Abstract
In normal non-exercised skeletal muscles in mice, the activity of histidine decarboxylase (HDC), the enzyme which forms histamine, was very low.
HDC activity in the quadriceps femoris muscle was markedly elevated following contractions evoked by even a few minutes of direct electrical stimulation, peaking at 8-12 h following contraction lasting 10 min, and gradually decreasing during the 24 h following contraction. The elevation in HDC activity depended on the duration and strength of stimulation.
Direct electrical stimulation induced a quantitatively similar elevation of HDC activity in the muscles of mast-cell-deficient mice (W/Wv mice).
Prolonged walking at a speed of 6 m min−1 for up to 6 h with a 30 min rest period at 3 h also elevated muscle HDC activity, the magnitude of the elevation being related to the duration of the walking. Repeated exercise (training) for several days diminished the elevation of muscle HDC activity induced by walking. In contrast, starvation augmented the elevation of muscle HDC activity induced by walking.
Intraperitoneal injection of interleukin-1β (IL-1β) also elevated muscle HDC activity in a dose-dependent manner, as little as 1 μg kg−1 of IL-1 producing a significant elevation of muscle HDC activity.
IL-1β was immunohistochemically detected in normal non-exercised quadriceps femoris muscle. We could not detect a significant increase in IL-1β after exercise in the muscle or in serum: it may be below the level of detection.
On the basis of these results, together with those reported previously and the known actions of histamine, we propose that an elevation of HDC activity and generation of histamine occur in skeletal muscle following muscle contraction possibly as a result of induction by IL-1β and that the histamine may be involved in fatigue in skeletal muscle as part of a defence mechanism preventing damage to the muscle.
Unaccustomed hard and/or prolonged exercise may lead to pain in skeletal muscles, often several hours or even a few days after the period of exercise. However, it has not been established what factors are responsible for this phenomenon.
Inflammatory mediators, such as histamine, serotonin, bradykinin and prostaglandins, are known to be pain-producing substances (Garrison & Rall, 1990). Of these, histamine, in addition to its role in the production of pain, is a potent vasodilator of precapillary arterioles and a potent stimulator of capillary permeability (Garrison & Rall, 1990). It is known that histamine may be pooled in large quantities in mast cells in the tissues. Moreover, in man, eccentric exercise, which has been shown to damage the fine structure of skeletal muscles (Friden, Seger, Sjostrom & Ekblom, 1983; O'Reilly, Warhol, Fielding, Frontera, Meredith & Evans, 1987), has also been reported to cause an increase in histamine in blood, the blood being taken immediately before the termination of 18-24 min exercise at maximal load on a bicycle ergometer) (Dunér & Pernow, 1958). Further, Morganroth, Young & Sparks (1977) showed that histamine may be released during powerful electrical stimulation of skeletal muscle in the dog (square-wave pulses, 85 mA, 0.2 ms duration, for 20 min). On the basis of such findings it is a reasonable hypothesis that histamine is released from mast cells during exercise that causes muscle damage and that it is an important mediator in inducing pain and/or vasodilatation in the muscle (Morganroth, Young & Sparks, 1977).
However, it must be emphasized that the results described above were obtained over a short period during, or immediately after, fairly brief exercise or electrical stimulation. Thus, whilst histamine release can be implicated in the production of pain during a bout of exercise, there is as yet no evidence of histamine release from mast cells at the time of onset of the muscle pain that occurs hours or days after eccentric exercise.
Histidine decarboxylase (HDC), the enzyme that forms histamine, is an adaptive enzyme which is induced in response to appropriate stimuli and it has been suggested that histamine, newly formed by the induced HDC, plays a role in the regulation of the microcirculation (Schayer, 1974, 1978) and in anabolic processes during rapid cell growth (Kahlson & Rosengren, 1968). Graham, Kahlson & Rosengren (1964) have already shown that running on a treadmill for 3 h enhances HDC activity in the skeletal muscles of mice. But the cytokines interleukin-1 (IL-1) and tumour necrosis factor (TNF) can also induce HDC in various tissues in mice (Endo, Suzuki & Kumagai, 1986; Endo, 1989; Endo, Kikuchi, Takeda, Nitta, Rikiishi & Kumagai, 1992c; Endo, Nakamura, Nitta & Kumagai, 1995). Moreover, IL-1 has been detected around capillaries in human skeletal muscles immediately after, and for up to 5 days after eccentric exercise (Cannon, Fielding, Fiatarone, Orencole, Dinarello & Evans, 1989; Fielding, Manfredi, Ding, Fiatarone, Evans & Cannon, 1993).
Thus a reasonable working hypothesis is that, in addition to the release of histamine from mast cells that occurs during muscle contraction, there is also an induction of HDC activity and consequent release of histamine in the hours after muscle contraction, possibly caused by the action of IL-1. Given that histamine not only causes vasodilatation and increased permeability of capillary vessels, but also stimulates sensory nerve endings to produce pain and gastric acid secretion, it may be that the whole process of histamine release should be seen as a defensive response (Endo et al. 1995). The pain occurring in muscle during or immediately after exercise would help to protect the muscle from exhaustion and/or damage, while pain occurring in muscle hours after a bout of damaging exercise may deter the individual from engaging in further potentially damaging exercise. In the present study, as a step in the testing of our hypothesis, we have examined whether HDC activity is induced in skeletal muscle and other tissues of the mouse in the hours after direct electrical stimulation of the muscle under anaesthesia, and after prolonged walking. We have also compared the effects of such muscle contraction on HDC activity with the effects of injection of IL-1 in unanaesthetized animals. In addition, we also examined the effects on HDC activity of training (repeated exercise), which may make muscles better able to resist fatigue, and of starvation, which may accelerate muscular exhaustion. Because histamine is generally considered to be released from mast cells, we also examined whether HDC activity can be induced in the same way in the muscles of mice that are mast cell deficient.
METHODS
Animals
BALB/c mice were bred at the animal facility of our university and used as a normal strain in the present study. WBB6F1(B-W/+× C57BL/6-Wv/+)-W/Wv mice (mast-cell-deficient mice) were obtained from the Shizuoka Agricultural Association for Laboratory Animals (Shizuoka, Japan); such mice were used only for electrical stimulation experiments. The mice (male) were 6 weeks old (23-26 g body weight): there were approximately six mice in each of the experimental groups described below. All experiments complied with Guidelines for Care and Use of Laboratory Animals issued by Tohoku University.
Materials
Recombinant human IL-1α was provided by Dainippon Pharmaceutical Co. (Osaka, Japan). Recombinant human IL-1β was from Ohtsuka Pharmaceutical Co. (Tokushima, Japan). Rabbit anti-mouse IL-1α, anti-mouse IL-1β and anti-mouse TNFα antisera were prepared as described by Fujioka et al. (1995).
Electrical stimulation of skeletal muscle
Some experiments were performed on normal or mast-cell-deficient mice anaesthetized by an intraperitoneal injection of sodium pentobarbitone (50 mg kg−1) without supplement. In these experiments, bipolar needle electrodes made from stainless-steel wire (tip diameter 0.08 mm, tip exposure 1.0-1.5 mm, interpolar distance 5-6 mm) were inserted into the right quadriceps femoris muscle as follows. First, the electrode was inserted into a hypodermic needle and the tip of the electrode was bent like a hook; then, the needle was inserted into the muscle without an incision being made in the skin. After insertion, the needle was pulled out, leaving the electrode embedded in the muscle. The muscle was stimulated with square-wave, constant-current pulses (2 mA or less, pulse duration 1 ms, 50 Hz) for a fixed period in each experiment (see Results). At various times after the start of the period of electrical stimulation (0.4-50 min; see Results) the mice were decapitated and the quadriceps femoris of both right (stimulated) and left (non-stimulated) legs were rapidly removed and stored at -80°C until they were assayed for HDC activity. As a sham experiment, electrodes were inserted into the same muscle in other mice and left in place for the time indicated (0.4-50 min; see Results) without electrical stimulation.
Physical exercise
Forced walking was imposed on other mice at room temperature (22-25°C) in a cylindrical cage (37 cm diameter, 37 cm width), the floor of which was made of stainless-steel rods (2 mm diameter, 1 cm apart). The cage was turned around a horizontal axis at 5.2 r.p.m. by an electric motor, giving a walking speed of 6 m min−1. We were anxious that the mice should not suffer exhaustion or undue distress as a result of the walking, although it would have frustrated the aim of the experiment if their muscles had not experienced some degree of fatigue. For this reason, when walking was to be prolonged for more than 3 h, the mice were given a 30 min period of rest. For this purpose, after 3 h walking, the mice were taken back to their home cage, where they had free access to water and food. In fact, after 3 h walking, the mice began eating and drinking within 5 min and they had resumed their normal activity level by the end of the rest time. They were then returned to the walking cage, where they resumed walking as before. Although they lost about 7 % of their initial body weight during 6 h of such walking (the maximum time in the present experiments), they resumed normal activity in the home cage within 1 h of the end of 6 h walking. Throughout the period of walking, each mouse was closely observed for signs of distress (inability to maintain walking and their normal standing posture). However, there were no such signs in any of the mice. In our experience, such signs are seen only in aged mice with a heavy body weight. For the assay of HDC activity, mice were decapitated immediately after walking for the indicated period (0-6 h; see Results), or up to 5 h later, and the quadriceps femoris and other tissues, namely liver, spleen, lung and bone (tibia and femur), were removed and stored at -80°C until assayed.
Physical exercise after training
Forced walking for 3 h was imposed on some mice on each of 5 successive days; 3 days later forced walking for 6 h was imposed on some of the ‘trained’ mice while others were allowed to rest for 6 h. The forced walking was carried out under the conditions described above. The mice were decapitated immediately after the final period of walking (or the equivalent rest period) and the quadriceps femoris was removed and stored as described above.
Physical exercise after starvation
On the evening (about 17.00 h) before the day on which they were to perform forced walking, in order to ensure complete fasting some mice were moved to a cage (5-6 mice per cage) with a false floor made of wire netting (7 mm mesh) some 2.5 cm above the real floor of the cage. This was done because the mice, when starved, would otherwise eat the bedding (wood shavings) and even their droppings. In some of these mice, forced walking for 6 h was started at about 10.00 h under the conditions described above; the remaining animals served as controls.
Effects of IL-1
In other experiments, human IL-1 was dissolved in sterile saline (0.01-1 μg ml−1) and injected into conscious mice intraperitoneally in a volume of 0.1 ml per 10 g body weight. After the indicated period (0-15 h; see Results), mice were decapitated and tissues (quadriceps femoris muscle, liver, spleen and bone) were removed for the assay of HDC activity.
Assay for HDC activity
HDC activity was assayed using our previously described method (Endo, 1983) with a slight modification (Endo, Kikuchi & Nakamura, 1992a). Briefly, mice were decapitated at 0-24 h after application of one of the various forms of stimulation used (see Results), and tissues were rapidly removed and stored in a jar with dry ice. Each tissue sample (less than 250 mg) was put into a cooled Teflon tube with phosphorylated cellulose (50 mg) and 2.5 ml of ice-cold 0.02 M phosphate buffer (pH 6.2) containing pyridoxal 5′-phosphate (20 μM) and dithiothreitol (200 μM), and homogenized using an Ultra Turrax homogenizer (Janke & Kunkel Co., Germany). The supernatant obtained after centrifugation of the homogenate (10 000 g for 15 min at 4°C) was used as the enzyme solution. The histamine in the tissues was bound to the phosphorylated cellulose and was removed almost completely from the enzyme solution by the centrifugation. The reaction mixture (1 ml) containing the enzyme solution was incubated at 37°C for 14 h with histidine (initial concentration 1 mM). After the enzyme reaction had been terminated by adding 2 ml of 0.5 M HClO4, the histamine formed during the incubation was separated by chromatography on a small phosphorylated cellulose column and quantified fluorometrically as previously described (Endo, 1983). HDC activity was expressed as nanomoles of histamine formed during a 1 h period of incubation by the enzyme contained in 1 g (wet weight) of each tissue (nmol h−1 g−1). Enzyme activity is usually expressed as a standard unit (U) equivalent to micromoles per minute, but the HDC activity induced in tissues is markedly lower than that of many other enzymes. HDC activity in the bone marrow was expressed as the activity in 1 g (wet weight) of tibia plus femur, because these tissues were subjected to the assay without separating the bone marrow (Endo, Kikuchi, Nakamura & Shinoda, 1992b).
Determination of histamine
In the experiments involving electrical stimulation or prolonged walking (see Results), the histamine in the blood, quadriceps femoris muscle and other tissues (spleen, lung and ear) was determined as follows. Mice were decapitated and six to eight drops of blood were collected into a pre-weighed test-tube containing 3 ml of 0.4 M HClO4, and then tissues were removed and stored at -80°C until assayed. Histamine extracted with 0.4 M HClO4 was separated by chromatography and determined fluorometrically as previously described (Endo, 1983).
Immunohistochemical staining of cytokines
Quadriceps femoris muscles were removed from some non-exercised or exercised mice immediately after decapitation. The exercised mice were decapitated at the end of a 6 h period of forced walking (which was performed under the conditions described above). The muscles were fixed with 4 % paraformaldehyde in phosphate-buffered saline (PBS) and samples taken subsequently were frozen in a mixture of dry ice and acetone. Sections were cut at a thickness of 10 μm on a cryostat and immunohistochemically stained with antisera against IL-1α, IL-1β and TNFα as follows. Sections were incubated with normal goat serum at 37°C for 30 min, rinsed with PBS, incubated with each antiserum at 37°C for 120 min, and then incubated with fluorescein isothiocyanate (FITC)-goat anti-rabbit IgG (diluted to 1/500 with PBS) at 37°C for 60 min. After rinsing with PBS, sections were mounted in PermaFluor Aqueous Mounting Medium (Immunon) and observed under a fluorescence microscope (Zeiss Axioskop).
Data analysis
Experimental values are given as means ± standard deviation. The statistical significance of the difference between two means was evaluated using Student's unpaired t test. For analysing multiple mean values, Dunnet's multiple comparison test was used after ANOVA. In these tests, P values less than 0.05 were considered to be significant. Statistical analyses were carried out using a Macintosh computer with InStat software.
RESULTS
Elevation of muscle HDC activity following electrical stimulation
HDC activity in the quadriceps femoris of control mice was very low. Electrode insertion without electrical stimulation (sham experiment) elevated HDC activity slightly, but significantly (Fig. 1). Electrical stimulation of the quadriceps femoris at 2 mA for 10 min markedly elevated HDC activity in the muscle: it began to rise somewhere between 2 and 5 h after the stimulation, peaked at 8-12 h and then declined. There was no significant increase in HDC activity in the non-stimulated quadriceps femoris of the contralateral leg.
Figure 1. Time course of the increase in HDC activity in quadriceps femoris after electrical stimulation.
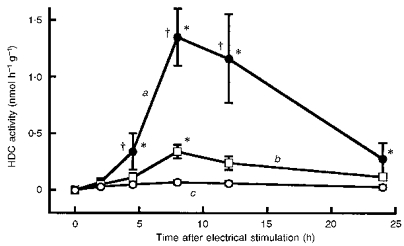
Ordinate indicates HDC activity measured in muscle at various times after the end of the stimulation or sham period (as indicated on the abscissa): a, muscles that were electrically stimulated for 10 min; b, muscles in which the electrode was in place for 10 min; c, muscles that were contralateral to the stimulated muscles. Each value represents the mean ±s.d. from 6 mice. *P < 0.01vs. time 0; †P < 0.01vs.b at the corresponding time.
Prolonging the period of electrical stimulation (at 2 mA) progressively increased the magnitude of the elevation in HDC activity seen 7 h after the start of the period of stimulation (Fig. 2A). The HDC activity induced by electrode insertion alone (sham experiment) was independent of the time for which the electrode was in place (Fig. 2A). The elevation of HDC activity seen at 7 h after the start of a 10 min period of electrical stimulation of the muscle proved to be current dependent (Fig. 2B).
Figure 2. Influence of the duration of electrical stimulation and magnitude of electric current on muscle HDC activity.
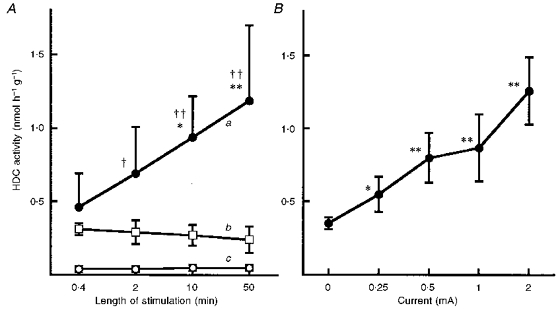
A, ordinate shows HDC activity in quadriceps femoris muscles removed 7 h after the start of periods of electrical stimulation (each at 2 mA, but of various durations, see abscissa): a, stimulated muscle; b, electrode insertion alone; c, non-stimulated muscle from contralateral leg. Each value represents the mean ±s.d. from 7 mice. *P < 0.05 and **P < 0.01vs. the value for 0.4 min; †P < 0.05 and ††P < 0.01vs. corresponding values in b. B, ordinate shows HDC activity in quadriceps femoris muscles removed 7 h after the start of 10 min periods of electrical stimulation at various current intensities. Each value represents the mean ± s. d. from 9 mice. *P < 0.05 and **P < 0.01vs. the value at 0 mA.
Induction by electrical stimulation of HDC activity in skeletal muscle in mast-cell-deficient W/Wv mice
The amount of histamine in the tissues, including the quadriceps femoris, of W/Wv mice was markedly lower than in the control BALB/c mice (Table 1). However, electrical stimulation elevated HDC activity in the quadriceps femoris of W/Wv mice, the magnitude of the increase seen at 7 h after a 10 min period of electrical stimulation being similar to that seen in BALB/c mice (Fig. 3). Neither an increase nor a decrease in histamine was detected in the blood or muscles of either W/Wv or BALB/c mice 1 or 6 h after 10 min electrical stimulation (data not shown).
Table 1.
Histamine content of skeletal muscle and other tissues in BALB/c mice and mast-cell-deficient W/Wv mice at rest
| Histamine (nmol (g wet tissue)−1) | ||||
|---|---|---|---|---|
| Muscle * | Spleen | Lung | Ear | |
| BALB/c | 10.5 ± 1.9 | 18.3 ± 4.3 | 5.0 ± 0.5 | 354 ± 24 |
| W/Wv | 0.11 ± 0.04 | 1.0 ± 0.1 | 0.5 ± 0.1 | 1.1 ± 0.3 |
* Quadriceps femoris. Values represent the means ±s.d. of results from 4 mice.
Figure 3. HDC activity in the electrically stimulated muscles of BALB/c mice and mast-cell-deficient W/Wv mice.
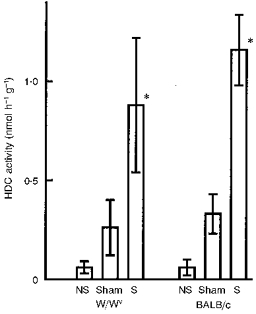
HDC activity is shown for the quadriceps femoris of both the non-stimulated leg (NS) and the stimulated, contralateral leg (S; removed 7 h after electrical stimulation at 2 mA for 10 min) and for muscles removed 7 h after electrode insertion alone for 10 min (sham experiment). Each value represents the mean ±s.d. from 6 mice. *P < 0.01vs. the corresponding sham experiment.
Induction of HDC activity by prolonged walking
HDC activity in the quadriceps femoris of BALB/c mice was also elevated by prolonged walking (Fig. 4), although the effect was smaller than that induced by electrical stimulation (Figs 1-3). The elevated HDC activity in the muscle gradually declined after the walking had ended. In these experiments, neither an increase nor a decrease in histamine was detected in blood or skeletal muscle at the end of 6 h walking (data not shown).
Figure 4. Induction of HDC activity in the quadriceps femoris by prolonged walking.
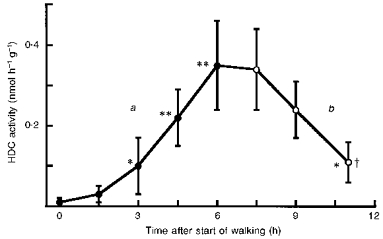
Ordinate shows HDC activity in the quadriceps femoris of BALB/c mice: a, immediately after a period of walking (see Methods) of the indicated duration (•); b, at the indicated times after the start of a 6 h period of walking (○). ‘Time after start of walking’ excludes the 30 min rest time allowed when walking was prolonged for more than 3 h (see Methods). Each value represents the mean ±s.d. from 6 mice. *P < 0.05 and **P < 0.01vs. time 0; †P < 0.01vs. HDC activity at 6 h.
Effects of training and starvation on the elevation of HDC activity induced by physical exercise
Next, we examined the effect of training (see Methods) on the elevation of muscle HDC activity induced by prolonged walking. The elevation of HDC activity in the ‘trained mice’ was significantly lower than that seen in untrained mice (Fig. 5A). By contrast, in mice starved overnight, prolonged walking induced a higher level of HDC activity in the quadriceps femoris muscle than that induced in control non-starved mice (Fig. 5B). Starvation itself slightly, but significantly, elevated HDC activity in the muscle.
Figure 5. Effects of training and starvation on the elevation of muscular HDC activity induced by prolonged walking.
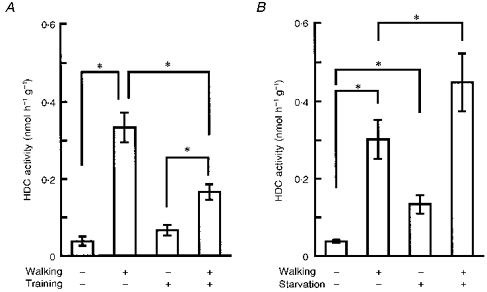
A, as described in Methods, the three groups of control mice underwent (i) neither training nor walking, (ii) a 6 h period of walking but no prior training, or (iii) training but not the 6 h period of walking. The fourth group underwent both training and 6 h walking. The values shown for HDC activity were obtained from quadriceps femoris muscles removed immediately after the final period of walking. Each value represents the mean ±s.d. from 5 mice. *P < 0.01. B, as described in Methods, the 3 groups of control mice underwent (i) neither starvation nor walking, (ii) a 6 h period of walking but no prior starvation, or (iii) starvation but not the 6 h period of walking. The fourth group underwent both starvation and 6 h walking. The values shown for HDC activity were obtained from quadriceps femoris muscles removed immediately after the period of walking (or starvation if they were not to perform walking). Each value represents the mean ±s.d. from 5 mice. *P < 0.01.
Induction of HDC activity by IL-1
Intraperitoneal injection of IL-1β increased HDC activity in the quadriceps femoris (Fig. 6). After injection of IL-1β at 1 μg kg−1, HDC activity began to rise at 2.5 h, peaked at 5 h and had disappeared 15 h after the injection (Fig. 6A). The time to onset of this increase was shorter than that seen in response to walking (Fig. 4). As little as 1 μg kg−1 (25 ng per mouse) of IL-1β significantly elevated HDC activity in the muscle at 5 h after its injection (Fig. 6B). The effects of IL-1α on HDC activity in the quadriceps femoris muscle were essentially the same as those of IL-1β in terms of dose dependency and time course (data not shown).
Figure 6. Time course and dose dependence of increase in HDC activity evoked by IL-1 in skeletal muscle and liver.
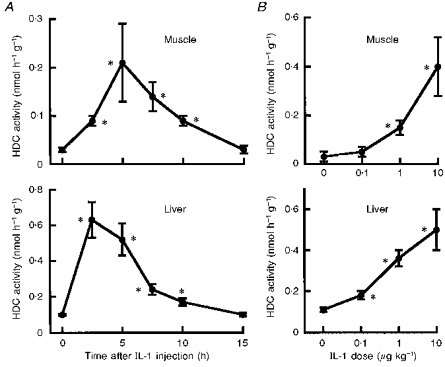
A, IL-1β (1 μg kg−1) was injected intraperitoneally into BALB/c mice. Ordinate shows HDC activity in quadriceps femoris muscle and liver removed after the indicated time intervals. Each value represents the mean ±s.d. from 6 mice. *P < 0.01vs. time 0. B, various doses of IL-1β were injected intraperitoneally into BALB/c mice. Ordinate shows HDC activity in quadriceps femoris muscle and liver removed 5 h later. Each value represents the mean ±s.d. from 6 mice. *P < 0.01vs. dose 0.
Comparison between ability of electrical stimulation, prolonged walking and injection of IL-1β to induce HDC activity
Prolonged walking (6 h) and intraperitoneal injection of IL-1β (1 μg kg−1) elevated HDC activity in all tissues tested (Fig. 7). In the muscle, although walking for 6 h produced a rather higher level of HDC activity than that seen 5 h after injection of 1 μg kg−1 of IL-1β, the levels of HDC activity induced in other tissues by walking were markedly lower than those induced by the injection of IL-1β. Unlike walking and injection of IL-1β, electrical stimulation did not elevate HDC activity in tissues other than the stimulated muscle. However, the HDC activity induced in muscle 7 h after electrical stimulation was the highest of those induced in this tissue by the various stimuli.
Figure 7. HDC activity induced in muscle and in other tissues by electrical stimulation, prolonged walking and administration of IL-1.
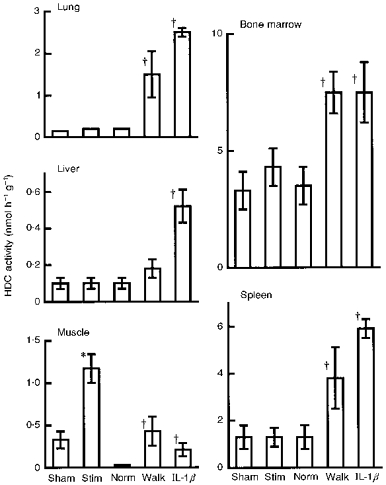
HDC values shown were obtained from quadriceps femoris and various other tissues removed either 7 h after electrical stimulation at 2 mA for 10 min (Stim), immediately after forced walking for 6 h (Walk; see Methods), or 5 h after intraperitoneal injection of IL-1β (1 μg kg−1). Each value represents the mean ±s.d. from 6 mice. *P < 0.01vs. the sham experiment; †P < 0.01vs. normal (Norm; non-exercised) mice.
Detection of IL-1β in the muscle
IL-1β immunostaining was positive in non-exercised normal quadriceps femoris muscles (Fig. 8). In cross-sections of muscle fibres (Fig. 8A), positive reactions for IL-1β formed a dotted pattern in the cytoplasm of smaller-sized fibres. Stronger positive staining was also seen under the sarcolemma of some larger fibres. In longitudinal sections (Fig. 8B), positive staining could be seen along muscle fibres and in cross-sections of fine blood vessels. However, we could not detect qualitatively a significant increase in the intensity of this reaction in the muscle after a 6 h period of walking. Control specimens (non-exercised quadriceps femoris muscles treated with non-immunized serum from the rabbit) were negative for this fluorescence reaction. No positive immunoreactions were detected for IL-1α or TNFα in exercised or non-exercised muscle.
Figure 8. Immunohistochemical detection of IL-1 in resting muscle.
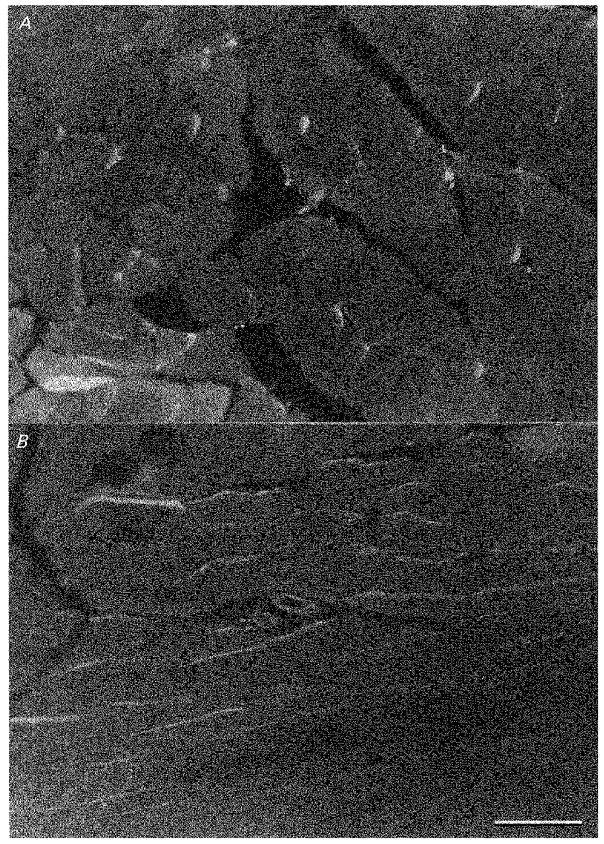
A, cross-section of muscle fibres. Positive reactions for IL-1β immunostaining formed a dotted pattern in small-sized fibres and were seen under the sarcolemma of some larger fibres. × 220. B, longitudinal section of muscle fibres. Positive reactions for IL-1β immunostaining can be seen along muscle fibres and in cross-sections of fine blood vessels. × 220. Bar: 100 μm (this scale is the same as in A).
DISCUSSION
In the present study, we found the following. (i) Although HDC activity is very low in normal non-exercised skeletal muscles in mice, HDC activity in the quadriceps femoris muscle was markedly elevated by just a few minutes of contraction evoked by direct electrical stimulation. (ii) Electrical stimulation induced a similar elevation of muscle HDC activity in mast-cell-deficient mice. (iii) Prolonged walking elevated HDC activity in muscle and in other tissues. (iv) The elevation of muscle HDC activity induced by prolonged walking was diminished by prior training (repeated exercise). (v) In contrast, prior starvation augmented the elevation of muscle HDC activity induced by prolonged walking. (vi) Intraperitoneal injection of IL-1β also elevated HDC activity in the muscle and in other tissues. (vii) IL-1β or substances related to IL-1β, but not IL-1α or TNFα, were detected in normal, non-exercised muscle. However, (viii) there was no detectable increase in IL-1β or histamine in the muscle or blood after 6 h of exercise under the conditions of the present study and (ix) there was also no detectable increase in histamine in muscle or blood 1 or 6 h after a 10 min period of contraction evoked by electrical stimulation of the muscle.
The present study showed that HDC activity in the skeletal muscle of mice began to rise at about 3-5 h after either electrical stimulation or the start of walking. Unlike the release of histamine that occurs during a 20 min or so period of muscular work or electrical stimulation (Dunér & Pernow, 1958; Morganroth et al. 1977; see Introduction), the enhancement of HDC activity seen here was later in onset and longer lasting, continuing for several hours after the end of electrical stimulation or muscular work. The magnitude of the elevation depended on the strength and duration of the electrical stimulation and on the duration of the walking. To our knowledge, this is the first demonstration of a quantitative correlation between muscular work and the induction of HDC activity, a mechanism known to produce a substance (histamine) that is capable of both inducing pain and producing vasodilatation. Interestingly, the elevation of HDC activity induced by prolonged walking was diminished in ‘trained mice’. In contrast, starvation augmented the HDC elevation induced by prolonged walking. These results suggest that the induction of HDC activity in skeletal muscle can be modulated by factors that also influence the degree of fatigue.
Histamine is known to be pooled in large quantities in mast cells, which are distributed widely in the tissues. However, W/Wv mice are deficient in mast cells (Kitamura, Go & Hatanaka, 1978; Suda, Suda, Spicer & Ogawa, 1985). The amount of histamine in their tissues, including skeletal muscle, was markedly lower than that found in BALB/c mice (Endo & Nakamura, 1992; Table 1 in this study), indicating that the skeletal muscle of W/Wv mice also lacks mast cells. Thus, our finding that electrical stimulation elevated HDC activity in the skeletal muscle of W/Wv mice to an extent similar to that seen in the control BALB/c mice suggests that HDC was induced in cells other than mast cells.
IL-1 and TNF are known to be produced in a variety of cells, including macrophages and vascular endothelial cells (Oppenheim, Elizabeth, Matsushima & Durum, 1986; Dinarello, 1991). We previously reported that IL-1 can induce HDC activity in the liver, lung, spleen and bone marrow in mice (Endo et al. 1986, 1992c; Endo, 1989): the elevation of HDC activity results from an enhanced formation of HDC enzyme following the induced formation of new HDC mRNA (Kikuchi, Watanabe & Endo, 1997). The induction of HDC activity by IL-1 or inflammatory stimuli occurs to a great extent in tissues that are rich in capillaries (i.e. rich in vascular endothelial cells), such as the liver and lungs. Indeed, on the basis of this and other evidence, we have proposed that, in such tissues, endothelial cells are the major site at which HDC is induced (Endo et al. 1995). Since the present study shows that both the time course and the dose dependence of the elevation of HDC activity induced in skeletal muscle by IL-1 were similar to those seen in the liver (Fig. 6), this is coincident with the idea that the cells in which HDC is induced in skeletal muscle are endothelial cells.
Induction of HDC activity is known to lead to histamine production (Reilly & Schayer, 1968; Endo, 1982; Endo et al. 1986), and the magnitude of the HDC activity determines the rate of histamine formation (Reilly & Schayer, 1968; Schayer, 1974). However, because the newly formed histamine is freely diffusible from the site of its formation (Kahlson & Rosengren, 1968; Schayer, 1974; Beaven, 1978), it disappears rapidly without being accumulated there (Endo, 1979, 1982; Endo et al. 1986). This may be why we could not detect an elevation in newly formed histamine in blood or muscle even after electrical stimulation in either BALB/c or mast-cell-deficient mice. However, the more important explanation for our failure to detect histamine may be that the HDC activity induced in the muscle was comparatively low (less than 1.5 nmol h−1 g−1). Indeed, our previous data suggest that an elevation of more than 3-5 nmol h−1 g−1 of HDC activity may be needed to produce a fluorometrically detectable amount of newly formed histamine (Endo et al. 1986). This being so, a more powerful stimulus than that provided by prolonged walking might be needed to produce a detectable increase in histamine in the blood or muscle.
In our study, intraperitoneal injection of as little as 1 μg kg−1 of IL-1β or prolonged walking were each capable of elevating HDC activity in the various tissues tested (Fig. 7). On the other hand, electrical stimulation induced HDC activity only in the stimulated muscle and the level so induced was the highest of those induced by the stimuli tested. If IL-1 acts as the natural stimulus for induction of HDC activity following muscle contraction, these results may be explained in the following way. The mouse uses many skeletal muscles for walking and it may be that the production or release of IL-1 from those muscles results in an increase in IL-1 in the blood and a subsequent elevation of HDC activity in various tissues (even though we have not detected this IL-1 at present, see below). On the other hand, direct electrical stimulation of the skeletal muscle may lead to the production or release of IL-1 that gives a high local concentration in the stimulated muscle itself, but this does not increase IL-1 in the blood to a level sufficient to induce HDC activity in other tissues. This must, of course, remain a tentative explanation until we have actual evidence of an exercise-induced increase in IL-1 in blood.
The immunohistochemistry of the present study suggests that in skeletal muscle, IL-1β (or related substances) is largely present in the muscle fibres themselves. In fact, the positive reaction for IL-1β occurred as a dotted pattern in small-sized fibres (Fig. 8A), this pattern being very similar to the distribution of mitochondria (Y. Endo, H. Kuroda, M. Shibazaki, A. Suzuki, T. Yamaguchi & M. Watanabe, unpublished data). However, as Fig. 8B shows, positive reactions for IL-1β were also seen in cross-sections of fine blood vessels: as yet we have no data on the distribution of IL-1β within the blood vessel and so we do not know whether it is present in the endothelium or other cells of the vessel wall.
In a previous study in which immunohistochemistry was used, Cannon et al. (1989) detected a light staining for IL-1β before exercise and an increase in its intensity immediately after, and up to 5 days after, eccentric exercise around capillaries in human skeletal muscles. However, they could not detect IL-1α. They also reported that eccentric exercise resulted in a slight increase (to 130 % of the basal value) in the activity of IL-1 in extracts from muscle tissues as determined by bioassay (see below). In a later study on the basis of the results obtained by a quantitative image-analysis technique for assessing immunohistochemical staining, the same group (Fielding et al. 1993) discussed the possibility that eccentric exercise induces not only the accumulation in the muscle of IL-1β that has been preformed in other tissues, but also the production by the muscle itself of IL-1β to result in a slight, but detectable increase in the level of IL-1β in muscle, to about 2 times its normal level at most. In a preliminary study, in which we used an enzyme-linked immunosorbent assay (ELISA), we could not detect an increase in IL-1β after prolonged walking in extracts from the quadriceps femoris muscle or in the serum (data not shown). Cannon, Evans, Hughes, Meredith & Dinarello (1986), using a bioassay method, reported that exercise in man increases IL-1 activity in the blood, too. On the other hand, other groups, using ELISA, recently reported that eccentric exercise enhanced human plasma IL-6, but did not change IL-1α, IL-1β or TNFα (Ullum, Haahr, Diamant, Palmo, Halkjaer-Kristensen & Pedersen, 1994; Bruunsgaard, Galbo, Halkjaer-Kristensen, Johansen, Maclean & Pedersen, 1997). In view of this differential effect, it may be that the older, bioassay technique, being non-specific, actually detected IL-6 or others as well as IL-1. We have already shown that human recombinant IL-6 at a dose of 20 μg kg−1 does not induce HDC activity in a variety of mouse tissues (Endo et al. 1992c). The fact that an increase in IL-1β has been detected in muscle tissues following exercise by immunostaining methods (see above), even though an increase in the serum was not detectable by current ELISA methods, may suggest that even if the serum level of IL-1β actually is increased by exercise (by IL-1β originating from working skeletal muscle), the level is still very low and below the level of detection with current methods. Possibly, the intensity or type of exercise may also be a factor.
Unfortunately, we do not have the ability to measure fatigue, pain or actual blood flow in the muscles of mice in their natural state. Moreover, as is discussed above, we do not yet have evidence of an exercise-induced increase in IL-1 or histamine in working muscle or blood at the appropriate time. Nevertheless, we believe the present results are consistent with our working hypothesis and allow us to elaborate it. Thus, we have shown that after muscle contraction evoked by electrical stimuation or during prolonged walking, there is a late-onset induction of HDC activity beginning at about 3-5 h, the magnitude of this increase directly related to the strength and duration of contraction or period of walking. Since the increase in HDC activity was inhibited by training and accentuated by starvation which, respectively, reduce and enhance the fatigue of musular exercise, the present results are consistent with the idea that induction of HDC activity is determined by some factor(s) that is (are) related to fatigue. Given that the time course, tissue distribution and magnitude of the HDC activity that was induced by exercise were similar to that induced by i.p. injections of IL-1β, this strengthens the proposal that IL-1β is a factor, possibly the major factor, that induces HDC activity in exercise. On the basis of the available evidence, it seems likely that IL-1β is released as part of the inflammatory process in hard or prolonged exercise and that it stimulates HDC activity and histamine release by the vascular endothelium of the muscle itself. The newly formed histamine, by enhancing capillary permeability and causing vasodilatation, may contribute to supplying O2 and nutrients and to removing CO2 and other waste products, and may help the recovery from fatigue, but may also stimulate sensory nerve endings, producing pain and so deterring the individual from engaging in further exercise.
Acknowledgments
We are grateful to Akiko Tamura, Chiyoko Tanihira and Masayuki Miyazawa of Tohoku College of Pharmacy, Yukiko Yamaguchi, Yoshiko Yamato, Hiromi Funayama, Masayuki Itoh and Nozomi Yamaguchi of the School of Dentistry, Tohoku University, and Masahiko Shibazaki of the Faculty of Agriculture, Tohoku University, for help with the experiments. This work was supported by a grant-in-aid for Scientific Research from the Ministry of Education of Japan (No. 06454530) and a grant from Taisho Pharmaceutical Co. (Ohmiya, Japan).
References
- Beaven MA. Monographs in Allergy. Vol. 13. Basel: S. Karger; 1978. Histamine: its role in physiological and pathological processes. [PubMed] [Google Scholar]
- Bruunsgaard H, Galbo H, Halkjaer-Kristensen J, Johansen TL, Maclean DA, Pedersen BK. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. The Journal of Physiology. 1997;499:833–841. doi: 10.1113/jphysiol.1997.sp021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JG, Evans WJ, Hughes VA, Meredith CN, Dinarello CA. Physiological mechanisms contributing to increased interleukin-1 secretion. Journal of Applied Physiology. 1986;61:1869–1874. doi: 10.1152/jappl.1986.61.5.1869. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Fielding RA, Fiatarone MA, Orencole SF, Dinarello CA, Evans WJ. Increased interleukin 1β in human skeletal muscle after exercise. American Journal of Physiology. 1989;257:R451–455. doi: 10.1152/ajpregu.1989.257.2.R451. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- Dunér H, Pernow B. Histamine and leukocytes in blood during muscular work. Scandinavian Journal of Clinical and Laboratory Investigation. 1958;10:391–396. doi: 10.3109/00365515809051243. [DOI] [PubMed] [Google Scholar]
- Endo Y. Elevation of histamine in rat and mouse tissues by the deacetylation of administered N-acetylhistamine. European Journal of Pharmacology. 1979;60:299–305. doi: 10.1016/0014-2999(79)90233-4. 10.1016/0014-2999(79)90233-4. [DOI] [PubMed] [Google Scholar]
- Endo Y. Simultaneous induction of histidine and ornithine decarboxylases and changes in their product amines following the injection of Escherichia coli lipopolysaccharide into mice. Biochemical Pharmacology. 1982;31:1643–1647. doi: 10.1016/0006-2952(82)90394-x. 10.1016/0006-2952(82)90394-X. [DOI] [PubMed] [Google Scholar]
- Endo Y. A simple method for the determination of polyamines and histamine and its application to the assay of ornithine decarboxylase and histidine decarboxylase activities. Methods in Enzymology. 1983;94:42–47. doi: 10.1016/s0076-6879(83)94008-9. [DOI] [PubMed] [Google Scholar]
- Endo Y. Induction of histidine and ornithine decarboxylase activities in mouse tissues by recombinant interleukin-1 and tumor necrosis factor. Biochemical Pharmacology. 1989;38:1287–1292. doi: 10.1016/0006-2952(89)90335-3. 10.1016/0006-2952(89)90335-3. [DOI] [PubMed] [Google Scholar]
- Endo Y, Kikuchi T, Nakamura M. Ornithine and histidine decarboxylase activities in mice sensitized to endotoxin, interleukin-l or tumor necrosis factor by D-galactosamine. British Journal of Pharmacology. 1992a;107:888–894. doi: 10.1111/j.1476-5381.1992.tb14542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Kikuchi T, Nakamura M, Shinoda H. Determination of histamine and polyamines in calcified tissues of mice: contribution of mast cells and histidine decarboxylase to the amount of histamine in the bone. Calcified Tissue International. 1992b;51:67–71. doi: 10.1007/BF00296220. [DOI] [PubMed] [Google Scholar]
- Endo Y, Kikuchi T, Takeda Y, Nitta Y, Rikiishi H, Kumagai K. GM-CSF and G-CSF stimulate the synthesis of histamine and putrescine in the hematopoietic organs in vivo. Immunology Letters. 1992c;33:9–14. doi: 10.1016/0165-2478(92)90086-4. 10.1016/0165-2478(92)90086-4. [DOI] [PubMed] [Google Scholar]
- Endo Y, Nakamura M. The effect of lipopolysaccharide, interleukin-1 and tumor necrosis factor on the hepatic accumulation of 5-hydroxytryptamine and platelets in the mouse. British Journal of Pharmacology. 1992;105:613–619. doi: 10.1111/j.1476-5381.1992.tb09028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Nakamura M, Nitta Y, Kumagai K. Effects of macrophage depletion on the induction of histidine decarboxylase by lipopolysaccharide, interleukin-1 and tumor necrosis factor. British Journal of Pharmacology. 1995;114:187–193. doi: 10.1111/j.1476-5381.1995.tb14924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Suzuki R, Kumagai K. Macrophages can produce factors capable of inducing histidine decarboxylase, a histamine-forming enzyme, in vivo in the liver, spleen, and lung of mice. Cellular Immunology. 1986;97:13–22. doi: 10.1016/0008-8749(86)90370-9. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG. Acute phase response in exercise. III. Neutrophil and IL-1{bold on}β{bold} accumulation in skeletal muscle. American Journal of Physiology. 1993;265:R166–172. doi: 10.1152/ajpregu.1993.265.1.R166. [DOI] [PubMed] [Google Scholar]
- Friden J, Seger J, Sjostrom M, Ekblom B. Adaptive response in human skeletal muscle subjected to prolonged eccentric training. International Journal of Sports Medicine. 1983;4:177–183. doi: 10.1055/s-2008-1026031. [DOI] [PubMed] [Google Scholar]
- Fujioka N, Mukaida N, Harada A, Akiyama M, Kasahara T, Kuno K, Ooi A, Mai M, Matsushima K. Preparation of specific antibodies against murine IL-Ra and the establishement of IL-ra as an endogenous regulator of bacteria-induced fulminant hepatitis in mice. Journal of Leukocyte Biology. 1995;58:90–98. doi: 10.1002/jlb.58.1.90. [DOI] [PubMed] [Google Scholar]
- Garrison JC, Rall TW. Autacoids: drug therapy of inflammation. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8. London: Pergamon Press; 1990. pp. 574–599. [Google Scholar]
- Graham P, Kahlson G, Rosengren E. Histamine formation in physical exercise, anoxia and under the influence of adrenaline and related substances. The Journal of Physiology. 1964;172:174–188. doi: 10.1113/jphysiol.1964.sp007411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlson G, Rosengren E. New approaches to the physiology of histamine. Physiological Reviews. 1968;48:155–196. doi: 10.1152/physrev.1968.48.1.155. [DOI] [PubMed] [Google Scholar]
- Kikuchi H, Watanabe M, Endo Y. Induction by interleukin-1 of the mRNA of histidine decarboxylase, the histamine-forming enzyme, in the lung of mice in vivo and the effect of actinomycin D. Biochemical Pharmacology. 1997;53:1383–1388. doi: 10.1016/s0006-2952(96)00887-8. 10.1016/S0006-2952(96)00887-8. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase in bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- Morganroth ML, Young EW, Sparks HV. Prostaglandin and histaminergic mediation of prolonged vasodilatation after exercise. American Journal of Physiology. 1977;233:H27–33. doi: 10.1152/ajpheart.1977.233.1.H27. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, Elizabeth JK, Matsushima K, Durum SK. There is more than one interleukin 1. Immunology Today. 1986;7:45–56. doi: 10.1016/0167-5699(86)90124-6. [DOI] [PubMed] [Google Scholar]
- O'Reilly KP, Warhol MJ, Fielding RA, Frontera WR, Meredith CN, Evans WJ. Eccentric exercise-induced muscle damage impairs muscle glycogen repletion. Journal of Applied Physiology. 1987;63:252–256. doi: 10.1152/jappl.1987.63.1.252. [DOI] [PubMed] [Google Scholar]
- Reilly MA, Schayer RW. Further studies on the histidine-histamine relationship in vivo: effects of endotoxin and of histidine decarboxylase inhibitors. British Journal of Pharmacology. 1968;34:551–563. doi: 10.1111/j.1476-5381.1968.tb08484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schayer RW. Histamine and microcirculation. Life Science. 1974;15:391–401. doi: 10.1016/0024-3205(74)90338-5. 10.1016/0024-3205(74)90338-5. [DOI] [PubMed] [Google Scholar]
- Schayer RW. Handbook of Experimental Pharmacology. Vol. 18. Berlin: Springer-Verlag; 1978. Metabolism and excretion of histamine; pp. 109–129. part 2. [Google Scholar]
- Suda T, Suda J, Spicer SS, Ogawa M. Proliferation and differentiation in culture of mast cell progenitors derived from mast cell-deficient mice of genotype W/Wv. Journal of Cellular Physiology. 1985;122:187–192. doi: 10.1002/jcp.1041220204. [DOI] [PubMed] [Google Scholar]
- Ullum H, Haahr PM, Diamant M, Palmo J, Halkjaer-Kristensen J, Pedersen BK. Bicycle exercise enhances plasma IL-6 but does not change IL-1α, IL-1β, IL-6, or TNFα pre-mRNA in BMNC. Journal of Applied Physiology. 1994;77:93–97. doi: 10.1152/jappl.1994.77.1.93. [DOI] [PubMed] [Google Scholar]


