Abstract
The mechanism of pHi recovery from an intracellular alkali load (induced by acetate prepulse or by reduction/removal of ambient PCO2) was investigated using intracellular SNARF fluorescence in the guinea-pig ventricular myocyte.
In Hepes buffer (pHo 7.40), pHi recovery was inhibited by removal of extracellular Cl−, but not by removal of Na+o or elevation of K+o. Recovery was unaffected by the stilbene drug DIDS (4,4-diisothiocyanatostilbene-disulphonic acid), but was slowed dose dependently by the stilbene drug DBDS (dibenzamidostilbene-disulphonic acid).
In 5 % CO2/HCO3− buffer (pHo 7.40), pHi recovery was faster than in Hepes buffer. It consisted of an initial rapid recovery phase followed by a slow phase. Much of the rapid phase has been attributed to CO2-dependent buffering. The slow phase was inhibited completely by Cl− removal but not by Na+o removal or K+o elevation.
At a test pHi of 7.30 in CO2/HCO3− buffer, the slow phase was inhibited 70 % by DIDS. The mean DIDS-inhibitable acid influx was equivalent in magnitude to the HCO3−-stimulated acid influx. Similarly, the DIDS-insensitive influx was equivalent to that estimated in Hepes buffer.
We conclude that two independent sarcolemmal acid-loading carriers are stimulated by a rise of pHi and account for the slow phase of recovery from an alkali load. The results are consistent with activation of a DIDS-sensitive Cl−-HCO3− anion exchanger (AE) to produce HCO3− efflux, and a DIDS-insensitive Cl−-OH− exchanger (CHE) to produce OH− efflux. H+-Cl− co-influx as the alternative configuration for CHE is not, however, excluded.
The dual acid-loading system (AE plus CHE), previously shown to be activated by a fall of extracellular pH, is thus activated by a rise of intracellular pH. Activity of the dual-loading system is therefore controlled by pH on both sides of the cardiac sarcolemma.
Intracellular pH (pHi) exerts considerable influence on cardiac contractility and rhythm (Orchard & Kentish, 1990; Orchard & Cingolani, 1994). It is controlled in mammalian cardiac cells by means of sarcolemmal acid-extrusion and acid-loading carriers. The acid-equivalent extrusion carriers are Na+-H+ exchange (Deitmer & Ellis, 1980) and Na+-HCO3− symport (Dart & Vaughan-Jones, 1992; Lagadic-Gossmann, Buckler & Vaughan-Jones, 1992, but cf. Liu, Piwnica-Worms & Lieberman, 1990) while the loading carriers are Cl−-HCO3− exchange (Vaughan-Jones, 1979; Xu & Spitzer, 1994) and a novel Cl−-dependent carrier, proposed to be Cl−-OH− exchange (Sun, Leem & Vaughan-Jones, 1996). We have shown recently that, in mammalian ventricular myocytes, the two sarcolemmal acid loaders are stimulated by a fall of extracellular pH, and that this stimulation accounts for the subsequent fall of pHi (Sun et al. 1996). In addition to this extracellular pH sensitivity, earlier reports showed that Cl−-HCO3− exchange, one of the two acid loaders, is stimulated by a rise of intracellular pH, leading to pHi recovery from an alkali load (Vaughan-Jones, 1982; Xu & Spitzer, 1994). The possibility, however, of high pHi activation of Cl−-OH− exchange has not so far been investigated.
In the preceding paper (Leem & Vaughan-Jones, 1998), we showed that much of the initial rapid phase of pHi recovery from an intracellular alkali load is due to slow CO2-dependent buffering. In the present work, we examine the contribution to pHi recovery made by the acid loaders. In particular, we investigate the possible role played by the novel Cl−-OH− exchanger (CHE). The high pHi stimulation of Cl−-HCO3− anion exchange (AE) is also re-assessed, since its contribution to pHi recovery has not previously been distinguished from that of CHE.
Preliminary reports of this work have appeared (Leem, Loh & Vaughan-Jones, 1996; Leem & Vaughan-Jones, 1996).
METHODS
Details are given in full in the Methods of the preceding paper (Leem & Vaughan-Jones, 1998). Briefly, ventricular myocytes were isolated enzymically from hearts of albino guinea-pigs (killed by cervical dislocation) weighing 350-450 g. Intracellular pH was recorded ratiometrically from single myocytes, AM-loaded with carboxy SNARF-1. Unless otherwise stated, cells were superfused with either Hepes buffer (20 mM) or 5 % CO2-22 mM HCO3−-buffered Tyrode solution, pH 7.40 at 37°C. Intracellular alkali loads were induced by acetate prepulsing (40-80 mM) or by reduction/removal of PCO2 at pHo 7.40, as specified. Composition of solutions is also given in the Methods of the preceding paper (Leem & Vaughan-Jones, 1998). All drugs and chemicals were obtained from Sigma apart from DIDS (diisothiocyanatostilbene disulphonic acid) which was from Boehringer Mannheim and Hoe 694 which was a gift from Dr U. Albus, Hoechst Akitengesellschaft (Germany). These drugs were added as the solid to solutions shortly before use. DIDS solutions were protected from light and used for no more than 4 h.
Calculation of sarcolemmal acid-equivalent flux
This was calculated from the pHi record as: JH=βtot× dpHi/dt, where JH is net sarcolemmal flux, and βtot (total intracellular buffering power) =[βi (intrinsic buffering power) +βCO2 (CO2-dependent buffering power)]. When superfusates are Hepes buffered, βCO2= 0, and thus βtot=βi. The βi at any given pHi was estimated from the equation, determined previously by Lagadic-Gossmann et al. (1992): βi= -28 pHi+ 222.6. The βCO2 at any given pHi was determined from the equation: βCO2= 2.3 [HCO3−]i, where [HCO3−]i is the intracellular concentration of bicarbonate anion, assuming CO2 is at equilibrium across the sarcolemma, and assuming that intracellular CO2 hydration is also at equilibrium. [HCO3−]i was calculated from a rearrangement of the Henderson- Hasselbalch equation:
where [HCO3−]o is the bicarbonate concentration of the extracellular solution. This assumes that the CO2 solubility coefficient and the apparent pK of CO2 hydration do not vary between the extracellular and intracellular fluid. The previous paper (Leem & Vaughan-Jones, 1998) showed that CO2 hydration is out of equilibrium (OOE) for periods of up to 2.5 min following the imposition of an intracellular alkali load (by weak acid prepulse). Estimates of sarcolemmal acid flux were therefore always made > 2.5 min following such a prepulse.
Rates of change of pHi (dpHi/dt) at any given pHi were obtained by computer from the first time differential of the best-fit polynomial equation (Sigmaplot, Jandel Corp.) to experimental data points sampled at 0.5 s intervals. The computer fit was assessed by comparing it with the original data using Student's paired t test (acceptable if P > 0.99).
All statistical data were expressed as means ±s.e.m.
RESULTS
Intracellular pH recovery from an alkali load
Figure 1A illustrates pHi recovery from an intracellular alkali load, observed in the nominal absence of CO2/HCO3− (Hepes buffer). A rise in pHi of about 0.25 units was induced by using the acetate prepulse technique. The pHi subsequently recovered (n= 42). A similar recovery in Hepes-buffered medium is evident in the later part of the trace shown in Fig. 1B. In this case the alkali load was induced by switching from CO2/HCO3− to Hepes buffer. An identical result was observed in six other cells. A similar recovery in Hepes buffer was also seen following an alkali load induced by a 40 mM propionate prepulse (n= 6; not shown). The property of pHi recovery is therefore independent of the method of alkali loading.
Figure 1. Intracellular pH recovery from an alkali load.
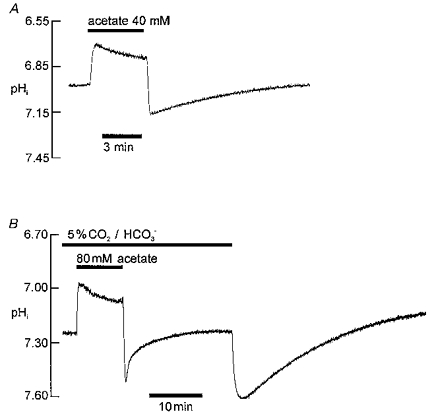
Extracellular pH 7.40 throughout. A, intracellular alkalosis induced by 40 mM acetate prepulse in Hepes-buffered solution. Trace shows ratiometric SNARF recording of pHi in an isolated ventricular myocyte. B, comparison of time course of pHi recovery from alkalosis in presence (first section of pHi recording) and absence (second section of pHi recording) of 5 % CO2/HCO3− buffer. First alkali load induced by 80 mM acetate prepulse; second alkali load induced by replacement of 5 % CO2-22 mM HCO3− buffer by 20 mM Hepes.
Figure 1B compares pHi recovery from alkalosis in CO2/HCO3− buffer (induced by acetate prepulse) with that seen in Hepes buffer (induced by CO2 removal). The initial pHi recovery was considerably faster in CO2/HCO3− buffer (n= 43). In the preceding paper (Leem & Vaughan-Jones, 1998) we showed that much of this initial recovery (< 2.5 min after acetate removal) is caused by CO2-dependent buffering. Inspection of the trace shown in Fig. 1B reveals that the time course of the later phase of pHi recovery in CO2/HCO3− medium (> 2.5 min after acetate removal) also differs from that seen in Hepes buffer. A comparison of rates, however, should take into account the different intracellular buffering power in the two situations, as detailed later in the Results. The pHi in Fig. 1B stabilized more quickly in CO2/HCO3− (n= 7), and at a slightly more alkaline pHi. Variable differences of steady-state pHi in HCO3−versus Hepes-buffered solutions have been reported extensively for ventricular cells (e.g. Bountra, Powell & Vaughan-Jones, 1990; Blank et al. 1992; Sun et al. 1996), and so are not analysed in detail here. Figure 1B illustrates that CO2/HCO3− influences both the early (rapid) and the late (slower) phases of pHi recovery from alkalosis.
In the remainder of the Results, we examine the ionic dependence and drug sensitivity of the slow phase of pHi recovery from alkalosis, while also comparing data obtained in the presence and nominal absence of CO2/HCO3−.
Effect of Hoe 694 and of Na+o removal
Hoe 694 is a high-affinity inhibitor of cardiac Na+-H+ exchange (Scholz, Albus, Lang, Martorana, Englert & Scholkens, 1993; Loh, Sun & Vaughan-Jones, 1996). Figure 2A shows that in Hepes buffer, pHi recovery from alkalosis was unimpaired by 30 μM Hoe 694, a dose sufficient to inhibit cardiac Na+-H+ exchange maximally (Loh et al. 1996). In a total of five experiments, pHi recovered at a rate of 0.0202 ± 0.003 pH units min−1 in the presence of 30 μM Hoe, compared with 0.019 ± 0.004 pH units min−1 under control conditions (measured at pHi 7.31 in both cases; no significant difference, P > 0.05, paired t test). This indicates that modulation of Na+-H+ exchange activity is not responsible for the recovery. This conclusion is reinforced by the observation (not illustrated) that recovery was not impaired by complete removal of extracellular Na+ (replaced isosmotically by N-methyl-D-glucamine (NMDG)) a manoeuvre which, like Hoe 694 addition, inhibits Na+-H+ exchange (note that significant reversal of Na+-H+ exchange in Na+-free solution does not occur in the ventricular myocyte; see, for example, Loh et al. 1996): recovery rate at pHi 7.28 was 0.027 ± 0.004 pH units min−1 in Na+-free solution compared with 0.022 ± 0.003 pH units min−1 under control conditions (n= 5; P > 0.05, paired t test)
Figure 2. Recovery from alkali load is independent of Na+-H+ exchange and of Na+o.
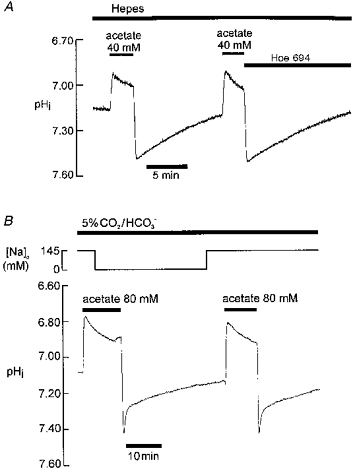
pHo 7.40 throughout. A, Hepes buffer; application of 30 μM Hoe 694 (an Na+-H+ exchange (NHE)-1 inhibitor) indicated by bar above trace B, 5 % CO2-22 mM HCO3− buffer; Na+o replaced by NMDG as indicated at top of figure.
Na+o removal (replaced by NMDG) also had no inhibitory effect on pHi recovery observed in the presence of CO2/HCO3− buffer (Fig. 2B; n= 5; recovery rate (slow, late phase) at pHi 7.23 in Na+-free conditions was 0.145 ± 0.001 pH units min−1 compared with 0.141 ± 0.003 pH units min−1 in control conditions; P > 0.05, paired t test). Thus, neither the early (Leem & Vaughan-Jones, 1998) nor the late phase of recovery in CO2/HCO3− buffer is Na+o dependent. It should be noted that Na+i in cardiac cells declines within a couple of minutes to levels << 1 mM following Na+o removal (Ellis, 1977) thus also excluding a dependence on intracellular Na+.
Replacing Na+o with K+
Recovery of pHi (from a 40 mM acetate prepulse) was also unimpaired by isosmotic replacement of Na+ with K+ (instead of NMDG). This was the case in both Hepes buffer and CO2/HCO3−-buffered solution (not illustrated; recovery rate not significantly different when measured at pHi 7.36 in Hepes buffer, in 4.5 and 144.5 mM K+o, P > 0.05, paired t test, n= 5; recovery rate not significantly different in CO2/HCO3− buffer in 4.5 and 144.5 mM K+o, measured at pHi 7.22, n= 3, P > 0.05, paired t test). Since membrane potential is depolarized to approximately 0 mV in the high K+ solution, this suggests that the recovery mechanism is voltage insensitive, at least in the physiological range of membrane potential.
Effect of Cl−o removal
Hepes buffer
Recovery was abolished entirely by removal of extracellular chloride (replaced by gluconate and/or glucuronate). Figure 3A shows an experiment where pHi had initially been elevated by using an acetate (40 mM) prepulse. Na+o and Cl−o had also been removed (see legend for details). Intracellular pH was stable at 7.55, showing no tendency to move towards less alkaline levels. Figure 3 shows that re-adding Na+o had no effect on pHi which remained at the elevated level of 7.55. Re-adding Cl−o, however, prompted an immediate recovery at a rate identical to that seen in the control recovery observed in the last part of the experiment. Figure 3B shows pooled data from seven such experiments, showing that recovery is inhibited equally well by removal of Na+o plus Cl−o, or by removal of Cl−o alone. This (i) confirms that recovery is not Na+ dependent and (ii) demonstrates an absolute dependence of recovery on Cl−o.
Figure 3. Recovery in Hepes buffer is Cl−o dependent.
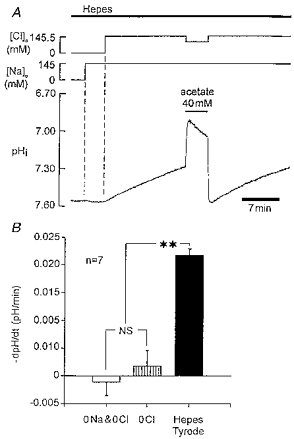
A, trace begins after cell has been exposed to Cl−-free (gluconate substituted), Na+-free (NMDG substituted) solution for about 10 min. An acetate prepulse (40 mM) was used to raise pHi to 7.55. Note lack of pHi recovery. Re-addition of Na+o and then Cl−o is indicated at the top of the figure. B, histogram showing pHi recovery rate (measured at pHi 7.38 ± 0.03, mean ±s.e.m.) in normal Hepes-Tyrode solution (black column), Cl−-free Tyrode solution (hatched column) and Na+-free, Cl−-free Tyrode solution. pHo 7.40 throughout. NS, not significant, P > 0.05, Student's paired t test; ** significant difference, P < 0.00005.
CO2/HCO3− buffer
Under these conditions, Cl−-HCO3− exchange is functional, and would be expected to contribute to pHi recovery (Vaughan-Jones, 1982; Xu & Spitzer, 1994). The traces shown in Fig. 4 show that recovery was again abolished in the absence of extracellular Cl−. Two different methods of alkali loading are illustrated, the acetate prepulse (simulating metabolic alkalosis; Fig. 4A; same result in 21 experiments) and reduction of PCO2 (simulating respiratory alkalosis; PCO2 reduction from 10 to 5 %; Fig. 4B; same result in 17 experiments). Note that, in Fig. 4A, an initial rapid phase of pHi recovery following acetate removal was evident in Cl−-free solution (Leem & Vaughan-Jones, 1998) even though the slow phase was abolished (n= 21). This is consistent with CO2-dependent buffering contributing significantly to the rapid but not the slow phase.
Figure 4. Slow recovery phase in CO2/HCO3− buffer is also Cl−o dependent.
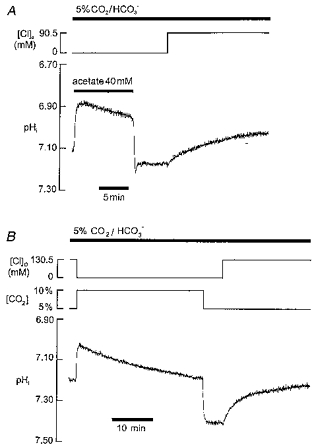
Two types of alkali load are investigated, simulated metabolic (A) and respiratory (B) alkalosis. A, acetate pre-pulsing to raise pHi. Note slow recovery phase in Cl−-free solution on Cl−o re-admission; in this case only 90.5 mM Cl−o re-admitted (remaining anion balance being gluconate) B, PCO2 was elevated to 10 % (extracellular bicarbonate raised to 44 mM; constant pHo of 7.40) for several minutes and then reduced back to 5 % (22 mM HCO3−) to induce intracellular alkalosis. Recovery only occurred upon readmission of Cl−o.
We conclude that the sarcolemmal acid-loading mechanisms active in Hepes or CO2/HCO3− buffer are Cl−o dependent.
Effect of stilbenes
Recovery of pHi from an alkali load in heart has been attributed previously to activation of Cl−-HCO3− exchange (AE). Figure 5A shows that 500 μM DIDS, a high-affinity AE inhibitor (Cabantchik, Knauf & Rothstein, 1978), had no effect on pHi recovery in Hepes-buffered solution (n= 6, 0.1-0.5 mM DIDS), but it slowed considerably the recovery observed in CO2/HCO3−-buffered solution, notably during the secondary recovery phase (Fig. 5B; n= 7; 0.1-0.5 mM DIDS). The effect of DIDS is analysed quantitatively later in the Results.
Figure 5. DIDS attenuates the slow phase of recovery in CO2/HCO3− but not in Hepes buffer.
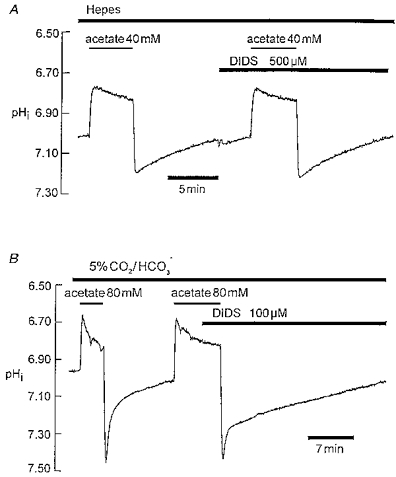
A, Hepes buffer; acetate prepulsing with and without 0.5 mM DIDS. B, CO2/HCO3− buffer; acetate prepulsing with and without 100 μM DIDS.
The trace shown in Fig. 6A shows that another stilbene, dibenzamidostilbene-disulphonic acid (DBDS), which slows the DIDS-insensitive Cl−-OH− exchanger (CHE) (Sun et al. 1996), also slowed pHi recovery in Hepes buffer. Slowing was dose dependent in the range up to 300 μM DBDS, which is close to the maximum solubility of the drug. At this concentration, DBDS inhibited recovery by 60 %, which is similar to its inhibition (70 %) of low pHo-activated CHE (Sun et al. 1996). The effect of DBDS on recovery in CO2/HCO3− buffer was not tested.
Figure 6. DBDS blocks recovery in Hepes buffer dose dependently.
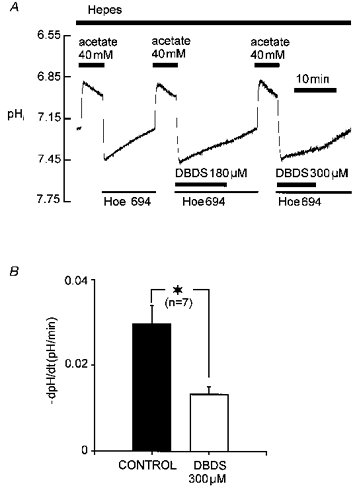
A, experimental protocol; Hoe 694 applied to remove possible influence on pHi recovery from Na+-H+ exchanger. B, histogram comparing mean pHi recovery rate recorded at pHi 7.43 ± 0.015 (mean ±s.e.m.), with and without 300 μM DBDS (close to maximum solubility of drug).
Dual acid loading mediates pHi recovery
The trace shown in Fig. 7A compares pHi recovery from alkalosis in CO2/HCO3− buffer in the presence and absence of DIDS, with recovery in Hepes buffer. As before, the secondary recovery in CO2/HCO3− buffer was slowed by DIDS, and it was slowed following a switch from CO2/HCO3− to Hepes buffer. The left-hand couplet of the histogram in Fig. 7B summarizes data from several experiments where recovery rate was compared, at a common pHi (7.30), in Hepes and bicarbonate buffer (without any intermediate exposure to DIDS). Note that all rate measurements in CO2/HCO3− buffer were made > 2 min following acetate removal, to permit out-of-equilibrium buffer conditions to subside. Net acid influx was stimulated nearly 3-fold in CO2/HCO3− compared with Hepes buffer. Comparison of the left- and right-hand couplets of Fig. 7B shows that reduction of net acid loading caused by removal of CO2/HCO3− (70 % decrease) is virtually identical to the reduction caused by addition of DIDS in the presence of CO2/HCO3− buffer. Thus all HCO3−-stimulated acid loading must be DIDS inhibitable. In contrast, acid loading in HCO3−-free buffer (Hepes) is equivalent to the DIDS-resistant acid loading observed in CO2/HCO3− buffer. As discussed below, these results are consistent with the presence of two independent acid-loading mechanisms in the mammalian cardiac cell.
Figure 7. DIDS-sensitive acid influx is HCO3− dependent; DIDS-insensitive influx is HCO3− independent.
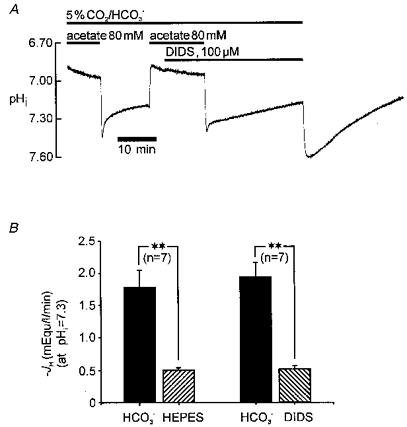
A, pHi recovery from alkalosis in CO2/HCO3− buffer, with and without DIDS (first part of trace) should be compared with that observed in Hepes buffer (last part of trace). B, histograms pooling data from several experiments. Left-hand couplet compares, in the same cell (n= 7 cells), net acid-equivalent influx (JH) measured during recovery from intracellular alkalosis at pHi 7.30 in CO2/HCO3− (left column) with Hepes-buffered (right column) conditions. Right-hand couplet compares, in the same cell (n= 7 cells), net acid influx (JH) measured during recovery from intracellular alkalosis at pHi 7.30 in CO2/HCO3−-buffered conditions in the absence (left column) and presence (right column) of 100 μM DIDS. Acid-equivalent influx (JH) was calculated as described in Methods. ** Significant difference (Student's paired t test, P < 0.001).
DISCUSSION
Intracellular pH recovery from alkalosis: role for AE and CHE
The present work indicates that the slow phase of pHi recovery from an alkali load in the cardiac cell is mediated through two independent, sarcolemmal acid-loading carriers, one HCO3− dependent and DIDS sensitive, the other HCO3− independent and DIDS insensitive. Both carriers are independent of Na+ but dependent on Cl−o, and appear to be insensitive to membrane depolarization (K+o induced). These results are consistent with the dual acid-loading mechanism proposed recently for the ventricular myocyte (Sun et al. 1996). This comprises a DIDS-sensitive Cl−-HCO3− exchanger (AE) and a DIDS-insensitive but DBDS-inhibited Cl−-OH− exchanger (CHE).
While we have not entirely eliminated the possibility that pHi recovery in Hepes buffer (putative CHE) is dependent on trace levels of HCO3− generated from metabolic or atmospheric CO2 (rather than upon OH−), its similarity with acid loading observed previously (Sun et al. 1996) during CHE stimulation by low pHo (Hepes buffer), is consistent with the same mechanism. In the latter case, the putative CHE component was not dependent on HCO3− since it continued to function as an acid loader in the complete absence of metabolic or atmospheric CO2 (Leem & Vaughan-Jones, 1997). This result, however, while excluding HCO3−, does not distinguish between H+ and OH− as the transported species (see Sun et al. 1996).
When combined with the results of the preceding paper (Leem & Vaughan-Jones, 1998), we see that pHi recovery from an alkali load is a combination of two processes. The first is buffering, and here intrinsic buffering is essentially instantaneous (at least on the present time scale of experiments) while CO2-dependent buffering is notably slow. The second process is acid equivalent influx through sarcolemmal carriers. Although these carriers must contribute to both the early and late phases of pHi recovery, their initial participation is hard to quantify from the pHi record, because at this time the CO2/HCO3− buffer is out of equilibrium (Leem & Vaughan-Jones, 1998). Once the out-of-equilibrium period is over, pHi recovery occurs only through sarcolemmal transport and it is here, during the slow recovery phase, that carrier activity can be readily quantified.
The pHi dependence of acid loading
The equilibrium pHi for both AE and CHE carriers is approximately 6.70 (Sun et al. 1996). Therefore, at normal steady-state pHi (about 7.10), the carriers are thermodynamically energized in favour of acid-equivalent influx. Intracellular alkalosis increases the driving force for influx and, as shown in Fig. 7B, influx is clearly stimulated at pHi 7.30. About 70 % of this influx is mediated through AE and 30 % through CHE. This is to be compared with the effect of reducing extracellular pH from 7.40 to 6.40 which also stimulates acid loading, but in this case there are roughly equal contributions from AE and CHE (Sun et al. 1996). The dual acid-loading system in the myocyte is therefore a major mechanism for the trafficking of acid into the cardiac cell, activated by a fall of pHo and a rise of pHi.
Elucidating the full pHi dependence of acid loading will require measuring it under conditions where the acid extruders have been inhibited (any residual acid extruder activity during an alkali load may offset the slow phase of pHi recovery). In the present work the effect of the extruders (Na+-H+ exchange and Na+-HCO3− symport) on pHi recovery from alkalosis was not systematically eliminated, although it will have been negligible at pHi 7.30 (where our measurements were made) since recovery was similar in the presence and absence of Na+o (Fig. 2; see also Xu & Spitzer, 1994). At less alkaline values close to normal pHi, however, the influence of the extruders will become significant. For example, we have previously estimated that acid loading in Hepes buffer is about 0.06 mM min−1 at the resting pHi level, but because this is counterbalanced by extrusion through Na+-H+ exchange, net acid loading at this pHi is zero. This emphasizes that, at steady state, both acid-extrusion and acid-loading processes exert an equal influence on pHi.
Acid influx in the guinea-pig myocyte (in CO2/HCO3− buffer) has recently been estimated over the pHi range 7.2 to 7.6 (Xu & Spitzer, 1994). It was proposed that influx activated steeply with rising pHi. In that work, however, the acid extruders were not inactivated. The pHi recovery from alkalosis was also assumed to reflect only sarcolemmal acid influx but, as pointed out in the preceding paper (Leem & Vaughan-Jones, 1998), flux measurements calculated from the pHi record shortly after establishing an alkali load will be distorted by slow CO2-dependent buffering. In addition, the present work shows that recovery is by two rather than one type of sarcolemmal carrier. In future, it will therefore be important to characterize the pHi dependence of acid influx under clear buffer equilibrium conditions and under conditions where individual carrier activities can be distinguished.
The involvement of AE in pHi recovery from alkalosis is consistent with earlier findings in cardiac tissue (Vaughan-Jones, 1982; Desilets, Puceat & Vassort, 1994; Xu & Spitzer, 1994). The present work, however, is the first to identify significant contributions from an additional acid loader, CHE. Interestingly, although not commented on at the time, there is some evidence in earlier work for dual acid loading during pHi recovery (Xu & Spitzer, 1994). In addition to a bicarbonate-stimulated acid loader (AE), these authors reported a small bicarbonate-independent component of pHi recovery in the guinea-pig myocyte, and this may correspond to CHE. The evidence, however, is inconclusive since the bicarbonate-independent component appeared to be insensitive to changes of pHi and its fractional contribution to recovery was small (about 10 % at pHi 7.30). In our own work, CHE is clearly activated by a raised pHi and its contribution to recovery in CO2/HCO3− buffer is at least 30 % (note, however, that if the pHi sensitivity of AE and CHE differs, the precise fractional contribution from CHE will vary with pHi).
Alkali regulation in heart and the AE gene family
The molecular basis of alkali regulation in heart cannot be established until the protein sequences producing functional, wild-type cardiac Cl−-HCO3− and Cl−-OH− exchange have been formally identified. One obvious possibility is that the two transporters are part of the same gene family of acid loaders, in which case Cl−-OH− exchange would be a member of the AE family. There is considerable uncertainty about the AE isoform(s) responsible for alkali regulation in heart. Three principal isoforms are known (AE1-3) (Alper, 1991), and all three have been implicated at various times as being the functional Cl−-HCO3− exchanger in heart (Kudrycki, Newman & Shull, 1990; Yannoukakos, Stuart-Tilley, Fernandez, Fey, Duyk & Alper, 1994; Puceat, Korichneva, Cassoly & Vassort, 1995; Sekler, Kobayashi & Kopito, 1996). Activity of these isoforms appears to be DIDS inhibited, albeit with differing inhibition constant (Ki) values. CHE, however, is DIDS insensitive (at least at doses up to 0.5 mM) and HCO3− independent (Sun et al. 1996; Leem & Vaughan-Jones, 1997, plus the present study). CHE may therefore represent a new DIDS-insensitive AE isoform. Alternatively the carrier may be a member of an entirely different family of acid transporters. We do not have direct proof, for example, that CHE is indeed an hydroxyl ion transporter, although its requirement for Cl−o and its sensitivity to the stilbene DBDS is suggestive of an anion exchanger. As pointed out previously (Sun et al. 1996), the alternative formulation for the carrier would be an inwardly directed H+-Cl− symport.
Conclusions
Intracellular pH recovery from alkalosis in the guinea-pig cardiomyocyte is mediated through two independent acid-loading carriers, Cl−-HCO3− exchange (AE) and a putative Cl−-OH− exchange (CHE). Hence the dual acid-loading system in this cell, which is stimulated by a fall of extracellular pH (Sun et al. 1996), is also stimulated by a rise of intracellular pH. Traffic of acid equivalents through the system is consequently regulated by pH at the extracellular and intracellular sites of both carriers. The physiological advantage to the cardiac cell of possessing two rather than one type of acid-loading carrier is unclear. What is clear, however, is that both carriers make significant contributions to sarcolemmal acid influx.
Acknowledgments
We thank Ms Anna Clark for excellent technical assistance during experiments and for helping to prepare the figures. This work was funded by a grant (to R. D. V.-J.) from the British Heart Foundation.
References
- Alper SL. The band 3-related anion exchanger (AE) gene family. Annual Review of Physiology. 1991;53:549–564. doi: 10.1146/annurev.ph.53.030191.003001. [DOI] [PubMed] [Google Scholar]
- Blank PS, Silverman HS, Chung OY, Hogue BA, Stern MD, Hansford RG, Lakatta EG, Capogrossi MC. Cytosolic pH measurements in single cardiac myocytes using carboxy-seminapthorhodafluor-1. American Journal of Physiology. 1992;263:H276–284. doi: 10.1152/ajpheart.1992.263.1.H276. [DOI] [PubMed] [Google Scholar]
- Bountra C, Powell TP, Vaughan-Jones RD. Comparison of intracellular pH transients in single ventricular myocytes and isolated ventricular muscle of guinea-pig. The Journal of Physiology. 1990;424:343–365. doi: 10.1113/jphysiol.1990.sp018071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik ZA, Knauf PA, Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of ‘probes’. Biochimica et Biophysica Acta. 1978;515:239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Dart C, Vaughan-Jones RD. Na+-HCO3− symport in the sheep cardiac Purkinje fibre. The Journal of Physiology. 1992;451:365–385. doi: 10.1113/jphysiol.1992.sp019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. The Journal of Physiology. 1980;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desilets M, Puceat M, Vassort G. Chloride dependence of pH modulation by β-adrenergic agonist in rat cardiomyocytes. Circulation Research. 1994;75:862–869. doi: 10.1161/01.res.75.5.862. [DOI] [PubMed] [Google Scholar]
- Ellis D. The effect of external cations and ouabain on the sodium activity in sheep heart Purkinje fibres. The Journal of Physiology. 1977;273:211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrycki KE, Newman PR, Shull GE. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl−/HCO3− exchanger. Journal of Biological Chemistry. 1990;265:462–471. [PubMed] [Google Scholar]
- Lagadic-Gossmann D, Buckler KJ, Vaughan-Jones RD. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. The Journal of Physiology. 1992;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem CH, Loh SH, Vaughan-Jones RD. Mechanism of alkali regulation in HCO3−-free conditions in single guinea-pig ventricular myocytes. The Journal of Physiology. 1996;491:159. P. [Google Scholar]
- Leem CH, Vaughan-Jones RD. Alkali regulation in HCO3−-buffered conditions in single guinea-pig ventricular myocytes. The Journal of Physiology. 1996;494:110. P. [Google Scholar]
- Leem CH, Vaughan-Jones RD. Chloride-hydroxyl exchange in the guinea-pig ventricular myocyte: no role for bicarbonate. Journal of Molecular and Cellular Cardiology. 1997;29:2483–2489. doi: 10.1006/jmcc.1997.0485. 10.1006/jmcc.1997.0485. [DOI] [PubMed] [Google Scholar]
- Leem CH, Vaughan-Jones RD. Out-of-equilibrium pH transients in the guinea-pig ventricular myocyte. The Journal of Physiology. 1998;509:471–485. doi: 10.1111/j.1469-7793.1998.471bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Piwnica-Worms D, Lieberman M. Intracellular pH regulation in cultured embryonic chick heart cells, Na+-dependent Cl−/HCO3− exchange. Journal of General Physiology. 1990;96:1247–1269. doi: 10.1085/jgp.96.6.1247. 10.1085/jgp.96.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh S-H, Sun B, Vaughan-Jones RD. Effect of Hoe 694, a novel Na+-H+ exchange inhibitor, on intracellular pH regulation in the guinea-pig ventricular myocyte. British Journal of Pharmacology. 1996;118:1905–1912. doi: 10.1111/j.1476-5381.1996.tb15623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard CH, Cingolani HE. Acidosis and arrhythmias in cardiac muscle. Cardiovascular Research. 1994;28:1312–1319. doi: 10.1093/cvr/28.9.1312. [DOI] [PubMed] [Google Scholar]
- Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. American Journal of Physiology. 1990;258:C967–981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Puceat M, Clement O, Scamps F, Vassort G. Extracellular ATP-induced acidification leads to cytosolic calcium transient rise in single rat cardiac myocytes. Biochemical Journal. 1991;274:55–62. doi: 10.1042/bj2740055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puceat M, Korichneva I, Cassoly R, Vassort G. Identification of band 3-like proteins and Cl−/HCO3− exchange in isolated cardiomyocytes. Journal of Biological Chemistry. 1995;270:1315–1322. doi: 10.1074/jbc.270.3.1315. 10.1074/jbc.270.3.1315. [DOI] [PubMed] [Google Scholar]
- Scholz W, Albus U, Lang HJ, Martorana PA, Englert HC, Scholkens BA. Hoe 694, a new Na+/H+ exchange inhibitor and its effects in cardiac ischaemia. British Journal of Pharmacology. 1993;109:562–568. doi: 10.1111/j.1476-5381.1993.tb13607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekler I, Kobayashi S, Kopito RR. A cluster of cytoplasmic histidine residues specifies pH-dependence of the AE2 plasma membrane anion exchanger. Cell. 1996;86:929–935. doi: 10.1016/s0092-8674(00)80168-3. [DOI] [PubMed] [Google Scholar]
- Sun B, Leem CH, Vaughan-Jones RD. Novel chloride-dependent acid loader in the guinea-pig ventricular myocyte: part of a dual acid-loading mechanism. The Journal of Physiology. 1996;495:65–82. doi: 10.1113/jphysiol.1996.sp021574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones RD. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive microelectrodes. The Journal of Physiology. 1979;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones RD. Chloride activity and its control in skeletal and cardiac muscle. Philosophical Transactions of the Royal Society. 1982;299:537–548. doi: 10.1098/rstb.1982.0150. B. [DOI] [PubMed] [Google Scholar]
- Xu P, Spitzer KW. Na-independent Cl−-HCO3− exchange mediates recovery of pHi from alkalosis in guinea pig ventricular myocytes. American Journal of Physiology. 1994;267:H85–91. doi: 10.1152/ajpheart.1994.267.1.H85. [DOI] [PubMed] [Google Scholar]
- Yannoukakos D, Stuart-Tilley A, Fernandez HA, Fey P, Duyk G, Alper SL. Molecular cloning, expression, and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circulation Research. 1994;75:603–614. doi: 10.1161/01.res.75.4.603. [DOI] [PubMed] [Google Scholar]


