Abstract
In vivo extracellular and intracellular recordings were performed from thalamocortical (TC) neurones in a genetic model of absence epilepsy (genetic absence epilepsy rats from Strasbourg) during spontaneous spike and wave discharges (SWDs).
Extracellularly recorded single units (n= 14) fired either a single action potential or a high frequency burst of up to three action potentials, concomitantly with the spike component of the spike-wave complex.
Three main events characterized the intracellular activity of twenty-six out of twenty-eight TC neurones during SWDs: a small amplitude tonic hyperpolarization that was present throughout the SWD, rhythmic sequences of EPSP/IPSPs occurring concomitantly with the spike-wave complexes, and a small tonic depolarization at the end of the SWD. The rhythmic IPSPs, but not the tonic hyperpolarization, were mediated by activation of GABAA receptors since they reversed in polarity at -68 mV and appeared as depolarizing events when recording with KCl-filled electrodes.
The intracellular activity of the remaining two TC neurones consisted of rhythmic low threshold Ca2+ potentials, with a few EPSP/IPSP sequences present at the start of the SWD.
These results obtained in a well-established genetic model of absence epilepsy do not support the hypothesis that the intracellular activity of TC neurones during SWDs involves rhythmic sequences of GABAB IPSPs and low threshold Ca2+ potentials.
Absence seizures, the main hallmark of absence epilepsy, a primary generalized epilepsy, are characterized by a brief loss of consciousness associated with electroencephalographic (EEG) recordings of 3 Hz, bilaterally synchronous spike and wave discharges (SWDs), which are not followed by post-ictal depression (Malafosse, Genton, Hirsch, Marescaux, Broglin & Bernasconi, 1994; Niedermeyer, 1996). Our present understanding of the mechanisms involved in the generation of SWDs highlights a key role for cortical and thalamic neurones (Gloor & Fariello, 1988; Avoli, Gloor, Kostopoulos & Naquet, 1990; Avoli & Gloor, 1992), and is supported by extensive data from different experimental models of this disease (Avoli et al. 1990; Marescaux, Vergnes & Depaulis, 1992; Snead, 1995).
However, the detailed sequence of events leading to the expression of SWDs in the EEG remains unclear (Niedermeyer, 1996). Several electrophysiological studies in the thalamocortical (TC) system of feline generalized penicillin epilepsy (FGPE) (Prince & Farrel, 1969; Avoli et al. 1990), a pharmacological model of absence epilepsy, have suggested that the generation of SWDs mainly involves an enhancement of GABA-mediated inhibition (Gloor & Fariello, 1988), while rhythmic IPSPs superimposed on a tonic hyperpolarization have been observed in the majority of TC neurones during spike-wave complexes in ketamine- xylazine anaesthetized cats (Steriade & Contreras, 1995). Experiments with GABAA antagonists in a ferret thalamic slice preparation have shown the transformation of the sleep spindle into a paroxysmal activity with high frequency burst firing, resembling earlier extracellular data obtained in the FGPE, and consisting of rhythmic GABAB IPSPs and low threshold Ca2+ potentials (LTCPs) (von Krosigk, Bal & McCormick, 1993; Bal, von Krosigk & McCormick, 1995a,b). On the other hand, γ-hydroxybutyrate, an endogenous analogue of GABA that induces SWDs when injected systemically, has recently been shown in vitro to elicit a tonic GABAB receptor-mediated hyperpolarization leading to the expression of rhythmic intrinsic LTCPs (Williams, Turner & Crunelli, 1995).
In view of the absence of in vivo data on the cellular events occurring during SWDs in TC and other types of thalamic neurones from any of the established experimental models of absence epilepsy, we have now made intracellular recordings in vivo from TC neurones of genetic absence epilepsy rats from Strasbourg (GAERS) (Marescaux et al. 1992; Vergnes & Marescaux, 1994). This inbred strain is a well-characterized genetic model of human absence epilepsy that reproduces most of its clinical and EEG features, including spontaneous SWDs that occur all over the TC system, and, in contrast to other genetic models (Dung & Swigert, 1972; Noebels & Sidman, 1979), is free of additional neuropathologies (Marescaux et al. 1992; Vergnes & Marescaux, 1994).
METHODS
Experiments were performed on adult rats (4-5 months old) from the GAERS strain that were initially anaesthetized with pentobarbitone (40 mg kg−1i.p.; Sanofi) and ketamine (100 mg kg−1i.m.; Imalgène, Rhone Mérieux, Lyon, France) and placed in a stereotaxic frame. The skull and the dura above parts of the cortex were removed to allow insertion of stimulating and recording electrodes, a cannula was inserted in the trachea, and cisternal drainage was performed. All holes in the skull were filled with 4 % agar, wounds and pressure points were infiltrated with xylocaine 2 % (Astra, Paris) (repeated every 2 h), and body temperature was maintained at 37°C with a homeothermic blanket. Once the surgical procedures had been completed, ear bars were removed and the head was held via a metallic rod cemented to the skull. Neurolept analgesia was then initiated with an i.v. injection of Fentanyl (3 μg kg−1i.v.; Janssen, Boulogne-Billancourt, France), that was repeated every 20-30 min, and haloperidol (1 mg kg−1i.p.; Haldol, Janssen) (Flecknell, 1996). Rats were immobilized with d-tubocurarine chloride (1 mg kg−1i.v., repeated every 2 h; Sigma) and artificially ventilated while continuously monitoring EEG, heart rate and the state of the pupils. At the end of the experiments, animals were killed with an overdose of pentobarbitone, and the position of the electrodes was verified in 80 μm sections using standard histological procedures (Pinault, 1996; Charpier & Deniau, 1997).
A tungsten bipolar electrode (1 mm tip separation) was used to record the EEG and to stimulate deep layers in the sensory motor cortex, while glass electrodes containing 0.5 M NaCl and 1.5 % biocytin were used for extracellular recordings. Glass electrodes filled with 3 M potassium acetate (40-70 MΩ) or 3 M KCl (25-60 MΩ) were used for intracellular recordings in the ventroposterior nuclei. Cells were characterized as TC neurones following orthodromic and antidromic responses to cortical stimulation (Fig. 1A), and also, in intracellular recordings, by the presence of a large amplitude low threshold Ca2+ potential (LTCP) at the offset of hyperpolarizing voltage responses to negative current steps. Voltage and current records were amplified with an Axoclamp 2B (Axon Instruments), and stored on a Biologic DAT recorder (Intracel, Royston, UK) for off-line analysis. Extracellular single unit activity and intracellular activity were filtered at 1-10 kHz and 30 kHz, respectively, and analysed using Spike 2 (Cambridge Electronic Design, Cambridge, UK). Numerical data are expressed in the text as means ±s.d.
Figure 1. Properties of extracellularly recorded TC neurones and power spectra of the EEG in immobilized GAERS.
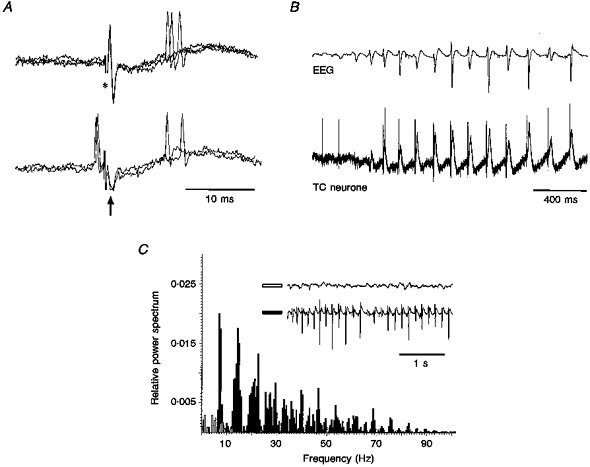
A, orthodromic response of a TC neurone (top, 3 superimposed traces) to stimulation of the sensory motor cortex (*), and collision test (bottom, 3 superimposed traces) (arrow indicates the antidromic field potential). Note the failure of an antidromic response due to collision with a spontaneous action potential. B, extracellular recordings (bottom trace) from the same unit as in A show the action potential firing to become time-locked with the spike component of the spike-wave complex in the EEG (top trace). Note the large field potential occurring in close time relationship with the EEG spike-wave complex. C, EEG power spectra before (open bars) and during (filled bars) a SWD show the marked increase in power during, and the shift towards the dominant frequency (6-9 Hz) of, spike-wave complexes. Note the change in the amplitude of the spike-wave complexes throughout a SWD.
RESULTS
The results of this study are based on fourteen and twenty-eight TC neurones recorded extracellularly and intracellularly, respectively, during spontaneous SWDs. Power spectra analysis of the EEG indicated that spike-wave complexes had a frequency of 6.9 ± 0.7 Hz (n= 11) (Fig. 1B and C). The duration of SWDs ranged from 600 ms to 2-3 min, and even in the same animal we could observe from a few short SWDs per minute up to a SWD every 5-10 min. The properties of SWDs recorded here, therefore, were similar to those observed in freely moving GAERS (Marescaux et al. 1992).
Extracellular recordings
The appearance of a SWD dramatically changed the mostly irregular firing pattern of the majority of the spontaneously active, extracellularly recorded TC neurones, time-locking their firing close to the spike component of the spike-wave complex (Fig. 1B). Independently from the pattern of firing occurring before a SWD, the firing during a SWD consisted of only single action potentials (n= 3 out of 14 neurones) (Fig. 1B), only bursts of up to three action potentials with a frequency of 346 ± 78 Hz (n= 26) (n= 3 out of 14 neurones), or an equal proportion of these two firing patterns (n= 8 neurones). The single action potential, or the first action potential of a burst preceded the negative component of the EEG spike by 10.6 ± 1.6 ms (n= 42). The latency of the orthodromic response to cortical stimulation was 7.4 ± 1.8 ms (n= 93).
Intracellular recordings
The resting membrane potential and steady-state input resistance (calculated from 10 mV, 300 ms, hyperpolarizing voltage responses) of TC neurones in GAERS were -56 ± 2 mV (n= 18) and 32 ± 14 MΩ (n= 26), respectively. Three main events occurred in twenty-six out of the twenty-eight TC neurones recorded intracellularly during spontaneous SWDs in the EEG: (i) a small tonic hyperpolarization present throughout the SWD (Figs 2Aa and Ab, 3Aa and Ab), (ii) a rhythmic sequence of synaptic potentials consisting of an EPSP closely followed by two to six IPSPs (Figs 2Aa and Ad, 2B and 3Aa-c), which was also present throughout the SWD and occurred at the same frequency (7.9 ± 0.2 Hz) as the spike-wave complexes, and (iii) a small tonic depolarization that started at the end of the SWD and could last for up to 800 ms (Figs 2Ab and 3Ac). The start of both the tonic hyperpolarization and the rhythmic synaptic potential sequence could precede the full expression of a SWD in the EEG (Fig. 2Ab and C), as it was particularly evident during depolarization (Fig. 2B) and hyperpolarization (Fig. 3Ac) of the neurones by DC injection. Both the tonic hyperpolarization and the synaptic potential sequence could end either before or after the termination of a SWD in the EEG. The frequency of the EPSP/IPSP sequence was not affected by hyperpolarization or depolarization of the neurone by DC injection (Fig. 2A and C).
Figure 2. Intracellularly recorded activity of TC neurones during spontaneous SWDs.
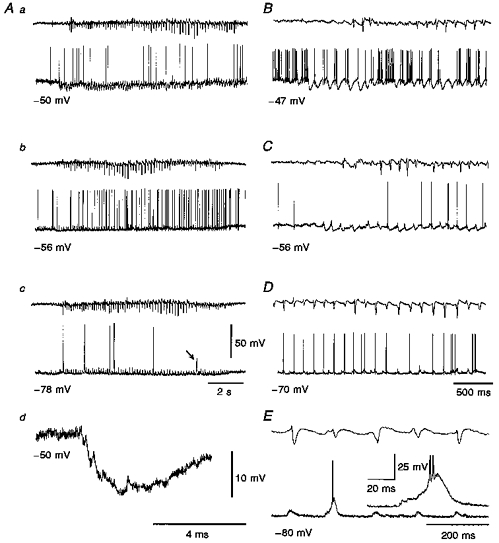
In this and following figures, the upper and lower trace in each pair are the EEG and the intracellular voltage record, respectively. A, examples of intracellular activity recorded during spontaneous SWDs in a TC neurone. Note the rhythmic EPSP/IPSP sequences at -50 mV (a) and how the IPSPs are fully reversed at -78 mV (c). The enlarged record in Ad clearly shows the EPSP/IPSP sequence. The tonic hyperpolarization is visible in Aa and b (cf. Fig. 3Aa-c), but not in Ac. The arrow in Ac indicates a LTCP that does not reach firing threshold. B-E, records from the same neurone as in A show that the firing is sculpted by the rhythmic synaptic potential sequences. In particular, at -47 mV (B) the activity consists of high frequency firing (around 100 Hz) intermixed with IPSP-mediated periods of inhibition, but no LTCP is present. At -56 mV (C), the firing is brought about by the EPSP/partially reversed IPSPs sequences. At -70 mV (D) the IPSPs are fully reversed but no LTCP is evoked. At -80 mV (E), the summation of the EPSP and reversed IPSPs provides a depolarization sufficient to elicit an LTCP with two action potentials (enlarged in the inset). Voltage calibration for the intracellular trace in Ac applies to all other intracellular traces except those in Ad and in the inset in E.
Figure 3. The rhythmic IPSPs, but not the tonic hyperpolarization, are mediated by GABAA receptors.
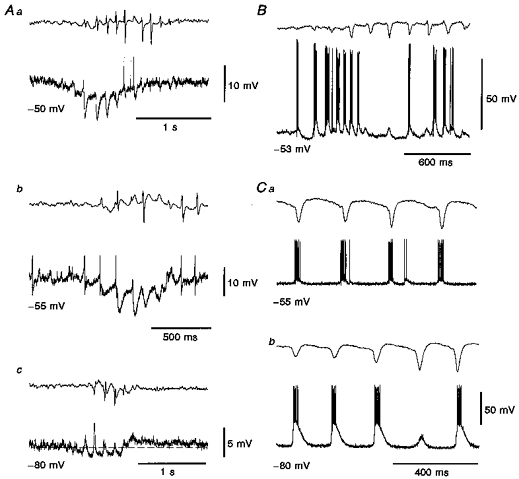
A, intracellular voltage records show the tonic hyperpolarization present during spontaneous SWDs (b and c are from the same neurone). At -80 mV (c) the tonic hyperpolarization and the tonic depolarization present at the end of SWD are clearly visible. B, intracellular activity recorded with a KCl-filled electrode show the lack of any rhythmic hyperpolarizing potentials at -53 mV, and the presence of the tonic hyperpolarization starting well before the first large spike in the EEG. C, intracellular records from another TC neurone recorded with a KCl-filled electrode show full depolarizing envelopes at -55 mV (a), which at -80 mV (b) become very large depolarizations (i.e. EPSP, reversed IPSPs and LTCP) evoking robust action potential bursts. In Aa and b, the amplitude of the action potentials has been truncated for clarity.
The rhythmic EPSP/IPSP sequences present during the SWD limited the firing to the period close to the spike component of the spike-wave complex, so that at resting membrane potential the first action potential in each period of firing preceded the negative component of the EEG spike by 10.4 ± 2.7 ms (n= 38). At potentials ≤ -70 mV, the rhythmic sequence of EPSP and reversed IPSPs (see below) could evoke a LTCP, often with a burst of action potentials (Fig. 2Ac and E). The first action potential of this LTCP-evoked burst could either precede (by up to 15 ms) or follow (by up to 20 ms) the spike component of the spike-wave complex, because of the variable delay in the ability of the synaptic potentials to evoke a LTCP (Fig. 2E; cf. Fig. 3Cb).
The tonic hyperpolarization decreased in amplitude with steady hyperpolarization of the neurone (Fig. 2A), and was absent at potentials more negative than about -80 mV (Fig. 2Ac). We were unable, however, to observe a clear reversal of the tonic hyperpolarization. The rhythmic IPSPs became smaller following steady hyperpolarization of the neurone by DC injection, and reversed in polarity at -68 ± 3 mV (n= 4) (Fig. 2Ac, D and E). Thus, at potentials ≤ -70 mV all the rhythmic IPSPs already appeared as depolarizing events, while the tonic hyperpolarization had not changed its polarity (Figs 2Ac and 3Ac). Furthermore, in the five TC neurones recorded with KCl-filled electrodes the rhythmic postsynaptic potential sequence was always depolarizing even at potentials close to -55 mV (Fig. 3B and C), while the tonic hyperpolarization was still present. These results, together with the value of the reversal potential, indicated that the rhythmic IPSPs, but not the tonic hyperpolarization, were mediated by the activation of GABAA receptors.
The tonic depolarization (Figs 2Ab and Ac and 3Ac) was not seen at the end of every SWD. However, even when the tonic depolarization was absent, more action potentials were often present immediately after the end of a SWD than before the start of the same SWD. The tonic depolarization was also observed in neurones recorded with KCl-filled electrodes.
In two TC neurones, with a steady-state input resistance higher (65 and 85 MΩ) than the other twenty-six neurones, the intracellular events accompanying the SWDs were different from those described above (Fig. 4). Although the tonic hyperpolarization was present, the intracellular activity appeared to consist mainly of rhythmic LTCPs (occurring in phase with the spike component of the spike-wave complex), with little evidence for the presence of rhythmic sequences of EPSP/IPSPs. A few clear sequences, however, could be observed at the start of some of the SWDs (Fig. 4C). The membrane potential oscillation brought about by the rhythmic LTCPs (Fig. 4Ab and Bb) closely resembled the intrinsic pacemaker oscillation described in rat TC neurones (cf. Fig. 8B in Leresche, Lightowler, Soltesz, Jassik-Gerschenfeld & Crunelli, 1991): in particular, the overall rounded shape of their hyperpolarizing phase (Fig. 4Ab and D) was similar to the waveform of the pacemaker oscillation recorded at the most depolarized potentials of their voltage region of existence (cf. Fig. 1 in Leresche et al. 1991; and Fig. 7 in Turner, Anderson, Williams & Crunelli, 1997). The first action potential evoked by the LTCP preceded the spike component of the spike-wave complex by 13.7 ± 5.5 ms (n= 10), a value similar to the one obtained for the firing recorded extracellularly and to the one observed in the other TC neurones recorded intracellularly.
Figure 4. Rhythmic LTCPs during SWDs.
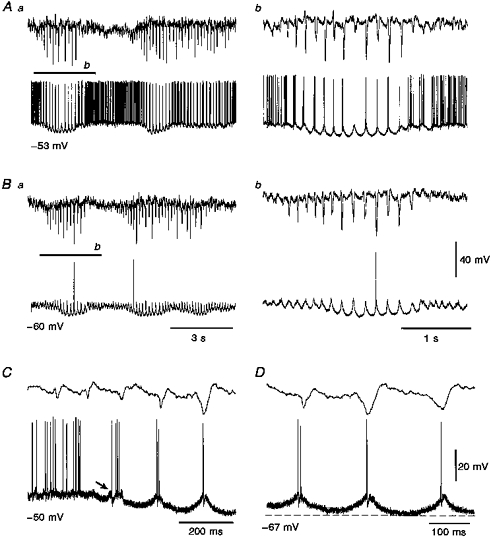
A-D, intracellular voltage records from 1 of the 2 TC neurones where the voltage waveform during the main part of the SWDs appeared to consist of rhythmic LTCPs with no clear evidence of synaptic potentials. Note, however, the presence of EPSP/IPSPs sequence at the start of the SWDs (arrow in C). Ab and Bb are enlargements of the indicated portion of the records in Aa and Ba, respectively.
DISCUSSION
The main conclusion of this investigation conducted in a well-characterized rat genetic model of absence epilepsy is that the cellular counterpart of SWDs in TC neurones is a rhythmic EPSP/GABAA IPSP sequence superimposed on a small tonic hyperpolarization. These findings, therefore, do not support the hypothesis that SWDs involve rhythmic sequences of GABAB IPSPs and LTCPs in TC neurones.
Comparison of our data with those obtained in freely moving GAERS (Marescaux et al. 1992) indicates that the surgical procedures used in this study had no substantial effect on the properties of SWDs recorded in the EEG. It is unlikely, therefore, that the drug regimen used in the present study had drastically altered the cellular mechanisms responsible for the generation and expression of SWDs within the TC loop.
Our recordings with acetate- and chloride-filled electrodes showed no rhythmic hyperpolarizing potentials at membrane potentials more hyperpolarized than -70 and -50 mV, respectively, suggesting the absence of GABAB-mediated IPSPs in these phasic events occurring in TC neurones concomitantly with spontaneous SWDs in the EEG. On the other hand, the tonic hyperpolarization, which did not reverse even at potentials of about -90 mV and was insensitive to an increase in [Cl−]i, is likely to represent a synaptic, K+-mediated process. Although on the basis of in vivo data from GAERS and other experimental models of absence epilepsy (Hosford et al. 1992; Liu, Vergnes, Depaulis & Marescaux, 1992; Snead, 1992), activation of thalamic postsynaptic GABAB receptors would appear as the most likely explanation for the origin of the tonic hyperpolarization, any of the neurotransmitter receptors known to mediate K+-dependent hyperpolarizing effects in TC neurones (McCormick, 1992) should be considered at present as a likely candidate. As far as the tonic depolarization present at the end of a SWD is concerned, the slow deactivation of the hyperpolarization-activated inward current, Ih (Bal & McCormick, 1996), and/or the activation of metabotropic glutamate receptors by corticothalamic afferents (McCormick & von Krosigk, 1992) should be considered as possible underlying mechanisms.
Whatever the origin of the tonic hyperpolarization and depolarization observed in the GAERS, however, substantial differences exist between the intracellular activity of TC neurones recorded in vivo in this genetic model of absence epilepsy and the bicuculline-induced paroxysmal in vitro activity (i.e. rhythmic sequences of GABAB IPSPs and LTCPs at 2-4 Hz) (Bal et al. 1995a,b), resembling earlier extracellular data obtained in vivo in the FGPE model (Giarretta, Avoli & Gloor, 1987; Gloor & Fariello, 1988).
On the other hand, our findings are remarkably similar (except for the frequency) to those reported in cats showing spontaneous transitions from sleep to a hypersynchronous epileptic state, though under ketamine-xylazine anaesthesia (Steriade & Contreras, 1995). In 60 % of these cat TC neurones in vivo, in fact, the observed intracellular events consisted of phasic (2-4 Hz) IPSPs superimposed on a tonic hyperpolarization, and a depolarization with increased firing was present at the end of the paroxysmal period (cf. Figs 11B, 11C, 12A and 13 in Steriade & Contreras, 1995). The remaining 40 % of cat TC neurones were described as discharging action potential bursts at 2-4 Hz in close time relation with the spike component of the SWD (Steriade & Contreras, 1995). Although we did not observe an identical firing pattern in our sample, the only illustrated example of intracellularly recorded activity from the latter group of TC neurones (cf. Fig. 7B1 and B2 in Steriade & Contreras, 1995) looks remarkably similar to the one recorded in our group of twenty-six rat TC neurones at relatively depolarized membrane potentials (Fig. 2B and C).
The finding of two TC neurones that showed an intracellular activity different from all the other TC neurones in our sample is intriguing. It is unlikely that they were not TC neurones or that we were recording outside the thalamus, since above and below this neurone along the same electrode tract TC neurones with an intracellular activity similar to all other TC neurones were found. Our ability to observe few EPSP/IPSP sequences only at the start of a SWD, therefore, suggests that these sequences were either absent during the main part of a SWD or fully masked by the LTCP, i.e. they occurred in perfect synchrony with the rising and falling phase of each LTCP. An alternative possibility is that a very small number (5 %) of TC neurones displays a different intracellular activity during SWDs, consisting of a few initial EPSP/IPSP sequences followed by rhythmic LTCPs resembling intrinsic pacemaker oscillations (Leresche, Jassik-Gerschenfeld, Haby, Soltesz & Crunelli, 1990; McCormick & Pape, 1990; Leresche et al. 1991; Steriade, Curró Dossi & Nuñez, 1991). The relatively higher input resistance of these TC neurones would have clearly contributed to the expression of this intrinsic activity, and in this respect, it is worth noting that in the cat ventrobasal thalamus in vitro a group (30 %) of TC neurones with a higher input resistance show a higher proportion of pacemaker oscillating neurones (Turner et al. 1997). It is also important to note that the GABAB receptor-mediated hyperpolarization evoked by the postsynaptic action of γ-hydroxybutyrate in TC neurones in vitro elicits an intrinsic 1-3 Hz pacemaker oscillation similar in waveform and presence of LTCPs to the activity observed in these two TC neurones (cf. Fig. 3 in Williams, Turner & Crunelli, 1995).
In summary, the present results demonstrate the lack of rhythmic GABAB IPSPs and LTCPs in a well-established rat genetic model of absence epilepsy. The different frequency of SWDs between different species/models may indicate the presence of different underlying mechanisms, some of which might represent the processes involved in the human condition.
Acknowledgments
We would like to thank Professor M. Collard for his generous support (funds from the Centre de Recherches de Neurologie, Hôpital Civil, Strasbourg) in setting up the INSERM laboratory of anatomo-electrophysiology. The work was supported by the European Union (Biomed 2, grant 97-2093), The Wellcome Trust (grant 37089), the CNRS (URA 1488), and the British Council (Alliance).
References
- Avoli M, Gloor P. Role of the thalamus in generalized penicillin epilepsy: observations on decorticate cats. Experimental Neurology. 1992;77:386–402. doi: 10.1016/0014-4886(82)90252-7. 10.1016/0014-4886(82)90252-7. [DOI] [PubMed] [Google Scholar]
- Avoli M, Gloor P, Kostopoulos G, Naquet R. Generalized Epilepsy: Neurobiological Approaches. Boston: Birkhäuser; 1990. [Google Scholar]
- Bal T, McCormick DA. What stops synchronized thalamocortical oscillations. Neuron. 1996;17:297–308. doi: 10.1016/s0896-6273(00)80161-0. [DOI] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret LGNd in vitro. The Journal of Physiology. 1995a;483:641–663. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. The Journal of Physiology. 1995b;483:665–685. doi: 10.1113/jphysiol.1995.sp020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S, Deniau JM. In vivo activity-dependent plasticity at cortico-striatal connections: Evidence for physiological long-term potentiation. Proceedings of the National Academy of Sciences of the USA. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dung HC, Swigert RH. Histo-pathological observations of the nervous and lymphoid tissues of ‘lethargic’ mutant mice. Texas Reports on Biology and Medicine. 1972;30:23–39. [PubMed] [Google Scholar]
- Flacknell P. Laboratory Animal Anaesthesia. London: Academic Press; 1996. [Google Scholar]
- Giarretta D, Avoli M, Gloor P. Intracellular recordings in pericruciate neurons during spike and wave discharges of feline generalized penicillin epilepsy. Brain Research. 1987;405:68–79. doi: 10.1016/0006-8993(87)90990-5. 10.1016/0006-8993(87)90990-5. [DOI] [PubMed] [Google Scholar]
- Gloor P, Fariello G. Generalized epilepsy: Some of its cellular mechanisms differ from those of focal epilepsy. Trends in Neurosciences. 1988;11:63–68. doi: 10.1016/0166-2236(88)90166-x. [DOI] [PubMed] [Google Scholar]
- Hosford DA, Clark S, Cao Z, Wilson WA, Lin F-H, Morrisett RA, Huin A. The role of GABAB receptor activation in absence seizures of lethargic (lh/lh) mice. Science. 1992;257:398–401. doi: 10.1126/science.1321503. [DOI] [PubMed] [Google Scholar]
- Leresche N, Jassik-Gerschenfeld D, Haby M, Soltesz I, Crunelli V. Pacemaker-like and other types of spontaneous membrane potential oscillations of thalamocortical cells. Neuroscience Letters. 1990;113:72–77. doi: 10.1016/0304-3940(90)90497-w. 10.1016/0304-3940(90)90497-W. [DOI] [PubMed] [Google Scholar]
- Leresche N, Lightowler S, Soltesz I, Jassik-Gerschenfeld D, Crunelli V. Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. The Journal of Physiology. 1991;441:155–174. doi: 10.1113/jphysiol.1991.sp018744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Vergnes M, Depaulis A, Marescaux C. Involvement of intrathalamic GABAB neurotransmission in the control of absence seizures in the rat. Neuroscience. 1992;48:87–93. doi: 10.1016/0306-4522(92)90340-8. 10.1016/0306-4522(92)90340-8. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Progress in Neurobiology. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape H-C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. The Journal of Physiology. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through activation of glutamate metabotropic receptors. Proceedings of the National Academy of Sciences of the USA. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafosse A, Genton P, Hirsch E, Marescaux C, Broglin D, Bernasconi R. Idiopathic Generalized Epilepsies. London: John Libbey; 1994. [Google Scholar]
- Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy in rats from Strasbourg - A review. Journal of Neural Transmission. 1992;35:37–69. doi: 10.1007/978-3-7091-9206-1_4. suppl. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E. Primary idiopathic generalized epilepsy and underlying mechanisms. Clinical Electroencephalography. 1996;27:1–21. doi: 10.1177/155005949602700103. [DOI] [PubMed] [Google Scholar]
- Noebels JL, Sidman RL. Inherited epilepsy: spike-wave and focal motor seizure in the mutant mouse tottering. Science. 1979;204:1334–1336. doi: 10.1126/science.572084. [DOI] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin and neurobiotin. Journal of Neuroscience Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Prince DA, Farrell D. ‘Centrencephalic’ spike-wave discharges following parenteral penicillin injection in the cat. Neurology. 1969;19:309–310. [Google Scholar]
- Snead OC. Evidence for GABAB-mediated mechanisms in experimental generalized absence seizures. European Journal of Pharmacology. 1992;213:343–349. doi: 10.1016/0014-2999(92)90623-c. 10.1016/0014-2999(92)90623-C. [DOI] [PubMed] [Google Scholar]
- Snead OC. Basic mechanisms of generalized absence seizures. Annals of Neurology. 1995;37:146–157. doi: 10.1002/ana.410370204. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D. Relations between cortical and thalamic cellular events during transition from sleep patterns to paroxysmal activity. Journal of Neuroscience. 1995;15:623–642. doi: 10.1523/JNEUROSCI.15-01-00623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Curró Dossi R, Nuñez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. Journal of Neuroscience. 1991;11:3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JP, Anderson CM, Williams SR, Crunelli V. Morphology and membrane properties of neurones in the cat ventro-basal thalamus in vitro. Journal of Physiology. 1997;505:707–726. doi: 10.1111/j.1469-7793.1997.707ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C. Pathophysiological mechanisms underlying genetic absence epilepsy in rats. In: Malafosse A, Genton P, Hirsch E, Marescaux C, Broglin D, Bernasconi R, editors. Idiopathic Generalized Epilepsies. London: John Libbey; 1994. pp. 151–168. [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- Williams SR, Turner JP, Crunelli V. Gamma-hydroxybutyrate promotes oscillatory activity of rat and cat thalamocortical neurons by a tonic GABAB receptor-mediated hyperpolarization. Neuroscience. 1995;66:133–141. doi: 10.1016/0306-4522(94)00604-4. 10.1016/0306-4522(94)00604-4. [DOI] [PubMed] [Google Scholar]


