Abstract
The influence of the transverse-tubular (T-) system Cl− conductance on membrane excitability in skeletal muscle fibres of toad and rat was examined because of conflicting conclusions of previous studies on Cl− conductance. A mechanically skinned fibre preparation was used that permitted investigation of Ca2+ release via the normal T-system voltage-sensor mechanism after complete removal of the surface membrane, which thereby allowed estimation of the T-system potential from force measurements.
When a skinned fibre was bathed in a high-[K+] solution, the sealed T-system became polarized and could be rapidly depolarized by replacing the K+ with Na+, thereby eliciting Ca2+ release from the sarcoplasmic reticulum. In rat skinned fibres, addition of 20 mM Cl− to the ‘myoplasm’ (i.e. bathing solution) partially depolarized the T-system, inducing Ca2+ release and subsequent voltage-sensor inactivation. These effects were completely abolished with 100 μM of the Cl− channel blocker 9-anthracene carboxylic acid (9-AC). Voltage-sensor inactivation increased in a graded manner over the range 3-20 mM myoplasmic Cl−.
In toad fibres, voltage-sensor inactivation was only detectable at > 10 mM myoplasmic Cl−, and 20 mM Cl− was only able to depolarize the T-system sufficiently to trigger Ca2+ release if the myoplasmic [K+] was reduced by 50 %. In toad fibres, 100 μM 9-AC caused little if any block of the T-system Cl− conductance.
It was also found that when skinned fibres were obtained from muscles that had been bathed in a zero Cl− extracellular solution, the initial Na+ substitutions were more effective at depolarizing the T-system. This is consistent with Cl− trapped in the sealed T-system exerting a polarizing effect on T-system potential.
These results unequivocally demonstrate that there is a large 9-AC-sensitive Cl− conductance in the T-system of rat fibres, and a smaller, though still appreciable, Cl− conductance in the T-system of toad fibres, which is relatively insensitive to 9-AC. The results are important for understanding the basis of the Cl− channel aberration in myotonia.
The total Cl− conductance is large in both mammalian and amphibian skeletal muscle fibres and plays an important role in stabilizing the membrane potential (Bryant & Morales-Aguilera, 1971; Palade & Barchi, 1977; Dulhunty, 1979; Rüdel & Lehmann-Horn, 1985; Bretag, 1987; Fahlke & Rüdel, 1995). Conductance experiments in intact and detubulated fibres have indicated that virtually all of the Cl− conductance in frog fibres is located in the surface membrane, with very little or none in the transverse-tubular (T-) system (Eisenberg & Gage, 1969). In contrast, other investigators using potentiometric dyes have concluded that a Cl− current contributes substantially to the total T-system outward current during the rapid phase of repolarization of an action potential in frog fibres, indicating that there is a significant Cl− conductance in the T-system (Heiny, Valle & Bryant, 1990). In agreement with earlier work in frog fibres (Foulks, Pacey & Perry, 1965), Cl− withdrawal experiments in toad fibres (Dulhunty, 1982) found a small degree of T-tubule swelling, indicative of the presence of a Cl− permeability, although the relative amount could not be reliably calculated. Thus, the relative contribution of the T-system to the total Cl− conductance in amphibian muscle fibres is quite unclear.
In rat muscle fibres, detubulation experiments have indicated that the great majority of the Cl− conductance is located in the T-system (Palade & Barchi, 1977; Dulhunty, 1979). However, such experiments are open to question, because glycerol treatment is often incomplete and causes depolarization in mammalian fibres (Dulhunty, 1982; Bretag, 1987). Further support for a large T-system Cl− conductance in rat fibres comes from the substantial T-tubule swelling observed upon Cl− withdrawal (Dulhunty, 1982) and from capacitance measurements (Dulhunty, Carter & Hinrichsen, 1984). It is said that the ClC-1 channel is the major Cl− channel in mammalian skeletal muscle (Steinmeyer, Ortland & Jentsch, 1991b), and Fahlke & Rüdel (1995) have suggested that a single population of channels dominates the Cl− conductance. ClC-1 is blocked by 100 μM 9-anthracene carboxylic acid (9-AC) (Steinmeyer et al. 1991b), as is most if not all of the Cl− conductance in mammalian muscle (Fahlke & Rüdel, 1995). However, using immunofluorescence microscopy and fractionation of muscle membranes, it was concluded that in mouse fibres ClC-1 is confined exclusively to the surface membrane (Gurnett, Kahl, Anderson & Campbell, 1995). Assuming that rat and mouse fibres are comparable, this would imply that either ClC-1 is not the major contributor to the Cl− conductance or there is actually relatively little Cl− conductance in the T-system in mammalian fibres.
This study investigates the presence and relative importance of the T-system Cl− conductance in both rat and toad fibres, using a mechanically skinned fibre preparation in which the normal excitation-contraction (E-C) coupling mechanism is still functional (Lamb & Stephenson, 1990). As the surface membrane was completely absent, we were able to separately identify the effect of the T-system Cl− conductance on T-system potential by its effect on voltage-sensor activation and inactivation. These experiments show that there is a large Cl− conductance in the T-system of rat fibres, which is blocked by 100 μM 9-AC, and a smaller, though still appreciable, Cl− conductance in the T-system of toad fibres, which is insensitive to 9-AC. The findings suggest that a substantial part of the Cl− conductance in mammalian muscle may not be attributable to ClC-1, and this is important in understanding the role of Cl− conductance aberrations in myotonia.
METHODS
Isolation of skinned fibres
Tropical cane toads (Bufo marinus), which had been kept at 16°C for 1 to 5 weeks before use, were immobilized by a blow to the head and killed by pithing, and then both iliofibularis muscles were removed. Long Evans hooded rats (Rattus norvegicus) were killed by asphyxia under halothane anaesthesia (2 % v/v) before removal of both extensor digitorum longus (EDL) muscles. The muscles were placed in paraffin oil immediately after excision (and kept cool on ice), except for some experiments (see below) where they were first placed in a particular extracellular solution in order to modify the ionic environment in the T-system. Single muscle fibres were isolated from the whole muscle (under paraffin oil) and mechanically skinned. The skinned fibre was mounted onto a force transducer (AME875; SensoNor, Horten, Norway), and stretched to 120 % of its resting length. The fibre was then placed into a 2 ml Perspex bath containing a potassium 1,6-diaminohexane-N,N,N',N'-tetraacetic acid (HDTA) solution for 2 min, to allow the sealed T-system to polarize (Lamb, Junankar & Stephenson, 1995), before being stimulated by rapid substitution of an appropriate solution. All experiments were performed at room temperature (23 ± 2°C).
Solutions
All chemicals were obtained from Sigma unless stated otherwise. The potassium HDTA (K-HDTA) solution consisted of (mM): K+, 117 (toad) or 130 (rat); Na+, 37; HDTA2− (Fluka, Buchs, Switzerland), 50; total ATP, 8.0; total creatine phosphate, 10.0; total Mg2+, 8.5; Hepes, 60 (toad) or 90 (rat); NaN3, 1.0; total EGTA, 0.025; pCa (-log10[Ca2+]) 6.7, pH 7.10 ± 0.01. The T-system of a skinned fibre was depolarized by replacing the K-HDTA solution with a matched sodium HDTA (Na-HDTA) solution or in some cases with a choline chloride (ChCl) solution. The Na-HDTA solution used for rat or toad fibres was identical to the corresponding K-HDTA solution except that all K+ was replaced with equimolar Na+. The ChCl solution contained (mM): ChCl, 100; Na+, 42 (toad) or 50 (rat); total ATP, 8.0; total creatine phosphate, 10.0; total Mg2+, 8.15; Hepes, 20 (toad) or 50 (rat); NaN3, 1.0; total EGTA, 0.025. These solutions all contained 1.0 mM free Mg2+ and had an osmolality of approximately 255 ± 5 and 295 ± 5 mosmol kg−1 for the toad and rat solutions, respectively. Relaxing and maximum Ca2+-activating solutions were similar in composition to the corresponding K-HDTA solutions, except that all HDTA (50 mM) was replaced with EGTA or Ca-EGTA, respectively, and the total magnesium was adjusted to maintain a free [Mg2+] of 1 mM (Stephenson & Williams, 1981). A K+-based stock solution with 120 mM Cl−, which had identical pH and ATP, creatine phosphate, and free Mg2+ concentrations as the K-HDTA solution, was mixed with the K-HDTA solution to give solutions with different myoplasmic [Cl−]. This KCl stock solution was (mM): KCl (Ajax), 103; Na2ATP, 8.0; sodium creatine phosphate, 10.0; MgCl2, 8.3; Hepes, 20. A corresponding NaCl solution in which all K+ was replaced with Na+ was used for mixing the Na-HDTA solution.
The [Cl−] in the T-system was modified by incubating a whole muscle for 30 min in a Ringer-like solution in which Cl− was replaced with methanesulphonate (CH3SO3−). The composition of the NaCH3SO3 solution used for the toad was (mM): NaCH3SO3 (Aldrich), 110; KOH, 4; CaBr2, 2; MgBr2, 1; Hepes, 15; glucose, 10.0; adjusted to pH 7.10 ± 0.01 with NaOH. A muscle soaked in a matched NaCl solution was used as control. Similar solutions for rat experiments were made using 140 mM NaCH3SO3 or NaCl.
Stock solutions of 9-AC (Aldrich) were prepared on the day of use in either of two ways. In the first, a 0.4 M 9-AC stock was prepared in dimethyl sulphoxide (DMSO) and diluted 4000-fold to produce a [9-AC] of 100 μM in the final solution. Alternatively, a 5 mM 9-AC stock was prepared in double-distilled water and adjusted to pH 7.10 ± 0.01 with 4 M NaOH. This was diluted 50-fold to also produce a [9-AC] of 100 μM in the final solution. An equal amount of DMSO or double-distilled water was added to all corresponding ‘control’ solutions not containing 9-AC. DMSO and double-distilled water at these concentrations had no noticeable effect on depolarization-induced force responses in either toad or rat fibres. The effects of 9-AC were the same regardless of the way the stock was prepared.
Contractile apparatus experiments
The effects of Cl− and 9-AC on the Ca2+ sensitivity and the maximum Ca2+-activated force of the contractile apparatus were determined by directly activating the fibre in a series of solutions in which the [Ca2+] was very heavily buffered at progressively higher levels, as described by Bakker, Lamb & Stephenson (1996). These solutions were made by mixing appropriate proportions of the 50 mM EGTA and 50 mM Ca-EGTA solutions. Control solutions had the same volume of DMSO added (0.025 %) as the solutions containing 9-AC. The force produced at each pCa was expressed as a percentage of the maximum Ca2+-activated force in that fibre. Plots of force versus pCa for each fibre were fitted with Hill curves by the analysis program GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA) to obtain the slope and the pCa50, i.e. the pCa value giving half-maximum force.
Force traces and analysis of results
In all traces showing depolarization-induced force responses, skinned muscle fibres were bathed in the standard K-HDTA solution, unless otherwise indicated. In the text, mean values ±s.e.m. are shown and statistical probability (P) was determined with Student's t test (paired or unpaired, as appropriate), or where indicated with a two-way ANOVA on the first four force responses of a data set, and considered significant if P < 0.05.
RESULTS
Depolarization-induced force responses in skinned muscle fibres
As described previously (Lamb & Stephenson, 1990, 1994), when a skeletal muscle fibre is mechanically skinned, the T-system seals off and evidently becomes polarized if the fibre is placed into a high-[K+] solution that mimics the normal myoplasmic environment (see Methods). Transferring the skinned fibre to a Na+-based solution with no K+ shifts the equilibrium potential for K+ across the T-system membrane from a strongly negative value (defined as myoplasm relative to T-system lumen, as in intact fibres) to a positive value, causing depolarization of the membrane, which in turn triggers Ca2+ release from the sarcoplasmic reticulum (SR) and a rapid, transient force response (e.g. Fig. 1A). These responses are highly analogous to potassium contractures in intact fibres. The [Cl−] in the sealed T-system is unknown, but initially should be quite high owing to the entrapment of extracellular fluid, and as there is no Cl− in the standard bathing solutions, this will have a polarizing effect on the T-system potential. This may explain why replacing K+ with Na+ is initially not fully effective in eliciting depolarization-induced responses (see later for discussion of ‘work-up’, e.g. Fig. 5). Many depolarization-induced responses (typically 15 to 30) can be elicited before the fibre eventually becomes ‘run down’, provided that the fibre is returned to the high-[K+] bathing solution for 30 s to 1 min to repolarize between successive depolarizations.
Figure 1. Effect of 20 mM myoplasmic Cl− on activation and inactivation in a rat EDL fibre.
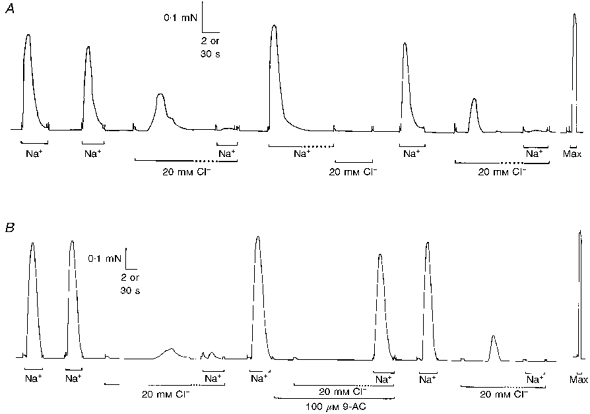
A, replacement of the K-HDTA solution bathing a skinned EDL fibre with a Na-HDTA solution (for the periods indicated by the horizontal bars labelled Na+) depolarized the sealed T-system and elicited Ca2+ release and a large force response. When the fibre was again polarized (in K-HDTA solution, no bar), addition of 20 mM Cl− to the myoplasm (with isosmotic reduction of K-HDTA) elicited a substantial force response, and then caused the fibre to become largely unresponsive to Na+ depolarization. This indicates that the Cl− addition caused substantial depolarization of the T-system. Consistent with this, Cl− did not elicit any force response when applied after prolonged (1 min) depolarization in the Na+ solution, which was sufficient to inactivate the voltage sensors in the T-system. B, the depolarizing effect of myoplasmic Cl− seen in another EDL fibre was reversibly blocked by the presence of 100 μM 9-AC. Time scale: 2 s during periods indicated by continuous horizontal bars, and 30 s during periods indicated by the horizontal dotted bars and elsewhere. In this and subsequent figures, Max indicates exposure to the Ca-EGTA solution (pCa 4.5), which gives maximum Ca2+ activation of the contractile apparatus.
Figure 5. Initial Na+ substitutions are less effective at eliciting a response.
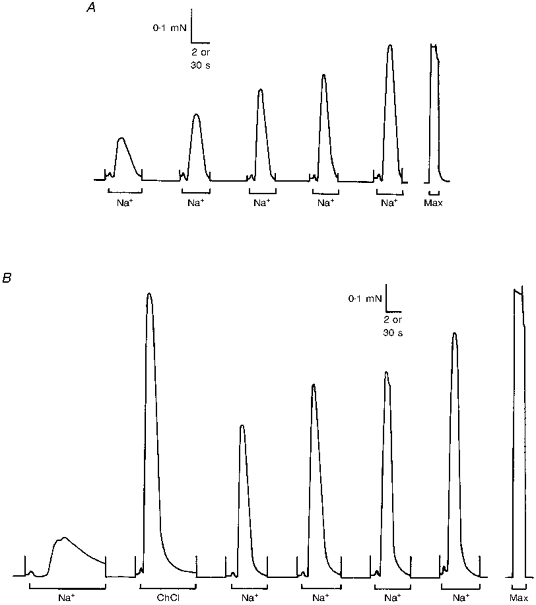
A, typical responses of a toad iliofibularis fibre showing the progressive increase (‘work-up’) in the force response to successive depolarizations by Na+ substitution. The fibre was equilibrated after skinning for 2 min in K-HDTA solution before the first Na+ substitution. B, ChCl substitution produced a near-maximal force response when used in another toad fibre in place of the second Na+ substitution. Time scale: 2 s during Na+ and ChCl substitution (bars) and 30 s elsewhere.
Depolarizing effect of myoplasmic Cl− in rat EDL fibres
In order to investigate whether there is a substantial Cl− permeability in the T-system of toad and rat fibres, we examined the ability of ‘myoplasmic’ Cl− (i.e. Cl− in the bathing solution) to depolarize the T-system in skinned fibres and hence cause activation and inactivation of the voltage sensors. Adding Cl− to the myoplasm will make the Cl− equilibrium potential across the T-system less negative/more positive (irrespective of the exact [Cl−] in the T-system), which will cause depolarization of the T-system if the Cl− permeability is appreciable relative to the K+ permeability (see Discussion). The skinned fibre preparation is ideally suited to such a study because the complete absence of the surface membrane allows rapid change of the myoplasmic conditions. Preliminary experiments on the properties of the contractile apparatus showed that the presence of 10 or 20 mM Cl− in the myoplasm had no noticeable effect on maximum Ca2+-activated force, and caused a slight increase in Ca2+ sensitivity (ΔpCa50 was 0.017 and 0.081, in 10 and 20 mM Cl−, respectively) in rat fibres, but no significant change in toad fibres (Coonan & Lamb, 1998). In the depolarization experiments, fibres were first stimulated repeatedly by successive Na+ substitutions (in the absence of myoplasmic Cl−) until near-maximal depolarization-induced force responses were achieved (see later for discussion of ‘work-up’ of initial responses). Then, following 1 min of repolarization in the standard K+ solution, each fibre was exposed to a similar K+ solution containing 20 mM myoplasmic Cl− (with isosmotic reduction of K-HDTA, see Methods). In all ten rat fibres examined, this resulted in the production of a substantial force response (e.g. left-hand section of Fig. 1A), with the mean size being 37 ± 6.3 % of the force response to the preceding Na+ substitution in the same fibre (18 observations in 10 fibres). Several lines of evidence indicate that this response upon rapid addition of 20 mM Cl− to the myoplasm was due to the Cl− causing depolarization of the T-system membrane. Firstly, if a fibre was maintained in the 20 mM Cl− solution for a further 30-60 s, the response to depolarization by Na+ substitution (with 20 mM myoplasmic Cl− still present) was substantially attenuated, with the mean size being 57 ± 10 % of the force response to the preceding Na+ substitution in the same fibre (18 observations in 10 fibres; e.g. Fig. 1A, which shows a case of marked attenuation). Even when the response to depolarization was strongly attenuated by prolonged exposure to 20 mM myoplasmic Cl−, lowering the [Mg2+] (from 1 to 0.015 mM) invariably elicited a near-maximal force response, showing that the Ca2+ release channels could still be activated directly. Thus, prolonged exposure to 20 mM myoplasmic Cl− inhibited voltage-sensor-mediated responses without inhibiting direct activation of the release channels. Furthermore, if the Cl− remained in the myoplasm, it continued to exert its inhibitory effect long after its initial activating effect, or even when such activation was avoided (see later, Fig. 2), showing that the inhibition depended on the presence of myoplasmic Cl− rather than its ability to induce Ca2+ release. Thus, these data are consistent with the voltage sensors in the T-system becoming inactivated during the prolonged exposure to the 20 mM myoplasmic Cl− solution, which in turn implies that the Cl− depolarized the T-system to some extent.
Figure 2. Effect of myoplasmic Cl− on depolarization-induced force responses.
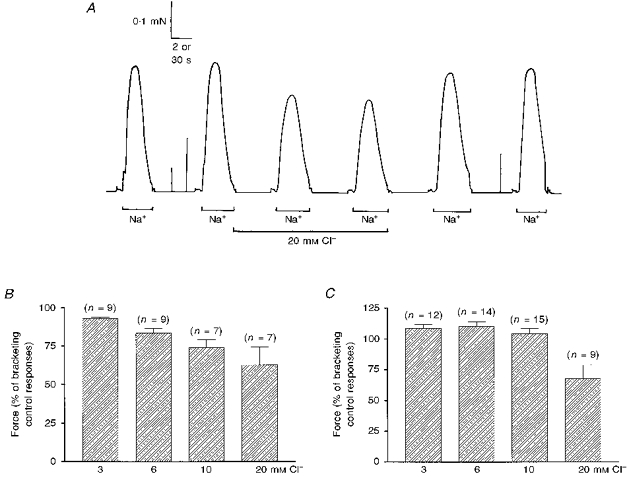
A, force responses in a rat EDL fibre showing the protocol used. Cl− was added to the myoplasm whilst the fibre was refractory following a control depolarization, and the responses to two consecutive Na+ depolarizations in the presence of Cl− were compared with bracketing pairs of responses in the absence of Cl−. Time scale: 2 s during Na+ depolarizations and 30 s elsewhere. B, mean (±s.e.m.) force response of rat EDL fibres to Na+ depolarization in the presence of the indicated myoplasmic [Cl−] relative to bracketing control responses; all values were significantly lower than 100 %. C, corresponding mean data in toad fibres; values indicate a small but significant potentiation of the response in 3 and 6 mM Cl−, no significant change in 10 mM Cl− and a substantial reduction of the response in 20 mM Cl−.
Further evidence that the 20 mM myoplasmic Cl− solution elicited responses by depolarizing the T-system membrane was the finding that the same solution did not elicit any response if it was applied when the voltage sensors were already in the inactivated state. In these experiments, the fibre was depolarized by Na+ substitution, eliciting the usual large force response, and then kept in that solution for a further 30-60 s to ensure that voltage sensors were inactivated (Lamb & Stephenson, 1990). In all five cases examined, when the inactivated fibre was exposed to the 20 mM Cl− solution, no force response was observed (e.g. middle section of Fig. 1A). In each case it was shown that the SR was still loaded with Ca2+ because a large response could be evoked by Na+ substitution after repolarizing the fibre for 30 s. Furthermore, when fibres were repolarized for at least 30 s and then exposed to the same 20 mM Cl− solution as described earlier, they once again gave a substantial force response in every case (e.g. right-hand section of Fig. 1A). Thus, the 20 mM Cl− solution only elicited a response when the voltage sensors could be activated, strongly suggesting it acted by depolarizing the T-system.
Finally in this regard, in all six rat fibres examined, the force response to the 20 mM Cl− solution was completely blocked by the presence of 100 μM 9-AC (e.g. middle section of Fig. 1B), an agent known to block all Cl− conductance in mammalian fibres (Bretag, 1987; Fahlke & Rüdel, 1995). When fibres treated with 9-AC were depolarized by Na+ substitution at the end of a 1 min period in the 20 mM Cl− solution, they showed little or no evidence of inactivation (e.g. Fig. 1B), giving a response of 82 ± 11 % of the preceding Na+ depolarization. At a concentration of 100 μM, 9-AC had no detectable effect on the contractile apparatus properties of either toad or rat fibres (see Methods), i.e. there was no significant change in the maximum Ca2+-activated force or the pCa50 (toad: ΔpCa50= 0.013 ± 0.007, n= 7; rat: ΔpCa50= 0.003 ± 0.003, n= 7). Thus, 9-AC stopped 20 mM myoplasmic Cl− both from eliciting Ca2+ release and inhibiting the response to Na+ substitution.
The data on the effects of Cl− detailed in the above sections can only be readily explained by concluding that: (a) there was a substantial Cl− conductance in the T-system of rat EDL fibres, (b) addition of 20 mM Cl− to the myoplasm of rat fibres depolarized the T-system sufficiently to appreciably activate and then inactivate the voltage sensors, and (c) 100 μM 9-AC prevented this depolarization by blocking the T-system Cl− conductance.
Dependence of voltage-sensor inactivation on myoplasmic [Cl−]
We then examined the manner in which steady-state inactivation of the voltage sensors varied with myoplasmic [Cl−]. Each skinned fibre was first depolarized by Na+ substitution several times to ensure the response was maximal. Whilst still inactivated following a maximal Na+ depolarization, each fibre was exposed to a K+ solution containing either 3, 6, 10 or 20 mM Cl−, and then depolarized twice by Na+ substitution (at 1 min intervals), before the responses in the absence of Cl− were again examined (e.g. Fig. 2A). This protocol enabled examination of the effect of myoplasmic Cl− on steady-state inactivation in the absence of any SR depletion that might occur when a polarized fibre was first exposed to Cl− (e.g. Fig. 1A). In most cases, the effect of either two or three different concentrations of Cl− were examined successively on the same fibre (using intervening control responses), with the order of examination randomized between fibres. The summarized results in Fig. 2B show that the response in the presence of Cl− was significantly smaller than the bracketing control responses at every [Cl−] examined (all P < 0.025), with the size of the reduction increasing with higher myoplasmic [Cl−]. Corresponding data in toad fibres (Fig. 2C) showed that the response to Na+ depolarization was slightly potentiated (∼10 %) in both 3 and 6 mM Cl− (P < 0.005), was not significantly changed in 10 mM Cl−, and substantially inhibited in 20 mM Cl− (P < 0.005).
In order to confirm that the inhibitory effect of myoplasmic Cl− on the response was due to chronic partial depolarization of the T-system with resultant partial inactivation of the voltage sensors, some rat EDL fibres were examined both in the absence and presence of 100 μM 9-AC (e.g. Fig. 3A). The presence of 9-AC completely reversed the inhibitory effect of myoplasmic Cl−, with the response to Na+ depolarization actually being potentiated by 9 ± 1 % (n= 11, P < 0.0005) in 10 mM Cl− and 5 ± 1 % (n= 4, P < 0.025) in 20 mM Cl− (Fig. 3B). The size of the response to Na+ depolarization in Cl− and 9-AC was expressed relative to the bracketing control responses with 9-AC alone (see Fig. 3A), and consequently the small potentiation was not due to some effect of 9-AC but instead must be due to an effect of Cl− on the contractile apparatus (see above) or on Ca2+ release itself.
Figure 3. Effect of myoplasmic Cl− on depolarization-induced force responses in rat EDL fibres in the presence of 100 μM 9-AC.
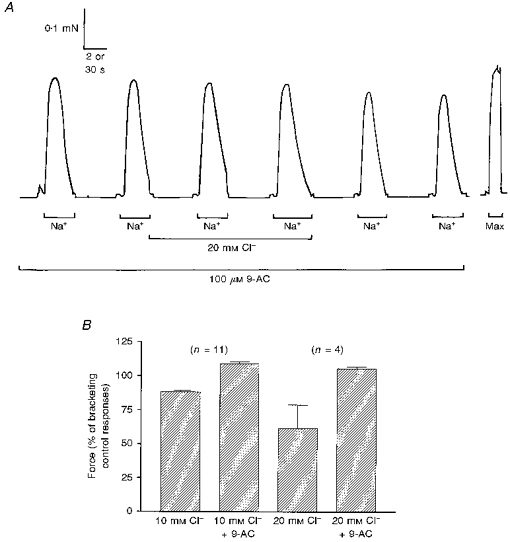
A, when 100 μM 9-AC was present, addition of 20 mM Cl− to the myoplasm had little effect on the force responses to Na+ depolarization; compare the effect of Cl− in same fibre in the absence of 9-AC in Fig. 2A. Time scale: 2 s during Na+ depolarizations and 30 s elsewhere. B, effect of 10 or 20 mM myoplasmic Cl− on the mean (±s.e.m.) normalized force response to Na+ depolarization in the absence and then the presence of 100 μM 9-AC. In each fibre, the response in Cl− was expressed relative to the bracketing control responses under the same conditions (i.e. with 9-AC absent or present accordingly). Both 10 and 20 mM Cl− caused a significant reduction in the response when 9-AC was absent, and a small but significant enhancement of the response when 9-AC was present.
Depolarizing effect of myoplasmic Cl− in toad fibres
The finding that inhibition of the response to Na+ depolarizations appeared to require a higher myoplasmic [Cl−] in toad fibres (> 10 mM; Fig. 2C) than in rat fibres (≥ 3 mM; Fig. 2B) might be consistent either with myoplasmic Cl− causing proportionately less depolarization of the T-system in toad fibres or with voltage-sensor inactivation requiring a greater level of T-system depolarization in toad fibres (see Discussion concerning the likely resting potential in these skinned fibres). This was investigated further by examining the ability of myoplasmic Cl− to itself cause depolarization-induced Ca2+ release in toad fibres. Using the same protocol as in Fig. 1A with rat fibres, it was found that when a toad fibre was polarized in the standard K+ solution, addition of 20 mM Cl− to the myoplasm did not induce any force response at all in any of the four fibres examined (e.g. Fig. 4A), although as expected it caused partial inhibition of the response to Na+ depolarization (reduced to 32 ± 20 % of control response; cf. Fig. 2C). Its inability to trigger Ca2+ release is consistent with the myoplasmic Cl− being less effective at depolarizing the T-system in toad fibres than in rat fibres (see Discussion). When the [K+] of the bathing solution was reduced by 50 % (by isotonic replacement with Na+), in order to partially depolarize the T-system (see Lamb & Stephenson, 1990), subsequent addition of 20 mM Cl− to the myoplasm induced a force response in all twelve fibres examined (e.g. Fig. 4B), with the mean response being 26 ± 8 % of the response to the initial Na+ depolarizations. Such Cl− addition did not induce any response if the voltage sensors had been inactivated by prolonged (1 min) depolarization in Na+ solution (n= 3, subset of above fibres; not shown). The response to Na+ depolarization in the 50 % K+ solution (in the absence of Cl−) was reduced to 79 ± 10 % of that in full K+ in the subset of seven fibres examined, confirming that the T-system was indeed partially depolarized in 50 % K+ solution. The response to Na+ depolarization after 1 min exposure to the 20 mM Cl− in the presence of 50 % K+ (e.g. Fig. 4B) was reduced to 39 ± 13 % of the response to Na+ depolarization under control conditions (n= 8), showing that the presence of Cl− in the myoplasm considerably increased the level of steady-state inactivation. Thus, addition of Cl− to the myoplasm does depolarize the T-system in toad fibres, but this effect is not sufficient to trigger Ca2+ release unless the polarizing effect of K+ has been reduced by lowering the myoplasmic [K+]. Interestingly, in contrast to rat fibres, the ability of Cl− to cause depolarization-induced Ca2+ release was not noticeably reduced in the presence of 100 μM 9-AC in the two toad fibres examined (not shown). Thus, it appears that 100 μM 9-AC causes little if any inhibition of the T-system Cl− conductance in toad fibres. In another toad fibre the response to 20 mM Cl− was reduced by < 50 % in the presence of 500 μM 9-AC.
Figure 4. Effect of myoplasmic Cl− on activation and inactivation in toad iliofibularis fibres in standard and low [K+] solutions.
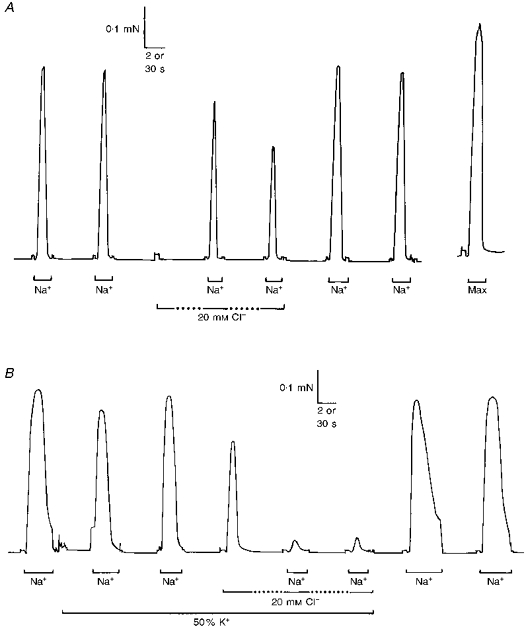
A, addition of 20 mM Cl− to the myoplasm did not elicit any force response when a toad fibre was well polarized by the presence of the standard, high myoplasmic [K+], although the presence of Cl− did reduce the response to Na+ depolarizations. B, when a fibre was bathed in reduced myoplasmic [K+] (50 % K+), addition of 20 mM Cl− elicited a large force response and caused almost complete inhibition of the response to Na+ substitution. Time scale: 2 s during periods indicated by the continuous horizontal bars, and 30 s during periods indicated by the dotted horizontal bars and elsewhere.
Effect of T-system Cl− on the response to Na+ substitution
Further evidence of a substantial T-system Cl− conductance comes from the behaviour of skinned fibres in response to the first few Na+ substitutions after skinning. With both toad and rat fibres, it was noted that such Na+ substitutions were often not fully effective in eliciting a force response. Typically, it took approximately three to five repetitions before the force response had ‘worked up’ to a stable level close to the maximum Ca2+-activated force in that fibre (e.g. Fig. 5A). Further experiments indicated that this ‘work-up’ phenomenon depended on repeated depolarization of the fibre, rather than simply on time alone, because the response to the first Na+ substitution was not different when toad fibres were allowed to equilibrate after skinning for either 2 min or 4 min in the K-HDTA solution (45.6 ± 6.0 % of maximum Ca2+-activated force (n= 6) and 47.1 ± 9.2 % (n= 6), respectively), with both groups showing an indistinguishable increase in force response to the next three to four Na+ substitutions. (It was also noted that the rate of ‘work-up’ depended more on the number of Na+ substitutions than on their duration.) These poor responses to the initial Na+ substitutions could be due to the effect of a high [Cl−] remaining in the T-system following skinning. If the T-system membrane is appreciably permeable to Cl−, as suggested by the experiments described above, the presence of a high [Cl−] in the T-system lumen should exert a polarizing effect which would tend to counter the depolarizing effect of replacing myoplasmic K+ with Na+, thereby giving a submaximal net stimulus.
To further test this hypothesis, toad fibres were depolarized by ChCl substitution instead of by Na+ substitution, in order to observe the effect of a stronger depolarizing stimulus on the force response. ChCl substitution not only lowers the [K+] in the myoplasm, but also simultaneously increases the myoplasmic [Cl−]. Consequently, if the T-system has any appreciable permeability to Cl−, the depolarization resulting from ChCl substitution will be significantly more potent than that produced by Na+ substitution. The effect of depolarization by ChCl substitution was investigated in a subset of toad fibres by following the initial Na+ substitution with a single ChCl substitution, before continuing with a series of further Na+ substitutions (e.g. Fig. 5B). Control fibres were subjected to depolarization by Na+ substitution only (e.g. Fig. 5A). To ensure accurate determination of the relative effect of depolarization by ChCl substitution, stimulation sequences of the types shown in Fig. 5A and B were used alternately for consecutive fibres from each muscle. When a depolarization by ChCl was interposed in this manner, it elicited a near-maximal force response in each fibre (e.g. Fig. 5B), with the subsequent Na+ substitutions continuing to progressively ‘work up’ in a manner similar to control fibres (i.e. compare Fig. 5A with B). The mean response to the ChCl substitution was 99 ± 1 % of the maximum response to Na+ substitution in the same fibre. This was significantly larger than the corresponding (i.e. second) mean response to Na+ substitution (66 ± 8 %) elicited in control fibres (P < 0.001; Student's t test; n= 11). The near-maximal force response elicited by ChCl substitution at a time when the response to Na+ substitution was still ‘working up’ indicates that the normal E-C coupling mechanism was quite functional in the fibres at that time. Consequently, these results suggest that the poor responses elicited by Na+ substitution are due to the ineffectiveness of the depolarizing stimulus, i.e. that Na+ substitution was failing to fully depolarize the T-system. This would be consistent with a high [Cl−] within the T-system helping to keep the membrane partially polarized during the Na+ substitutions.
If the effectiveness of the initial Na+ depolarizations is being reduced by the presence of Cl− in the T-system, replacement of this Cl− with an impermeant anion should lead to larger initial force responses. To test this, we compared the responses of skinned fibres from muscles that had been incubated in a NaCl extracellular solution with those from the contralateral muscles that had been incubated in a matched solution in which all Cl− had been replaced with methanesulphonate (CH3SO3−; see Methods); in each experiment, skinned fibres were obtained alternately from the two muscles. The responses to successive Na+ substitutions in fibres from muscles bathed in the NaCl solution (e.g. Fig. 6) were not noticeably different from those in fibres from muscles placed in paraffin oil immediately after excision (e.g. Fig. 5A). In contrast, in both toad and rat, fibres from muscles that had been bathed in CH3SO3− gave significantly larger responses to the initial Na+ substitutions than did fibres from muscles bathed in normal extracellular [Cl−] (P < 0.0001; two-way ANOVA). The potentiating effects of replacing T-system Cl− with CH3SO3− on the initial responses to Na+ substitution supports the hypothesis that the presence of Cl− in the T-system inhibits the depolarizing effect of decreasing myoplasmic [K+], which in turn implies that there must be a considerable Cl− conductance in the T-system in both toad and rat fibres.
Figure 6. Effect of replacing T-system Cl− with CH3SO3− on the response to Na+ substitution in skinned fibres.
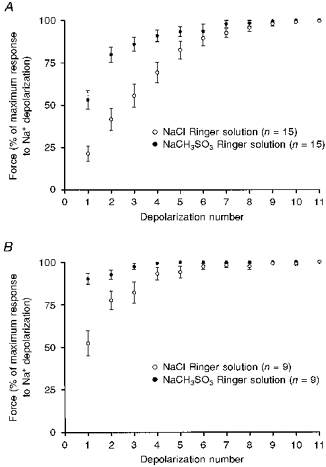
In both toad (A) and rat (B), the force responses to the initial depolarizations by Na+ substitution were significantly larger in skinned fibres obtained from muscles bathed in extracellular CH3SO3− solution compared with fibres from the contralateral muscles bathed in normal extracellular Cl− (P < 0.0001; two-way ANOVA). Data points show the mean (±s.e.m.) of the normalized force response in each fibre for the indicated conditions; n is the number of fibres examined.
DISCUSSION
In this study we present evidence that there is a considerable Cl− conductance in the T-system of both rat and toad skeletal muscle fibres. Firstly, we showed that addition of Cl− to the myoplasm caused both activation and inactivation of the normal voltage-sensor mechanism controlling SR Ca2+ (Figs 1-4), indicating that it caused substantial depolarization of the T-system. Secondly, we showed that removal of T-system Cl− apparently increased the level of T-system depolarization produced by reducing myoplasmic [K+] (Fig. 6), suggesting that T-system Cl− exerted a substantial polarizing effect on the membrane potential. Thus, both types of experiments strongly suggest that the Cl− concentration gradient across the T-system plays an important role in determining the T-system potential, which in turn means that the T-system must be appreciably permeable to Cl−.
It might be argued that the increase in the response to the initial Na+ depolarizations in fibres from muscle bathed in zero extracellular [Cl−] (i.e. Fig. 6) was due to T-tubule swelling caused by Cl− withdrawal. However, it is not apparent why this would increase the effectiveness of the Na+ depolarizations and, even if it were the case, it would still imply that the T-system was quite permeable to Cl− (Dulhunty, 1982). Furthermore, this explanation seems unlikely because: (a) the degree of T-system swelling is quite small in toad fibres, and (b) in most fibres studied, the T-system should have returned to its normal size by the end of the 30 min incubation period in low [Cl−] or by the time the fibres were actually dissected and used (Dulhunty, 1982). It is also very unlikely that T-system swelling could explain the effects of adding Cl− to the myoplasm, because Cl− could induce Ca2+ release in a matter of seconds (e.g. Fig. 4B), whereas in situations in which T-system swelling does occur, it does so over a time scale of minutes (Dulhunty, 1982).
There are two points worth mentioning in regard to the ‘work-up’ of Na+ depolarizations (Figs 5 and 6). Firstly, although increased, the initial responses in skinned fibres from toad muscles bathed in low [Cl−] were not maximal (i.e. they still showed ‘work-up’; Fig. 6A). This might have been caused by there still being some Cl− present in the sealed T-system, perhaps because it moved there from the myoplasm while the fibre was in paraffin oil before being skinned. Secondly, one must ask what causes a fibre to ‘work-up’? As T-system Cl− appears to exert a polarizing effect on T-system potential, it might be thought that over the ‘work-up’ period either the lumenal [Cl−] became substantially lower or the relative permeability of the T-system to Cl− became smaller (perhaps as the permeability to K+ increased). If the latter were true, it would mean that the relative permeability of the T-system to Cl− was initially even higher than that indicated in the experiments in which Cl− was added to the myoplasm (i.e. Figs 1-4), which were performed after the fibre had ‘worked up’. Alternatively, it is possible that the polarizing effect of T-system Cl− remained constant over time, and that the ‘work-up’ phenomenon was due to a progressive decrease in an additional polarizing effect on T-system potential exerted by some other factor (e.g. Na+-K+ exchange).
Comparison with alteration of [Cl−] in intact fibres
The depolarizing effect of raising myoplasmic [Cl−] in skinned fibres is somewhat analagous to experiments in intact fibres in which extracellular [Cl−] was lowered (Hodgkin & Horowicz, 1959), but there are some crucial differences. In the intact fibre experiments, the membrane potential under control conditions was close to the equilibrium potential for K+ (EK) and the Cl− distributed passively such that the equilibrium potential for Cl− (ECl) was close to the resting potential. When the extracellular [Cl−] was lowered, ECl became less negative and the membrane potential rapidly decreased to a level between EK and ECl determined by the reciprocal of the respective membrane conductances. Over some minutes, equal amounts of K+ and Cl− moved out of the fibre down their respective electrochemical gradients, and as the intracellular [Cl−] was initially much lower than the [K+], after only a small amount of Cl− efflux ECl moved back towards a largely unchanged EK, and consequently the fibre repolarized to close to its original resting level (Hodgkin & Horowicz, 1959). In the case of the skinned fibre experiments reported here, raising the myoplasmic [Cl−] should cause the T-system membrane to rapidly depolarize to between EK and the new ECl, as occurred in intact fibres. Again, there will be a tendency for both K+ and Cl− to move down their electrochemical gradients. However, in contrast to the intact fibre case, the K+ and Cl− will be moving into a sealed T-system, and as such, even a small influx of K+ will cause a large reduction in EK. Furthermore, the matched absolute influx of Cl− will have little effect on ECl unless the [Cl−] in the T-system lumen is initially extremely low. Thus, unlike in the intact fibre, the membrane will not repolarize towards the original resting level, but instead will remain relatively unchanged or become even more depolarized over time. In apparent agreement with this, the level of voltage-sensor inactivation apparent from the force response to successive depolarizations was found to remain steady after Cl− addition (e.g. Fig. 2A) or increase slightly over the 1-2 min (e.g. Fig. 4A) before reaching a steady level (not shown).
Estimation of relative Cl− conductance
A rough estimate of the relative permeability of the T-system membrane to Cl− can be obtained from the present data as follows. Comparison with voltage-clamp data in intact and cut fibres from rat EDL muscle indicates that the T-system potential of the rat skinned fibres used here must be more negative than -80 mV when the fibres are in the standard K+ solution, in order to account for the responsiveness, rapid repriming of the voltage sensors after a depolarization, and the observed resistance of such fibres to the effects of nifedipine (Lamb, 1986; Chua & Dulhunty, 1988; Dulhunty, 1992; Posterino & Lamb, 1998). It can be similarly concluded that addition of 20 mM Cl− to the myoplasm must depolarize the T-system to about -50 to -30 mV, if one is to explain the degree of activation and inactivation observed (Figs 1 and 2). The graded increase in apparent inactivation seen here with even small increases in myoplasmic [Cl−] fits well with the observation that inactivation in intact EDL fibres becomes steadily larger at any potential less negative than -90 mV (Dulhunty, 1992). The Goldman-Hodgkin- Katz equation allows estimation of the T-system potential (EM) as follows, assuming that the membrane is only permeable to K+, Na+ and Cl−:
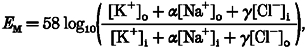 |
(1) |
where [X]o and [X]i are the concentrations of ion X in the T-system and myoplasm, respectively, and α and γ are the permeabilities of the T-system to Na+ and Cl−, respectively, relative to that to K+. In these experiments, [K+]i is 125 mM, [Na+]i is 36 mM, and [Na+]o should be ∼120-160 mM as Na+ must be the predominant cation in the T-system, and α is presumably a relatively small number (∼0.01-0.03) (Dulhunty, 1978; DeCoursey, Bryant & Owenburg, 1981). From the above, when [Cl−]i is 0 and 20 mM, EM is ∼-80 and ∼-40 mV, respectively. If, in the extreme case, the [Cl−] in the T-system ([Cl−]o) was 0, γ is ∼1, irrespective of the exact values of [K+]o and α. If instead, [Cl−]o is 50 or 100 mM, γ is ∼1.7 and ∼4.8, respectively. Thus, the Cl− permeability of the T-system in rat EDL fibres appears to be at least as large as the permeability to K+, and possibly ∼2-5 times larger. This agrees well with changes in relative permeabilities observed upon detubulation of rat EDL fibres, which implied that the Cl− conductance in the T-system is 4 or more times higher than the K+ conductance (Dulhunty, 1979).
Similar calculations can be made for the toad skinned fibres. In the standard K+ solution, the repriming rate is very similar to that in intact fibres of frog at -70 mV (Caputo, Bolaños & Gonzalez, 1984), and the effect of increased myoplasmic [K+] on activation and steady-state inactivation (Lamb & Stephenson, 1990) implies a similar resting potential (i.e. -70 mV) when compared with corresponding data for frog fibres (Bolaños, Caputo & Velaz, 1986; Melzer, Herrmann-Frank & Lüttgau, 1995). Similarly, the lack of any activation (Fig. 4A) and the level of inactivation occurring in 20 mM myoplasmic Cl− (Fig. 2C) indicates that the T-system was depolarized to only ∼-55 mV. The steady-state inactivation in 50 % K+ in the absence of Cl−, and the substantial activation and inactivation occurring upon addition of 20 mM Cl−, likewise imply T-system potentials of ∼-55 and ∼-40 mV, respectively. Substitution into eqn (1) gives ∼8 and ∼50 mM for T-system [K+] and [Cl−], respectively, and 0.35 for γ, with the exact values of α (in the range 0.01-0.03) and [Na+]o being of little importance. The fact that the calculated T-system [Cl−] is substantially lower than the normal extracellular concentration could be consistent either with the [Cl−] decreasing during ‘work-up’ (see above) or with there being a substantial density of fixed negative charges in the T-system that act as countercharges for cations. The latter has been proposed previously as an explanation of low apparent T-system Cl− conductance (see Eisenberg & Gage, 1969) and might also explain the large amount of divalent cation binding found in the T-system (Dulhunty, 1989; Owen, Lamb, Stephenson & Fryer, 1997). However, the above calculations would be altered if the T-system were substantially permeable to another anion present. In particular, it has been reported that frog fibres are permeable to the ionized form of Hepes (Venosa, Kotsias & Horowicz, 1994), which was present here in the myoplasm at about 20 mM. This would mean that Cl− was exerting a larger effect on the T-system potential than indicated above, and if, for example, Hepes− is half as permeant as Cl−, the above data could be reasonably fitted with [Cl−]o and γ being 120 mM and 0.8, respectively. In the case of rat fibres, however, the potent depolarizing effect of myoplasmic Cl− implies that other myoplasmic anions such as Hepes− must have only little effect on the potential and hence are relatively impermeant. In the above estimates, we have not attempted to take into account the polarizing effect of the Na+-K+ pump; the pump rate was presumably similar in the presence and absence of Cl−, and so its effect on the T-system potential seems unlikely to substantially alter the conclusions about the relative depolarization upon raising myoplasmic [Cl−].
Despite considerable uncertainties about the exact values, it is apparent that the relative Cl− permeability of the T-system in toad fibres is lower than that in rat fibres, but nevertheless is still substantial. The relative permeability obtained here in toad fibres is quite comparable with the observation of Heiny et al. (1990) that, during the early rapid phase of repolarization of an action potential, when the delayed K+ conductance is maximal, the Cl− current constitutes 9-15 % of the total outward current. Thus, in contrast to the conclusion of Eisenberg & Gage (1969), it seems that there is an appreciable Cl− conductance in amphibian fibres. The reason for the difference in conclusions is not certain, but could involve: (a) difficulties with the detubulation technique, (b) their use of low pH to block Cl− conductance, (c) a large increase in Cl− conductance with an increase in membrane potential from more negative than -70 mV (Eisenberg & Gage, 1969) to ∼-55 mV (this study), or (d) seasonal or cold-adaptation differences in membrane conductance (Dulhunty & Gage, 1973). In any case, we point out that the divergence in conclusions could be due to a relatively small error in the estimated absolute value of the T-system Cl− conductance in the study of Eisenberg & Gage (1969), because if the true conductance was even 10 % of the total fibre Cl− conductance (220 μS cm−2), this would be equal to ∼40 % of the estimated T-system K+ conductance (55 μS cm−2).
Effect of 9-AC
It was found here that 100 μM 9-AC largely or completely blocked the T-system Cl− conductance in rat fibres, but had little if any effect on the T-system conductance in toad fibres. The former is consistent with the reported ability of 100 μM 9-AC to block all of the Cl− conductance in rat fibres (Bretag, 1987; Fahlke & Rüdel, 1995), and the latter with the insensitivity of amphibian muscle to other Cl− channel blockers (Bretag, 1987). Allard & Rougier (1994) found that the force response of frog fibres to brief voltage-clamp steps was potentiated both by addition of 1 mM 9-AC and by replacement of extracellular Cl− with methanesulphonate. However, their current recordings show that 9-AC caused a far smaller reduction in the total outward current than did Cl− replacement, suggesting that even 1 mM 9-AC was insufficient to fully block the Cl− conductance in frog fibres.
ClC-1 and myotonia
As the total membrane area of the T-system is approximately 4 times that of the surface membrane in rat EDL fibres (Dulhunty, Gage & Lamb, 1986), it appears that T-system Cl− channels probably contribute the major portion of the total Cl− conductance (see also Palade & Barchi, 1977; Dulhunty, 1979). Consequently, if the ClC-1 channel in rat muscle is present only in the surface membrane, as has been concluded for mouse muscle (Gurnett et al. 1995), another type of Cl− channel besides ClC-1 must be of considerable importance too. We show here that the T-system Cl− channel, like ClC-1, is blocked by 100 μM 9-AC. Thus, it is possible that the 9-AC-sensitive Cl− current recorded in EDL fibres by Fahlke & Rüdel (1995) may have been generated by two types of Cl− channels: ClC-1 in the surface membrane and another type of Cl− channel in the T-system. This latter channel type is possibly the one observed in lipid bilayer recordings of rabbit T-tubular vesicles (Ide, Hidaka & Kasai, 1995). This channel has a much higher conductance (40 pS in 300 mM Cl−) than does ClC-1 (1 pS in 140 mM Cl−; Pusch, Steinmeyer & Jentsch, 1994), and is blocked by 100 μM 9-AC from the myoplasmic side but not from the extracellular (i.e. T-system lumenal) side, whereas ClC-1 is blocked by 9-AC only from the extracellular side (Rychov, Astill, Bennetts, Hughes, Bretag & Roberts, 1997). Interestingly, Fahlke & Rüdel (1995) reported that 100 μM 9-AC took 10 min to fully block the Cl− current when applied extracellularly to a single EDL fibre. This could suggest that a substantial part of the Cl− current was blocked by 9-AC acting at an intracellular site, which would again indicate an important role of a Cl− channel other than ClC-1. In our experiments, 9-AC was added to the myoplasm (i.e. ‘intracellular’ environment) and caused rapid (< 30 s) block of the T-system Cl− conductance, and this effect could also be rapidly reversed (< 30 s) by washout of 9-AC. However, we are unable to conclude that 9-AC necessarily exerted its action on the intracellular side, because it may have rapidly equilibrated inside the T-system given the large surface to volume ratio.
Myotonia can be induced in a variety of mammals by replacing extracellular Cl− with an impermeant anion or addition of Cl− channel blockers, such as 9-AC (Bryant & Morales-Aguilera, 1971; Rüdel & Lehmann-Horn, 1985; Bretag, 1987). Congenital myotonia in humans and goats is apparently due to a decrease in Cl− conductance (Bryant & Morales-Aguilera, 1971; Rüdel & Lehmann-Horn, 1985; Franke, Iaizzo, Hatt, Spittelmeister, Ricker & Lehmann-Horn, 1991) and is associated with mutations in ClC-1 (Koch et al. 1992; Beck, Fahlke & George, 1996). Importantly, although expression of ClC-1 is completely absent in ‘ADR’ myotonic mice (Steinmeyer et al. 1991a), the Cl− conductance is still substantial, estimated as 26 % of the total conductance (Mehrke, Brinkmeier & Jockusch, 1988), and this will be a considerable underestimate if the conductance is located primarily in the T-system (see Dulhunty et al. 1984). This again suggests that a large fraction of the total Cl− conductance in mouse fibres is not due to ClC-1, and may instead be due to an unaffected Cl− conductance in the T-system. A similar situation may prevail in human myotonia congenita, given that a substantial Cl− conductance still remains (Rüdel & Lehmann-Horn, 1985). It has been proposed previously that myotonia arises when a train of action potentials raises the [K+] in the T-system, slightly depolarizing the T-system, which in turn depolarizes the surface membrane sufficiently to generate further action potentials if the Cl− conductance there is low (Adrian & Bryant, 1974). We point out that, although the original proposal had not considered the possibility that the surface and T-system Cl− conductances in myotonic animals might be differentially affected, such a mechanism can still account for the occurrence of myotonia in muscles that retain a high T-system Cl− conductance. A high Cl− conductance in the T-system may well reduce the [K+] increase occurring with each action potential (Dulhunty, 1979), as well as reducing the level of T-system depolarization it causes. Nevertheless, if the depolarization still becomes substantial after repetitive activity, it will trigger further action potential generation at the surface membrane, causing myotonia. This phenomenon might explain why the relative Cl− conductance in the T-system of rats is apparently substantially higher than in frogs and toads, because this would be a way of reducing the consequences of the greater K+ accumulation that presumably occurs with activity because of the relatively high surface to volume ratio of the T-system in rat fibres (Dulhunty, 1984). Finally, we note that models of myotonia that utilize Cl− replacement or Cl− channel blockers may well induce a disproportionately large myotonic effect, because they block the Cl− conductance in the T-system as well as in the surface membrane.
Acknowledgments
We thank Professor D. G. Stephenson for helpful comments and the National Health and Medical Research Council of Australia for financial support.
References
- Adrian RH, Bryant SH. On the repetitive discharge in myotonic muscle fibres. The Journal of Physiology. 1974;240:505–515. doi: 10.1113/jphysiol.1974.sp010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B, Rougier O. The effects of chloride ions in excitation-contraction coupling and sarcoplasmic reticulum calcium release in twitch muscle fibre. Journal of Muscle Research and Cell Motility. 1994;15:563–571. doi: 10.1007/BF00121162. [DOI] [PubMed] [Google Scholar]
- Bakker AJ, Lamb GD, Stephenson GD. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. Journal of Muscle Research and Cell Motility. 1996;17:55–67. doi: 10.1007/BF00140324. [DOI] [PubMed] [Google Scholar]
- Beck CL, Fahlke C, George AL. Molecular basis for decreased muscle chloride conductance in the myotonic goat. Proceedings of the National Academy of Sciences of the USA. 1996;93:11248–11252. doi: 10.1073/pnas.93.20.11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños P, Caputo C, Velaz L. Effects of calcium, barium and lanthanum on depolarization-contraction coupling in skeletal muscle fibres of Ranapipiens. The Journal of Physiology. 1986;370:39–60. doi: 10.1113/jphysiol.1986.sp015921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretag AH. Muscle chloride channels. Physiological Reviews. 1987;67:618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- Bryant SH, Morales-Aguilera A. Chloride conductance in normal and myotonic muscle fibres and the action of monocarboxylic aromatic acids. The Journal of Physiology. 1971;219:367–383. doi: 10.1113/jphysiol.1971.sp009667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C, Bolaños P, Gonzalez GF. Effect of membrane polarization on contractile threshold and time course of prolonged contractile responses in skeletal muscle fibres. Journal of General Physiology. 1984;84:927–943. doi: 10.1085/jgp.84.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua M, Dulhunty AF. Inactivation of excitation- contraction coupling in rat extensor digitorum longus and soleus muscles. Journal of General Physiology. 1988;91:737–757. doi: 10.1085/jgp.91.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonan JR, Lamb GD. Effect of chloride on Ca2+ release from the sarcoplasmic reticulum of mechanically skinned skeletal muscle fibres. Pflügers Archiv. 1998. in the Press. [DOI] [PubMed]
- DeCoursey TE, Bryant SH, Owenburg KM. Dependence of membrane potential on extracellular ionic concentrations in myotonic goats and rats. American Journal of Physiology. 1981;240:C56–63. doi: 10.1152/ajpcell.1981.240.1.C56. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF. The dependence of membrane potential on extracellular chloride concentration in mammalian skeletal muscle fibres. The Journal of Physiology. 1978;276:67–82. doi: 10.1113/jphysiol.1978.sp012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty AF. Distribution of potassium and chloride permeability over the surface and T-tubule membrane of mammalian skeletal muscle. Journal of Membrane Biology. 1979;45:293–310. doi: 10.1007/BF01869290. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF. Effect of chloride withdrawal on the geometry of the T-tubules in amphibian and mammalian muscle. Journal of Membrane Biology. 1982;67:81–90. doi: 10.1007/BF01868650. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF. Heterogeneity of T-tubule geometry in vertebrate skeletal muscle fibres. Journal of Muscle Research and Cell Motility. 1984;5:333–347. doi: 10.1007/BF00713111. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF. Feet, bridges, and pillars in triad junctions of mammalian skeletal muscle: their possible relationship to calcium buffers in terminal cisternae and T-tubules and to excitation- contraction coupling. Journal of Membrane Biology. 1989;109:73–83. doi: 10.1007/BF01870792. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF. The voltage-activation of contraction in skeletal muscle. Progress in Biophysics and Molecular Biology. 1992;57:181–223. doi: 10.1016/0079-6107(92)90024-z. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Carter G, Hinrichsen C. The membrane capacity of mammalian skeletal muscle fibres. Journal of Muscle Research and Cell Motility. 1984;5:315–332. doi: 10.1007/BF00713110. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Gage PW. Electrical properties of toad sartorius muscle fibres in summer and winter. The Journal of Physiology. 1973;230:619–641. doi: 10.1113/jphysiol.1973.sp010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty AF, Gage PW, Lamb GD. Differential effects of thyroid hormone on T-tubules and terminal cisternae in rat muscles: an electrophysiological and morphometric analysis. Journal of Muscle Research and Cell Motility. 1986;7:225–236. doi: 10.1007/BF01753555. [DOI] [PubMed] [Google Scholar]
- Eisenberg RS, Gage PW. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibres. Journal of General Physiology. 1969;53:279–297. doi: 10.1085/jgp.53.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Rüdel R. Chloride currents across the membranes of mammalian skeletal muscle fibres. The Journal of Physiology. 1995;484:355–368. doi: 10.1113/jphysiol.1995.sp020670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulks JG, Pacey JA, Perry FA. Contractures and swelling of the transverse tubules during chloride withdrawal in frog skeletal muscle. The Journal of Physiology. 1965;180:96–115. [PMC free article] [PubMed] [Google Scholar]
- Franke C, Iaizzo PA, Hatt H, Spittelmeister W, Ricker K, Lehmann-Horn F. Altered Na+ channel activity and reduced Cl− conductance cause hyperexcitability in recessive generalized myotonia (Becker) Muscle and Nerve. 1991;14:762–770. doi: 10.1002/mus.880140811. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, Kahl SD, Anderson RD, Campbell KP. Absence of the skeletal muscle sarcolemma chloride channel ClC-1 in myotonic mice. Journal of Biological Chemistry. 1995;270:9035–9038. doi: 10.1074/jbc.270.16.9035. [DOI] [PubMed] [Google Scholar]
- Heiny JA, Valle JR, Bryant SH. Optical evidence for a chloride conductance in the T-system of frog skeletal muscle. Pflügers Archiv. 1990;416:288–295. doi: 10.1007/BF00392065. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Horowicz P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. The Journal of Physiology. 1959;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T, Hidaka J, Kasai M. An anion channel from transverse tubular membranes incorporated into planar bilayers. Biochimica et Biophysica Acta. 1995;1237:115–120. doi: 10.1016/0005-2736(95)00091-g. [DOI] [PubMed] [Google Scholar]
- Koch M, Steinmeyer K, Lorenz C, Ricker K, Wolf F, Otto M, Zoll B, Lehmann-Horn F, Grzeschik K, Jentsch TJ. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992;257:797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- Lamb GD. Components of charge movement in rabbit skeletal muscle: the effects of tetracaine and nifedipine. The Journal of Physiology. 1986;376:85–100. doi: 10.1113/jphysiol.1986.sp016143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. The Journal of Physiology. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. The Journal of Physiology. 1990;423:495–517. doi: 10.1113/jphysiol.1990.sp018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. The Journal of Physiology. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G, Brinkmeier H, Jockusch H. The myotonic mouse mutant ADR: electrophysiology of the muscle fibre. Muscle and Nerve. 1988;11:440–446. doi: 10.1002/mus.880110505. [DOI] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Lüttgau H., Ch. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochimica et Biophysica Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Owen VJ, Lamb GD, Stephenson DG, Fryer MW. Relationship between depolarization-induced force responses and Ca2+ content in skeletal muscle fibres of rat and toad. The Journal of Physiology. 1997;498:571–586. doi: 10.1113/jphysiol.1997.sp021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade PT, Barchi RL. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. Journal of General Physiology. 1977;69:325–342. doi: 10.1085/jgp.69.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino GS, Lamb GD. Effect of nifedipine on depolarization-induced force responses in skinned skeletal muscle fibres of rat and toad. Journal of Muscle Research and Cell Motility. 1998;19:53–65. doi: 10.1007/BF03257390. [DOI] [PubMed] [Google Scholar]
- Pusch M, Steinmeyer K, Jentsch TJ. Low single channel conductance of the major skeletal muscle chloride channel, ClC-1. Biophysical Journal. 1994;66:149–152. doi: 10.1016/S0006-3495(94)80753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdel R, Lehmann-Horn F. Membrane changes in cells from myotonia patients. Physiological Reviews. 1985;65:310–356. doi: 10.1152/physrev.1985.65.2.310. [DOI] [PubMed] [Google Scholar]
- Rychkov GY, Astill D, St J., Bennetts B, Hughes BP, Bretag AH, Roberts ML. pH-dependent interactions of Cd2+ and a carboxylate blocker with the rat ClC-1 chloride channel and its R304E mutant in the Sf-9 insect cell line. The Journal of Physiology. 1997;501:355–362. doi: 10.1111/j.1469-7793.1997.355bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmeyer K, Klocke R, Ortland C, Gronemeier M, Jockusch H, Gründer S, Jentsch TJ. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Science. 1991a;354:304–308. doi: 10.1038/354304a0. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991b;354:301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. The Journal of Physiology. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venosa RA, Kotsias BA, Horowicz P. Frog striated muscle is permeable to hydroxide and buffer anions. Journal of Membrane Biology. 1994;139:57–74. doi: 10.1007/BF00232675. [DOI] [PubMed] [Google Scholar]


