Abstract
Voltage-dependent Ca2+ currents of dissociated rat supraoptic nucleus (SON) neurones were measured using the whole-cell configuration of the patch-clamp technique to examine direct postsynaptic effects of GABAB receptor activation on SON magnocellular neurones.
The selective GABAB agonist baclofen reversibly inhibited voltage-dependent Ca2+ currents elicited by voltage steps from a holding potential of −80 mV to depolarized potentials in a dose-dependent manner. The ED50 of baclofen for inhibiting Ca2+ currents was 1.4 × 10−6 M. Baclofen did not inhibit low threshold Ca2+ currents elicited by voltage steps from −120 to −40 mV.
Inhibition of high threshold Ca2+ currents by baclofen was rapidly and completely reversed by the selective GABAB antagonists, CGP 35348 and CGP 55845A, when the antagonists were added at the molar ratio vs. baclofen of 10 : 1 and 0.01 : 1, respectively. It was also reversed by a prepulse to +150 mV lasting for 100 ms.
The inhibition of Ca2+ currents was abolished when the cells were pretreated with pertussis toxin for longer than 20 h or with N-ethylmaleimide for 2 min. It was also abolished when GDPβS was included in the patch pipette. When GTPγS was included in the patch pipette, baclofen produced irreversible inhibition of Ca2+ currents and this inhibition was again reversed by the prepulse procedure.
The inhibition of N-, P/Q-, L- and R-type Ca2+ channels by baclofen (10−5 M) was 24.1, 10.5, 3.1 and 3.6 %, respectively, of the total Ca2+ currents. Only the inhibition of N- and P/Q-types was significant.
These results suggest that GABAB receptors exist in the postsynaptic sites of the SON magnocellular neurones and mediate selective inhibitory actions on voltage-dependent Ca2+ channels of N- and P/Q-types via pertussis toxin-sensitive G proteins, and that such inhibitory mechanisms may play a role in the regulation of SON neurones by the GABA neurones.
Magnocellular neurones in the supraoptic nucleus (SON) of the hypothalamus that produce and secrete vasopressin or oxytocin are under inhibitory control by GABAergic neurones (Randle & Renaud, 1987; Wuarin & Dudek, 1993) that make direct synaptic contact (Decavel & Van den Pol, 1990). The inhibitory actions of GABA in the SON have been thought to be exerted primarily by fast IPSPs mediated by GABAA receptors (Wuarin & Dudek, 1993). Several lines of evidence suggest that GABAB receptors are not present or, if present, do not play a major role in the SON. The selective GABAB agonist baclofen had no effect on action potential firing of guinea-pig SON neurones (Ogata, 1987) and baclofen injected into the SON region did not inhibit the milk-ejection reflex in lactating rats (Voisin, Herbison, Chapman & Poulain, 1996). In cultured oxytocin neurones, neither baclofen nor the GABAB antagonist hydroxysaclofen affected membrane potential and input resistance (Jourdain, Poulain, Theodosis & Israel, 1996). Moreover, in slice patch-clamp recordings of rat SON neurones, there was no slow spontaneous outward current, indicative of K+ current from activation of GABAB receptors (Wuarin & Dudek, 1993). Recently, we and others reported that GABAB receptor activation caused inhibition of spontaneous and electrically evoked excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs) in the SON (Kombian, Zidichoudki & Pittman, 1996; Kabashima, Shibuya, Ibrahim, Ueta & Yamashita, 1997; Mouginot, Kombian & Pittman, 1998). The studies revealed that GABAB receptor antagonists increased synaptic currents through presynaptic mechanisms, indicating that GABAB receptors are present at the presynaptic sites in the SON, and function to suppress the synaptic inputs to SON magnocellular neurones.
The GABAB receptor has recently been cloned, and has proved to be a member of the seven transmembrane receptor superfamily (Kaupmann et al. 1997). In the hippocampus and other regions of the CNS, GABAB receptors are found at postsynaptic as well as at presynaptic sites (Thompson & Gähwiler, 1992). The cellular mechanisms coupled to postsynaptic GABAB receptor activation are well documented: GABAB receptors are known to activate G proteins, which, in turn, cause inhibition of voltage-dependent Ca2+ channels, activation of K+ channels and inhibition of adenylate cyclases (Bowery, 1989). However, to date, there is no evidence as to whether GABAB receptors exist in the postsynaptic site of magnocellular neurones of the SON.
In SON neurones, Ca2+ influx through voltage-dependent Ca2+ channels during action potentials is important in the genesis of the characteristic phasic bursting of vasopressin cells, and in vasopressin and oxytocin release from the dendrites or soma into the SON (Hu & Bourque, 1992). Four distinct subtypes of high threshold Ca2+ currents (N-, P-, L- and R-type) and one type of low threshold Ca2+ currents (T-type) have been identified in the soma of rat SON neurones (Fisher & Bourque, 1995). However, little is known about the regulation of these Ca2+ currents.
The purpose of the present study was to examine whether GABAB receptors are present in the postsynaptic site of the SON and, if so, through what mechanisms they influence SON neurones. For this purpose, we dissociated magnocellular neurones from ‘punch-out’ (1 mm diameter) slice preparations containing the SON and examined the effects of selective GABAB agonists and antagonists on voltage-dependent Ca2+ currents of these neurones by the whole-cell patch-clamp technique.
METHODS
Cell preparations
Rat SON neurones were enzymatically dissociated by a slightly modified method of Ishibashi et al. (Ishibashi & Akaike, 1995). In short, young male Wistar rats weighing 30-80 g (9-25 days old) were stunned by a blow to the back of the neck and rapidly decapitated. The brains were quickly removed and cooled in a bathing medium at 4°C for approximately 1 min. The bathing medium contained (mM): NaCl, 124; KCl, 5; MgSO4, 1.3; KH2PO4, 1.24; CaCl2, 2; NaHCO3, 25.9; and glucose, 10; continuously oxygenated with a mixture of 95 % O2-5 % CO2. A block containing the hypothalamus was cut from the brain and was glued to the stage of vibratome-type slicer (DSK-2000, Kyoto, Japan). Coronal slices of 300 μm thickness were cut from the block and the slices carefully trimmed with a circular punch (inner diameter, 1 mm). The slices were incubated in bathing solution containing pronase (0.05 mg ml−1, Sigma) for 20 min and then in bathing solution containing thermolysin (0.1 mg ml−1, Sigma) for 20 min, at 30°C. The slices were then mechanically dissociated by trituration with fire-polished glass pipettes (tip inner diameter ranging from 250 to 650 μm). When dissociating cells for pertussis toxin (PTX) experiments, in which cells were maintained for up to 24 h, all procedures were carried out under sterilized conditions to avoid bacterial contamination. The purity of the dissociated cells was examined by a previously described immunocytochemical method (Ison et al. 1993) using vasopressin and oxytocin antibodies (Incstar, Stillwater, MN, USA). All cells with a surface area of > 200 μm2 (n= 52) were positively stained with the antibodies. In this study, only cells with a large soma (surface area, > 200 μm2) and dendrites were used.
Electrophysiology
Cells were plated in a culture dish and used more than 5 min later when the cells had attached to the bottom of the dish. Standard perfusion medium (Hepes-buffered solution, HBS) contained (mM): NaCl, 140; KCl, 5; CaCl2, 2; MgCl2, 1; Hepes, 10; and glucose, 11.1 (pH 7.4 adjusted with NaOH). HBS was oxygenated with 100 % O2 throughout the experiments. The electrodes were made with a puller (P-87; Sutter Instrument Co.) from thick-walled borosilicate glass (GD-1.5; Narishige, Tokyo, Japan) and had a final resistance of between 3 and 6 MΩ when filled with the electrode solution. The volume of the recording chamber was 1 ml and the flow rate of the perfusion medium 1.5 ml min−1. The solution level was kept constant by a low pressure aspiration system. Electrophysiological recordings were carried out at a room temperature of 23°C. Whole-cell tight-seal recordings were made from microscopically identified cells. Membrane currents were recorded with a patch-clamp amplifier (AxoPatch 200A; Axon Instruments Inc.) and were digitized using pCLAMP software (version 6.0.3; Axon Instruments Inc.) for subsequent off-line analysis. Data were analysed using AxoGraph software (version 3.5; Axon Instruments Inc.). The pipette solution contained (mM): CsCl, 140; EGTA, 10; CaCl2, 1; MgCl2, 1; Mg-ATP, 2; GTP, 0.3; and Hepes, 10 (pH 7.2 adjusted with Tris base). After making whole-cell access, the perfusion medium was switched to a solution containing (mM): CaCl2, 2; TEA-Cl, 15; 4-aminopyridine (4-AP), 5; NaCl, 125; KCl, 5; Hepes, 10; glucose, 11.1; plus 1 μM tetrodotoxin (pH 7.4 adjusted with NaOH). Voltage-dependent Ca2+ currents were elicited by voltage steps from the holding potential of -80 mV to various depolarized test potentials. Leak and capacitative currents were cancelled by off-line subtraction of Cd2+ (200 μM)-insensitive currents. The sampling rate was 10 kHz. Unless otherwise noted, Ca2+ currents recorded between 5 and 10 ms after depolarizing voltage steps were averaged and used for further analysis. As Ca2+ currents showed run-down (2.5 ± 0.3 % min−1 in 49 neurones), the magnitude of Ca2+ current inhibition was expressed as percentage inhibition of the total currents that were measured just before application of drugs. All chemicals except PTX were added by changing the bath solution with a peristaltic pump, and the time required for the complete change of the solution was estimated to be a few seconds. Because limited diffusion of PTX into brain slices has been reported (Knott, Maguire, Moratalla & Bowery, 1993), we dissociated SON neurones first, and then pretreated them with PTX (obtained from two different commercial sources) in distilled HBS continuously oxygenated with humidified 100 % O2 before measurement of Ca2+ currents.
Statistics
The values in this text are expressed as means ±s.e.m. unless otherwise noted. Student's unpaired t test was used for statistical analysis and P < 0.05 was regarded as significant.
Drugs
CGP 35348 and CGP 55845A were generously provided by Ciba-Geigy (Basel, Switzerland). (±)-Baclofen and nicardipine were purchased from Sigma, tetrodotoxin was from Sankyo (Tokyo, Japan), pertussis toxin was from List Biological Laboratories (Campbell, CA, USA) and Kaken-seiyaku (Tokyo, Japan), all the peptide toxin Ca2+ channel blockers were from Peptide Institute (Osaka, Japan) and other chemicals were from Nacalai tesque (Kyoto, Japan).
RESULTS
Voltage-dependent Ca2+ currents were measured from 288 SON neurones dissociated from SON slices from forty-three rats. SON neurones were readily identified under a phase-contrast microscope by their large soma and attached dendritic processes.
Effects of baclofen on voltage-dependent Ca2+ currents
Figure 1A shows typical examples of voltage-dependent Ca2+ currents elicited from a holding potential of -80 mV to depolarized test potentials (-60 to 30 mV) and the effects of baclofen (10−5 M) on these currents. Baclofen inhibited Ca2+ currents with a clear ‘kinetic slowing’ of the currents as well as ‘steady-state inhibition’. Current-voltage relations of Ca2+ currents measured before, during and after baclofen application are shown in Fig. 1B. In ten of twenty-three neurones examined, the I-V curve shifted towards negative voltage after baclofen washout. Voltage dependency of the inhibition of Ca2+ currents by baclofen was calculated from the current-voltage relation of the inhibition obtained from fifteen neurones (Fig. 1C). Baclofen significantly inhibited Ca2+ currents elicited by the test potentials ranging from -20 to 30 mV but had little or no effects on the Ca2+ currents elicited by a voltage step to -60 to -30 mV (Fig. 1C). To demonstrate more clearly the effect of baclofen on the low threshold Ca2+ currents, effects of baclofen on the Ca2+ currents elicited by voltage steps from -120 to -40 mV were observed (Fig. 1D). Such low threshold Ca2+ currents showed rapid inactivation: the peak inward currents during application of baclofen (10−5 M) were 106.8 ± 6.6 % of control (n= 7; no significant difference).
Figure 1. Time courses and current-voltage relations of Ca2+ currents measured before, during and after application of baclofen.
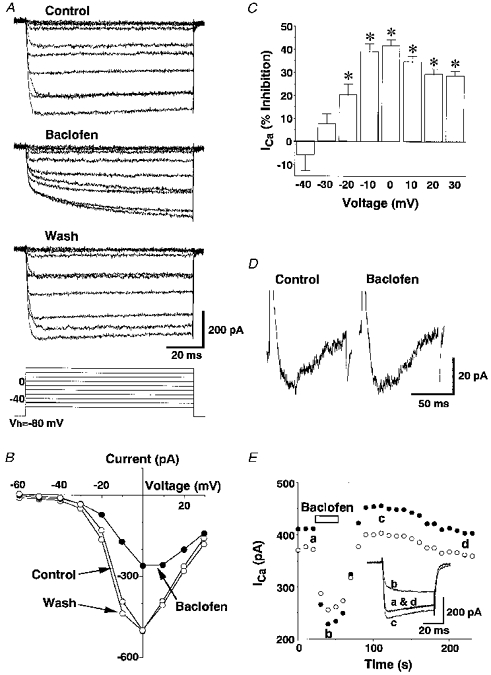
A, representative traces of leak-subtracted Ca2+ currents elicited by voltage steps to the command potentials (Vc) of -60 to 30 mV (10 mV intervals, 90 ms) from the holding potential (Vh) of -80 mV before, during and after application of 10−5 M baclofen. The baclofen response was obtained 30 s after application of baclofen and the wash response was obtained 1 min after removal of baclofen. B, the current-voltage relation of Ca2+ currents before, during and after baclofen (10−5 M) application, calculated from the results shown in A. C, the voltage dependency of baclofen (10−5 M)-induced inhibition of Ca2+ currents (ICa) obtained from 15 neurones. The asterisks represent significant inhibition (P < 0.05). The averaged Ca2+ currents evoked by the voltage step to 0 mV were 398.2 ± 30.4 pA. D, representative traces of Ca2+ currents elicited by voltage steps from -120 mV to -40 mV (for 90 ms) in the absence (Control) and presence of baclofen (10−5 M). Similar results were obtained from 6 other experiments. E, a representative time course of baclofen (10−5 M)-induced inhibition of Ca2+ currents. Ca2+ currents between 5 and 10 ms (•) and between 40 and 45 ms (○) after depolarizing voltage steps were averaged. The Vc was 0 mV and the Vh was -80 mV. Inset, Ca2+ current traces obtained at times a-d are superimposed.
The time course of inhibition of the high threshold Ca2+ currents by baclofen was examined by applying a voltage command from -80 mV to 0 mV (where the inhibition by baclofen was maximal) at 10 s intervals (Fig. 1E). Ca2+ currents measured between 5 and 10 ms after the voltage step were reduced rapidly upon application of baclofen and recovered rapidly upon withdrawal of baclofen. A similar time course was observed when Ca2+ currents were measured between 40 and 45 ms after the voltage step, although the magnitude of the inhibition was smaller. During the course of the recovery, Ca2+ currents often (25 of 64 tests) showed a rebound increase for several minutes before they returned to the pre-inhibition level, as has been observed with Ca2+ currents of the neuroblastoma/glioma cell line NG108-15 in response to an opioid agonist (Kasai, 1991) (Fig. 1E). For further analysis, Ca2+ currents elicited by a voltage command from -80 mV to 0 mV lasting for 50 ms was used.
The dose-response relationship of baclofen-induced inhibition of Ca2+ currents was studied using voltage-step commands to 0 mV at 10 s intervals (Fig. 2A). Baclofen at concentrations between 10−7 and 10−4 M inhibited the Ca2+ currents and the maximal inhibition was observed at 10−4 M. No inhibition was observed at 10−8 M. The EC50 and the maximum values of the baclofen-induced inhibition were estimated to be 9.3 × 10−7 M and 43.7 %, respectively, from the dose-response curve calculated using the Hill equation (Fig. 2B). Baclofen (10−5 or 10−4 M) inhibited Ca2+ currents elicited by the voltage step from -80 mV to 0 mV in all 264 SON neurones examined. In SON neurones from rats aged 9-13, 14-18 and 19-25 days, amplitudes of Ca2+ currents were 353.9 ± 18.9, 375.3 ± 16.8 and 371.3 ± 29.9 pA, respectively, and magnitudes of Ca2+ current inhibition by baclofen (10−5 M) were 42.9 ± 1.8, 39.0 ± 1.6 and 38.3 ± 1.9 %, respectively. There was no significant difference in the two parameters between the three groups, suggesting that voltage-dependent Ca2+ channels and function of GABAB receptors in SON neurones do not undergo major changes during the postnatal period.
Figure 2. Dose-dependent inhibition of Ca2+ currents by baclofen.
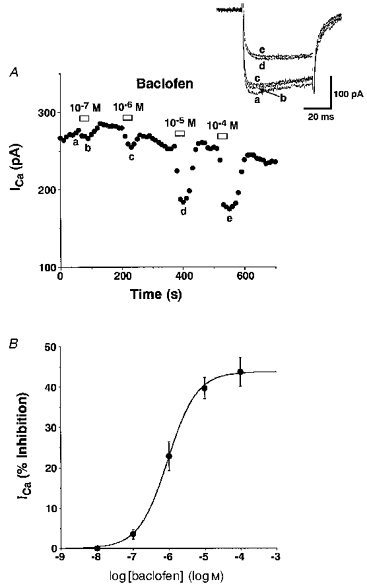
A, a representative time course of dose-dependent inhibition of Ca2+ currents by increasing concentrations of baclofen. Inset, Ca2+ current traces obtained at times a-e are superimposed. The Vc was 0 mV and the Vh was -80 mV. B, the dose-response curve of the baclofen-induced inhibition (percentage of the total currents measured just before each baclofen application) of Ca2+ currents. The curve was calculated by the least-squares method using the Hill equation. The data are shown as means ±s.e.m. of the values obtained from 4 (10−8 M), 4 (10−7 M), 14 (10−6 M), 25 (10−5 M) and 9 (10−4 M) experiments. The averaged Ca2+ currents were 408.5 ± 34.0 pA (n= 25).
Effects of selective GABAB antagonists on inhibition of Ca2+ currents by baclofen
The inhibition of Ca2+ currents by baclofen was rapidly reversed by addition of the selective and competitive GABAB antagonist CGP 35348 in a dose-dependent manner. A complete reversal of inhibition of Ca2+ currents by 10−5 M baclofen was obtained when CGP 35348 was used at 10−4 M (Fig. 3A). CGP 35348 (10−4 M) by itself did not significantly affect Ca2+ currents in eleven independent tests (the change was 1.1 ± 1.1 % of the total Ca2+ current). The ED50 of CGP 35348 in reversing baclofen-induced inhibition was estimated to be 1.1 × 10−5 M from the dose-response curve (Fig. 3B). A more recently introduced GABAB antagonist, CGP 55845A, also completely reversed inhibition of Ca2+ currents by 10−5 M baclofen when CGP 55845A was used at 10−7 M. The ED50 of CGP 55845A in reversing baclofen-induced inhibition was estimated to be 1.1 × 10−8 M.
Figure 3. Reversal of baclofen-induced inhibition of Ca2+ currents by CGP 35348 and CGP 55845A.
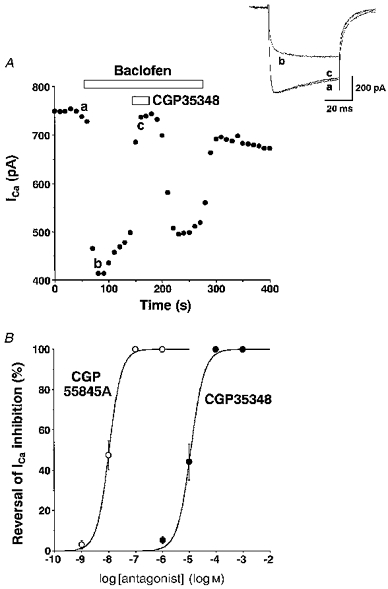
A, a representative time course of inhibition of Ca2+ currents by baclofen (10−5 M) and its reversal by CGP 35348 (10−4 M). The Vc was 0 mV and the Vh was -80 mV. Inset, Ca2+ current traces obtained at times a-c are superimposed. B, dose-dependent reversal of baclofen(10−5 M)-induced inhibition of Ca2+ currents by CGP 35348 and CGP 55845A. The data are shown as means ±s.e.m. of the values obtained from 4-7 experiments. The averaged Ca2+ currents in cells used for CGP 35348 and CGP 55845A experiments were 464.2 ± 130.7 (n= 9) and 409.2 ± 25.2 pA (n= 17), respectively.
Effects of prepulse on inhibition of Ca2+ currents by baclofen
Figure 4A illustrates representative effects of baclofen (10−5 M) on Ca2+ currents with and without a prepulse to +150 mV for 100 ms. The prepulse potently reversed the majority of baclofen-induced kinetic slowing of Ca2+ currents; however, inhibition by baclofen appeared gradually during the test pulse. The inhibition of Ca2+ currents in the third command was similar to that observed in the first command, indicating that the effect of prepulse was entirely reversible. The time and voltage dependencies of the effects of prepulses were examined by changing the prepulse voltage in 20 mV increments from -70 to 150 mV (Fig. 4B), the interval between the prepulse and the test command from 0 to 115 ms (Fig. 4C), and the duration of the prepulse from 5 to 115 ms (Fig. 4D). The prepulses to 0 mV or higher voltages produced significant reversal compared with inhibition by baclofen observed without a prepulse and the magnitude of reversal reached a plateau at around +70 mV (Fig. 4B), indicating that the reversal is due to voltage-dependent relief of baclofen-induced inhibition of Ca2+ currents but not to Ca2+ entry-dependent inactivation of the currents. A maximum reversal (91.3 ± 0.4 %, n= 4) was obtained with 0 ms interval between the prepulse and the test pulse and increasing the interval steeply reduced the magnitude of the reversal (Fig. 4C). A prepulse of approximately 100 ms produced a maximum reversal and shortening the duration also reduced the magnitude of the reversal (Fig. 4D). From these relations, it appeared that the prepulse to +150 mV for 100 ms with 0 ms interval produced a maximum reversal of baclofen-induced inhibition of Ca2+ currents.
Figure 4. Effects of prepulses on baclofen-induced inhibition of Ca2+ currents.
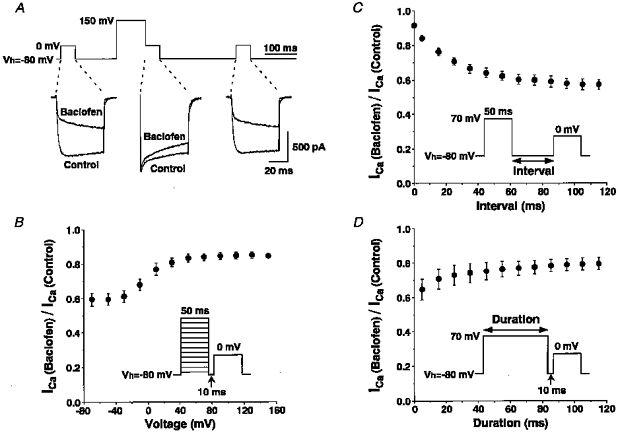
A, 3 consecutive command pulses from -80 mV to 0 mV were added and only the middle command was preceded by a prepulse to 150 mV for 100 ms. Two leak-subtracted current traces obtained in the presence and the absence of baclofen 10−5 M are superimposed. Similar reversal by the prepulse of baclofen-induced inhibition was observed in 7 other SON neurones. B, effects of changing the voltage of prepulse on baclofen-induced inhibition of Ca2+ currents. As shown in the inset, Ca2+ currents were measured with prepulses to various depolarizing voltages from -70 to 150 mV for 50 ms with an interval of 10 ms before the test pulse. Ca2+ currents in the presence of baclofen (10−5 M) were normalized by those in the absence of baclofen and plotted against the prepulse voltages (n= 4). C, effects of changing the interval between the prepulse and the test pulse on baclofen-induced inhibition of Ca2+ currents (n= 4). D, effects of changing the duration of the prepulse on baclofen-induced inhibition of Ca2+ currents (n= 4).
Effects of GTP and GDP analogues on inhibition of Ca2+ currents by baclofen
Effects of baclofen on Ca2+ currents were analysed using a pipette containing 0.3 mM GTPγS, a non-hydrolysable GTP analogue (Fig. 5A). Inclusion of GTPγS in the pipette rendered baclofen (10−5 M)-induced inhibition of Ca2+ currents irreversible, as previously shown in dorsal root ganglion cells (Dolphin, 1995), without significantly changing the magnitude (43.6 ± 6.4 %) or onset of inhibition (20.8 ± 5.2 % inhibition in 10 s after baclofen application, as compared with 24.8 ± 4.6 % without GTPγS) (n= 4). Ca2+ currents measured 1 min after baclofen removal were 39.0 ± 7.2 % of the total Ca2+ currents measured before baclofen application (451.8 ± 130.5 pA, n= 4). The prepulse procedure greatly reduced the inhibition observed with GTPγS in a rapidly reversible manner (Fig. 5A). When Ca2+ currents were measured without adding baclofen, Ca2+ currents decreased progressively (Fig. 5B) and reached a plateau in 3-5 min after whole-cell access was gained. The maximum decrease measured after a plateau was reached was 41.6 ± 3.5 % of the initial Ca2+ currents (n= 4). The decreased Ca2+ currents were again increased by the prepulse procedure. The time course of Ca2+ currents observed with GTPγS showed both kinetic slowing and steady-state inhibition (Fig. 5B, inset) as observed in the presence of baclofen. After Ca2+ currents reached a plateau, application of baclofen slightly decreased the Ca2+ currents to 47.0 ± 4.4 % of the total currents (n= 4), but the effect of baclofen was not significant. By contrast, baclofen did not cause significant inhibition of Ca2+ currents when GDPβS, a non-hydrolysable GDP analogue, was included in the pipette. The inhibition by baclofen with GDPβS in the pipette was 2.5 ± 1.6 % of the total Ca2+ current of 534.8 ± 122.0 pA (n= 4). Ca2+ current recorded with GDPβS was stable after whole-cell access was gained but showed run-down of 1.9 ± 0.9 % min−1 (n= 6).
Figure 5. Effects of including GTPγS in the patch pipette on baclofen-induced inhibition of Ca2+ currents.
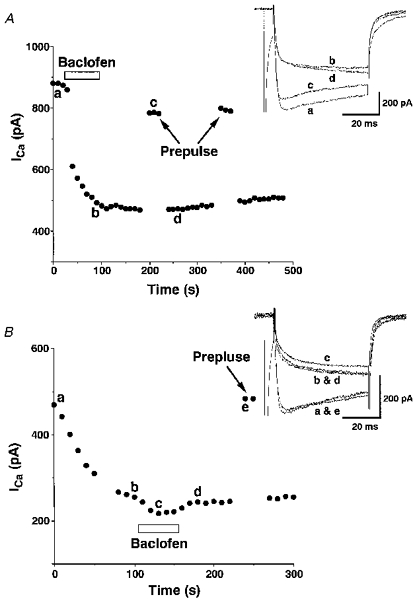
A, a representative time course of Ca2+ currents in response to baclofen (10−5 M) obtained with GTPγS (0.3 mM) included in the patch pipette. The first Ca2+ current shown in this figure was obtained immediately after gaining whole-cell access. The Vc was 0 mV and the Vh was -80 mV. The prepulse was to +150 mV from Vh for 100 ms with a 5 ms interval. Similar results were obtained from 3 other SON neurones. Inset, Ca2+ current traces obtained at times a-d are superimposed. B, a representative time course of spontaneous inhibition of Ca2+ currents observed with GTPγS (0.3 mM) included in the patch pipette. The first Ca2+ current shown in this figure was obtained 1 min after gaining whole-cell access. Similar results were obtained from 3 other SON neurones. Inset, Ca2+ current traces obtained at times a-e are superimposed.
Effects of pretreatment with PTX and N-ethylmaleimide on inhibition of Ca2+ currents by baclofen
Pretreatment with PTX is known to block the inhibition of Ca2+ channels by G proteins of the Gi superfamily (Dolphin, 1995). When cells were pretreated with PTX for 6-20 h, inhibition of Ca2+ currents by baclofen (10−5 M) in fifteen of twenty cells became smaller than 26.6 %, which is the mean - 1 s.d. of Ca2+ current inhibition by baclofen (10−5 M) obtained in Fig. 2B (Fig. 6A). Pretreatment for 20 h or longer abolished the inhibition by baclofen in all seven cells examined. By contrast, Ca2+ currents measured more than 20 h after dissociation without PTX pretreatment were still susceptible to the inhibition by baclofen (n= 7) and the magnitude of inhibition was 38.1 ± 3.0 %. The time course and the amplitude of Ca2+ currents were unaffected by PTX pretreatment or an incubation for longer than 20 h: the total Ca2+ currents were 472.9 ± 96.2 pA in the seven cells pretreated with PTX for longer than 20 h and 454.6 ± 64.7 pA in the seven cells maintained in the PTX-free solution for longer than 20 h.
Figure 6. Effects of pretreatment with pertussis toxin (PTX) or N-ethylmaleimide (NEM) on the inhibition of Ca2+ currents by baclofen.
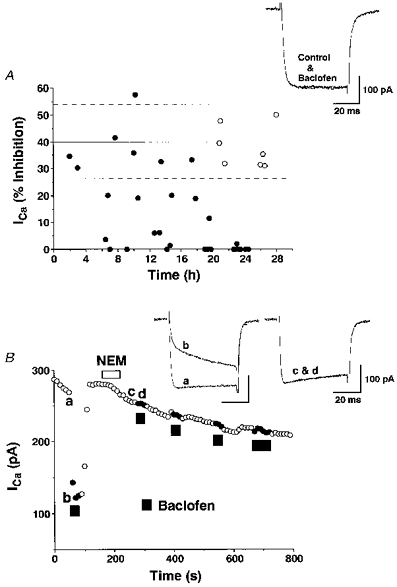
A, SON neurones were dissociated and then incubated in sterilized standard solution containing PTX (1 μg ml−1) gassed with humidified 100 % O2. The percentage inhibition of Ca2+ currents by baclofen (10−5 M) is plotted against the time during which cells were incubated in the PTX-containing solution (•). Control cells were dissociated and incubated in PTX-free standard solution for the time indicated (○). The continuous and dashed lines represent means and ±1 s.d., respectively, of the inhibition of Ca2+ currents by baclofen (10−5 M) taken from the results shown in Fig. 2B. The Vc was 0 mV and the Vh was -80 mV. Inset, representative traces of Ca2+ currents before and during baclofen application in a PTX-pretreated SON neurone are shown. B, a representative time course of Ca2+ currents in response to baclofen (10−5 M) before and after NEM (10−4 M) treatment. Similar results were obtained in 6 other SON neurones. Inset, Ca2+ current traces obtained at times a-d are superimposed.
N-Ethylmaleimide (NEM), a sulfhydryl alkylating agent, was reported to abolish inhibition of Ca2+ currents by PTX-sensitive G proteins (Shapiro, Wollmuth & Hille, 1994). Pretreatment with NEM (10−4 M) for 1 or 2 min eliminated the inhibition of Ca2+ currents by baclofen (10−5 M) and the effect of NEM was irreversible (Fig. 6B). NEM significantly accelerated run-down of Ca2+ currents to 12.9 ± 4.1 % min−1 (n= 7) as previously reported (Shapiro et al. 1994). When cells were pretreated with NEM (5 × 10−5 M) for 2 min, baclofen (10−5 M) caused inhibition of Ca2+ currents by 15.7 ± 8.1 % (n= 3).
Effects of blockers of Ca2+ channels on inhibition by baclofen
Rat SON neurones possess several types of high-threshold Ca2+ currents, namely, N-, P-, L-type, and other remaining (R-type) Ca2+ currents (Fisher & Bourque, 1995). We examined which type of the Ca2+ currents are influenced by baclofen by blocking each type of currents with the selective inhibitors, 10−6 M ω-conotoxin GVIA (N-type), 10−7 M ω-agatoxin IVA plus 10−6 M ω-conotoxin MVIIC (P/Q-type) and 10−5 M nicardipine (L-type) (Fig. 7A and B). As reported by others (Fisher & Bourque, 1995), the block of Ca2+ currents by ω-conotoxin GVIA or by a combination of ω-agatoxin IVA and ω-conotoxin MVIIC was irreversible, while the block by nicardipine was partially reversible. For this reason, the inhibitors were added in the order ω-conotoxin GVIA, ω-agatoxin IVA-ω-conotoxin MVIIC, and nicardipine. Both Ca2+ currents and the inhibition by baclofen were greatly reduced after application of ω-conotoxin GVIA, and moderately reduced after application of ω-agatoxin IVA-ω-conotoxin MVIIC, whereas they were largely unaffected by application of nicardipine (Fig. 7A). The Ca2+ currents sensitive to the inhibitors and the percentage of the inhibition by baclofen are summarized in Fig. 7B, where Ca2+ currents of N-, P/Q-, L- and R-types were 41.6 ± 3.4 % (n= 14), 21.0 ± 2.9 % (n= 14), 16.1 ± 3.2 % (n= 12), and 19.8 ± 3.4 % (n= 11), respectively, of the total current, and the Ca2+ currents of each type inhibited by baclofen were 24.1 ± 3.2 % (n= 14), 10.5 ± 1.9 % (n= 12), 3.1 ± 1.4 % (n= 12), and 3.6 ± 0.7 % (n= 9), respectively, of the total current. Only the inhibition of Ca2+ currents of N- and P/Q-types was significant (P < 0.05).
Figure 7. Analysis of Ca2+ channel subtypes susceptible to inhibition by baclofen.
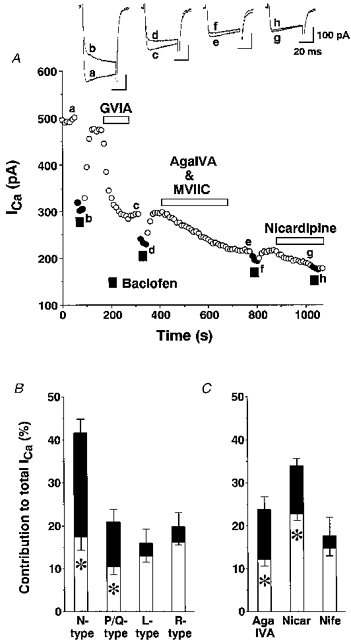
A, a representative time course of Ca2+ currents in response to baclofen (10−5 M) before and after blockade of N-, P/Q- and L-type Ca2+ channels. Each type of Ca2+ channel was blocked by the selective blockers for N-, P/Q-, and L-types, ω-conotoxin GVIA (10−6 M), a combination of ω-agatoxin IVA (AgaIVA, 10−7 M) and ω-conotoxin MVIIC (10−6 M), and nicardipine (10−5 M), respectively. The Vc was 0 mV and the Vh was -80 mV. B, fractional components of N-, P/Q-, L- and R-types of Ca2+ channels and of those inhibited by baclofen (4). The asterisks indicate significance (P < 0.05). C, fractional components of P/Q- and L-types of Ca2+ channels and of those inhibited by baclofen (4) examined by applying ω-agatoxin IVA (10−6 M), nicardipine (Nicar, 10−5 M) or nifedipine (Nife, 10−5 M) as a first blocker.
The contribution of P/Q- and L-type Ca2+ currents to the total Ca2+ current and baclofen-induced inhibition was further examined by blocking each type of Ca2+ current. ω-Agatoxin IVA at 10−6 M was used to block P/Q-type currents because ω-conotoxin MVIIC blocks N-type currents as well (McDonough, Swartz, Mintz, Boland & Bean, 1996). ω-Agatoxin IVA at 10−6 M blocked Ca2+ currents by 23.8 ± 3.0 % (n= 5) and baclofen-induced inhibition sensitive to block by ω-agatoxin IVA was 11.5 ± 1.7 % of the total Ca2+ currents (n= 4), both of which were in good agreement with the results obtained when ω-Agatoxin IVA and ω-conotoxin MVIIC were added after ω-conotoxin GVIA. Nicardipine at 10−5 M, blocked the total Ca2+ currents by 33.9 ± 1.9 % (n= 12), and baclofen-induced inhibition sensitive to block by nicardipine was significant (11.1 ± 1.6 %, n= 12). On the other hand, another dihydropyridine antagonist, nifedipine, at 10−5 M inhibited Ca2+ currents by 17.7 ± 4.3 % (n= 5) and baclofen-induced inhibition sensitive to block by nifedipine was 2.8 ± 1.9 % (not significant, n= 5), which is consistent with the results obtained when nicardipine was added after blocking N- and P/Q-type Ca2+ currents. These results suggest that nicardipine produced non-selective block of N- or P/Q-type Ca2+ currents in SON neurones.
DISCUSSION
The present study provides the first direct evidence that functional GABAB receptors are present in the postsynaptic sites of SON magnocellular neurones. Our present results, together with the results that SON neurones receive massive synaptic inputs from GABA neurones (Decavel & Van den Pol, 1990) and that GABAB receptors are present also in the presynaptic site of the SON (Kombian et al. 1996; Kabashima et al. 1997), indicate the major importance of GABAB receptors in regulation of the SON.
The pharmacological properties of postsynaptic GABAB receptors in the SON
The effective doses of baclofen in inhibiting Ca2+ currents in SON neurones were similar to those observed with other CNS neurones such as nucleus tractus solitarii neurones (Rhim, Toth & Miller, 1996). The selective and competitive GABAB antagonist CGP 35348 completely reversed baclofen-induced inhibition of Ca2+ currents at the dose ratio vs. baclofen of 10 : 1, whereas another selective but more potent antagonist, CGP 55845A, did so at the dose ratio of 0.01 : 1, indicating that the inhibition of Ca2+ currents by baclofen is mediated entirely through GABAB receptors. The result is also in good agreement with previous reports that CGP 55845A is approximately three orders of magnitude more potent than CGP 35348 in pre- and post-synaptic GABAB receptors in the hippocampus (Davies, Pozza & Collingridge, 1993). However, the report that inhibition of R-type Ca2+ channels by baclofen is reversed by CGP 55845A but not by CGP 35348 in thalamocortical neurones (Guyon & Leresche, 1995) indicates the presence of different subclasses of postsynaptic GABAB receptors. Two molecular forms of GABAB receptors have recently been cloned (Kaupmann et al. 1997) and distinct subclasses were suggested for GABAB receptors from pharmacological data obtained from various neuronal preparations (see Bonanno & Raiteri, 1993).
The mechanism of inhibition of Ca2+ channels
Activation of GABAB receptors leads to inhibition of Ca2+ channels through G proteins and such inhibition can be partially or entirely removed by applying a depolarizing prepulse (Dolphin, 1995). The mechanism underlying the phenomenon is believed to be mediated, at least in part, by a membrane-delimited interaction between voltage-dependent Ca2+ channels and G proteins (Pollo, Lovallo, Sher & Carbone, 1992). In some neuronal preparations, the steady-state inhibition of Ca2+ channels has been ascribed to voltage-independent mechanisms involving protein kinases that are downstream of the G protein activation. For example, in baclofen-induced inhibition of Ca2+ currents observed in sensory neurones, staurosporine, a protein kinase C inhibitor, selectively eliminated steady-state inhibition while a depolarizing prepulse selectively eliminated kinetic slowing of the currents (Diversé-Pierluissi, Goldsmith & Dunlap, 1995). In the present study, the prepulse reversibly and potently occluded the majority of both kinetic slowing and steady-state inhibition of Ca2+ currents induced by baclofen, indicating that the membrane-delimited inhibition of Ca2+ channels mediated by G proteins accounts for the major inhibitory mechanism exerted upon GABAB receptor activation in SON neurones. The suggestion is supported by the observation that inclusion of GTPγS in the pipette closely mimicked the baclofen-induced inhibition in a prepulse-sensitive manner.
Inhibition of neuronal Ca2+ channels is mediated through either PTX-sensitive or -insensitive G proteins (Hille, 1994). To investigate which G protein pathway the GABAB receptor-mediated inhibitory action in the SON utilizes, we examined effects of baclofen in SON neurones pretreated with PTX or NEM. The results that the baclofen-induced inhibition of Ca2+ currents was abolished when the cells were pretreated with PTX are consistent with results obtained from other types of neurones (Kobrinsky, Pearson & Dolphin, 1994; Rhim et al. 1996) and indicate that postsynaptic GABAB receptors in the SON also couple with G proteins of the Gi/Go superfamily. As for PTX sensitivity of receptor-mediated modulation of SON neurones, it has been reported that intracerebroventricular PTX prevented morphine-induced inhibition of spontaneous electrical activity of oxytocin neurones (Pumford, Leng & Russell, 1993). Because the study was conducted in vivo, the site of the PTX-sensitive mechanism was unclear. Our present results extended their results and revealed that the soma or dendrites of SON neurones possess such a mechanism. The inhibition of Ca2+ currents induced by baclofen persisted in some SON neurones for more than 10 h, even though we used a relatively high concentration of PTX and ensured the access of PTX to the plasma membrane by directly adding PTX into dissociated neurones. In other preparations, PTX eliminated baclofen-induced inhibition within 6 h in adrenal chromaffin cells at the same concentration as we used in the present study (Doroshenko & Neher, 1991). It is also reported that PTX prevented inhibition by baclofen within 16 h in dorsal root ganglion cells (Kobrinsky et al. 1994). In this regard, the inhibition by baclofen in the SON neurones seems to be relatively resistant to PTX and, therefore, caution should be taken to investigate the effect of PTX in these cells. By contrast with the slow time course of the effect of PTX, NEM quite rapidly eliminated baclofen-induced inhibition of Ca2+ currents in SON neurones. Since it is reported that NEM selectively uncoupled inhibition of Ca2+ channels mediated by PTX-sensitive G-proteins in rat sympathetic neurones (Shapiro et al. 1994), NEM would be a useful tool to analyse involvement of PTX-sensitive G proteins in receptor-mediated modulation of ion channels in brain slice preparations, where the effect of PTX cannot be readily obtained because of the limited diffusion of PTX (Knott et al. 1993).
The Ca2+ channel subtypes susceptible to inhibition by GABAB receptor activation
In the present study, four distinct subtypes of high threshold Ca2+ currents were identified in rat SON neurones by the use of the selective inhibitors of Ca2+ channels. The contribution of the four subtypes to the total Ca2+ currents of SON neurones was in good agreement with the previous report that N-, P- and L-type currents were 39, 20 and 23 %, respectively, of the total inactivating high threshold Ca2+ currents (Fisher & Bourque, 1995). The present results indicate that, among the high-threshold Ca2+ channels of rat SON neurones, only N- and P/Q-type Ca2+ channels receive inhibitory control of GABAB receptors and that N-type channels are inhibited to a greater extent. The lack of effect of baclofen on the low-threshold Ca2+ channels suggests that T-type and a novel low threshold L-type channels found in SON neurones (Fisher & Bourque, 1995) are insensitive to GABAB receptor activation. Although GABAB receptors inhibit different types of neuronal Ca2+ in different preparations, the most common targets of the modulation by GABAB receptors appear to be N- and P/Q-type Ca2+ channels (see Rhim et al. 1996). This is consistent not only with the present results but also with results obtained from cells expressing a subunits of cloned neuronal Ca2+ channels which indicate that Ca2+ currents in cells expressing α1A (P/Q-type) or α1B (N-type) were inhibited by neurotransmitters, whereas those in cells expressing α1C (L-type) or α1E (R-type) were unresponsive (Toth, Shekter, Ma, Philipson & Miller, 1996; Zhang, Ellinor, Aldrich & Tsien, 1996).
Physiological significance of Ca2+ channel modulation by GABAB receptors
N- and P/Q-type Ca2+ channels play a major role in triggering transmitter release in axon terminals (Turner, Adams & Dunlap, 1993) and these Ca2+ channels are involved in baclofen-induced suppression of synaptic transmission (Doze, Cohen & Madison, 1995). However, it is unlikely that such mechanisms function in the axon terminals of SON neurones, because voltage-dependent Ca2+ currents in the terminals are reported to be insensitive to baclofen (Zhang & Jackson, 1995), although N- and P/Q-type Ca2+ channels have been found in neurosecretosomes obtained from the neural lobe (see Fisher & Bourque, 1996). These reports, together with the present results, suggest that GABAB receptors are confined to the soma or dendrites of SON. Such an inhibitory mechanism could regulate somatodendritic release of vasopressin and oxytocin inside the SON, which has been observed in response to osmotic and various other stimuli, and which critically depends on Ca2+ influx (Shibuya et al. 1997). Furthermore, there are indications that Ca2+ entry through voltage-dependent Ca2+ channels during action potentials importantly contributes to the electrophysiological function of SON neurones. Ca2+ influx is required for the expression of spike broadening, which occurs during the phasic bursts characteristic of magnocellular vasopressin neurones (Kirkpatrick & Bourque, 1991), that the current underlying the depolarizing after-potential carried by Ca2+ influx promotes burst initiation by forming plateau potential and that Ca2+-activated K+ conductance regulates the steady-state firing of SON neurones (Andrew & Dudek, 1984). Taken together, inhibition of N- and P/Q-type Ca2+ channels by GABAB receptors may modulate the Ca2+ influx during action potentials and thereby influence electrophysiological and other functions of SON neurones.
GABAB receptor-mediated postsynaptic actions in the SON
In other CNS preparations, postsynaptic GABAB receptor activation causes a large increase in K+ conductance and resultant hyperpolarization, and this appears to be a common mechanism whereby postsynaptic GABAB receptors inhibit neurones at the level of the soma or dendrites (Gage, 1992). However, several studies in the SON have demonstrated that GABAB receptor agonists did not cause hyperpolarization, or induce slow currents due to an increase in K+ conductance in these neurones (see Kabashima et al. 1997). The present results suggest instead that postsynaptic GABAB receptors in the SON selectively couple with voltage-dependent Ca2+ channels but not with K+ channels. In this regard, the results obtained from SON neurones show a clear contrast with results obtained from hippocampal CA3 neurones that baclofen increased K+ permeability but did not decrease Ca2+ permeability (Gähwiler & Brown, 1985). The difference between SON and the other CNS neurones could be explained if the population of ion channels sensitive to GABAB receptors is different. The K+ currents activated by GABAB receptors are inward rectifying K+ currents and transient K+ currents (A currents) (Gage, 1992). Of these two currents, the former is responsible for hyperpolarization or slow IPSPs observed in the soma of various neuronal preparations. SON neurones possess three distinct types of outward rectifying K+ currents, namely, delayed rectifying K+ currents, A currents and Ca2+-activated K+ currents (see Mason, Cobbett, Inenaga & Legendre, 1988), whereas there is no report of a G protein-activated inward rectifying K+ (GIRK) current. One possible explanation for the lack of hyperpolarization in response to a GABAB agonist in the SON is that these neurones do not express many functional inward rectifying K+ channels sensitive to activation by GABAB receptors.
In conclusion, GABAB receptor activation leads to the inhibition of voltage-dependent Ca2+ currents of rat SON neurones. The result clearly indicates that there are functional GABAB receptors in the postsynaptic sites of SON neurosecreotry cells. The postsynaptic GABAB receptors may play a role in the regulation of the function of these neurones.
Acknowledgments
This work was supported by research grants from the Ministry of Education, Science and Culture, Japan to I. S. (09470020) and H. Y. (07507004 and 08457022).
References
- Andrew RD, Dudek FE. Intrinsic inhibition in magnocellular neuroendocrine cells of rat hypothalamus. The Journal of Physiology. 1984;353:171–185. doi: 10.1113/jphysiol.1984.sp015330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno G, Raiteri M. Multiple GABAB receptors. Trends in Pharmacological Sciences. 1993;14:259–261. doi: 10.1016/0165-6147(93)90124-3. [DOI] [PubMed] [Google Scholar]
- Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends in Pharmacological Sciences. 1989;10:401–407. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Davies CH, Pozza MF, Collingridge GL. CGP 55845A: a potent antagonist of GABAB receptors in the CA1 region of rat hippocampus. Neuropharmacology. 1993;32:1071–1073. doi: 10.1016/0028-3908(93)90073-c. 10.1016/0028-3908(93)90073-C. [DOI] [PubMed] [Google Scholar]
- Decavel C, Van den Pol A. GABA: a dominant neurotransmitter in the hypothalamus. Journal of Comparative Neurology. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Diversé-Pierluissi M, Goldsmith PK, Dunlap K. Transmitter-mediated inhibition of N-type calcium channels in sensory neurons involves multiple GTP-binding proteins and subunits. Neuron. 1995;14:191–200. doi: 10.1016/0896-6273(95)90254-6. 10.1016/0896-6273(95)90254-6. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Voltage-dependent calcium channels and their modulation by neurotransmitters and G proteins. Experimental Physiology. 1995;80:1–36. doi: 10.1113/expphysiol.1995.sp003825. [DOI] [PubMed] [Google Scholar]
- Doroshenko P, Neher E. Pertussis-toxin-sensitive inhibition by (-)baclofen of Ca signals in bovine chromaffin cells. Pflügers Archiv. 1991;419:444–449. doi: 10.1007/BF00370786. [DOI] [PubMed] [Google Scholar]
- Doze VA, Cohen GA, Madison DV. Calcium channel involvement in GABAB receptor-mediated inhibition of GABA release in area CA1 of the rat hippocampus. Journal of Neurophysiology. 1995;74:43–53. doi: 10.1152/jn.1995.74.1.43. [DOI] [PubMed] [Google Scholar]
- Fisher TE, Bourque CW. Voltage-gated calcium currents in the magnocellular neurosecretory cells of the rat supraoptic nucleus. The Journal of Physiology. 1995;486:571–580. doi: 10.1113/jphysiol.1995.sp020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher TE, Bourque CW. Calcium-channel subtypes in the somata and axon terminals of magnocellular neurosecretory cells. Trends in Neurosciences. 1996;19:440–444. doi: 10.1016/0166-2236(96)10034-5. [DOI] [PubMed] [Google Scholar]
- Gage PW. Activation and modulation of neuronal K+ channels by GABA. Trends in Neurosciences. 1992;15:46–51. doi: 10.1016/0166-2236(92)90025-4. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Brown DA. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proceedings of the National Academy of Sciences of the USA. 1985;82:1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Leresche N. Modulation by different GABAB receptor types of voltage-activated calcium currents in rat thalamocortical neurones. The Journal of Physiology. 1995;485:29–42. doi: 10.1113/jphysiol.1995.sp020710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends in Neurosciences. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Hu B, Bourque CW. NMDA receptor-mediated rhythmic bursting activity in rat supraoptic nucleus neurones in vitro. Journal of Physiology. 1992;458:667–687. doi: 10.1113/jphysiol.1992.sp019440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi H, Akaike N. Somatostatin modulates high-voltage-activated Ca2+ channels in freshly dissociated rat hippocampal neurons. Journal of Neurophysiology. 1995;74:1028–1036. doi: 10.1152/jn.1995.74.3.1028. [DOI] [PubMed] [Google Scholar]
- Ison A, Yuri K, Ueta Y, Leng G, Koizumi K, Yamashita H, Kawata M. Vasopressin- and oxytocin-immunoreactive hypothalamic neurones of inbred polydipsic mice. Brain Research Bulletin. 1993;31:405–414. doi: 10.1016/0361-9230(93)90234-3. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Poulain DA, Theodosis DT, Israel JM. Electrical properties of oxytocin neurons in organotypic cultures from postnatal rat hypothalamus. Journal of Neurophysiology. 1996;76:2772–2785. doi: 10.1152/jn.1996.76.4.2772. [DOI] [PubMed] [Google Scholar]
- Kabashima N, Shibuya I, Ibrahim Y, Ueta Y, Yamashita H. Inhibition of spontaneous EPSCs and IPSCs by presynaptic GABAB receptors on rat supraoptic magnocellular neurons. The Journal of Physiology. 1997;503:103–126. doi: 10.1111/j.1469-7793.1997.113bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H. Tonic inhibition and rebound facilitation of a neuronal calcium channel by a GTP-binding protein. Proceedings of the National Academy of Sciences of the USA. 1991;88:8855–8859. doi: 10.1073/pnas.88.19.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Bourque CW. Dual role for calcium in the control of spike duration in rat supraoptic neuroendocrine cells. Neuroscience Letters. 1991;133:271–274. doi: 10.1016/0304-3940(91)90586-i. [DOI] [PubMed] [Google Scholar]
- Knott C, Maguire JJ, Moratalla R, Bowery NG. Regional effects of pertussis toxin in vivo and in vitro on GABAB receptor binding in rat brain. Neuroscience. 1993;52:73–81. doi: 10.1016/0306-4522(93)90183-g. [DOI] [PubMed] [Google Scholar]
- Kobrinsky EM, Pearson HA, Dolphin AC. Low- and high-voltage-activated calcium channel currents and their modulation in the dorsal root ganglion cell line ND7–23. Neuroscience. 1994;58:539–552. doi: 10.1016/0306-4522(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Zidichoudki JA, Pittman QJ. GABAB receptors presynaptically modulate excitatory synaptic transmission in the rat supraoptic nucleus in vitro. Journal of Neurophysiology. 1996;76:1166–1179. doi: 10.1152/jn.1996.76.2.1166. [DOI] [PubMed] [Google Scholar]
- Mason WT, Cobbett P, Inenaga K, Legendre P. Ionic currents in cultured supraoptic neurons: actions of peptides and transmitters. Brain Research Bulletin. 1988;20:757–764. doi: 10.1016/0361-9230(88)90088-3. [DOI] [PubMed] [Google Scholar]
- McDonough SI, Swartz KJ, Mintz IM, Boland LM, Bean BP. Inhibition of calcium channels in rat central and peripheral neurons by omega-conotoxin MVIIC. Journal of Neuroscience. 1996;16:2612–2623. doi: 10.1523/JNEUROSCI.16-08-02612.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouginot D, Kombian SB, Pittman QJ. Activation of presynaptic GABAB receptors inhibits evoked IPSCs in rat magnocellular neurons in vitro. Journal of Neurophysiology. 1998;79:1508–1517. doi: 10.1152/jn.1998.79.3.1508. [DOI] [PubMed] [Google Scholar]
- Ogata N. γ-Aminobutyric acid (GABA) causes consistent depolarization of neurons in the guinea pig supraoptic nucleus due to an absence of GABAB recognition sites. Brain Research. 1987;403:225–233. doi: 10.1016/0006-8993(87)90059-x. [DOI] [PubMed] [Google Scholar]
- Pollo A, Lovallo M, Sher E, Carbone E. Voltage-dependent noradrenergic modulation of ω-conotoxin-sensitive Ca2+ channels in human neuroblastoma IMR32 cells. Pflügers Archiv. 1992;422:75–83. doi: 10.1007/BF00381516. [DOI] [PubMed] [Google Scholar]
- Pumford KM, Leng G, Russell JA. A pertussis toxin-sensitive G protein mediates inhibition by morphine of spontaneous electrical activity of oxytocin neurones in anaesthetized rats. Experimental Brain Research. 1993;94:247–251. doi: 10.1007/BF00230292. [DOI] [PubMed] [Google Scholar]
- Randle JC, Renaud LP. Actions of γ-aminobutyric acid on rat supraoptic nucleus neurosecretory neurones in vitro. Journal of Physiology. 1987;387:629–647. doi: 10.1113/jphysiol.1987.sp016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim H, Toth PT, Miller RJ. Mechanism of inhibition of calcium channels in rat nucleus tractus solitarius by neurotransmitters. British Journal of Pharmacology. 1996;118:1341–1350. doi: 10.1111/j.1476-5381.1996.tb15543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MS, Wollmuth LP, Hille B. Modulation of Ca2+ channels by PTX-sensitive G-proteins is blocked by N-ethylmaleimide in rat sympathetic neurons. Journal of Neuroscience. 1994;14:7109–7116. doi: 10.1523/JNEUROSCI.14-11-07109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya I, Noguchi J, Tanaka K, Harayama N, Inoue Y, Kabashima N, Ueta Y, Hattori Y, Yamashita H. PACAP increases the cytosolic Ca2+ concentration and stimulates somatodendritic vasopressin release in rat supraoptic neurons. Journal of Neuroendocrinology. 1997;10:31–42. doi: 10.1046/j.1365-2826.1998.00168.x. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Gähwiler BH. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. Journal of Physiology. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth PT, Shekter LR, Ma GH, Philipson LH, Miller RJ. Selective G-protein regulation of neuronal calcium channels. Journal of Neuroscience. 1996;16:4617–4624. doi: 10.1523/JNEUROSCI.16-15-04617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TJ, Adams ME, Dunlap K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proceedings of the National Academy of Sciences of the USA. 1993;90:9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin DL, Herbison AE, Chapman C, Poulain DA. Effects of central GABAB receptor modulation upon the milk ejection reflex in the rat. Neuroendocrinology. 1996;63:368–376. doi: 10.1159/000126977. [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Patch-clamp analysis of spontaneous synaptic currents in supraoptic neuroendocrine cells of the rat hypothalamus. Journal of Neuroscience. 1993;13:2323–2331. doi: 10.1523/JNEUROSCI.13-06-02323.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JF, Ellinor PT, Aldrich RW, Tsien RW. Multiple structural elements in voltage-dependent Ca2+ channels support their inhibition by G proteins. Neuron. 1996;17:991–1003. doi: 10.1016/s0896-6273(00)80229-9. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Jackson MB. Properties of the GABAA receptor of rat posterior pituitary nerve terminals. Journal of Neurophysiology. 1995;73:1135–1144. doi: 10.1152/jn.1995.73.3.1135. [DOI] [PubMed] [Google Scholar]


