Cell cultures were loaded with 5 μM BCECF AM for 5-10 min at room temperature (20-22 °C) in Ringer solution and then washed with plain Ringer solution 3 times. Bath application of NH4Cl resulted in concentration-dependent intracellular alkalinization of 1-day-old Xenopus cultured myocytes (A and C). Substitution of all extracellular NaCl with sodium acetate caused intracellular acidification (B and C). Changes of the extracellular pH between pH 6.6 and 8.6 had no significant effect on intracellular pH (A-C). Data are presented as means ± s.e.m (n). *P < 0.05 compared with pH 7.6 normal Ringer solution.
Since the channel open probability caused by ACh release from myocyte was influenced by the pH changes, we further investigated the effect of pH changes on the kinetics of ACh channels when the patch pipette was filled with 1 nM ACh. Bath application of NH4Cl slightly reduced the open duration of a single ACh channel (2.5 ± 0.1 ms, n= 8 compared with control 3.7 ± 0.4 ms, n= 18). Extracellular alkalinization by replacing normal Ringer solution with pH 8.6 Ringer solution increased the open duration of the single ACh channel (6.6 ± 0.8 ms, n= 10), which probably resulted from the increase of pipette ACh concentration via the potentiation of ACh release from the myocyte. Further bath application of NH4Cl in pH 8.6 Ringer solution to decrease ACh release from the myocyte restored the open duration of the single ACh channel to 3.3 ± 0.3 ms (n= 10). On the other hand, intracellular acidification with sodium acetate substitution also did not significantly affect the open duration of single ACh channels (3.0 ± 0.4 ms, n= 8).
Evidence for the existence of choline acetyltransferase in myocytes
It has been reported that cardiac carnitine acetyltransferase (CarAT) is mainly responsible for the synthesis of ACh in skeletal muscles of the rat (Tucek, 1982). We thus further examined the type of enzymes responsible for synthesizing ACh in embryonic Xenopus cultured myocytes. As shown in Fig. 7A and B, both neurons and myocytes were positively stained with monoclonal antibody directed against choline acetyltransferae (ChAT; n= 10). For comparison, co-cultured fibroblasts showed no significant immunoreactive staining (Fig. 7C).
Figure 7. Immunoreactivity of choline acetyltransferase in 1-day-old Xenopus cultured myocytes.
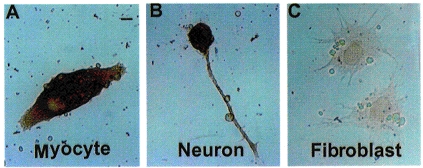
Immunoreactivity for choline acetyltransferase appeared in the cultured myocyte (A) as well as in the neuron (B) but not in the fibroblast (C). Scale bar, 5 μm.
DISCUSSION
ACh release from myocytes
Xenopus muscle cells, at 1 or 2 days in culture, have a relatively high uniform density (about 100 μm−2) of extrajunctional ACh receptors (Kidokoro & Greuner, 1982). We here used cell-attached single ACh channel recordings to measure directly the characteristics of ACh release from the embryonic myocytes. When the patch pipette was filled with Ringer solution alone, a few channels opened, and this was sensitive to inhibition by d-tubocurarine, indicating that there is spontaneous ACh channel opening in Ringer solution. These channel openings may result from the spontaneous opening of ACh channels without ligand binding or the ACh release from myocytes. Addition of anticholinesterase drugs such as neostigmine or physostigmine into the patch pipette markedly enhanced the open probability of ACh channels, due to the accumulation of ACh in the patch pipette. This activity can be blocked by agents such as d-tubocurarine and α-bungarotoxin, preventing ACh action on nicotinic ACh receptors, indicating that ACh is released from myocytes and that a substantial amount of ACh is hydrolysed upon release into the patch pipette. We did not detect any pulsatile ACh release and the ACh release from myocyte was insensitive to CCCP, and thus ACh is released in a non-quantal manner that is Ca2+ independent. When the myocyte was whole-cell clamped, that the membrane hyperpolarization was not observed upon the addition of d-tubocurarine or α-bungarotoxin (data not shown; in contrast to the natural synapse) probably resulted from the rapid diffusion of released ACh in the bathing medium, which is different from that from the narrow synaptic cleft into the natural synapses.
The monoclonal anti-ChAT antibodies used in the present study were reported to react specifically with bovine ChAT, which is similar to reported human ChAT (Grosman, Lorenzi, Trinidad & Strauss, 1994). Immunocytochemical demonstration of ChAT immunorectivity in the myocyte further supports the notion that the myocyte is capable of synthesizing ACh. It has been reported that cardiac carnitine acetyltransferase (CarAT) catalyses some synthesis of ACh in muscle homogenates (Tucek, 1982). In the present study, we demonstrated the presence of ChAT in the myocyte, which is mainly responsible for the synthesis of ACh in these embryonic myocytes. Whether CarAT co-exists with ChAT in these myocytes needs further investigation.
The characteristics of ACh release from myocytes
Vesamicol and quinacrine are known to inhibit the vesicular transporter responsible for the accumulation of ACh in presynaptic vesicles (Marshall & Parsons, 1987). It was found that vesamicol diminishes both the spontaneous release of ACh from the diaphragm and the d-tubocurarine-induced hyperpolarization in its endplate area (Edwards et al. 1985; Vyskocil, 1985). It was proposed that ACh carriers in the presynaptic membrane, incorporated into the membrane during vesicular exocytosis, are responsible for the non-quantal release of ACh from nerve terminals (Vyskocil et al. 1989). The release of ACh from myocytes is also sensitive to the inhibition by these two agents, suggesting that the similar carriers or some vesamicol-sensitive proteins are responsible for the non-quantal release of ACh from myocytes. Since no change in the kinetics of exogenous ACh-induced single channels was observed at these concentrations of vesamicol and quinacrine, the reduction of open probability caused by ACh release from myocytes did not result from a direct curare-like action. In Xenopus cultured myocytes, it has been reported that when ACh is loaded into an isolated myocyte, there is spontaneous and depolarization-evoked quantal release of ACh from the myocyte with a Ca2+ dependence reminiscent of ACh secretion from presynaptic nerve terminals (Dan & Poo, 1992). It is possible that there is also an ACh vesicular transporter existing in the cytoplasm of myocytes. However, we cannot detect any quantal release in the resting condition without loading with exogenous ACh. The conclusion of ‘non-quantal’ ACh release by myocyte was further strengthened by the finding that there was an immediate elevation of the opening rates of single ACh channels at the myocyte surface following intracellular loading of ACh by a whole-cell recording pipette prior to the appearance of quantal secretion. In physiological conditions, whether the ACh transporter proteins in the muscle cytoplasm are mainly responsible for non-quantal but not quantal ACh secretion needs further investigation.
Transport of ACh from the nerve terminal cytoplasm into the lumen of synaptic vesicles is linked to the movement of protons in the opposite direction, as defined as the vesicular ACh-H+ antiporter (Usdin, Eiden, Bonner & Erickson, 1995). A striking difference between the transport of ACh in neurons and myocytes is the H+ sensitivity. If the non-quantal leakage from the myocyte similarly required a proton gradient, an increase in the bath pH should at some level inhibit the release by setting up a proton gradient in the direction opposite to that requried for H+-ACh exchange. However, alkalinization of extracellular solution in the cell-attached patch pipette to pH 8.6 increased the secretion of ACh from myocytes, whereas intracellular alkalinization with NH4Cl decreased the release of ACh from myocytes. Although the open duration of ACh channel was slightly reduced by intracellular alkalinization upon the addition of 15 mM NH4Cl, the greater reduction of NPo may result from the inhibition of the ACh release. On the other hand, with an acidic pipette pH had no significant effect on the release of myocyte ACh (data not shown). Therefore, there is a transport system to move ACh across the muscle membrane, and the nature of the transporter in myocyte is different from that in synaptic vesicles.
Possible physiological function of non-quantal ACh release from myocytes
The non-quantal leakage of ACh is probably an important factor during synaptogenesis. We recently reported that there are presynaptic nicotinic receptors at developing motoneurons and the activation of these receptors potentiates the spontaneous release of ACh (Fu & Liu, 1997). Furthermore, endogenously released ACh in concert with released ATP is involved in this positive feedback regulation of spontaneous ACh release at high-activity neuromuscular synapses (Fu & Huang, 1994; Fu, 1995). ACh release from myocytes may partly contribute to this potentiation. It is well known that the activity of neuromuscular transmission at developing synapses is crucial in synaptic maturation and competition as well as in the differentiation of postsynaptic properties (Balice-Gordon & Lichtman, 1993; Dan & Poo, 1994). Voltage-gated Ca2+ channels and the nicotinic ACh receptors provide biologically important pathways for Ca2+ influx into presynaptic or postsynaptic muscle cells (Decker & Dani, 1990). The elevation of cytosolic Ca2+ levels in the postsynaptic cell is critical for the induction of many forms of activity-dependent synaptic modulation (Laufer & Changeux, 1989; Lo & Poo, 1994). We recently found that chronic treatment of the Xenopus nerve-muscle co-cultures with d-tubocurarine for 2 days resulted in a marked reduction of the ACh quantal size of natural synapses (Liou & Fu, 1997), suggesting that synaptic activity plays an important role in the maintenance and/or regulation of synaptic function in the developing nervous system. At present, we cannot distinguish the origins of ACh contributing to the regulation of quantal size. It is possible that a portion of endogenously released ACh comes from myocytes. The activity of acetylcholinesterase of embryonic synaptic regions is much lower than that in mature neuromuscular junctions. The decay time of SSCs is only slightly increased upon bath application of 0.1 μM neostigmine. Thus, ACh released from either presynaptic nerve terminals or postsynaptic muscle cells can accumulate in the vicinity of the synaptic cleft to a higher level. On the other hand, it has been shown that there are positive turning responses of the nerve growth cone of cultured Xenopus spinal neurons in a defined extracellular gradient of ACh, suggesting that ACh may possibly serve as a specific chemoattractant for growth cone guidance (Zheng, Felder, Connor & Poo, 1994). That the target of the motoneuron is skeletal muscle cells and that ACh can be synthesized and non-quantally released from embryonic myocytes further supports this notion. It is possible that localized secretion of ACh by the target tissue such as myocytes may influence the direction of neurite growth in the embryonic stages.
Acknowledgments
This work was supported by a grant from the National Science Council (NSC 86-2314-B002-299). We also thank Mr I. S. Peng for the preparation of the manuscript.
References
- Anderson DC, King SC, Parsons SM. Proton gradient linkage to activate uptake of 3H-acetylcholine by Torpedo electric organ synaptic vesicles. Biochemistry. 1982;21:3037–3043. doi: 10.1021/bi00256a001. [DOI] [PubMed] [Google Scholar]
- Anderson DC, King SC, Parsons SM. Inhibition of 3H-acetylcholine active transport by tetraphenylborate and other anions. Molecular Pharmacology. 1983a;24:55–59. [PubMed] [Google Scholar]
- Anderson DC, King SC, Parsons SM. Pharmacological characterization of the acetylcholine transport system in purified Torpedo electric organ synaptic vesicles. Molecular Pharmacology. 1983b;24:48–54. [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. Journal of Neuroscience. 1993;13:834–855. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JJ, Forrest JW, Hubbard JI. Evidence for the role of non-quantal acetylcholine in the maintenance of the membrane potential of rat skeletal muscle. The Journal of Physiology. 1982;326:285–296. doi: 10.1113/jphysiol.1982.sp014192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP. Vesicle hypothesis of the release of quanta of acetylcholine. Physiological Reviews. 1980;60:396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo M. Quantal transmitter secretion from myocytes loaded with aceytlcholine. Nature. 1992;359:733–736. doi: 10.1038/359733a0. 10.1038/359733a0. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Retrograde interactions during formation and elimination of neuromuscular synapses. Current Opinion in Neurobiology. 1994;4:95–100. doi: 10.1016/0959-4388(94)90037-x. 10.1016/0959-4388(94)90037-X. [DOI] [PubMed] [Google Scholar]
- Decker ER, Dani JA. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. Journal of Neuroscience. 1990;10:3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal V, Tucek S. The synthesis and release of acetylcholine in normal and denervated rat diaphragms during incubation in vitro. Journal of Physiology. 1983;334:461–474. doi: 10.1113/jphysiol.1983.sp014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DB, Stanley EF, Pestronk A, Griffin JW, Price DL. Neurotrophic regulation of two properties of skeletal muscle by impulse-dependent and spontaneous acetylcholine transmission. Journal of Neuroscience. 1982;2:232–243. doi: 10.1523/JNEUROSCI.02-02-00232.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C, Dolezal V, Tucek S, Zemkova H, Vyskocil F. Is an acetylcholine transport system responsible for non-quantal release of acetylcholine at the rodent myoneural junction. Proceedings of the National Academy of Sciences of the USA. 1985;82:3514–3518. doi: 10.1073/pnas.82.10.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu WM. Regulatory role of ATP at developing neuromuscular junctions. Progress in Neurobiology. 1995;47:31–39. doi: 10.1016/0301-0082(95)00019-r. 10.1016/0301-0082(95)00019-R. [DOI] [PubMed] [Google Scholar]
- Fu WM, Huang FL. Endogenously released ATP potentiates spontaneous transmitter release at developing neuromuscular synapses. British Journal of Pharmacology. 1994;111:880–886. doi: 10.1111/j.1476-5381.1994.tb14820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu WM, Liu JJ. Regulation of acetylcholine release by presynaptic nicotinic receptors at developing neuromuscular synapses. Molecular Pharmacology. 1997;51:390–398. [PubMed] [Google Scholar]
- Grosman DD, Lorenzi MV, Trinidad AC, Strauss WL. The human choline acetyltransferase gene encodes two proteins. Journal of Neurochemistry. 1994;65:484–491. doi: 10.1046/j.1471-4159.1995.65020484.x. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cell and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. Journal of Histochemistry and Cytochemistry. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Katz B. The Release of Neural Transmitter Substances. Liverpool, UK: Liverpool University Press; 1969. [Google Scholar]
- Katz B, Miledi R. Transmitter leakage from motor nerve endings. Proceedings of the Royal Society of London. 1977;196:59–72. doi: 10.1098/rspb.1977.0029. B. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. Does the motor nerve impulse evoke non-quantal transmitter release. Proceedings of the Royal Society of London. 1981;212:131–137. doi: 10.1098/rspb.1981.0029. B. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y, Greuner R. Distribution and density of α-bungarotoxin binding sites on innervated and noninnervated Xenopus muscle cells in culture. Developmental Biology. 1982;91:78–85. doi: 10.1016/0012-1606(82)90010-0. [DOI] [PubMed] [Google Scholar]
- Laufer R, Changeux JP. Activity-dependent regulation of gene expression in muscle and neuronal cells. Molecular Neurobiology. 1989;3:1–53. doi: 10.1007/BF02935587. [DOI] [PubMed] [Google Scholar]
- Liou JC, Fu WM. Regulation of quantal secretion form developing motoneurons by postsynaptic activity-dependent release of NT-3. Journal of Neuroscience. 1997;17:2459–2468. doi: 10.1523/JNEUROSCI.17-07-02459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YJ, Poo MM. Heterosynaptic suppression of developing neuromuscular synapse in culture. Journal of Neuroscience. 1994;14:4684–4693. doi: 10.1523/JNEUROSCI.14-08-04684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall IG, Parsons SM. The vesicular acetylcholine transport system. Trends in Neurosciences. 1987;10:174–177. [Google Scholar]
- Mathers DA, Thesleff S. Studies on neurotrophic regulation of murine skeletal muscle. The Journal of Physiology. 1978;282:105–114. doi: 10.1113/jphysiol.1978.sp012451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R, Molenaar PC, Polak RL, Tas JWM, van der Laaken T. Neural and non-neural acetylcholine in the rat diaphragm. Proceedings of the Royal Society of London. 1982;214:153–168. doi: 10.1098/rspb.1982.0002. B. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis. 2. North Holland: Amsterdam; 1967. [Google Scholar]
- Rink TJ, Tsien RY, Pozzan T. Cytosolic pH and free Mg2+ in lymphocytes. Journal of Cell Biology. 1982;95:189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A, Boron F. Intracellular pH. Physiological Reviews. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Tabti N, Poo MM. Culturing spinal cord neurons and muscle cells from Xenopus embryos. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cambridge, MA, USA: MIT Press; 1991. pp. 137–154. [Google Scholar]
- Tucek S. The synthesis of acetylcholine in skeletal muscles of the rat. The Journal of Physiology. 1982;322:53–69. doi: 10.1113/jphysiol.1982.sp014022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin TB, Eiden LE, Bonner TI, Erickson JD. Molecular biology of the vesicular ACh transporter. Trends in Neurosciences. 1995;18:218–224. doi: 10.1016/0166-2236(95)93906-e. [DOI] [PubMed] [Google Scholar]
- Vyskocil F. Inhibition of non-quantal acetylcholine leakage by 2-(4-phenylpiperidino) cyclohexanol in the mouse diaphragm. Neuroscience Letters. 1985;59:277–280. doi: 10.1016/0304-3940(85)90144-2. [DOI] [PubMed] [Google Scholar]
- Vyskocil F, Zemkova H, Edwards C. Non-quantal acetylcholine release. In: Sellin LC, Libelius R, Thesleff S, editors. Neuromuscular Junction. Amsterdam: Elsevier; 1989. pp. 197–205. [Google Scholar]
- Wu ML, Tsai MC, Tseng YZ. DIDS-sensitive pHi-regulation in single rat cardiac myocytes in nominally HCO3−-free conditions. Circulation Research. 1994;75:123–132. doi: 10.1161/01.res.75.1.123. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]


