Abstract
Hypoxia rapidly reduces force in many smooth muscles and we review recent data that shed light on the mechanisms involved. As many regulated cellular processes are integrated to co-ordinate smooth muscle contractility, the processes responsible for decreased force output with altered metabolism are also likely to be many, acting in concert, rather than the actions of one altered parameter. Nevertheless the aim of this study is to elucidate the hierarchical series of events that contribute to reduced smooth muscle force production during altered metabolism. We conclude that in many phasic smooth muscles the decrease in force can be attributed to impaired electro-mechanical coupling whereby the Ca2+ transient is reduced. A direct effect of hypoxia on the Ca2+ channel may be of key importance. In tonic vascular smooth muscles KATP channels may also play a role in the integrated functional responses to hypoxia. There are also many examples of force being reduced, in tonically activated preparations, without a fall in steady-state Ca2+; indeed it usually increases. We examine the roles of altered [ATP], pH, myosin phosphorylation, inorganic phosphate and proteolytic activity on the [Ca2+]-force relationship during hypoxia. We find no defining force-inhibitory role for any one factor acting alone, and suggest that force most probably falls as a result of the combination of myriad factors.
Smooth muscle contraction is essential for many homeostatic functions, e.g. the regulation of blood pressure, gastrointestinal motility and the behaviour of the urinogential tract. It is now apparent that many smooth muscles are markedly affected by hypoxia on a rapid time scale. The resulting effect on contraction may be beneficial, e.g. the dilatation of a blood vessel, thereby increasing blood flow to a hypoxic region or preservation of ATP during times of metabolic stress. However, hypoxia can also cause dysfunction, which has been associated with many disease states including atherosclerosis, vasospasm, cerebral haemorrhage and uterine dystocia. The purpose of this review is to draw together the recent data pertaining to hypoxia (or metabolic inhibition with cyanide, which will not generally be distinguished from true hypoxia, apart from when direct effects of low O2 on ion channels are discussed), and develop mechanistic explanations for its effects. We do not consider hypoxic contraction, the most notable example of which is that which occurs in the lungs, or hypoxic pulmonary vasoconstriction, but confine ourselves to considering how hypoxia reduces force. We begin therefore with a brief overview of smooth muscle force production.
Contraction in smooth muscle
For many of the details and references to this section we recommend the review by Horowitz, Menice, LaPorte & Morgan (1996). The pattern of contractile activity in smooth muscles can be divided into two sorts: phasic, where cycles of contraction and relaxation occur, and tonic, where a maintained level of force exists. Phasic contractions are due to electrical excitation at the surface membrane, leading to action potential generation, and spreading from cell to cell via gap junctions. This depolarization results in Ca2+ entry, via L-type Ca2+ channels and in the presence of agonists, and IP3-induced Ca2+ release from the sarcoplasmic reticulum (SR) (Somlyo & Somlyo, 1994). Tonic smooth muscles lack spontaneous action potential generation, and force follows a rise in Ca2+ following stimulation by neurohormonal agonists. Sustained depolarization can often cause tonic contraction in phasic muscles. The relationship between electrical activity, [Ca2+] and force has not been clearly established in intact smooth muscle, as simultaneous measurement of these three parameters has only recently been made (Burdyga & Wray, 1997). When [Ca2+]i rises it combines with calmodulin and activates myosin light chain kinase, which phosphorylates myosin light chains (MLC). There is, then, significant actin-activated myosin ATPase activity for cross-bridge cycling and force production and MLC phosphorylation is thus an important determinant of contractile initiation. During periods of sustained tone MLC phosphorylation levels often decline and force is maintained by slowly cycling cross-bridges (Murphy, 1994). Upon stimulus cessation, [Ca2+]i declines, primarily by plasmalemmal Ca2+ extrusion via the Ca2+-ATPase (Kosterin, Burdyga, Fomin & Grover, 1994), with possible contributions from the Na+-Ca2+ exchanger (Suzuki, 1991; McCarron, Walsh & Fay, 1994; Taggart & Wray, 1997b) and sequestration by intracellular organelles such as the SR and mitochondria (see later). As [Ca2+]i declines, MLC20 dephosphorylation is accomplished by a MLC phosphatase and precedes relaxation (Khromov, Somlyo & Somlyo, 1996).
The effect of hypoxia on smooth muscle force
Figure 1 shows typical examples of the effects of hypoxia (simulated with cyanide) in a phasically contracting (Fig. 1A) and tonically activated preparation (Fig. 1B). The effects of hypoxia on force are both rapid and marked, and in this example from the ureter, such effects would reduce the flow of urine from the kidneys to the bladder (Bullock & Wray, 1997). A similar rapid attenuation of force with hypoxia or cyanide occurs in many phasic smooth muscles including uterus (Wray, Duggins, Iles, Nyman & Osman, 1992; Earley & Wray, 1993), stomach (Huang, Chowdhury, Kobayashi & Tomita, 1993) and portal vein (Sward, Josefsson, Lydrup & Hellstrand, 1993) and decline in force such as seen in Fig. 1B can be seen in many tonically activated preparations including uterus (Taggart, Menice, Morgan & Wray, 1997), taenia caeci (Ishida & Paul, 1990), portal vein (Sward et al. 1993) and cerebral arterioles (Taguchi, Heistad, Kitazono & Faraci, 1997). Given the dependence of force on Ca2+ it is natural to ask if the contractile inhibition seen in Fig. 1 is due to a reduction in [Ca2+].
Figure 1. Simultaneous force (top) and intracellular [Ca2+] (bottom) measurements before, during and after cyanide application to guinea-pig ureter.
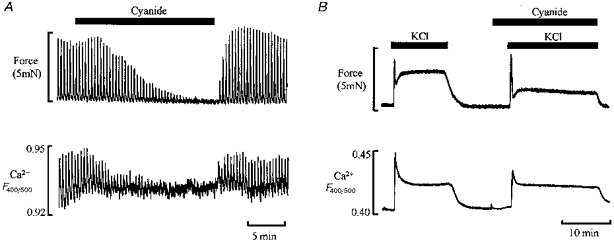
A, phasic contractions produced by action potentials following electrical stimulation. B, tonic contraction produced by high K+ depolarization. The Ca2+ measurements were made from the ratio of the fluorescence signals of indo-1 emitted at 400 and 500 nm. Adapted from Bullock & Wray, 1998.
What are the effects of hypoxia on [Ca2+] in smooth muscle?
Several studies have shown that changes in the Ca2+ transient amplitude or duration cause similar changes in the phasic contractions (Himpens, Lydrup, Hellstrand & Casteels, 1990; Huang et al. 1993; Taggart, Burdyga, Heaton & Wray, 1996). Figure 1 also shows the simultaneously recorded intracellular Ca2+ changes associated with the contractile activity during control and hypoxic conditions. Cyanide concomitantly reduces the Ca2+ transients along with the phasic contractions; similar finding have been reported for several different smooth muscles (Sward et al. 1993; Taggart et al. 1997; Bullock & Wray, 1997). Thus we can explain the fall of force in Fig. 1A by a reduction in the Ca2+ transient. In Fig. 1B, however, contraction is also reduced in hypoxic conditions but under conditions of unchanged steady-state [Ca2+]. Similar findings of unchanged or even increased [Ca2+] (see Fig. 3) with hypoxia have been reported in many smooth muscles (see Table 1). It is therefore clear that we must discuss two main questions: (1) what are the mechanisms underlying the decreased Ca2+ and force transients in phasic tissue? and (2) what are the mechanisms disrupting the Ca2+-force relationship in tonically activated smooth muscle? To answer the first question we require a detailed appreciation of the control of smooth muscle membrane excitability, as this underlies the Ca2+ transient.
Figure 3. Changes in uterine force and myosin phosphorylation with cyanide.
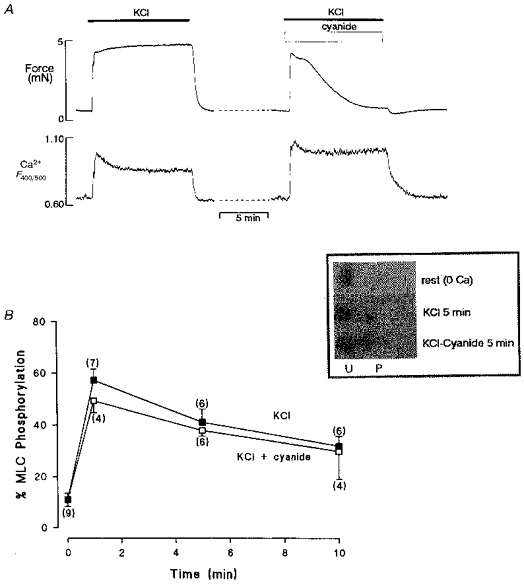
A, simultaneous force (top) and Ca2+ (bottom) records showing the effect of hypoxia on a depolarized rat uterine preparation. Note the increased Ca2+ record in hypoxia, but the fall of force. B, MLC phosphorylation measurements during contraction for 10 min produced by high K+, in the presence and absence of hypoxia. There was no significant difference in the amount of MLC phosphorylation at any time point; mean data from densiotometric scans of silver stained 2-D gels (see inset). U, unphosphorylated; P, phosphorylated. Figure adapted from Taggart et al. 1997.
Table 1.
Reported changes in smooth muscle steady-state or basal [Ca2+]i during conditions of altered metabolism
| Intervention | Smooth muscle | Change steady-state in calcium | Reference |
|---|---|---|---|
| Hypoxia | Coronary | ⇄ | Paul et al. 1991 |
| Small pulmonary | ↑ | Salvaterra & Golfman, 1993; Post et al. 1995; Urena et al. 1996 | |
| Conduit pulmonary | ↓ | Urena et al. 1996 | |
| Cerebral and mesenteric | ⇄ | Aalkjaer & Lombard, 1995 | |
| Cerebral | ⇄ | Gebremedhin et al. 1994 | |
| Taenia coli | ⇄ | Obara et al. 1997 | |
| Metabolic inhibition | Ileum | ↑ | Hori et al. 1989 |
| Aorta | ↑ | Ishida & Honda, 1991 | |
| Stomach | ↑/⇄ | Huang et al. 1993; Drummond & Fay, 1996 | |
| Portal vein | ↑ | Sward et al. 1993; Lydrup et al. 1994 | |
| Pulmonary | ↑ | Yuan, 1995 | |
| Uterus | ↑ | Taggart et al. 1997 | |
| Ureter | ↑/⇄ | Bullock & Wray, 1997 |
Membrane excitability
Many topics (and references) that can only be touched on here can be found in the recent book on smooth muscle excitation, edited by Bolton & Tomita (1996). Resting membrane potential in smooth muscles (approximately -75 to -45 mV) is dependent upon the balance of K+, Ca2+ and Cl− channel activity, which may change during hypoxia. Many different K+ channels have been described in smooth muscle cells including those modulated by ATP, O2, Ca2+ and voltage. The activity and distribution of ion channels depend not just upon the particular smooth muscle preparation, but on developmental and gestational state (Khan, Smith, Morrison & Ashford, 1993; Weir & Archer, 1995; Urena, Franco-Obregon & Lopez-Barneo, 1996). Such differences may well account for much of the diversity found between smooth muscles. Any effect of hypoxia on these channels will affect resting membrane potential and is therefore likely to affect force. A general point to consider is that many ion channels require a basal level of phosphorylation for activation, and hence a fall in [ATP] may reduce current flow (Tewari & Simard, 1994; Hilgemann, 1997), although some Ca2+ channels do not appear to be activated by phosphorylation (Klockner & Isenberg, 1985; Ohya, Kitamura & Kuriyama, 1988; Sperelakis, Xiong, Haddad & Musuda, 1994). The changes in metabolites and ions that occur with hypoxia and which may influence ion channel activity are summarized in Table 1. As will become apparent, in many smooth muscles increased outward K+ current and decreased inward Ca2+ current can be demonstrated with hypoxia or cyanide, along with increased K+ efflux and decreased Ca2+ entry.
What are the effects of hypoxia on K+ channels?
ATP-gated K+ channels (KATP) are present in smooth muscle and have been proposed as a critical link between cell metabolism and electrical activity, and in particular as forming part of the mechanism underlying the dilatation of coronary and cerebral arterioles during hypoxia (Standen, Quayle, Davies, Brayden, Huang & Nelson, 1989; Daut, Maier-Rudolph, Von Beckerath, Mehrke, Gunther & Goedel-Meinen, 1990; Dart & Standen, 1995; Taguchi et al. 1997). Teleologically this makes sense; this channel is inhibited by intracellular ATP (inhibition constant, ki, ∼10-100 μM). In hypoxia, the fall in [ATP] and increase in [ADP], along with a decrease in intracellular pH (pHi) (see Table 2), will be expected to lead to the opening of the channel and hyperpolarization of the smooth muscle cell. This will result in relaxation of the vessel and increased blood flow to the hypoxic region. Recent work has drawn attention to the gating of similar channels by other nucleotide diphosphates (Zhang & Bolton, 1996), but their role in hypoxia remains to be investigated. So, do KATP channels open in hypoxia? There is good evidence that they do in the vascular studies quoted above. However, the reliance in several studies on glibenclamide to block these channels and show their involvement in the hypoxic process, has been questioned (Zhang & Bolton, 1996). It is also difficult to control for a direct effect of low oxygen on channels. It is also clear that a small membrane depolarization, rather than the predicted hyperpolarization, occurs with hypoxia or cyanide in several phasic smooth muscles (Huang et al. 1993; Nakayama, Chihara, Clark, Huang, Horiuchi & Tomita, 1997). In these tissues and others (Heaton, Wray & Eisner, 1993) glibenclamide is also unable to reverse the functional effects of hypoxia. Finally as can be seen in Fig. 1A, cyanide reduced the amplitude of the contractions but not their frequency. As KATP channel opening should hyperpolarize the membrane, an effect on frequency would be expected. Thus for phasic muscles there is little compelling evidence for KATP channel opening, and hyperpolarization being responsible for the fall in Ca2+ and force. This may reflect a difference between phasic and tonic smooth muscle responsiveness to hypoxia, but it would also be useful to have more measurements of membrane potential, Ca2+, force and KATP activity in these microvessels. There is also a discrepancy between the ki for ATP and the [ATP] found in smooth muscle during hypoxia, i.e. does it fall low enough to cause significant channel opening? (see Table 2).
Table 2.
A summary of the changes in some important metabolites and ions occurring as a consequence of hypoxia
| Substance | Control | Hypoxia |
|---|---|---|
| ATP | 3 mm | 1 mm |
| ADP | 100 μm | 200 μm |
| Pi | 2 mm | 7 mm |
| PCr | 4 mm | < 1 mm |
| pH | 7.2 | 6.9 |
| Mg2+ | 100 μm | 1 mm |
| Ca2+ (basal) | 50 nm | 200 nm |
These values are representative of those reported in many studies. Pi, inorganic phosphate. PCr, phosphocreatine.
There are K+ (and Cl−) channels that are activated by a rise in Ca2+ (Carl, Lee & Sanders, 1996; Mironneau, Arnaudeau, Macrez-Lepretre & Boittin, 1996; Large & Wang, 1996). Opening of both these channels can occur spontaneously, due to brief focal releases of Ca2+ from the SR, referred to as Ca2+ sparks, and can be enhanced by Ca2+ entry. The resulting currents are referred to, respectively, as spontaneous transient outward currents (STOCS) associated with hyperpolarization and relaxation (Benham & Bolton, 1986; Nelson et al. 1995) and spontaneous transient inward currents (STICS) associated with depolarization and contraction (Large & Wang, 1996). Clearly if there is a significant alteration in STOC activity due to hypoxia affecting SR Ca2+ release (see later) or calcium-activated K+ current (IK(Ca)), due, e.g. to the rise in basal Ca2+ commonly observed (see Fig. 3A), then this could explain the fall of force in hypoxia. However, Large & Wang (1996) suggested that there is a heterogeneous distribution of K+ and Cl− channels and that when Ca2+ rises and activates both IK(Ca) and calcium-activated Cl− current (ICl(Ca)), depolarization is the net result. Little is known about the effect of hypoxia on these currents but in pulmonary vessels the Cl− current is activated and the K+ current is inhibited, and depolarization is seen (Greenwood, Helliwell & Large, 1997). The effects of hypoxia on K+ channels and hence membrane potential, will influence the gating of L-type Ca2+ channels. It is also likely that hypoxia will have direct effects on Ca2+ channels, and these effects will be discussed next.
Calcium channels
Before intracellular [Ca2+] or Ca2+ currents could be measured during hypoxia, there was good evidence that it reduced Ca2+ entry into smooth muscle cells (van Breemen, Wuytack & Casteels, 1975; Pearce, Ashwal, Long & Cuevas, 1989). Recent more direct approaches have shown reductions in Ca2+ currents and relaxation by cyanide, hypoxia or oxidation (Ohya & Sperelakis, 1989; Okashiro, Tokuno, Fukumitsu, Hayashi & Tomita, 1992; Chiamvimonvat et al. 1995; Urena et al. 1996; Rekalov, Juranek, Malekova & Bauer, 1997). The first two groups of authors consider the effect of hypoxia in reducing Ca2+ current to be a direct one on the channel, i.e. low O2 directly inhibits smooth muscle Ca2+ channels. Interestingly the evidence for such effects has followed the demonstration that low oxygen inhibits K+ currents in pulmonary myocytes, causing depolarization and Ca2+ entry, which could explain hypoxic pulmonary contraction (Yuan, Goldman, Tod, Rubin & Blaustein, 1993; Franco-Obregon & Lopez-Barneo, 1996; Haddad & Jiang, 1997). An oxygen-sensitive Ca2+ channel as a mechanism for vasodilatation is appealing, especially as it is rapidly effective, and sensitive to low oxygen in a dose-dependent manner and over the physiological range.
Reductions in Ca2+ currents with secondary effects of hypoxia (e.g. changes in ATP, pH, Ca2+ and Mg2+) have also been reported; for example, in portal vein the Ca2+ current was reduced when ATP was decreased (Lorenz & Paul, 1997) and acidification also reduces Ca2+ current in many smooth muscle cells (Shmigol, Smith, Taggart, Wray & Eisner, 1995). Basal Ca2+ is elevated during hypoxia in many smooth muscles (see Fig. 1B) and elevated [Ca2+] can also inhibit L-type Ca2+ channels (Ohya et al. 1988). It is, of course, as Mg-ATP that ATP is found within the cell and a decrease in [ATP] will increase [Mg2+]. McHugh & Beech (1996) suggested that, during blockade of oxidative metabolism, in basilar artery Mg2+ block on the channels is responsible for the reductions in current seen. Interestingly they found no effect of inhibiting glycolysis on the inward current. Thus it differs from another report (Lorenz & Paul, 1997) which indicates a role for glycolysis in supporting Ca2+ channel activity and directly altering Ca2+ entry. Both studies provide a link between Ca2+ channel function and energy production, although which mechanism predominates may well depend upon the particular smooth muscle.
Thus it appears that hypoxia, by both its direct effect on the Ca2+ current and via secondary changes in other parameters, e.g. low pH inhibits gap junction conductance (Turin & Warner, 1980), will act to reduce the Ca2+ current (see Fig. 2). These effects will also be reinforced by any increases in K+ conductance produced by hypoxia, which will favour a decrease in L-type channel activity. The net result of these processes in phasic smooth muscles will be a decrease or abolition of the Ca2+ transient in the cell and a decrease in force (Fig. 2). Consistent with this mechanism is the fact that if the decrease in membrane excitability is overcome, e.g. by application of high-K+ depolarizing solution or agonist, then contraction, albeit of reduced magnitude, can be initiated in the continued presence of cyanide or hypoxia (Paul, 1989; Heaton et al. 1993). This makes it unlikely that the rapid effects of hypoxia on phasic force are due to critical changes in ATP, pHi or inorganic phosphate (Pi) around the myofilaments. These effects on the initiation of Ca2+ transients, which are dependent upon electrical activity, may be distinct from those changes occurring in basal or steady-state [Ca2+]i which are discussed next.
Figure 2. A scheme to show the possible mechanism underlying the fall of phasic contractions in hypoxia.
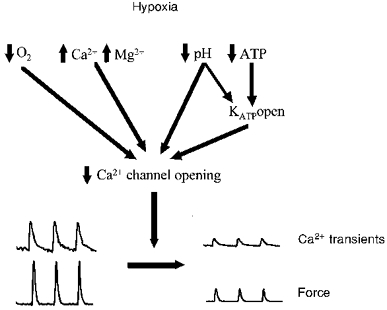
Hypoxia reduces O2, pH and ATP, and increases Ca2+, Mg2+ and ADP. These changes affect the opening of Ca2+ channels, which underly the Ca2+ transient.
What causes the rise in calcium?
As shown in Table 1, basal or steady-state Ca2+ increases with hypoxia or cyanide in many smooth muscles. There is as yet no clear indication of the source of this. Several intracellular organelles are capable of storing and releasing Ca2+ i.e. the SR, nucleus and mitochondria, and may therefore be considered likely candidates. All three organelles are often found close to one another within the cytosol (Broderick & Broderick, 1990; Nixon, Migneri & Somlyo, 1994), offering the possibility that Ca2+ movements from each organelle may be important in co-ordinating [Ca2+]i signalling, energy provision and gene expression with contractile activity.
Sarcoplasmic reticulum
The SR is the major intracellular storage site of releasable Ca2+ for contractile activation (Somlyo & Somlyo, 1994). The SR is found throughout the cell and forms a continuous tubular network from regions closely apposed to the plasma membrane (peripheral SR) to deeper in the cell (central SR) and the perinuclear space (Nixon et al. 1994; Golivina & Blaustein, 1997; Arnaudeau, Boittin, Macrez, Lavie, Mironneau & Mironneau, 1997). Ca2+ is accumulated in the SR by the action of a Ca2+-ATPase. Agonist- and IP3-dependent release of SR Ca2+ can directly activate the contractile apparatus in the absence of trans-sarcolemmal Ca2+ influx (Somlyo & Somlyo, 1990). Additionally, there is evidence for [Ca2+]i amplification by ryanodine-sensitive Ca2+-induced Ca2+ release from some (Ganitkevich & Isenberg, 1992; Kamishima & McCarron, 1996; Arnaudeau et al. 1997; Kamishima & McCarron, 1997) but not all investigations (Ganitkevich & Isenberg, 1995; Kamishima & McCarron, 1996; Taggart & Wray, 1997a). Thus Ca2+ released from the SR may play a role in smooth muscle contractility by directly activating the myofilaments. The direct influence of hypoxia or metabolic inhibition on SR Ca2+ handling in smooth muscle has not been extensively studied. An early study (van Breemen et al. 1975) suggested that iodoacetate or dinitrophenol increased the fractional loss of 45Ca2+ from intracellular stores. Ishida & Honda (1991) reported that [Ca2+]i and tension responses to either noradrenaline or caffeine were inhibited by cyanide application. Indeed, conditions expected to occur with hypoxia such as decreased [ATP] and pH, and increased Mg2+ and [Pi] and altered cellular redox state, may lead to a reduction in SR-releasable Ca2+ (Iino, 1991; Tsukioka, Iino & Endo, 1994; Kuemmerle & Makhlouf, 1995; Bootman, Missiaen, Parys, De Smedt & Casteels, 1995; Kosterin, Babich, Shlykov & Rovenets, 1996). Presently, however, there are few data to support a major role for the SR in either the rise in Ca2+ or fall in force, during hypoxia.
Mitochondria
The mitochondrion is a high capacity, low affinity Ca2+ storage site which several recent studies have suggested may play a role in removing Ca2+ from the cytosol upon cell stimulation. In non-muscle cells, oxidizable substrates that increase mitochondrial membrane potential and respiration, and presumably Ca2+ uptake, were found to alter the IP3-induced Ca2+ wave profile (Jouaville, Ichas, Holmuhamedov, Camacho & Lechlelter, 1995). In stomach smooth muscle cells, mitochondrial Ca2+ increased upon SR Ca2+ release by caffeine (Drummond & Fay, 1996). In addition, it has recently been reported that the decay of smooth muscle [Ca2+]i transients, evoked by membrane depolarization, is impaired by a variety of agents interfering with mitochondrial function (Drummond & Fay, 1996; McGeown, Drummond, McCarron & Fay, 1996). Furthermore, inhibition of oxidative phosphorylation slowed the time course of decay of Ca2+-activated chloride currents elicited by membrane depolarization, again suggesting that Ca2+ signals may be regulated by mitochondrial uptake mechanisms (Greenwood et al. 1997).
Ca2+ accumulation by the mitochondria may be a means of limiting cytosolic Ca2+ increases and thereby protecting against deleterious protease activity. It may also be a mechanism linking energy supply to demand as Ca2+ has been implicated in the regulation of several key mitochondrial enzymes (Broderick & Broderick, 1990). Organellar swelling, and increased Ca2+ leakiness, with mitochondrial inhibition has been reported (Somlyo & Somlyo, 1990; Piper, Null & Siegmund, 1994) and may therefore result in several important consequences for the smooth muscle cell including contributing to the rise in steady-state Ca2+ both directly, and indirectly by activation of depolarizing currents (Greenwood et al. 1997).
Nucleus
Evidence from a large number of cell types, including smooth muscle, indicates that the nucleus can also act as a Ca2+ store. Changes in smooth muscle nuclear Ca2+ concentration ([Ca2+]n) following depolarization or agonist stimulation have been reported (Williams, Becker & Fay, 1987; Burnier, Centeno, Burki & Brunner, 1994; Bkaily et al. 1997). It has been suggested that IP3 receptors are located on the inner surface of the nuclear envelope membrane (Gerasimenko, Gerasimenko, Tepikin & Petersen, 1995) such that any agonist-induced release of stored Ca2+ will be directed into the nucleoplasm (Fay, Shlevin, Granger & Taylor, 1979; Himpens, De Smedt & Casteels, 1994; Gerasimenko et al. 1995). Although the SR forms a continuous tubular network with the perinuclear space, Ca2+ may not be stored homogeneously throughout this region (Golivina & Blaustein, 1997). Given a dual role in Ca2+ homeostasis and gene expression, the nucleus may perform crucial functions in the cellular responses to hypoxia, e.g. regulation of transcription of genes encoding carbohydrate metabolism (Bunn & Poyton, 1996). We can, however, find no study examining the contribution of nuclear Ca2+ to changes in cytosolic Ca2+ found in hypoxia. We will now discuss the possible causes of the reduction of force in hypoxia when [Ca2+] is not reduced.
Altered [Ca2+]-force relationship during hypoxia
In a variety of different smooth muscles hypoxia, or metabolic inhibition, has resulted in a significant reduction in force, but no change or increases in [Ca2+]i either at rest or in activated tissues (see Table 1 for references) as illustrated in Figs 1B and 3A. These studies thus led to the conclusion that hypoxia can produce a dissociation of the [Ca2+]i-force relationship in smooth muscle (Taggart et al. 1997) and indirectly supported earlier observations that raising extracellular Ca2+ under such conditions did not prevent hypoxic-induced reductions in force (Ishida & Paul, 1990). Therefore we will now review which other factors may be important in causing the reduction of force.
Myosin light chain (MLC) phosphorylation
As previously described the phosphorylation of MLCs is a critical determinant of smooth muscle force production (Somlyo & Somlyo, 1994). As hypoxia or metabolic inhibition result in a reduction of cytosolic [ATP], it is possible that the phosphorylation potential of the tissue declines, especially as MLC20 phosphorylation has been suggested to account for up to 50 % of the ATP cost associated with contraction (Wingard, Paul & Murphy, 1994). However, the findings of recent studies suggest that neither mitochondrial inhibition, nor hypoxia, result in significant changes in levels of MLC20 phosphorylation (Taggart et al. 1997; Obara, Bowman, Ishida & Paul, 1997; and see Fig. 3B). Thus, altered smooth muscle metabolism results in a dissociation of both the [Ca2+]i-force and MLC20-force relationships, as shown in Fig. 3.
Myosin ATPase activity
The above experiments and Fig. 3 illustrate that the fall in force with altered metabolism is unrelated to the phosphorylation of MLC20. As cytosolic [ATP] during hypoxia or as a result of cyanide addition remains > 1 mM (Wray, 1990), in the absence of microdomains of extremely low ATP, it is unlikely that [ATP] surrounding the myofilaments is limiting the myosin ATPase (Arner & Hellstrand, 1985) and hence contraction. Furthermore as creatine kinase is localized to smooth muscle myofilaments it will also help maintain ATP : ADP (Clark, Khuchua, Kuznetsov, Saks & Ventura-Clapier, 1993; Clark & Dillon, 1995). Several studies are thus in agreement that energy supply to the myofilaments is not inhibiting force during hypoxia (Hardin, Wiseman & Kushmerick, 1992; Harrison, Larcombe-McDouall, Earley & Wray, 1994; Clark & Dillon, 1995; Nakayama et al. 1997; Taggart et al. 1997).
Intracellular pH
Significant decreases in intracellular pH occur with hypoxia and metabolic inhibition and often can be correlated with an increase in lactate production and efflux (Vogel, Lilja & Hellstrand, 1983; Wray, 1990; Ishida & Paul, 1990; Harrison et al. 1994; Taggart & Wray, 1995). For example, both in vivo and in vitro uterine hypoxia cause pHi to fall by around 0.3 pH units and lactate efflux to increase fivefold (Wray, 1990). Hypoxia and ATP depletion both inhibit Na+-H+ exchange, which would also contribute to a reduction in pHi (Burns, Homma & Harris, 1991; Noel & Pouyssegur, 1995). Decreases in pHi with metabolic inhibition were suggested to contribute to impaired mechanical output especially in spontaneously active tissues (Wray, 1990). However, altered pHi appears not to be the prime factor responsible for force inhibition for several reasons: (a) when cyanide-induced pHi changes are removed, force is still attenuated (Taggart & Wray, 1995); (b) the tonic force of many smooth muscles is often promoted by intracellular acidification (Taggart et al. 1996); (c) pH-induced alterations in Ca2+-activated force of permeabilized smooth muscles cannot explain hypoxic-induced force inhibition (Crichton, Taggart, Wray & Smith, 1993; Wu & Vaughan-Jones, 1994); and (d) hypoxia can result in force inhibition without concomitant reductions in pHi (Aalkjaer & Lombard, 1995; Obara et al. 1997).
Inorganic phosphate (Pi)
Hypoxic-induced elevations of [Pi] have been reported in both in vivo and in vitro situations (Ishida & Paul, 1990; Harisson et al. 1994). Pi inhibits Ca2+-activated force of permeabilized smooth muscle fibres (Itoh, Kanmura & Kuriyama, 1986; Crichton et al. 1993) independently of the level of MLC20 phosphorylation (Gagelman & Guth, 1987; Osterman & Arner, 1995). Pi also accelerates the relaxation from rigor upon photolysis of caged ATP, even in the presence of increased [MgADP], an effect attributable to Pi driving the actomyosin equilibrium to a condition favouring weakly attached or detached cross-bridges (Somlyo & Somlyo, 1990; Nishiye, Somlyo, Torok & Somlyo, 1993). Thus, as suggested by Taggart et al. (1997), the hypoxia-induced increase in [Pi] appears to be a suitable candidate for modulating force in the face of elevated [MgADP] (which would promote the lifespan of attached cross-bridges; Nishiye et al. 1993) and unaltered MLC phosphorylation. Indeed, when the increases in [Pi] with altered metabolism were reduced, the resultant inhibition of force (Ishida & Paul, 1990), and rates of relaxation (Hardin et al. 1992; Taggart & Wray, 1997c), were also less. The absolute (Hardin et al. 1992) reduction in Ca2+-activated force of permeabilized smooth muscle with elevated [Pi], however, is less than the inhibition of force in intact arteries during metabolic alteration (e.g. compare uterine smooth muscle studies of Crichton et al. 1993 and Taggart et al. 1997). It is unlikely, therefore, that [Pi] changes alone can account for tonic force attenuation with metabolic inhibition.
Cytoskeleton
An additional possible force-modulatory consequence of hypoxia/metabolic inhibition of smooth muscle, especially as [Ca2+]i remains elevated, is the activation of Ca2+-dependent actin-capping proteins and/or proteases (Gailly, Lejuene, Capony & Gillis, 1990) which have been suggested to contribute to attenuated cardiac contractility following ischaemic reperfusion (Gao, Liu, Mellgren & Marban, 1995). Consistent with this notion are the findings that actin-capping agents inhibit tonic smooth muscle contraction independently of [Ca2+]i and MLC20 phosphorylation (Hori, Saito, Shin, Ozaki, Fusetani & Karaki, 1993; Obara & Yabu, 1994). Additionally, the extent of force inhibition is similar to that observed during mitochondrial inhibition (Taggart et al. 1997). Changes in the cytoskeleton will also affect ion channel activity (Hilgemann, 1997) and therefore also influence the response to hypoxia.
Conclusions
There are many potential regulatory sites of the excitation- contraction coupling pathway in smooth muscle during conditions of altered metabolism. Hypoxia and cyanide can inhibit force production either with or without altering the profile of the activating [Ca2+]. Hypoxic changes of plasma membrane channel activity contribute to reduced contractility. We propose that in phasic smooth muscles decreased electromechanical coupling and reduction of the Ca2+ transient are responsible for the decline in spontaneous force seen in hypoxic conditions. We further speculate that this is predominantly a direct effect on the Ca2+ channels, rather than via an increase in K+ conductance. In tonically active smooth muscle, a dissociation between Ca2+ and force is commonly encountered with hypoxia and cyanide. The decrease in force appears not to be due to limitation of ATP around the myofilaments. We suggest rather, that a combination of the factors, including increased [Pi], acting directly on the myofilaments is the most likely cause. The SR, nucleus and mitochondria may play a wider role in smooth muscle Ca2+ homeostasis than previously appreciated, and their involvement in the acute and chronic changes occurring with hypoxia pose many intriguing questions for smooth muscle homeostasis.
Acknowledgments
We are grateful to The Wellcome Trust, BHF, Royal Society NKRF and the MRC for supporting our work, Dr Paul Kerr (University of Bristol) for advice, Professor D. Eisner for comments, Mr Bill Franks for technical assistance and Anthony Bullock for help with the figures. M. J. T. is a Wellcome Trust career development fellow.
References
- Aalkjaer C, Lombard JH. Effect of hypoxia on force, intracellular pH and Ca2+ concentration in rat cerebral and mesenteric small arteries. The Journal of Physiology. 1995;482:409–419. doi: 10.1113/jphysiol.1995.sp020528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaudeau S, Boittin FX, Macrez N, Lavie JL, Mironneau C, Mironneau J. L-type and Ca2+ release channel-dependent hierarchial Ca2+ signalling in rat portal vein myocytes. Cell Calcium. 1997;22:399–411. doi: 10.1016/s0143-4160(97)90024-5. [DOI] [PubMed] [Google Scholar]
- Arner A, Hellstrand P. Effects of calcium and substrate on force-velocity relation and energy turnover in skinned smooth muscle of the guinea-pig. The Journal of Physiology. 1985;360:347–365. doi: 10.1113/jphysiol.1985.sp015621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Bolton TB. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. The Journal of Physiology. 1986;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bkaily G, Jaalouk D, Jacques D, Economous D, Simaan M, Regoli D, Pothier P. Bradykini activates R-, T- and L-type Ca2+ channels and induces a sustained increase of nuclear Ca2+ in aortic vascular smooth cells. Canadian Journal of Physiology and Pharmacology. 1997;75:652–660. [PubMed] [Google Scholar]
- Bolton TB, Tomita T. Smooth Muscle Excitation. London: Academic Press; 1996. [Google Scholar]
- Bootman MD, Missiaen L, Parys JB, De Smedt H, Casteels R. Control of inositol 1,4,5-trisphosphate-induced Ca2+ release by cytosolic Ca2+ Biochemical Journal. 1995;306:445–451. doi: 10.1042/bj3060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick R, Broderick KA. Ultrastructure and calcium stores in the myometrium. In: Carsten ME, Miller JD, editors. Uterine Function: Molecular and Cellular Aspects. New York: Plenum Press; 1990. pp. 1–70. [Google Scholar]
- Bullock AJ, Wray S. The effects of metabolic inhibition on force, Ca2+ and pHi in guinea-pig ureteric smooth muscle. Pflügers Archiv. 1998;435:240–246. doi: 10.1007/s004240050507. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiological Reviews. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Burdyga TV, Wray S. Simultaneous measurements of electrical activity, intracellular [Ca2+] and force in intact smooth muscle. Pflügers Archiv. 1997;435:182–184. doi: 10.1007/s004240050499. [DOI] [PubMed] [Google Scholar]
- Burnier M, Centeno G, Burki E, Brunner HR. Confocal microscopy to analyze cytosolic and nuclear calcium in cultured vascular cells. American Journal of Physiology. 1994;266:C1118–1127. doi: 10.1152/ajpcell.1994.266.4.C1118. [DOI] [PubMed] [Google Scholar]
- Burns KD, Homma T, Harris RC. Regulation of Na/H exchange by ATP depletion and calmodulin antagonism in renal epithelial cells. American Journal of Physiology. 1991;261:F607–616. doi: 10.1152/ajprenal.1991.261.4.F607. [DOI] [PubMed] [Google Scholar]
- Carl C, Lee HK, Sanders KM. Regulation of ion channels in smooth muscles by calcium. American Journal of Physiology. 1996;271:C9–34. doi: 10.1152/ajpcell.1996.271.1.C9. [DOI] [PubMed] [Google Scholar]
- Chiamvimonvat N, O'Rourke B, Kamp TJ, Kallen RG, Hofmann F, Flockerzi V, Marban E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circulation Research. 1995;76:325–334. doi: 10.1161/01.res.76.3.325. [DOI] [PubMed] [Google Scholar]
- Clark JF, Dillon PF. Phosphocreatine and creatine kinase in enegetic metabolism of porcine carotid artery. Journal of Vascular Research. 1995;32:24–30. doi: 10.1159/000159074. [DOI] [PubMed] [Google Scholar]
- Clark JF, Khuchua Z, Kuznetsov A, Saks VA, Ventura-Clapier R. Compartmentation of creatine kinase isoenzymes in myometrium of gravid guinea-pig. The Journal of Physiology. 1993;466:553–572. [PMC free article] [PubMed] [Google Scholar]
- Crichton CA, Taggart MJ, Wray S, Smith GL. Effects of pH and inorganic phosphate on force production in α-toxin-permeabilized isolated rat uterine smooth muscle. The Journal of Physiology. 1993;465:629–645. doi: 10.1113/jphysiol.1993.sp019697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart C, Standen NB. Activation of ATP-dependent K+ channels by hypoxia in smooth muscle cells isolated from the pig coronary artery. The Journal of Physiology. 1995;483:29–39. doi: 10.1113/jphysiol.1995.sp020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J, Maier-Rudolph W, Von Beckerath N, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990;247:1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Drummond RM, Fay FS. Mitochondria contribute to Ca2+ removal in smooth muscle cells. Pflügers Archiv. 1996;431:473–482. doi: 10.1007/BF02191893. [DOI] [PubMed] [Google Scholar]
- Earley L, Wray S. Effects of hypoxia on force produced by agonists and depolarization and arising spontaneously in the rat uterus. Journal of Reproduction and Fertility. 1993;99:539–544. doi: 10.1530/jrf.0.0990539. [DOI] [PubMed] [Google Scholar]
- Fay FS, Shlevin HH, Granger WC, Jr, Taylor SR. Aequorin luminescence during activation of single isolated smooth muscle cells. Nature. 1979;280:506–508. doi: 10.1038/280506a0. [DOI] [PubMed] [Google Scholar]
- Franco-Obregon A, Lopez-Barneo J. Differential oxygen sensitivity of calcium channels in rabbit smooth muscle cells of conduit and resistance pulmonary arteries. The Journal of Physiology. 1996;491:511–518. doi: 10.1113/jphysiol.1996.sp021235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagelman M, Guth K. Effect of inorganic phosphate on the Ca2+ sensitivity in skinned taenia coli smooth muscle fibers. Biophysical Journal. 1987;51:457–463. doi: 10.1016/S0006-3495(87)83367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailly Ph, Lejuene Th, Capony JP, Gillis JM. The action of brevin, an F-actin severing protein, on the mechanical properties and ATPase activity of skinned smooth muscle. Journal of Muscular Research and Cell Motility. 1990;11:293–301. doi: 10.1007/BF01766667. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Contribution of calcium-induced calcium release to [Ca2+]i transients in myocytes from guinea-pig urinary bladder. The Journal of Physiology. 1992;458:119–137. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Efficacy of peak calcium currents as triggers of sarcoplasmic Ca2+ release in myocytes from guinea-pig coronary artery. The Journal of Physiology. 1995;484:287–306. doi: 10.1113/jphysiol.1995.sp020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W-D, Liu Y, Mellgren R, Marban E. Intrinsic myofilament alteration underlying the decreased contractility of stunned myocardium. Circulation Research. 1995;78:455–465. doi: 10.1161/01.res.78.3.455. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Bonnet P, Greene AS, England SK, Rusch NJ, Lombard JH, Harder DR. Hypoxia increases the activity of Ca2+-sensitive K+ channels in cat cerebral arterial muscle cell membrane. Pflügers Archiv. 1994;428:621–630. doi: 10.1007/BF00374586. [DOI] [PubMed] [Google Scholar]
- Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose mediated release of Ca2+ from the nuclear envelope. Cell. 1995;80:1–20. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- Golivina VA, Blaustein MP. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Helliwell RM, Large WA. Modulation of Ca2+-activated Cl− currents in rabbit portal vein smooth muscle by an inhibitor of mitochondrial Ca2+ uptake. The Journal of Physiology. 1997;505:53–64. doi: 10.1111/j.1469-7793.1997.053bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad GG, Jiang C. O2-sensing mechanisms in excitable cells: Role of plasma membrane K+ channels. Annual Review of Physiology. 1997;59:23–43. doi: 10.1146/annurev.physiol.59.1.23. [DOI] [PubMed] [Google Scholar]
- Hardin CD, Wiseman RW, Kushmerick MJ. Tension response of sheep aorta to simultaneous decreases in phosphocreatine, inorganic phosphate and ATP. The Journal of Physiology. 1992;458:139–150. doi: 10.1113/jphysiol.1992.sp019410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N, Larcombe-McDouall JB, Earley L, Wray S. An in vivo study of the effects of ischaemia on uterine contraction, intracellular pH and metabolites in the rat. The Journal of Physiology. 1994;476:349–354. doi: 10.1113/jphysiol.1994.sp020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RC, Wray S, Eisner DA. Effects of metabolic inhibition and changes of intracellular pH on potassium permeability and contraction of rat uterus. The Journal of Physiology. 1993;465:43–56. doi: 10.1113/jphysiol.1993.sp019665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW. Cytoplasmic ATP-dependent regulation of ion transporters and channels: mechanisms and messengers. Annual Review of Physiology. 1997;59:193–220. doi: 10.1146/annurev.physiol.59.1.193. [DOI] [PubMed] [Google Scholar]
- Himpens B, De Smedt H, Casteels R. Subcellular Ca2+-gradients in A7r5 vascular smooth muscle. Cell Calcium. 1994;15:55–65. doi: 10.1016/0143-4160(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Himpens B, Lydrup ML, Hellstrand P, Casteels R. Free cytosolic calcium during spontaneous contractions in smooth muscle of the guinea-pig mesotubarium. Pflügers Archiv. 1990;417:404–409. doi: 10.1007/BF00370660. [DOI] [PubMed] [Google Scholar]
- Hori M, Saito S, Shin YZ, Ozaki H, Fusetani N, Karaki H. Mycalolide-B, a novel and specific inhibitor of actomyosin ATPase isolated from marine sponge. FEBS Letters. 1993;322:151–154. doi: 10.1016/0014-5793(93)81557-g. [DOI] [PubMed] [Google Scholar]
- Hori M, Shimizu K, Nakajyo S, Urakawa N. The effect of trifluoperazine on muscle tension and cytoplasmic Ca2+ level in guinea-pig ileum. Japanese Journal of Pharmacology. 1989;49:540–543. doi: 10.1254/jjp.49.540. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Menice CB, LaPorte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiological Reviews. 1996;76:967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- Huang S-M, Chowdhury JU, Kobayashi K, Tomita T. Inhibitory effects of cyanide on mechanical and electrical activities in the circular muscle of gastric antrum of guinea-pig stomach. Japanese The Journal of Physiology. 1993;43:229–238. doi: 10.2170/jjphysiol.43.229. [DOI] [PubMed] [Google Scholar]
- Iino M. Effects of adenine nucleotides on inositol 1,4,5-trisphosphate-induced calcium release in vascular smooth muscle cells. Journal of General Physiology. 1991;98:681–698. doi: 10.1085/jgp.98.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Honda H. Efects of cyanide on fura-2 fluorescence and tension responses to norepinephrine and K in the guinea-pig aorta. Japanese Journal of Pharmacology. 1991;55:254. [Google Scholar]
- Ishida Y, Paul RJ. Effects of hypoxia on high-energy phosphagen content, energy metabolism and isometric force in guinea-pig taenia caeci. The Journal of Physiology. 1990;424:41–56. doi: 10.1113/jphysiol.1990.sp018054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Kanmura Y, Kuriyama H. Inorganic phosphate regulates the contraction-relaxation cycle in skinned muscles of the rabbit mesenteric artery. The Journal of Physiology. 1986;376:231–252. doi: 10.1113/jphysiol.1986.sp016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechlelter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- Kamishima T, McCarron JG. Depolarization-evoked increases in cytosolic calcium concentration in isolated smooth muscle cells in rat portal vein. The Journal of Physiology. 1996;492:61–74. doi: 10.1113/jphysiol.1996.sp021289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamishima T, McCarron JG. Regulation of the cytosolic Ca2+ concentration by Ca2+ stores in single smooth muscle cells from cerebral arteries. The Journal of Physiology. 1997;501:497–508. doi: 10.1111/j.1469-7793.1997.497bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RN, Smith SK, Morrison JJ, Ashford MLJ. Properties of large-conductance K+ channels in human myometrium during pregnancy and labour. Proceedings of the Royal Society. 1993;251:9–15. doi: 10.1098/rspb.1993.0002. B. [DOI] [PubMed] [Google Scholar]
- Khromov AS, Somlyo AV, Somlyo AP. Nucleotide binding by actomyosin as a determinant of relaxation kinetics of rabbit phasic and tonic smooth muscle. The Journal of Physiology. 1996;492:669–673. doi: 10.1113/jphysiol.1996.sp021336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockner U, Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig) Pflügers Archiv. 1985;405:329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Kosterin SA, Babich LG, Shlykov SG, Rovenets NA. Mg2+, ATP-dependent Ca2+ transport in endoplasmic reticulum of myometrial cells. Biochemistry. 1996;61:53–58. [PubMed] [Google Scholar]
- Kosterin SA, Burdyga TV, Fomin VP, Grover AK. Mechanisms of Ca2+ transport in myometrium. In: Garfield RE, Tubb TN, editors. Control of Uterine Contractility. Boca Raton: CRC Press; 1994. pp. 130–159. [Google Scholar]
- Kuemmerle JF, Makhlouf GM. Agonist-stimulated cyclic ADP ribose. Journal of Biological Chemistry. 1995;270:25488–25494. doi: 10.1074/jbc.270.43.25488. [DOI] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. American Journal of Physiology. 1996;271:C435–454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Lorenz JN, Paul RJ. Dependence of Ca2+ channel currents on endogenous and exogenous sources of ATP in portal vein smooth muscle. American Journal of Physiology. 1997;272:H987–994. doi: 10.1152/ajpheart.1997.272.2.H987. [DOI] [PubMed] [Google Scholar]
- Lydrup M-L, Sward K, Hellstrand P. Effect of glibenclamide on membrane response to metabolic inhibition in smooth muscle of rat portal vein. Journal of Vascular Research. 1994;31:82–91. doi: 10.1159/000159034. [DOI] [PubMed] [Google Scholar]
- McCarron JG, Walsh JV, Fay FS. Sodium/calcium exchange regulates cytoplasmic calcium in smooth muscle. Pflügers Archiv. 1994;426:199–205. doi: 10.1007/BF00374772. [DOI] [PubMed] [Google Scholar]
- McGeown JG, Drummond RM, McCarron JG, Fay FS. The temporal profile of calcium transients in voltage clamped gastric myocytes from Bufo marinus. The Journal of Physiology. 1996;497:321–336. doi: 10.1113/jphysiol.1996.sp021771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Beech DJ. Modulation of Ca2+ channel activity by ATP metabolism and internal Mg2+ in guinea-pig basilar artery smooth muscle cells. The Journal of Physiology. 1996;492:359–376. doi: 10.1113/jphysiol.1996.sp021314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J, Arnaudeau S, Macrez-Lepretre N, Boittin FX. Ca2+ sparks and Ca2+ waves activate different Ca2+-dependent ion channels in single myocytes from rat portal vein. Cell Calcium. 1996;20:153–160. doi: 10.1016/s0143-4160(96)90104-9. [DOI] [PubMed] [Google Scholar]
- Murphy RA. What is special about smooth muscle? The significance of covalent crossbridge regulation. FASEB Journal. 1994;8:311–318. doi: 10.1096/fasebj.8.3.8143937. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Chihara S, Clark JF, Huang S-M, Horiuchi T, Tomita T. Consequences of metabolic inhibition in smooth muscle isolated from guinea-pig stomach. The Journal of Physiology. 1997;505:229–240. doi: 10.1111/j.1469-7793.1997.229bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Nishiye E, Somlyo AV, Torok K, Somlyo AP. The effects of MgADP on cross-bridge kinetics: a laser flash photolysis study of guinea-pig smooth muscle. The Journal of Physiology. 1993;260:247–271. doi: 10.1113/jphysiol.1993.sp019470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon GF, Migneri GA, Somlyo AV. Immunogold localisation of inositol 1,4,5-triphosphate receptors and characterisation of ultrastructural features of the sarcoplasmic reticulum in phasic and tonic smooth muscle. Journal of Muscle Research and Cell Motility. 1994;15:682–700. doi: 10.1007/BF00121075. [DOI] [PubMed] [Google Scholar]
- Noel J, Pouyssegur J. Hormonal regulation, pharmacology and membrane sorting of vertebrate Na/H exchanger isoforms. American Journal of Physiology. 1995;268:C283–296. doi: 10.1152/ajpcell.1995.268.2.C283. [DOI] [PubMed] [Google Scholar]
- Obara K, Bowman PG, Ishida Y, Paul RJ. Effects of hypoxia on Ca2+, pHi and myosin light chain phosphorylation in guinea-pig taenia caeci. The Journal of Physiology. 1997;503:426–433. doi: 10.1111/j.1469-7793.1997.427bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K, Yabu H. Effect of cytochalasin B on intestinal smooth muscle cells. European Journal of Pharmacology. 1994;255:139–147. doi: 10.1016/0014-2999(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Ohya Y, Kitamura K, Kuriyama H. Regulation of calcium current by intracellular calcium in smooth muscle cells of rabbit portal vein. Circulation Research. 1988;62:375–383. doi: 10.1161/01.res.62.2.375. [DOI] [PubMed] [Google Scholar]
- Ohya Y, Sperelakis N. ATO regulation of the slow calcium channels in vascular smooth muscle cells of guinea-pig mesenteric artery. Circulation Research. 1989;64:145–154. doi: 10.1161/01.res.64.1.145. [DOI] [PubMed] [Google Scholar]
- Okashiro T, Tokuno H, Fukumitsu T, Hayashi H, Tomita T. Effects of intracellular ATP on calcium current in freshly dispersed single cells of guinea-pig portal vein. Experimental Physiology. 1992;77:719–731. doi: 10.1113/expphysiol.1992.sp003638. [DOI] [PubMed] [Google Scholar]
- Osterman A, Arner A. Effects of inorganic phosphate on cross-bridge kinetics at different activation levels in skinned guinea-pig smooth muscle. The Journal of Physiology. 1995;484:369–383. doi: 10.1113/jphysiol.1995.sp020671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RJ. Smooth muscle energetics. Annual Review of Physiology. 1989;51:331–349. doi: 10.1146/annurev.ph.51.030189.001555. [DOI] [PubMed] [Google Scholar]
- Paul RJ, Bowman PS, Close LA. Hypoxia relaxes porcine coronary artery without changing [Ca2+] FASEB Journal. 1991;5:1737. abstract. [Google Scholar]
- Pearce WJ, Ashwal S, Long DM, Cuevas J. Hypoxia inhibits calcium influx in rabbit basilar and carotid arteries. American Journal of Physiology. 1989;262:H106–113. doi: 10.1152/ajpheart.1992.262.1.H106. [DOI] [PubMed] [Google Scholar]
- Piper HM, Noll T, Siegmund B. Mitochondrial function in the oxygen depleted and reoxygenated myocardial cell. Cardiovascular Research. 1994;28:1–15. doi: 10.1093/cvr/28.1.1. [DOI] [PubMed] [Google Scholar]
- Post JM, Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circulation Research. 1995;77:131–139. doi: 10.1161/01.res.77.1.131. [DOI] [PubMed] [Google Scholar]
- Rekalov V, Juranek I, Malekova L, Bauer V. Hypoxia-induced inhibition of calcium channels in guinea-pig taenia caeci smooth muscle cells. The Journal of Physiology. 1997;505:107–119. doi: 10.1111/j.1469-7793.1997.107bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvaterra CG, Golfman WF. Acute hypoxia increases cystolic calcium in cultured pulmonary arterial myocytes. American Journal of Physiology. 1993;264:L323–328. doi: 10.1152/ajplung.1993.264.3.L323. [DOI] [PubMed] [Google Scholar]
- Shmigol AV, Smith RD, Taggart MJ, Wray S, Eisner DA. Changes of pH affect calcium currents but not outward potassium currents in rat myometrial cells. Pflügers Archiv. 1995;431:135–137. doi: 10.1007/BF00374388. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Flash photolysis studies of excitation-contraction coupling, regulation and contraction in smooth muscle. Annual Review of Physiology. 1990;52:857–874. doi: 10.1146/annurev.ph.52.030190.004233. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Sperelakis N, Xiong Z, Haddad G, Masuda H. Regulation of slow calcium channels of myocardial cells and vascular smooth muscle cells by cyclic nucleotides and phosphorylation. Molecular and Cellular Biochemistry. 1994;140:103–117. doi: 10.1007/BF00926749. [DOI] [PubMed] [Google Scholar]
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Contraction and prostaglandins in pregnant rabbits in response to adenosine 5-triphosphate. European Journal of Pharmacology. 1991;195:93–99. doi: 10.1016/0014-2999(91)90385-4. [DOI] [PubMed] [Google Scholar]
- Sward K, Josefsson M, Lydrup M-L, Hellstrand P. Effects of metabolic inhibition on cytoplasmic calcium and contraction in smooth muscle of rat portal vein. Acta Physiologica Scandinavica. 1993;148:265–272. doi: 10.1111/j.1748-1716.1993.tb09557.x. [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Burdyga Th, Heaton RC, Wray S. Stimulus-dependent modulation of smooth muscle intracellular calcium and force by altered intracellular pH. Pflügers Archiv. 1996;432:803–811. doi: 10.1007/s004240050202. [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Menice CB, Morgan KG, Wray S. Effect of metabolic inhibition on intracellular Ca2+, phosphorylation of myosin regulatory light chain and force in rat smooth muscle. The Journal of Physiology. 1997;499:485–496. doi: 10.1113/jphysiol.1997.sp021943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart MJ, Wray S. The effect of metabolic inhibition on rat uterine intracellular pH and its role in contractile failure. Pflügers Archiv. 1995;430:125–131. doi: 10.1007/BF00373847. [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Wray S. Effects of modulators of sarcoplasmic reticular function on intracellular Ca2+ and force of rat isolated myometrium. The Journal of Physiology. 1997a;499.P:8. P. [Google Scholar]
- Taggart MJ, Wray S. Agonist mobilization of sarcoplasmic reticular calcium during inhibition of Na+-Ca2+ exchange in isolated rat uterine smooth muscle. The Journal of Physiology. 1997b;501.P:113. P. [Google Scholar]
- Taggart MJ, Wray S. The effects of metabolic inhibition on intracellular Ca2+ and force in isolated myometria of pregnant rats. The Journal of Physiology. 1997c;505.P:103. doi: 10.1113/jphysiol.1997.sp021943. P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi H, Heistad DD, Kitazono T, Faraci FM. ATP-sensitive K+ channels mediate dilation of cerebral arerioles during hypoxia. Circulation Research. 1997;74:1005–1008. doi: 10.1161/01.res.74.5.1005. [DOI] [PubMed] [Google Scholar]
- Tewari K, Simard JM. Protein kinase A increases availibilty of calcium channels in smooth muscle cells from guinea pig basilar artery. Pflügers Archiv. 1994;428:9–16. doi: 10.1007/BF00374746. [DOI] [PubMed] [Google Scholar]
- Tsukioka M, Iino M, Endo M. pH dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in permeabilized smooth muscle cells of the guinea-pig. The Journal of Physiology. 1994;475:369–375. doi: 10.1113/jphysiol.1994.sp020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin L, Warner AE. Intracellular pH in early Xenopus embryos: its effect on current flow between blastomeres. The Journal of Physiology. 1980;300:489–504. doi: 10.1113/jphysiol.1980.sp013174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urena J, Franco-Obregon A, Lopez-Barneo J. Contrasting effects of hypoxia on cytosolic Ca2+ spikes in conduit and resistance myocytes of the rabbit pulmonary artery. The Journal of Physiology. 1996;496:103–109. doi: 10.1113/jphysiol.1996.sp021668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C, Wuytack F, Casteels R. Stimulation of 45Ca efflux from smooth muscle cells by metabolic inhibition and high K depolarisation. Pflügers Archiv. 1975;359:183–196. doi: 10.1007/BF00587378. [DOI] [PubMed] [Google Scholar]
- Vogel HJ, Lilja H, Hellstrand P. Phosphorus-31 NMR studies of smooth muscle from guinea-pig taenia coli. Bioscience Reports. 1983;3:863–870. doi: 10.1007/BF01133785. [DOI] [PubMed] [Google Scholar]
- Weir EK, Archer SL. The mechanism of acute pulmonary vasoconstriction: the tale of two channels. FASEB Journal. 1995;9:183–189. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- Williams DA, Becker PL, Fay FS. Regional changes in calcium underlying contraction of single smooth muscle cells. Science. 1987;235:1644–1647. doi: 10.1126/science.3103219. [DOI] [PubMed] [Google Scholar]
- Wingard CJ, Paul RJ, Murphy RA. Dependence of ATP consumption on cross-bridge phosphorylation in swine carotid smooth muscle. The Journal of Physiology. 1994;481:111–117. doi: 10.1113/jphysiol.1994.sp020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S. The effects of metabolic inhibition on uterine metabolism and intracellular pH in the rat. The Journal of Physiology. 1990;423:411–423. doi: 10.1113/jphysiol.1990.sp018030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Duggins K, Iles R, Nyman L, Osman VA. The effects of metabolic inhibition and intracellular pH on rat uterine force production. Experimental Physiology. 1992;77:307–319. doi: 10.1113/expphysiol.1992.sp003590. [DOI] [PubMed] [Google Scholar]
- Wu M, Vaughan-Jones RD. Effect of metabolic inhibitors and second messengers upon Na+-H+ exchange in the sheep cardiac Purkinje fibre. The Journal of Physiology. 1994;478:301–313. doi: 10.1113/jphysiol.1994.sp020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X-J. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circulation Research. 1995;77:370–378. doi: 10.1161/01.res.77.2.370. [DOI] [PubMed] [Google Scholar]
- Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. American Journal of Physiology. 1993;264:L116–123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]
- Zhang H-L, Bolton TB. ATP-sensitive potassium channels and their modulation by nucleotides and potassium channel openers in vascular smooth muscle cells. In: Bolton TB, Tomita T, editors. Smooth Muscle Excitation. London: Academic Press; 1996. pp. 139–154. [Google Scholar]


