Abstract
We used intact single fibres from a mouse foot muscle to study the role of oxidation-reduction in the modulation of contractile function.
The oxidant hydrogen peroxide (H2O2, 100-300 μM) for brief periods did not change myoplasmic Ca2+ concentrations ([Ca2+]i) during submaximal tetani. However, force increased by 27 % during the same contractions.
The effects of H2O2 were time dependent. Prolonged exposures resulted in increased resting and tetanic [Ca2+]i, while force was significantly diminished. The force decline was mainly due to reduced myofibrillar Ca2+ sensitivity. There was also evidence of altered sarcoplasmic reticulum (SR) function: passive Ca2+ leak was increased and Ca2+ uptake was decreased.
The reductant dithiothreitol (DTT, 0.5-1 mM) did not change tetanic [Ca2+]i, but decreased force by over 40 %. This was completely reversed by subsequent incubations with H2O2. The force decline induced by prolonged exposure to H2O2 was reversed by subsequent exposure to DTT.
These results show that the elements of the contractile machinery are differentially responsive to changes in the oxidation-reduction balance of the muscle fibres. Myofibrillar Ca2+ sensitivity appears to be especially susceptible, while the SR functions (Ca2+ leak and uptake) are less so.
Reactive oxygen species (ROS) such as the superoxide anion and hydrogen peroxide (H2O2) are continuously generated inside cells as by-products of oxidative metabolism (Chance, Sies & Boveris, 1979). Skeletal muscle, like most mammalian cell types, generates ROS as demonstrated by a variety of techniques (e.g. Jackson, Edwards & Symons, 1985; Reid, Haack, Franchek, Valber, Kobzik & West, 1992; Phung, Ezieme & Turrens, 1994).
While interest in the function of ROS in skeletal muscle has concentrated on their putative role in the development of fatigue and/or injury (Davies, Quintanilha, Brooks & Packer, 1982; Barclay & Hansel, 1991; Anzueto et al. 1992), there are data suggesting that they may also be involved in more basic physiological processes like excitation- contraction coupling. Recently, it was shown that H2O2, acting as an oxidant, induces Ca2+ release from isolated sarcoplasmic reticulum (SR) vesicles and stimulates the activity of single Ca2+ release channels reconstituted in lipid bilayers (Favero, Zable & Abramson, 1995). Accordingly, it has been proposed that excitation- contraction coupling depends on the redox balance of the SR Ca2+ release channels (Salama, Abramson & Pike, 1992). However, studies in more complete systems have found that oxidants do not activate Ca2+ release from the SR directly, and may actually inhibit depolarization-induced Ca2+ release (Brotto & Nosek, 1996; Posterino & Lamb, 1996). These conflicting findings may be explained by multiple sulfhydryl subpopulations in the Ca2+ release channel tetramer, with distinct sensitivities to redox probes and opposing functional effects (Aghdasi, Zhang, Wu, Reid & Hamilton, 1997). Furthermore, changes of the function of isolated SR Ca2+ release channels might reflect altered SR Ca2+ leak rather than changes of depolarization-induced Ca2+ release (Lamb, Recupero & Stephenson, 1992).
There are data demonstrating that the contractile elements themselves are also sensitive to oxidants and reductants. It is a well known fact that alkylation of the sulfhydryl groups of myosin has significant functional effects (Crowder & Cooke, 1984). Apparently, H2O2 does not alter force production or myofibrillar Ca2+ sensitivity in skinned single fibres (Brotto & Nosek, 1996). However, the sulfhydryl oxidants 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) and 2,2′-dithiodipyridine (DTDP) have complex effects on maximal Ca2+-activated force and myofibrillar Ca2+ sensitivity, effects that are at least partially reversed by subsequent exposure to DTT (Wilson, Dos Remedios, Stephenson & Williams, 1991; Posterino & Lamb, 1996).
Studies on intact muscle have shown that the cellular redox balance may have an important influence on contractile function. That is, optimal contractile function may depend on the redox state of some, or all the participating cellular elements. In accordance with this, a study by Reid, Khawli & Moody (1993) demonstrated in rat diaphragm bundles that brief exposures to H2O2 increased twitch forces and prolonged the time to peak tension and the half-relaxation time. In contrast, the antioxidant enzyme catalase had the opposite effects: it shortened time to peak tension and half-relaxation time, and decreased twitch and submaximal tetanic forces. Furthermore, in intact, single frog fibres it has been shown that H2O2 has a biphasic effect on twitch force. Initially, twitch force increases, but as the exposure to H2O2 continues, twitch force declines significantly (Oba, Koshita & Yamaguchi, 1996).
We postulated that ROS would reversibly increase myoplasmic [Ca2+] ([Ca2+]i) during tetanic stimulation, and thereby increase force production in skeletal muscle. To test this hypothesis, we studied the influence of changes in cellular redox balance on contractile function and [Ca2+]i in intact, single skeletal muscle fibres. We found that brief exposures to H2O2 or DTT do not alter [Ca2+]i during submaximal tetani. Force, on the other hand, significantly increases with H2O2, and decreases with DTT. H2O2 reverses the effects of DTT. Furthermore, the response to H2O2 is biphasic: longer exposure times result in [Ca2+]i increases and drastic force decreases, which are fully reversible with DTT. These results indicate that force production in skeletal muscle fibres is very sensitive to changes in cellular redox balance, while Ca2+ release is much less so. The variations of force in response to shifts in redox balance seem to be mediated mainly by changes in myofibrillar Ca2+ sensitivity.
METHODS
Male mice (NMRI strain, weight about 30 g) were killed by rapid neck disarticulation and intact, single fibres were dissected from the flexor brevis muscle of the hindlimb following a procedure described elsewhere (Lännergren & Westerblad, 1987). The isolated fibres were mounted between an Akers 801 force transducer and an adjustable holder, and their length adjusted to that giving maximum tetanic force. Stimulation was achieved by supra-maximum current pulses (duration 0.5 ms) delivered via platinum plate electrodes lying parallel to the fibres. Electrolytic effects, if any, should be very small since the maximal current required to activate the fibres (0.6 mA) and the duration of the stimuli are three to four orders of magnitude less than those used to produce detectable amounts of ROS by electrolysis in saline solutions (Jackson, Mickelson, Stringer, Rao & Lucchesi, 1986; Reid, Shoji, Moody & Entman, 1992).
Solutions and drugs
Experiments were performed at room temperature (22°C). The fibre was superfused with a Tyrode solution of the following composition (mM): NaCl, 121; KCl, 5.0; CaCl2, 1.8; MgCl2, 0.5; NaH2PO4, 0.4; NaHCO3, 24.0; glucose, 5.5; 0.2 % fetal calf serum was added to the solution. The solution was bubbled with 5 % CO2-95 % O2 to maintain pH at 7.4 throughout the experiment. H2O2, DTT and caffeine were obtained from Sigma Chemical Co. (Sweden), and prepared daily as concentrated stocks in Tyrode solution and added to the perfusing solution as needed.
[Ca2+]i measurements
[Ca2+]i was measured with the fluorescent Ca2+ indicator indo-1 (Molecular Probes Europe B.V., Leiden, The Netherlands), which was microinjected into the fibre. The fluorescence of indo-1 was measured with a system consisting of a xenon lamp, a mono-chromator and two photomultiplier tubes (PTI, Photo Med GmbH, Wedel, Germany). The excitation light was set to 360 ± 5 nm and the light emitted at 405 ± 5 and 495 ± 5 nm was measured. The ratio of the light emitted at 405 nm to that at 495 nm (R) was translated to [Ca2+]i using the following equation (Grynkiewicz, Poenie & Tsien, 1985):
| (1) |
where KD is the apparent dissociation constant of indo-1, β is the ratio of the 495 nm signals at very low and saturating [Ca2+]i, Rmin is the ratio at very low [Ca2+]i, and Rmax is the ratio at saturating [Ca2+]i.
β, Rmin, and Rmax will vary from one experimental set-up to another depending on the filters used, photomultiplier gains, etc. Furthermore, they are not the same in simple salt solutions and in the intracellular environment. These three parameters were therefore established intracellularly with the equipment used in the present study. Rmin was assessed by repeated injections of 0.5 M EGTA into a fibre until a stable ratio was obtained. This was performed in four fibres and gave an Rmin of 0.495 ± 0.015. Rmax was estimated by injections of 1 M CaCl2. The contracture induced by this treatment was minimized by adding 20 mM 2,3-butanedione monoxime (BDM) to the bath solution prior to the injections. Rmax amounted to 4.42 ± 0.30 (n= 6). β was obtained following the method described by Bakker, Head, Williams & Stephenson (1993; see also Westerblad & Allen, 1996). Thus, we first plotted the 495 nm signal vs. the 405 nm signal from tetanic contractions at 100 Hz produced in ten fibres. Linear regression was then used to get the slope of this plot (m) in each fibre. The fit was generally good (regression coefficient = 0.86 ± 0.02) and m amounted to -1.55 ± 0.11. Mean values of m, Rmin and Rmax were then used to establish β using the following equation:
| (2) |
which gave a value for β of 4.44.
The value of KD, in contrast to β, Rmin, and Rmax, depends on the Ca2+ binding properties of indo-1 and should not vary from one experimental equipment to another. KD is affected by the intracellular environment, which makes it difficult to measure and values presented from different laboratories show marked differences. We have previously reported a mean KD of 182 nM (n= 3) in the present mouse fibre preparation (Westerblad & Allen, 1993). In that study we used injections of a Ca2+-EGTA/EGTA solution with a [Ca2+] of 101 nM. More recently, we estimated KD using injections of an EGTA-buffered solution with [Ca2+] set to 300 nM (contractures minimized by adding 20 mM BDM to the bath) in a experimental set-up similar to that used in the present study and obtained a mean KD of 373 nM (n= 3). Finally, we estimated KD in the present set-up using injections of 300 nM [Ca2+] and got a mean value of 287 nM (n= 5). We believe that the most reliable measure of KD is obtained by taking the global mean from all these three studies and this gives a KD equal to 283 ± 26 nM (n= 11), which we used in the present study. When translating fluorescence ratios into [Ca2+]i, KD simply acts as a scaling factor (see eqn (1)) and consequently the [Ca2+]i values reported in this study will be about 50 % higher than those of our previous studies. However, altering KD has no effect on relative changes of [Ca2+]i, i.e. the percentage change of [Ca2+]i with some manoeuvres is independent of KD. Thus the conclusions in our previous studies are not affected by the fact that the KD used might have been too low.
In vitro calibration experiments, performed as described in Westerblad & Allen (1993), showed no effect of H2O2 or DTT on indo-1 fluorescence; likewise, these chemicals did not have any immediate effect on the intracellular fluorescence of indo-1. In contrast, application of caffeine rapidly attenuated the intracellular fluorescence signals of indo-1 without affecting the ratio, in agreement with previous studies (O'Neill, Donoso & Eisner, 1990; Allen & Westerblad, 1995).
Fluorescence and force signals were sampled online, and stored on a desktop computer system for subsequent data analysis. [Ca2+]i and force (in kPa) were measured as the mean over a number of integral stimulation cycles with a total duration of about 100 ms (20-60 Hz) or as the mean over the last 100 ms of stimulation (70-100 Hz).
Experimental procedure
Tetanic contractions of 350 ms duration were produced at 1-2 min intervals. In most experiments, the stimulation frequency during tetani was kept constant at a frequency that under control conditions gave approximately 50 % of the maximum force. This frequency ranged between 30-50 Hz and these contractions will be referred to as submaximal tetani. At the beginning of each experiment, the fibre was stimulated at constant intervals to verify that tetanic force and [Ca2+]i were stable. H2O2 or DTT was then added and the resulting changes in force and [Ca2+]i were followed with contractions at regular intervals. In some fibres, one chemical was changed for the other or was washed out as force and [Ca2+]i were measured. We also studied the effects of H2O2 on the force-[Ca2+]i relationship during selected experiments. Tetani were then produced at 20-100 Hz and the following Hill equation was fitted to the force-[Ca2+]i data points (for details see Westerblad & Allen, 1993):
| (3) |
where P is the relative force, Pmax is the force at saturating [Ca2+]i, Ca50 is the [Ca2+]i giving 50 % of Pmax, and N is a constant which describes the steepness of the function. In this curve fitting, Pmax was assumed to be 8 % higher than the force during 100 Hz control tetani (Allen & Westerblad, 1995). Force and [Ca2+]i were often still increasing at the end of the 350 ms stimulation period, especially at lower stimulation frequencies, and this is a potential problem. Control experiments showed that since both force and [Ca2+]i are increasing relatively slowly, the tetanus duration has little effect on the force-[Ca2+]i relationship. In these experiments, [Ca2+]i and force were measured at 50 ms intervals after 250 ms of stimulation (i.e. from 250 to 300 ms, and from 300 to 350 ms), and fitted to eqn (3). Ca50 was exactly the same in both sets of results, 0.47 ± 0.03 μM (P > 0.8; n= 7 fibres). N, the steepness of the curve, was also very similar (5.3 ± 0.7 and 5.3 ± 0.6; P > 0.85). Nonetheless, the changes in submaximal force that we report will depend on the tetanus duration and the values provided refer exclusively to 350 ms tetani.
Statistics
Values are presented as means ±s.e.m. Statistically significant differences were determined using Student's paired t tests or with one-way repeated measures analysis of variance, followed by Dunnett's test for multiple comparisons vs. a control group (SigmaStat, Jandel Corp., San Rafael, CA, USA). The significance level (P) was set at 0.05 throughout.
RESULTS
Brief exposures to H2O2
First, we studied the response of single fibres to brief exposures to the oxidant H2O2. Figure 1A shows typical 40 Hz tetani before and after a 3 min incubation with 150 μM H2O2. H2O2 had a very small effect on [Ca2+]i (top), increasing it by 2.5 % from 0.40 to 0.41 μM. Meanwhile, force (bottom) increased from 93.8 to 130.8 kPa, a 39.4 % change.
Figure 1. Brief exposure to H2O2 increases force in single skeletal muscle fibres.
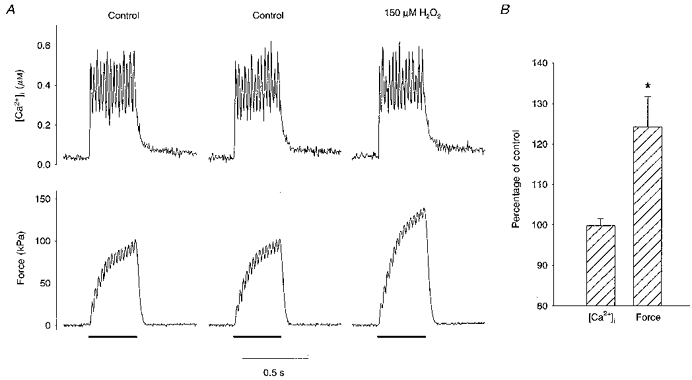
A, original [Ca2+]i (top) and force (bottom) records from a single skeletal muscle fibre during 40 Hz tetani. The bars under the force tracings represent the 350 ms stimulation periods. Shown are 2 responses before (Control, 4 min apart) and after being exposed to 150 μM H2O2 for 3 min. Observe that the control responses are virtually identical. H2O2 increased force whereas [Ca2+]i was almost unchanged. B, mean data from 10 single fibres show that tetanic [Ca2+]i in single skeletal muscle fibres remained unaffected during short exposures to H2O2 (100-300 μM) whereas force increased. * Significant difference from control (P < 0.05).
The two control contractions in Fig. 1A show that the muscle was in a stable condition before application of H2O2; these tetani were separated by 4 min and tetanic [Ca2+]i and force measured in the second contraction were within 2 % of the first. Mean data (n= 17) showed that [Ca2+]i and force declined by 0.2 and 0.4 % of initial control values per minute. These slopes were not significantly different from zero (P= 0.75 and 0.5, respectively). All subsequent comparisons were made against the control tetanus immediately prior to H2O2 exposure.
Experiments with brief exposure to H2O2 (100-300 μM) were performed in a total of ten fibres. The results from these experiments were rather similar and seemed independent of the H2O2 concentration used. Figure 1B summarizes the results obtained at the time of the maximum force increase, which occurred after 6 ± 2 min of exposure to H2O2. In these submaximal tetani, [Ca2+]i remained unchanged at 100 ± 2 % of the control level and force increased to 124 ± 7 % of control (P < 0.05).
Time dependence of H2O2 effects
In general, the single fibres responded to H2O2 in a time-dependent manner, showing biphasic changes. Figure 2A presents the typical response to incubation with 300 μM H2O2 for prolonged periods. It presents [Ca2+]i (top) and force (bottom) during 50 Hz tetani before (Control), and during incubation with 300 μM H2O2 for 4 and 8 min. At the 4 min time point, H2O2 increased tetanic [Ca2+]i by 2 % from 0.49 to 0.50 μM while force increased from 209 to 265 kPa, a 22 % change. Extending the exposure for a further 4 min gave different results; [Ca2+]i increased to 0.52 μM (6 % greater than control), and force decreased to 155 kPa, a 26 % drop from the initial control level. This time dependence is summarized in Fig. 2B: tetanic [Ca2+]i remained unchanged (100 ± 3 % of control) during the first 2-4 min of incubation with 300 μM H2O2, while force increased to 126 ± 4 % of control (P < 0.05; n= 8 fibres). These were very much like the results seen with a range of H2O2 concentrations in Fig. 1B. As exposure to 300 μM H2O2 was extended to 6-16 min, tetanic [Ca2+]i increased to 114 ± 8 % of control and force decreased to 57 ± 10 % (P < 0.05). These changes were not spontaneously reversible, and after 4-20 min of wash-out [Ca2+]i was 135 ± 7 % of control (P < 0.05) and force was only 21 ± 4 % (P < 0.05). At lower H2O2 concentrations, it usually took longer exposures to obtain similar effects.
Figure 2. Prolonged exposure to H2O2 decreases force.
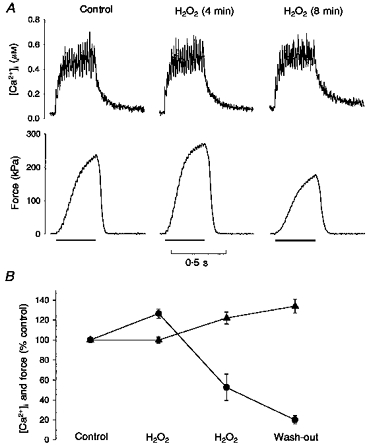
A, original [Ca2+]i (top) and force (bottom) records from a single fibre, presenting 50 Hz tetani before (Control), and at 4 and 8 min in 300 μM H2O2. The bars under the force tracings represent the stimulation periods. H2O2 for 4 min increased [Ca2+]i by only 1 % and force by 22 %. After 8 min in H2O2, [Ca2+]i increased 6 %, and force decreased to 74 % of control. B, biphasic effects of H2O2 on single fibre function. During the first 2-4 min, H2O2 (300 μM) increased force (•) during submaximal tetani without altering [Ca2+]i (▴; n= 8). As exposure extended to 6-16 min, [Ca2+]i increased, while force declined by over 40 %. These trends continued during the wash-out period.
Effects of H2O2 on force-[Ca2+]i relationship
Initially, [Ca2+]i did not change and force increased in response to H2O2. As time of exposure to H2O2 was prolonged, [Ca2+]i increased and force declined. To better grasp the time dependence of these changes, we obtained the force-[Ca2+]i relationship in four fibres before the exposure to H2O2. Figure 3A presents a typical example of the responses obtained from 40 Hz tetani at different time points during incubation with 100 μM H2O2, referenced to the control force-[Ca2+]i relationship. Two 40 Hz control tetani (3 min before (▵), and 8 min after (▿), establishing the force-[Ca2+]i relationship) show that the preparation is stable over time under the control conditions. The first response to H2O2, at 2 min, was a 26 % increase in force, and the data point is to the left of the control force-[Ca2+]i relationship. Over the next 8 min, [Ca2+]i increased while force steadily declined, such that at 10 min [Ca2+]i was 26 % greater and force was only 35 % of the initial control value, clearly to the right of the force-[Ca2+]i relationship. Similar results were obtained in the three other fibres tested. Changes of the mid-point of the force-[Ca2+]i relationship (Ca50 in eqn (3)) were estimated assuming that the steepness of the relationship (N) and the force at saturating [Ca2+]i (Pmax) stay constant during the individual experiments. This assumption might not be correct, but minor changes of N and Pmax will have relatively little effect on the estimated Ca50 since we used tetanic contractions with forces close to 50 % of the maximum (see below). Under control conditions, Ca50 was 0.51 ± 0.04 μM. Early on during exposure to H2O2, Ca50 decreased to 0.42 ± 0.03 μM (83 ± 4 % of control, P < 0.05, n= 4 fibres). Prolonged exposure to H2O2 increased Ca50 by 43 ± 13 % over control to 0.76 ± 0.11 μM (P < 0.05).
Figure 3. H2O2 alters myofibrillar Ca2+ sensitivity and SR function.
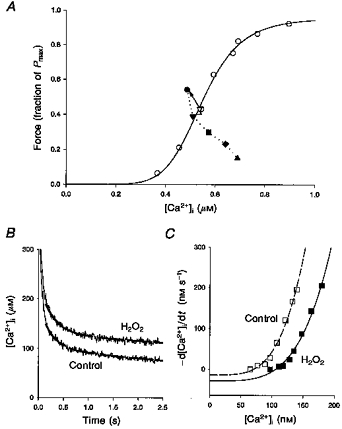
A, force-[Ca2+]i relationship obtained from a single fibre under control conditions (○). The continuous line represents the Hill equation fitted to these data points (Ca50= 0.51 μM, N= 7.2). Open triangles show [Ca2+]i and force during 40 Hz tetani 3 min before (▵) and 8 min after (▿) producing the force-[Ca2+]i curve. Filled symbols represent [Ca2+]i and force during 40 Hz tetani at selected time points during incubation with 100 μM H2O2 (2 min, •; 6 min, ▾; 7 min, ▪; 8 min, ♦; 10 min, ▴). The arrow shows the step change from the control 40 Hz tetanus (▿) to the first time point during H2O2 exposure. B, averaged records from 8 fibres of [Ca2+]i after the end of stimulation. The dashed curves represent the double exponential functions fitted to them. C, plots of the rate of decline in [Ca2+]i (d[Ca2+]i/dt) vs.[Ca2+]i taken from the double exponential fits shown in B (control, □; H2O2, ▪). The lines represent the curve fitting of the data points to eqn (4).
To study further the role of altered myofibrillar function on the force deficit seen after prolonged H2O2 exposure, we incubated three fibres with caffeine. First, we exposed the fibres to 300 μM H2O2, until force during submaximal tetani was only 21 ± 9 % of control. Then, we perfused the fibres with 5 mM caffeine in regular Tyrode solution and force during 100 Hz tetani increased to 77 ± 13 % of the initial control value. [Ca2+]i during caffeine-induced tetani was 1.3 ± 0.1 μM, which is noticeably less than values previously obtained (about 3 μM after accounting for the higher KD of indo-1 used in the present study; Allen & Westerblad, 1995). Thus it is possible that force production was limited by Ca2+ release, even in the presence of caffeine, and 77 % might be seen as the lower limit of Pmax after prolonged exposure to H2O2. The lower limit of Ca50 after prolonged exposure to H2O2 can then be calculated using this lower Pmax (77 %) and this gives a mean Ca50 of 0.75 μM, which is somewhat less than the value obtained with Pmax set to 100 % but still 39 % higher than the control value.
Effects of H2O2 on SR function
Prolonged exposure to H2O2 (100-300 μM, 15 ± 3 min) also increased resting [Ca2+]i by 56 %, from 64.8 ± 9.1 to 98.5 ± 12.4 nM (P < 0.05). We also observed a slowing of the [Ca2+]i decline to resting levels after the end of stimulation (Figs 3B and 4B). Both facts suggested an increase in the SR Ca2+ leak or a decrease in SR Ca2+ uptake. These parameters can be estimated by a closer analysis of the final slow decline of [Ca2+]i after the end of stimulation (Klein, Kovacs, Simon & Schneider, 1991; Westerblad & Allen, 1993). The noisy lines in Fig. 3B show the decline in [Ca2+]i after the end of stimulation, averaged from eight experiments. As in Fig. 4B, it is easily seen that H2O2 slowed the return of [Ca2+]i to its resting level. To estimate changes in SR Ca2+ leak and uptake, these [Ca2+]i tails were first fitted to double exponential functions, shown by the dashed lines in Fig. 3B. Then, d[Ca2+]i/dt was measured at selected [Ca2+]i on these exponential fits, the [Ca2+]i at rest was used to determine the zero value for d[Ca2+]i/dt, and the results were plotted in Fig. 3C. At any given [Ca2+]i, the rate of [Ca2+]i decline was markedly lower with H2O2 than in control. The following equation was fitted to these data points:
| (4) |
where A reflects the rate of SR Ca2+ uptake and L is the SR Ca2+ leak. N is a power function and was set to 4, which gave a good fit under all conditions studied (Klein et al. 1991; Westerblad & Allen, 1993). A was about 60 % smaller in H2O2 (2.29 × 10−7vs. 5.67 × 10−7 nM−3 s−1 for control). Furthermore, L was twice as large in H2O2 (28.9 vs. 14.1 nM s−1 for control).
Figure 4. The effects of H2O2 are reversible.
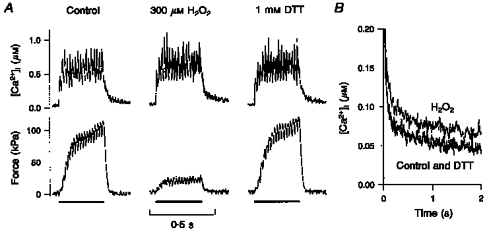
A, original [Ca2+]i (top) and force (bottom) records from a single fibre, presenting 50 Hz tetani before (Control), after 6 min in 300 μM H2O2, and after 10 min exposure to 1 mM DTT. H2O2 increased [Ca2+]i by 10 % and decreased force by 78 %. DTT restored [Ca2+]i and force to 105 and 107 % of control, respectively. B, decline of [Ca2+]i to resting levels, after the end of stimulation, for the three experimental conditions. The prolonged exposure to H2O2 displaced the [Ca2+]i tail upwards and to the right. Incubation with DTT completely reversed this change.
DTT reverses H2O2 effects
Obviously, the changes in force and [Ca2+]i at the end of long exposures to H2O2 could represent irreversible oxidation of essential cellular components or loss of viability. Therefore, we tested whether fibres could be ‘rescued’ from such oxidative stress. The deleterious effects of H2O2 were fully reversed by DTT. This is demonstrated in Fig. 4. Figure 4A presents [Ca2+]i (top) and force (bottom) before (Control), during incubation with 300 μM H2O2, and during incubation with 1 mM DTT. First, we duplicated the typical response to incubation with 300 μM H2O2 for prolonged periods. H2O2 for 8 min at this high concentration increased [Ca2+]i from 0.59 to 0.65 μM, while force decreased from 100 to 22 kPa. Then, to ‘rescue’ the fibre, DTT was used immediately after H2O2 exposure, and it restored [Ca2+]i to 0.62 μM and force to 107 kPa. It was noticed again that prolonged exposure to H2O2 slowed the decline of [Ca2+]i to the resting level after the end of stimulation. This is presented in Fig. 4B, using data from the contractions shown in Fig. 4A: the tail of [Ca2+]i after the end of stimulation was slower in the contraction during exposure to H2O2, and it was restored to normal with DTT. We tried DTT in three more fibres exposed to 300 μM H2O2 for 6-12 min (Fig. 5). [Ca2+]i was not significantly affected by H2O2 (100 ± 9 % of control), while force decreased to 36 ± 5 % of control (P < 0.05). Subsequent exposure to 0.5-1 mM DTT for 6-10 min had no significant effect on [Ca2+]i (108 ± 6 %) and restored force to 90 ± 7 % of control. When DTT exposure continued 6-9 min more, [Ca2+]i declined slightly to 95 ± 2 %, and force decreased to 60 ± 12 %.
Figure 5. DTT reverses the effects of H2O2.
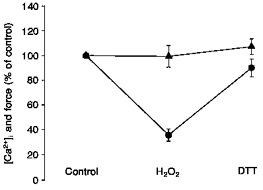
Incubation with 300 μM H2O2 for 6-16 min markedly decreased force (•), while tetanic [Ca2+]i (▴) was not significantly affected during submaximal tetani (n= 4). DTT (0.5-1 mM for 6-10 min) applied immediately following the exposure to H2O2 almost restored force to the initial control value, whereas [Ca2+]i remained virtually unaffected.
Brief exposures to DTT
The last step was to explore the contractile response during exposure to the reductant DTT, i.e. the response to the reductant starting from the baseline condition. Figure 6 exemplifies the effects of DTT on [Ca2+]i (top) and force (bottom). Exposure to 1 mM DTT decreased [Ca2+]i during a 40 Hz tetanus from 0.77 to 0.74 μM, while force decreased from 116 to 75 kPa. This was not spontaneously reversible by wash-out; [Ca2+]i held steady at 0.76 μM and force decreased even further to 45 kPa. Expecting that the effects of a reductant would be reversed with an oxidant, we exposed the fibre to 150 μM H2O2 for 6 min; [Ca2+]i increased slightly to 0.78 μM; force increased to 92 kPa. These findings were replicated in a total of six fibres. Figure 7 shows changes in [Ca2+]i and force as percentage of control during exposure to 0.5-1 mM DTT for 6-12 min, during 8-15 min of wash-out, and during incubation with 25 to 150 μM H2O2. Tetanic [Ca2+]i was unchanged throughout the protocol, being 92 ± 2, 91 ± 4, and 104 ± 5 % of control during DTT, wash-out, and H2O2, respectively. Force decreased to 57 ± 6 % of control with DTT (P < 0.05). This was not reversible upon wash-out as force remained depressed at 42 ± 5 % of control (P < 0.05). Finally, incubation with H2O2 reversed the effects of DTT and restored force to the initial control level (95 ± 7 %).
Figure 6. Typical effects of DTT on [Ca2+]i and force transients.
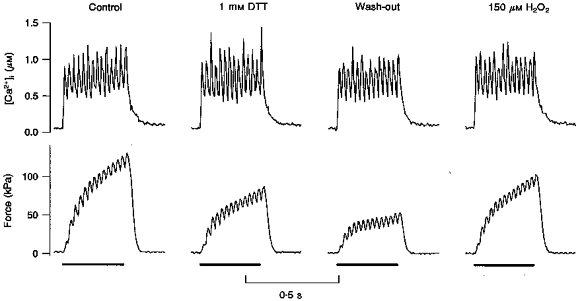
Original records presenting [Ca2+]i (top traces) and force (bottom traces) from a single fibre during 40 Hz tetani. The bars under the force tracings represent the stimulation periods. Four sequential conditions are shown: control, incubation with 1 mM DTT for 6 min, wash-out for 8 min, and incubation with 150 μM H2O2 for 6 min. DTT barely decreased [Ca2+]i by 4 %, while force was reduced by 35 %. Force dropped a further 25 % in the subsequent wash-out period. Finally, a short incubation with 150 μM H2O2 partially restored force, to 79 % of the control level.
Figure 7. DTT does not change [Ca2+]i but decreases force.
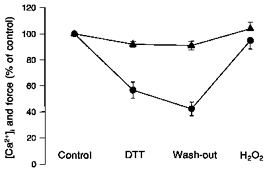
[Ca2+]i (▴) and force (•) during submaximal tetani as percentage of control values and under the same conditions as in Fig. 6. [Ca2+]i was not significantly affected during incubation with DTT. The wash-out period and subsequent exposure to H2O2 did not significantly alter [Ca2+]i either. On the other hand, DTT decreased force by over 40 % (P < 0.05). The wash-out period did not reverse this change, but incubation with H2O2 restored force to within 6 % of the control level.
The depression of force induced by DTT could be due to a reduction in the ability of cross-bridges to produce force (i.e. reduced Pmax) and/or in the myofibrillar Ca2+ sensitivity (i.e. reduced Ca50). To distinguish between these, 100 Hz tetani were produced in three of the fibres under control conditions and during wash-out of DTT. In these 100 Hz tetani neither force (94 ± 3 %) nor [Ca2+]i (95 ± 10 %) was significantly reduced during the wash-out of DTT compared with the control. Thus the lack of a significant decline in force during 100 Hz tetani is clearly different from the marked decline during 40 Hz tetani, indicating that the impaired contractile performance with DTT was mainly due to decreased myofibrillar Ca2+ sensitivity.
DISCUSSION
Reid et al. (1993) showed that ROS improved force during submaximal tetani in isolated muscle bundles, and that the antioxidant enzymes superoxide dismutase and catalase had the opposite effect. These findings suggested ROS are necessary for optimal contractile function. Arguing along these lines, Salama et al. (1992) proposed that excitation- contraction coupling in skeletal muscle is mediated via changes in the redox state of the SR Ca2+ release channels. According to their model, sarcolemmal depolarization induces sulfhydryl groups on the voltage sensors, located in the T-tubular membranes, to oxidize specific sulfhydryl groups on the Ca2+ release channels, leading to their opening and Ca2+ efflux to the cytosol. These two studies in combination would predict that modulation of force production by oxidants and reductants is secondary to changes in Ca2+ release and mean tetanic [Ca2+]i. That is, oxidants should improve Ca2+ release, increase tetanic [Ca2+]i, and consequently, increase force. Reductants would have the opposite effect. Our results present a different picture: (1) brief exposures to H2O2 and DTT do not change [Ca2+]i during submaximal tetani; (2) force is very sensitive to H2O2 and DTT; and (3) altered Ca2+ release and uptake are late events during exposure to H2O2.
The continuous interaction between ROS and the cellular environment determines the cellular ‘redox balance.’ This term refers to the state of the different cellular redox pools, including sulfhydryls (glutathione, cysteine and accessible protein sulfhydryls) and pyridine nucleotides (Chance et al. 1979; Kehrer & Lund, 1994). For example, glutathione is maintained predominantly in the reduced state (Gilbert, 1990). Its depletion, or alteration of its redox state, can result in structural and functional abnormalities in skeletal muscle (Mårtensson & Meister, 1989; Anzueto et al. 1992; Posterino & Lamb, 1996). However, the redox state of the different cellular redox pools is not necessarily the same, as their metabolic roles differ (Kehrer & Lund, 1994). Furthermore, there is evidence that the cellular redox balance is a dynamic condition that fluctuates from reduction to oxidation, depending on functional state (Gilbert, 1990; Hwang, Sinskey & Lodish, 1992; Kehrer & Lund, 1994). It is then possible to define an optimal cellular redox state for contractile function, such that submaximal force is highest at this point (Fig. 8). Our findings suggest that the cellular redox balance in resting fibres is in a relatively reduced state and that this is not optimal for contractile function (see below).
Figure 8. Force as a function of cellular redox balance.
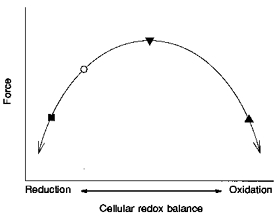
Model of force production as a function of cellular redox balance. The baseline redox balance (○) is to the left of the peak, into the reduction range. DTT shifts the reduction state further to the left and decreases force (▪). A short exposure to H2O2 changes the baseline redox balance to the right, into the oxidation range and increases force during submaximal tetani (▾). As exposure to H2O2 continues, the redox balance moves further into the oxidation state, and force decreases (▴). The positioning of ‘reduction’ and ‘oxidation’ is arbitrary. For further explanation, see Discussion.
Brief exposure to H2O2
H2O2 was used to shift the cellular redox balance towards an oxidized state. Short exposures to H2O2 did not change tetanic [Ca2+]i, but significantly increased force in the single fibres during submaximal tetani (Fig. 1). Because force increased, we conclude that the baseline cellular redox balance is not optimal for contractile function. In addition, considering that force increased in response to an oxidant (H2O2), the baseline redox balance must be relatively reduced (○ in Fig. 8). A brief exposure to H2O2 brings the cellular redox balance to the right of this baseline reduced state and force increases (▾ in Fig. 8). The anticipated change had been an increase in force secondary to an increase in tetanic [Ca2+]i as proposed previously (Salama et al. 1992; Reid et al. 1993; Oba et al. 1996). However, our findings indicate that brief exposures to H2O2 augment force independently of corresponding changes in Ca2+ release from the SR.
Prolonged exposure to H2O2
The time course of the extended exposures to H2O2 show the contractile response to graded changes in cellular redox balance as the single fibres become more and more oxidized. During the first few minutes of incubation, comparable with the brief exposure experiments described above, the cellular redox balance becomes more oxidized, reaches its optimum and force increases substantially (Fig. 2B, and ▾ in Fig. 8). As exposure to H2O2 continues, the cell becomes further oxidized, moving past the optimal redox state, and force declines drastically (Figs 2B and 5, and ▴ in Fig. 8). This is similar to data published by Oba et al. (1996), who show that twitch force in frog single fibres first increases and then decreases during incubation with H2O2 (see Fig. 1 in Oba et al. 1996). The extreme shift in redox state is reversed when the fibres are incubated with DTT and force is restored to the baseline level (Figs 4 and 5). As in the experiments discussed above, the variations in force at the different intervals during H2O2 exposure cannot be explained by corresponding changes in tetanic [Ca2+]i. A more stringent oxidant treatment, on the other hand, might disrupt excitation-contraction coupling and compound the force deficit (Brotto & Nosek, 1996; Oba et al. 1996; Posterino & Lamb, 1996).
After prolonged exposure to H2O2, there was a relatively smaller decline of force at high [Ca2+]i (i.e. 100 Hz tetani in the presence of caffeine) compared with that during submaximal tetani (mean decline, 23 vs. 79 %). This indicates that H2O2 primarily alters myofibrillar Ca2+ sensitivity. Our analysis of the changes in Ca50 support this notion. The initial increase in force corresponds with a decrease in Ca50 from the control value of about 15 %. When the exposure is extended for a longer period, this change is reversed and Ca50 increases by almost 50 %. However, it must be noted that complete force-[Ca2+]i curves could not be obtained during exposure to H2O2 since force and [Ca2+]i were not stable over time in the presence of this substance. This means, for instance, that if there was a reduction of the steepness of the force-[Ca2+]i relationship (reduced N in eqn (3)), as has been described in skinned muscle fibres (Wilson et al. 1991; Posterino & Lamb, 1996), this would not have been detected. Furthermore, the absolute magnitude of the change in Ca50 depends on whether or not Pmax is assumed to decrease, but its influence is rather small (see Results). Thus, H2O2 exposure induced marked and time-dependent changes of the myofibrillar Ca2+ sensitivity, but there are some uncertainties concerning the absolute changes of Ca50 that we report.
Brief exposure to DTT
The reductant DTT was used to drive cellular redox balance towards a reduced state. Incubation with DTT did not change [Ca2+]i during submaximal tetani, but decreased force (Figs 6 and 7). Since the baseline state of the fibres is relatively reduced, DTT shifts the redox balance further from its optimum and decreases force (▪ in Fig. 8). As in brief H2O2 exposures, force changes without a corresponding change in tetanic [Ca2+]i. Moreover, the force during 100 Hz tetani was not significantly reduced by DTT, indicating that DTT had little effect on Pmax. The observed decline in force during submaximal tetani with DTT would then mainly be due to reduced myofibrillar Ca2+ sensitivity.
Redox modulation of muscle function
If the observed effects of H2O2 and DTT reflected changes in the cellular redox balance, it would be expected that oxidants and reductants would antagonize and reverse the effects of one another. This is true for the effects of DTT, which were reversed by H2O2 (Figs 6 and 7), and for the effects of H2O2, which were reversed by DTT (Figs 4 and 5). Cells control their redox balance, and can restore it to its normal state when it is altered (Chance et al. 1979; Kehrer & Lund, 1994). However, the observed changes with H2O2 and DTT did not spontaneously reverse upon wash-out (see Figs 2 and 7 for H2O2 and DTT, respectively). We suspect that the fibres did not have time to restore the redox shifts induced by H2O2 and DTT within the brief wash-out periods used in the present study.
An earlier study proposed that contractile function is dependent on the cellular redox state: resting muscles have a very low rate of ROS production and cellular redox balance is in a relatively reduced state, depressing contractile function. At the other extreme, intense muscular activity increases ROS production and drives the redox balance to the oxidized state, which also depresses force (Reid et al. 1993). Implicit in this scheme is an intermediate cellular redox state that optimizes force production in skeletal muscle (see also Oba et al. 1996). Our findings are consistent with such a model. The changes in force seen when single fibres are exposed to H2O2 and DTT can be visualized in terms of variations of the cellular redox balance, as presented above (and in Fig. 8). Moreover, it is likely that the observed effects are secondary to the overall change in cellular redox balance, as H2O2 and DTT apparently do not markedly affect the contractile function of skinned skeletal muscle fibres (Brotto & Nosek, 1996; Posterino & Lamb, 1996). Our data also downplay the role of redox effects on Ca2+ release; they suggest that force modulation is primarily accomplished at sites distal to SR Ca2+ release (a main determinant of tetanic [Ca2+]i). This is in agreement with a recently published report that an oxidant increased myofibrillar Ca2+ sensitivity in skinned single fibres and the effect was reversed with DTT (Posterino & Lamb, 1996).
Force changes independent of [Ca2+]i
Brief exposures to H2O2 increase force during submaximal tetani, and DTT and prolonged incubation with H2O2 decrease it. One thing in common between these two opposite effects is that force changes are independent of alterations in tetanic [Ca2+]i. Even during prolonged H2O2 exposure, when tetanic [Ca2+]i may increase marginally, force changes in the opposite direction and decreases (Fig. 2B). DTT and brief exposures to H2O2 have opposite redox capacities and effects on force, and could affect different components of the contractile machinery. However, the fact that one reverses the effect of the other (e.g. Figs 5 and 7) suggests that they have opposing effects on the same processes. At a given tetanic [Ca2+]i, force is set by myofibrillar Ca2+ sensitivity and cross-bridge kinetics (Brenner, 1988). One or both of these two processes are likely to mediate the changes caused by the redox state. Of the proteins that integrate the contractile machinery, actin and tropomyosin seem to be the most resistant to redox alterations (Liu, Wang & Stracher, 1990; Williams & Swenson, 1982). Furthermore, moderate modification of the reactive myosin sulfhydryl groups does not have drastic functional consequences (Crowder & Cooke, 1984). The targets that determine the Ca2+ sensitivity of the contractile process are troponin and the regulatory myosin light chains (Brenner, 1988; Metzger & Moss, 1992). Both proteins have significant primary sequence homology as Ca2+-binding proteins (Collins, 1976). A possible, albeit speculative, consequence of this arrangement could be variable sensitivity to Ca2+ of either one of these proteins in response to changes in redox state. This mechanism has already been shown for cardiac troponin C, which becomes insensitive to Ca2+ when an intramolecular disulphide bridge is formed (Putkey, Dotson & Mouawad, 1993). Another possibility is that changes in Ca2+ sensitivity occur via changes of the redox status of glutathione. In line with this argument, excess glutathione has been shown to reduce the myofibrillar Ca2+ sensitivity of skinned rat muscle fibres (Posterino & Lamb, 1996). This could also explain why H2O2 produced a large change in Ca2+ sensitivity in our experiments, but had no effect on the Ca2+ sensitivity in skinned rat fibres (Brotto & Nosek, 1996).
The SR as a redox-sensitive target
Our results suggest that SR function in the intact fibre is not particularly sensitive to changes in cellular redox balance. The SR was unaffected by DTT. Only the final stages of prolonged H2O2 exposure provide any evidence of altered Ca2+ transients, seen as increased resting [Ca2+]i and slower return of [Ca2+]i to baseline (Figs 3B and 4B). These data establish that SR Ca2+ uptake decreases and the passive Ca2+ leak increases following prolonged incubation with H2O2; this last finding confirms data published previously (Posterino & Lamb, 1996). Furthermore, when we exposed single fibres to caffeine following prolonged exposure to H2O2, tetanic [Ca2+]i was not as high as we have observed in the past, suggesting that Ca2+ release was impaired, and that the level of tetanic [Ca2+]i was maintained, at least in part, because of slower reuptake. Two other groups have obtained evidence which argues against modulation by the redox state of SR function in skinned fibres; oxidants did not enhance excitation-contraction coupling in these studies; rather, they inhibited depolarization-induced Ca2+ release (Posterino & Lamb, 1996; Brotto & Nosek, 1996).
Conclusions
We have presented data showing that contractile function in intact, single skeletal muscle fibres is sensitive to changes in cellular redox balance. Brief exposures to H2O2 and DTT do not change tetanic [Ca2+]i but increase and decrease force, respectively. Prolonged exposures to H2O2 decrease force, independently of changes in tetanic [Ca2+]i. The divergent results obtained with H2O2 and DTT and the time dependence of the H2O2 effects indicate that the presumed cellular targets have optimal redox states. Contrary to our expectations, SR function was fairly insensitive to changes in redox balance. However, myofibrillar Ca2+ sensitivity appears to be most susceptible to variations of the cellular redox balance.
Acknowledgments
The present study was supported by the Swedish Medical Research Council (project no. 10842), the Swedish National Centre for Sports Research, the Harald and Greta Jeanssons Foundation, the Magnus Bergvalls Foundation, the Baylor College of Medicine-Karolinska Institute Research Exchange Program, and the National Institutes of Health (grant HL45721).
References
- Aghdasi B, Zhang J-Z, Wu Y, Reid MB, Hamilton SL. Multiple classes of sulfhydryls modulate the skeletal muscle Ca2+ release channel. Journal of Biological Chemistry. 1997;272:3739–3748. doi: 10.1074/jbc.272.6.3739. 10.1074/jbc.272.6.3739. [DOI] [PubMed] [Google Scholar]
- Allen DG, Westerblad H. The effects of caffeine on intracellular calcium, force and the rate of relaxation of mouse skeletal muscle. The Journal of Physiology. 1995;487:331–342. doi: 10.1113/jphysiol.1995.sp020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzueto A, Andrade FH, Maxwell LC, Levine SM, Lawrence RA, Gibbons WJ, Jenkinson SG. Resistive breathing activates the glutathione redox cycle and impairs performance of rat diaphragm. Journal of Applied Physiology. 1992;72:529–534. doi: 10.1152/jappl.1992.72.2.529. [DOI] [PubMed] [Google Scholar]
- Bakker AJ, Head SI, Williams DA, Stephenson DG. Ca2+ levels in myotubes grown from the skeletal muscle of dystrophic (mdx) and normal mice. The Journal of Physiology. 1993;460:1–13. doi: 10.1113/jphysiol.1993.sp019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay JK, Hansel M. Free radicals may contribute to oxidative skeletal muscle fatigue. Canadian The Journal of Physiology and Pharmacology. 1991;69:279–284. doi: 10.1139/y91-043. [DOI] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proceedings of the National Academy of Sciences of the USA. 1988;85:3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotto MAP, Nosek TM. Hydrogen peroxide disrupts Ca2+ release from the sarcoplasmic reticulum of rat skeletal muscle fibers. Journal of Applied Physiology. 1996;81:731–737. doi: 10.1152/jappl.1996.81.2.731. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiological Reviews. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Collins JH. Homology of myosin DTNB light chain with alkali light chains, troponin C and parvalbumin. Nature. 1976;259:699–700. doi: 10.1038/259699a0. [DOI] [PubMed] [Google Scholar]
- Crowder MS, Cooke R. The effect of myosin sulfhydryl modification on the mechanics of fiber contraction. Journal of Muscle Research and Cell Motility. 1984;5:131–146. doi: 10.1007/BF00712152. [DOI] [PubMed] [Google Scholar]
- Davies KJA, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochemical and Biophysical Research Communications. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- Favero TG, Zable AC, Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. Journal of Biological Chemistry. 1995;270:25557–25563. doi: 10.1074/jbc.270.43.25557. 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Advances in Enzymology. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Jackson CV, Mickelson JK, Stringer K, Rao PS, Lucchesi BR. Electrolysis-induced myocardial dysfunction. A novel method for the study of free radical mediated tissue injury. Journal of Pharmacological Methods. 1986;15:305–320. doi: 10.1016/0160-5402(86)90010-0. 10.1016/0160-5402(86)90010-0. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Edwards RHT, Symons MCR. Electron spin resonance studies of intact mammalian skeletal muscle. Biochimica et Biophysica Acta. 1985;847:185–190. doi: 10.1016/0167-4889(85)90019-9. 10.1016/0167-4889(85)90019-9. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Lund LG. Cellular reducing equivalents and oxidative stress. Free Radical Biology and Medicine. 1994;17:65–75. doi: 10.1016/0891-5849(94)90008-6. 10.1016/0891-5849(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Klein MG, Kovacs L, Simon BJ, Schneider MF. Decline of myoplasmic Ca2+, recovery of calcium release and sarcoplasmic Ca2+ pump properties in frog skeletal muscle. The Journal of Physiology. 1991;441:639–671. doi: 10.1113/jphysiol.1991.sp018771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Recupero E, Stephenson DG. Effect of myoplasmic pH on excitation-contraction coupling in skeletal muscle fibres of the toad. The Journal of Physiology. 1992;448:211–224. doi: 10.1113/jphysiol.1992.sp019037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. The Journal of Physiology. 1987;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DF, Wang D, Stracher A. The accessibility of the thiol groups on G- and F-actin of rabbit muscle. Biochemical Journal. 1990;266:453–459. doi: 10.1042/bj2660453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J, Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proceedings of the National Academy of Sciences of the USA. 1989;86:471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Moss RL. Myosin light chain 2 modulates calcium-sensitive cross-bridge transitions in vertebrate skeletal muscle. Biophysical Journal. 1992;63:460–468. doi: 10.1016/S0006-3495(92)81614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba T, Koshita M, Yamaguchi M. H2O2 modulates twitch tension and increases P0 of Ca2+ release channel in frog skeletal muscle. American Journal of Physiology. 1996;271:C810–818. doi: 10.1152/ajpcell.1996.271.3.C810. [DOI] [PubMed] [Google Scholar]
- O'Neill SC, Donoso P, Eisner DA. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. The Journal of Physiology. 1990;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phung CD, Ezieme JA, Turrens JF. Hydrogen peroxide metabolism in skeletal muscle mitochondria. Archives of Biochemistry and Biophysics. 1994;315:479–482. doi: 10.1006/abbi.1994.1528. 10.1006/abbi.1994.1528. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Lamb GD. Effects of reducing agents and oxidants on excitation-contraction coupling in skeletal muscle fibres of rat and toad. The Journal of Physiology. 1996;496:809–825. doi: 10.1113/jphysiol.1996.sp021729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putkey JA, Dotson DG, Mouawad P. Formation of inter- and intramolecular disulfide bonds can activate cardiac troponin C. Journal of Biological Chemistry. 1993;268:6827–6830. [PubMed] [Google Scholar]
- Reid MB, Haack KE, Franchek KM, Valber PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle I. Intracellular oxidant kinetics and fatigue in vitro. Journal of Applied Physiology. 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle III. Contractility of unfatigued muscle. Journal of Applied Physiology. 1993;75:1081–1087. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle II. Extracellular release of free radicals. Journal of Applied Physiology. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- Salama G, Abramson JJ, Pike GK. Sulphydryl reagents trigger Ca2+ release from the sarcoplasmic reticulum of skinned rabbit psoas fibres. The Journal of Physiology. 1992;454:389–420. doi: 10.1113/jphysiol.1992.sp019270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. The Journal of Physiology. 1993;466:611–628. [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Intracellular calibration of the calcium indicator indo-1 in isolated fibers of Xenopus muscle. Biophysical Journal. 1996;71:908–917. doi: 10.1016/S0006-3495(96)79294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Jr, Swenson CA. Disulfide bridges in tropomyosin. European Journal of Biochemistry. 1982;127:495–499. [PubMed] [Google Scholar]
- Wilson GJ, Dos Remedios CG, Stephenson DG, Williams DA. Effects of sulphydryl modification on skinned rat skeletal muscle fibres using 5,5′-dithiobis(2-nitrobenzoic acid) The Journal of Physiology. 1991;437:409–430. doi: 10.1113/jphysiol.1991.sp018603. [DOI] [PMC free article] [PubMed] [Google Scholar]


