Abstract
The hydraulic resistance of the synovial lining to fluid outflow from a joint cavity (
 ) is important for the retention of intra-articular lubricant. The resistance has been attributed in part to extracellular glycosaminoglycans, including hyaluronan and chondroitin sulphates. Increased permeability in joints infused with testicular hyaluronidase, which digests both chondroitin sulphates and hyaluronan, supports this view. In this study the importance of interstitial hyaluronan per se was assessed using leech and Streptomyces hyaluronidases, which degrade only hyaluronan.
) is important for the retention of intra-articular lubricant. The resistance has been attributed in part to extracellular glycosaminoglycans, including hyaluronan and chondroitin sulphates. Increased permeability in joints infused with testicular hyaluronidase, which digests both chondroitin sulphates and hyaluronan, supports this view. In this study the importance of interstitial hyaluronan per se was assessed using leech and Streptomyces hyaluronidases, which degrade only hyaluronan.Ringer solution was infused into the knee joint cavity of anaesthetized rabbits for 30 min, with or without hyaluronidase, after which intra-articular pressure (Pj) was raised and the relation between pressure and outflow determined.
Treatment with Streptomyces, leech or testicular hyaluronidases increased the fluid escape rates by similar factors, namely 4- to 6-fold. After Streptomyces hyaluronidase treatment the slope d
 /dPj, which at low pressures represents synovial hydraulic conductance, increased from a control of 0.90 ± 0.20 μl min−1 cmH2O−1 (mean ± s.e.m., n = 6) to 4.52 ± 0.70 μl min−1 cmH2O−1. The slope d
/dPj, which at low pressures represents synovial hydraulic conductance, increased from a control of 0.90 ± 0.20 μl min−1 cmH2O−1 (mean ± s.e.m., n = 6) to 4.52 ± 0.70 μl min−1 cmH2O−1. The slope d /dPj increased to a similar level after testicular hyaluronidase, namely to 4.14 ± 1.06 μl min−1 cmH2O−1 (control, 0.54 ± 0.24 μl min−1 cmH2O−1). Streptomyces and leech hyaluronidases were as effective as testicular hyaluronidase (no statistically significant differences) despite differences in substrate specificity.
/dPj increased to a similar level after testicular hyaluronidase, namely to 4.14 ± 1.06 μl min−1 cmH2O−1 (control, 0.54 ± 0.24 μl min−1 cmH2O−1). Streptomyces and leech hyaluronidases were as effective as testicular hyaluronidase (no statistically significant differences) despite differences in substrate specificity.It was shown using histochemical and immunohistochemical techniques that hyaluronan was removed from the synovium by leech, Streptomyces and testicular hyaluronidases. The binding of antibodies 2-B-6 and 3-B-3 showed that the core proteins of the chondroitin sulphate proteoglycans remained intact after treatment with hyaluronidases, and the binding of 5-D-4 showed that keratan sulphate was unaffected. An azocasein digestion assay confirmed that the hyaluronidase preparations had no significant proteolytic activity.
The effect of the hyaluronidases was four times greater than predicted from the low concentration of interstitial hyaluronan and its resistivity. Factors that might amplify the effect of hyaluronan depletion include the matrix-organizing role of hyaluronan, and/or non-uniformity of hyaluronan distribution. It is concluded that interstitial hyaluronan makes a major contribution to synovial hydraulic resistance, but the mechanisms are as yet poorly understood.
Diarthrodial joints contain a small, but important, volume of synovial fluid that lubricates and nourishes the articular cartilage. The fluid is retained within the joint cavity by a thin layer of specialized connective tissue called synovium that lines the cavity. Synovium is unlike an epithelium, because its cells lack intercellular junctions: there are micrometre-wide gaps between the cells. Despite this, the resistance to fluid leakage out of the joint cavity is substantial. The hydraulic resistance is an important physiological parameter because it conserves intra-articular fluid.
The chief source of the hydraulic resistance is thought to be the interstitial matrix occupying the intercellular gaps. This matrix contains type VI collagen microfibrils and bundles of types I, III and V collagen fibrils. Various large, polymeric macromolecules occur in the intervening, i.e. extrafibrillar, spaces, namely the giant, non-sulphated glycosaminoglycan hyaluronan; proteoglycans with sulphated glycosaminoglycan sidechains of chondroitin, heparan or keratan sulphate; numerous glycoproteins; and pericellular type IV collagen (Revell, Al-Saffar, Fish & Osei, 1995; Levick, Price & Mason, 1996; Coleman, Kavanagh, Mason, Levick & Ashhurst, 1998). It has been argued, on quantitative, biophysical grounds, that the glycosaminoglycans should contribute significantly, but not exclusively, to synovial hydraulic resistance (Price, Levick & Mason, 1996). This inference was tested recently by intra-articular injection of testicular hyaluronidase.
Testicular hyaluronidase is an endo-N- acetylhexosaminidase of molecular size 43-61 kDa and is active at neutral pH. Its primary substrate, hyaluronan, is a polymer of the disaccharide formed by N- acetyl-D-glucosamine and D-glucuronic acid, but, in addition, testicular hyaluronidase hydrolyses chondroitin sulphates, though at a slower rate. This is because it cleaves β(1→4) bonds between hexosamine and glucuronyl residues, whether the hexosamine group be N- acetylglucosamine, as in hyaluronan, or N- acetylgalactosamine, as in chondroitin sulphates (Meyer, 1971; Knepper, Farbman & Telser, 1984). The end products are chiefly tetrasaccharides. Testicular hyaluronidase injected into the cavity of the rabbit knee causes an ∼5-fold increase in the rate of escape of intra-articular saline across the synovial lining, which confirms the hydraulic importance of interstitial glycosaminoglycans (Scott, Coleman, Mason & Levick, 1997). The previous literature was confused on this issue, probably because the method used to assess synovial permeability was very indirect, namely the rate of urinary appearance of intra-articularly injected phenolsulphthalein. Treatment of the joint lining with testicular hyaluronidase was reported either to increase the urinary excretion of phenolsulphthalein (Seifter, Baeder & Begany, 1949), or not affect it significantly (Paul, Hodges, Knouse & Wright, 1952). Rodnan & MacLachan (1960) found no change in the rate of clearance of intra-articularly injected radiolabelled gamma globulin from a broad region of the rabbit knee after treatment with intra-articular testicular hyaluronidase.
Quantitative biochemical analysis of rabbit synovium shows that chondroitin sulphate, which occurs as chondroitin 4- and 6-sulphates, and hyaluronan are present at comparable concentrations, namely 1.16 and 0.78 mg (ml extrafibrillar space)−1, respectively (Price, Levick & Mason, 1996). Since testicular hyaluronidase hydrolyses chondroitin sulphates in addition to hyaluronan, the question arose as to the contribution of each glycosaminoglycan individually to synovial hydraulic resistance. This was investigated in the present study by selectively depleting the synovial lining of hyaluronan alone, using two highly specific hyaluronidases, Streptomyces hyaluronidase (from Streptomyces hyalurolyticus) and leech hyaluronidase (from Hirudo medicinalis), which are both active at neutral pH (Meyer, 1971; Knepper et al. 1984). Streptomyces hyaluronidase is without action on chondroitin sulphate or any glycosaminoglycan other than hyaluronan (Ohya & Kaneko, 1970; Knepper et al. 1984). Streptomyces hyaluronidase (or hyaluronan lyase) is an N- acetylhexosamine β-eliminase that specifically eliminates the β(1→4) glycosidic bond between the two monosaccharides of hyaluronan, namely N-acetyl-D-glucosamine and D-glucuronic acid. The end products are unsaturated tetra- and hexasaccharides. By contrast, leech hyaluronidase (a β-endoglucuronidase or hydrolase; Meyer, 1971) specifically attacks the β(1→3) bond that alternates with the β(1→4) bond along the polysaccharide chain, yielding tetrasaccharides. It, too, does not attack chondroitin sulphates. The effects of these two enzymes on the hydraulic conductance of the joint lining in rabbit knees is compared here with the effect of testicular hyaluronidase. The removal of hyaluronan by the three enzymes was confirmed histochemically.
METHODS
Materials
The intra-articular infusate and enzyme vehicle was sterile, non-pyrogenic Baxter Ringer solution, a commercial preparation for intravenous infusion containing 147 mM Na+, 4 mM K+, 2 mM Ca2+and 156 mM Cl−, pH 7.2 (Baxter Healthcare Ltd, Thetford, UK). Bovine testicular hyaluronidase, type III (EC 3.2.1.35), Streptomyces hyalurolyticus hyaluronidase (EC 4.2.2.1) and leech hyaluronidase (EC 3.2.1.36) were supplied by Sigma. Chondroitinase ABC (EC 4.2.2.4) was from ICN Flow (High Wycombe, UK). Histochemical reagents and labelled antibodies were from Sigma. The sources of the monoclonal antibodies and probe for hyaluronan are given below.
Assessment of hyaluronidase activity by viscometry
Relative viscosities of solutions of 4 mg ml−1 rooster comb hyaluronan (Sigma) in Baxter Ringer solution at 35°C, the normal intra-articular temperature, were measured in a cylinder-in-cup rotational torque viscometer (Contraves Low-Shear 30, Contraves AG, Zürich, Switzerland) at a fixed shear rate. Measurements were made before, and at various times after, addition of 125 units (U) of hyaluronidase.
Animal preparation
New Zealand White rabbits weighing 2-3 kg were anaesthetized via the marginal ear vein by i.v. pentobarbitone (30 mg kg−1) plus urethane (500 mg kg−1), tracheostomized and maintained by smaller half-hourly doses. Core temperature was controlled by a Harvard animal blanket and rectal thermistor. With the animal supine the hindlimbs were secured with the knees at an unforced angle, viz. 100 -130 deg extension. Two cannulae were inserted into the suprapatellar joint space as described previously (Levick, 1979). One, a 21-gauge hypodermic needle with lateral perforations drilled near the tip, was connected to one port of a Validyne CD 15 differential pressure transducer (Linton Instruments, Diss, UK) to measure intra-articular fluid pressure (Pj, ± 0.1 cmH2O). The transducer was zeroed by connecting a fluid column to the opposite port and setting the column meniscus level with the joint. The other intra-articular cannula was a 20-gauge polypropylene Medicut (Argyle-Sherwood, Tullamore, Ireland) with a lateral aperture cut just proximal to the tip. This cannula was connected to a saline-filled infusion reservoir, the vertical height of which controlled intra-articular pressure. Flow of solution into the joint cavity, Q̇in, was recorded by a photoelectric drop counter whose drop size was 8 μl over the range of flows observed. Conversion of Q̇in to trans-synovial flow,  , is described below. Pressures and flows were recorded continuously on a chart recorder. Procedures conformed to UK legislation and animals were killed at the end of the experiment by an overdose of i.v. sodium pentobarbitone (Euthatal, Rhône Mérieux, Ireland).
, is described below. Pressures and flows were recorded continuously on a chart recorder. Procedures conformed to UK legislation and animals were killed at the end of the experiment by an overdose of i.v. sodium pentobarbitone (Euthatal, Rhône Mérieux, Ireland).
Determination of the pressure-flow relation
Net trans-synovial flow was measured over imposed values of Pj ranging from ∼2 cmH2O (a pressure reached in a normal flexed joint, and the minimum pressure at which flow is measurable) to ∼22-25 cmH2O (levels reached in chronic joint effusions). The intra-articular pressure was raised in steps of ∼2-4 cmH2O by raising the infusion reservoir 2-4 cm at 15 min intervals. Trans-synovial flow was calculated in the steady state at the end of each interval.
A step elevation of the infusion reservoir initiates a transient fast inflow which, over ∼2 min, raises Pj to a new, stable level. Beyond this point the smaller but sustained inflow from the reservoir is due chiefly to trans-synovial absorption of the infused fluid. A small fraction of the inflow in this phase, however, is due to viscous creep of the stressed cavity walls. The volume creep rate (Q̇creep, μl min−1) has been evaluated previously by infusing a non-absorbed oil at controlled pressures and is summarized by the expression Q̇creep = 0.23Pj+ 0.4 (Levick, 1979). To correct for wall creep, Q̇creep at a given pressure was subtracted from the measured flow, Q̇in, to give trans-synovial flow,  . The creep correction is small (< 10 %) for most values of Q̇in.
. The creep correction is small (< 10 %) for most values of Q̇in.
Enzymatic digestion protocols
Protocol A
Initial studies were carried out to assess the time course of action of hyaluronidase on synovial hydraulic conductance. The synovial cavity was continuously infused with Ringer solution to maintain a stable intra-articular pressure, and trans-synovial flow was measured at that pressure in the steady state. Some of the intra-articular fluid was then withdrawn, replaced by a solution containing 250 U of hyaluronidase, and the pressure quickly brought back to its former level. The time course of the increase in trans-synovial flow was then followed as the hyaluronidase acted on the synovial lining.
Protocol B
For the main series of experiments the protocol was designed to determine the full pressure-flow relation. In each animal one knee was used as a control and the other received intra-articular enzyme. At first the control joints were studied before enzyme-infused joints, in order to prevent any putative interference by absorbed, circulating hyaluronidase. Assay of the plasma for hyaluronidase activity, however, using the viscosity-reducing action of hyaluronidase on hyaluronan, showed no detectable circulating activity after intra-articular enzyme injection. Consequently the order of ‘treatment’ (control or enzyme) was varied in later experiments.
The volume initially injected into the joint cavity was either 500 μl of Ringer solution (control knee) or 500 U of hyaluronidase in 500 μl Ringer solution (opposite knee). This raised the intra-articular pressure to a few centimetres of water above atmospheric pressure. The joint was then left undisturbed for 30 min. With all three hyaluronidases it was noted that, within a few minutes of injection, the intra-articular pressure was decaying faster in the enzyme-containing cavity than in the control cavity. This indicated that fluid absorption was already occurring faster in the enzyme-injected joint than in the control joint. The observation confirmed that enzyme action was rapid and well within with 30 min allowed (see also results of protocol A below). After 30 min the pressure- flow relation was determined as above. Using this protocol, testicular hyaluronidase was studied in seven rabbits, Streptomyces hyaluronidase in seven rabbits and leech hyaluronidase in two rabbits. The pressure-flow relation was also studied in two joints that had received only 5 U of Streptomyces hyaluronidase.
In addition one animal received chondroitinase ABC (10 U) plus Streptomyces hyaluronidase (500 U) in one knee, and Streptomyces hyaluronidase alone in the opposite knee, the remainder of the protocol being as described above. Chondroitinase ABC is so named because it degrades chondroitin 4-sulphate (formerly chondroitin sulphate A), dermatan sulphate (formerly chondroitin sulphate B), chondroitin 6-sulphate (formerly chondroitin sulphate C) and, with reduced activity, hyaluronan. This experiment was introduced because it clarified the interpretation of the result with testicular hyaluronidase (see Discussion). Further studies using chondroitinase ABC alone are reported by Scott, Coleman, Abiona, Ashhurst, Mason & Levick (1998).
Proteolysis assessments
Significant proteolytic activity has in the past occurred in some commercial preparations of Streptomyces hyaluronidase (e.g. Calbiochem-Behring Corp. preparation at 1000 U ml−1, the concentration used here), but not others (e.g. Sigma testicular hyaluronidase at the same concentration) (Harrisson, Van Hoof & Vanroelen, 1986). If present in significant quantity in the enzymes used here, protease would deplete interstitial proteoglycans and glycoproteins, confusing the interpretation of results. To assess this possibility, three controls were carried out.
(1) Enzyme-treated tissues were assessed immunohistologically for the presence of sulphated glycosaminoglycans that are bound to core protein as proteoglycans (chondroitin and keratan sulphates, see below).
(2) The action of Streptomyces hyaluronidase on the pressure-flow curve was determined in the presence of a cocktail of protease inhibitors, namely phosphoramidon (200 μg ml−1, a metalloproteinase inhibitor), phenylmethanesulphonylfluoride (17 μg ml−1, a serine protease inhibitor) and N-ethylmaleimide (1250 μg ml−1, a cysteine protease inhibitor).
(3) Enzymes were assessed for proteolytic activity in vitro using the colorimetric azocasein digestion assay (Wilson et al. 1987). Enzyme solution at the concentration used in vivo was mixed with 4 mg azocasein (Sigma) in 1 ml Ringer solution and incubated with agitation for 1 h at 37°C. Protein and bound dye were precipitated by 100 μl 80 % trichloracetic acid. After centrifugation at 10000 r.p.m. for 3 min, the supernatant, which became orange after proteolysis, was aspirated and its absorbance read at 340 nm in a Beckman DU-62 spectrophotometer. Ringer solution was incubated with azocasein as a negative control and 10 U of chymopapain, a protease, was incubated as a positive control.
Preparation of tissue for microscopy
After determining the pressure-flow relation on each side, the rabbit was killed as described earlier. The synovium, subsynovium and underlying tissue were excised from the lateral and medial sides of the quadriceps tendon and suprapatella in the suprapatellar bursa. The tissue was fixed in 4 % paraformaldehyde in 0.05 M Tris-HCl buffer, pH 7.2, overnight at room temperature. After washing in buffer, the tissue was dehydrated in graded ethanols, cleared in methyl salicylate and embedded in paraffin wax. Sections were cut at 7 μm.
Detection of hyaluronan
The distribution of hyaluronan was determined using the biotinylated hyaluronan binding region (HABR) of aggrecan, namely the G1 domain (supplied by M. Bayliss, Royal Veterinary College, London); the procedure is described fully by Coleman et al. (1998). In brief, sections were incubated with HABR. The bound HABR was detected by incubation with streptavidin-peroxidase, followed by a standard peroxidase substrate.
Immunohistochemistry
The persistent presence of chondroitin sulphate attachment regions and keratan sulphate (both present on proteoglycans) after hyaluronidase treatment was assessed immunohistochemically to check for non-specific proteolytic activity in the hyaluronidases (see ‘Proteolysis assessments’ above). The mouse monoclonal antibodies (supplied by B. Caterson, University of Wales, Cardiff, UK) were as follows.
(1) 2-B-6. This recognizes the delta unsaturated disaccharide of chondroitin 4-sulphate that remains at the region of attachment of the glycosaminoglycan chain to the core protein after treatment with chondroitinase ABC (Caterson, Christner, Baker & Couchman, 1985). To generate the epitope, tissue sections were treated with chondroitinase ABC. The antibody does not recognize the main chondroitin sulphate chain.
(2) 3-B-3. This recognizes the delta unsaturated tetrasaccharide of chondroitin 6-sulphate at the core protein attachment region, left after digestion of the tissue sections with chondroitinase ABC. Like 2-B-6, this antibody does not recognize native chondroitin sulphate chains (Caterson et al. 1985).
(3) 5-D-4. This recognizes highly sulphated octa- and dodecasaccharides of keratan sulphate. Enzymatic treatment is not needed to expose the epitopes (Mehmet, Scudder, Tang, Hounsell, Caterson & Feizi, 1986).
The immunohistochemical procedure is described in detail by Coleman et al. (1998). In brief, sections for the localization of chondroitin sulphates were treated with 0.5 U ml−1 chondroitinase ABC before incubation with the 2-B-6 or 3-B-3 antibodies. Sections for the localization of keratan sulphate received no pretreatment before exposure to the 5-D-4 antibody. The second antibody was goat anti-mouse IgG conjugated with alkaline phosphatase. The label was visualized using standard alkaline phosphatase substrate.
For controls, some sections were not exposed to specific antibody. None showed a positive reaction.
Statistical analyses
In control joints the Pj- relation usually shows a marked steepening at ∼7-14 cmH2O (‘yield pressure’) with little or inconsistent curvature above or below this. Consequently the relation is usually summarized empirically, for purposes of numerical comparison, by two linear regression lines; one is fitted to results in the high pressure range, i.e. above yield pressure, and the other to results in the low pressure range, i.e. below yield pressure (Levick, 1979). Yield pressure was determined by inspection. Regression slopes were compared by Student's paired t test using Graphpad Prism (Graphpad Software Inc., San Diego, CA, USA), with P < 0.05 accepted as a significant difference. The infusion-driven intra-articular pressures varied a little between experiments, so in order to compare flows at identical pressures between experiments, the flows were interpolated when necessary to standard values at 2.5 cmH2O intervals (2.5, 5.0, 7.5 cmH2O, etc.), using linear interpolation between the two bounding measurements. Means are given with the standard error of the mean (s.e.m.) throughout. Ratios were compared using non-parametric tests.
relation usually shows a marked steepening at ∼7-14 cmH2O (‘yield pressure’) with little or inconsistent curvature above or below this. Consequently the relation is usually summarized empirically, for purposes of numerical comparison, by two linear regression lines; one is fitted to results in the high pressure range, i.e. above yield pressure, and the other to results in the low pressure range, i.e. below yield pressure (Levick, 1979). Yield pressure was determined by inspection. Regression slopes were compared by Student's paired t test using Graphpad Prism (Graphpad Software Inc., San Diego, CA, USA), with P < 0.05 accepted as a significant difference. The infusion-driven intra-articular pressures varied a little between experiments, so in order to compare flows at identical pressures between experiments, the flows were interpolated when necessary to standard values at 2.5 cmH2O intervals (2.5, 5.0, 7.5 cmH2O, etc.), using linear interpolation between the two bounding measurements. Means are given with the standard error of the mean (s.e.m.) throughout. Ratios were compared using non-parametric tests.
RESULTS
Biochemical results
Hyaluronidase activity
The activity of all three commercial preparations was confirmed in vitro by the rapid reduction of the viscosity of hyaluronan solutions. Testicular hyaluronidase is reported to act more slowly than Streptomyces hyaluronidase (Knepper et al. 1984). With the preparations used here, 125 U of Streptomyces hyaluronidase reduced the relative viscosity of 4 mg ml−1 rooster comb hyaluronan from 29.6 to 1.7 by 10 min and to 1.3 by 30 min. The sample of testicular hyaluronidase also acted rapidly, although a little less efficiently, reducing the relative viscosity to 2.8 by 10 min and 1.7 by 30 min. For both enzymes most of the effect was completed in < 10 min.
Azocasein assay for proteolytic activity in hyaluronidases
The supernatant after azocasein incubation with chymopapain (positive control) was deep orange while that after azocasein incubation with Ringer solution, though not visibly coloured, possessed a small, but reproducible, absorbance relative to the Ringer solution blank (negative control absorbance). Further studies indicated that the latter was due to traces of free dye in the preparation. Supernatants after incubation with Streptomyces, leech or testicular hyaluronidase or chondroitinase ABC lacked visible colouration and, scaling their absorbances relative to the increased absorbance after chymopapain, their proteolytic activities were respectively 0.011, 0.001, 0.031 and 0.006 times that of chymopapain. Expressed as the percentage digestion of azocasein, the corresponding results were 0.5, 0.03, 1.3 and 0.2 %, while for chymopapain itself 41 % digestion of the azocasein occurred over the incubation period. Similar minimal levels of azocasein digestion by hyaluronidases and chondroitinase ABC were reported by Meyer, Laver-Rudich & Tanenbaum (1983). The enzyme preparations thus had little proteolytic activity at the concentrations used here. Leech hyaluronidase, with virtually undetectable proteolytic activity, had no less an effect on synovial lining conductance than had testicular hyaluronidase (Table 1 and below).
Table 1.
Effect of three hyaluronidases on hydraulic conductance of synovial lining, dQs/dPj, below yield pressure and at higher pressures
| Below yield pressure | Above yield pressure | |||
|---|---|---|---|---|
| Control | After enzyme | Control | After enzyme | |
| Streptomyces hyaluronidase | ||||
| Mean of slopes* | 0.90 ± 0.20 (5) | 4.52 ± 0.70 (6) | 2.30 ± 0.13 (6) | 10.72 ± 1.14 (7) |
| Pooled regression† | 0.78 ± 0.22 (23) | 3.43 ± 0.56 (25) | 2.17 ± 0.27 (26) | 10.23 ± 0.80 (32) |
| Leech hyaluronidase | ||||
| Mean of slopes* | 1.29 (1) | 4.79 (2) | 3.14 (1) | 16.84 (2) |
| Pooled regression† | 1.29 ± 0.07 (4) | 5.10 ± 0.60 (7) | 3.14 ± 0.26 (5) | 16.15 ± 1.57 (11) |
| Testicular hyaluronidase | ||||
| Mean of slopes* | 0.54 ± 0.24 (4) | 4.14 ± 1.06 (7) | 2.28 ± 0.14 (5) | 13.84 ± 1.69 (7) |
| Pooled regression† | 0.53 ± 0.13 (21) | 3.82 ± 0.55 (31) | 2.06 ± 0.39 (22) | 13.06 ± 1.64 (27) |
Values are means ± S.E.M. in μl min−1 cmH2O−1.
Mean of slopes fitted by regression analysis to each individual pressure-flow plot; values in parentheses, number of animals.
Regression slope fitted to a pooled plot of all pressure-flow values; values in parentheses, total number of pooled points.
Time course of action of hyaluronidase in vivo
All three hyaluronidases rapidly and dramatically increased the fluid escape rate across the synovial lining. The time course of action was assessed using protocol A, where trans-synovial flow was recorded in the same joint at the same pressure before and immediately after enzyme infusion. As shown in Fig. 1, incubation of the joint lining with 250 U of Streptomyces hyaluronidase increased the trans-synovial flow from the control level (8 μl min−1 at the low intra-articular pressure illustrated) to a flow several times greater (27 μl min−1 in the case illustrated) within 3 min, after which no further increase in permeability occurred over 30 min. Control experiments showed that the act of draining and refilling the cavity with Ringer solution did not itself increase the lining permeability. The actions of testicular and leech hyaluronidases were similarly rapid. These results showed that 30 min, the period of exposure of synovium to enzyme under protocol B, was sufficient for completion of the action of the enzyme.
Figure 1. Assessment of the rate of action of Streptomyces hyaluronidase on synovial hydraulic permeability.
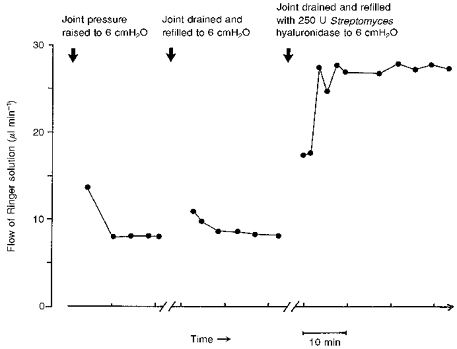
The first group of points shows the inflow of Ringer solution into the cavity of the rabbit knee from the infusion reservoir after a step rise in infusion pressure. The initial high inflow is due to joint expansion as intra-articular pressure rises to its new set level. After pressure in the joint has stabilized (here at 6 cmH2O) inflow settles to a steady rate that reflects rate of fluid absorption across the synovial lining. Aspiration and refilling with Ringer solution did not significantly change the steady state absorption rate (second curve). Aspiration and refilling with Ringer solution containing 250 U Streptomyces hyaluronidase increased the absorption rate 3.5-fold within 3 min of the first measurement, with no further rise over 30 min (third curve). The first measurement was several minutes after enzyme injection due to the time needed to re-establish control pressure (interrupted time axis).
Trans-synovial flows following hyaluronidase treatments
In protocol B the entire pressure-trans-synovial flow relation of the control joint was compared with that of the opposite joint of the same animal after hyaluronidase digestion. All three forms of hyaluronidase increased the trans-synovial absorption rate,  , severalfold at any given intra-articular pressure. When the flow after enzyme digestion is expressed relative to the paired control flow at the same pressure, Streptomyces hyaluronidase raised
, severalfold at any given intra-articular pressure. When the flow after enzyme digestion is expressed relative to the paired control flow at the same pressure, Streptomyces hyaluronidase raised  by 6.22 ± 0.56 times the control flow, averaged over the entire pressure range (45 paired measurements, 7 animals). The increases in flow at individual pressure levels and the statistical significances are summarized in Fig. 2.
by 6.22 ± 0.56 times the control flow, averaged over the entire pressure range (45 paired measurements, 7 animals). The increases in flow at individual pressure levels and the statistical significances are summarized in Fig. 2.
Figure 2. Effect of Streptomyces hyaluronidase on trans-synovial flow (Qs) at various intra-articular pressures (Pj) in 7 rabbits.
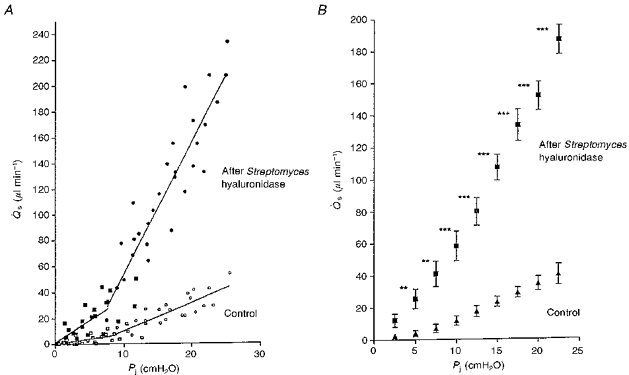
A, pooled results from control knee joints (open symbols) and contralateral joints after treatment with Streptomyces hyaluronidase (filled symbols). Square symbols show results below yield pressure (identified by inspection of individual plots) and circles show results above yield pressure. The regression lines are described in Table 1. B, means ± s.e.m. of flows interpolated to standard pressures for statistical comparison of control and post-enzymatic flows at the same pressure. * P = 0.05; **P = 0.01; ***P = 0.001 - Student's t test. The change in slope at yield pressure is blunted by this averaging process and is better seen in individual joints, as in Fig. 3.
Leech hyaluronidase was studied in less detail, but again it increased the trans-synovial flow markedly, namely by 4.09 ± 0.37 times the control values overall (n = 9) (Fig. 3).
Figure 3. Synovial pressure (Pj)-flow (Qs) relations in rabbit knee joints treated with intra-articular leech hyaluronidase in Ringer solution (left knee, filled symbols) or plain Ringer solution (right knee of same animal, open symbols).
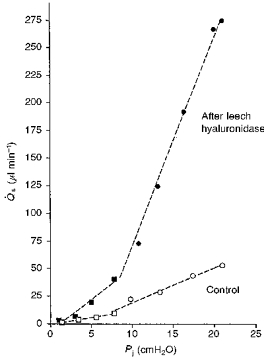
The dashed regression lines were fitted above and below yield pressures. The latter were identified by inspection. Slopes are given in the text. Squares show results below yield pressure and circles show results above yield pressure.
Testicular hyaluronidase raised  by 4.93 ± 0.49 times the control flow over the entire pressure range (n = 42). The effect of testicular hyaluronidase was thus no bigger than that of Streptomyces hyaluronidase, despite the broader substrate range of testicular hyaluronidase. When the absolute increases in flow were analysed, the difference between the two enzymes was not significant (P = 0.38), and for the ratio of flows the effect of testicular hyaluronidase was actually less than that of Streptomyces hyaluronidase (P = 0.02, Mann-Whitney U test). The increases in
by 4.93 ± 0.49 times the control flow over the entire pressure range (n = 42). The effect of testicular hyaluronidase was thus no bigger than that of Streptomyces hyaluronidase, despite the broader substrate range of testicular hyaluronidase. When the absolute increases in flow were analysed, the difference between the two enzymes was not significant (P = 0.38), and for the ratio of flows the effect of testicular hyaluronidase was actually less than that of Streptomyces hyaluronidase (P = 0.02, Mann-Whitney U test). The increases in  at individual pressures after testicular hyaluronidase are shown in Fig. 4.
at individual pressures after testicular hyaluronidase are shown in Fig. 4.
Figure 4. Effect of testicular hyaluronidase on trans-synovial flow (Qs) at various intra-articular pressures (Pj) in 7 rabbits.
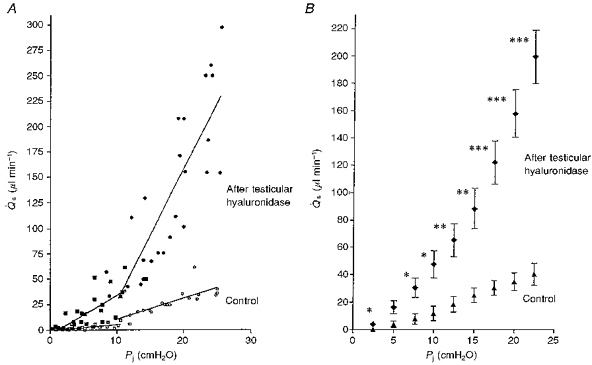
A, pooled results from control knee joints (open symbols) and contralateral joints after treatment with testicular hyaluronidase (filled symbols). Squares show results below yield pressure and circles show results above yield pressure. Regression slopes are given in Table 1. B, means ± s.e.m. of flows interpolated to standard pressures for comparison of control and post-enzymatic flows at same pressure. * P = 0.05; **P = 0.01; ***P = 0.001 - t test.
Changes in tissue hydraulic conductance and composition following hyaluronidase treatments
The relation between intra-articular pressure and trans-synovial flow of Ringer solution in the control joint is non-linear (Figs 2-4); it steepens at intra-articular pressures around 7-14 cmH2O (yield phenomenon, Levick, 1979; Price, Levick & Mason, 1996). The slope of the pressure- flow plot at low pressures, d /dPj, represents the synovial hydraulic conductance in the control state, i.e. below yield pressure. The slope above the yield pressure reflects the rate of increase of conductance with pressure (Levick et al. 1996). The determination of the slopes below and above the yield pressure by linear regression is illustrated for a leech hyaluronidase-treated joint in Fig. 3. All three hyaluronidases increased the slope d
/dPj, represents the synovial hydraulic conductance in the control state, i.e. below yield pressure. The slope above the yield pressure reflects the rate of increase of conductance with pressure (Levick et al. 1996). The determination of the slopes below and above the yield pressure by linear regression is illustrated for a leech hyaluronidase-treated joint in Fig. 3. All three hyaluronidases increased the slope d /dPj of the relation below yield pressure, and also above it (Figs 2-4). The pressures at which steepening occurred, determined by inspection, were not significantly changed by enzyme treatment; they averaged 8.9 ± 1.0 cmH2O for all hyaluronidase-treated joints and 9.9 ± 0.5 cmH2O for the control joints (P = 0.28, paired t test).
/dPj of the relation below yield pressure, and also above it (Figs 2-4). The pressures at which steepening occurred, determined by inspection, were not significantly changed by enzyme treatment; they averaged 8.9 ± 1.0 cmH2O for all hyaluronidase-treated joints and 9.9 ± 0.5 cmH2O for the control joints (P = 0.28, paired t test).
Streptomyces hyaluronidase
Results in vivo
Results after Streptomyces hyaluronidase treatment are shown in Fig. 2. The enzyme increased d /dPj fivefold over the low pressure range, i.e. below yield pressure. The control conductance was 0.90 ± 0.20 μl min−1 cmH2O−1 (n = 5) and the post-enzyme conductance was 4.52 ± 0.70 μl min−1 cmH2O−1 (n = 6, P < 0.002); see Table 1. Similarly, over the higher pressure range, Streptomyces hyaluronidase raised d
/dPj fivefold over the low pressure range, i.e. below yield pressure. The control conductance was 0.90 ± 0.20 μl min−1 cmH2O−1 (n = 5) and the post-enzyme conductance was 4.52 ± 0.70 μl min−1 cmH2O−1 (n = 6, P < 0.002); see Table 1. Similarly, over the higher pressure range, Streptomyces hyaluronidase raised d /dPj approximately fivefold, from a control conductance of 2.30 ± 0.13 μl min−1 cmH2O−1 (n = 6) to a post-enzyme conductance of 10.72 ± 1.14 μl min−1 cmH2O−1 (n = 7, P < 0.001). Similar results were obtained in the two joints treated with only 5 U of Streptomyces hyaluronidase: in both cases the absorption rates exceeded 200 μl min−1 at 20 cmH2O, and the values for d
/dPj approximately fivefold, from a control conductance of 2.30 ± 0.13 μl min−1 cmH2O−1 (n = 6) to a post-enzyme conductance of 10.72 ± 1.14 μl min−1 cmH2O−1 (n = 7, P < 0.001). Similar results were obtained in the two joints treated with only 5 U of Streptomyces hyaluronidase: in both cases the absorption rates exceeded 200 μl min−1 at 20 cmH2O, and the values for d /dPj above yield pressure were 14.6 and 17.9 μl min−1 cmH2O−1, respectively. The relative increase in d
/dPj above yield pressure were 14.6 and 17.9 μl min−1 cmH2O−1, respectively. The relative increase in d /dPj in the high pressure range following enzyme treatment, namely to 4.95 ± 0.52 times the value in control joints, was not significantly different from the increase in the low pressure range, namely to 5.95 ± 2.28 times control (P = 0.65).
/dPj in the high pressure range following enzyme treatment, namely to 4.95 ± 0.52 times the value in control joints, was not significantly different from the increase in the low pressure range, namely to 5.95 ± 2.28 times control (P = 0.65).
The pressure-flow relation after enzymatic digestion still showed a pronounced increase in slope d /dPj with pressure. The magnitude of the pressure-related increase in d
/dPj with pressure. The magnitude of the pressure-related increase in d /dPj in the enzyme-treated joints was 2.4-fold, expressed as slope above yield/slope below yield (P = 0.001). This was not significantly different from the pressure-related increase in slope in the control joints, namely 2.6-fold (P < 0.001). Thus the yield phenomenon was not significantly attenuated by Streptomyces hyaluronidase. Although the yield pressure tended to be a little lower in the Streptomyces hyaluronidase-treated joint (mean, 7.8 ± 1.1 cmH2O) than in the paired control joint (10.5 ± 0.9 cmH2O), the difference did not reach statistical significance (P = 0.13, paired t test). The pressure-dependent increase in d
/dPj in the enzyme-treated joints was 2.4-fold, expressed as slope above yield/slope below yield (P = 0.001). This was not significantly different from the pressure-related increase in slope in the control joints, namely 2.6-fold (P < 0.001). Thus the yield phenomenon was not significantly attenuated by Streptomyces hyaluronidase. Although the yield pressure tended to be a little lower in the Streptomyces hyaluronidase-treated joint (mean, 7.8 ± 1.1 cmH2O) than in the paired control joint (10.5 ± 0.9 cmH2O), the difference did not reach statistical significance (P = 0.13, paired t test). The pressure-dependent increase in d /dPj in the control joint is caused by stretch of the lining and increased hydration of the interstitial matrix (Levick et al. 1996). The present results imply that similar processes operate after hyaluronidase treatment.
/dPj in the control joint is caused by stretch of the lining and increased hydration of the interstitial matrix (Levick et al. 1996). The present results imply that similar processes operate after hyaluronidase treatment.
The investigation of Streptomyces hyaluronidase in the presence of protease inhibitors was limited to one animal because the increase in synovial lining conductance was clearly unaltered by protease inhibitors, in keeping with the biochemical evidence of only a slight protease activity. After treatment with enzyme plus protease inhibitors, the conductance d /dPj below yield pressure (11.5 cmH2O) was 4.64 ± 0.08 μl min−1 cmH2O−1, which is close to the mean for joints receiving Streptomyces hyaluronidase without protease inhibitors (4.50 ± 0.85 μl min−1 cmH2O−1, n = 5). The post-enzyme slope was 3.5 times the control slope in the contralateral knee (1.33 ± 0.11 μl min−1 cmH2O−1). Above yield pressure d
/dPj below yield pressure (11.5 cmH2O) was 4.64 ± 0.08 μl min−1 cmH2O−1, which is close to the mean for joints receiving Streptomyces hyaluronidase without protease inhibitors (4.50 ± 0.85 μl min−1 cmH2O−1, n = 5). The post-enzyme slope was 3.5 times the control slope in the contralateral knee (1.33 ± 0.11 μl min−1 cmH2O−1). Above yield pressure d /dPj was 12.91 ± 0.86 μl min−1 cmH2O−1 after treatment with enzyme plus protease inhibitors (mean result without protease inhibitors, 10.35 ± 1.28 μl min−1 cmH2O−1), which was 5.7 times the control slope in the contralateral knee (2.28 ± 0.69 μl min−1 cmH2O−1). These results support the view that the permeability-enhancing action of Streptomyces hyaluronidase is unlikely to be an artifact of contamination by a protease.
/dPj was 12.91 ± 0.86 μl min−1 cmH2O−1 after treatment with enzyme plus protease inhibitors (mean result without protease inhibitors, 10.35 ± 1.28 μl min−1 cmH2O−1), which was 5.7 times the control slope in the contralateral knee (2.28 ± 0.69 μl min−1 cmH2O−1). These results support the view that the permeability-enhancing action of Streptomyces hyaluronidase is unlikely to be an artifact of contamination by a protease.
Histochemistry and immunohistochemistry
The control synovium was strongly positive for hyaluronan (Fig. 5Aa). After treatment with Streptomyces hyaluronidase in vivo, staining for hyaluronan was abolished (Fig. 5Ab).
Figure 5. Photomicrographs of synovium (S) and subsynovium (SS) overlying muscle (M) from the suprapatellar bursa of the knee joint cavity (JC), showing distribution of hyaluronan (A) and sulphated glycosaminoglycan epitopes (B).
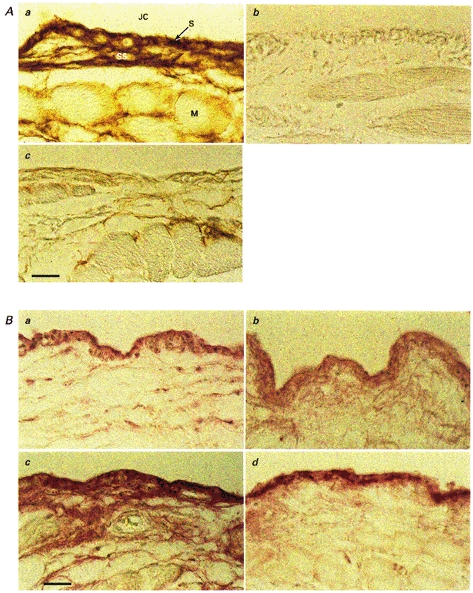
A, hyaluronan has been demonstrated using the HABR probe. a, control tissue from a Ringer-infused joint. Synovium and subsynovium have a higher concentration of hyaluronan than the connective tissue around the muscle fibres. b, the synovial lining of a joint treated with 500 U of Streptomyces hyaluronidase in vivo, excised at the end of the experiment. All the hyaluronan has been removed. c, the synovial lining of a joint treated with 500 units of intra-articular testicular hyaluronidase in vivo. Binding of the HABR probe is greatly reduced, compared with control, but a small amount of hyaluronan remains in the synovium and subsynovium. B, the distribution of chondroitin 4-sulphate core protein and keratan sulphate chains. a, the binding of the 2-B-6 antibody to chondroitin 4-sulphate attachment region in control synovium. The antibody is bound by synovium and to a lesser degree by subsynovium. b,2-B-6 binding after treatment of the joint with 500 units of intra-articular testicular hyaluronidase in vivo. Antibody binding is similar to that in control tissue. c, shows the binding of the 5-D-4 antibody to keratan sulphate in control synovium. The antibody is bound by synovium and to a lesser extent by subsynovium. d,5-D-4 binding after treatment of the joint with 500 units of intra-articular testicular hyaluronidase in vivo. The binding of antibody by the synovium is similar to that by control synovium. Scale bar, 25 μm.
Control synovium bound the antibodies 2-B-6 (Fig. 5Ba) and 3-B-3 (not shown) after treatment of the tissue sections with chondroitinase ABC (see Methods). Subsynovium bound less antibody than synovium. Control synovium also bound the antibody 5-D-4 (Fig. 5Bc).After Streptomyces hyaluronidase treatment in vivo, 2-B-6, 3-B-3 and 5-D-4 were still bound (not shown). Thus, Streptomyces hyaluronidase treatment did not deplete the tissue of chondroitin sulphate proteoglycan or keratan sulphate proteoglycan.
Leech hyaluronidase
Results in vivo
The effect of leech hyaluronidase was studied in only two animals because its effects were very similar to those of Streptomyces and testicular hyaluronidases. After leech hyaluronidase treatment, flows rose to > 200 μl min−1 at pressures of ∼20 cmH2O (Fig. 3), a magnitude of flow never seen in control joints. Below yield pressure the conductance d /dPj was increased from 1.29 ± 0.07 (control) to 5.64 ± 0.91 (same animal) and 3.94 ± 0.31 μl min−1 cmH2O−1 (second animal) after leech hyaluronidase digestion. Above yield pressure the enzyme raised d
/dPj was increased from 1.29 ± 0.07 (control) to 5.64 ± 0.91 (same animal) and 3.94 ± 0.31 μl min−1 cmH2O−1 (second animal) after leech hyaluronidase digestion. Above yield pressure the enzyme raised d /dPj from 3.14 ± 0.26 (control) to 20.21 ± 1.69 (same animal) and 13.48 ± 0.76 μl min−1 cmH2O−1 (second animal). The flows and d
/dPj from 3.14 ± 0.26 (control) to 20.21 ± 1.69 (same animal) and 13.48 ± 0.76 μl min−1 cmH2O−1 (second animal). The flows and d /dPj values after leech hyaluronidase were within the range seen in the larger series of experiments with Streptomyces and testicular hyaluronidases (Table 1).
/dPj values after leech hyaluronidase were within the range seen in the larger series of experiments with Streptomyces and testicular hyaluronidases (Table 1).
Histochemistry and immunohistochemistry
After treatment with leech hyaluronidase in vivo, synovium no longer bound the HABR probe for hyaluronan (not shown). The antibodies 2-B-6 and 3-B-3 were still bound by sections treated with chondroitinase ABC (not shown). The 5-D-4 antibody too was still bound.
Testicular hyaluronidase
Results in vivo
Testicular hyaluronidase, like Streptomyces and leech hyaluronidases, increased the hydraulic conductance of the joint lining both below and above yield pressure (Fig. 4). Over the lower pressure range testicular hyaluronidase increased d /dPj from 0.54 ± 0.24 (control, n = 4) to 4.14 ± 1.06 μl min−1 cmH2O−1 (n = 7, P = 0.03). Over the higher pressure range the enzyme raised d
/dPj from 0.54 ± 0.24 (control, n = 4) to 4.14 ± 1.06 μl min−1 cmH2O−1 (n = 7, P = 0.03). Over the higher pressure range the enzyme raised d /dPj from 2.28 ± 0.14 (control, n = 5) to 13.87 ± 1.69 μl min−1 cmH2O−1 (n = 7, P < 0.001). The increase in slope caused by enzyme at the high pressures, namely to 6.68 ± 1.9 times control (n = 5), was not significantly different from the increase in slope at low pressures, namely to 5.36 ± 1.86 times control (n = 4, P = 0.55). The pressure-flow relation after testicular hyaluronidase again showed a significant increase in slope with pressure (P = 0.001), as in the control joints (P < 0.001) (Table 1).
/dPj from 2.28 ± 0.14 (control, n = 5) to 13.87 ± 1.69 μl min−1 cmH2O−1 (n = 7, P < 0.001). The increase in slope caused by enzyme at the high pressures, namely to 6.68 ± 1.9 times control (n = 5), was not significantly different from the increase in slope at low pressures, namely to 5.36 ± 1.86 times control (n = 4, P = 0.55). The pressure-flow relation after testicular hyaluronidase again showed a significant increase in slope with pressure (P = 0.001), as in the control joints (P < 0.001) (Table 1).
Histochemistry and immunohistochemistry
After treatment with testicular hyaluronidase in vivo, the synovial staining for hyaluronan was greatly reduced, but not completely eliminated (Fig. 5Ac).The antibodies 2-B-6 (Fig. 5Bb) and 3-B-3 (not shown) were still bound by synovium in tissue sections treated with chondroitinase ABC. Antibody 5-D-4 (Fig. 5Bd) was likewise still bound by synovium after testicular hyaluronidase treatment in vivo.
Comparison of the effects of testicular hyaluronidase, Streptomyces hyaluronidase, and Streptomyces hyaluronidase plus chondroitinase ABC
Since testicular hyaluronidase degrades chondroitin sulphates as well as hyaluronan, it might be expected, a priori, to have a greater effect than Streptomyces hyaluronidase on trans-synovial flows and conductance. Comparison of the flows after testicular hyaluronidase with those after Streptomyces hyaluronidases, however, revealed no significant differences at any intra-articular pressure, irrespective of whether the comparison was based on absolute flow after treatment (Fig. 6), increase in absolute flow (post-enzymatic flow minus control flow) or factorial increases in flow (post-enzymatic flow divided by control flow). Similarly, the conductances after testicular hyaluronidase were not significantly different from those after leech and Streptomyces hyaluronidases, either in the low or high pressure ranges. This was the case whether the comparison was based on absolute conductance, increase in absolute conductance caused by enzyme or factorial increase in conductance (post-enzymatic conductance divided by control conductances; Table 2).
Figure 6. Trans-synovial flows at 2.5 cmH2O intervals of intra-articular pressure after testicular hyaluronidase treatment compared with flows at the same pressures after Streptomyces hyaluronidase treatment.
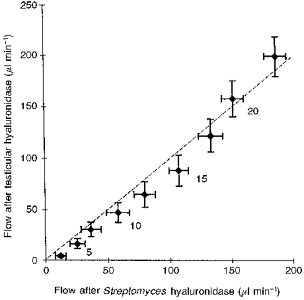
Flows (means ± s.e.m.) were interpolated to standard intra-articular pressures, which are shown in cmH2O alongside alternate points of the graph. The dashed line is the line of equality (slope = 1). The effects of the two enzymes do not differ significantly, even though testicular hyaluronidase removes chondroitin sulphate as well as hyaluronan.
Table 2.
Comparison of effects of testicular hyaluronidase and highly specific hyaluronidases (Streptomyces and leech) on synovial lining conductance dQs/dPj at low and high intra-articular pressures
| Below yield pressure | Above yield pressure | |||||
|---|---|---|---|---|---|---|
| Testicular hyaluronidase | Highly specific hyaluronidases | P | Testicular hyaluronidase | Highly specific hyaluronidases | P | |
| Absolute slopes* | 4.14 ± 1.06 (7) | 4.59 ± 0.54 (8) | 0.70† | 13.84 ± 1.69 (7) | 11.98 ± 1.37 (9) | 0.40† |
| Increase in slope** | 2.34 ± 1.16 (4) | 3.37 ± 0.52 (6) | 0.39† | 12.60 ± 2.19 (5) | 10.09 ± 1.55 (7) | 0.36† |
| Factorial increase*** | 5.36 ± 1.86 (4) | 5.69 ± 1.88 (6) | 0.76‡ | 6.68 ± 1.19 (5) | 5.16 ± 0.49 (7) | 0.34‡ |
Values are means ± s.e.m. in μl min−1 cmH2O−1. Values in parentheses, number of joints.
Value of slope dQ̇s/dPj after enzyme treatment.
Difference between control slope and slope after enzyme treatment in same animal.
Ratio of slope after enzyme treatment to control slope in same animal.
Unpaired t test
non-parametric test on ratios, Mann-Whitney U test.
DISCUSSION
Composition of synovial matrix and change after hyaluronidases
The major fibrillar components of synovial linings are collagen fibrils (types I, III and V collagens), microfibrils of type VI collagen and peri-synoviocyte type IV collagen (Ashhurst, Bland & Levick, 1991; Revell et al. 1995). In the extrafibrillar space, human synovium contains hyaluronan, the chondroitin sulphate proteoglycans biglycan and decorin, heparan sulphate proteoglycans, fibronectin, vitronectin, laminin, entactin, tenascin and fibrillin (Worrall, Bayliss & Edwards, 1991; review, Levick et al. 1996). Rabbit synovium contains hyaluronan; chondroitin 4- and 6-sulphate proteoglycans, including biglycan and decorin; and keratan sulphate proteoglycan, including fibromodulin (Coleman et al. 1998, and present results).
The immunohistochemical results show that all three hyaluronidases remove hyaluronan from the synovium (Fig. 5Ab and c), confirming previous work by Okada, Nakanishi & Kajikawa (1981). The hyaluronidases probably exerted close to their maximal effect, since (1) after the first few minutes of treatment there were no further increases in permeability with time (Fig. 1), (2) histochemistry indicated total depletion of hyaluronan by leech and Streptomyces hyaluronidases, and (3) the units used were more than sufficient to degrade 8 mg hyaluronan in the viscometer, whereas the total mass of hyaluronan in the entire synovium, which is ≤ 20 μm thick, is only of the order of 16 μg (Price et al. 1996).
Antibodies 2-B-6 and 3-B-3 recognize short stubs remaining on the core protein after treatment by chondroitinase ABC in vitro and not the main chondroitin sulphate chains (see Methods). The continued binding of these antibodies by synovium after hyaluronidase treatment in vivo shows, therefore, that the proteoglycan core proteins are still present. The persistent synovial binding of the anti-keratan sulphate antibody, 5-D-4, shows that the hyaluronidases do not deplete synovium of keratan sulphate.
Quantitative biochemical analyses show that the interstitial matrix of rabbit synovium contains 0.8 mg hyaluronan, 1.2 mg chondroitin sulphate and 2 mg heparan sulphate per millilitre of extrafibrillar space (Price et al. 1996). The total concentration of non-fibrillar biopolymers, including proteoglycans and glycoproteins, that is needed to account for synovial hydraulic resistance is ∼13.9 mg (ml extrafibrillar space)−1 according to a modelling study (Levick, 1994). Since hyaluronan constitutes only ∼6 % of the putative 13.9 mg ml−1, and chondroitin sulphates another ∼9 %, the effects of the hyaluronidases were expected, a priori, to be modest, and certainly less than observed (see below).
Increases in conductance in response to hyaluronidases
The fivefold increase in synovial hydraulic conductance caused by Streptomyces and leech hyaluronidases established that hyaluronan is a major contributor to synovial hydraulic resistance, despite its relatively low concentration. The importance of hyaluronan in determining tissue hydraulic resistance was first recognized from studies of connective tissue treated with testicular hyaluronidase, e.g. Day (1952). Hedbys (1963) found that testicular hyaluronidase raises the hydraulic conductance of corneal stroma 1.9 times on average, although a protease, trypsin, causes even bigger increases. In the trabecular drainage system of the rabbit eye, 10 U of Streptomyces hyaluronidase causes a twofold increase in permeability, while 100 U of testicular hyaluronidase causes only a 1.4-fold increase (Knepper et al. 1984). In pulmonary perivascular tissue, testicular hyaluronidase increases the permeation of albumin and dextran solutions within seconds, by between 1.6 and 269 times (Lai-Fook, Rochester & Brown, 1989). Hyaluronidase does not affect endothelial permeability (Sunnergren, Fairman, DeBlois & Glauser, 1987). The results for synovium thus fall within the wide range reported for other interstitia.
Contamination by protease was unlikely to have contributed significantly to the action of the hyaluronidases because (1) little protease activity could be demonstrated in vitro, (2) the hyaluronidases did not deplete the tissue of chondroitin sulphate proteoglycans or keratan sulphate proteoglycan, as shown by the immunohistochemical results, and (3) inclusion of protease inhibitors did not prevent the permeability-increasing effect of hyaluronidase.
A modified trans-synovial flow model and the predicted limit for conductance increases
Synovial conductance after hyaluronidase treatment is limited by the hydraulic drag of the remaining proteoglycans, glycoproteins, fibrils and cell surfaces. It is useful to estimate the maximum conductance that would arise if all extrafibrillar biopolymers were removed, leaving just cells and collagen to create hydraulic drag, because the observed effects of the hyaluronidases should fall within this limit if the model is valid. To calculate the limit, and also to enable quantitative prediction of the effect of hyaluronan depletion, the model of trans-synovial flow developed by Levick (1994) was expanded to include the three surfaces that generate the limiting hydraulic drag, namely the surfaces of collagen fibrils, microfibrils and cells; see Appendix. The last two of these were not included in the 1994 model because their contribution to total resistance is negligible when the concentration of extrafibrillar biopolymer is normal. The revised model predicted that removal of all non-collagenous biopolymers should raise synovial lining conductance below yield pressure by 106 times, to 188 μl min−1 cmH2O−1. The observed increases in lining conductance after hyaluronidase treatments were 5-6 times, to 4-5 μl min−1 cmH2O−1, and thus fell well inside the theoretical limit.
Comparison of observed and predicted conductance increases after hyaluronan removal
Although well within the theoretical limit, the conductances after hyaluronidase treatment were nevertheless puzzlingly large. Hyaluronan and chondroitin sulphate together add up to ∼2 mg (ml extrafibrillar space)−1, which is about half of the measured glycosaminoglycan mass (Price et al. 1996) and a smaller fraction of the predicted total proteoglycan- glycoprotein mass of 13.9 mg ml−1 (Levick, 1994). The revised model predicts that removal of 2 mg ml−1 of uniformly distributed glycosaminoglycan chains, simulating the action of testicular hyaluronidase, should increase synovial lining conductance 1.43 times (Fig. 7). A much larger rise, namely 5.4 times, was observed experimentally.
Figure 7. Relation between extrafibrillar biopolymer concentration and increase in synovial hydraulic conductance predicted by trans-synovial flow model (continuous curve), compared with experimental results (data points).
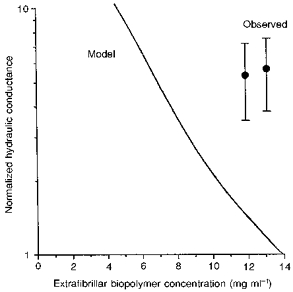
Conductances were normalized by dividing by control conductance and are plotted on a logarithmic scale since they cover an order of magnitude. The observed increase in conductance after Streptomyces hyaluronidase treatment (data point on right, means ± s.e.m.) is plotted against control biopolymer concentration minus the concentration of hyaluronan in the synovial extrafibrillar space. The observed increase in conductance after testicular hyaluronidase treatment (left point) is plotted against control concentration minus hyaluronan and chondroitin sulphate concentrations. Reading from the model curve, an effective depletion of ≈7 mg ml−1 uniformly distributed extrafibrillar biopolymer is needed to generate a 5-fold increase in conductance.
The contrast is even more striking for leech and Streptomyces hyaluronidases. The model predicts that removal of hyaluronan alone (concentration 0.8 mg ml−1 in saline-washed synovium) should increase the lining conductance 1.19 times, or by 0.33 μl min−1 cmH2O−1 (Fig. 7). The observed effect, by contrast, was 5.7 times, an increase of 3.37 μl min−1 cmH2O−1 in absolute terms (Table 2). For unwashed human synovium/subsynovium samples, Pitsillides et al. (1994) reported a 2.9 times higher concentration of hyaluronan; but even given this higher starting concentration of hyaluronan, the predicted rise in conductance upon hyaluronan removal is only 1.45 times.
The comparison of experiment and model thus indicates that the hyaluronidases have an ‘excess’ effect on hydraulic conductance, meaning one that is over and above the removal of the hydraulic drag of uniformly distributed hyaluronan chains. The identification of the ‘excess’ effect does not dependent critically on the estimate of total extrafibrillar biopolymer concentration (∼14 mg ml−1). For example, if the total extrafibrillar biopolymer concentration were only 4.1 mg ml−1, which is the glycosaminoglycan concentration reported by Price et al. (1996), the removal of 0.8 mg ml−1 hyaluronan would be a proportionately bigger (20 %) depletion of the matrix; but even so the computed increase in synovial conductance is only 1.54 times. This is because, given a lower control concentration, extrafibrillar resistance is lower, so the hydraulic drag of the microfibrils and collagen bundles exerts a non-trivial influence on overall conductance.
Hyaluronan and interstitial matrix organization
The ‘excess’ effect of hyaluronidases identified above may arise from one or more of the following factors.
Uniform versus non-uniform distribution of hyaluronan
If hyaluronan distribution were non-uniform, as reported for human synovium by Pitsillides et al. (1994) and Carley, Szczepanski & Gerritsen (1996), the hydraulic resistance would be high in regions of locally high concentration. Depending on the particular pattern of distribution, depletion might then cause a greater rise in conductance than predicted from a uniform distribution. Pitsillides et al. (1994) found, by biochemical analysis, that there was a 4-fold variation in hyaluronan concentration in human synovium/subsynovium samples from the same knee. Some sections in the present study showed uneven hyaluronan staining, but the tissue cellularity and low resolution make this difficult to interpret. A flow-induced gradient of interstitial matrix composition was proposed by Bert & Martinez (1995), but this should dissipate when flow stops, so would not be preserved in conventional histochemical sections. The concentration polarization of hyaluronan at the synovial interface during outflow, as proposed earlier (Levick et al. 1996, ‘filtercake hypothesis’), is not applicable here because the cavity contained Ringer solution, not hyaluronan solution.
Hyaluronan and the integrity of microfibrils of type VI collagen
Microfibrils composed of type VI collagen are the major structural elements in the 2-3 μm thick layer of interstitium that abuts the joint cavity (Okada et al. 1990; Levick & McDonald, 1990; Ashhurst et al. 1991). Such microfibrils bind hyaluronan. Leech or Streptomyces hyaluronidase treatment of microfibril-rich fetal skin, or of microfibrils in vitro, degrades the microfibrils to tetramers of type VI collagen and short microfibrillar sections; and the disruption of the microfibrils releases other proteins from the tissue (Kielty, Whittaker, Grant & Shuttleworth, 1992). The microfibrils partially reform on restoration of hyaluronan. Since microfibrils probably have a pivotal role in matrix organization, via interactions with fibronectin, decorin and synoviocytes (Wolf & Carsons, 1991), their disaggregation and the release of other matrix proteins could contribute to the ‘excess’ conductance increase after hyaluronidase treatment.
Other interactions between hyaluronan and matrix components
Hyaluronan plays a major role in organizing the three-dimensional structure of some interstitial matrices, e.g. cartilage, by binding to hyaluronan-binding proteins (HABPs) such as aggrecan, link protein, versican and hyaluronectin (Mason, Crossman & Sweeney, 1989; Knudson & Knudson, 1993). Disruption of the hyaluronan- aggrecan-link protein interaction in cartilage by Streptomyces hyaluronidase causes marked ultrastructural changes (Poole, Pidoux, Reiner & Rosenberg, 1982). Versican, a chondroitin sulphate proteoglycan present in many connective tissues, but not yet studied in synovium, also binds strongly to hyaluronan. In umbilical cord, hyaluronan degradation by Streptomyces hyaluronidase leads to the release of sulphated glycosaminoglycans from the tissue (Meyer et al. 1983). Our positive immunohistochemical results for sulphated glycosaminoglycans after Streptomyces hyaluronidase do not preclude a partial escape of proteoglycans, because the method is qualitative rather than quantitative.
Hyaluronan as a cell-to-matrix link
Hyaluronan can link matrix components to the cell surface, because some cell surface receptors are HABPs (Knudson & Knudson, 1993). One example is the glycoprotein CD44 on synoviocytes (Henderson, Pitsillides, Edwards & Worrall, 1993). However, in pilot experiments on two rabbits, intra-articular injections of mouse anti-rabbitCD44 monoclonal antibody (10 μg; Serotec, Kiddlington, UK) to block the receptor did not significantly increase trans-synovial flow (test flow/control flow = 1.4 ± 0.3, n = 16; P = 0.6, paired t test; authors’ unpublished results).
Altered matrix mechanics and hydration
Matrix hydration is a major determinant of hydraulic conductance (Bert & Martinez, 1995). The degree of hydration depends on the balance between glycosaminoglycan swelling pressure and a restraining force exerted by fibrils or microfibrils. In Wharton's jelly, for example, proteolytic disruption of a network of glycoprotein microfibrils causes marked tissue swelling (Meyer et al. 1983). If microfibrils of type VI collagen have a comparable role in synovium, their disruption by hyaluronidase would allow tissue hydration and conductance to increase.
Hyaluronidase thus has the potential, albeit unproven as yet in synovium, to disrupt tissue organization at several levels, and so raise the hydraulic permeability by additional mechanisms besides direct elimination of the hydraulic drag of hyaluronan chains.
Testicular versus leech and Streptomyces hyaluronidases
Testicular hyaluronidase hydrolyses chondroitin sulphate chains as well as hyaluronan (Meyer, 1971; Knepper et al. 1984), yet its effect on conductance was no greater than that of leech or Streptomyces hyaluronidase. This cannot be attributed to the traces of hyaluronan remaining after testicular hyaluronidase treatment (Fig. 5Ac), because chondroitinase ABC and Streptomyces hyaluronidase together had no greater effect on permeability than did testicular hyaluronidase, or Streptomyces hyaluronidase alone. The inference from these results is that chondroitin sulphate depletion lacks the ‘amplifying’ effects of hyaluronan depletion, so the latter dominates the response to testicular hyaluronidase, or to chondroitinase ABC and Streptomyces hyaluronidase together. In keeping with this interpretation, chondroitin sulphate depletion by chondroitinase ABC does not depolymerize type VI collagen microfibrils (Kielty et al. 1992), and has less effect than testicular or Streptomyces hyaluronidase on synovial conductance (Scott et al. 1998).
Conclusions
(1) Interstitial hyaluronan makes a major contribution to the hydraulic resistance of synovium, and hence to the role of synovium in retaining synovial fluid within the joint cavity.
(2) The contribution of hyaluronan to the hydraulic resistance of synovium greatly exceeds the hydraulic drag of evenly distributed hyaluronan chains.
Acknowledgments
We thank Professor B. Caterson (University of Wales, Cardiff, UK) for gifts of monoclonal antibodies, Professor M. Bayliss (Royal Veterinary College, London) for the gift of the biotinylated hyaluronan binding region of aggrecan, and Miss Y. S. Bland (St George's Hospital Medical School, London) for assistance with the immunohistochemistry. The work was supported by The Wellcome Trust grant 039033/Z/93.
APPENDIX
Additions to models of trans-synovial flow
The model of Levick (1994) uses biophysical data on the resistivity of glycosaminoglycans in vitro and, for other elements, resistivities calculated by the Happel-Carman- Kozeny theory in order to predict trans-synovial flow at a given intra-articular pressure (Levick, 1994). Flows are calculated from the pressure gradients and resistances in the system using Darcy's law for interstitial flow and the Starling principle for transcapillary fluid exchange. The model is a distributed one, i.e. it incorporates tissue geometry (path lengths, areas, capillary density, etc.). The modifications to the 1994 model were as follows.
Collagen fibril organization
This has a strong impact on interstitial porosity and hydraulic resistance (Bert & Martinez, 1995). Synovial collagen fibrils were formerly modelled in a random distribution, but later work showed that most synovial fibrils are clustered in bundles (fibres) of diameter ∼500 nm. The fibrils are packed so tightly in a bundle that the resistance to flow through the bundle exceeds the resistance to flow around it (Price, Mason & Levick, 1995). To incorporate this into the model, the interstitial space available for flow was reduced by subtracting the bundle volume fraction (0.4), and the hydraulic drag of cylindrical collagen bundles of radius 250 nm and volume fraction 0.4 was calculated by the Happel-Carman-Kozeny theory. This modification only affects the model output significantly when extrafibrillar glycosaminoglycans, etc. are depleted. In agreement with Bert & Martinez (1995), the model predicted that, in the absence of extrafibrillar biopolymer, the conductance of synovium with collagen as bundles is ∼175 times greater than the conductance of synovium with randomly distributed fibrils.
Synovial microfibrils
The hydraulic drag created by the microfibrils, radius 4.65 nm, was calculated to be 1.5 × 199 dyn s cm−4 at 20°C (Levick & McDonald, 1990). This is considerably greater than the drag attributable to the collagen bundles, 0.06 × 109 dyn s cm−4, and was incorporated in the model.
Cell surfaces
The hydraulic drag due to the cell surfaces was calculated from the measured cell volume fraction of 0.34 and an idealized cylindrical geometry (radius, 3 μm; length, 10 μm), using the Happel-Carman-Kozeny theory (Levick, 1987). The additional drag due to the cells is so low as to be almost negligible, namely 0.0005 × 109 dyn s cm−4.
References
- Ashhurst DE, Bland Y, Levick JR. An immunohistochemical study of the collagens of rabbit synovial interstitium. Journal of Rheumatology. 1991;18:1669–1672. [PubMed] [Google Scholar]
- Bert JL, Martinez M. Interstitial fluid transport. In: Reed RK, McHale NG, Bert JL, Winlove CP, Laine GA, editors. Interstitium, Connective Tissue and Lymphatics. London: Portland Press; 1995. pp. 101–117. [Google Scholar]
- Carley WW, Szczepanski A, Gerritsen ME. Cytokeratin expression and hyaluronic acid production in cultures of human synovial microvascular endothelial cells; influence of cytokines and growth factors. Microcirculation. 1996;3:359–370. doi: 10.3109/10739689609148308. [DOI] [PubMed] [Google Scholar]
- Caterson B, Christner JE, Baker JR, Couchman D. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Federation Proceedings. 1985;44:386–393. [PubMed] [Google Scholar]
- Coleman PJ, Kavanagh E, Mason RM, Levick JR, Ashhurst DE. The proteoglycans and glycosaminoglycan chains of rabbit synovium. Histochemical Journal. 1998;30 doi: 10.1023/a:1003291303380. in the Press. [DOI] [PubMed] [Google Scholar]
- Day TD. The permeability of interstitial connective tissue and the nature of the interfibrillary substance. The Journal of Physiology. 1952;117:1–8. [PMC free article] [PubMed] [Google Scholar]
- Harrisson F, Van Hoof J, Vanroelen C. On the presence of proteolytic activity in glycosaminoglycan-degrading enzyme preparations. Journal of Histochemistry and Cytochemistry. 1986;34:1231–1235. doi: 10.1177/34.9.3525669. [DOI] [PubMed] [Google Scholar]
- Hedbys BO. Corneal resistance to the flow of water after enzymatic digestion. Experimental Eye Research. 1963;2:112–121. doi: 10.1016/s0014-4835(63)80002-0. [DOI] [PubMed] [Google Scholar]
- Henderson KJ, Pitsillides AA, Edwards JCW, Worrall JG. Reduced expression of CD44 in rheumatoid synovial cells. British Journal of Rheumatology. 1993;32(suppl. 1):25. [Google Scholar]
- Kielty CM, Whittaker SP, Grant ME, Shuttleworth CA. Type VI collagen microfibrils: evidence for a structural association with hyaluronan. Journal of Cell Biology. 1992;118:979–990. doi: 10.1083/jcb.118.4.979. 10.1083/jcb.118.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepper PA, Farbman AI, Telser AG. Exogenous hyaluronidases and degradation of hyaluronic acid in the rabbit eye. Investigative Ophthalmology and Visual Science. 1984;25:286–293. [PubMed] [Google Scholar]
- Knudsen CB, Knudsen W. Hyaluronan-binding proteins in development, tissue homeostasis and disease. FASEB Journal. 1993;7:1233–1241. [PubMed] [Google Scholar]
- Lai-Fook SJ, Rochester NL, Brown LV. Effects of albumin, dextran and hyaluronidase on pulmonary interstitial conductivity. Journal of Applied Physiology. 1989;67:606–613. doi: 10.1152/jappl.1989.67.2.606. [DOI] [PubMed] [Google Scholar]
- Levick JR. The influence of hydrostatic pressure on trans-synovial fluid movement and on capsular expansion in the rabbit knee. The Journal of Physiology. 1979;289:69–82. doi: 10.1113/jphysiol.1979.sp012725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick JR. Flow through interstitium and other fibrous matrices. Quarterly Journal of Experimental Physiology. 1987;72:409–438. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- Levick JR. An analysis of the interaction between extravascular plasma protein, interstitial flow and capillary filtration; application to synovium. Microvascular Research. 1994;47:90–125. doi: 10.1006/mvre.1994.1007. 10.1006/mvre.1994.1007. [DOI] [PubMed] [Google Scholar]
- Levick JR, McDonald JN. The microfibrillar meshwork of the synovial lining and associated broad-banded collagen – a clue to identity. Annals of Rheumatic Diseases. 1990;49:31–36. doi: 10.1136/ard.49.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick JR, Price FM, Mason RM. Synovial matrix-synovial fluid system of joints. In: Comper WD, editor. Extracellular Matrix, Tissue Function. Vol. 1. Amsterdam: Harwood Academic Publishers; 1996. pp. 328–377. [Google Scholar]
- Mason RM, Crossman MV, Sweeney C. Hyaluronan and hyaluronan-binding proteins in cartilaginous tissues. In: Evered D, Whelan J, editors. The Biology of Hyaluronan, Ciba Foundation Symposium. Vol. 143. Chichester, UK: John Wiley & Sons; 1989. pp. 107–120. [DOI] [PubMed] [Google Scholar]
- Mehmet H, Scudder P, Tang PW, Hounsell EF, Caterson B, Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve hepta- or larger oligosaccharides of the poly-(N-acetyllactosamine) series. European Journal of Biochemistry. 1986;157:385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- Meyer FA, Laver-Rudich Z, Tanenbaum R. Evidence for a mechanical coupling of glycoprotein microfibrils with collagen fibrils in Wharton's jelly. Biochimica et Biophysica Acta. 1983;755:376–387. doi: 10.1016/0304-4165(83)90241-6. [DOI] [PubMed] [Google Scholar]
- Meyer K. Hyaluronidases. In: Boyer PD, editor. The Enzymes. Vol. 5. New York: Academic Press; 1971. pp. 307–320. chap. 11. [Google Scholar]
- Ohya T, Kaneko Y. Novel hyaluronidase from Streptomyces. Biochimica et Biophysica Acta. 1970;198:607–609. doi: 10.1016/0005-2744(70)90139-7. 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Okada Y, Naka K, Minamoto T, Ueda Y, Oda Y, Nakanishi I, Timpl R. Localization of type VI collagen in the lining cell layer of normal and rheumatoid synovium. Laboratory Investigation. 1990;63:647–656. [PubMed] [Google Scholar]
- Okada Y, Nakanishi I, Kajikawa K. Ultrastructure of the mouse synovial membrane; development and organisation of the extracellular matrix. Arthritis and Rheumatism. 1981;24:835–843. doi: 10.1002/art.1780240611. [DOI] [PubMed] [Google Scholar]
- Paul WD, Hodges RE, Knouse RW, Wright CS. Effect of anti-rheumatic drugs on synovial membrane permeability. Proceedings of the Society for Experimental Biology and Medicine. 1952;79:68–71. doi: 10.3181/00379727-79-19275. [DOI] [PubMed] [Google Scholar]
- Pitsillides AA, Worrall JG, Wilkinson LS, Bayliss MT, Edwards JCW. E0yaluronan concentration in non-inflamed and rheumatoid synovium. British Journal of Rheumatology. 1994;33:5–10. doi: 10.1093/rheumatology/33.1.5. [DOI] [PubMed] [Google Scholar]
- Poole AR, Pidoux I, Reiner A, Rosenberg L. An immunoelectron microscope study of the organization of proteoglycan monomer, link protein and collagen in the matrix of articular cartilage. Journal of Cell Biology. 1982;93:921–937. doi: 10.1083/jcb.93.3.921. 10.1083/jcb.93.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price FM, Levick JR, Mason RM. Glycosaminoglycan concentration in synovium and other tissues of rabbit knee in relation to hydraulic resistance. The Journal of Physiology. 1996;495:803–820. doi: 10.1113/jphysiol.1996.sp021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price FM, Mason RM, Levick JR. Radial organization of interstitial exchange pathway and influence of collagen in synovium. Biophysical Journal. 1995;69:1429–1439. doi: 10.1016/S0006-3495(95)80012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell PA, Al-Saffar N, Fish S, Osei D. Extracellular matrix of the synovial intimal cell layer. Annals of the Rheumatic Diseases. 1995;54:404–407. doi: 10.1136/ard.54.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnan GP, MacLachlan MJ. The absorption of serum albumin and gammma globulin from the knee joint of man and rabbit. Arthritis and Rheumatism. 1960;3:152–157. doi: 10.1002/art.1780030206. [DOI] [PubMed] [Google Scholar]
- Scott D, Coleman PJ, Abiona A, Ashhurst DE, Mason RM, Levick JR. Effects of depletion of glycosaminoglycans and non-collagenous proteins on interstitial hydraulic permeability in rabbit knee synovium. The Journal of Physiology. 1998 doi: 10.1111/j.1469-7793.1998.629bh.x. in the Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Coleman PJ, Mason RM, Levick JR. Glycosaminoglycan depletion greatly raises the hydraulic permeability of the joint synovial lining. Experimental Physiology. 1997;82:603–606. doi: 10.1113/expphysiol.1997.sp004050. [DOI] [PubMed] [Google Scholar]
- Seifter J, Baeder DH, Begany AJ. Influence of hyaluronidase and steroids on permeability of synovial membrane. Proceeding of the Society for Experimental Biology and Medicine. 1949;72:277–282. doi: 10.3181/00379727-72-17406. [DOI] [PubMed] [Google Scholar]
- Sunnergren KP, Fairman RP, DeBlois GG, Glauser FL. Effects of protamine, heparinase and hyaluronidase on endothelial permeability and surface charge. Journal of Applied Physiology. 1987;63:1987–1992. doi: 10.1152/jappl.1987.63.5.1987. [DOI] [PubMed] [Google Scholar]
- Wilson R, Pitt T, Taylor G, Watson D, MacDermot J, Sykes D, Roberts D, Cole P. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. Journal of Clinical Investigation. 1987;79:221–229. doi: 10.1172/JCI112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J, Carsons S. Distribution of type VI collagen expression in synovial tissue and cultured synoviocytes: relation to fibronectin expression. Annals of the Rheumatic Diseases. 1991;50:493–496. doi: 10.1136/ard.50.7.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall JG, Bayliss MT, Edwards JCW. E0orphological localization of hyaluronan in normal and diseased synovium. Journal of Rheumatology. 1991;18:1466–1472. [PubMed] [Google Scholar]


