Abstract
In paired-pulse cortical stimulation experiments, conditioning subthreshold stimuli suppress the electromyographic (EMG) responses of relaxed muscles to suprathreshold magnetic test stimuli at short interstimulus intervals (ISIs) (1-5 ms) and facilitate them at long ISIs (8-15 ms).
We made paired-pulse magnetic stimulation studies on the response of the first dorsal interosseous muscle (FDI) produced by I1 or I3 waves using our previously reported method which preferentially elicits one group of I waves when subjects make a slight voluntary contraction. In some experiments the conditioning and test stimuli were oppositely directed, in the others they were oriented in the same direction. Single motor unit responses were recorded with a concentric needle electrode, and surface EMG responses with cup electrodes.
In post-stimulus time histograms (PSTHs) of the firing probability of motor units, the peaks produced by I3 waves were decreased by a subthreshold conditioning stimulus that preferentially elicited I1 or I3 waves at an ISI of 4 ms. The amount of decrement depended on the intensity of the conditioning stimulus. The stronger the conditioning stimulus, the greater the suppression. In contrast, the peaks produced by I1 waves were little affected by any type of subthreshold conditioning stimulus, given 4 ms prior to the test stimulus. At an ISI of 10 ms, a subthreshold conditioning stimulus slightly decreased the size of the peak produced by the I3 waves, but did not affect the peaks evoked by I1 waves.
Surface EMGs showed that a subthreshold conditioning stimulus suppressed the responses produced by I3 waves irrespective of its current direction (anterior or posterior). Both the amount and duration of suppression depended on the intensity of the conditioning stimulus, but not on its current direction. Both parameters increased when the intensity increased. At a high intensity conditioning stimulus, suppression was evoked at ISIs of 1-20 ms, compatible with the duration of GABA-mediated inhibition found in animal experiments. Responses produced by I1 waves were little affected by any type of subthreshold conditioning stimulus.
We conclude that a subthreshold conditioning stimulus given over the motor cortex moderately suppresses I3 waves but does not affect I1 waves. The duration of suppression of the I3 waves supports the idea that this is an effect of GABAergic inhibition within the motor cortex.
A conditioning subthreshold transcranial magnetic stimulus is reported to suppress the EMG response evoked by a succeeding suprathreshold test stimulus when given 1-5 ms prior to the test stimulus (Kujirai et al. 1993). This suppression was designated ‘cortico-cortical inhibition’ because the effect was considered to be produced by inhibitory connections at the cortical level. Kujirai et al. (1993) proposed that this effect is produced by activation of the intracortical GABAergic inhibitory system by the conditioning stimulus. One inconsistency in their findings compared with those of animal experiments (Krnjevic, Randic & Straughan, 1964, 1965; Matsumura, Sawaguchi & Kubota, 1992; Bekenstein, Rempe & Lothman, 1993) is the duration of inhibition. The duration reported by Kujirai et al. (1993) seemed to be only 5 ms, whereas inhibition in animals sometimes lasted 100 ms. They concluded that in humans simultaneous activation of excitatory and inhibitory connections by a conditioning stimulus makes it difficult to estimate the true duration of inhibition. Later findings on changes in this type of suppression induced by various drugs (Ziemann, Lönnecker, Steimhoff & Paulus 1996a,b) support the supposition that suppression is produced by GABAergic action within the motor cortex. The preferential current direction for intracortical inhibition differs from that for intracortical facilitation at late intervals (Ziemann, Rothwell & Ridding 1996c). Corticospinal volleys evoked by paired-pulse magnetic stimulation have shown that greater suppression of the late I waves (indirect waves: descending volleys produced by indirect activation of pyramidal tract neurones via presynaptic neurones) occurs (Nakamura, Kitagawa, Kawaguchi & Tsuji, 1997). Those authors used higher intensity cortical stimuli than we did because they elicited discernible descending volleys in the spinal recordings. Moreover, they only studied patients for whom recordings of descending volleys had to be made. To confirm that there is a difference in inhibitory effects among different I waves and to estimate the true duration of the inhibitory effect in normal subjects, we investigated cortico-cortical inhibition of responses produced selectively by I1 or I3 waves using a technique by which one group of I waves is preferentially activated with a figure-of-eight-shaped coil (Sakai, Ugawa, Terao, Hanajima, Furubayashi & Kanazawa 1997). We conclude that I3 waves are moderately affected by cortico-cortical inhibitory systems, whereas I1 waves are little affected, and that the duration of this inhibitory effect is longer than 20 ms. Our evidence provides a strong indication that this inhibition is mediated by GABAergic inhibitory mechanisms.
METHODS
Subjects
Nine healthy volunteers (8 men and 1 woman, 29-42 years old) were the subjects. Informed consent was obtained from all the subjects. The experimental procedures used were approved by the Ethics Committee of the University of Tokyo. No side effects were noted in any of the individuals tested.
Electromyographic (EMG) recordings
Single motor units were recorded from the right FDI with a concentric needle electrode (Medelec, disposable type DML25). Signals were amplified through filters set at 100 Hz and 3 kHz. The subjects were instructed to fire the unit voluntarily at about 10 Hz with the aid of audiovisual feedback. Care was taken to record the same motor unit during each experimental session by using an on-line oscilloscope monitor. Twenty-three motor units from nine subjects were studied. Post-stimulus time histograms (PSTHs) were made from the single motor unit recording data under various conditions.
Surface EMGs were recorded from the first dorsal interosseous muscles (FDIs) with Ag-AgCl surface cup electrodes (9 mm in diameter). The active electrode was placed over the muscle belly, and the reference electrode over the metacarpophalangeal joint of the index finger. Responses were amplified (Biotop, NEC San-Ei, Japan) through filters set at 100 Hz and 3 kHz then recorded by a computer (Signal Processor DP-1200, NEC San-Ei, Japan). During the experiments the subjects maintained slight contraction of the FDI (5-10 % of the maximum voluntary contraction) with the aid of an oscilloscope monitor.
Stimulation
Transcranial electrical stimulation (TES) was done with a high voltage electric stimulator D180A (Digitimer, UK) to determine the D wave (direct waves: descending volleys produced by direct activation of pyramidal tract neurones) latency for each muscle studied. Stimuli were given through two Ag-AgCl cup electrodes (9 mm in diameter) fixed to the scalp; the cathode was placed at the vertex, the anode over the hand motor area (about 5-6 cm lateral to the vertex). The D wave latency was measured as a reference for both single motor unit and surface EMG recordings during slight contraction of the target muscle.
Transcranial magnetic stimulation (TMS) was done with a Magstim 200 magnetic stimulator (The Magstim Company, UK). A figure-of-eight-shaped coil was placed over the hand motor area and held at eight different orientations, each separated by 45 deg, to determine the current directions at which I1 or I3 waves could be preferentially elicited. We chose two current directions induced in the brain for further experiments; the direction of the current for eliciting responses about 1.5 ms later than those evoked by TES (I1 waves) and the direction for producing responses about 4.5 ms later than D waves (I3 waves). In most subjects, anteriorly directed induced current preferentially elicited I1 waves, and posteriorly directed current I3 waves. For descriptive purposes only, when presenting the mean data obtained for all the subjects as one group, we refer to the induced current that preferentially activates I1 waves as anteriorly directed current (A) and that which activates I3 waves as posteriorly directed current (P).
Paired-pulse magnetic stimulation
We studied the ipsilateral cortico-cortical inhibitory effect (Kujirai et al. 1993) on two different I waves evoked by magnetic stimulation with the selected current directions, using a technique similar to methods reported elsewhere (Kujirai et al. 1993; Hanajima et al. 1996). Conditioning and test stimuli were given through the same figure-of-eight-shaped coil (external diameter at each wing 9 cm) by connecting two magnetic stimulators linked with a Bistim module (Magstim). In some parts of the experiments we also used a new instrument which reverses the direction of the current flowing in the coil (Magstim). When using this instrument with the Bistim module, we could give two successive, oppositely directed stimuli, which were separated by given intervals (3 ms at the shortest), through the same coil. With this system, we could study the effect of an anteriorly directed conditioning stimulus on responses produced by a posteriorly directed test stimulus, and vice versa.
Single motor unit studies
We first determined the threshold that produced a peak in the PSTH for each current direction by changing the intensity of stimulation in steps of 2 % of the maximum stimulator output. We defined the threshold as the lowest intensity that evoked a small peak in the PSTH. The intensity of the test stimulus was adjusted to produce a 20-30 % firing probability, which was 10-15 % above the threshold, and the conditioning stimulus was set 5 % below the threshold. In some experiments, the intensity of the conditioning stimulus was changed from -20 to -5 % to investigate the dependence of suppression on the intensity of the conditioning stimulus. Several PSTHs were recorded simultaneously. One was the PSTH evoked by a test stimulus given alone (control PSTH), the others were PSTHs evoked by a test stimulus preceded 4 or 10 ms by a conditioning stimulus (conditioned PSTHs). These representative interstimulus intervals (ISIs) for suppression and facilitation were chosen because in previous studies (Kujirai et al. 1993; Ridding, Taylor & Rothwell, 1995; Ziemann et al. 1996c; Hanajima, Ugawa, Terao, Ogata & Kanazawa, 1996) clear suppression and facilitation, respectively, were shown at these intervals. Control and conditioned trials were intermixed randomly by the computer until 100 trials had been collected for each condition. A PSTH of the unit discharges was constructed for each condition. The conditioned PSTH was compared with the control PSTH in the same session.
In addition to the control PSTHs, four conditioned PSTHs were made for the different combinations of conditioning and test stimuli: (1) both stimuli were oriented in the direction in which I3 waves were elicited preferentially, usually the posterior direction (P-P); (2) the conditioning stimulus was oriented for I1 waves and the test stimulus for I3 waves (A-P); (3) both stimuli were oriented for I1 waves (A-A); (4) the conditioning stimulus was oriented for I3 waves and the test stimulus for I1 waves (P-A). To estimate the effect of the conditioning stimulus on the response to the test stimulus, we calculated the ratio of the firing probability during the peak of interest in the conditioned PSTH to that in the control PSTH (firing probability ratio). We compared these values for various combinations of the two stimuli.
Surface EMG recordings
To confirm the PSTH findings and to clarify the time course of the effect, we also studied the effects of several different kinds of conditioning stimuli on the surface EMG responses to a test stimulus.
We first determined the threshold for each current direction using the averaged rectified EMGs for active muscles (average of at least 10 responses). The intensity of stimulation was changed in steps of 2 % of the maximum stimulator output. We defined the threshold as the lowest intensity that evoked a small response (about 50 μV) compared with the pre-stimulus background activities. The thresholds were almost the same as those for the PSTH peaks when using the same stimulating current direction. Differences in the same muscle were less than 5 % of the maximum stimulator output. The intensity of the conditioning stimulus was fixed at 5 % below the threshold in the main parts of the experiments. The test stimulus was adjusted to evoke a response with an amplitude of approximately 0.2-0.4 mV peak to peak in the active FDI, which was 10-15 % above the threshold. To investigate the effect of the intensity of the conditioning stimulus, in some experiments we used several intensities for the conditioning stimulus. The response latencies were measured from the superimposed responses. We confirmed that the latencies of the control responses elicited by stimulation with the two selected currents were compatible with the I1 or I3 waves. We used a randomized conditioning-test design similar to that reported previously (Hanajima et al. 1996). In short, various conditions (a test or conditioning stimulus given alone, or a test stimulus preceded by a conditioning stimulus at various ISIs) were intermixed randomly in one block. Several blocks of trials were performed to investigate the complete time course of the effect studied. As in the single motor unit studies, we studied the time courses for four different combinations of the conditioning and test stimuli.
Interstimulus intervals (ISIs) between 1 and 20 ms (1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 15 and 20 ms) were used. Eight to ten responses were collected and averaged for each condition in which both stimuli were given, and twenty responses were collected for a control condition in which the test stimulus alone was given. The amplitude of each single response under each condition was measured to compare statistically the amplitudes of the control and conditioned responses in the same block using Student's unpaired t test in each single subject. We calculated the ratio of the mean amplitude of the conditioned response to that of the control response for each condition in every subject. We then calculated the mean of these from all subjects for every ISI. We designated this value the mean size ratio. The time course of the effect of the conditioning shock was plotted with this mean size ratio on the ordinate and with the ISI on the abscissa. We compared the time courses for different combinations of the conditioning and test stimuli using a repeated measures analysis of variance (ANOVA) test and Tukey's method for post hoc analysis.
RESULTS
Current direction and response latency
In seven subjects, in the surface EMG recordings, TMS with anteriorly directed current in the brain produced responses whose latencies were about 1.5 ms later (I1 wave) than the responses produced by ES (D wave). In the other two subjects, stimulation with anteriorly directed current produced responses whose latencies were 3.0 ms later (I2 wave) than the responses elicited by ES. Responses with latencies about 4.5 ms later (I3 wave) than the D wave were evoked by posteriorly directed current in the brain in seven subjects and by laterally directed current in one. In the remaining one subject, the latency of the responses elicited by posteriorly directed current was 3.0 ms later (I2 wave) than the D wave. The latencies did not change under any of the stimulation conditions when the amplitudes of responses were less than 0.5 mV. These findings were consistent with those of single motor unit studies. Stimulation at an intensity evoking surface EMG responses of less than 0.5 mV produced a single peak in the PSTHs in all twenty-three motor units studied. In all the units studied, this initial single PSTH peak was produced by the same I wave as that first recruited in the surface EMG recordings in the same muscle. Stimulation at an intensity evoking surface EMG responses of more than 1.0 mV often produced two or more peaks in the PSTHs. These suggest that the EMG responses of less than 0.5 mV evoked by stimulating current flowing in the selected direction were almost purely produced by one group of descending volleys. These findings indicate that anteriorly directed current in the brain preferentially evokes I1 waves and that posteriorly directed current evokes I3 waves in most subjects, and are consistent with previous results (Sakai et al. 1997).
In the present study, we performed paired-pulse magnetic stimulation experiments using current directions that produce I1 or I3 waves in order to investigate selectively the cortico-cortical inhibition of I1 or I3 waves. We used anteriorly directed current for seven subjects in order to study their I1 waves, and posteriorly directed current for seven subjects and laterally directed current for one to study their I3 waves. We did not make paired-pulse stimulation studies of the I2 waves because responses produced by I2 waves were seen in only three of the subjects. Since these three motor units were not used in the paired-pulse stimulation study, the paired-pulse magnetic stimulation was performed on twenty motor units. We selected the test stimulus intensity that could elicit control surface EMG responses of 0.2-0.4 mV (described in Methods) because one group of descending volleys can be evoked almost purely by stimulation at this intensity. The intensity of the conditioning stimulus usually was fixed at 5 % less than the threshold (-5 %), at which intensity the magnetic stimulation produced no peaks in the PSTHs.
Paired-pulse magnetic stimulation
Single motor unit studies
Figure 1 shows the control and conditioned PSTHs obtained from a single subject for four combinations of the conditioning and test stimuli. The conditioning stimulus was given 4 ms prior to the test stimulus (ISI = 4 ms) in the conditioned PSTHs. The test stimulus with posteriorly directed current produced one peak (firing probability: 31/100) about 4.5 ms later (I3 wave) than that evoked by TES (dotted lines indicate the latency of the peak produced by TES) (Fig. 1Aa). The posteriorly directed conditioning stimulus of -5 % intensity strongly suppressed the peak elicited by the posteriorly directed test stimulus (Fig. 1Ab). The firing probability changed from 31/100 to 4/100. The ratio of the firing probability of the conditioned to the control PSTHs was 0.13 (4/31). The peak evoked by the posteriorly directed test stimulus was moderately reduced by the anteriorly directed conditioning stimulus of -5 % intensity (firing probability reduced from 35/100 to 22/100; Fig. 1B). The firing probability ratio for this condition was 0.63 (22/35). An anteriorly directed test stimulus evoked a peak about 2 ms later than the D wave (firing probability: 28/100) (Fig. 1Ca). The anteriorly directed conditioning stimulus of -5 % intensity did not reduce the size of this peak (firing probability: 26/100) (Fig. 1Cb). The peak produced by the I1 waves was not affected by the posteriorly directed conditioning stimulus (Fig. 1D). These differences among the various combinations of conditioning and test stimuli were observed in all the motor units studied. The mean (±s.d.) firing probability ratios obtained from all the motor units at ISI = 4 ms were 0.14 ± 0.11 for P-P, 0.31 ± 0.21 for A-P, 0.94 ± 0.3 for A-A and 0.98 ± 0.36 for P-A. The threshold for the I1 waves was about 15-20 % less than that for the I3 waves in most of the subjects. The mean absolute intensity of the posteriorly directed conditioning stimulus therefore was 18.6 ± 8.2 % more than that for the anteriorly directed conditioning stimulus when the intensity relative to the threshold of both stimuli was the same.
Figure 1. Pairs of control and conditioned PSTHs for four different combinations of the conditioning and test stimuli in a single subject.
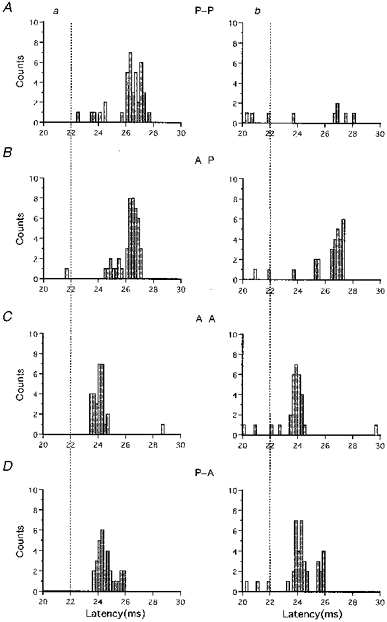
Control PSTHs are shown on the left (a), and conditioned PSTHs at an ISI of 4 ms on the right (b). Each PSTH was constructed from 100 trials. The abscissa shows the latency after the magnetic stimulation for 20-30 ms. A, P-P is the condition under which both the conditioning and test stimuli were posteriorly directed current in the brain. B, in A-P, the conditioning stimulus was anteriorly directed current, and the test stimulus was posteriorly directed current. C, in A-A, both stimuli were anteriorly directed current. D, in P-A, the conditioning stimulus was posteriorly directed current, and the test stimulus anteriorly directed. In the control PSTHs (Aa and Ba) the posteriorly directed test stimulus elicited a single peak with a latency about 4.5 ms later than that of the peak produced by electrical stimulation (D wave, dotted lines). The peak was much reduced by the conditioning stimulus under the P-P condition (Ab), and moderately so under the A-P condition (Bb). In the control PSTHs, the anteriorly directed test stimulus produced a single peak 2 ms later than the D wave (Ca and Da). These peaks were not reduced by both the anteriorly and posteriorly directed conditioning stimuli (Cb and Db).
In the control PSTHs of a few motor units, an anteriorly directed test stimulus of high intensity evoked two or three peaks (I1 to I3 waves) (Fig. 2A). A posteriorly directed conditioning stimulus of -5 % intensity given 4 ms prior to the test stimulus (ISI = 4 ms) greatly reduced the peak corresponding to the I3 waves and moderately reduced that for the I2 waves (Fig. 2B). In contrast, I1 waves were not affected by the same conditioning stimulus. The peaks for I1 waves were also not affected by a conditioning stimulus of -5 % intensity given at an ISI of 7 ms (not shown).
Figure 2. Effect of a conditioning stimulus on three peaks in a PSTH (total 100 trials) evoked by an anteriorly directed test stimulus of high intensity.
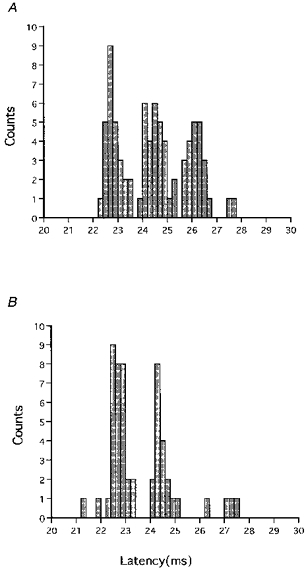
A, the anteriorly directed test stimulus of high intensity produced three peaks in a PSTH which had latencies compatible with I1, I2 and I3 waves. B, the anteriorly directed conditioning stimulus of -5 % intensity given at an ISI of 4 ms markedly decreased the size of the I3 wave, moderately decreased that of the I2 wave, but did not affect the I1 wave.
These results suggest that I3 waves are reduced by a preceding subthreshold conditioning stimulus, whereas I1 waves are negligibly affected. This suppressive effect on I3 waves is evoked by both the posteriorly and anteriorly directed conditioning stimulus.
Figure 3 shows differences in the suppressive effects produced by conditioning stimuli of various intensities. Both the conditioning and test stimuli were directed posteriorly (P-P). In the control PSTHs (Fig. 3A), one peak was produced at the latency corresponding to the I3 wave (dashed lines, the D wave latency). A conditioning stimulus of -5 % intensity reduced the peak produced by the test stimulus at ISIs of 4 and 10 ms, the firing probability ratios being 0.12 for 4 ms and 0.67 for 10 ms (Fig. 3B). In the case of -10 % intensity, the firing probability ratios were 0.46 for 4 ms and 0.89 for 10 ms (Fig. 3C). A conditioning stimulus of -15 % intensity reduced the peak somewhat at the ISI of 4 ms (firing probability ratio: 0.77), but did not affect it at the ISI of 10 ms (firing probability ratio: 0.94) (Fig. 3D). The dependence of the amount and duration of suppression on the intensity of the conditioning stimulus was found for all five units studied at the various conditioning stimulus intensities. This indicates that the stronger the conditioning stimulus, the deeper and longer the suppressive effect.
Figure 3. Dependence of inhibition on the intensity of the conditioning stimulus under the P-P condition.
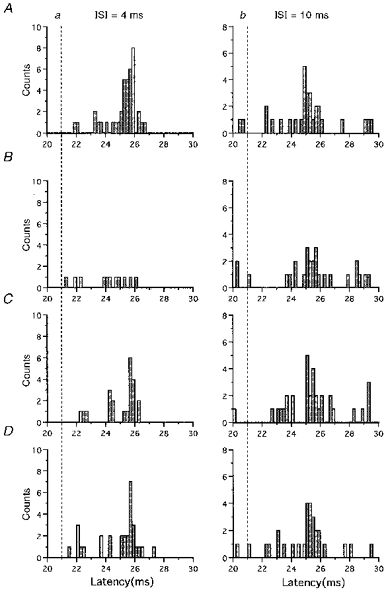
A shows the control PSTHs; B-D show conditioned PSTHs. Total trial number was 100 for every PSTH. Conditioned PSTHs at an ISI of 4 ms are given on the left (a) and those at an ISI of 10 ms on the right (b). The test stimulus evoked a peak that corresponded to the I3 wave. Conditioning stimuli of -5, -10 and -15 % intensity reduced the peak size produced by the test stimulus at an ISI of 4 ms. In the PSTH for the -5 % conditioning stimulus given 10 ms prior to the test stimulus, the peak produced by the test stimulus was smaller than that in the control PSTH. This peak was not affected by conditioning stimuli of -10 or -15 % intensity given at an ISI of 10 ms.
The peak produced by the electrical test stimulation was not affected by the conditioning stimulus even though the same conditioning stimulus suppressed the peak produced by magnetic cortical stimulation. In this experiment, we used ISIs of 4, 5, 6, 7, 8, 9 and 10 ms between magnetic conditioning and electrical test stimulus in order to compare the effect on electrical cortical responses with that on magnetic cortical responses at an ISI of 4 ms because there was 4-5 ms difference in the onset latency between magnetically and electrically evoked test responses. This confirms previous findings (Kujirai et al. 1993) and suggests that the effect studied occurs at the cortical level.
Surface EMG recordings
To confirm the above findings for the single motor unit studies and to investigate the entire time course of the effect, we did similar experiments using surface EMG recordings.
Figure 4 shows the effects of a conditioning stimulus on responses produced by a posteriorly directed test stimulus. The test stimulus given alone evoked a control response about 0.2 mV in amplitude. Its onset latency of 25.4 ms corresponded to the latency of the I3 wave. The posteriorly directed conditioning stimulus of -5 % intensity (55 %) reduced the response sizes to the test stimulus at ISIs of 3, 4, 8 and 10 ms (Fig. 4Aa) (Student's unpaired t test: P < 0.01). Conditioned responses for an anteriorly directed conditioning stimulus of -5 % intensity (40 %) were significantly smaller than the control response at ISIs of 3 and 4 ms (Student's unpaired t test: P < 0.02), smaller at an ISI of 8 ms but not significant (Student's unpaired t test: P > 0.2), and almost the same size at an ISI of 10 ms (Student's unpaired t test: P > 0.5) (Fig. 4Ab). Similar patterns of suppression were observed in all the subjects. The mean (±s.e.m.) time courses of the effect on the response to a posteriorly directed test stimulus were obtained from the results for all the subjects (Fig. 4B). The mean values of the size ratios were calculated from the size ratios of each subject for a -5 % conditioning stimulus. There was significant suppression when a posteriorly directed conditioning stimulus was used (•; ANOVA test: P < 0.01) at all ISIs (Tukey's method: P < 0.02, ISI = 1-6 ms, P < 0.05, ISI = 7, 10 and 12 ms). The amounts of suppression at ISIs of 1- 4 ms were smaller than the values for relaxed muscles reported previously (Kujirai et al. 1993; Ridding et al. 1996; Hanajima et al. 1996). In contrast, for an anteriorly directed conditioning stimulus (○), significant suppression (ANOVA test: P < 0.05) occurred at ISIs of 3-6 ms (Tukey's method: P < 0.02), but there were no significant changes at later ISIs. No facilitation was elicited at long ISIs. These two time courses between ISIs of 3-12 ms were significantly different (ANOVA: P < 0.05). There was significantly less suppression in the time course for the anteriorly directed conditioning stimulus compared with that for the posteriorly directed one.
Figure 4. Averaged surface EMG potentials (A) and mean (±s.e.m.) time courses (B) for responses evoked by a posteriorly directed test stimulus.
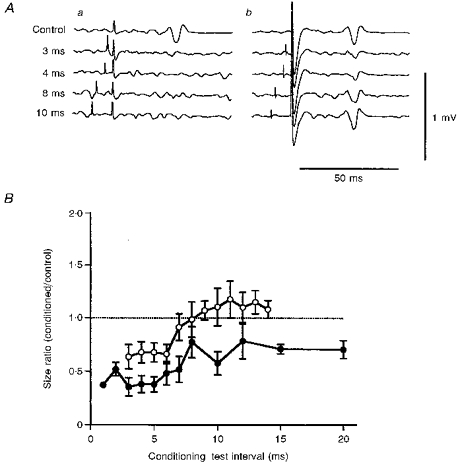
Aa, responses under the P-P condition; Ab, responses under the A-P condition. Top traces show the control responses and the four lower traces show responses to a test stimulus preceded by a conditioning stimulus by 3, 4, 8 and 10 ms. The conditioning stimulus was fixed at 5 % less than the threshold for the active muscle. The sizes of control responses were about 0.2 mV. Conditioned responses for the P-P condition were significantly smaller than the control responses at all the ISIs (Student's unpaired t test: P < 0.01). Those for the A-P condition were significantly smaller than the control at ISIs of 3 and 4 ms (Student's unpaired t test: P < 0.02), but not significantly different at ISIs of 8 and 10 ms (P > 0.2). B, mean (±s.e.m.) time courses of the effect on the response to a posteriorly directed test stimulus were obtained from the results for all the subjects. •, P-P condition; ○, A-P condition. Each point represents the mean (±s.e.m.) size ratio for all the subjects. The abscissa shows the ISIs and the ordinate the size ratios. In both time courses, significant suppression occurred (ANOVA test: P < 0.01 for P-P, P < 0.02 for P-A). At short ISIs (1-5 ms), the size ratios for both conditions were significantly less than 1.0 (Tukey's method: P < 0.01 for P-P, P < 0.05 for A-P). Significant suppression continued up to an ISI of 20 ms in the time course for the P-P condition (P < 0.05 for ISI of 20 ms), but not in that for the A-P condition. Significantly deeper suppression occurred under the P-P condition than under the A-P condition (ANOVA test: P < 0.05)
The effects on the responses produced by an anteriorly directed test stimulus are shown in Fig. 5. The onset latency of the control responses was 22.6 ms, corresponding to the latency of the I1 wave (same subject as in Fig. 4), and their sizes were about 0.2 mV. The intensity of the conditioning stimulus was 5 % less than the threshold. The anteriorly directed conditioning stimulus slightly suppressed responses to the test stimulus at an ISI of 3 ms, but not significantly (Student's unpaired t test: P > 0.1) (Fig. 5Aa). The other conditioned responses were almost the same in size as the control response (Fig. 5Ab). The mean (±s.e.m.) time courses for the anteriorly (•) and posteriorly (○) directed conditioning stimuli are shown in Fig. 5B. Slight suppression was evoked at ISIs of 1 and 2 ms by the anteriorly directed conditioning stimulus (Student's unpaired t test: P < 0.05), but only at an ISI of 3 ms by the posteriorly directed conditioning stimulus (Student's unpaired t test: P < 0.05). These two time courses were not significantly different (ANOVA test: P > 0.2). The repeated measures ANOVA test for the time courses of four different combinations of the conditioning and test stimuli showed that both time courses for P-P (P < 0.01) and A-P (P < 0.05) conditions differed significantly from those for A-A or P-A.
Figure 5. Averaged surface EMG responses (A) and mean (±s.e.m.) time courses (B) for the A-A and P-A conditions.
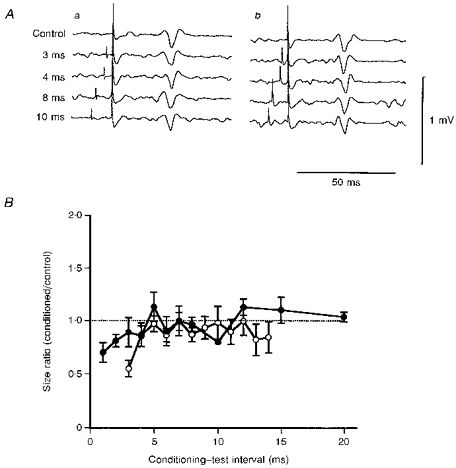
The arrangement is the same as in Fig. 4. Aa, responses under the A-A condition; Ab, responses under the P-A condition. The intensity of the conditioning stimulus was -5 %. The conditioned response was smaller than the control response only at an ISI of 3 ms under the A-A condition (Aa). This suppression was not significant (Student's unpaired t test: P > 0.1). No suppression occurred at the other intervals under the A-A condition (Aa). No suppression was evoked at any intervals under the P-A condition (Ab). B, slight but significant suppression occurred at ISIs of 1 and 2 ms in the time course for the A-A condition (•; Student's unpaired t test: P < 0.05), and only at an ISI of 3 ms for the P-A condition (○; Student's unpaired t test: P < 0.05).
Suppressive effects evoked by three conditioning stimulus intensities (-5, -10 and -15 %) were studied in four different combinations of the conditioning and test stimuli (P-P, A-P, A-A and P-A). Figure 6 shows the responses in a single subject at ISIs of 4 and 10 ms. For each intensity, the conditioned responses at an ISI of 4 ms were much smaller than the control response under the P-P conditions (Student's unpaired t test: P < 0.01). Under the A-P condition, however, the conditioned response was slightly smaller than the control only at -5 % intensity (Student's unpaired t test: P < 0.05). The threshold for I1 waves was about 15 % less than that for the I3 waves in this subject. The amount of suppression under the P-P condition at a -20 % intensity conditioning stimulus (not shown) was about the same as that for the A-P condition at -5 % intensity. The average results obtained from all the subjects showed that the average size ratios under the P-P condition with a -20 % conditioning stimulus did not differ significantly (ANOVA test: P > 0.2) from those under the A-P condition with a -5 % conditioning stimulus at ISIs of 3, 4 and 5 ms. Because of the threshold difference between the two current directions, the absolute values of these two conditioning stimulus intensities were almost the same. This suggests that the present suppressive effect depends on the intensity of the conditioning stimulus, but not on its current direction. All the conditioned responses at an ISI of 4 ms were almost the same size as the control responses under the A-A and P-A conditions (Student's unpaired t test: P > 0.1). Conditioned responses at an ISI of 10 ms were significantly smaller than the control response only under the P-P condition at -5 % intensity (Student's unpaired t test: P < 0.01). Similar results were obtained for all the subjects. Significant suppression was evoked by conditioning stimuli of -5, -10 and -15 % intensities at ISIs of 3-6 ms under the P-P condition (Tukey's method: -5 %, P < 0.01 for all ISIs; -10 %, P < 0.01 for 3 and 4 ms, P < 0.05 for 5 and 6 ms; -15 %, P < 0.01 for 3 ms, P < 0.05 for 4, 5 and 6 ms) and this suppression was still present at an ISI of 12 ms when a -5 % conditioning stimulus was used (Tukey's method: P < 0.05). Significant suppression occurred at ISIs of 3-6 ms for -5 % intensity conditioning stimuli under the A-P condition (Tukey's method: P < 0.02). No significant suppression was found under any of the A-A conditions. Only at an ISI of 3 ms was the conditioned response significantly smaller than the control response under the P-A condition at a -5 % conditioning stimulus (Student's unpaired t test: P < 0.01). This suppression at an ISI of 3 ms was considered to be caused by the collision between the conditioning and test stimuli at the cortex. Because the latency difference between the I1 and I3 waves was about 3 ms, the impulse from a posteriorly directed conditioning stimulus that precedes an anteriorly directed test stimulus by 3 ms must arrive at the pyramidal cell at about the same time as that of the test stimulus.
Figure 6. Typical responses of a single subject to a given test stimulus conditioned by stimuli of different intensities.
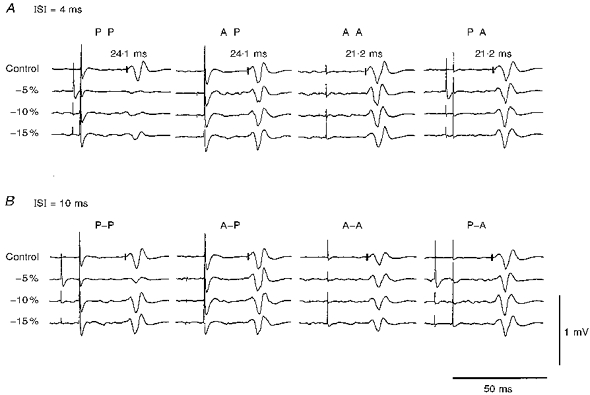
Averaged control and conditioned responses at ISIs of 4 ms (A) and 10 ms (B) are shown. The left column shows responses for the P-P condition, and the other columns responses for the A-P, A-A and P-A conditions. The conditioning stimulus intensities were 5, 10 and 15 % less than the threshold for the active muscles. All the control responses were similar in size. A, significant suppression occurred under the P-P conditions at all the intensities used (Student's unpaired t test: P < 0.01) and under the A-P condition with a -5 % conditioning stimulus given at an ISI of 4 ms (Student's unpaired t test: P < 0.05).B, at an ISI of 10 ms, however, there was significant suppression only for a -5 % conditioning stimulus under the P-P condition (Student' s unpaired t test: P < 0.01).
These surface EMG recording findings confirm the deduction from the single motor unit experiments that I3 waves are reduced by a subthreshold conditioning stimulus, whereas I1 waves are little affected.
DISCUSSION
This study has produced three main findings. (1) Responses produced by I3 waves are suppressed by a subthreshold conditioning stimulus directed anteriorly or posteriorly. Both the amount and duration of suppression depend on the intensity of the conditioning stimulus. The higher the intensity of the conditioning stimulus, the larger and longer the effect. This suppression continues for more than 20 ms when a high intensity conditioning stimulus is used. (2) Responses produced by I1 waves are negligibly affected by a subthreshold conditioning stimulus, except for suppression at an ISI of 3 ms under the P-A condition which is probably caused by the collision of the conditioning and test stimuli. (3) There was no facilitation at long intervals (8-15 ms) for any combination of the conditioning and test stimuli.
Comparisons with previous reports
The striking contrast between the suppressive effects on responses produced by the I3 and I1 waves which we have demonstrated is consistent with a previous report that later I waves recorded with epidural electrodes implanted during surgery were more affected by the paired-pulse magnetic stimulation (Nakamura et al. 1997). We obtained the same results for paired-pulse stimulation in normal subjects, even when the stimulation intensities used were lower than those in the epidural recordings. Our observation that the responses to I3 waves were suppressed by both posteriorly and anteriorly directed conditioning stimuli is also compatible with a previous report that the inhibitory effect elicited by paired-pulse stimulation is independent of the direction of the conditioning stimulus (Ziemann et al. 1996c). Ziemann et al. (1996c) used a conditioning stimulus directed perpendicular to the test stimulus, whereas we used an oppositely directed conditioning stimulus. Taking into account all these findings, we speculate that the direction of the conditioning stimulus does not affect the extent of inhibition of paired-pulse stimulation. We showed that parameter changes in the conditioning stimulus did not have the same effect on early inhibition (ISI = 1-5 ms) as on late facilitation (ISI = 8-15 ms). This supports the previous conclusion that separate independent neural mechanisms are responsible for inhibition and facilitation in the paired-pulse stimulation technique (Ziemann et al. 1996c). Our finding that a subthreshold conditioning stimulus did not affect electrical cortical responses is also consistent with an earlier report (Kujirai et al. 1993) that suggested that the suppression occurs at the cortical level.
The previous and present findings are explained as follows. The neurones responsible for I3 waves are suppressed by the conditioning stimulus in the paired-pulse magnetic stimulation method, but those for I1 waves are little affected. The inhibitory interneurones responsible for the present effect are similarly activated by both anteriorly and posteriorly directed stimuli. One major finding of our study is that the suppression of responses produced by I3 waves continued for more than 20 ms. This long duration of suppression is compatible with the GABAergic inhibitory mechanism reported in the animal motor cortex (Krnjevic et al. 1964, 1965; Matsumura et al. 1992; Bekenstein et al. 1993) which was proposed to be responsible for this effect by Kujirai et al. (1993). The fact that suppression increased and its duration lengthened when the conditioning stimulus was stronger also is consistent with GABAergic inhibition (Matsumura et al. 1992; Bekenstein et al. 1993). In previous studies on the responses from relaxed muscles, as mentioned by Kujirai et al. (1993), the true duration of inhibition could not be estimated because both the excitatory and inhibitory mechanisms were activated by the conditioning stimulus. Because we recorded responses from active muscles that were elicited by a figure-of-eight shaped coil at a low intensity, a relatively pure effect of inhibition was obtained.
The above hypothesis explains other earlier findings as follows. One reason for the moderate suppression evoked at short ISIs, even under the A-A condition in previous reports (Kujirai et al. 1993; Ziemann et al. 1996c; Hanajima et al. 1996), must be that the responses were recorded from relaxed muscles. During relaxation, in addition to I1 waves, activation of I3 waves by an anteriorly directed test stimulus is necessary to evoke the control EMG responses. Because an anteriorly directed conditioning stimulus affects I3 waves, the sizes of the responses in relaxed muscles were reduced by the conditioning stimulus. Ridding et al. (1995) reported that the amount of suppression was reduced, compared with the value for relaxed muscles, by voluntary contraction of the target muscle. This is partly explained by the fact that during voluntary contraction the control EMG responses evoked by an anteriorly directed test stimulus are mediated by I1 waves that are only slightly affected by the conditioning stimulus. In contrast, during relaxation of the target muscle, I3 as well as I1 waves contribute to the generation of the control EMG responses. Because the I3 waves are greatly affected by the conditioning stimulus, the responses to the test stimulus are suppressed. Our finding that even for I3 waves the amount of inhibition during voluntary contraction was smaller than that evoked in relaxed muscles supports the hypothesis proposed by Ridding et al. (1995) that voluntary contraction reduces the excitability of the intracortical inhibitory mechanisms of the motor cortex. These two mechanisms (contribution of the I3 waves to the control EMG responses and decreased intracortical inhibitory function by voluntary contraction) are considered to contribute to the finding that inhibition of the paired-pulse stimulation is reduced by voluntary contraction of the target muscle in responses produced by an anteriorly directed test stimulus.
Why I3 waves are more affected?
There are at least two possible explanations for why I3 waves are much more affected by the cortical inhibitory interneurones than are I1 waves. One is that different groups of interneurones, which have different physiological characteristics, in particular for susceptibility to the intracortical inhibition, are responsible for these waves. The other is that the motor cortical interneurones responsible for the I1 and I3 waves reach the corticospinal neurones by way of the same group of interneurones. The process which produces I3 waves, however, has more synapses than that which produces I1 waves. I3 waves, therefore, are more susceptible to inhibition because they are generated through more synapses at the cortical level than are I1 waves.
Facilitation at late ISIs
The other important finding is that neither the responses produced by I1 waves nor those produced by I3 waves were facilitated by a conditioning stimulus given at long interstimulus intervals (8-15 ms), even though facilitation is reported to be evoked in relaxed muscles at these ISIs (Kujirai et al. 1993; Ridding et al. 1995; Ziemann et al. 1996c; Hanajima et al. 1996). Significant suppression of the responses produced by I3 waves occurred, even at these long intervals. A previous report of less facilitation in responses evoked by an anteriorly directed test stimulus at long ISIs during tonic contraction (Ridding et al. 1995) is compatible with our findings for responses evoked by I1 waves. Ridding et al. (1995) speculated that this reduced facilitation might be due to changes in the excitability of intracortical circuits induced by a voluntary contraction. Another possible explanation is that facilitation at long intervals (8-15 ms) occurs only when more than two groups of descending volleys, such as I1 and I3 waves, contribute to the generation of the control EMG responses. Facilitation at long intervals occurred only in relaxed muscles because EMG responses can be evoked by a combination of different groups of I waves when subjects do not contract the target muscle. Because activation of one group of descending volleys (such as I1 or I3 waves) is sufficient to elicit EMG responses during tonic voluntary contraction, there was no facilitation at long intervals in active muscles. Whatever the mechanism for late facilitation, our findings support Ziemann's conclusion (Ziemann et al. 1996c) that this facilitation is not a rebound phenomenon that follows inhibition at early intervals, rather it occurs at the cortical level independently of early inhibition.
We conclude that I3 waves are affected by the cortical inhibition produced by paired-pulse magnetic stimulation, whereas I1 waves are little affected. The inhibitory interneurones activated by the conditioning stimulus are not positioned in a single direction. They are probably randomly directed within the motor cortex.
Acknowledgments
Part of this work was supported by grants from the Research Committee on Ataxic Disease of the Ministry of Health and Welfare of Japan, the Brain Science Foundation, the Life Science Foundation of Japan, the Nakabayashi Trust for ALS Research and by research project Grant-in-Aid for Scientific Research No. 9670640 from the Ministry of Education, Science, Sports and Culture of Japan. Two of the authors (R. H. and Y. T.) are supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
References
- Bekenstein J, Rempe D, Lothman E. Decreased heterosynaptic and homosynaptic paired-pulse inhibition in the rat hippocampus as a chronic sequel to limbic status epilepticus. Brain Research. 1993;601:111–120. doi: 10.1016/0006-8993(93)91701-s. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Ogata K, Kanazawa I. Ipsilateral cortico-cortical inhibition of the motor cortex in various neurological disorders. Journal of the Neurological Sciences. 1996;140:109–116. doi: 10.1016/0022-510x(96)00100-1. 10.1016/0022-510X(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Randic M, Straughan DW. Cortical inhibition. Nature. 1964;201:1294–1296. doi: 10.1038/2011294a0. [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Randic M, Straughan DW. Pharmacology of cortical inhibition. The Journal of Physiology. 1965;184:78–105. doi: 10.1113/jphysiol.1966.sp007904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Cortico-cortical inhibition in human motor cortex. The Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M, Sawaguchi T, Kubota K. GABAergic inhibition of neuronal activity in the primate motor and premotor cortex during voluntary movement. Journal of Neurophysiology. 1992;68:692–702. doi: 10.1152/jn.1992.68.3.692. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. The Journal of Physiology. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. The Journal of Physiology. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-eight shaped coil. Experimental Brain Research. 1997;113:24–32. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Experimental Brain Research. 1996a;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: A transcranial magnetic stimulation study. Annals of Neurology. 1996b;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding M. Interaction between intracortical inhibition and facilitation in human motor cortex. The Journal of Physiology. 1996c;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]


