Abstract
In vivo extracellular recordings were made of 171 dorsal horn cells in both superficial and deep laminae in urethane-anaesthetized newborn rats aged 3, 6, 10 and 21 days, and their response to single and repeated stimuli to primary afferent fibres investigated.
No long-latency spike responses were evoked in response to C fibre stimulation in pups at postnatal day 3 (P3) or P6, while by P10, 35 % of cells had a C fibre response. Latencies of response to A fibre skin stimulation were very long and varied widely in the youngest animals, particularly in superficial cells, but mean latencies decreased with postnatal age, from 33.1 ± 2.78 ms at P3 to 7.3 ± 0.3 ms at P21. The mean number of spikes evoked by a single A fibre skin stimulus was remarkably consistent between cells and not significantly different in superficial and deep laminae at each age. The mean value of 5.1 ± 0.6 at P3 increased to 7.0 ± 1.4 at P10.
Repeated stimulation of cutaneous A fibres at 0.5 Hz at twice the threshold level did not significantly alter the magnitude of the evoked response but led to shifts in latency, or ‘latency jitter’, which decreased with age. Deeper cells displayed more latency jitter than superficial cells.
Repeated stimulation of cutaneous A fibres at 0.5 Hz at twice the threshold level produced considerable sensitization in a population of dorsal horn cells in the neonate. This sensitization was unlike the classic C fibre-evoked ‘wind-up’ observed in adult dorsal horn. The direct A fibre-evoked activity did not increase, but the background activity increased during repetitive stimulation leading to a prolonged after-discharge beyond the stimulation period. At P6, 33 % of cells were sensitized, displaying a mean after-discharge of 70.6 ± 18 s. At P10, only 6 % were sensitized, with a mean after-discharge of 63 s, and by P21, sensitization was no longer observed.
The present study demonstrates that the postsynaptic activity evoked in neonatal dorsal horn cells by cutaneous afferents differs considerably from that in adults. The results may account for the known behavioural reflex sensitization to low-intensity cutaneous stimulation observed in neonatal rats and man.
In the neonatal rat, cat and human, the cutaneous flexion withdrawal reflex can be evoked by low-threshold mechanical stimuli (Ekholm, 1967; Fitzgerald & Gibson, 1984; Fitzgerald, Shaw & MacIntosh, 1988), although the reflex has been shown to be purely nociceptive in the adult (Sherrington, 1910; Woolf, 1983). Since the thresholds of cutaneous mechanosensitive primary afferents are generally the same in the adult and the neonatal rat (Fitzgerald, 1987a), it has been proposed that this difference in the reflex sensitivity is due to a difference in central processing. One possibility is that in the neonatal spinal cord both low- and high-intensity stimuli can activate spinal pathways that are purely nociceptive in the adult. In support of this, low-intensity mechanical or A fibre stimulation in the neonatal rat has been shown to evoke c-fos expression in lamina II cells, whereas in the adult such expression is selectively evoked by nociceptive stimulation (Jennings & Fitzgerald, 1996a). The anatomical substrate for this may be the presence, in early postnatal life, of large myelinated A fibre terminals and synapses in lamina II that, in the adult, are restricted to the deep dorsal horn (Fitzgerald, Butcher & Shortland, 1994; Mirnics & Koerber, 1995; Coggeshall, Jennings & Fitzgerald, 1996a). Any postnatal developmental changes in A fibre synaptic connections are likely to be reflected in a change in the pattern of afferent-evoked activity in dorsal horn cells at this time, but to date there has been only limited electrophysiological analysis of the A fibre- and C fibre-evoked responses in neonatal dorsal horn cells.
In the rat fetus, A fibre afferents from the hindlimb grow into the spinal cord at embryonic day 15 (E15), before C fibres, which do not enter lamina II until E18-19 (Fitzgerald, 1987b; Fitzgerald, Reynolds & Benowitz, 1991; Mirnics & Koerber, 1995). Dorsal root stimulation evokes polysynaptic ventral root activity at E15.5 (Saito, 1979), while responses to electrical stimulation can be recorded in individual lumbar dorsal horn cells from E17 (Fitzgerald, 1991). Natural cutaneous sensory input from the hindpaw begins to evoke dorsal horn activity at E19 (Fitzgerald, 1991). The afferent input and receptive field properties of dorsal horn cells in the newborn rat and cat have been surveyed (Fitzgerald, 1985; Wilson & Snow, 1988) and compared with those of the adult. A number of postnatal changes were observed, including the delayed maturation of C fibre input to the spinal cord, which does not evoke postsynaptic activity until the second postnatal week (Fitzgerald, 1988). The aim of this study, however, was to examine the physiological responses of the superficial and deep dorsal horn cells to A fibre stimuli during postnatal development, especially in the context of the transient A fibre synapses in the neonatal superficial dorsal horn. Preliminary results have been published in abstract form (Jennings & Fitzgerald, 1996b).
METHODS
Sprague-Dawley rat pups of both sexes at postnatal day 3 (P3), P6, P10 and P21 were anaesthetized with 2.5 g kg−1 urethane i.p. (Sigma). Animals given this dose of urethane remain anaesthetized for at least 8 h, and our experiments always lasted less than 6 h. The trachea was cannulated and the pup was held firmly with small ear bars and a small clamp at L1 to stabilize the cord. The lumbar cord was exposed by laminectomy, the dura removed, and the surface of the cord bathed in warm mineral oil to prevent it from drying. When the pup was deeply anaesthetized, as shown by areflexia, it was paralysed with 0.1 ml Flaxedil (May & Baker Ltd, Dagenham, UK) and ventilated. The pup was kept warm with a heated blanket, and the heart rate was monitored throughout the experiment and maintained within the range of 350-500 beats min−1. Animals were killed by an overdose of anaesthetic at the end of the experiment.
Extracellular recordings were made from cells in the dorsal horn of the L4-L5 lumbar cord using glass-coated tungsten microelectrodes. Recordings were made throughout the dorsal horn and the depth noted from the surface of the cord. Receptive fields on the hindlimb of single cells were mapped using natural mechanical stimuli, i.e. light brush, touch and pinch. Electrical stimulation of the skin was applied through subcutaneous pin electrodes in the centre of the receptive field at stimulus intensities of 100 μA to 10 mA, 50-500 μs. In five cells recorded from three P10 animals, the electrical stimulation was applied directly to the exposed sciatic nerve in the hindlimb. The A fibre threshold was defined as the minimum electrical stimulus needed to produce a short-latency response from the dorsal horn cell. All cells were also tested at higher stimulus intensities to test for a longer-latency C fibre input. The appropriate stimulus parameters required to excite A fibres only, or both A and C fibres at different ages have been established previously in this laboratory using compound action potential recordings from dorsal roots (Fitzgerald, 1985), and these were confirmed in the present study. Repetitive stimuli were applied with a train of sixteen stimuli at a frequency of 0.5 Hz. Spike recordings were captured by computer using a Maclab interface and software, and peristimulus time histograms were computed. Statistical analysis was carried out using Student's t test, or ANOVA factorial tests. All results are presented as means ± s.e.m., unless otherwise stated.
RESULTS
General properties of extracellularly recorded units
Single unit recordings (171) were made from the lumbar dorsal horn at different postnatal ages: n = 21 at P3, n = 57 at P6, n = 48 at P10, and n = 31 at P21. Cells were recorded from both superficial laminae I and II (200 μm from the dorsal surface of the spinal cord at P3 and P6, 250 μm at P10, and 300 μm at P21) and deeper laminae III-V. At P3, n = 13 were from superficial laminae and n = 8 from deep laminae; at P6, n = 45 were superficial and n = 12 deep; at P10, n = 36 were superficial and n = 12 were deep; and at P21, n = 20 were superficial and n = 11 were deep.
The receptive fields of the cells used in this study were located on the distal hindlimb and were all cutaneous mechanoreceptive fields of the slowly adapting or rapidly adapting type. Of these, 34 % were responsive to light touch or brush only, and the remaining 66 % were also responsive to high-intensity mechanical stimulation such as pinch, giving a more intense response when pinched than when brushed. Receptive field sizes were not accurately measured in this study but previous observations of an inverse relationship between receptive field size and postnatal age were confirmed (Fitzgerald, 1985). Background activity was generally absent when cells were initially isolated for recording.
Latencies of A fibre-evoked responses: single stimuli
The spike activity evoked in individual cells to low-intensity electrical skin stimulation (100 μA to 3.5 mA, 50-200 μs) sufficient to recruit A fibres was investigated at each postnatal age. The latency of response to A fibre stimulation was defined as the latency to the first spike after a single stimulus at twice the threshold level. Figure 1 shows that this latency progressively decreased with age, in both superficial and deep cells. At P3, the mean latency of the A fibre-evoked response was 33.1 ± 2.78 ms (n = 21), compared with 19.1 ± 1.32 ms (n = 57) at P6, 13.5 ± 0.8 ms (n = 48) at P10 and 7.3 ± 0.3 ms (n = 31) at P21. There was no significant difference between the mean A fibre-evoked latencies of superficial and deep cells.
Figure 1. Postnatal latencies of A fibre-evoked response.
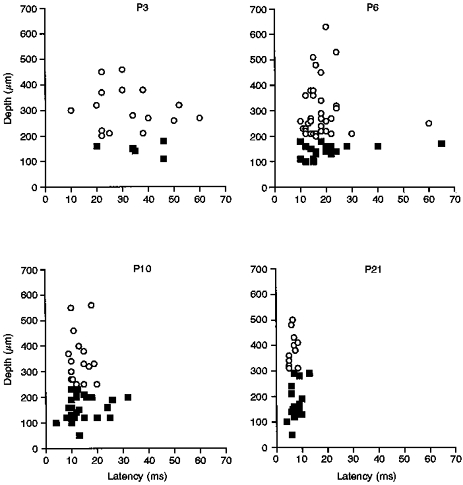
Scatter plots showing the latency of the A fibre-evoked response plotted against depth of the cell from the cord surface, for the different postnatal ages examined. ○, deep cells; ▪, cells in the superficial laminae. The mean latencies ( ± s.e.m.) for the deep cells at P3, P6, P10 and P21, respectively, were: 28.0 ± 4.5, 17.9 ± 1.2, 13.1 ± 1.0 and 6.45 ± 0.4 ms. The latencies for the superficial cells were: 36.3 ± 3.4, 19.4 ± 1.2, 13.7 ± 0.9, and 7.8 ± 0.5 ms, respectively.
Figure 1 also shows that the variation in the A fibre latencies within the population of recorded cells decreased with age. The standard deviation of the latencies was taken as a measure of the variation for this comparison (Fig. 2). The standard deviation for the total cell population at P3 was 12.8 ms compared with 10.0 ms at P6, 5.3 ms at P10 and 1.9 ms at P21. Figure 2 shows that the A fibre-evoked latency variation between superficial cells (▪) was consistently greater than that of the deeper cells in the dorsal horn (○), except at P3 where the standard deviation for both the deep and the superficial cells was the same and both were very large.
Figure 2. Postnatal variation in evoked latencies (s.d.).
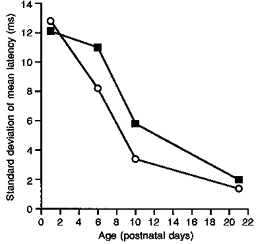
The change in variation of response latency in individual cells on repeated A fibre stimulation with postnatal age. The variation is expressed as the standard deviation of the mean latency for the population of cells at each age. ▪, cells recorded in superficial laminae; ○, cells recorded in deep laminae.
Latencies of A fibre-evoked responses: repetitive stimulation
Repetitive 0.5 Hz electrical skin or sciatic nerve stimulation, at twice the A fibre threshold, leads to considerable variation in latency in the evoked response, or ‘latency jitter’, of individual cells in the neonatal dorsal horn. Figure 3 shows this variability, expressed as the standard deviation of the latencies over sixteen stimuli, in both superficial and deep cells at P3, P6 and P10. Despite the variation in initial latency between individual superficial cells at P3, as shown in Fig. 1, repeated stimulation of these cells produces significantly less latency jitter than for deeper cells. A similar pattern appears at P6 but the difference is not significant at this age, and at P10 the latency jitter is very much reduced in both superficial and deep cells.
Figure 3. Stability of evoked latency on repeated stimulation.
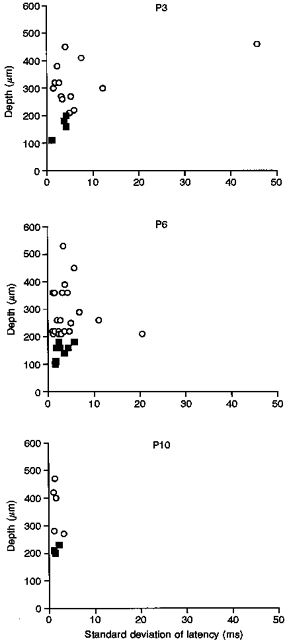
The ‘latency jitter’ of dorsal horn cells at P3, P6 and P10. Latency jitter is the variation in the response latency recorded from an individual cell, when comparing the evoked response to each stimulus in the train (16 stimuli at 0.5 Hz). This is different from the variation in latency of the whole-cell population at a specific age (Fig. 2). ▪, cells in the superficial dorsal horn; ○, cells in the deep dorsal horn.
Latencies of C fibre-evoked responses
The spike activity evoked in individual cells to high-intensity skin stimulation (1-5 mA, 500 μs), which recruits both A and C fibre afferents, was investigated at each postnatal age. No long-latency spike responses were evoked in response to C fibre stimulation in pups at P3 or P6, consistent with previous reports (Fitzgerald, 1988). Cells responding with long-latency activity to a C fibre electrical stimulus were found in both superficial and deep laminae in animals at P10 and P21. At P10, 35 % of cells had a C fibre input with a mean latency of 97.65 ± 4.44 ms (n = 17), and at P21 the value was 32 % with a mean latency of 107.0 ± 10.12 ms (n = 10) (not shown).
Magnitude of afferent-evoked activity: single stimuli
Figure 4 shows the mean number of spikes evoked by each stimulus in a train of sixteen stimuli. The latency ranges between which the evoked responses were measured were: 0-70 ms for P3, 0-40 ms for P6, 0-35 ms for P10, and 0-15 ms for P21. The mean response to the first stimulus of each was remarkably consistent between cells at a given age, as shown by the low standard error values. The mean values at P3 (not shown), P6 and P10 were not significantly different at 5.1 ± 0.6, 3.8 ± 0.7 and 5.64 ± 0.9 spikes per stimulus, respectively. The mean evoked response was also not significantly different in superficial and deep laminae at each age. C fibre-evoked responses ranged from one to four spikes evoked by the first C fibre stimulus.
Figure 4. Magnitude of evoked response.
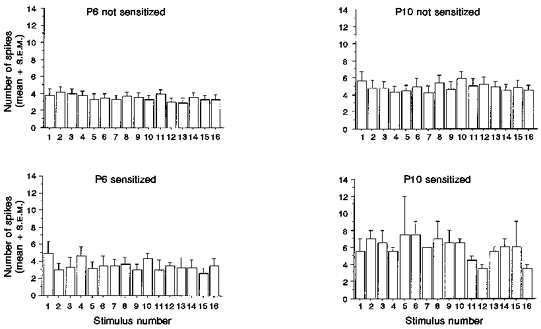
Bar charts representing the mean number of spikes (+s.e.m.) evoked by each stimulus in a train of 16 A fibre stimuli (twice threshold; 0.5 Hz). P6 and P10 cells are divided into those that sensitized on repeated A fibre stimulation and those that did not.
Magnitude of afferent-evoked activity: repetitive stimulation
Figure 4 also illustrates that the mean number of A fibre-evoked spikes did not significantly alter on repetitive stimulation at all ages. Despite the latency jitter illustrated in Fig. 3, the number of evoked spikes over sixteen stimuli stays remarkably consistent at all ages.
A fibre-evoked sensitization
Repetitive A fibre electrical skin or sciatic nerve stimulation, at 0.5 Hz at twice the A fibre threshold, could produce considerable sensitization of the dorsal horn cells in the neonate. This sensitization is illustrated in Fig. 5, and took the form of a build-up of background activity in the cells during repetitive stimulation that outlasted the stimulation period, thereby producing a prolonged after-discharge of up to 138 s. It was particularly apparent in younger animals, and at P6, nineteen out of fifty-seven cells (33 %) displayed background firing during, and a prolonged after-discharge of 70.6 ± 18 s following repetitive A fibre stimulation. At P10, three out of forty-eight cells showed this type of sensitization (6 %) with an after-discharge of 63 s whereas at P21, sensitization was not seen in any cells (n = 31) (not shown).
Figure 5. Time histogram of a sensitized cell.
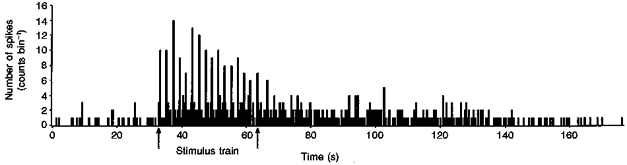
A ratemeter record of the firing of a single P6 dorsal horn cell illustrating the sensitization seen following a train of 16 stimuli (0.5 Hz) at twice the A fibre threshold. Bin size, 0.2 s. Note the low background rate before the stimulus train and the long after-discharge.
A fibre-induced sensitization was not accompanied by an increase in the direct A fibre-evoked spike discharge, as described above (Fig. 4). However, it can be seen in Fig. 6 that, during the stimulation period, the sensitized units showed a significant increase in activity outside of this short-latency-evoked burst. The mean background activity during the stimulation period, measured in the 40-2000 ms period between stimuli, was 2.6 ± 0.16 spikes in P6 sensitizing cells, significantly greater than the 0.4 ± 0.04 spikes in non-sensitizing cells. At P10 there is a similar pattern; the mean background activity for sensitizing cells was 15.7 ± 0.84 spikes while that of non-sensitizing cells was 1.3 ± 0.13 spikes.
Figure 6. Magnitude of the after-discharge.
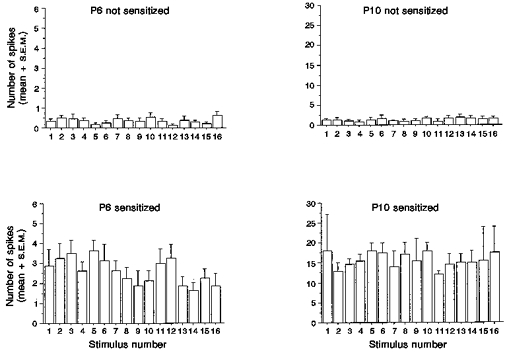
Bar charts representing the mean number of spikes (+s.e.m.) in the 40-2000 ms range after each stimulus, in a train of 16 stimuli (twice threshold, 0.5 Hz). This illustrates sensitization of dorsal horn cells not reflected in the directly evoked responses occurring within a 40 ms latency (Fig. 4).
Repetitive C fibre stimulation at three times the C fibre threshold produced a classical ‘wind-up’ as reported in the adult dorsal horn (Mendell & Wall, 1965). This C fibre- induced central sensitization was observed in three out of seventeen cells at P10 (18 %), and four out of ten cells at P21 (40 %) (not shown).
DISCUSSION
The present study demonstrates that the postsynaptic activity evoked in neonatal dorsal horn cells by cutaneous afferents undergoes a number of developmental changes in the postnatal period. C fibre-evoked postsynaptic spike activity is absent before P10 and, therefore, A fibre-evoked activity is of particular importance. Despite long and variable response latencies at younger ages, we show here that A fibre input to both superficial and deep dorsal horn cells is robust and repeatable. Furthermore, repeated activation of cutaneous A fibres produces sensitization in dorsal horn cells in neonatal spinal cord which disappears with increasing age. Since sensitization is not produced by A fibre input in the adult dorsal horn, these results demonstrate fundamentally differing central sensory processing in the immature spinal cord.
The absence of C fibre-evoked activity in dorsal horn cells before postnatal day 10 (P10) has been reported elsewhere (Fitzgerald & Gibson, 1984; Hori & Watanabe, 1987; Fitzgerald, 1988). Responses to noxious mechanical stimulation or chemical irritants, such as formalin, produce clear reflex activity and induction of c-fos expression from birth, although both of these stimuli also activate A fibres (Fitzgerald & Gibson, 1984; Williams, Evan & Hunt, 1990; Jennings & Fitzgerald, 1996a). Responses to pure C fibre inputs remain subthreshold until the second postnatal week (Fitzgerald & Gibson, 1984; Fitzgerald, 1988), and here we confirm this and show that even at P21, the percentage of dorsal horn cells that can be excited by peripheral C fibre activation is still relatively low.
It appears, therefore, that all the direct cutaneous-evoked dorsal horn spike activity in the first postnatal week results from activation of A fibres. In the present study, electrical stimulation of the receptive field allowed us to identify clearly low-threshold A fibre-evoked responses in both superficial and deep cells from P3 to P21. Previous studies involving dorsal root recordings of afferent volleys (Fitzgerald, 1985, 1988) show that these responses were evoked by the large diameter, myelinated Aβ fibres. The mean magnitude of the A fibre-evoked response does not change substantially over the postnatal period, but there are considerable changes in the response latencies, which are longer and much more variable in younger animals. To some extent the long latencies are due to low cutaneous afferent conduction velocities in the newborn animal, which increase in the rat hindlimb from 3.0 m s−1 at P3 to over 18 m s−1 at P21 (Fitzgerald, 1985, 1987a). These, in turn, will be influenced by the absence of myelin in rat sciatic nerves at birth, which appears within 3 days and grows rapidly for 2 weeks (Webster, 1971). The diameter of sciatic nerve fibres continues to increase until 6 months of age, with the largest fibres doubling in diameter between P5 and P20.
The long response latencies are also likely to arise from prolonged central, synaptic delays and very slowly rising EPSPs in the neonatal dorsal horn. These results are consistent with the reported I a afferent monosynaptic reflex central delay in P3 lumbar cord of 5.7 ± 0.5 ms and polysynaptic cutaneous reflex delay of 14.2 ± 3.6 ms (Fitzgerald, King, Thompson & Woolf, 1987). Such long central delays may also lead to the latency variation between cells particularly in younger animals.
Some differences were detected in the responses of superficial versus deeper dorsal horn cells. In particular, superficial cells generally displayed a wider range of response latencies to A fibre stimulation than deeper cells, while their ability to respond to repeated stimulation was superior, with lower latency jitter than deeper cells. At birth, the rat spinal cord is still immature. While the maturation of motoneurones, intermediate neurones and deep dorsal horn projection neurones is well underway, the superficial neurones of substantia gelatinosa (SG) are born late in fetal life and their axodendritic growth only begins postnatally (Altman & Bayer, 1984). Synaptogenesis in the cord peaks in the first 2 postnatal weeks and is concentrated in the deep dorsal horn at P4-5 and in SG at P7-9 (Cabalka, Ritchie & Coulter, 1990). The large, future-myelinated, cutaneous afferents begin to enter the lumbar spinal cord at E15 (Fitzgerald et al. 1991; Mirnics & Koerber, 1995), but C fibre afferents grow into the spinal cord only a few days before birth, at E18-19, and the formation of C fibre synaptic connections is almost entirely a postnatal event (Fitzgerald, 1987b; Pignatelli, Ribeiro da Silva & Coimbra, 1989). C fibres terminate in SG, and in the adult there is no other afferent input to this region, but in the neonate, A fibres terminate much more superficially in the dorsal horn and therefore SG is occupied by both A and C fibres in the first 3 weeks of life (Fitzgerald et al. 1994; Mirnics & Koerber, 1995). At the ultrastructural level, these transient A fibre terminals in the superficial laminae can be seen to have formed synapses (Coggeshall et al. 1996). Competitive interaction apparently takes place between the two afferent groups, and if C fibres are selectively destroyed at birth with the neurotoxin capsaicin, A fibres remain in the superficial dorsal horn until adulthood (Shortland, Molander, Fitzgerald & Woolf, 1990). It is tempting to speculate that the apparent stability of the A fibre input in the superficial laminae at P3 and P6, where the response latencies on repeated stimulation are less variable than in deep laminae, is a reflection of transient monosynaptic connections between A fibres and superficial dorsal horn neurones at this time. Subsequent withdrawal of A fibres to deeper laminae may lead to later maturation of those connections and subsequent maturation of the polysynaptic A fibre input to SG observed in the adult (Willis & Coggeshall, 1978). Intracellular recording is required to resolve these issues.
A major finding here is that, in contrast to the normal adult spinal cord, central sensitization occurs in the immature spinal cord in response to electrical stimulation of large-diameter Aβ primary afferents, and this disappears with postnatal maturation. The ability of some cells in the newborn rat to display long-lasting excitation to natural stimulation of receptive fields and for repeated natural stimuli to result in the build-up of considerable background activity was reported in an earlier study (Fitzgerald, 1985), but this was not analysed quantitatively nor was the afferent component identified. Such central sensitization in the adult spinal cord is normally evoked by high-intensity C fibre input. Low-frequency, repetitive stimulation of peripheral nerves at C fibre strength results in ‘wind-up’, a progressive increase in the amplitude of the C fibre-evoked response and a build-up of background activity (Mendell & Wall, 1965). This is due to a progressive depolarization of the postsynaptic membrane with each incoming C fibre stimulus and is not produced by Aβ fibre stimulation in either adult cord ‘in vivo’ or young P12-14 cords ‘in vitro’ (Woolf, Thompson & King, 1988; Woolf & Thompson, 1991; Thompson, Woolf & Sivlotti, 1993). In adults, Aβ fibre stimulation only produces such central excitability in pathological states, such as neuropathy or chronic inflammation (Woolf & Doubell, 1994; Neuman, Doubell, Leslie & Woolf, 1997). Here we report that in neonates, where all C fibre-evoked activity is subthreshold, repetitive Aβ fibre stimulation can induce central sensitization but that this differs from wind-up. The magnitude of the evoked response remains unchanged with each progressive stimulus, only the background discharge increases followed by a prolonged after-discharge that outlasts the stimulus. Wind-up is, however, only one manifestation of central sensitization, which can be more generally described as an expression of increased excitability of dorsal horn neurones (Woolf, 1983). The key to central sensitization is an increase in [Ca2+]i, and this can occur in a number of ways (Woolf, 1996). Summation of synaptic potentials may occur at a rate below that necessary to change the evoked response of the cells (Thompson et al. 1993) but sufficient to unblock NMDA receptor ion channels and allow direct Ca2+ influx. This may be particularly true in neonates, where the rate of rise of EPSPs is already low. Furthermore, recent studies in Aplysia indicate that excitation during and after stimulation can be differentially regulated, in that the two phases of response have differential Ca2+ dependence (Fischer, Blazis, Priver & Carew, 1997).
If the A fibre-induced sensitization reported here is NMDA dependent, as has been reported for the C fibre-induced wind-up of dorsal horn cells (Dickenson & Sullivan, 1990) and the central excitability of the flexion reflex (Woolf & Thompson, 1991), it may be a reflection of the ability of such central excitability to be more freely induced in the neonatal cord. The properties of the NMDA receptor in the postnatal spinal cord are different from those in the adult. NMDA binding studies in the mouse lumbar spinal cord show that [3H]glutamate binding peaks around P6-10 and is labelled fairly evenly throughout the grey matter until P10-12 when labelling begins to increase in SG. The labelling then decreases in other areas so that the adult pattern is visible by P30 (Gonzalez, Fuchs & Droge, 1993). The affinity of receptors for NMDA and NMDA-induced elevations of [Ca2+]i are also markedly elevated in SG neurones in the first postnatal week, while neither the AMPA response nor the resting [Ca2+]i show these developmental changes (Hori & Kanda, 1994).
A fibre activation of adult dorsal horn cells involves glutamatergic AMPA receptors (King, Lopez-Garcia & Cumberbatch, 1992) and these are also more widespread in the developing spinal cord compared with that of the adult. The subunit composition of the non-NMDA receptor in the neonatal spinal cord maximizes non-NMDA Ca2+ influx (Jakowec, Fox, Martin & Kalb, 1995). Other maturational changes in the postnatal spinal cord include the onset of descending inhibition, via the dorsolateral funiculus, which only begins to function in the second postnatal week (Fitzgerald & Koltzenburg, 1986), presumably dampening this central excitability.
The ability of A fibres to produce central sensitization in neonatal spinal cord is consistent with behavioural and c-fos studies. Cutaneous reflexes in the newborn rat, kitten and human are exaggerated compared with those of the adult. In rat pups, kittens, and human neonates, flexion reflexes can be elicited with light touch, rather than a noxious stimulus which is required in the adult (Ekholm, 1967; Fitzgerald et al. 1988). Thresholds are lower and the reflex responses more synchronized and longer lasting. Repeated skin stimulation results in considerable hyperexcitability or sensitization with generalized movements of all limbs. Significantly shorter latencies to paw withdrawal from heat have also been observed in neonates (Lewin, Ritter & Mendell, 1993), and pain behaviour following formalin injection into the hindpaw, measured as frequency of paw flexion and paw licking, is greatly augmented in the first postnatal week (Guy & Abbott, 1992). This period of hyperexcitability begins to decline after the first postnatal week in the rat (and after 30 weeks gestation in humans), gradually falling to adult levels at P20-30 (Hammond & Ruda, 1991). Furthermore, we have previously shown that, in contrast to adults, neonatal rat dorsal horn cells express c-fos in response to an innocuous or Aβ-strength skin stimulus (Jennings & Fitzgerald, 1996a). In the normal adult, c-fos expression is only induced by noxious skin or Aδ and C fibre nerve stimulation, and c-fos induction by innocuous stimulation occurs only under pathological conditions (Ma & Woolf, 1996).
These results show that sensory mechanisms in the neonatal spinal dorsal horn are not simply immature or incomplete versions of those in the adult. A fibre inputs are able to produce postsynaptic excitatory effects in dorsal horn cells that are not seen in the normal adult cord but which are likely to be important for normal sensory function in the intact developing mammalian nervous system.
Acknowledgments
We thank Jacqueta Middleton and Andrew Allchorne for technical support and Qing-Ping Ma, Tim Doubell and Clifford Woolf for useful discussions. The work was supported by the Wellcome Trust and the MRC. Ernest Jennings is an MRC research student.
References
- Altman J, Bayer SA. The development of the rat spinal cord. Advances in Anatomy, Embryology and Cell Biology. 1984;85:1–166. doi: 10.1007/978-3-642-69537-7. [DOI] [PubMed] [Google Scholar]
- Cabalka LM, Ritchie TC, Coulter JD. Immunolocalization of a novel nerve terminal protein in spinal cord development. Journal of Comparative Neurology. 1990;295:83–91. doi: 10.1002/cne.902950108. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Jennings EA, Fitzgerald M. Evidence that large myelinated primary afferent fibers make synaptic contacts in lamina II of neonatal rats. Developmental Brain Research. 1996;92:81–90. doi: 10.1016/0165-3806(95)00207-3. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Differential effects of excitatory amino acid antagonists on dorsal horn nociceptive neurones in the rat. Brain Research. 1990;506:31–39. doi: 10.1016/0006-8993(90)91195-m. 10.1016/0006-8993(90)91195-M. [DOI] [PubMed] [Google Scholar]
- Ekholm J. Postnatal changes in cutaneous reflexes and in the discharge pattern of cutaneous and articular sense organs. A morphological and physiological study in the cat. Acta Physiologica Scandinavica. 1967;(suppl. 297):1–130. [PubMed] [Google Scholar]
- Fischer TM, Blazis DI, Priver NA, Carew TJ. Metaplasticity at identified inhibitory synapses in Aplysia. Nature. 1997;389:860–865. doi: 10.1038/39892. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The post-natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. The Journal of Physiology. 1985;364:1–18. doi: 10.1113/jphysiol.1985.sp015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. Cutaneous primary afferent properties in the hindlimb of the neonatal rat. The Journal of Physiology. 1987a;383:79–92. doi: 10.1113/jphysiol.1987.sp016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. Prenatal growth of fine-diameter primary afferents into the rat spinal cord: a transganglionic tracer study. Journal of Comparative Neurology. 1987b;261:98–104. doi: 10.1002/cne.902610108. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of activity evoked by fine diameter cutaneous fibres in the spinal cord of the newborn rat. Neuroscience Letters. 1988;86:161–166. doi: 10.1016/0304-3940(88)90564-2. 10.1016/0304-3940(88)90564-2. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. A physiological study of the prenatal development of cutaneous sensory inputs to dorsal horn cells in the rat. The Journal of Physiology. 1991;432:473–482. doi: 10.1113/jphysiol.1991.sp018395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Butcher T, Shortland P. Developmental changes in the laminar termination of A fibre cutaneous sensory afferents in the rat spinal cord dorsal horn. Journal of Comparative Neurology. 1994;348:225–233. doi: 10.1002/cne.903480205. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Gibson S. The postnatal physiological and neurochemical development of peripheral sensory C fibres. Neuroscience. 1984;13:933–944. doi: 10.1016/0306-4522(84)90107-6. 10.1016/0306-4522(84)90107-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, King AE, Thompson SW, Woolf CJ. The postnatal development of the ventral root reflex in the rat; a comparative in vivo and in vitro study. Neuroscience Letters. 1987;78:41–45. doi: 10.1016/0304-3940(87)90558-1. 10.1016/0304-3940(87)90558-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Koltzenburg M. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Research. 1986;389:261–270. doi: 10.1016/0165-3806(86)90194-x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Reynolds ML, Benowitz LI. GAP-43 expression in the developing rat lumbar spinal cord. Neuroscience. 1991;41:187–199. doi: 10.1016/0306-4522(91)90209-7. 10.1016/0306-4522(91)90209-7. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Shaw A, MacIntosh N. Postnatal development of the cutaneous flexor reflex: comparative study of preterm infants and newborn rat pups. Developmental Medicine and Child Neurology. 1988;30:520–526. doi: 10.1111/j.1469-8749.1988.tb04779.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez DL, Fuchs JL, Droge MH. Distribution of NMDA receptor binding in developing mouse spinal cord. Neuroscience Letters. 1993;151:134–137. doi: 10.1016/0304-3940(93)90004-5. 10.1016/0304-3940(93)90004-5. [DOI] [PubMed] [Google Scholar]
- Guy ER, Abbott FV. The behavioural response to formalin pain in preweanling rats. Pain. 1992;51:81–90. doi: 10.1016/0304-3959(92)90012-Z. 10.1016/0304-3959(92)90012-Z. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Ruda MA. Developmental alterations in nociceptive threshold, immunoreactive calcitonin gene-related peptide and substance P, and fluoride-resistant acid phosphatase in neonatally capsaicin-treated rats. Journal of Comparative Neurology. 1991;312:436–450. doi: 10.1002/cne.903120310. [DOI] [PubMed] [Google Scholar]
- Hori Y, Kanda K. Developmental alterations in NMDA receptor-mediated [Ca2+]i elevation in substantia gelatinosa neurons of neonatal rat spinal cord. Developmental Brain Research. 1994;80:141–148. doi: 10.1016/0165-3806(94)90098-1. 10.1016/0165-3806(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Hori Y, Watanabe S. Morphine-sensitive late components of the flexion reflex in the neonatal rat. Neuroscience Letters. 1987;78:91–96. doi: 10.1016/0304-3940(87)90567-2. 10.1016/0304-3940(87)90567-2. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Fox AJ, Martin LJ, Kalb RG. Quantitative and qualitative changes in AMPA receptor expression during spinal cord development. Neuroscience. 1995;67:893–907. doi: 10.1016/0306-4522(95)00026-f. 10.1016/0306-4522(95)00026-F. [DOI] [PubMed] [Google Scholar]
- Jennings E, Fitzgerald M. C-fos can be induced in the neonatal rat spinal cord by both noxious and innocuous peripheral stimulation. Pain. 1996a;68:301–306. doi: 10.1016/s0304-3959(96)03194-6. 10.1016/S0304-3959(96)03194-6. [DOI] [PubMed] [Google Scholar]
- Jennings E, Fitzgerald M. Postnatal changes in A fibre evoked responses in rat dorsal cord. Abstracts of the 8th World Congress on Pain. 1996b;237:237. A66. [Google Scholar]
- King AE, Lopez-Garcia JA, Cumberbatch M. Antagonism of synaptic potentials in ventral horn neurones by cyano-7-nitroquinoxaline-2,3-dione: a study in the rat spinal cord in vitro. Journal of Pharmacology. 1992;107:375–381. doi: 10.1111/j.1476-5381.1992.tb12754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. Journal of Neuroscience. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Basal and touch-evoked fos-like immunoreactivity during experimental inflammation in the rat. Pain. 1996;67:307–316. doi: 10.1016/0304-3959(96)03132-6. 10.1016/0304-3959(96)03132-6. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Wall PD. Responses of dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature. 1965;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Koerber HR. Prenatal development of rat primary afferent fibers: II. Central projections. Journal of Comparative Neurology. 1995;355:601–614. doi: 10.1002/cne.903550409. [DOI] [PubMed] [Google Scholar]
- Neumann S, Doubell TP, Leslie TA, Woolf CJ. Inflammatory pain hypersensitivity mediated by a phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- Pignatelli D, Ribeiro da Silva A, Coimbra A. Postnatal maturation of primary afferent terminations in the substantia gelatinosa of the rat spinal cord. An electron microscope study. Brain Research. 1989;491:33–44. doi: 10.1016/0006-8993(89)90085-1. 10.1016/0006-8993(89)90085-1. [DOI] [PubMed] [Google Scholar]
- Saito K. Development of spinal reflexes in the rat fetus studied in vitro. Of Physiology. 1979;294:581–594. doi: 10.1113/jphysiol.1979.sp012947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CL. Flexion reflex of the limb, crossed extension reflex, and reflex stepping and standing. The Journal of Physiology. 1910;40:28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortland P, Molander C, Fitzgerald M, Woolf CJ. Neonatal capsaicin treatment induces invasion of the substantia gelatinosa by the arborizations of HFA's in the rat dorsal horn. Journal of Comparative Neurology. 1990;296:23–31. doi: 10.1002/cne.902960103. [DOI] [PubMed] [Google Scholar]
- Thompson SWN, Woolf CJ, Sivilotti LG. Small caliber afferent inputs produce a heterosynaptic facilitation of the synaptic responses evoked by primary afferent A-fibers in the neonatal rat in vitro. Journal of Neurophysiology. 1993;69:2116–2128. doi: 10.1152/jn.1993.69.6.2116. [DOI] [PubMed] [Google Scholar]
- Webster HD. The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. Journal of Cell Biology. 1971;48:348–367. doi: 10.1083/jcb.48.2.348. 10.1083/jcb.48.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Evan GI, Hunt SP. Changing patterns of c-fos induction in spinal neurons following thermal cutaneous stimulation in the rat. Neuroscience. 1990;36:73–81. doi: 10.1016/0306-4522(90)90352-5. 10.1016/0306-4522(90)90352-5. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 2. New York: Plenum Press; 1978. [Google Scholar]
- Wilson P, Snow PJ. Somatotopic organization of the dorsal horn in the lumbosacral enlargement of the spinal cord in the neonatal cat. Experimental Neurology. 1988;101:428–444. doi: 10.1016/0014-4886(88)90054-4. 10.1016/0014-4886(88)90054-4. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66:105–108. 10.1016/0304-3959(96)03114-4. [PubMed] [Google Scholar]
- Woolf CJ, Doubell TP. The pathophysiology of chronic pain – increased sensitivity to low threshold A beta-fibre inputs. Current Opinion in Neurobiology. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW, King AE. Prolonged primary afferent-induced alterations in dorsal horn neurones, an intracellular analysis in vivo and in vitro. The Journal of Physiology (Paris) 1988;83:255–266. [PubMed] [Google Scholar]


