Abstract
In anaesthetized rats, we have examined the role of adenosine in vasodilatation evoked in the cerebral cortex by systemic hypoxia (breathing 8 % O2). Red cell flux was recorded from the surface of the exposed parietal cortex (CoRCF) by a laser Doppler probe, cortical vascular conductance (CoVC) being computed as CoRCF divided by mean arterial blood pressure. All agonists and antagonists were applied topically to the cortex.
Systemic hypoxia or adenosine application for 5 or 10 min, respectively, induced an increase in CoRCF and CoVC. These responses were substantially reduced by 8-phenyltheophylline (8-PT), an adenosine receptor antagonist which is non-selective between the adenosine A1 and A2A receptor subtypes. By contrast, the adenosine receptor antagonist 8-sulphophenyltheophylline (8-SPT) which is similarly non-selective, but unlike 8-PT, does not cross the blood-brain barrier, reduced the increases in CoRCF and CoVC induced by adenosine, but had no effect on those induced by hypoxia.
The A2A receptor agonist CGS21680 produced a substantial increase in CoRCF and CoVC, but the A1 receptor agonist 2-chloro-N6-cyclopentyladenosine had minimal effects.
The A2A receptor antagonist ZM241385 reduced the increase in CoRCF and CoVC induced by adenosine and reduced the increase in CoRCF induced by hypoxia.
We propose that exogenous adenosine that is topically applied to the cerebral cortex produces vasodilatation by acting on A2A receptors on the vascular smooth muscle. However, during systemic hypoxia, we propose that adenosine is released from endothelial cells and acts on endothelial A2A receptors to produce the major part of the hypoxia-induced dilatation in the cerebral cortex.
It is generally accepted that systemic hypoxia exerts strong dilator influences upon the vasculature of skeletal muscle and the brain due to the action of locally released vasodilator substances. Our studies on the rat indicated that in skeletal muscle, adenosine plays a major part in this vasodilatation (Mian & Marshall, 1991; Thomas & Marshall, 1994; Skinner & Marshall, 1996). However, for the cerebral circulation, the observations reported to date are equivocal. Thus, Winn, Rubio & Berne (1981) showed that adenosine levels in the cerebral cortex of rats rose within a few seconds of the onset of systemic hypoxia, while theophylline given systemically reduced the hypoxia-induced dilatation of the pial vessels (Morii, Ngai, Ko & Winn, 1987). This finding must be treated with caution for theophylline is not only an adenosine receptor antagonist, but can inhibit phosphodiesterase activity so leading to an increase in cAMP levels (Smellie, Davis, Daly & Wells, 1979). However, adenosine deaminase has also been shown to attenuate the increase in pial vessel diameter induced in the rat by systemic hypoxia (Simpson & Phillis, 1991), so supporting a role for adenosine. On the other hand, experiments on cats and human subjects showed that theophylline, given locally or systemically, had no effect on dilatation induced in the pial circulation or cerebral cortex by systemic hypoxia (Haller & Kuschinsky, 1987; Bowton, Haddon, Prough, Adair, Alford & Stump, 1988). Similarly, experiments on newborn piglets, (McPhee & Maxwell, 1987) showed that theophylline had no effect on hypoxia-induced cerebral vasodilatation. By contrast, Laudignon, Farri, Beharry, Rex & Aranda (1990) who also used newborn piglets reported that the adenosine receptor antagonist 8-phenyltheophylline (8-PT), which does not have phosphodiesterase inhibitory activity (Smellie et al. 1979), substantially reduced hypoxia-induced cerebral vasodilatation.
Our own studies are also inconclusive (see Thomas & Marshall, 1994). In experiments on rats, we recorded blood flow from the common carotid artery with the external carotid artery ligated, as an index of forebrain blood flow. The increase in carotid vascular conductance (CVC) induced by systemic hypoxia was reduced by 8-PT given systemically. However, 8-PT also reduced the hypoxia-induced fall in systemic arterial pressure. Thus, even though the effect on CVC might be attributed to blockade of the effect of adenosine on cerebral vessels, it could also be explained by a smaller cerebral myogenic response to a smaller fall in perfusion pressure.
In view of these results, a primary aim of the present study was to more rigorously test whether adenosine contributes to vasodilatation induced in the cerebral cortex by systemic hypoxia. To this end, we made recordings of red cell flux in the cerebral cortex (CoRCF) of anaesthetized rats by using a laser Doppler meter and probe. This method does not provide absolute measurements of blood flow per unit volume of tissue, but it does indicate changes in perfusion that correlate very well with changes in blood flow recorded using other established methods (Skarphedinsson, Hårding & Thorén, 1988; Fabricus & Lauritzen, 1996). In this way, we tested the effect of 8-PT (a selective adenosine receptor antagonist, but with no real specificity for any one adenosine receptor subtype) when applied topically to the cerebral cortex upon the cortical vasodilatation induced by systemic hypoxia and by topically applied adenosine.
Having obtained evidence that adenosine does make a substantial contribution to the hypoxia-induced cerebral vasodilatation, a second aim was to investigate the mode of action of adenosine. It has been assumed that adenosine is released from tissue parenchyma during hypoxia and that it causes vasodilatation by acting on adenosine receptors on the vascular smooth muscle (Olsson & Pearson, 1990). However, more recent studies demonstrate that adenosine can also induce dilatation by acting on endothelial receptors in an nitric oxide-dependent manner (see Skinner & Marshall, 1996). Further, in isolated hearts, radiolabelled adenosine that was taken up by the coronary endothelium was shown to be released during hypoxic perfusion (Deussen, Möser & Schrader, 1986), while endothelial cell cultures were also shown to release adenosine under hypoxic conditions (Nees & Gerlach, 1983). Thus, it is a reasonable hypothesis that the adenosine that mediates cerebral vasodilatation during systemic hypoxia is released from the endothelium and acts via endothelial receptors. Indeed, this is exactly what our evidence suggests for skeletal muscle circulation (see Skinner & Marshall, 1996). To test this hypothesis, 8-sulphophenyltheophylline (8-SPT), was topically applied to the cortex: 8-SPT is an adenosine receptor antagonist which is similar to 8-PT in having no selectivity for either the A1 or A2 adenosine receptor subtype (see below), but unlike the 8-PT does not cross the blood-brain barrier (Evoniuk, Von Borstel & Wurtman, 1987).
Until recently, it was accepted that adenosine generally induces vasodilatation by acting on the A2 subtype of adenosine receptor (Olsson & Pearson, 1990) which can be further subdivided into A2A and A2B receptors. The EC50 values for adenosine are in the high nanomolar range for the A2A receptor and in the micromolar range for the A2B subtype (Daly, 1985), suggesting that the A2A receptors are likely to be of greater physiological importance. However, more recent studies indicate that adenosine can also induce dilatation by acting on A1 receptors, for example in porcine coronary artery (Dart & Standen, 1993). In fact, our evidence indicates that in skeletal muscle circulation of the rat, the dilatation induced by systemic hypoxia is mediated by A1 receptors (see Bryan & Marshall, 1996).
Thus, a third aim of the present study was to establish the adenosine receptor subtype that is important in mediating cerebral vasodilatation induced by systemic hypoxia. For this purpose, we applied the following substances topically to the cerebral cortex: 2-chloro-N6-cyclopentyladenosine (CCPA) which is a highly selective A1 receptor agonist (Lohse, Klotz, Schwabe, Cristalli, Vittori & Grifantini, 1988), CGS21680 which is a relatively selective agonist for A2A receptors (Hutchison, Webb, Oei, Ghai, Zimmerman & Williams, 1989) and ZM241385 which is a non-xanthine adenosine receptor antagonist with high affinity at the A2A receptor (Poucher et al. 1995). Some of the results of these studies have already been reported in brief (Coney & Marshall, 1995a,b; 1996a,b).
METHODS
Experiments were performed on male Wistar rats that were anaesthetized and prepared for recording cardiovascular variables largely as we have described previously (Thomas & Marshall, 1994). In brief, after induction of anaesthesia with 3.5 % halothane in O2, anaesthesia was maintained by a continuous i.v. infusion of Saffan (Alphaxolone/Alphadolone; Pitman-Moore, Uxbridge, Middlesex, UK) at 12-20 mg kg−1 h−1 during surgery and at 7-12 mg kg−1 h−1 during the experimental period (see below). At the end of the experiments the animals were killed by overdose of anaesthetic. The trachea was cannulated with a T-shaped cannula and connected via tubing to an air pump such that the animal either breathed air or a hypoxic mixture of 8 % O2 in N2, which was directed across the end of the sidearm. Arterial pressure (ABP) was recorded from the femoral artery and the left brachial artery was cannulated to allow anaerobic sampling of blood for analysis of the partial pressures of O2 and CO2 (Pa,O2 and Pa,CO2, respectively) by a Nova Stat Profile Analyser (Stat 3, V. A. Howe, Waltham, MA, USA).
In Series 1 (see below) carotid artery blood flow (CBF) was recorded using a cuff-type electromagnetic probe placed on the left common carotid artery after vascular isolation of the internal carotid artery (Thomas & Marshall, 1994). At this stage in Series 1, and after the cannulations had been completed in the other series of experiments (see below), the animal was placed in a stereotaxic frame and the parietal bone removed by gradually thinning the bone bilaterally between the temporal and transverse suture lines using a dental burr: bone wax was used to achieve haemostasis. The dura mater was carefully removed from the left parietal cortex and the surface of the cortex was superfused with a mock cerebrospinal fluid (CSF) containing (mM): NaCl, 128.3; KCl, 3.3; CaCl2, 1.7; MgCl2, 1.2; NaHCO3, 25; glucose, 10. It was bubbled with CO2 to give a PCO2 of ∼40 mmHg (38-42 mmHg) as measured by the gas analyser. A plasticine barrier was fixed caudal to the craniotomy so that when a drug was applied topically to the cortex it bathed the surface for the whole period required by the protocol (see below). A laser Doppler PF310 probe connected to a Periflux PF3 meter (Perimed Ltd, Stockholm, Sweden) was then positioned on the left parietal cortex for recording CoRCF, care being taken to ensure that the probe tip was as far away as possible from larger pial vessels.
The recordings of ABP, CBF and CoRCF were made either on a pen recorder or on an Apple PowerMac 6100 computer via MacLab (ADInstruments Ltd, Hastings) at a sampling frequency of 40 Hz. Heart rate (HR) was computed on-line from the ABP recording and carotid and cortical vascular conductances (CVC and CoVC, respectively) were computed instantaneously on-line as the means of CBF and CoRCF divided by mean ABP (MABP), respectively.
Experimental protocols
After surgery, the animal was allowed to equilibrate at the lighter level of anaesthesia (see above) until the cardiovascular variables had stabilized.
Series 1
In six rats (305 ± 4 g body weight), recordings were made during air breathing, during a 5 min period of hypoxia (breathing 8 % O2) and for a 10 min period of air breathing during which adenosine (10 mM) in mock CSF was topically applied to the cortex. The CSF was then aspirated and replaced with mock CSF containing 1 mM 8-PT which was dissolved under sonication. This dose of 8-PT is just higher than its Ki at A2 receptors and 10 times its Ki at A1 receptors (Bruns, Lu & Pugsley, 1986), where the Ki is the affinity constant of the drug. After an equilibrium period of ∼10 min, recordings were again made during air breathing, hypoxia and adenosine application as just described. Samples for blood gas analysis were taken during air breathing just prior to the hypoxic period, in the last minute of hypoxia, during air breathing just prior to topical application of adenosine and in the 10th minute of the application, both before and after 8-PT.
Series 2
Recordings were made in nine rats (313 ± 3 g) using the same protocol as in Series 1, except that rather than 8-PT, 1 mM 8-SPT dissolved in mock CSF was topically applied to the cortex: topical application of this dose of 8-SPT was previously shown to attenuate dilatation induced by adenosine and by systemic hypoxia in skeletal muscle microcirculation (Mian & Marshall, 1996).
Series 3a
In six rats (320 ± 5 g), CCPA was topically applied to the cortex at 100 μM: CCPA was dissolved in mock CSF with 1 % DMSO. This concentration was chosen because pilot studies showed that lower concentrations had no detectable effect on CoRCF. CCPA has a Ki at A1 receptors of 0.4 nM, thus it did not seem appropriate to apply CCPA at doses higher than 100 μm, particularly as this would have required a concomitant increase in the concentration of DMSO.
Series 3b
In seven rats (310 ± 6 g), CGS21680 dissolved in mock CSF was topically applied to the cortex at a range of concentrations from 10 nM to 10 μM (see Results), such that the induced increase in CoRCF was of similar magnitude to that induced by adenosine in Series 1 and 2. In Series 3a and 3b the drugs were applied topically for 5 min and samples for blood gas analysis were taken just prior to, and in the 5th minute of drug application.
Series 4
Recordings were made in nine rats (316 ± 4 g) as described in Series 1, except that rather than 8-PT, the selective A2A antagonist ZM241385 was applied topically to the cortex at a concentration of 150 μM: it was dissolved in 0.5 % 0.1 M NaOH and 0.5 % polyethylene glycol (average molecular weight, 400) in physiological saline. The vehicle was also topically applied to ensure it had no effect on any of the measured variables. This concentration of ZM241385 was calculated from that used previously as an intravenous dose in the rat to reverse the effect of an intra-arterial infusion of the A2A agonist CGS21680 (see Bryan & Marshall, 1996) assuming the drug was freely distributed in plasma. In order to test the effect of the vehicle, the vehicle alone was topically applied to the cortex after the control responses to hypoxia and adenosine had been recorded and the responses to these stimuli were retested: the vehicle was then replaced with vehicle containing ZM241385.
Statistical analysis
All data are presented as means ±s.e.m. In Series 1, 2 and 4, analysis of the responses to hypoxia and adenosine application, before and after application of an antagonist, was performed by taking the values at 1 min intervals over the 5 min period of hypoxia and at 2 min intervals over the 10 min period of adenosine application and applying a two-way ANOVA for time effects and drug effects. The effect of topical application of drug on baseline values was analysed by Student's paired t test. In Series 3, the effect on the recorded variables of a 5 min period of agonist application was analysed by Student's paired t test. The values of mean arterial pressure (MABP) and HR are expressed in absolute terms. Baseline values of CoRCF were in the range of 66-140 perfusion units in all groups where 100 perfusion units are equivalent to a voltage signal of 1 V. CoRCF and CoVC are expressed as a percentage of the value recorded immediately before each stimulus: CBF and CVC are also expressed as a percentage of the baseline immediately before the stimulus so that the vascular responses recorded in the cerebral cortex and in the whole forebrain may be compared.
Drugs
8-PT was supplied by Sigma; 8-SPT, CCPA and CGS21680 were supplied by Research Biochemicals Inc (Semat Technical (UK) Ltd, Herts); ZM241385 was a kind gift from Dr Simon Poucher at Zeneca Pharmaceuticals.
RESULTS
Series 1
A 5 min period of hypoxia (breathing 8 % O2) evoked a similar pattern of change in ABP, HR, CBF and CVC to that described previously (Thomas & Marshall, 1994) with CVC increasing as ABP fell, indicating vasodilatation in the forebrain. Meanwhile, CoRCF increased as did CoVC, indicating vasodilatation in the cerebral cortex (Figs 1 and 2). Topical application of adenosine onto the cortex (Fig. 3) evoked no change in ABP or HR and no significant change in CBF or CVC. However, adenosine induced an increase in CoRCF and CoVC indicating cortical vasodilatation (Fig. 3).
Figure 1. Responses evoked by systemic hypoxia (breathing 8 % O2) before and after topical application of 8-PT to the cerebral cortex.
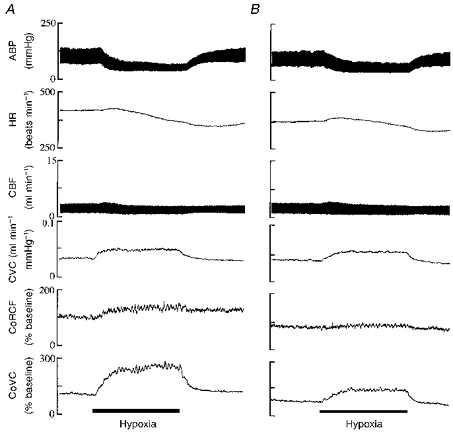
Traces from above downwards are arterial blood pressure (ABP), heart rate (HR), carotid blood flow (CBF), carotid vascular conductance (CVC), cortical red cell flux (CoRCF) and cortical vascular conductance (CoVC). Continuous bars beneath traces indicate 5 min periods of hypoxia. 8-PT at 1 μM was given between A and B.
Figure 2. Effects of topical application of 8-PT to the cerebral cortex on responses induced by systemic hypoxia (breathing 8 % O2).
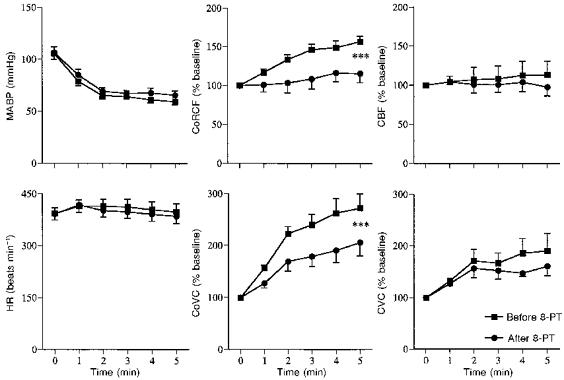
Abbreviations as in Fig. 1 except MABP indicates mean arterial blood pressure. In each graph values are means ±s.e.m. during air breathing (time 0) and over 5 min of hypoxia as indicated by abscissa. ▪, before 8-PT; •, after 8-PT. CoRCF, CoVC, CBF and CVC are expressed as percentages of values recorded before hypoxic stimulus (time 0). *** Significant difference between response recorded before and after 8-PT; P < 0.001.
Figure 3. Effects of topical application of 8-PT to the cerebral cortex on responses induced by topically applied adenosine.
Abbreviations and symbols as in Fig. 2.
Topical application of 8-PT had no effect on the baseline values of any of the recorded variables (Figs 2 and 3, Table 3), nor on the changes induced in MABP and HR by hypoxia, but the increases in CoRCF and CoVC were attenuated (Figs 1 and 2). Similarly, the increases in CoRCF and CoVC evoked by adenosine were attenuated by 8-PT, without an effect on MABP or HR (Fig. 3). Values of arterial blood gases recorded during air breathing and hypoxia are shown in Table 1, whilst those recorded during adenosine application are shown in Table 2: it can be seen the values recorded were fully comparable before and after 8-PT.
Table 3.
Effect of topical application of antagonist
| Series | MABP (mmHg) | HR (beats min−1) | CoRCF (% baseline) | CoVC (% baseline) | CBF (% baseline) | CVC (% baseline) | |
|---|---|---|---|---|---|---|---|
| 1 | Control | 108 ± 6 | 382 ± 17 | 100 | 100 | 100 | 100 |
| + 8-PT | 106 ± 6 | 391 ± 17 | 111 ± 7 | 118 ± 6 | 100 ± 5 | 102 ± 5 | |
| 2 | Control | 117 ± 4 | 425 ± 14 | 100 | 100 | — | — |
| + 8-SPT | 117 ± 5 | 420 ± 16 | 94 ± 5 | 91 ± 8 | — | — | |
| 4 | Control | 126 ± 5 | 387 ± 11 | 100 | 100 | — | — |
| + ZM241385 | 125 ± 5 | 377 ± 11 | 96 ± 6 | 98 ± 5 |
Effect of topical application of antagonist in Series 1 (8-PT), Series 2 (8-SPT) or Series 4 (ZM241385) on baseline values of the measured variables.
Table 1.
Effect of antagonist on changes in arterial blood gases induced by hypoxia
| Pa,O2 (mmHg) | Pa,CO2 (mmHg) | ||||
|---|---|---|---|---|---|
| Series | Before antagonist | After antagonist | Before antagonist | After antagonist | |
| 1 | Control | 91.6 ± 2.2 | 92.7 ± 2.0 | 41.6 ± 1.4 | 41.5 ± 1.3 |
| Hypoxia | 34.2 ± 1.0 | 33.5 ± 1.3 | 31.5 ± 1.1 | 30.8 ± 1.2 | |
| 2 | Control | 86.9 ± 2.4 | 91.4 ± 3.6 | 40.7 ± 0.9 | 40.0 ± 1.1 |
| Hypoxia | 30.5 ± 1.1 | 31.8 ± 0.8 | 28.0 ± 0.7 | 28.0 ± 0.7 | |
| 4 | Control | 95.2 ± 1.4 | 94.9 ± 1.5 | 41.3 ± 1.2 | 40.7 ± 1.6 |
| Hypoxia | 31.7 ± 0.6 | 31.7 ± 0.6 | 29.5 ± 1.0 | 30.1 ± 1.2 | |
Arterial blood gases (Pa,O2 and Pa,CO2) measured during air breathing (Control) and when breathing 8% O2 (Hypoxia) both before and after topical application of the antagonists in Series 1 (8-PT),Series 2 (8-SPT) and Series 4 (ZM241385).
Table 2.
Effect of agonist on arterial blood gases
| Pa,O2 (mmHg) | Pa,CO2 (mmHg) | ||||
|---|---|---|---|---|---|
| Series | Stimulus | Before agonist | During agonist | Before agonist | During agonist |
| 1 | Adenosine | 91.6 ± 2.2 | 93.2 ± 1.8 | 41.6 ± 1.4 | 41.2 ± 1.6 |
| Adenosine +8-PT | 92.7 ± 2.0 | 93.8 ± 1.6 | 41.5 ± 1.3 | 40.9 ± 1.4 | |
| 3a | CCPA | 87.4 ± 3.5 | 86.6 ± 2.6 | 43.2 ± 1.8 | 46.5 ± 1.6 |
| 3b | CGS21680 | 90.7 ± 1.9 | 91.4 ± 1.2 | 40.6 ± 1.0 | 39.8 ± 1.3 |
Arterial blood gases (Pa,O2 and Pa,CO2) measured before and during topical application of adenosine both before and after application of 8-PT (Series 1) and during topical application of either a selective A1 (CCPA; Series 3a) or A2A (CGS21680; Series 3b) agonist.
Series 2
The control responses induced by hypoxia and by adenosine (Figs 4 and 5) were similar to those seen in Series 1. Like 8-PT, 8-SPT did not affect the baseline values or the changes induced by hypoxia in MABP or HR (Figs 4 and 5, Table 3). However, in contrast to the 8-PT, 8-SPT had no effect on the increases in CoRCF and CoVC induced by hypoxia (Fig. 4). On the other hand, 8-SPT, like 8-PT, did attenuate the increases in CoRCF and CoVC induced by adenosine (Fig. 5). The values of arterial blood gases recorded during air breathing and hypoxia were not affected by 8-SPT (Table 1).
Figure 4. Effects of topical application of 8-SPT to the cerebral cortex on responses induced by systemic hypoxia (breathing 8 % O2).

Abbreviations and symbols as in Fig. 2.
Figure 5. Effects of topical application of 8-SPT to the cerebral cortex on responses induced by topically applied adenosine.

Abbreviations and symbols as in Fig. 2; *P < 0.05.
Series 3
In Series 3a, topical application of CCPA at 100 μM did not affect MABP or HR and only induced a small increase in CoRCF with no significant increase in CoVC (Fig. 6). However, in Series 3b, topical application of CGS21680 at 10 nM to 10 mM had no significant effect on MABP or HR, but induced substantial increases in CoRCF and CoVC (Fig. 6). In practice, the concentrations used to produce an increase in CoRCF similar to that induced by adenosine in Series 1 were 10 nM in one rat, 100 nM in three rats, 1 μM in two rats and 10 μM in one rat (see Discussion). Neither CCPA, nor CGS21680 affected blood gas values (Table 2).
Figure 6. Responses induced by topical application to the cerebral cortex of the A1 and A2 adenosine receptor agonists CCPA and CGS21680, respectively.

Abbreviations as in Fig. 2. □, baseline values; shaded columns indicate values recorded at the end of 5 min topical application of the agonist ( , CCPA;
, CCPA;  , CGS21680). Values for CoRCF and CoVC during agonist application are shown as percentage of baseline values. ** and * indicate a significant difference between values recorded at baseline and at the 5th minute of agonist application of P < 0.01 and P < 0.05, respectively.
, CGS21680). Values for CoRCF and CoVC during agonist application are shown as percentage of baseline values. ** and * indicate a significant difference between values recorded at baseline and at the 5th minute of agonist application of P < 0.01 and P < 0.05, respectively.
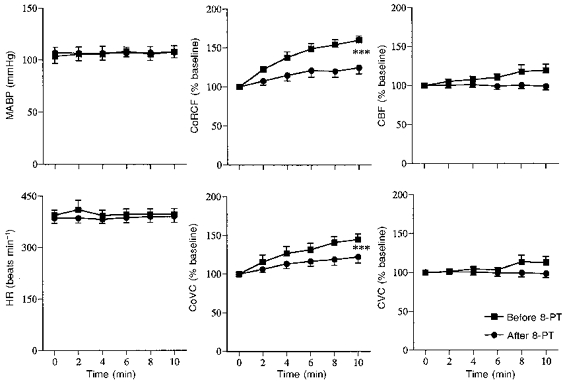
Series 4
Hypoxia induced similar responses to those seen in Series 1 and 2 (See Fig. 7 and Table 1). Topical application of ZM241385 had no effect on baseline values, but the increase in CoRCF induced by hypoxia was significantly attenuated (Fig. 7 and Table 3): there was no significant effect on the hypoxia-induced increase in CoVC though it tended to be reduced. ZM241385 significantly attenuated both the increases in CoRCF and CoVC that were induced by topical application of adenosine without affecting MABP or HR (Fig. 8). Topical application of the vehicle had no significant effect on baseline values nor the response induced by hypoxia or adenosine (data not shown).
Figure 7. Effects of topical application to the cerebral cortex of the A2A adenosine receptor antagonist ZM241385 on responses induced by systemic hypoxia (breathing 8 % O2).

Abbreviations and symbols as in Fig. 2. **P < 0.01.
Figure 8. Effects of topical application to the cerebral cortex of the A2A adenosine receptor antagonist ZM241385 on responses induced by topically applied adenosine.
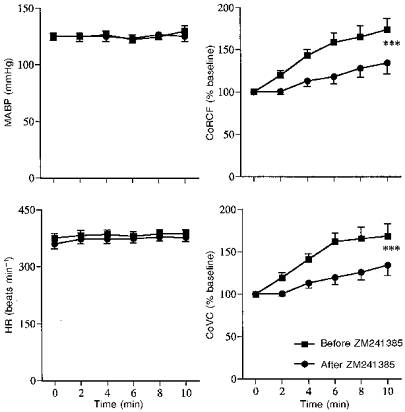
Abbreviations and symbols as in Fig. 2.
DISCUSSION
The present results substantiate the idea that adenosine makes a major contribution to the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia. They provide the first evidence that the adenosine released by hypoxia acts at adluminal, rather than abluminal receptors on the cerebral vessels to produce dilatation and that it acts via the A2A subtype of adenosine receptor. They further suggest that exogenous adenosine, when applied to the abluminal side, can also induce dilatation in the cerebral cortex, by acting on abluminal A2A receptors: stimulation of A1 adenosine receptors produces little or no vascular response.
In Series 1 in which total forebrain blood flow and vascular conductance (CBF and CVC) were assessed from recordings of common carotid blood flow with all but the internal carotid artery ligated, the percentage changes in CBF and CVC induced by breathing 8 % O2 were comparable with those we reported previously (see Thomas & Marshall, 1994). The hypoxia-induced increase in vascular conductance in the cerebral cortex (CoVC) calculated as a percentage change from the cortical red cell flux (CoRCF) and arterial pressure recording, was substantially larger than the increase in CVC (172 ± 28 vs. 91 ± 34 %) because CoRCF increased substantially, whereas CBF remained more or less constant. This suggests that the vasodilatation that occurred in the superficial grey matter of the cerebral cortex was much greater than that which occurred in the forebrain as a whole. This is consistent with evidence gained from previous studies involving different techniques (microspheres: Marshall & Metcalfe, 1990; xenon clearance: Ponte & Purves, 1974). It is also consistent with the evidence that the magnitude of the vasodilatation that occurs in different brain regions during hypoxia is related to metabolic requirements and is such as to maintain O2 delivery at a constant level (Koehler, Traystman & Jones, 1986).
Our finding that topical application of adenosine to the cerebral cortex also induced an increase in CoVC and CoRCF is in accord with previous observations that adenosine can dilate pial vessels (e.g. Morii, Ngai & Winn, 1986). The concentration of adenosine we applied in the mock CSF to achieve an increase in CoRCF comparable with that induced by hypoxia (∼60 % from baseline) was high (10 mM). However, the concentration achieved at the adenosine receptor sites must have been much lower, for the adenosine applied in the mock CSF would have been diluted by the endogenous CSF and extracellular fluid that could not be aspirated, while the arachnoid membrane which was not removed with the dura, may have served as a barrier to diffusion. In fact, in previous studies in which a similar preparation of the cerebral cortex was used, an adenosine concentration of at least 1 mM was required to elicit any cortical dilatation (Van Wylen, Park, Rubio & Berne, 1989) whereas, in a skeletal muscle preparation, where no barrier would be expected and the dilution effect is much less, we observed substantial arteriolar dilatation on topical application of adenosine at a concentration as low as 0.2 μM (Mian & Marshall, 1991). The fact that changes in CoVC and CoRCF induced by adenosine were greatly reduced by topical application of 8-PT confirms that adenosine was acting in a receptor-dependent manner (Thomas & Marshall, 1994). Seen in this light, the fact that topically applied 8-PT had a similarly large effect on the increase in CoRCF induced by hypoxia, provides strong evidence that the vasodilatation recorded in the cerebral cortex was mainly mediated by adenosine, in agreement with the results of some previous studies (see Introduction). Given that CoRCF was maintained during hypoxia after 8-PT application, it seems that the persisting, albeit smaller, increase in CoVC during hypoxia could be explained simply as a myogenic response to the fall in systemic arterial pressure. The fact that hypoxia continued to evoke a fall in arterial pressure after topical application of 8-PT to the cortex is not surprising, for even if the whole dose had diffused into the blood stream, the concentration reached would have been far below the dose required to produce effective blockade of systemic adenosine receptors (∼0.6 μg kg −1vs. 10 mg kg−1; Thomas & Marshall, 1994).
In previous functional studies, 8-SPT was reported to be 20-30 times less potent than 8-PT as an antagonist at adenosine receptors (see Bruns, Lu & Pugsley, 1986). Indeed, in our previous studies on skeletal muscle microcirculation, 8-PT and 8-SPT when topically applied at 8 × 10−5 and 10−3 M, respectively, had similar blocking actions on the dilatation induced in skeletal muscle by hypoxia and by adenosine (Mian & Marshall, 1991; 1996). In the present study, 8-SPT was topically applied at 1 mM, 1000 times higher than the concentration at which we applied 8-PT. Therefore, we have confidence that 8-SPT applied at 1 mM was able to produce an effective blockade of the adenosine receptors to which it had access and, in accord with this, 8-SPT at 1 mM reduced the cortical vasodilatation evoked by 10 mM adenosine by a similar extent as that achieved by 8-PT at 1 μM. Yet, 8-SPT had no effect on the increase in CoRCF or CoVC that was induced by systemic hypoxia. Given that 8-SPT, in contrast to 8-PT, does not cross the blood-brain barrier (Evoniuk et al. 1987) and since the capillary endothelium acts as a functional barrier to the movement of adenosine (Pardridge, Yoshikawa, Kang & Miller, 1994) the simplest conclusions to draw from our results are that adenosine, when applied topically to the cortex produces dilatation by acting on adenosine receptors on the brain side of the blood-brain barrier, and that the adenosine that mediates the hypoxia-induced cortical dilatation originates from, and acts via, the blood side of the blood-brain barrier.
It seems most likely that the topically applied adenosine acts directly on adenosine receptors on the vascular smooth muscle. However, there is a theoretical possibility that it acts on excitatory A2 adenosine receptors on cortical neurones to increase their activity such that the dilatation is mediated by locally released metabolites. This is highly unlikely since adenosine has only been shown to have an excitatory influence on cortical A2A receptors under ischaemic conditions and because the net effect of adenosine in the cortex is one of neuro-inhibition (see review of Latini, Pazzagli, Pepeu & Pedata, 1996). As far as the hypoxia-induced dilatation is concerned, it seems most likely that adenosine is released from the endothelial cells by the local actions of hypoxia (see Introduction), and that it acts on adenosine receptors on the endothelial cells. Since the cortical vasodilatation that is induced in the rat by systemic hypoxia has been shown to be nitric oxide (NO)-dependent (Reid, Davies, Ashcroft & Paterson, 1995) and given that the source of the NO in hypoxia was found to be the endothelium rather than neurones (Heinert, Nye & Paterson, 1997), this raises the possibility that the adenosine that is released by hypoxia produces dilatation by acting on the endothelium to increase the synthesis of NO, just as we have proposed for the dilatation that occurs in skeletal muscle during systemic hypoxia (Skinner & Marshall, 1996). Recent experiments performed on human umbilical vein endothelial cells (HUVEC) provide the direct evidence that adenosine can act on endothelial adenosine receptors to increase the synthesis of NO (Sobrevia, Yudilevich & Mann, 1997).
In Series 3, CGS21680, which is a selective A2A receptor agonist (Hutchison et al. 1989) applied topically at concentrations that were nominally in the range 10 nM to 10 μM (see Results) was able to produce cortical vasodilatation (an increase in CoRCF and CoVC of ∼40 %) that was as large as that induced by adenosine and by systemic hypoxia. The higher concentrations of CGS21680 were required when the drug had been stored in solution overnight in a refrigerator at 4-5°C in accord with the suppliers instructions. Since we completed these experiments the suppliers have changed the instructions for storage and now recommend that the drug should be stored desiccated at -20°C, but should be stable overnight in solution at 4°C. It seems reasonable to assume that our need to use a higher concentration of CGS21680 to produce a similar level of cortical dilatation correlated with loss of activity of the agonist. As argued above, even if the whole of the nominal concentration of 10 μM CGS21680 (∼1 ml applied) diffused into the blood stream over the 5 min period of application, the systemic dose would have been ∼1.6 μg kg−1 which would not have produced a fall in systemic arterial pressure if given as a systemic infusion (P. T. Bryan & J. M. Marshall, unpublished observations); if the actual active concentration was only ∼10 nM then clearly the systemic dose would have been even lower. Thus, it is not surprising that application of CGS21680 to the cortex had no obvious systemic effects. By contrast with CGS21680, CCPA, which is a selective A1 receptor agonist (Lohse et al. 1988) was only able to increase CoRCF by < 10 % even when applied at 100 μM. This strongly suggests that the receptors at which adenosine acts when it is topically applied or released by hypoxia are of the A2A subtype, particularly as CGS21680 is 40-fold less active at A2A receptors (Ki 15 nM) than CCPA is at A1 receptors (Ki 0.4 nM; Lohse et al. 1988; Hutchison et al. 1989). In other words, in accord with the conclusions drawn above, our results suggest that the topically applied adenosine acts on A2A receptors on the vascular smooth muscle and that adenosine released in hypoxia acts on A2A receptors on the endothelium. In agreement with the latter suggestion, the adenosine receptors that were able to stimulate NO synthesis in HUVEC were identified as being of the A2A subtype (Sobrevia et al. 1997). Since CCPA only has a 104-fold selectivity for A1 over A2A receptors (Lohse et al, 1988) the small cortical dilatation that was produced by CCPA may, in fact, have been due to an action at A2A receptors. Certainly it seems likely that the functional importance of A1 receptors in mediating dilatation in the cortical circulation is minimal.
In Series 4, we used ZM241385 which is the most selective A2A receptor antagonist available to date with a pA2 of 9.02 for vasodilatation induced by CGS21680 (Poucher et al. 1995), where pA2 is -log10 of concentration of antagonist that produces a 2-fold rightward shift of a dose-response curve. The fact that ZM241385 reduced the cortical dilatation evoked by topically applied adenosine to a similar extent as 8-PT, which is non selective between the A1 and A2 receptor subtypes (Bruns, Lu & Pugsley, 1986), is entirely consistent with the conclusion drawn above that the receptors that mediate the cortical dilator response to topically applied adenosine are A2A receptors. The effects of ZM241385 on the cortical dilatation induced by hypoxia were not as conclusive as might have been expected in view of the discussion above. It reduced the hypoxia-induced increase in CoRCF, but had no significant effect on the concurrent increase in CoVC. Looking in more detail at these results, firstly, ZM241385 only seemed to reduce the later part of the increase in CoRCF (from 3 min onwards) and secondly, the lack of significant effect on the computed increase in CoVC is, at least partly, explained by the fact that the hypoxia-induced fall in arterial pressure tended to be greater after ZM241385 than before, particularly after the 3rd minute, while the heart rate was lower throughout the period of hypoxia. If, as we have proposed, the adenosine released in hypoxia acts on receptors on the endothelium, then it may be that ZM241385 diffused less readily to these receptors than 8-PT: as far as we are aware there is as yet no published evidence on the ability of ZM241385 to penetrate the blood-brain barrier. The fall in arterial pressure is mainly due to vasodilatation in skeletal muscle (Thomas & Marshall, 1994), and this, in turn, is mainly due to the action of locally released adenosine on A1 receptors: systemically administered ZM241385 has no effect on this response (Bryan & Marshall, 1996). Rather, it seems likely that in this particular group of rats there was a time-dependent effect, perhaps attributable to gradually deepening of anaesthesia (see Marshall & Metcalfe, 1988) which meant that, during the second hypoxic period, the sympathetic nervous system was less effective in maintaining arterial pressure by causing peripheral vasoconstriction and tachycardia than during the first. Indeed, on balance, it seems reasonable to conclude that the results we obtained with ZM241385 on the cortical vasodilator response to hypoxia are in accord with our other findings and suggest that adenosine released in hypoxia induces cortical vasodilatation by acting on A2A receptors.
In summary, the results of the present study lead us to propose that in the superficial layer of the cerebral cortex there are A2A receptors on the vascular smooth muscle which can be stimulated to cause vasodilatation by topically applied adenosine and, presumably, by endogenous adenosine that is released into the interstitial space. These receptors may become functionally important during severe hypoxia or ischaemia when the interstitial concentrations of adenosine reach suprathreshold levels at the receptor sites. However, we propose that there are also A2A receptors on the vascular endothelium and that during systemic hypoxia, adenosine is released from the endothelium and acts on these receptors to cause the major part of the cortical vasodilatation induced by hypoxia.
Acknowledgments
This work was supported by the British Heart Foundation.
References
- Bowton DL, Haddon WS, Prough DS, Adair N, Alford PT, Stump DA. Theophylline effect on the cerebral blood flow response to hypoxemia. Chest. 1988;94:371–375. doi: 10.1378/chest.94.2.371. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Lu GH, Pugsley TA. Characterization of the A2adenosine receptor labelled by [3H]-NECA in rat striatal membranes. Molecular Pharmacology. 1986;29:331–346. [PubMed] [Google Scholar]
- Bryan PT, Marshall JM. The role of adenosine A2A receptors in vasodilatation induced in skeletal muscle of the rat by systemic hypoxia. The Journal of Physiology. 1996;494.P:106. doi: 10.1111/j.1469-7793.1998.507bn.x. P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coney A, Marshall JM. Effect of systemic hypoxia upon circulation of the cerebral cortex in the anaesthetized rat. The Journal of Physiology. 1995a;483.P:88. P. [Google Scholar]
- Coney A, Marshall JM. The role of adenosine in cerebral cortical vasodilatation induced by acute systemic hypoxia in normoxic and chronically hypoxic rats under anaesthesia. The Journal of Physiology. 1995b;487.P:174–175. P. [Google Scholar]
- Coney A, Marshall JM. Effect of ZM241385 on the responses to acute systemic hypoxia and adenosine in the cerebral cortex of the anaesthetized rat. The Journal of Physiology. 1996a;494.P:105–106. P. [Google Scholar]
- Coney A, Marshall JM. The effect of 8-sulphophenyltheophylline on the vasodilatation induced in the cerebral cortex of the anaesthetized rat by acute systemic hypoxia and exogenous adenosine. The Journal of Physiology. 1996b;497.P:80. P. [Google Scholar]
- Daly JW. Adenosine Receptors. Advances in Cyclic Nucleotide and Protein Phosphorylation Research. 1985;19:29–46. [PubMed] [Google Scholar]
- Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. The Journal of Physiology. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussen A, Möser G, Schrader J. Contribution of coronary endothelial cells to cardiac adenosine production. Pflügers Archiv. 1986;406:608–614. doi: 10.1007/BF00584028. [DOI] [PubMed] [Google Scholar]
- Evoniuk G, Von Borstel RW, Wurtman RJ. Antagonism of the cardiovascular effects of adenosine by caffeine or 8-(p-sulphophenyl)-theophylline. Journal of Pharmacology and Experimental Therapeutics. 1987;240:428–432. [PubMed] [Google Scholar]
- Fabricius M, Lauritzen M. Laser Doppler evaluation of rat brain microcirculation - comparison with the [14C] iodoantipyrine method suggests discordance during cerebral blood flow increases. Journal of Cerebral Blood Flow and Metabolism. 1996;16:156–161. doi: 10.1097/00004647-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Haller C, Kuschinsky W. Moderate hypoxia: reactivity of pial arteries and local effect of theophylline. Journal of Applied Physiology. 1987;63:2208–2215. doi: 10.1152/jappl.1987.63.6.2208. [DOI] [PubMed] [Google Scholar]
- Heinert G, Nye PCG, Paterson DJ. Effect of a neuronal nitric oxide synthase blocker on eucapnic, hypoxic and hypercapnic cerebral blood flow in the anaesthetised rat. The Journal of Physiology. 1997;501.P:66. P. [Google Scholar]
- Hutchison AJ, Webb RL, Oei HH, Ghai GR, Zimmerman MB, Williams M. CGS21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. Journal of Pharmacology and Experimental Therapeutics. 1989;251:47–55. [PubMed] [Google Scholar]
- Koehler RC, Traystman RJ, Jones MD. Influence of reduced oxyhemoglobin affinity on cerebrovascular response to hypoxic hypoxia. American Journal of Physiology. 1986;251:H756–763. doi: 10.1152/ajpheart.1986.251.4.H756. [DOI] [PubMed] [Google Scholar]
- Latini S, Pazzagli M, Pepeu G, Pedata F. A2 adenosine receptors: their presence and neuromodulatory role in the central nervous system. General Pharmacology. 1996;27:925–933. doi: 10.1016/0306-3623(96)00044-4. [DOI] [PubMed] [Google Scholar]
- Laudignon N, Farri E, Beharry K, Rex J, Aranda JV. Influence of adenosine on cerebral blood flow during hypoxic hypoxia in the newborn piglet. Journal of Applied Physiology. 1990;68:1534–1541. doi: 10.1152/jappl.1990.68.4.1534. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Klotz K-N, Schwabe U, Cristalli G, Vittori S, Grifantini M. 2-Chloro-N6-cyclopentyladenosine: a highly selective agonist at A1 adenosine receptors. Naunyn-Schmiedeberg's Archives of Pharmacology. 1988;337:687–689. doi: 10.1007/BF00175797. [DOI] [PubMed] [Google Scholar]
- Mcphee AJ, Maxwell GM. The effect of theophylline on regional cerebral blood flow responses to hypoxia in newborn piglets. Pediatric Research. 1987;21:573–578. doi: 10.1203/00006450-198706000-00014. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Metcalfe JD. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. The Journal of Physiology. 1988;407:385–403. doi: 10.1113/jphysiol.1988.sp017422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM, Metcalfe JD. Effects of systemic hypoxia on the distribution of cardiac output in the rat. The Journal of Physiology. 1990;426:335–353. doi: 10.1113/jphysiol.1990.sp018141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian R, Marshall JM. The role of adenosine in dilator responses induced in arterioles and venules of rat skeletal muscle by systemic hypoxia. The Journal of Physiology. 1991;443:499–511. doi: 10.1113/jphysiol.1991.sp018847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian R, Marshall JM. The behaviour of muscle microcirculation in chronically hypoxic rats: the role of adenosine. The Journal of Physiology. 1996;491:489–498. doi: 10.1113/jphysiol.1996.sp021233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii S, Ngai AC, Ko K, Winn HR. Role of adenosine in regulation of cerebral blood flow: effects of theophylline during normoxia and hypoxia. American Journal of Physiology. 1987;253:H165–175. doi: 10.1152/ajpheart.1987.253.1.H165. [DOI] [PubMed] [Google Scholar]
- Morii S, Ngai AC, Winn HR. Reactivity of rat pial arterioles and venules to adenosine and carbon dioxide: with detailed description of the closed cranial window technique in rats. Journal of Cerebral Blood Flow and Metabolism. 1986;6:34–41. doi: 10.1038/jcbfm.1986.5. [DOI] [PubMed] [Google Scholar]
- Nees S, Gerlach E. Adenosine nucleotide and adenosine metabolism in cultured coronary endothelial cells: formation and release of adenine compounds and possible functional implication. In: Berne RM, Rall TW, Rubio R, editors. Regulatory Function of Adenosine. Amsterdam: M. Nijhoff Publishers; 1983. pp. 347–360. [Google Scholar]
- Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiological Reviews. 1990;70:761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Yoshikawa T, Kang Y-S, Miller LP. Blood-brain barrier transport and brain metabolism of adenosine and adenosine analogs. Journal of Pharmacology and Experimental Therapeutics. 1994;268:14–18. [PubMed] [Google Scholar]
- Ponte J, Purves MJ. The role of the carotid body chemoreceptors and carotid sinus baroreceptors in the control of cerebral blood vessels. The Journal of Physiology. 1974;237:315–340. doi: 10.1113/jphysiol.1974.sp010484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poucher SM, Keddie JR, Singh P, Stoggall SM, Caulkett PWR, Jones G, Collis MG. The in vitro pharmacology of ZM241385, a potent, non-xanthine, A2A selective adenosine receptor antagonist. British Journal of Pharmacology. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JM, Davies AG, Ashcroft FM, Paterson DJ. Effect of L-NMMA, cromakalim and glibenclamide on cerebral blood flow in hypercapnia and hypoxia. American Journal of Physiology. 1995;269:H916–922. doi: 10.1152/ajpheart.1995.269.3.H916. [DOI] [PubMed] [Google Scholar]
- Simpson RE, Phillis JW. Adenosine deaminase reduces hypoxic and hypercapnic dilatation of rat pial arterioles: evidence for mediation by adenosine. Brain Research. 1991;553:305–308. doi: 10.1016/0006-8993(91)90839-n. [DOI] [PubMed] [Google Scholar]
- Skarphedinsson JO, Hårding H, Thorén P. Repeated measurements of cerebral blood flow in rats. Comparisons between the hydrogen clearance method and laser Doppler flowmetry. Acta Physiologica Scandinavica. 1988;134:133–142. doi: 10.1111/j.1748-1716.1988.tb08469.x. [DOI] [PubMed] [Google Scholar]
- Skinner MR, Marshall JM. Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia. The Journal of Physiology. 1996;495:553–560. doi: 10.1113/jphysiol.1996.sp021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smellie FW, Davis CW, Daly JW, Wells JN. Alkylxanthine: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sciences. 1979;24:2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Sobrevia L, Yudilevich DL, Mann GE. Activation of A2-purinoceptors by adenosine stimulates L-arginine transport (system y+) and nitric oxide synthesis in human fetal endothelial cells. The Journal of Physiology. 1997;499:135–140. doi: 10.1113/jphysiol.1997.sp021916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Marshall JM. Interdependence of respiratory and cardiovascular changes induced by systemic hypoxia in the rat: the roles of adenosine. The Journal of Physiology. 1994;480:627–636. doi: 10.1113/jphysiol.1994.sp020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wylen DGL, Park TS, Rubio R, Berne RM. The effect of local infusion of adenosine and adenosine analogues on local cerebral blood flow. Journal of Cerebral Blood Flow. 1989;9:556–562. doi: 10.1038/jcbfm.1989.79. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio R, Berne RM. Brain adenosine concentration during hypoxia in rats. American Journal of Physiology. 1981;241:H235–242. doi: 10.1152/ajpheart.1981.241.2.H235. [DOI] [PubMed] [Google Scholar]


