Abstract
Whole-cell recordings were made from rat nodose ganglion neurones in culture and from human embryonic kidney (HEK293) cells stably transfected to express P2X2, P2X3 or both receptor subunits. We examined the blocking actions of 2v′,3v′-O-trinitrophenyl-ATP (TNP-ATP) on currents evoked by the agonists ATP and α,β-methylene ATP.
In cells expressing only P2X2 or P2X3 receptor subunits, the inhibition by TNP-ATP was fitted by a single binding site model with half-maximal concentrations of about 3 μM and 3 nM, respectively. In cells expressing both P2X2 and P2X3 receptor subunits, currents showed little or no desensitization, thus excluding contributions from homomeric P2X3 receptors. When α,β-methylene ATP was the agonist (activating heteromeric P2X2/3 receptors), the inhibition by TNP-ATP conformed to a single binding site (half-maximal concentration about 3 nM). When ATP (30 μM) was the agonist, activating both heteromeric P2X2/3 as well as homomeric P2X2 receptors, the inhibition curve was biphasic (half-maximal concentrations about 3 nM and 3 μM); the proportion of high affinity sites in all six cells tested was about 40 %.
In nodose ganglion neurones, the inhibition by TNP-ATP of currents evoked by ATP (30 μM) was also clearly biphasic. In this case, individual neurones showed more variability in the proportion of high and low affinity sites for TNP-ATP.
We conclude that more than one form of multimeric P2X receptor channels are functionally expressed on the cell bodies of individual nodose ganglion neurones. On the basis of sensitivity to TNP-ATP, and other properties, one of these may correspond to the homomeric P2X2 receptor and the other(s) to heteromeric P2X2/3 receptors.
Ionotropic P2X receptors, gated by extracellular ATP, are expressed throughout the central and peripheral nervous system and mediate fast excitatory synaptic transmission at a number of neuromuscular and neuro-neuronal junctions (see review by Surprenant, Buell & North, 1995). There are currently seven P2X receptor subunits (P2X1-7). They share 35-50 % amino acid identity between any pairs, and all appear to have a similar membrane topology distinguished by two transmembrane domains, intracellular -NH2 and -COOH termini and a large, cysteine-rich extracellular domain (Collo et al. 1996; North & Barnard, 1997). Six of these subunits (P2X1-6) are neuronally located, showing distinct but often overlapping distributions; at the RNA level all are found in sensory ganglia (Collo et al. 1996). Given the possible role of P2X receptors in nociceptive signalling (Burnstock, 1996; Cook, Vulchanova, Hargreaves, Elde & McCleskey, 1997), the composition of functional P2X receptors in sensory neurones is of particular physiological relevance. For example, the RNA and protein for one of the subunits (P2X3) is found only in a specific subpopulation of neurones; these are cells that do not show immunofluorescence for the peptides calcitonin gene-related peptide and substance P but do show immunoreactivity for the isolectin B4, and which on this basis are thought to correspond to a set of unmyelinated nociceptors (Chen, Akopian, Sivilotti, Colquhoun, Burnstock & Wood, 1995; Vulchanova et al. 1997, 1998).
Both P2X2 and P2X3 receptor subunits readily form channels, presumably as homomultimers, when expressed individually from their cDNAs. These have distinct properties. Homomeric P2X2 receptors are not activated by the ATP analogue α,β-methylene ATP (α,β-meATP) and they show relatively little desensitization during agonist applications of several seconds. Homomeric P2X3 receptors are activated by α,β-meATP and desensitize within tens of milliseconds (Chen et al. 1995; Lewis, Neidhart, Holy, North, Buell & Surprenant, 1995; Virginio, Robertson, Surprenant & North, 1998b). However, co-expression of these two subunits results in channels that are sensitive to α,β-meATP but show little desensitization, obliging one to conclude that they assemble into novel heteromeric channel proteins (Lewis et al. 1995). Cells expressing homomeric P2X2 receptors are also about tenfold less sensitive to ATP than cells transfected with both the P2X2 and P2X3 subunits (half-maximal concentrations are about 10 μM compared with 1 μM; Brake, Wagenbach & Julius, 1994; Lewis et al. 1995).
Electrophysiological recordings made from sensory neurones have demonstrated responses that sometimes correspond to these properties of the heterologously expressed receptors. For example, in one study on neonatal rat dorsal root ganglion neurones ATP evoked non-desensitizing currents with concentration-response relations resembling the heteromeric P2X2/3 receptors (Bean, 1991) while in another study on the same preparation ATP and α,β-meATP evoked rapidly desensitizing currents with properties similar to the homomeric P2X3 receptor (Robertson, Rae, Rowan & Kennedy, 1996). Two populations of neurones have been described in rat trigeminal nociceptive neurones based on agonist pharmacology and kinetics; one has a rapidly desensitizing, α,β-meATP-activated (P2X3-like) current and the other has a slowly desensitizing, α,β-meATP-activated (P2X2/3-like) current (Cook et al. 1997).
In the sensory neurones of the nodose ganglion, most or all cells which express P2X3 immunoreactivity also stain with antibodies to the P2X2 subunit (Vulchanova et al. 1998; Virginio, North & Surprenant, 1998a;Kawashima et al. 1998). The purpose of the present experiments was to determine whether combinations of these two subunits might fully account for the properties of the ATP-evoked currents in nodose ganglion neurones, and whether individual neurones expressed more than one subunit combination. The situation is somewhat simplified, because the cells do not show any fast-desensitizing component to the current (Khakh, Humphrey & Surprenant, 1995; Lewis et al. 1995) and are therefore unlikely to express significant homomeric P2X3 receptor channels. One way to distinguish between homomeric P2X2 and heteromeric P2X2/3 receptors might be to measure the amplitudes of the currents evoked in a single cell by the two agonists ATP and α,β-meATP; heteromeric P2X2/3 receptors would be activated by both agonists and homomeric P2X2 only by ATP.
An alternative, and generally superior, approach to distinguishing receptor subtypes depends on discriminating antagonists. Suramin and pyridoxal-5-phosphate-6-azophenyl-2v′,4v′-disulphonic acid (PPADS) inhibit ATP-evoked currents in nodose ganglion cells (Khakh et al. 1995), but they do not distinguish between P2X2, P2X3 and P2X2/3 receptors heterogously expressed (Evans, Lewis, Buell, North & Surprenant, 1995; Lewis et al. 1995; Collo et al. 1996). Recently, we found that 2v′,3v′-O-trinitrophenyl-ATP (TNP-ATP) is a potent antagonist at heterologously expressed P2X3 and P2X2/3 receptors with half-maximal inhibitory concentrations (IC50s) of about 1 nM, but it is about a thousandfold less effective in inhibiting homomeric P2X2 and P2X4 receptors (IC50s > 1 μM; Virginio et al. 1998b). Therefore, in the present study we have studied the antagonism by TNP-ATP of currents in nodose ganglion neurones, and compared this with its effects on cells transfected with P2X2, P2X3 or both cDNAs.
METHODS
Cell culture
Methods for generating and culturing human embryonic kidney (HEK293) cells stably expressing homomeric rat P2X2 and homomeric rat P2X3 receptors have been described in detail previously (Evans et al. 1995; Virginio et al. 1998b). The generation of cells stably expressing pairs of receptor subunits using an internal ribosome entry site expression vector has been described (Kawashima et al. 1998); we used HEK293 cells stably transfected with the rat P2X2-IRES-P2X3 construct (P2X[2-3]). Rat nodose neurones were dissociated and cultured as previously described (Stansfield & Mathie, 1993; Khakh et al. 1995). Young adult rats (3-4 weeks old) were anaesthetized with halothane and guillotined; these methods have been approved by the Office Veterinaire Cantonal of Geneva. Recordings were obtained from nodose neurones 5-12 days after dissociation.
Electrophysiology
Whole-cell recordings were made with an Axopatch 200 amplifier, using pCLAMP and Axograph software (Axon Instruments) for data acquisition and analysis. Patch pipettes (4-7 MΩ) contained (mM): NaCl, 154; EGTA, 10; and Hepes, 10. External solution was (mM): NaCl, 165; KCl, 2; MgCl2, 1; CaCl2, 2; glucose, 12; and Hepes, 10. Osmolarity and pH of both solutions were maintained at 295-305 mosmol l−1 and 7.3, respectively. All recordings were made at room temperature (20-23°C) and at a holding potential of -60 mV. Tetrodotoxin (10 μM) was present in the external solution for all experiments on nodose neurones in order to block voltage-activated sodium currents. Agonists were applied by a fast-flow U-tube delivery system (Fenwick, Marty & Neher, 1982); the antagonist TNP-ATP was added to the bath superfusate, for at least 5 min prior to agonist application, and the fast-flow solution. Concentration-inhibition curves were fitted by least squares either to a single logistic function of the form 1/(1 + ([TNP-ATP]/IC50)), where IC50 is the concentration of TNP-ATP ([TNP-ATP]) at which inhibition is half-maximal, or to the sum of two such functions r/(1 +[TNP-ATP]/IC501) + (1 - r)/(1 +[TNP-ATP]/IC502)}, where r is the fraction of high affinity sites, and IC501 and IC502 are the IC50 values for high and low affinity sites, respectively. All results are expressed as the mean ±s.e.m. TNP-ATP (sodium salt) was obtained from Molecular Probes, and ATP and α,β-meATP were obtained from Sigma.
RESULTS
Homomeric receptors in HEK293 cells
In cells transfected with the P2X2 receptor subunit, ATP (30 or 100 μM) evoked rapidly rising inward currents of several nanoamps whereas α,β-meATP (30 μM) had little or no effect (up to 8 % of the amplitude of the current evoked by 100 μM ATP in 3/10 cells, and 0 % in 7/10 cells). For ATP, the EC50 at P2X2 receptors is approximately 10 μM, and 100 μM is close to a maximal concentration (Evans et al. 1995). TNP-ATP inhibited the current evoked by ATP; at 30 μM ATP the IC50 was 2800 ± 300 nM (n= 4) and at 100 μM ATP the IC50 was 3500 ± 600 nM (n= 3). In both cases the inhibition was well fitted by a single logistic function. The finding that the same concentrations of TNP-ATP were effective against 30 or 100 μM ATP is consistent with our previous interpretation that the antagonism is non-competitive. These experiments confirm and extend previous studies (Evans et al. 1995; Lewis et al. 1995; Virginio et al. 1998b).
In cells transfected with the P2X3 receptor subunit, both ATP (30 or 100 μM) and α,β-meATP (30 μM) were maximally effective; the respective EC50s are approximately 1 μM (Evans et al. 1995). The current elicited by α,β-meATP (30 μM) was 68 ± 12 % (n= 11; range, 30-130 %) of that evoked by ATP (30 μM); this fraction was not different for 100 μM ATP (71 ± 19 %, n= 5; range, 28-120 %). TNP-ATP inhibited the current evoked by α,β-meATP (30 μM) with an IC50 of 3.7 ± 0.4 nM (n= 4); we have previously shown that this inhibition is non- competitive and is well-described by a single TNP-ATP binding model (Virginio et al. 1998b).
P2X2 and P2X3 receptors co-expressed in HEK293 cells
These experiments were carried out on cells stably expressing the P2X[2-3] construct (see Methods; Kawashima et al. 1998). These cells might express homomeric P2X3 receptors, one or more forms of heteromeric P2X2/3 receptors, and homomeric P2X2 receptors (Kawashima et al. 1998); in these experiments, as in previous studies, there was no rapidly desensitizing current in response to α,β-meATP, suggesting that they formed few or no homomeric P2X3 receptors. The currents evoked by α,β-meATP (30 μM) were 59 ± 7 % (n= 11; range, 30-130 %) of those evoked by ATP (30 μM).
When α,β-meATP (30 μM) is the agonist, one would expect to activate only the heteromeric receptors; we found that TNP-ATP inhibited these currents at low concentrations (Figs 1 and 2). The inhibition curve was well fitted by a single logistic function (Fig. 2A); the IC50 was 4.5 nM. The same result (3.7 nM) was obtained when a low concentration of ATP (3 μM) was used as the agonist, consistent with the interpretation that only heteromeric P2X2/3 receptors are contributing to the current under these conditions. These values are very close to those observed with homomeric P2X3 receptors, in these experiments (Fig. 2B, and left dashed line in Fig. 2A, and Fig. 3), and in previous work (Virginio et al. 1998b).
Figure 1. Inhibition of P2X receptor currents by TNP-ATP.
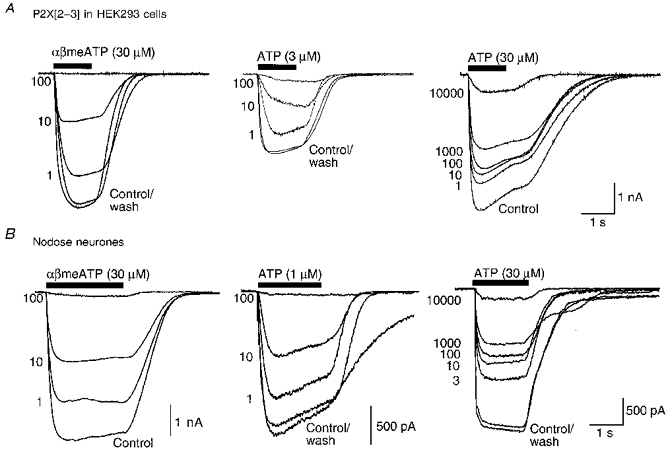
Membrane currents were recorded from HEK293 cells stably expressing the P2X[2-3] construct (A) or from rat cultured nodose neurones (B). Each set of superimposed traces are currents in response to a maximal concentration of α,β-meATP (30 μM, left-hand traces), to low concentrations of ATP (1 or 3 μM as indicated, middle traces), or to a near-maximal concentration of ATP (30 μM, right-hand traces), in the absence and presence of increasing concentrations of TNP-ATP. The concentration of TNP-ATP (in nM) is aligned with the peak of each current trace. Each set of traces was obtained from separate cells.
Figure 2. Summary of inhibitory action of TNP-ATP on HEK293 cells expressing the P2X[2-3] construct.
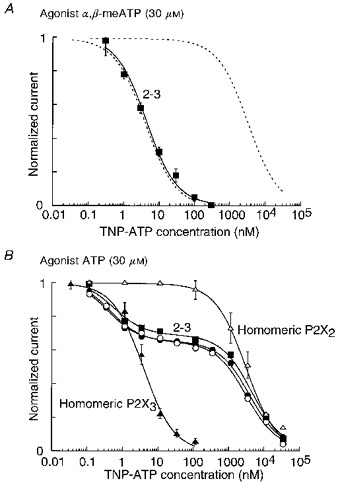
A, currents evoked by α,β-meATP (30 μM; ▪). Points are means ±s.e.m. for 8 cells. The continuous line is best fitted to a single hyperbolic function with a coefficient of 1. The result was not different when the agonist was a low concentration (3 μM) of ATP. Dashed lines depict results from cells expressing homomeric P2X3 and homomeric P2X2 receptors (see B). B, currents evoked by a high concentration of ATP (30 μM). Results from 3 cells are illustrated (•, ○, ▪), with lines fitted to the sum of two logistic functions. ▴ and ▵, results on cells expressing only P2X3 or P2X2 receptors, respectively; in this case the lines are fitted to single logistic functions. These fits are reproduced (dashed lines) in A, and Fig. 3.
Figure 3. Summary of inhibitory action of TNP-ATP on nodose ganglion neurones.
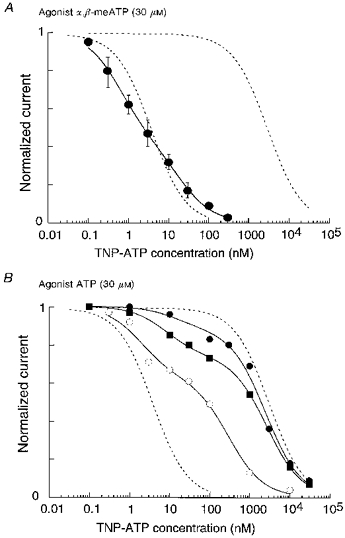
A, currents evoked by α,β-meATP (30 μM; •). Points are means ±s.e.m. for 3-5 cells. The continuous line is best fitted to the sum of two logistic functions. The result was not different when the agonist was a low concentration (1 μM) of ATP. B, currents evoked by a high concentration of ATP (30 μM). Results from 3 cells are illustrated (○, ▪, •), with lines fitted to the sum of two logistic functions. Dashed lines in A and B depict results from HEK293 cells expressing homomeric P2X3 or homomeric P2X2 receptors (see Fig. 2B).
When a high concentration of ATP (30 μM) was the agonist, TNP-ATP inhibited the currents with a clearly biphasic inhibition curve (Figs 1A and 2B). For the pooled data (6 cells) this was well fitted to the sum of two logistic functions with IC50 values of 2.5 and 3300 nM; the average contribution of the high affininity component was 37 %. In Fig. 2B we show the inhibition curves for three of the six cells, to illustrate the finding that all the cells showed very similar results; for example, at 100 nM TNP-ATP the fractional currents ranged from 0.50 to 0.62 (mean, 0.59; standard deviation, 0.05; n= 6).
Nodose ganglion neurones
The currents evoked by α,β-meATP (30 μM) were 54 ± 9 % (n= 11; range, 10-160 %) of those evoked by ATP (30 μM). When α,β-meATP (30 μM) was the agonist, the currents were inhibited by low concentrations of TNP-ATP (IC50 about 2 nM) (Figs 1B and 3A). However, the inhibition curve was somewhat less steep than that observed with the P2X[2-3]-expressing cells, and was better fitted by the sum of two components (0.57 at 0.6 nM, 0.43 at 20 nM). The same results were observed when a low concentration of ATP (1 μM) was used as agonist (0.52 at 0.4 nM, 0.48 at 20 nM).
When a higher concentration of ATP (30 μM) was the agonist, the TNP-ATP inhibition curve was markedly biphasic. This is illustrated for three cells in Fig. 3B, but a similar result was obtained in all ten neurones tested. In each case, there was a good fit to the sum of two logistic functions where IC501 ranged from 2.2 to 19 nM, and IC502 ranged from 300 to 6000 nM. In seven cells the low affinity component was in the range 2000-6000 nM, as would be expected for homomeric P2X2 receptors. Compared with the HEK293 cells expressing the P2X[2-3] construct, there was much variability among individual neurones. For example, the fractional inhibition by 100 nM TNP-ATP ranged from 0.36 to 0.83 (mean, 0.66; standard deviation, 0.17; n= 10).
DISCUSSION
Nodose ganglion cells express both P2X2 and P2X3 subunits at their plasma membrane (Vulchanova et al. 1997; Kawashima et al. 1998) and one purpose of the present work was to determine whether the functional responses of the individual cells could be accounted for by channels made from these subunits in various combinations. We excluded a contribution from homomeric P2X3 receptors because we recorded only currents which were well sustained for 1-2 s; during this time homomeric P2X3 channels would desensitize (Lewis et al. 1995; Virginio et al. 1998b).
In theory, it might be possible to distinguish the contributions of homomeric P2X2 receptors and heteromeric P2X2/3 receptors in nodose neurones by measuring the effectiveness of α,β-meATP to evoke currents relative to that of ATP. For example, Li, Peoples & Weight (1997) found that α,β-meATP evoked about 75 % of the maximal current elicited by ATP in bullfrog dorsal root ganglion cells. The ratio of the currents was about 50-60 % in the present experiments, but there was considerable variability. Unfortunately, this method seems to be unreliable because there was also similar variability when the experiment was carried out on HEK293 cells expressing the P2X[2-3] construct, or even P2X3 subunit alone; in some cells expressing homomeric P2X3 receptors, α,β-meATP evoked a larger current than ATP. It is possible that other unknown factors such as associated proteins or post-translational modifications are able to alter the efficacy of α,β-meATP relative to that of ATP.
Cells expressing the P2X[2-3] construct were more consistent with respect to antagonism by TNP-ATP. Thus, when a concentration of ATP was used which is sufficiently high to activate all P2X receptors, the TNP-ATP inhibition curve indicated the presence of two classes of receptor. The lower affinity component corresponds well to that of homomeric P2X2 receptors (Fig. 2B) (Virginio et al. 1998b); among the six cells tested this appeared to account for about 60 % of the total current, and this proportion was consistent among individual cells. The higher affinity component presumably corresponds to one or more subset of P2X2/3 heteromeric receptors, if we assume that homomeric P2X3 receptors do not contribute under these conditions. The finding that there appears to be only a single high affinity component to the inhibition, in the face of possible multiple channel forms (2n3(1-n), where n is the unknown stoichiometry), is consistent with the interpretation that binding of TNP-ATP to a single P2X3 subunit within the multimeric channel is sufficient to inhibit the current. It could also indicate, however, that only a single, preferred form of P2X2/3 heteromer is expressed.
The concentration-inhibition curves for TNP-ATP in nodose ganglion cells indicated that they express multiple forms of the P2X receptor. Even when the agonist was α,β-meATP (Fig. 3A), or a low concentration of ATP, the inhibition deviated somewhat from that expected for a single high affinity site (Fig. 3A). When tested with the higher ATP concentration, none of ten individual nodose ganglion neurones showed a monophasic inhibition curve with TNP-ATP. The minimum number of receptors required to fit these data was two but, taken together with the results using α,β-meATP, it is possible that there are several binding sites of intermediate affinity. All the neurones had a clearly resolvable low affinity component and, in seven of ten cells, this IC50 corresponded numerically to that seen with homomeric P2X2 receptors (IC50, 2-6 μM; Fig. 3B) (Virginio et al. 1998b). These results contrast with the data from HEK293 cells expressing the P2X[2-3] construct in two ways; the HEK293 cells were more uniform in their properties, and all appeared to express a single heteromeric P2X2/3 form along with the homomeric P2X2 receptor. The variability of the nodose ganglion neurones might arise from mixed populations of receptors made from P2X2 and P2X3 subunits (i.e. the multiple forms of 2n3(1-n)), or from the incorporation of subunits into the channels which are neither P2X2 nor P2X3. Homomeric P2X4 receptors are, like homomeric P2X2 receptors, relatively insensitive to TNP-ATP (Virginio et al. 1998b); however, they are unlikely to contribute because currents in nodose neurones are blocked by low micromolar concentrations of suramin and PPADS (Khakh et al. 1995) whereas currents in homomeric P2X4 receptors are not (Buell, Lewis, Collo, North & Surprenant, 1996).
Other ligand-gated ion channels are multimeric, and there is precedent for individual cells expressing channels assembled from different combinations of subunit. For example, ciliary ganglion neurones express and segregate nicotinic acetylcholine receptors which are α7 homomers and receptors which are α3α5β4 heteromers (Vernalis, Conroy & Berg, 1993; Conroy & Berg, 1995). The present experiments, using the antagonist TNP-ATP as a tool in the differentiation of P2X receptors, show that individual nodose ganglion neurones in culture express more than one type of P2X receptor channel. It will be important to extend these observations to the cell bodies within intact ganglia, as well as to discrete regions of the cell surface. Immunohistochemical studies with P2X2 and P2X3 subunit-specific antibodies suggest a different pattern of distribution between nodose ganglion cell bodies and terminals in the nucleus tractus solitarii (Vulchanova et al. 1997). Recent electrophysiological studies have focused on the presynaptic actions of ATP at P2X receptors (Gu & MacDermott, 1997) and TNP-ATP may provide one means to determine whether such presynaptic receptors are functionally distinct from their cell body counterparts.
Acknowledgments
We appreciate the skilled help of Danièle Estoppey with tissue culture and transfection.
References
- Bean BP. ATP-activated channels in rat and bullfrog sensory neurons: concentration-dependence and kinetics. Journal of Neuroscience. 1991;10:1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO Journal. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. A unifying hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. Journal of Biological Chemistry. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Buell G, North RA, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2X-purinoceptors) Molecular Pharmacology. 1995;48:178–183. [PubMed] [Google Scholar]
- Fenwick EM, Marty A, Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. The Journal of Physiology. 1982;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- Kawashima E, Estoppey D, Fahmi D, Virginio C, Rees S, Surprenant A, North RA. A novel and efficient method for the stable expression of heteromeric ion channels in mammalian cells. Receptors and Channels. 1998;5 in the Press. [PubMed] [Google Scholar]
- Khakh BS, Humphrey PPA, Surprenant A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurones. The Journal of Physiology. 1995;484:385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurones. Nature. 1995;377:432–434. doi: 10.1038/377432a0. 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Inhibition of ATP- activated currents by zinc in dorsal root ganglion neurones of bullfrog. The Journal of Physiology. 1997;505:641–653. doi: 10.1111/j.1469-7793.1997.641ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Barnard E. Nucleotide receptors. Current Opinion in Neurobiology. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. 10.1016/S0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Rae MG, Rowan EG, Kennedy C. Characterization of a P2X-purinoceptor in cultured neurones of the rat dorsal root ganglia. British Journal of Pharmacology. 1996;118:951–956. doi: 10.1111/j.1476-5381.1996.tb15491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield C, Mathie A. Recording membrane currents of peripheral neurones in short-term culture. In: Wallis DA, editor. Electrophysiology: a Practical Approach. Oxford, UK: Oxford University Press; 1993. pp. 3–30. [Google Scholar]
- Surprenant A, Buell G, North RA. P2X receptor brings new structure to ligand-gated ion channels. Trends in Neurosciences. 1995;18:224–228. doi: 10.1016/0166-2236(95)93907-f. 10.1016/0166-2236(95)93907-F. [DOI] [PubMed] [Google Scholar]
- Vernalis AB, Conroy WG, Berg DK. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron. 1993;10:451–464. doi: 10.1016/0896-6273(93)90333-m. 10.1016/0896-6273(93)90333-M. [DOI] [PubMed] [Google Scholar]
- Virginio C, North RA, Surprenant A. Calcium permeability and block at homomeric and heteromeric P2X2 and P2X3 receptors, and P2X receptors in rat nodose neurones. The Journal of Physiology. 1998a. in the Press. [DOI] [PMC free article] [PubMed]
- Virginio C, Robertson G, Surprenant A, North RA. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors. Molecular Pharmacology. 1998b. in the Press. [PubMed]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde RP. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in monkey and rat sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. 10.1016/S0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. The P2X3 receptor subunit is expressed by capsaicin-sensitive and capsaicin-insensitive, FRAP-containing sensory neurons. Journal of Neuroscience. 1998. in the Press.


