Abstract
This study was performed to elucidate the type of afferents that mediate the multiple actions of 5-hydroxytryptamine (5-HT) on mesenteric nerve discharge. Electrophysiological recordings were made from mesenteric afferents innervating the mid-jejunum of the urethane-anaesthetized rat. The discharge of single nerves within the whole nerve recording was monitored using waveform discrimination software.
Afferents responded to 5-HT in one of two ways: a short latency, transient excitation mediated by 5-HT3 receptors, or a delayed onset, more prolonged effect that was 5-HT2A receptor mediated. Afferents showing the 5-HT3-mediated response did not respond to luminal distension but were sensitive to intraluminal hydrochloric acid (150 mM) in twenty-eight of twenty-nine experiments. In eight experiments, the 5-HT3-mediated response was reversibly abolished by a 2 min exposure to intraluminal application of local anaesthetic (2 % Xylocaine).
Mechanosensitive afferents which responded to distension (< 10 cmH2O) did not show a 5-HT3-mediated response (P = 0.92, n = 14), and maintained this mechanosensitivity after luminal anaesthesia. Mechanosensitive afferents did show a secondary response to 5-HT that was significantly attenuated by atropine (100-200 μg kg−1), whereas hexamethonium (8 mg kg−1) had no effect.
In animals whose vagal afferent contribution to their mesenteric nerves had been eliminated by chronic truncal vagotomy, the 5-HT3-mediated response was absent in thirty-six of thirty-six nerve bundles. In contrast, mechanosensitivity to distension and the secondary response to 5-HT could still be evoked.
These results suggest that 5-HT stimulates mesenteric afferents by a direct action on 5-HT3 receptors that are present on vagal mucosal afferent terminals. The mucosal afferent response to luminal acid, however, was unaffected by treatment with granisetron (0.5 mg kg−1) indicating that endogenous 5-HT from enterochromaffin cells is not essential for transduction of this luminal signal. In contrast, mechanosensitivity in non-vagal afferents was modulated by 5-HT following an intestinal motor response which was influenced by cholinergic tone.
5-Hydroxytryptamine (5-HT) has widespread actions within the gastrointestinal tract with effects on both neural and non-neural target tissue. We have recently demonstrated that 5-HT stimulates mesenteric afferent nerve bundles supplying the rat jejunum via two different 5-HT receptor subtypes which appear to be located on two distinct populations of neurones innervating the intestine (Hillsley, Kirkup & Grundy, 1998). The most dramatic action of 5-HT is due to the stimulation of 5-HT3 receptors since the 5-HT response can be mimicked by the 5-HT3 receptor agonist 2-methyl-5-HT and abolished by the 5-HT3 receptor antagonist granisetron. This is likely to be a direct action on the afferent nerve terminal since the response to 5-HT persisted after treatment with L- and N-type calcium channel blockers (Hillsley et al. 1998) However, in approximately one-third of mesenteric bundles, this initial response was followed by a secondary response to 5-HT that was mediated by 5-HT2A receptors and attenuated by nifedipine, which also blunted the mechanical response to 5-HT (Hillsley et al. 1998).
Mesenteric bundles contain afferents which project to the spinal cord and brain stem via splanchnic and vagal pathways, respectively. In addition, there is a population of myenteric neurones whose axons project to prevertebral ganglia. These are predominantly second-order neurones synaptically driven by intrinsic sensory neurones, but for the purpose of the present study will be referred to as myenteric afferents. The relative contribution of these three different afferent populations to the mesenteric sensitivity to 5-HT has not been elucidated. In this respect, intestinal afferents include mechanosensitive afferents which respond to distension and contraction of the muscle wall. These mechanoreceptors have been described in both vagal and splanchnic pathways, and, while the stimulus response characteristics of these two populations have not been systematically compared, it is generally accepted that the latter have higher thresholds for activation than the former, and are responsible for sensations of gastrointestinal origin as well as the afferent limb for reflex activation (Sengupta & Gebhart, 1994). Myenteric afferents are also mechanosensitive, as inferred from the synaptic activity recorded from their target cells in the prevertebral ganglia (Anthony & Kreulen, 1990; Miller & Szurszewski, 1997). However, these myenteric afferents are themselves synaptically driven from other enteric neurones as demonstrated by the marked reduction in synaptic input to prevertebral ganglion cells after treatment with nicotinic receptor antagonists. Other afferent endings superficially located within the mucosa do not respond to distension but are sensitive to mechanical and chemical stimuli applied to the mucosa. These endings have multimodal sensitivity and respond whenever the luminal environment differs from neutral pH, isosmolarity or contain nutrients (Grundy, 1988). Recent confocal microscopic studies using fluorescent, lipophilic carbocyanine dyes injected into the nodose ganglia have revealed the detailed morphology of sensory nerve terminals within the gut wall (Berthoud, Kressel, Raybould & Neuhuber, 1995; Berthoud & Patterson, 1996). In the mucosa the terminal arborizations come close to the basal lamina but do not penetrate it to make contact with epithelial cells. This morphological arrangement has implications for the transduction process such that 5-HT released from enterochromaffin cells has been proposed as an intermediary step between the luminal signal and afferent impulse generation (Kirchgessner, Tamir & Gershon, 1992; Grider, Kuemmerle & Jin, 1996). Electrophysiological data obtained by intracellular recordings from myenteric sensory neurones which respond to mucosal application of 5-HT and acidic solutions would support this view (Bertrand, Kunze, Bornstein, Furness & Smith, 1997). However, a role for 5-HT in the chemosensitivity of extrinsic afferents has not been established.
The aim of the present study was therefore twofold. Firstly, to identify the relative sensitivity of the different mesenteric afferent subpopulations to 5-HT using a series of protocols designed to characterize afferents according to their mechanosensitivity and chemosensitivity, the relative location of the endings within the gut wall as gauged by sensitivity to luminal anaesthesia, the extent to which they are influenced by cholinergic blockade, and the pathways the afferent fibres follow to the CNS. Secondly, to examine the role of 5-HT in the signal transduction process for chemosensitivity to luminal acid.
METHODS
Experiments were performed on Sheffield strain male Wistar rats (350-400 g) allowed free access to food and water. Animals were anaesthetized with a single intraperitoneal injection of urethane (1.5 g kg−1). After tracheal cannulation, the left external jugular vein was cannulated to enable the administration of drugs and further anaesthetic (0.1 ml of 25 % urethane) if a limb withdrawal reflex developed. An overdose of anaesthetic was administered by this route to kill the animals at the end of the experiment. The exposed right carotid artery was cannulated in order to monitor systemic blood pressure. Body temperature was monitored (via a rectal thermometer) and maintained at around 37°C using a hot lamp. The neck incision was covered with cotton wool kept moist with 0.9 % saline.
Abdominal surgery
A mid-line laparotomy was performed and the caecum was removed to create a greater operating field. A 15 cm loop of mid-jejunum was located, typically 30 cm distally from the pylorus. The intestinal loop was cannulated with polythene tubing through stab incisions in the left side of the abdominal wall. A single cannula was inserted proximally to allow the infusion of isothermally maintained test solutions. A second double-bored cannula was inserted distally allowing drainage of luminal fluids aborally, with one port being connected to a pressure transducer (Elcomatic EM760) to allow the intraluminal pressure to be monitored. Test solutions were all perfused through the intestinal loop at a rate of 5 ml min−1. The muscle and skin comprising the abdominal wall were then sewn to a circular metal ring to create a well, and the abdominal cavity was filled with colourless light liquid paraffin prewarmed to 37°C. The stab incisions in the side of the abdominal wall were sutured to prevent leakage of the paraffin.
Nerve preparation and recording
A single neurovascular bundle was isolated from the surrounding connective tissue and placed upon a black Perspex platform. Under a viewing microscope, a mesenteric nerve was exposed by dissection of the overlying fat and surrounding blood vessels. The nerve was then sectioned at the proximal end of the bundle (approx. 10-15 mm from the serosal edge) and wrapped around one arm of a bipolar platinum electrode, with connective tissue wrapped around the second, indifferent electrode. The electrodes were connected to a Neurolog headstage (NL100) and then, via a 500 × preamplifier, the signal was differentially amplified (NL103) and filtered with a band width of 100-1000 Hz (NL125). The whole nerve signal was displayed on a storage oscilloscope (Tektronix 5111A) and also digitized (PCM-2 A/D VCR Adapter, Medical Systems Corp.), and stored on a VHS video recorder for postexperiment analysis. On-line, the signal was relayed to a spike processor (Digitimer D130) which discriminates action potentials from noise with a variable amplitude and polarity window allowing whole nerve discharge to be quantified. The output from the spike processor, along with systemic blood pressure and intestinal pressure, was displayed on the monitor of a computer running Spike 2 software (version 4.70, Cambridge Electronic Design (CED)) via a 1401+ interface board (CED).
Off-line analysis
The multiunit mesenteric afferent recordings contained action potentials of different amplitudes and waveforms, some of which were sufficiently different from each other to be accurately discriminated using computerized waveform analysis. This off-line analysis of the afferent activity in the mesenteric nerves was performed using Spike 2 software as described and validated previously (Richards, Hillsley, Eastwood & Grundy, 1996; Hillsley et al. 1998). The nerve signal was digitally sampled at 23 kHz which was sufficient to allow accurate spike discrimination. Each spike above a given amplitude was used to set up templates for the individual action potentials. Action potential waveforms were automatically averaged, DC offset arising from noise removed, and the resulting spike shapes assigned to different waveform templates. The afferent recording was subsequently analysed such that each action potential was compared with the waveform template and either matched to one or left unclassified. The timing of each template-matched spike was then used to calculate firing frequency and to plot discharge frequency against time. Tolerance was variable, but typically the allowed amplitude error was set at between 1 and 2 %, and for a spike to be matched at least 85 % of the data points had to fall within the template shape. These relatively rigid parameters were shown empirically to discriminate action potentials accurately, but at high firing frequencies a small proportion of individual spikes, typically < 5 %, could be missed because of summation. However, this under-estimate of spike frequency was obviously preferable to less rigorous discrimination configurations in which ‘cross-contamination’ could occur. The software allowed as many as eight templates to be simultaneously sampled but in our experience the system was most reliable dealing with fewer (typically 1-4 templates) of the relatively larger amplitude spikes. Once stored on computer, spike discrimination was checked manually by overlaying templates and spikes and compared by eye. Ambiguous spikes could be reassigned to a different template or ignored.
Chronic subdiaphragmatic vagotomy
In twelve cases, experiments were performed on rats 7-14 days after they had undergone chronic subdiaphragmatic vagotomy under aseptic conditions. The animals were anaesthetized with a single intraperitoneal injection of a mixture of 100 mg kg−1 ketamine and 7 mg kg−1 xylazine. If necessary during surgery the anaesthetic was added dropwise directly onto the mesenteric vasculature.
A mid-line laparotomy was performed and the abdominal wall retracted to expose the intestines. The stomach and liver were manoeuvred to expose the oesophagus as it emerged from the diaphragm. The two vagal branches running either side of the oesophagus were carefully separated from the oesophageal wall, ligated and sectioned. The abdominal wall was subsequently sutured using sterilized surgical catgut (B.P.68, Davis and Geck), as was the skin using Dexon (polyglycolic acid) (3-0, Davis and Geck). During the recovery period before experiments were performed, the body weight and general health of the animals were monitored daily.
To confirm that the subdiaphragmatic vagotomy had been effective in removing the vagal innervation of the jejunum, the peripheral end of the right cervical vagus nerve was stimulated at the end of the experiment. In control animals vagal stimulation caused a profound bradycardia, and in addition, an increase in the intestinal pressure, typically of 1-1.5 cmH2O. However, in vagotomized animals, although the bradycardia was still observed, vagal stimulation had no effect on jejunal pressure, demonstrating that the vagotomy was successful.
Experimental protocols
An initial test stimulus of 10 μg 5-HT was administered once a stable afferent recording had been secured. The intravenous cannula was preloaded with a 50 μg ml−1 solution, thus avoiding the need to flush in with saline. Subsequent administration of 2-methyl-5-HT (10 μg) was performed in the same way. If there was no initial response to 5-HT the nerve bundle was usually discarded and a different nerve was dissected out and the procedure repeated. However, in eight experiments the nerve was retained and the effects of distension were tested by intraluminal infusion of 2-3 ml saline. In all other experiments, distension was not normally performed until the end of the experiment since afferent recordings in such close proximity to the site of distension were unstable.
Responses to intraluminal acid were obtained by perfusing the loop with 150 mM HCl at 5 ml min−1 for 90 s. Excess fluid was allowed to drain from the distal cannula thereby minimizing any mechanical stimulation during this procedure. After 2 min the intestinal loop was perfused with 15 ml 0.9 % saline (at 5 ml min−1) during which time afferent discharge returned to baseline. Reproducible responses to acid could be evoked at 15 min intervals, which enabled responses to acid to be compared before and after treatment with the 5-HT3 receptor antagonist granisetron at a dose of 0.5 mg kg−1.
A recovery period of at least 5 min was allowed between successive injections of 5-HT or 2-methyl-5-HT except in the case of the luminal anaesthesia experiments in which three consecutive injections of 5-HT were delivered at 1 min intervals. However, in control studies we have shown that the afferent response to 5-HT does not desensitize in vivo even with injection intervals as low as 30 s, a finding similar to that seen in epithelial transport studies (Hardcastle, Hardcastle, Carstairs & Franks, 1994).
Drugs
The following drugs were obtained from Sigma: 5-hydroxytryptamine (5-HT), capsaicin, ethyl carbamate (urethane), hexamethonium HCl, xylazine, 2-methyl-5-HT, sodium chloride (NaCl), and hydrochloric acid (HCl). Atropine sulphate was purchased from BDH, Xylocaine (lignocaine hydrochloride) from Astra Pharmaceuticals and ketamine from Parke Davis. Granisetron was a gift from SmithKline Beecham. All drugs were dissolved in 0.9 % saline apart from capsaicin which was dissolved in Tween 80, ethanol and saline (20:20:80 v/v).
Data analysis
Baseline spike discharge in impulses per second was obtained by averaging thirty consecutive 1 s bins immediately before any experimental procedures. Values for discharge frequency following drug administration or during distension represent the mean discharge within the timeframe of the delineated response boundaries. The response to luminal acid was quantified as mean discharge in consecutive minutes from the onset of the perfusion. Data are shown as the mean ± standard error of the mean. Significance tests were carried out using the appropriate Student's paired or unpaired t test with Bonferroni corrections applied when multiple comparisons were made. P < 0.05 was considered significant.
RESULTS
Experiments were performed on thirty-six mesenteric nerve bundles dissected from control animals. The response to 10 μg 5-HT comprised an initial intense but transient burst of impulses which was present in twenty-three of thirty-six bundles, and a delayed, less powerful but more prolonged secondary activation in thirteen of thirty-six nerve bundles. Thus, in seventeen bundles only the initial 5-HT response was recorded, in seven bundles only the secondary response to 5-HT was present and in six bundles both the initial and secondary 5-HT responses were present (see Fig. 1). In six bundles there was no response to 5-HT whatsoever. Different populations of afferents, as determined by single unit analysis, mediated these two components of the response which have been further separated on the basis of their receptor pharmacology. The initial response is mimicked by the 5-HT3 receptor agonist 2-methyl-5-HT while the secondary activation is mimicked by 5-methoxytryptamine (Hillsley et al. 1998).
Figure 1. Representative traces of the cardiovascular, intestinal motor and mesenteric afferent responses to 5-HT (10 μg), distension and intraluminal acid (150 mM HCl).
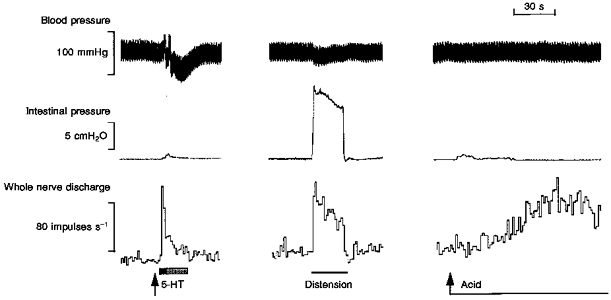
The afferent response to 5-HT consisted of a brief but pronounced initial increase in firing (highlighted by the filled area) followed by a more prolonged secondary component (highlighted by the shaded area), the latter occurring coincident with a small increase in intraluminal pressure. Rapid distension caused a slowly adapting increase in afferent discharge which returned rapidly to baseline on draining of the intestinal loop. Perfusion of the intestinal segment with acid caused a slowly developing increase in firing which was well maintained. The response to acid occurred in the absence of any increase in intestinal pressure.
All nerve bundles, irrespective of their sensitivity to 5-HT, contained mechanosensitive afferents. This was evident from the spontaneous oscillation in afferent discharge in phase with contractile activity (when present), and the marked immediate elevation in spike discharge in response to luminal distension with isotonic saline (Fig. 1). In all except one experiment (28/29), intraluminal perfusion with 150 mM HCl also evoked a marked elevation in afferent discharge after a mean latency of 39.7 ± 4.2 s (n = 28) from the onset of acid infusion. Afferent discharge persisted during the maintained acid infusion, often showing an intense bursting pattern which was unrelated to luminal pressure events, and continued for several minutes after the acid had been flushed out with isotonic saline (Fig. 1). Mean whole nerve discharge was typically increased 4- to 6-fold during the second minute of the acid response.
The mechanical and chemical sensitivity of the different 5-HT-sensitive populations was determined by comparing the sensitivity of single units, extracted from the whole nerve discharge using waveform analysis, to 5-HT, acid and distension.
Sensitivity of single units to distension
Single unit analysis was performed on fourteen distension-sensitive afferents whose spike shape and amplitude were such to enable their accurate discrimination. Baseline discharge in these units was 0.7 ± 0.1 impulses s−1 and increased 5-fold to 3.7 ± 0.9 impulses s−1 during distension (P < 0.005). None of these distension-sensitive afferents showed an initial 5-HT response or responded to the 5-HT3 agonist 2-methyl-5-HT. In contrast, single units which showed an initial response to 5-HT or responded to 2-methyl-5-HT were unaffected by distension (control, 1.2 ± 0.2 impulses s−1; distension, 1.2 ± 0.3 impulses s−1; n.s.). An example of mutually exclusive 5-HT-sensitive and distension-sensitive single units discriminated from the same nerve bundle is shown in Fig. 2.
Figure 2. Single unit analysis of mesenteric afferent responses to 5-HT and distension.
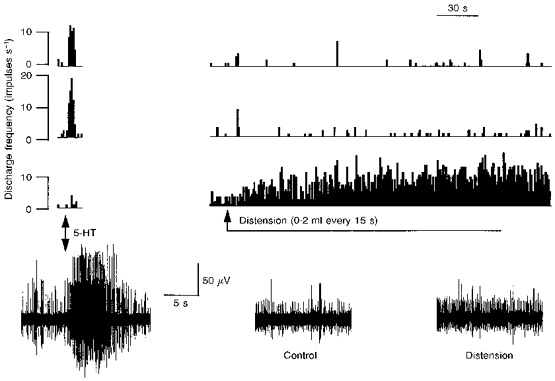
The bottom traces are snapshots of the raw nerve recordings showing (from left to right) the response to 5-HT (10 μg), baseline discharge (Control), and the response during distension. Note that two spikes can be readily recognized as dominating the 5-HT response while a smaller spike has a pronounced increase in firing during distension. These 3 ‘single’ units were isolated using waveform analysis and sequential rate histograms for each are plotted above the recordings. The upper two are the units which responded to 5-HT and these were unresponsive to distension. The lower of the 3 histograms shows the distension-sensitive fibre which did not respond to 5-HT.
Sensitivity of single units to luminal acid
The response to luminal acidification was analysed in fourteen single units. The spontaneous activity of these afferents was 0.9 ± 0.2 impulses s−1 and was significantly increased to 3.6 ± 0.7 impulses s−1 in the second minute of the acid flush (P < 0.01; Fig. 3). All fourteen acid-sensitive afferent units showed a short duration initial response to 5-HT (10 μg) with a mean response discharge of 14.9 ± 1.0 impulses s−1. In contrast, none of the distension-sensitive afferents were stimulated by luminal acid. The relative magnitude of the response of these units to 5-HT, acid and a saline flush is compared in Fig. 4.
Figure 3. Single unit analysis of the acid response.
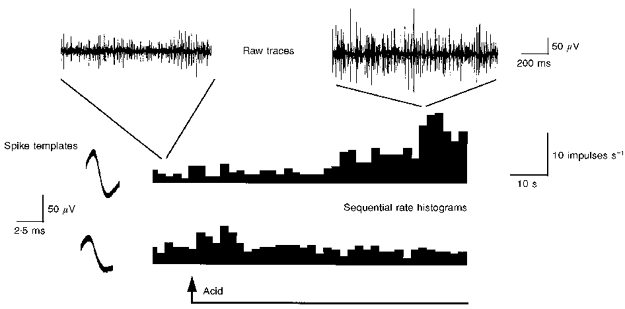
The top traces show brief excerpts of the raw mesenteric afferent recording just prior to and at the peak of the response to acid (150 mM HCl). Two single units (with distinctive spike waveforms that were used to create the spike templates illustrated) were extracted from the recording and the rate histograms for these two units are shown at the bottom. One of these units was sensitive to luminal perfusion with acid while the other showed a brief response at the onset of the perfusion and was subsequently confirmed to be distension sensitive.
Figure 4. Histogram showing the magnitude of the response of single afferent units to 5-HT and luminal perfusion with acid or saline.
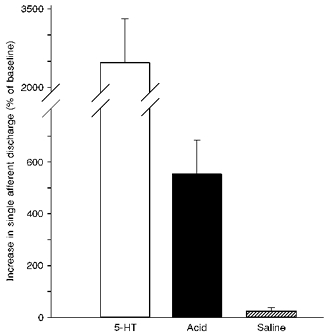
Data have been normalized to the baseline discharge of individual afferents. These afferents showed a more than 5-fold increase in afferent firing during the second minute of acid (150 mM HCl) perfusion while a similar perfusion with saline was without effect. The response to acid was considerably less than the peak discharge in response to 5-HT (10 μg) with a 25-fold increase in these afferents.
In four experiments the effect of 0.5 mg kg−1 granisetron on the acid response was examined. The initial response of nine single afferent units to 5-HT was completely abolished by granisetron, and the spontaneous discharge was reduced from 1.1 ± 0.4 to 0.7 ± 0.2 impulses s−1. The acid response was also slightly reduced following injection of granisetron but neither the decrease in baseline discharge nor the attenuation of the acid response reached significance (Fig. 5).
Figure 5. The response to acid is independent of 5-HT3 receptors.
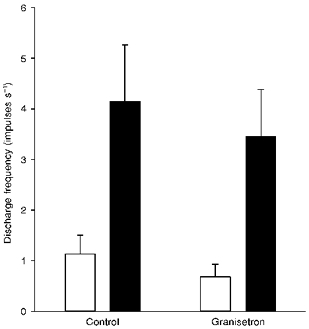
Comparison of the baseline discharge (□) and response to luminal acid (150 mM HCl; ▪) before (Control) and after treatment with granisetron (0.5 mg kg−1).
Effect of luminal anaesthesia
That 5-HT-sensitive afferents respond to luminal acid but not distension can be taken as evidence for receptive fields in close proximity to the mucosal epithelium. To further investigate this possibility, the response to 5-HT was examined in eight experiments after a brief exposure of the mucosa to local anaesthetic (3 ml flush with 2 % Xylocaine). In each experiment the response to 5-HT was abolished within 3 min of the onset of the Xylocaine perfusion (Fig. 6). The spontaneous firing of 5-HT-sensitive single afferents was attenuated by luminal anaesthesia but was still present at a reduced level at the time at which the 5-HT response had been completely abolished (control, 1.0 ± 0.1 impulses s−1; Xylocaine, 0.4 ± 0.1 impulses s−1; P < 0.01). In contrast, mechanosensitive afferents within the mesenteric bundles were still able to respond to the saline flush which was used to clear Xylocaine from the loop at the end of the experiment. Thus, 5-HT sensitivity was abolished by the local anaesthetic, while mechanosensitivity persisted indicating that the anaesthetic had not penetrated to the muscle layers where the afferent terminations of mechanoreceptors are located.
Figure 6. Response to 5-HT before and after intraluminal infusion of 2 % Xylocaine.
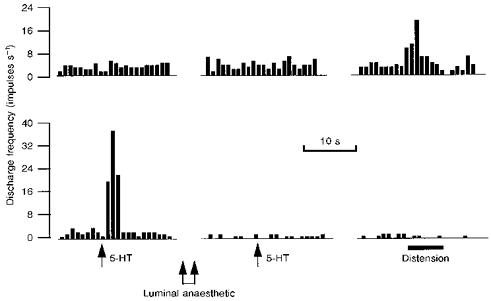
Each of the two rate histograms represents the discharge of a different single afferent fibre discriminated from within the same nerve bundle. The upper histogram shows the activity of an afferent which was unresponsive to 5-HT (10 μg) while the lower unit has a pronounced initial response. Two minutes after luminal exposure to Xylocaine, the level of spontaneous discharge in the responsive afferent had been reduced and the sensitivity to 5-HT was completely eliminated. In contrast, the firing of the upper unit was unaffected and was augmented when the loop was distended prior to flushing the anaesthetic from the lumen.
Chronic vagotomy
The discharge characteristics of thirty-six mesenteric bundles were examined from twelve vagotomized animals. All thirty-six bundles responded to luminal distension and to 5 μg capsaicin, which was used to confirm the presence of primary afferents in the bundles. A dose of 10 μg 5-HT was injected intravenously during each separate nerve recording, which failed to evoke an initial response in any of these animals. However, a secondary response to 5-HT was observed in seven bundles, which is a comparable proportion to that seen in control experiments.
Cholinergic blockade
Nicotinic transmission has been implicated in the stimulation of intestinofugal fibres projecting to the prevertebral ganglia. Hexamethonium (8 mg kg−1) was therefore used to determine the extent to which these afferents contributed to the sensitivity of mesenteric nerve bundles to 5-HT. However, hexamethonium had no effect on either the level of whole nerve spontaneous afferent activity (P = 0.66) or the initial response to 5-HT (P = 0.62) in four experiments. In contrast, atropine (100 μg kg−1) produced a significant decrease in the level of spontaneous whole nerve discharge from 19.9 ± 3.6 to 12.3 ± 2.3 impulses s−1 5 min after administration (P < 0.001, n = 9). However, the magnitude of the initial 5-HT response remained unchanged, as shown in Fig. 7.
Figure 7. Sequential rate histograms of whole nerve discharge in response to 5-HT in the absence and presence of atropine (100 μg kg−1).
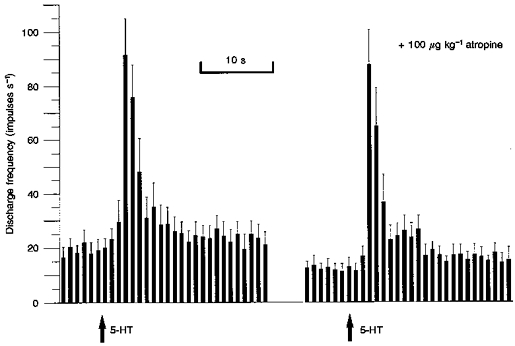
Note that the initial response to 5-HT (10 μg) was unchanged by atropine although the level of spontaneous firing was significantly reduced (P < 0.001, n = 9).
DISCUSSION
In the present study intestinal afferents were characterized as mechanoreceptors or chemoreceptors on the basis of their response to luminal distension with isotonic saline or luminal perfusion with HCl. The near 100 % correlation between the characteristic 5-HT3-mediated activation of mesenteric afferents and sensitivity to luminal acid would support the conclusion that 5-HT3 sensitivity is a property of chemosensitive afferents, with the action of 5-HT being a direct one on the afferent terminals. In contrast, the delayed response to 5-HT appears to be secondary to a contractile event which could arise from a direct action of 5-HT on the muscle, hence attenuation of both the contraction and afferent response by ketanserin (Hillsley et al. 1998). However, since in the same study treatment with the N-type calcium channel inhibitor ω-conotoxin GVIA markedly reduced the contractile response to 5-HT, this may suggest a neuronal site of 5-HT action in eliciting the contractile response. Indeed 5-HT may activate intrinsic sensory neurones to evoke a reflex contraction which in turn gives rise to a secondary response from extrinsic afferents. Both 5-HT1P and 5-HT4/5 receptors are implicated in the activation of intrinsic afferent responses to luminal stimuli (Kirchgessner et al. 1992; Grider et al. 1996).
The sensitivity of chemoreceptors to intraluminal acid has been described in the stomach and small intestine of a variety of species including rat (Clarke & Davison, 1978), ferret (Blackshaw & Grundy, 1993), sheep (Cottrell & Iggo, 1984) and cat (Iggo, 1957; Davison, 1972; El Ouazzani & Mei, 1981). In all except the study from Mei's group, this sensitivity to acid was a property of mucosal afferents with multimodal sensitivity, that is the afferent ending responded to both acid and mucosal deformation achieved by probing the mucosal epithelium, by which method the receptive field of the afferent terminals could be mapped. However, the use of a closed loop in the present study with the site of afferent recording within 10-20 mm of the intestinal border made mucosal probing difficult without running the risk of losing the recording. Nevertheless, the similarity of the mesenteric response to acid described above and that described previously for mucosal afferents would suggest that this sensitivity resided in mesenteric afferents which terminated in close proximity to the mucosal epithelium.
Only a subpopulation of mucosal afferents respond to acid. A little over 50 % of mucosal afferents in the ferret were stimulated by 150 mM HCl (Blackshaw & Grundy, 1993). In the present study, this sensitivity to acid was found to exist in more than 90 % of 5-HT-sensitive afferents. This high incidence of responses to acid in the present study may indicate a specific relationship between the two stimuli with 5-HT being involved in the signal transduction of luminal acid. Alternatively, the latency of the response to acid was also increased almost 4-fold compared with the earlier study in the ferret (Blackshaw & Grundy, 1993). Thus, the increased incidence of acid responses may simply reflect the longer exposure time necessary for the acid to penetrate through to the mucosa in this closed loop system compared with the direct application to the exposed mucosa in the earlier study. The variable extent to which mucus may limit acid penetration to the mucosa could be one possible explanation of the variation in response latency and magnitude following acid exposure. The concentration of acid used in this study reflects a compromise between the need for a robust method of stimulating mucosal afferents and a more physiological stimulus to these endings. Certainly, in pilot studies we observed effects of acid at lower concentrations but these were much less dramatic and occurred after a prolonged latency (K. Hillsley & D. Grundy, unpublished observation). Nevertheless, the ability of these afferents to maintain a response to acid in the 5-8 impulses s−1 range over a number of minutes while mechanoreceptor discharge is unchanged indicates this is indeed a potent and specific stimulus to mucosal chemoreceptors.
Most of the body's 5-HT is stored within the enterochromaffin cells of the gastrointestinal mucosa, which have a morphology that is consistent with a paracrine ‘sensory’ role (Gershon, Kirchgessner & Wade, 1994). Thus the apical membrane has specialized microvilli which are proposed to ‘sample’ luminal contents, while 5-HT is released across the basolateral membrane where afferent nerve terminals in the lamina propria are in close proximity. Some of these terminals are of neurones that are intrinsic to the bowel wall and may mediate the peristaltic reflex as proposed by Bülbring & Lin (1958). Intrinsic sensory neurones, visualized using c-fos expression as a marker of neuronal excitation, are stimulated by 5-HT acting on the 5-HT1P receptor (Kirchgessner et al. 1992). Using immunocytochemistry it was possible to stimulate certain cells in the submucosal and myenteric ganglia to ‘light up’ following both physiological stimulation by mechanical distortion and the luminal application of cholera toxin. The induction of c-fos immunoreactivity in neurones by both stimuli was prevented by a 5-HT1P receptor antagonist, N-acetyl-5-hydroxytryptophyl-5-hydroxytryptophan (5-HTDP), suggesting that endogenous 5-HT plays a role in the transduction of these luminal stimuli to intrinsic sensory activation. These observations have been recently extended using two novel activation markers (FM1-43 and FM2-10) visualized after luminal stimulation and emphasize the role of intrinsic sensory neurones within the submucosal plexus in reflex responses from the mucosa whereas distension provoked more widespread activation extending to both myenteric and submucosal plexuses (Kirchgessner, Liu & Gershon, 1996). In this respect there are recent data demonstrating that intrinsic sensory neurones in the myenteric plexus responded to mucosal application of acid and to 5-HT (Bertrand et al. 1997) although a direct involvement in signal transduction has yet to be proven. Similarly, extrinsic afferents are sensitive to 5-HT and respond to luminal acid. However, there are a number of differences between the activation of intrinsic and extrinsic afferents. Firstly, the nature of the receptor is different with 5-HT1P receptors on intrinsic afferents and 5-HT3 receptors on the extrinsic counterpart. Secondly, 5-HT release from enterochromaffin cells appears to be obligatory for activation of intrinsic afferents by luminal stimuli, at least when probed using fluorescence microscopic techniques (Kirchgessner et al. 1992, 1996), whereas the transduction of luminal acid into extrinsic mucosal afferent discharge recorded in the present study does not appear to depend on release of endogenous 5-HT. Thus responses to acid persisted after the response to 5-HT had been eliminated with the 5-HT3 receptor antagonist granisetron. Further experiments to elucidate the physiological role of endogenously released 5-HT on extrinsic afferents are necessary. It is certainly possible that acid might stimulate extrinsic afferents both by a direct action on the endings and via the release of 5-HT from the enterochromaffin cell and subsequent stimulation of 5-HT3 receptors on the afferent. If this were the case the former may obscure the contribution of the latter, especially given the concentration of acid employed in the present study, and it may be necessary to employ a more ‘physiological’ pH to address this issue.
That 5-HT sensitivity was a property of chemosensitive rather than distension-sensitive afferents would suggest that the terminal distribution of these 5-HT-sensitive fibres would be superficial to the mucosa rather than in the deeper muscle or serosal layers. This aspect was examined by applying local anaesthetic to the jejunal lumen. The 5-HT response was abolished within 3 min of exposure of the mucosa to 2 % Xylocaine. Spontaneous discharge in these afferents was similarly reduced while the response of mechanoreceptors to distension was still present, indicating that the anaesthetic had not penetrated through to the muscularis externa within this short timeframe. These experiments not only confirm that the nerves sensitive to 5-HT were mucosal receptors, but also provide some evidence to suggest that the 5-HT response was not mediated by mechanoreceptors either directly or triggered by axon reflexes of the kind described by Grider & Jin (1994). This conclusion is further supported by the observation that single units discriminated by their response to distension did not respond to 5-HT or 2-methyl-5-HT. Hence, all of the evidence discussed above demonstrates that the 5-HT response is mediated solely by afferent nerves that have a chemoreceptive function and which innervate the mucosa. That this is a direct action of 5-HT can be inferred from the very short latency of the response following systemic administration, which is very similar to the circulation time, its resistance to treatment with calcium channel blockers (Hillsley et al. 1998) and the observation in the present study that the response is unaffected by cholinergic blockade. The latter would attenuate any afferent activation secondary to 5-HT3 receptor stimulation of enteric neurones (Gershon, 1991).
At the level of the paravascular mesenteric nerve bundles, three distinct subpopulations of afferent fibre exist: those with cell bodies in the nodose ganglia and axons in the vagus nerve, dorsal root ganglion neurones following the splanchnic nerves and myenteric neurones projecting to the prevertebral ganglia. These intramural afferents have been shown to synaptically influence the firing of prevertebral neurones during bowel distension, a response which is markedly attenuated when hexamethonium is added to the gut side of a two-chambered in vitro set-up (Anthony & Kreulen, 1990; Miller & Szurszewski, 1997). As hexamethonium had no significant effect on the 5-HT response, it is unlikely that intestinofugal fibres contribute to the 5-HT sensitivity of mesenteric afferent bundles.
The mesenteric nerve bundles also contain primary afferents of vagal and splanchnic origin. Previous data would support the view that chemosensitivity is a property of vagal rather than spinal afferents although the co-localization of substance P and calcitonin gene-related peptide in nerve terminals within the gastrointestinal mucosa would support the possibility that splanchnic afferents do indeed supply the mucosa (Gibbins, Furness, Costa, Macintyre, Hillyard & Girgis, 1985). Although the sensory modality of these splanchnic afferents has not been established they may represent the so-called silent nociceptors that become active during inflammation or injury (Sengupta & Gebhart, 1994). Following chronic subdiaphragmatic vagotomy, although viable nerve recordings were still obtained, there was no initial response to 5-HT in any of the thirty-six mesenteric bundles tested. In control animals, such a 5-HT response was observed in about 64 % of nerve bundles. Thus vagal integrity is a prerequisite for 5-HT3 sensitivity which must reside in a subpopulation of vagal afferents.
In summary, through a series of experimental protocols in this and a previous study (Hillsley et al. 1998), we have established that the initial 5-HT response is due to a direct stimulation of 5-HT3 receptors located on vagal chemosensitive receptors. 5-HT has an additional effect on mesenteric afferent discharge, which our previous study had characterized as being mediated via an action on 5-HT2A receptors. This response was less pronounced than the 5-HT3-mediated effect, was less frequently encountered and had a slower onset than the initial response. This longer latency may be indicative of an indirect effect on nerve discharge secondary to mechanical events arising during intestinal contraction evoked by 5-HT. Indeed the attenuation of both the contractile response and the secondary afferent firing after nifedipine treatment would support this view. Both vagal and splanchnic mechanoreceptors may contribute to this secondary response to 5-HT. The latter are probably more numerous (Cervero & Sharkey, 1988) but the former are generally considered to have lower activation thresholds (Sengupta & Gebhart, 1994) and may respond more readily to the levels of distension and contraction encountered in the current study. Yet there were no dramatic differences in the frequency of occurrence of the secondary response to 5-HT in vagotomized animals. The sensitivity of spinal afferents to 5-HT has been reported previously but with no indication as to whether these actions were direct or indirect (Lew & Longhurst, 1986; Cervero & Sharkey, 1988; Akoev, Filippova & Sherman, 1996). It appears from the present data that this effect of 5-HT in vivo is secondary to mechanical activation.
Acknowledgments
K. H. was funded by a studentship from the SERC. We thank the BBSRC for their financial support.
References
- Akoev GN, Filippova LV, Sherman NO. Mast cell mediators excite the afferents of cat small intestine. Neuroscience. 1996;71:1163–1166. doi: 10.1016/0306-4522(95)00479-3. 10.1016/0306-4522(95)00479-3. [DOI] [PubMed] [Google Scholar]
- Anthony TL, Kreulen DL. Volume-sensitive synaptic input to neurons in guinea pig inferior mesenteric ganglion. American Journal of Physiology. 1990;259:G490–497. doi: 10.1152/ajpgi.1990.259.3.G490. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiL-tracing. Anatomy and Embryology. 1995;191:203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anatomica. 1996;156:123–131. doi: 10.1159/000147837. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the response of myenteric neurons in the small intestine to chemical stimulation of the mucosa. American Journal of Physiology. 1997;273:G422–435. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effect of 5-HT on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. Journal of the Autonomic Nervous System. 1993;45:41–50. doi: 10.1016/0165-1838(93)90360-7. [DOI] [PubMed] [Google Scholar]
- Bülbring E, Lin RCY. L0he effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. The Journal of Physiology. 1958;140:381–407. [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Sharkey KA. An electrophysiological and anatomical study of intestinal afferent fibres in the rat. The Journal of Physiology. 1988;401:381–397. doi: 10.1113/jphysiol.1988.sp017168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GD, Davison JS. Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. The Journal of Physiology. 1978;274:55–67. doi: 10.1113/jphysiol.1978.sp012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell DF, Iggo A. Mucosal enteroceptors with vagal afferent fibres in the proximal duodenum of sheep. The Journal of Physiology. 1984;354:497–522. doi: 10.1113/jphysiol.1984.sp015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison JS. Response of single vagal afferent fibres to mechanical and chemical stimulation of the gastric and duodenal mucosa in cats. Quarterly Journal of Experimental Physiology. 1972;57:405–416. doi: 10.1113/expphysiol.1972.sp002176. [DOI] [PubMed] [Google Scholar]
- El Ouazzani T, Mei N. Vagal acido- and glucoreceptors in the gastro-duodenal region. Experimental Brain Research. 1981;42:442–452. doi: 10.1007/BF00237509. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Serotonin: its role and receptors in enteric neurotransmission. Advances in Experimental Medicine and Biology. 1991;294:221–230. doi: 10.1007/978-1-4684-5952-4_20. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Kirchgessner AL, Wade PR. Functional anatomy of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 3. Vol. 1. New York: Raven Press; 1994. pp. 381–422. [Google Scholar]
- Gibbins IL, Furness JB, Costa M, Macintyre I, Hillyard CJ, Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neuroscience Letters. 1985;572:125–130. doi: 10.1016/0304-3940(85)90050-3. 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- Grider JR, Jin JG. Distinct populations of sensory neurons mediate the peristaltic reflex elicited by muscle stretch and mucosal stimulation. Journal of Neuroscience. 1994;14:2854–2860. doi: 10.1523/JNEUROSCI.14-05-02854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. American Journal of Physiology. 1996;270:G778–7782. doi: 10.1152/ajpgi.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- Grundy D. Speculations on the structure/function relationship for vagal and splanchnic afferent endings supplying the gastrointestinal tract. Journal of the Autonomic Nervous System. 1988;22:175–180. doi: 10.1016/0165-1838(88)90104-x. 10.1016/0165-1838(88)90104-X. [DOI] [PubMed] [Google Scholar]
- Hardcastle J, Hardcastle PT, Carstairs JW, Franks CM. Is desensitization of intestinal 5-hydroxytryptamine receptors an in-vitro phenomenon? Journal of Pharmacy and Pharmacology. 1994;46:322–325. doi: 10.1111/j.2042-7158.1994.tb03805.x. [DOI] [PubMed] [Google Scholar]
- Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. The Journal of Physiology. 1998;506:551–561. doi: 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Gastric mucosal receptors with afferent fibres in the cat. Quarterly Journal of Experimental Physiology. 1957;42:398–409. doi: 10.1113/expphysiol.1957.sp001284. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu MT, Gershon MD. In situ identification and visualization of neurons that mediate enteric and enteropancreatic reflexes. Journal of Comparative Neurology. 1996;371:270–286. doi: 10.1002/(SICI)1096-9861(19960722)371:2<270::AID-CNE7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. Journal of Neuroscience. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew WYW, Longhurst JC. Substance P, 5-hydroxytryptamine, and bradykinin stimulate abdominal visceral afferent fibre endings in cats. American Journal of Physiology. 1986;250:R465–473. doi: 10.1152/ajpregu.1986.250.3.R465. [DOI] [PubMed] [Google Scholar]
- Miller SM, Szurszewski JH. Colonic mechanosensory afferent input to neurons in the mouse superior mesenteric ganglion. American Journal of Physiology. 1997;272:G357–366. doi: 10.1152/ajpgi.1997.272.2.G357. [DOI] [PubMed] [Google Scholar]
- Richards W, Hillsley K, Eastwood C, Grundy D. Sensitivity of vagal mucosal afferents to cholecystokinin and its role in afferent signal transduction in the rat. The Journal of Physiology. 1996;497:473–481. doi: 10.1113/jphysiol.1996.sp021781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JM, Gebhart GF. Gastrointestinal afferent fibers and sensation. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 3. Vol. 1. New York: Raven Press; 1994. pp. 483–519. [Google Scholar]


