Abstract
Antioxidant and oxidative enzymes were examined in renal glomeruli and proximal tubules of healthy young rats (10-12 weeks old), and results were related to the superoxide anion generation of these tissues.
Activities of superoxide dismutases, catalase, and glutathione peroxidase were 3- to 6-fold higher in proximal tubules than in glomeruli. Similarly, enzyme levels and mRNA levels of superoxide dismutases and catalase were significantly higher in proximal tubules.
NADH- and NADPH-dependent oxidase activity and xanthine oxidase activity were not different in glomeruli and proximal tubules.
Measurements with lucigenin-enhanced chemiluminescence in vital tissues indicated 10-fold higher rates of superoxide anion in glomeruli than in tubules.
Compared with the young rats, tubules of 8-month-old rats had significantly higher superoxide anion rates and lower superoxide dismutase activity, whereas NADH- and NADPH-dependent oxidase activities were unchanged.
We conclude that considerable differences in the antioxidant-oxidant balance exist between the glomerulus and proximal tubule. Results from experiments using chemiluminescence in vital tissues suggest that changes in the antioxidant-oxidant balance have an effect on oxygen radical levels. The relevance of the observed differences to glomerular and tubulo-interstitial disease remains to be determined, but a greater susceptibility of the glomerulus to oxidant stress might be anticipated.
Oxygen radicals have been implicated as mediators of tissue injury in various diseases. To prevent excessive oxidant stress, eukaryotic cells have evolved a complex antioxidant defense including the superoxide dismutases, catalase and glutathione peroxidases as main components.
In the kidney, antioxidant enzyme expression has been shown to be particularly high compared with several other organs (Marklund, 1984; Lenzen, Drinkgern & Tiedge, 1996). However, despite this high antioxidant enzyme expression, oxidative injury has been reported in several models of renal disease, especially in glomerular diseases, suggesting the glomerulus as a main target for oxygen radical attack. Tubular injury by oxygen radicals has been observed less frequently and appears to be related mainly to the proximal tubule (Shah, 1989; Nath, Fischereder & Hostetter, 1994; Kiyama, Yoshioka, Burr, Kon, Fogo & Ichikawa, 1995).
This raises two important issues. First, are there differences in the antioxidant enzyme expression between glomeruli and proximal tubules? In the available studies (Marklund, 1984; Lenzen et al. 1996), antioxidant enzymes were examined in whole kidney samples, excluding further differentiation between glomeruli and proximal tubules. Second, based on the fact that the oxidant state of a tissue is determined by the balance between radical-generating enzymatic systems and the antioxidant defense (Janssen, van Houten, Borm & Mossman, 1993; Ichikawa, Kiyama & Yoshioka, 1994), are there differences in the oxidative enzyme activities between glomeruli and proximal tubules? With respect to oxidative enzymes, many previous studies have focussed on xanthine oxidase (Greene & Paller, 1991). More recently, components of NADPH- and NADH-dependent oxidases have been identified as potentially important sources of oxygen radicals in mesangial and glomerular epithelial cells (Neale, Ullrich, Ojha, Poczewski, Verhoeven & Kerjaschki, 1993; Jones, Hancock, Jones, Neubauer & Topley, 1995) Yet, no data are available about the activity of these enzymes in the glomerulus and in proximal tubules.
A comprehensive view of the physiological antioxidant- oxidant balance in the glomerulus and proximal tubule would aid in the understanding of oxygen radical-induced injury in glomerular and tubulo-interstitial disease. Therefore, the aim of the present study was to compare antioxidant and oxidative enzyme expression in glomeruli and proximal tubules in healthy rats. As a further aspect of the antioxidant-oxidant balance, results on enzymes were related to the levels of superoxide anion radicals in vital glomeruli and tubules as detected by enhanced chemiluminescence with lucigenin. Studies were performed on glomeruli and tubular tissue isolated by sieving of renal cortex. Tubular tissue obtained by this method contains 80 % proximal tubules by mass but inevitably there is some contamination with other components of the tubulo-interstitium (Gesek, Wolff & Strandhoy, 1987). Therefore, studies also included proximal tubules specifically isolated by collagenase digestion of renal cortex and subsequent centrifugation in Percoll.
METHODS
Animals and basic procedures
Male 10- to 12-week-old Sprague-Dawley rats, with a normal mean urinary protein excretion rate of 35 ± 7 mg day−1, were used. To assess age-dependent changes in the antioxidant-oxidant balance, 8-month-old male Sprague-Dawley rats (urinary protein excretion: 57 ± 12 mg day−1) were also examined. Animals were kept at 25°C, with free access to standard rat chow and water. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Protein concentrations were measured according to Lowry, Rosebrough, Farr & Randall (1951).
Isolation of renal tissues
Animals were killed by cervical dislocation. Kidneys were flushed with 20 ml ice-cold Krebs-Henseleit-saline buffer (KHS) (Vinay, Gougoux & Lemieux, 1981) via an aortal catheter.
Minced renal cortex was flushed through three steel sieves (300, 150, 75 μm) using 2 l ice-cold KHS. Glomeruli were recovered from the 75 μm sieve, washed and resuspended in KHS at +4°C. Evaluated by light microscopy, the purity of the glomeruli was more than 95 % in all preparations. Tubular tissue samples were taken from the 150 μm sieve, washed and resuspended in KHS at 4°C.
Specific isolation of proximal tubules (PT) was performed according to Vinay et al. (1981), with slight modifications. After flushing with KHS, kidneys were perfused with 10 ml 0.1 % Type IV collagenase in KHS (Worthington Biochemical Corp.). Minced renal cortices were incubated for 25 min at 37°C in 30 ml 0.1 % collagenase-KHS, continously gassed with 95 % O2- 5 % CO2 and gently agitated. Suspensions were filtered through a 425 μm sieve, washed and resuspended in KHS. PT were isolated in pre-gassed 45 % Percoll-KHS solution (Pharmacia) by centrifugation at 10 000 g in a fixed angle rotor head for 30 min at +4°C. The band enriched in PT was withdrawn and washed 2 times with ice-cold KHS.
Aliquots of tubular samples were prepared for light microscopy, by fixation with 2.5 % glutaraldehyde in 0.1 M sodium cacodylate-HCl, postfixation in 2 % osmium tetroxide and subsequent embedding in Epon 812 (Serva, Germany). One micrometre sections were cut, stained with Toluidine Blue (Sigma), and a mean number of sixty tubular segments of each PT sample were examined for the presence of a brush border. According to this criterion, 90 ± 2.5 % of the segments were identified as PT, and samples were found to be virtually free of renal vessels and glomeruli. Tubular samples isolated by sieving contained mainly proximal tubules but also significant amounts of small blood vessels, collecting ducts and distal tubules, and were free of glomeruli. Therefore, these preparations were designated as tubulo-interstitial (TIS).
Viability testing with 0.4 % Trypan Blue (Sigma)-KHS at 37°C in 95 % O2- 5 % CO2 in PT isolated by the collagenase-Percoll protocol revealed no Trypan Blue staining of tubules at 45 min. However, exclusion of the dye decreased to 55 % at 60 min, 43 % at 75 min, and 36 % at 90 min. To compare cellular integrity in PT and TIS samples quantitatively, release of lactate dehydrogenase (LDH) (Bernstein & Everse, 1975) into the medium was determined for different time intervals under the same conditions and related to the total LDH determined in the medium after freeze-thawing of the tissues. No differences were observed for the 20 min interval between PT and TIS samples (3 vs. 2 % of total LDH). Thereafter, LDH release was significantly higher in PT than in TIS samples (15 vs. 6 % by 40 min; 24 vs. 12 % by 60 min; P < 0.05, n= 8).
Antioxidant enzymes
For activity measurements, tissues were lysed at +4°C for 60 min in 50 mM potassium phosphate, 0.1 mM EDTA, and 1 % Triton X-100 (pH 7.8). From the homogenate, an aliquot was drawn for protein determination, and the remainder was centrifuged at 12 000 g for 20 min at +4°C. The supernatant was kept at -70°C until measurements were taken. Studies with different Triton concentrations showed that this procedure resulted in a maximal release of activities leaving no activity in the cellular debris. Sonication was not employed since this led to a severe loss of catalase activity, even at very low energy levels, and repeated freeze-thaw cycles were not used because catalase and glutathione peroxidase activities declined significantly after this treatment.
Superoxide dismutase (SOD) activity was determined by cytochrome c reduction (Crapo, McCord & Fridovich, 1978). Total SOD (copper/zinc and manganese SOD) was assayed in the presence of 10 μM KCN to avoid interference by cytochrome c oxidase. Manganese SOD was measured after inhibition of copper/zinc SOD by 1 mM KCN. Preliminary studies confirmed that 1 mM KCN was sufficient to inhibit copper/zinc SOD in the samples by more than 90 %. One unit of SOD activity was defined as inhibition of cytochrome c reduction by 50 % as specified by Crapo et al. (1978). Catalase activity was assayed spectrophotometrically by the conversion of 10 mM H2O2 in 50 mM potassium phosphate, 0.1 mM EDTA (pH 7.0). Calculations were based on ε= 0.0425 mM−1 cm−1 (where ε is the extinction coefficient) for H2O2 at 240 nm, and the change in absorbance during the first 60 s of the reaction (Aebi, 1982). Glutathione peroxidase (GSH-Px) activity was determined according to Beutler (1975), with minor changes. Measurements were performed in 0.1 M Tris-Cl, 0.5 mM EDTA, 20 mM glutathione, 2 mM NADPH, 0.5 U glutathione reductase (pH 7.7), using 70 μM tert-butyl hydroperoxide as substrate for GSH-Px (Sigma). Calculations were based on ε= 6.22 mM−1 cm−1 for NADPH at 340 nm, and the change in absorbance during the first 2 min of the reaction. Bovine liver catalase, erythrocyte SOD and GSH-Px (Sigma) were included in the assays to control interassay variation. Protein concentration in the samples containing Triton-X 100 was measured according to Wang & Smith (1975).
Antioxidant enzymes were detected by Western blotting as described (Gwinner, Tisher & Nick, 1995). Samples of glomeruli and PT containing 100 μg protein were separated in a SDS-12 % polyacrylamide gel and blotted to nitrocellulose. MnSOD was detected with an immune serum from rabbit prepared in this laboratory. For this purpose, a full-length rat MnSOD cDNA (Hurt, Hsu, Dougall, Visner, Burr & Nick, 1992) was cloned into a prokaryotic expression vector (Tanhauser, Jewell, Tu, Silverman & Laipis, 1992), expressed in E. coli, and the purified protein was used for the immunization. Copper/zinc SOD was detected with a polyclonal sheep antibody to human erythrocyte SOD and catalase with a polyclonal rabbit antibody to human erythrocyte catalase (both antibodies from Calbiochem). Bound primary antibodies were reacted with an anti-IgG sheep or an anti-IgG rabbit antibody from donkey coupled with horseradish peroxidase and protein bands were detected using a chemiluminescence kit (Amersham).
mRNA levels of antioxidant enzymes in PT and glomeruli were determined after electrophoresis of 10 μg total RNA per lane and Northern blotting (Gwinner et al. 1995). Rat catalase cDNA was from Furuta & Hayashi (1990), rat GSH-Px I cDNA from Reddy et al. (1988). As an internal control for loading variances, mRNA levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were determined.
Oxidative enzymes
Tissues were kept in buffer (136 mM NaCl, 4.7 mM KCl, 1.8 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 10 mM Hepes, 5 mM glucose, pH 7.4) at -70°C. After thawing, samples were homogenized on ice, subjected to a freeze-thaw cycle, and kept on ice for the measurements. In preliminary studies, this procedure was shown to result in a maximal release of enzyme activity, without the need for detergents which interfered with the assay.
NADH and NADPH oxidase activity was determined by lucigenin-enhanced chemiluminescence (CL) (Griendling, Minieri, Ollerenshaw & Alexander, 1994) in a multichannel luminometer (LB9505, Berthold Corp., Germany). Tissue homogenates containing 5 μg protein were pre-incubated for 5 min at 37°C in 50 mM potassium phosphate, 1 mM EGTA, 100 mM sucrose, 0.23 mM lucigenin, pH 7.0 (Sigma). Background CL of samples was recorded before the reaction was started with 100 μM NADH or NADPH. After an equilibration of 20 s, CL was recorded over 2 min. Oxidase activities were calculated as average counts per minute minus background counts per minute, and related to the protein concentration of the sample. Measurements were done in the linear range of the assays where NADH and NADPH was not rate-limiting for the reaction.
Xanthine oxidase activity was determined according to Mohazzab & Wolin (1994), with modifications. Samples of 50 μg protein were pre-incubated for 5 min at 37°C in 0.1 M Tris-Cl, 1 mM EDTA, 0.23 mM lucigenin (pH 9.0). Background CL of the samples was recorded before the reaction was started with 50 μM xanthine (Sigma) in 0.1 M Tris-Cl (pH 9). Subsequent steps were identical to that of NAD(P)H oxidase measurements. Oxypurinol at 0.1 mM completely inhibited the signal obtained but had no effect on background CL.
Detection of superoxide anion (O2−) in vital glomeruli and tubules
Measurements were performed in the multi-channel luminometer at 37°C in 500 μl Dulbecco's MEM for chemiluminescence (Boehringer-Mannheim, Germany) containing 0.23 mM lucigenin as a probe to detect extra- and intracellular O2− (Paky, Michael, Burke-Wolin, Wolin & Gurtner, 1993; Griendling et al. 1994). After recording background CL for 10 min, freshly isolated glomeruli or tubular samples containing approximately 100-150 μg protein were added and equilibrated for 1 min. Subsequently, CL activity was recorded for 20 min. In some experiments, scavengers of O2− (SOD, tiron) or an activator of NADPH oxidase (12-O-tetradecanoylphorbol-13-acetate, Sigma) were added to the samples. Basal generation of O2− was expressed as average counts per minute minus background counts per minute and related to the protein concentration determined in the sample after CL measurement. Inhibition of CL by scavengers was calculated as the percentage of basal CL. Phorbol ester-induced signals are presented as peak CL. In preliminary experiments, measurements were shown to be linear in the range of 50-250 μg protein per vial.
Statistics
Results were derived from at least n= 5 independent experiments, except for the results of mRNA analysis and are given as means ±s.d. Comparisons were performed with the Wilcoxon's U test. A P value of < 0.05 was considered as being statistically significant.
RESULTS
Antioxidant enzymes
The activities of the superoxide anion radical scavenging enzymes are depicted in Fig. 1. Total superoxide dismutase (SOD) activity (i.e. copper/zinc SOD and manganese SOD), as well as manganese SOD activity alone, was 4-fold higher in the proximal tubule (PT) and tubulo-interstitial (TIS) samples than in the glomeruli. Differences between glomeruli and PT or TIS samples were also observed for catalase and glutathione peroxidase (GSH-Px), which are involved in peroxide metabolism (Fig. 1). Further, TIS samples had higher GSH-Px activity than PT.
Figure 1. Antioxidant activities in glomeruli, proximal tubules and TIS samples.
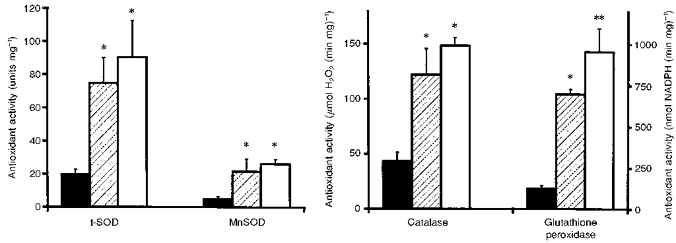
▪, glomeruli;  , proximal tubules; □, TIS samples. t-SOD, manganese + copper/zinc superoxide dismutase; MnSOD, manganese superoxide dismutase. *P < 0.01vs. glomeruli; **P < 0.01vs. glomeruli and vs. proximal tubules.
, proximal tubules; □, TIS samples. t-SOD, manganese + copper/zinc superoxide dismutase; MnSOD, manganese superoxide dismutase. *P < 0.01vs. glomeruli; **P < 0.01vs. glomeruli and vs. proximal tubules.
Analysis of superoxide dismutases and catalase by Western blotting revealed higher enzyme protein levels in PT than in glomeruli (Fig. 2). Similarly, steady-state mRNA levels of these enzymes were significantly higher in PT than in glomeruli (Fig. 3). Among the different glutathione peroxidases, mRNA analysis was confined to cellular GSH-Px I. In contrast to results for SOD and catalase, GSH-Px mRNA levels in proximal tubules and glomeruli were the same.
Figure 2. Enzyme protein levels of superoxide dismutases and catalase in proximal tubules and in glomeruli as detected by Western blotting.
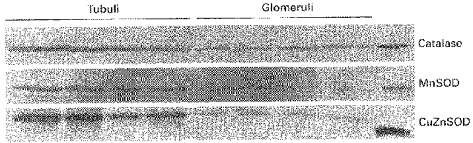
MnSOD, manganese SOD; CuZnSOD, copper/zinc SOD. The outermost right lane contains bovine liver catalase, rat MnSOD and bovine erythrocyte CuZnSOD.
Figure 3. Steady-state mRNA levels of antioxidant enzymes in proximal tubules and in glomeruli.
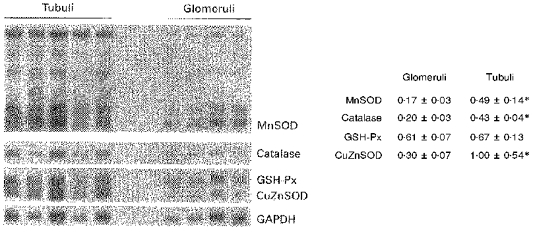
For MnSOD, all five transcripts present in rat tissue (Hurt et al. 1992) are shown, and densitometry was only performed on the largest transcript. CuZnSOD, copper/zinc SOD; GSH-Px, cellular glutathione peroxidase I. Numbers represent the results of densitometry relative to the GAPDH signal; *P < 0.02vs. glomeruli.
Oxidative enzymes
Using NADH as a substrate, similar superoxide anion radical (O2−) generation was observed in homogenates of glomeruli and PT (Fig. 4). TIS samples had significantly higher (44 %) NADH oxidase activity than glomeruli. With NADPH, O2− generation was generally lower than with NADH, and no major differences were present between glomeruli and tubular samples. With the substrate xanthine, very low rates of O2− were observed that were the same in glomerular and tubular samples.
Figure 4. NADH-, NADPH- and xanthine-dependent superoxide anion generation in homogenates of glomeruli, proximal tubules and of TIS samples.
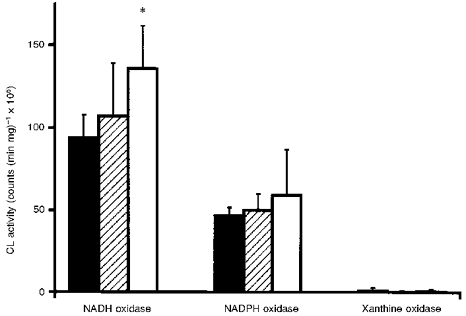
▪, glomeruli;  , proximal tubules; □, TIS samples. *P < 0.01vs. glomeruli.
, proximal tubules; □, TIS samples. *P < 0.01vs. glomeruli.
Detection of O2− in vital glomeruli and tubules
Under basal conditions (Fig. 5, left panel), glomeruli had a 10-fold higher CL activity than TIS samples. However, PT isolated by the collagenase-Percoll method also showed significantly higher CL activity than TIS samples. The scavenger SOD dose-dependently (10-100 units) decreased basal CL activity, maximally by 41 ± 5 % in glomeruli and 49 ± 7 % in PT, indicating that both glomeruli and tubules release a significant proportion of O2− extracellularly. With tiron (20 mM), a scavenger of O2− that permeates intracellularly (Ledenev, Konstantinov, Popova & Ruuge, 1986), the decrease in CL activity was 97 ± 2 % in glomeruli and 83 ± 7 % in PT, without further changes after increasing the dose. Following stimulation with phorbol ester, the increase in CL activity was greater in glomeruli than in PT or TIS samples (Fig. 5, right panel).
Figure 5. Superoxide anion generation in vital glomeruli, proximal tubules and in TIS samples as assessed by lucigenin-enhanced chemiluminescence (CL).
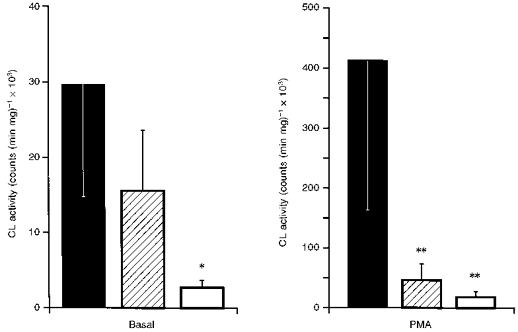
▪, glomeruli;  , proximal tubules; □, TIS samples. Left panel, basal, unstimulated CL activity; right panel, peak CL activity after 5 μM phorbol ester (PMA). *P < 0.01vs. glomeruli and vs. proximal tubules; **P < 0.01vs. glomeruli.
, proximal tubules; □, TIS samples. Left panel, basal, unstimulated CL activity; right panel, peak CL activity after 5 μM phorbol ester (PMA). *P < 0.01vs. glomeruli and vs. proximal tubules; **P < 0.01vs. glomeruli.
Age-dependent changes in enzymes and O2−
Based on reports of increased lipid peroxidation in the ageing kidney (Xia, Rao, van Remmen, Heydari & Richardson, 1995), levels of O2− and the activities of total SOD and NAD(P)H oxidases were examined in glomeruli and TIS samples of 8-month-old Sprague-Dawley rats (Table 1). CL activity in vital glomeruli did not differ in old and young rats. However, significantly higher CL activity was observed in TIS samples from old rats, associated with lower total SOD activity, whereas NAD(P)H oxidase activity was the same in both age groups. With 20 mM tiron, CL activity in TIS samples of old rats was decreased by 92 ± 9 %.
Table 1.
Comparison of the antioxidant-oxidant balance between young and old rats
| Glomeruli | TIS samples | |||
|---|---|---|---|---|
| Young rats | Old rats | Young rats | Old rats | |
| Superoxide anion generation (counts (min mg)−1× 103) | 30 ± 14 | 24 ± 7 | 3 ± 1 | 17 ± 7 * |
| NADH oxidase (counts (min mg)−1× 106) | 94 ± 14 | 93 ± 7 | 135 ± 26 | 115 ± 22 |
| NADPH oxidase (counts (min mg)−1× 106) | 47 ± 5 | 45 ± 3 | 59 ± 28 | 48 ± 14 |
| Total SOD (units mg−1) | 20 ± 3 | 23 ± 5 | 90 ± 22 | 52 ± 9 ** |
P < 0.025
P < 0.01 vs. young rats.
DISCUSSION
This study examined the antioxidant-oxidant balance in glomeruli and proximal tubules. The use of two different preparations of tubules deserves some discussion since it may be important for the interpretation of the results. First, the use of TIS samples obtained by sieving facilitates comparison with glomerular results because of the similar isolation procedure. However, sieved samples contain significant amounts of contaminants, such as blood vessels, distal tubules and collecting ducts, and contain a lower percentage of proximal tubules (80 %) than PT samples isolated by collagenase-Percoll (90 %). Conversely, use of the collagenase-Percoll method is certainly the most specific approach for obtaining relatively pure samples of proximal tubules. However, it involved prolonged collagenase treatment at 37°C, which affected cellular integrity as judged by an approximately 2-fold higher release of LDH within 40 min.
Major differences in antioxidant activity were demonstrated for all enzymes studied, with 3- to 6-fold lower values in the glomeruli than in the tubular samples. Previous histochemical studies in different mammalian species have reported intense staining for SOD, catalase and GSH-Px in proximal tubules but no or only faint staining of glomeruli (reviewed in Muse, Oberley, Sempf & Oberley, 1994). Our data expand these findings, enabling a comparison based on a quantitative and functional assessment of enzyme activities. Except for GSH-Px, antioxidant activities of the two tubular preparations were not different. Higher GSH-Px activity in TIS samples may be explained by the presence of vessels, since vascular smooth muscle cells in renal tissue are particularly rich in GSH-Px as shown by immunostaining (Muse et al. 1994).
Higher SOD and catalase activities of PT were associated with increased enzyme protein levels. This could be due to increased synthesis or decreased degradation of the enzymes. The fact that steady-state mRNA levels of the enzymes were similarly increased in PT indicates that increased synthesis may play a significant role in the regulation of antioxidant activities.
Despite 6-fold higher GSH-Px activity in PT, mRNA levels of cellular GSH-Px I were similar in glomeruli and PT. As one possible explanation, this could indicate that other forms of GSH-Px accounted for the high tubular GSH-Px activity. Previous studies in various tissues have reported the existence of genetically distinct forms besides cellular GSH-Px I, such as phospholipid hydroperoxide GSH-Px (Ursini, Maiorino & Gregolin, 1985), a second cellular GSH-Px (Chu, Doroshow & Esworthy, 1993), and an extracellular GSH-Px. Recently, renal tissue was shown to have the highest ratio of extracellular to cellular GSH-Px mRNA levels compared with several other organs, and expression of extracellular GSH-Px within the kidney was almost exclusively found in the proximal tubule (Avissar et al. 1994). On this basis, it is conceivable that the observed high GSH-Px activity of PT is related to their synthesis of extracellular GSH-Px. However, this does not preclude that the other forms of GSH-Px may contribute to the high GSH-Px activity in PT.
Generation of oxygen radicals occurs in glomerular and proximal tubular cells under basal conditions and in response to several stimuli (Shah, 1989; Rovin, Wurst & Kohan, 1990; Paller & Neumann, 1991). In addition, in certain pathophysiological states generation of radicals is greatly increased by leukocytes infiltrating the kidney (Shah, 1989; Nath et al. 1994). Cellular sources of radicals include cell membrane-bound, mitochondrial and microsomal enzymes, many of them being dependent on NADPH or NADH as a substrate (Cross & Jones, 1991). On a quantitative basis, NADPH- and NADH-dependent enzymes have been identified as a major source of superoxide anion radicals (O2−) in the vascular system (Griendling et al. 1994; Mohazzab & Wolin, 1994). With regard to the kidney, mesangial cells also possess a membrane-associated NADPH oxidase that shares some similarities with neutrophil NADPH oxidase (Radeke et al. 1991; Jones et al. 1995). In a model of human membranous nephropathy, expression of the NADPH oxidase subunit cytochrome b- 588was increased in glomerular epithelial cells, and this was associated with increased detection of hydrogen peroxide (Neale et al. 1993).
Therefore, O2−-generating sources were examined in homogenates of glomeruli and tubular samples using NADH and NADPH as a substrate. No major differences were found between glomeruli and tubular samples except higher NADH oxidase activity in TIS samples. This could be explained by the presence of renal vessels in these samples since NADH oxidase activity in arterial microvessels is considerably higher than NADPH oxidase activity (Mohazzab & Wolin, 1994). It should be pointed out that measurements in the homogenates reflect maximal rates of O2− generation, in the presence of excess NADH and NADPH. Under these conditions, O2− generation was independent of the different SOD activities present in glomeruli and tubular samples, as determined in experiments where exogenous SOD was added to glomerular homogenates to obtain activity levels of SOD equal to that of tubules (data not shown).
Compared with NADPH and NADH, xanthine caused very low generation of O2− in glomerular and tubular homogenates, suggesting that NAD(P)H-dependent systems may be more important sources of radicals in physiological conditions. In this line, a recent study showed that renal xanthine oxidase activity was low compared with several other organs that had up to 50-fold higher activities (Kurosaki, Li Calzi, Scanziani, Garattini & Terao, 1995). Yet, this does not preclude a role for xanthine oxidase in renal disease, as in ischaemia-reperfusion (Greene & Paller, 1991) where xanthine dehydrogenase converts to xanthine oxidase leading to increased radical generation (Hille & Nishino, 1995).
Similar oxidative enzyme activities in homogenates of glomerular and tubular samples, yet considerably lower antioxidant enzyme activities in the glomerular samples raised the question whether these differences had any relevance for the levels of oxygen radicals in vital tissues. Our results on lucigenin-enhanced CL in vital tissues may give some clues to this issue but also illustrate the difficulties related to such measurements. First, higher CL activity was obtained from glomeruli than from TIS samples. Second, TIS samples of older rats that displayed lower SOD activity than young rats showed increased CL activity. Both observations suggest that the observed differences in the balance between O2−-generating enzymes and SOD may be, in fact, relevant with respect to the superoxide anion levels. It should be noted that CL activity of PT was significantly higher than in TIS samples, which was unexpected because differences in the amount of proximal tubules (90 % in PT samples vs. 80 % in TIS samples) cannot explain the higher CL activity in PT. Although we are still a long way from elucidating the exact mechanism, we propose that the higher CL activity in PT was, most probably, related to the collagenase-Percoll treatment, which may have led to cellular integrity being less well retained, as is suggested by the increased rates of LDH release in PT. Thus, tubules isolated by this method may be not so suitable for assessment of O2− levels and TIS samples may in fact represent proximal tubular O2− more accurately.
It should also be noted that levels of O2− represent only one aspect of oxidant stress because O2− may give rise to further reactive oxygen species such as hydrogen peroxide, hydroxyl radicals, and peroxynitrite (Janssen et al. 1993). In particular, since the SOD reaction results in dismutation of O2− to hydrogen peroxide, one could even speculate that high tubular SOD activity promotes oxidant stress by hydrogen peroxide. However, the fact that tubular catalase and GSH-Px activities were similarly elevated suggests that dismutation of O2− by SOD is co-ordinately regulated with subsequent removal of hydrogen peroxide.
In summary, our data indicate significant differences in the antioxidant-oxidant balance between glomeruli and proximal tubules, even in the physiological state. The relevance of the observed differences to pathophysiological states remains to be determined, but a greater susceptibility of the glomerulus to oxidant stress and tissue damage might be anticipated.
Acknowledgments
This work was supported by a grant (GW 4/3-1) from the Deutsche Forschungsgemeinschaft to Dr Gwinner. The authors acknowledge the technical assistance of E. Gutjahr and H. Lindemann. We thank Dr Shuichi Furuta (Shinshu University School of Medicine, Matsumoto, Japan) for the catalase cDNA, and Dr Ambati P. Reddy (University of Michigan, MI, USA), for the GSH-Px cDNA.
References
- Aebi HE. Catalase. In: Bergmeyer HU, editor. Methods in Enzymatic Analysis. III. Germany: Verlag Chemie, Weinheim; 1982. pp. 273–286. [Google Scholar]
- Avissar N, Ornt DB, Yagil Y, Horowitz S, Watkins RH, Kerl EA, Takahashi K, Palmer IS, Cohen HJ. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. American Journal of Physiology. 1994;266:C367–375. doi: 10.1152/ajpcell.1994.266.2.C367. [DOI] [PubMed] [Google Scholar]
- Bernstein L, Everse J. Determination of the isoenzyme levels of lactate dehydrogenase. In: Wood WA, editor. Methods in Enzymology. Vol. 41. New York: Academic Press; 1975. pp. 47–52. [DOI] [PubMed] [Google Scholar]
- Beutler E. Red Cell Metabolism. A Manual of Biochemical Methods. New York: Grune & Stratton; 1975. Glutathione peroxidase (GSH-Px) pp. 71–73. [Google Scholar]
- Chu FF, Doroshow JH, Esworthy RS. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. Journal of Biological Chemistry. 1993;268:2571–2576. [PubMed] [Google Scholar]
- Crapo JD, McCord JM, Fridovich I. Preparation and assay of superoxide dismutases. In: Fleischer S, Packer L, editors. Methods in Enzymology. Vol. 53. San Diego: Academic Press; 1978. pp. 382–393. [DOI] [PubMed] [Google Scholar]
- Cross AR, Jones OT. Enzymic mechanisms of superoxide production. Biochimica et Biophysica Acta. 1991;1057:281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- Furuta S, Hayashi H. Purification and properties of recombinant rat catalase produced in Escherichia coli. Journal of Biochemistry. 1990;107:708–713. doi: 10.1093/oxfordjournals.jbchem.a123113. [DOI] [PubMed] [Google Scholar]
- Gesek FA, Wolff DW, Strandhoy JW. Improved separation method for rat proximal and distal renal tubules. American Journal of Physiology. 1987;253:F358–365. doi: 10.1152/ajprenal.1987.253.2.F358. [DOI] [PubMed] [Google Scholar]
- Greene EL, Paller MS. Oxygen free radicals in acute renal failure. Mineral and Electrolyte Metabolism. 1991;17:124–132. [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circulation Research. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Gwinner W, Tisher CC, Nick HS. Regulation of manganese superoxide dismutase in glomerular epithelial cells: Mechanisms for interleukin 1 induction. Kidney International. 1995;48:354–362. doi: 10.1038/ki.1995.303. [DOI] [PubMed] [Google Scholar]
- Hille R, Nishino T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB Journal. 1995;9:995–1003. [PubMed] [Google Scholar]
- Hurt J, Hsu JL, Dougall WC, Visner GA, Burr IM, Nick HS. Multiple mRNA species generated by alternate polyadenylation from the rat manganese superoxide dismutase gene. Nucleic Acids Research. 1992;20:2985–2990. doi: 10.1093/nar/20.12.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa I, Kiyama S, Yoshioka T. Renal antioxidant enyzmes: their regulation and function. Kidney International. 1994;45:1–9. doi: 10.1038/ki.1994.1. [DOI] [PubMed] [Google Scholar]
- Janssen YM, Van Houten B, Borm PJ, Mossman BT. Cell and tissue responses to oxidative damage. Laboratory Investigation. 1993;69:261–274. [PubMed] [Google Scholar]
- Jones SA, Hancock JT, Jones OT, Neubauer A, Topley N. The expression of NADPH oxidase components in human glomerular mesangial cells: detection of protein and mRNA for p47phox, p67phox, and p22phox. Journal of the American Society of Nephrology. 1995;5:1483–1491. doi: 10.1681/ASN.V571483. [DOI] [PubMed] [Google Scholar]
- Kiyama S, Yoshioka T, Burr IM, Kon V, Fogo A, Ichikawa I. Strategic locus for the activation of the superoxide dismutase gene in the nephron. Kidney International. 1995;47:536–546. doi: 10.1038/ki.1995.67. [DOI] [PubMed] [Google Scholar]
- Kurosaki M, Li Calzi M, Scanziani E, Garattini E, Terao M. Tissue- and cell-specific expression of mouse xanthine oxidoreductase gene in vivo: regulation by bacterial lipopolysaccharide. Biochemical Journal. 1995;306:225–234. doi: 10.1042/bj3060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledenev AN, Konstantinov AA, Popova E, Ruuge EK. A simple assay of the superoxide generation rate with Tiron as an EPR-visible radical scavenger. Biochemistry International. 1986;13:391–396. [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radical Biology & Medicine. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochemical Journal. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohazzab KM, Wolin MS. Sites of superoxide anion production detected by lucigenin in calf pulmonary artery smooth muscle. American Journal of Physiology. 1994;267:L815–822. doi: 10.1152/ajplung.1994.267.6.L815. [DOI] [PubMed] [Google Scholar]
- Muse KE, Oberley TD, Sempf JM, Oberley LW. Immunolocalization of antioxidant enzymes in adult hamster kidney. Histochemical Journal. 1994;26:734–753. doi: 10.1007/BF00158205. [DOI] [PubMed] [Google Scholar]
- Nath KA, Fischereder M, Hostetter TH. The role of oxidants in progressive renal injury. Kidney International. 1994;45:S111–115. suppl. [PubMed] [Google Scholar]
- Neale TJ, Ullrich R, Ojha P, Poczewski H, Verhoeven AJ, Kerjaschki D. Reactive oxygen species and neutrophil respiratory burst cytochrome b558 are produced by kidney glomerular cells in passive Heymann nephritis. Proceedings of the National Academy of Sciences of the USA. 1993;90:3645–3649. doi: 10.1073/pnas.90.8.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paky A, Michael JR, Burke-Wolin TM, Wolin MS, Gurtner GH. Endogenous production of superoxide by rabbit lungs: effects of hypoxia or metabolic inhibitors. Journal of Applied Physiology. 1993;74:2868–2874. doi: 10.1152/jappl.1993.74.6.2868. [DOI] [PubMed] [Google Scholar]
- Paller MS, Neumann TV. Reactive oxygen species and rat renal epithelial cells during hypoxia and reoxygenation. Kidney International. 1991;40:1041–1049. doi: 10.1038/ki.1991.312. [DOI] [PubMed] [Google Scholar]
- Radeke HH, Cross AR, Hancock JT, Jones OTG, Nakamura M, Kaever V, Resch K. Functional expression of NADPH oxidase components (alpha- and beta-subunits of cytochrome b558 and 45-kDa flavoprotein) by intrinsic human glomerular mesangial cells. Journal of Biological Chemistry. 1991;266:21025–21029. [PubMed] [Google Scholar]
- Reddy AP, Hsu BL, Reddy PS, Li NQ, Thyagaraju K, Reddy CC, Tam MF, Tu CPD. Expression of glutathione peroxidase I gene in selenium-deficient rats. Nucleic Acids Research. 1988;16:5557–5568. doi: 10.1093/nar/16.12.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovin BH, Wurst E, Kohan DE. Production of reactive oxygen species by tubular epithelial cells in culture. Kidney International. 1990;37:1509–1514. doi: 10.1038/ki.1990.142. [DOI] [PubMed] [Google Scholar]
- Shah SV. Role of reactive oxygen metabolites in experimental glomerular disease. Kidney International. 1989;35:1093–1106. doi: 10.1038/ki.1989.96. [DOI] [PubMed] [Google Scholar]
- Tanhauser SM, Jewell DA, Tu CK, Silverman DN, Laipis PJ. A T7 expression vector optimized for site-directed mutagenesis using oligodeoxyribonucleotide cassettes. Gene. 1992;117:113–117. doi: 10.1016/0378-1119(92)90498-e. 10.1016/0378-1119(92)90498-E. [DOI] [PubMed] [Google Scholar]
- Ursini F, Maiorino M, Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochimica et Biophysica Acta. 1985;839:62–70. doi: 10.1016/0304-4165(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. American Journal of Physiology. 1981;241:F403–411. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]
- Wang CS, Smith RL. Lowry determination of protein in the presence of triton x-100. Analytical Biochemistry. 1975;63:414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Xia E, Rao G, van Remmen H, Heydari AR, Richardson A. Activities of antioxidant enzymes in various tissues of male Fischer 344 rats are altered by food restriction. Journal of Nutrition. 1995;125:195–201. doi: 10.1093/jn/125.2.195. [DOI] [PubMed] [Google Scholar]


