Abstract
Motor units were characterized in partially denervated or completely denervated and reinnervated cat medial gastrocnemius (MG) muscles where the number of innervating motor axons was severely reduced to determine (1) to what extent the nerve and muscle properties are rematched in enlarged motor units, (2) whether the normal size relationships between axon size, unit tetanic force and contractile speed are re-established, and (3) whether the type of nerve injury and/or repair affects the re-establishment of nerve and muscle properties.
Single MG units were sampled in (1) partially denervated muscles and in reinnervated muscles after either (2) crushing or (3) transecting the nerve and suturing its proximal end to either the distal nerve stump (N-N), or (4) directly to the muscle fascia (N-M).
The majority (75-88 %) of motor units in all muscles were classified as S (slow), FR (fast fatigue resistant), FI (fast fatigue intermediate) and FF (fast fatigable). However, there was an increased number of FI and unclassifiable motor units compared to normal. These results suggest that motor unit properties are not entirely regulated by the reinnervating motoneurone.
Despite more overlap in the range of unit force between different motor unit types the tetanic force of each type increased in all muscles when reinnervated by few (< 50 %) motor axons. This increase in unit force was due to an expansion in motor unit innervation ratio.
The normal relationships between axon size, unit tetanic force, and contractile speed were re-established in all muscles except when reinnervated by < 50 % of their normal complement of motor units after N-M suture. This lack of correlation was due to the reduced fast glycolytic (FG) fibre size and the proportionately greater increase in force of the S units.
After reinnervation the ranges in fibre cross-sectional area within single FF units were very similar to those found within the entire FG fibre population.
These results show that when few axons make functional connections in partially denervated or reinnervated muscles the normal relationships between axon size and motor unit contractile properties are re-established provided the nerves regenerate within the distal nerve sheath. This rematching of motoneurone size and motor unit contractile properties occurs primarily because the size of the motor axon governs the number of muscle fibres it supplies.
Since the classical cross-reinnervation studies of Buller, Eccles & Eccles (1960), it has generally been accepted that there is considerable plasticity in the neuromuscular system (reviewed by Gordon & Pattullo, 1993; Vrbova, Gordon & Jones, 1995). Muscle fibre contractile properties are normally well matched to the electrical properties of the innervating motoneurone (reviewed by Kernell, 1992). Following a peripheral nerve injury many motor axons regenerate beyond the lesion site and make functional connections with the denervated muscle fibres (reviewed by Vrbova et al. 1995). Over time, the functional matching of motoneurone electrical and muscle fibre contractile properties are re-established (Gordon & Stein, 1982a, b). Because cut axons reinnervate fibres formerly belonging to several different types of motor units considerable conversion of muscle fibre properties must occur to convert the initial heterogeneous population of fibres into a homogeneous population that matches the electrical properties of the innervating motoneurone (Kugelberg, Edstrom & Abbruzzes, 1970).
Developmentally, the regulation of muscle fibre contractile and metabolic properties has generally been attributed to some property of the innervating motoneurone (Vrbova et al. 1995). However, following axonal regeneration, several studies at the single motor unit level have indicated that neural conversion of reinnervated muscle fibres properties is incomplete (Dum, O'Donovan, Toop, Tsairis, Pinter & Burke, 1985; Gordon, Stein & Thomas, 1986; Foehring, Sypert & Munson, 1986b; Gillespie, Gordon & Murphy, 1987; Gordon, Thomas, Stein & Erdebil, 1988; Cope, Webb & Botterman, 1991; Unguez, Roy, Pierotti, Bodine-Fowler & Edgerton, 1995). The factors limiting complete neural conversion of muscle fibre properties following regeneration in the adult are not known. One possibility is that, once denervated, the contractile and metabolic properties of muscle fibres are less conducive to regulation by the innervated motoneurone. Intrinsic differences may also exist between different muscle fibre types that limit the range in which their properties can be modulated by the innervating motor axon (Miller & Stockdale, 1987). Alternatively, incomplete conversion of motor unit properties may not be due to some aspect of the muscle fibres themselves, but due to the fact that injured motoneurones are compromised in their capacity to respecify muscle fibre contractile and metabolic properties.
One aspect of regeneration that is often overlooked is that following nerve injuries many axons fail to reinnervate denervated muscle fibres especially when the cut axons are sutured directly to the muscle (Gordon & Stein, 1982b; Fu & Gordon, 1995). Despite this fact, few regeneration studies have paid close attention to the success of reinnervation in terms of the number of reinnervated motor units and recovery of motor unit force. The number of muscle fibres innervated by a single motoneurone (i.e. innervation ratio) is closely correlated with axon size and motor unit force in both normal (Bodine, Roy, Eldred & Edgerton, 1987; Totosy de Zepetnek, Zung, Erdebil & Gordon, 1992; Rafuse & Gordon 1996a) and reinnervated muscles (Totosy de Zepetnek et al. 1992; Rafuse & Gordon, 1996a). Regenerating motor axons have the capacity to form enlarged motor units (i.e. increase motor unit innervation ratio) when few axons reinnervate the muscle, provided they regenerate along the distal nerve sheath (Rafuse & Gordon, 1996a). Whether regenerating motoneurones supplying an enlarged number of muscle fibres have a reduced capacity to re-establish normal motor unit properties compared with neurones reinnervating a normal complement of fibres is not known. Evidence for such a possibility comes from the study of Käser & Müntener (1989). In their study, omohyoid axons were forced to innervate sternomastoid muscles; a muscle that contains three times more fibres than the omohyoid muscle. Under such conditions, muscle fibre type conversion was significantly slower than would be expected in self-reinnervated muscles. This delay in fibre conversion could be explained if motoneurones supplying a highly enlarged peripheral field (i.e. increased innervation ratio) are compromised in their capacity to respecify muscle fibre properties (Käser & Müntener, 1989).
In this study we used three surgical protocols to systematically investigate (1) whether axotomy per se compromises the capacity of a motoneurone to fully respecify the properties of the muscle fibres it reinnervates and/or (2) whether intrinsic differences exist between adult muscle fibres which resist full conversion by the novel innervation. In the first surgical protocol cat medial gastrocnemius (MG) muscles were partially denervated. In partially denervated muscles, intact (i.e. non-axotomized) motoneurones not only innervate their original muscle fibres, but also sprout to reinnervate ‘foreign’ muscle fibres that were denervated at the time of the partial denervation. The number of foreign fibres reinnervated after sprouting increases with the extent of partial denervation. In the second surgical protocol the MG nerve was crushed. Crushed, axotomized motoneurones reinnervate their original muscle fibres because the regenerating axons follow their original pathways back to their original muscle fibres (Kugelberg et al. 1970). In addition to crushing the nerve, the number of regenerating axons was experimentally reduced to force the axotomized motoneurones to reinnervate more muscle fibres than normal (see Rafuse & Gordon, 1996a). Therefore, in this surgical protocol, the reinnervated motor units include original and foreign muscle fibres. In the third surgical protocol, the MG nerve was transected and either the proximal and distal nerve stumps were surgically reunited (nerve-nerve suture: N-N) or the proximal nerve was sutured directly to the denervated MG muscle (nerve-muscle suture: N-M). In both cases, regenerating axons reinnervate foreign muscle fibres because the regenerating axons do not follow their original pathways (Kugelberg et al. 1970). In previous studies (Rafuse & Gordon, 1996a, b) we showed that transected axons can only form enlarged motor units (i.e. increased innervation ratio) provided they grow along the distal endoneurial nerve sheath (i.e. after N-N suture, but not after N-M suture).
Comparing reinnervated motor unit properties after the first and second protocols tests the hypothesis that axotomy per se compromises the ability of motoneurones to regulate the properties of the denervated muscle fibres they reinnervate. In both cases, motor units include both original and foreign muscle fibres. However, motoneurones are only axotomized when the MG nerve is crushed. Comparing motor unit properties in reinnervated muscles after crush and N-N suture tests the second hypothesis that denervated muscle fibres retain their original properties and resist respecification by the novel innervation. In both conditions, motoneurones are axotomized but reinnervated motor units after crush injuries include many original muscle fibres in contrast to those after N-N suture. If motoneurone properties are more fully re-established after a crush than after N-N suture, we can conclude that intrinsic differences exist between muscle fibres which limit the neural influence of reinnervating motoneurones. Finally, comparing motor unit properties after N-N and N-M sutures tests whether the capacity of a motoneurone to respecify motor unit properties is compromised when it is forced to reinnervate a larger number of muscle fibres than normal. When few axons reinnervate a denervated muscle, regenerating axons can form enlarged motor units (i.e. innervate more muscle fibres than normal) after N-N sutures, but not after N-M sutures (Rafuse & Gordon, 1996a, b). Consequently, if the capacity of a motoneurone to convert muscle fibre properties is reduced when it supplies an enlarged number of muscle fibres then one would predict that motor unit properties should be more fully re-established in muscles reinnervated after N-M sutures than after N-N sutures.
METHODS
Initial surgery
A total of forty-four cats (21 females, 23 males), with a mean ( ± s.e.m.) weight of 3.2 ± 0.08 kg, were used in this study. Six of the cats were unoperated, control animals. Thirty-eight cats received an initial surgery between 3 and 18 months (mean ± s.e.m., 6.8 ± 0.7 months) prior to the final acute experiment. Under surgical anaesthesia (intraperitoneal injection of 25 mg kg−1 sodium pentobarbitone; Somnotol) and using sterile procedures, the MG muscle was either partially denervated (10 cats) or completely denervated by (1) crushing the MG nerve (7 cats) or (2) sectioning the nerve 15-20 mm from the muscle and suturing the proximal end to the distal nerve stump (N-N suture; 11 cats) or (3) directly to the muscle fascia approximately 5-10 mm caudal to the original nerve entry point (N-M suture; 10 cats). The number of motor axons capable of reinnervating the MG muscle was reduced by sectioning one of the two spinal roots (L7 and S1) that contribute innervation to the triceps surae muscles of the cat. The cats were housed in large cages that permitted normal walking and playful activities. All experiments were performed in strict accordance with Canadian animal Ethics Committee guidelines.
Motor unit isolation, characterization and glycogen depletion protocol
All animals were glucose loaded by adding glucose (5 %) to the drinking water 3-4 days prior to the final acute experiment to increase the contrast between glycogen-depleted and non-depleted fibres (see below). MG motor units were isolated and characterized in six control and thirty-five experimental cats. Motor units were isolated between 3 and 5 months (mean ± s.e.m., 4.1 ± 0.16 months; n = 16 cats) or 9-18 months (mean ± s.e.m., 11.4 ± 0.53 months; n = 17 cats) after the initial surgery. No significant difference in motor unit properties was found at the two different time points for any of the surgical manipulations performed (see Results). As a result, motor units characterized at the different time points can be directly compared with one another. The surgical isolation of the MG muscle and the contributing L7 and S1 ventral roots and the motor unit recording procedures have previously been described in detail (Rafuse, Gordon & Orozco, 1992; Rafuse & Gordon, 1996a). Briefly, the MG muscle and its innervation were surgically isolated for EMG and force recordings in response to stimulation of single axons or the MG nerve. Evoked action potentials of single MG motor nerves were recorded extracellularly using a triphasic electrode configuration in which EMG pickup was minimized (Gordon et al. 1986). The distal tendon of the MG muscle was fastened to a force transducer for recording isometric whole muscle (Grass FT 10) and motor unit force (Kulite transducer). Single motor units were isolated by teasing L7 and S1 ventral roots exposed by a dorsal laminectomy.
Criteria for isolation of a single motor unit were the recording of an all-or-none action potential, single EMG and twitch response upon stimulation of a teased ventral root filament. Motor units were classified as slow (S), fast fatigue resistant (FR), fast fatigue intermediate (FI) or fast fatigable (FF) on the basis of the ‘sag’ of unfused tetanic contractions and the degree of fatigue demonstrated during repetitive contractions, as previously described (Burke, Levine, Tsairis & Zajac, 1973). The motor units were subjected to a routine number of tests for recording twitch and tetanic forces, neural action potential peak-to-peak amplitude, conduction velocity, and for physiological classification. Nerve and muscle action potentials and isometric force were averaged on-line by a PDP 11 computer (Transduction Ltd) in response to (1) thirty stimuli at a 1 Hz repetition rate (twitch response), (2) six trains of twenty-one stimuli at 100 Hz (maximal tetanic response), (3) thirty stimuli at a 1 Hz repetition rate (potentiated twitch response), (4) an 800 ms tetanic train using interpulse intervals of 1.25 × contraction time (to demonstrate the presence or absence of ‘sag’ of the tetanic response for F (fast) and S motor units, respectively, (5) a 300 ms tetanic train of thirteen pulses at 40 Hz repeated every 1 s for 2 min (to assess the motor unit fatigue index calculated as a ratio of the tetanic forces measured at 2 and 0 min of the fatigue test). At least 10 % of the total motor unit population was sampled for adequate representation as described previously (Gordon et al. 1986; Rafuse et al. 1992; Rafuse & Gordon, 1996a). This represents more than twenty-eight motor units in control MG muscles. The percentage of the total motor unit population sampled in the experimental animals was higher (> 25 %) because the same absolute number of motor units was sampled in muscles that often contained far fewer motor units.
Because the proportion of MG motoneurones within the two contributing spinal nerves varies substantially between cats (Rafuse et al. 1992) we calculated the number of motor units in the reinnervated muscles (MUN) by dividing whole muscle tetanic force by the mean motor unit tetanic force (see also Rafuse & Gordon, 1996a, b). This method has previously been shown to be a reliable technique for calculating motor unit number in a number of different animal protocols (Jansen & Fladby, 1990). The proportion of reinnervated motor units (%MU) was then calculated to be:
where MUN is the estimated number of reinnervated motor units (i.e. whole muscle tetanic force/mean motor unit tetanic force) and 280 is the number of motor units that normally innervates the cat MG muscle (Boyd & Davey, 1968).
One to two hours after the physiological typing of motor units a single motor unit was chosen to be repetitively stimulated to deplete its muscle fibres of their glycogen stores. The procedure for depleting glycogen from muscle fibres belonging to a single cat MG motor unit has been previously described in detail (Rafuse & Gordon, 1996a). Briefly, the motor unit was stimulated by tetanic trains of ten pulses at 40 Hz. The interval between trains varied between 1 and 0.5 s. The amplitude of the EMG was carefully monitored and if it decreased by more than 20 % the number of pulses within a train was decreased or the interval between trains was increased. This pattern of stimulus was continuously applied until the motor unit force decreased to < 10 % of its original value. The interval between trains was then increased from 0.5 to 2 s for 2-5 min after which the inter-train interval was reduced to 0.5 s. This glycogen-depletion protocol was repeated 3 to 5 times. Immediately following the glycogen depletion protocol, the MG muscles in both hindlimbs were quickly excised, weighed and placed in cooled saline. All animals were killed with an overdose of Somnotol. The muscles were cut into five blocks along the muscle longitudinal axis, fixed to a piece of cork with tissue freezing medium, frozen in a pool of melted isopentane that was cooled in liquid nitrogen and immediately placed in a freezer (-70°C) for storage.
Muscle histochemistry and fibre measurements
Serial cross-sections, 10 μm thick, were cut from each muscle block and stained for myofibrillar ATPase with acid preincubation medium modified from Brooke & Kaiser (1970) and with alkaline preincubation medium modified from Guth & Samaha (1970) as described in detail by Gordon et al. (1988). Glycogen-depleted muscle fibres were identified as negatively stained fibres using the periodic acid-Schiff (PAS) stain. The nomenclature used for the histochemically identified muscle fibres (SO, slow oxidative; FOG, fast oxidative glycolytic; FG, fast glycolytic) is that of Peter, Barnard, Edgerton, Gillespie & Stempel (1972). Muscle fibre cross-sectional area (CSA) was measured with a microcomputer digitizing software program, (JAVA, Jandel Scientific). This system was linked from the computer via a colour video camera (Sony).
Statistical analysis
Statistical difference between two means was determined using Student's t test. Regression lines were fitted according to the least mean squares criterion and are only drawn through the data points if they are significantly different from 0 at the 95 % confidence level.
Data from different animals were pooled together only if the distributions of the parameters measured were not significantly different from each other, as determined by the non-parametric Kruskal-Wallis test (for example see Fig. 1, Rafuse & Gordon, 1996a). Significant differences between cumulative distributions were tested for by applying the Kolmogorov-Smirnov test.
Figure 1. Single motor unit contractile properties in normal, partially denervated, or reinnervated MG muscle.
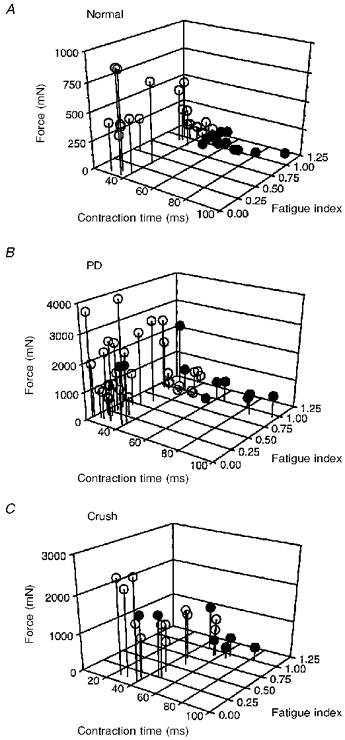
MG motor units in 1 normal (A), 3 partially denervated (PD; B), and 2 reinnervated muscles after MG nerve crush (C) plotted as a function of twitch contraction time, fatigue index and tetanic force on 3-dimensional graphs. •, motor units that did not ‘sag’ during unfused tetanic contractions (type S units); ○, motor units that ‘sagged’ during unfused tetanic contractions (type F units). Partially denervated (B) and reinnervated (C) muscles contain ≈20 % of their original complement of motor units. Despite a continuous distribution of unit twitch contraction times and tetanic force, S units in normal muscles show little overlap with F units with respect to these contractile properties. The distribution of motor units is similar to normal in partially denervated and reinnervated muscles after nerve crush apart from a few large motor units that show atypical ‘no sag’ characteristics with fast contraction times and/or low fatigue indices.
RESULTS
Motor unit properties in MG muscles reinnervated by few motor axons
Partially denervated muscles and reinnervated muscles after crush injuries
Motor axons in partially denervated muscles, and muscles reinnervated after a crush injury innervate their original muscle fibres plus ‘foreign’ muscle fibres left denervated by section of one of the two contributing spinal roots (see Methods for details). The main difference between the two conditions is that motoneurones are only axotomized after the crush injury. Interestingly, the reinnervated motor unit properties in both conditions were qualitatively distributed in the same characteristic way as normal (Figs 1-5). The most forceful motor units were the fastest, most fatigable, and were classified as FF on the basis of ‘sag’ (during unfused tetanic contractions) and fatigability. Motor units with intermediate forces were fast, fatigue resistant, and ‘sagged’ during unfused tetanic contractions (FR). The lowest forces were developed by the slow and fatigue-resistant motor units that did not exhibit ‘sag’ (i.e. type S units) (Fig. 1).
Figure 5. Distribution of motor unit fatigue index in normal and reinnervated muscles.
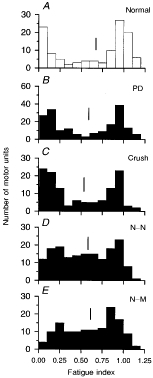
Frequency histograms of motor unit fatigue index sampled from normal (A), partially denervated (B) and reinnervated muscles after nerve crush (C), N-N (D) or N-M sutures (E). The distribution of fatigue index values is bimodally distributed in normal, partially denervated and reinnervated muscles after nerve crush, but is less discretely divided into 2 modes in muscles reinnervated by completely transected nerves (i.e. N-N and N-M sutures). Mean fatigue index is indicated by a vertical line.
Quantitatively, motor unit forces increased in these reinnervated muscles to compensate for the reduced number of motor units after section of one of the two contributing spinal roots (Fig. 2). There was little change in motor unit contractile speed, as measured by twitch contraction time (Fig. 3) and half-fall times (Fig. 4).
Figure 2. Distribution of motor unit tetanic forces in normal and reinnervated muscles.
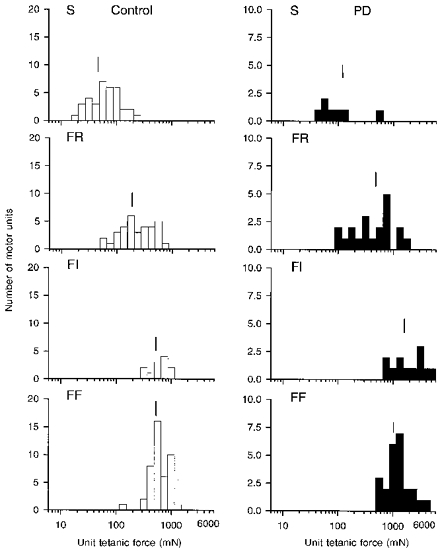
Frequency histograms of tetanic force developed by FF, FI, FR and S units sampled in 2 normal and 2 partially denervated (PD) muscles innervated by ≈20 % (mean ± s.e.m., 19.8 ± 3.7 %) of its normal complement of motor units. The force of all 4 motor unit types increased significantly in partially denervated muscles, but the range remained the same. Mean values are indicated by the vertical line to show that S < FR < FI = FF in normal and partially denervated muscles.
Figure 3. Distribution of unit twitch contraction times in normal and reinnervated muscles.
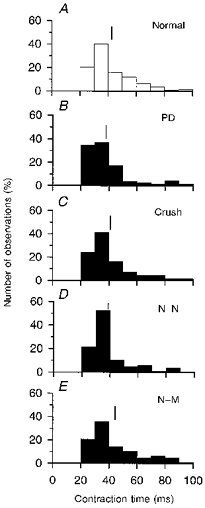
Frequency histograms of unit twitch contraction times (ms) in normal (A), partially denervated (B) and reinnervated muscles after nerve crush (C), nerve transection with N-N (D) or N-M sutures (E). Mean ( ± s.e.m.) number (%) of motor units in each experimental condition are: 73 ± 4.7 % (B),76 ± 6.9 % (C), 77 ± 5.2 % (D) and 80 ± 5.1 % (E). Motor units were sampled from 6, 10, 7, 9 and 7 cats in A, B, C, D and E, respectively. The mean and range in unit twitch contraction times are similar to normal in B-E. Means are shown by vertical lines and are ( ± s.e.m.; in ms): 42.2 ± 1.2 (A), 42 ± 2 (B), 41.6 ± 1.7 (C),42.4 ± 1.6 (D) and 46.3 ± 1.8 (E).
Figure 4. Distribution of motor unit half-fall times in normal and reinnervated muscles.
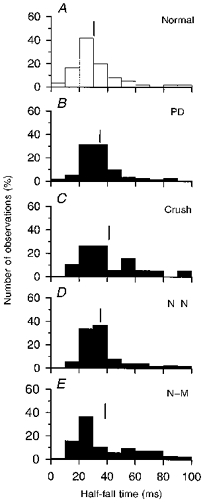
Frequency histograms of motor unit half-fall times in normal (A), partially denervated (B) and reinnervated muscles after nerve crush (C), N-N (D) or N-M sutures (E). Motor units were sampled from the same muscles as in Fig. 3. Means are shown by vertical lines and are ( ± s.e.m.; in ms): 30.2 ± 2.1 (A), 38.2 ± 2.1 (B), 39.6 ± 3.3 (C), 40.7 ± 2.3 (D) and 42.7 ± 2.1 (E).
However, there were noticeable differences between the normal and experimental muscles. Both partially denervated muscles and reinnervated muscles after nerve crush contained more motor units with fatigue indices between FF and FR motor units (i.e. FI units), particularly in muscles after MG nerve crush (Figs 1 and 5; Table 1). This becomes evident from the linking of the two peaks of the bimodal distribution of fatigue indices in Fig. 5. In addition, both partially denervated and reinnervated muscles contained some uncharacteristic S motor units which were unclassifiable using the criteria of ‘sag’ and fatigability (Fig. 1B and C, and Table 1). As shown in the examples in Fig. 6, normally when a motor unit twitch contraction time is < 40 ms (Fig. 6A) it exhibits ‘sag’ during an unfused tetanus (Fig. 6B). However, some reinnervated units with short contraction times (< 40 ms; Fig. 6C) did not ‘sag’ during an unfused tetanus (Fig. 6D). In all reinnervated muscle some S units (classified on the basis of ‘no sag’) had atypically short contraction times (< 40 ms) and/or were fatigable (fatigue index < 0.25). These abnormal motor units are labelled, for the purpose of this study, as unclassifiable (UC) motor units (Tables 1 and 2).
Table 1.
Percentage of motor unit types in cat MG muscles reinnervated by > 50 % or < 50 % of their normal complement of motoneurones
| Type | S | FR | FI | FF | UC | n | |
|---|---|---|---|---|---|---|---|
| > 50 % of MUs | Normal | 25 ± 4.6 | 26 ± 4.0 | 8 ± 4.0 | 42 ± 4.0 | – | 5 |
| PD | 13 ± 7.8 | 26 ± 11 | 15 ± 5.5 | 33 ± 5.7* | 14 ± 6.6 * | 4 | |
| Crush | 17 ± 4.5 | 8 ± 4.5 * | 18 ± 7.4 | 39 ± 5.5 | 17 ± 0.8 * | 3 | |
| N-N | 25 ± 11 | 7 ± 9.5 * | 15 ± 11 * | 34 ± 25 | 29 ± 24 * | 3 | |
| N-M | 14 na | 27 na | 32 na | 11 na | 16na | 1 | |
| < 50 % of MUs | Normal | 25 ± 4.6 | 26 ± 4.0 | 8 ± 4.0 | 42 ± 4.0 | – | 5 |
| PD | 23 ± 27 | 21 ± 14 | 17 ± 13 | 26 ± 13 * | 11 ± 8.7 * | 6 | |
| Crush | 18 ± 3.5 | 10 ± 14 * | 14 ± 10 | 26 ± 17 | 37 ± 13 * | 3 | |
| N-N | 17 ± 10 | 18 ± 12 | 37 ± 18 * | 21 ± 6.0 * | 8 ± 10 * | 5 | |
| N-M | 14 ± 13 | 19 ± 14 | 26 ± 20 * | 13 ± 13 * | 28 ± 25 * | 5 |
Means ± s.d.
Significantly different from normal at P < 0.05.
Insignificant number of samples to perform Student's two-tailed t test. UC, unclassifiable motor units. n, number of animals.
Figure 6. Single motor unit twitch and unfused tetanic contractions.
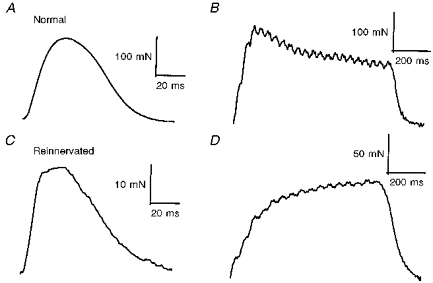
An example of a single fast motor unit (contraction time, 31 ms), sampled from a normal muscle (A) showing a typical ‘sag’ response during an unfused tetanic contraction (B). An example of a single fast contracting motor unit (contraction time, 28 ms), sampled from a reinnervated muscle after N-M suture (C) showing an atypical ‘no sag’ response (D). These atypical fast contracting motor units cannot be classified as fast or slow on the basis of ‘sag’ of unfused tetanic contraction and speed of contraction. They are therefore considered ‘unclassifiable’.
Table 2.
Percentage of motor unit types in cat MG muscles reinnervated (Reinn.) by motoneurones 3-5 or 9-14 months after surgery
| Type | S | FR | FI | FF | UC | n | |
|---|---|---|---|---|---|---|---|
| 3-5 months | Normal | 25 ± 4.6 | 26 ± 4.0 | 8 ± 4.0 | 42 ± 4.0 | – | 5 |
| PD | 15 ± 6.9 * | 26 ± 9.4 | 21 ± 13 * | 30 ± 10 | 12 ± 7.6 * | 6 | |
| Crush | 17 ± 3.6 | 5 ± 5.4 * | 16 ± 10 | 36 ± 12 | 26 ± 11 * | 5 | |
| Reinn. | 19 ± 9.6 | 18 ± 14 | 32 ± 16 * | 14 ± 11 * | 16 ± 24 * | 10 | |
| 9-14 months | Normal | 25 ± 4.6 | 26 ± 4.0 | 8 ± 4.0 | 42 ± 4.0 | – | 5 |
| PD | 11 ± 13 * | 25 ± 23 | 11 ± 6.9 | 25 ± 14 * | 16 ± 15 * | 4 | |
| Crush | 15 ± 8.0 na | 22 ± 15 na | 18 ± 3.5 na | 44 ± 30 na | 7 ± 7 na | 2 | |
| Reinn. | 19 ± 19 | 21 ± 17 | 23 ± 26 | 37 ± 36 | 13 ± 23 * | 4 |
Means ± s.d.
Significantly different from Normal at P < 0.05.
Insignificant number of samples to perform Student's two-tailed t test. UC, unclassifiable motor units. n, number of animals.
In summary, the motor unit properties, and how they differed from normal, were very similar in both partially denervated muscles and in reinnervated muscles after MG nerve crush. In both conditions, the motoneurones contain the same relative number of foreign and original muscle fibres when innervated by the same number of motor axons. Therefore, axotomy of motoneurones per se does not appear to be an important contributing factor to the incomplete recovery of normal motor unit properties following crush injuries.
N-N and N-M sutures
Regenerating motor axons do not reinnervate their original muscle fibres and only have the capacity to form enlarged motor units (i.e. increase innervation ratio) provided they regenerate along the distal endoneurial nerve sheath (Rafuse & Gordon, 1996a, b; see also Gordon & Stein, 1982b). Therefore, when few axons (∼20-30 % of the original complement) reinnervated the muscle, motor unit tetanic force only increased above normal values in muscles reinnervated after N-N, but not N-M suture. This is clearly evident in the examples shown in Fig. 7. Muscles reinnervated after N-N sutures contained a high number of motor units that produce > 1000 mN of force, while those reinnervated after N-M sutures did not (Figs 7 and 8; note that the scale of the y-axis in Fig. 7A is twice that of Fig. 7B). In addition, the different motor unit types were more similar in tetanic force output such that the normal order of motor units according to force (i.e. S < FR < FI = FF) was less evident, particularly in muscles reinnervated after N-M suture (see also Rafuse & Gordon, 1996a).
Figure 7. Motor unit contractile properties in reinnervated muscles.
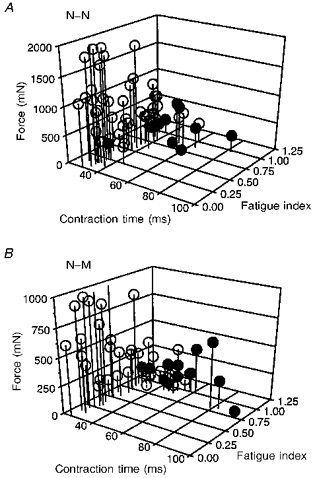
Motor units sampled from reinnervated muscles after complete MG nerve transection and repair with either N-N (A) or N-M sutures (B) plotted as in Fig. 1. In each case, motor units were sampled from 2 muscles reinnervated by 36 ± 2.8 % (A) and 41 ± 6.7 % (B) of their normal complement of motor units. Unit force increased in reinnervated muscles after N-N, but not after N-M suture (note z-axis is 2 times greater in A than B). Generally, the distribution of motor units in reinnervated muscles is similar to normal with the exception of a marked increase in FI units (fatigue index < 0.25 < 0.75) and a number of atypical motor units that did not ‘sag’ but had relatively fast contraction times (< 40 ms) or low fatigue indices (< 0.75).
Figure 8. Distribution of unit tetanic force from reinnervated muscles reinnervated by few motoneurones.
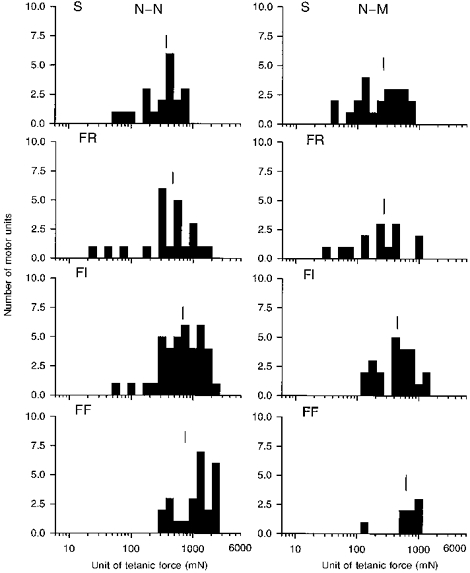
Frequency histogram of tetanic force developed by each of FF, FI, FR and S units in muscles reinnervated by ≈25 % of their normal complement of motor units after complete nerve transection and repair with N-N or N-M sutures. The force of the S units increased by a greater extent than the F units. Mean values are indicated by the vertical line to show that S < FR < FI = FF in normal and in reinnervated muscles after N-N and N-M suture.
Even though the general features of the normal interrelationships of motor unit force, contraction time and fatigue index were preserved in the reinnervated muscles after MG nerve transection and either N-N or N-M sutures (Fig. 7) and the distributions of parameters of contractile speed were very similar to normal (Figs 3 and 4), there were several striking deviations from normal. The increased number of FI units observed in partially denervated muscles and reinnervated muscles of MG nerve crush was even more pronounced in muscles reinnervated after either N-N or N-M suture. The large number of FI motor units, interposed between the two peaks of fatigable and non-fatigable motor units, dramatically altered the normal bimodal distribution of fatigue indices (Fig. 5). Many motor units were unclassifiable because they had contraction times in the S range, but either evoked a ‘sag’ response or were fatigable (Fig. 7 and Table 1).
Therefore, under conditions where reinnervated motor units contain predominantly ‘foreign’ muscle fibres, many motor units have intermediate fatigue characteristics and/or ‘mixed’ properties that make them unclassifiable in the normal sense. These findings suggest that reinnervating motor axons do not have the capacity to completely convert muscle fibre properties. As a result, there remains a heterogeneous population of muscle fibre properties within a single reinnervated motor unit. Because the motor unit populations were very similar in reinnervated muscles after N-N and N-M sutures, it is apparent that the heterogeneity in muscle fibre properties is related more to the surgical injury, namely nerve transection, which leads to reinnervation of foreign muscle fibres. Finally, because motor units increased in size (i.e. increased innervation ratio) in the former, but not in the latter condition, these results also indicate that motoneurones, which are forced to reinnervate an enlarged number of muscle fibres, have the same capacity to re-establish motor unit properties as motoneurones which supply a normal complement of muscle fibres. In agreement with these results, motor unit property profiles were similar in reinnervated muscles containing either a large (> 50 % of the normal complement of motor units) or small (< 50 %) number of motor units (Table 1).
Time course of reinnervation
Motor units were characterized in reinnervated muscles between 3 and 5 months (4.1 ± 0.16 months) and between 9 and 14 months (11.4 ± 0.53 months) after the initial surgery. As shown in Table 2 there was some conversion of FI units with time indicating that motor unit fatigue properties are not fully converted by 3 to 5 months. However, even after 9-14 months of reinnervation there remained a significantly higher proportion of UC units compared to normal and there was still a trend for there to be more FI units than normal. Consequently, reinnervating motor axons are unable to completely respecify muscle fibre contractile properties even after 9-14 months. Whether further conversion of some motor unit contractile properties would occur with longer reinnervation times is not known.
In summary, when the number of regenerating axons is experimentally reduced and motor units are forced to increase in size (i.e. increase innervation ratio), to compensate for a smaller motor unit number, the majority of motor units can be classified normally as S, FR, FI and FF. These results support the view that motor unit characteristics are, at least in part, regulated by the reinnervating axon. However, the higher proportion of FI and UC motor units in reinnervated muscles after N-N and N-M suture, supports the hypothesis that reinnervating motor axons cannot completely convert foreign muscle fibre properties (see Discussion). Finally, the capacity for motoneurones to re-establish motor unit properties is not compromised even when they are forced to reinnervate 4-5 times more muscle fibres than normal.
Motor unit contractile force and size relationships
Partially denervated muscles and reinnervated muscles after crush injuries
Rafuse et al. (1992) demonstrated that, in partially denervated muscles, the tetanic force of all motor units increased by the same factor to compensate for the loss of motoneurones. In agreement with this data, tetanic force of each motor unit type increased by a similar extent in partially denervated muscles containing only ∼20 % of their original complement of motor units. Consequently, the normal difference between S, FR and FF unit tetanic force was maintained (PD; Fig. 2). When only ∼20 % of the original complement of motor units reinnervated the muscle after a crush injury the normal order of unit force (i.e. S < FR < FI = FF) was re-established because the force of each unit type increased (data not shown; see Rafuse & Gordon, 1996a). However, unlike the observations in partially denervated muscles, the force of the S units increases proportionately more than F units (Rafuse & Gordon, 1996a; see also Fig. 1).
The normal size relationship between axon potential amplitude and motor unit tetanic force is shown in Fig. 9A. Because the range in motor unit force cannot be accounted for solely by differences in size of the SO, FOG and FG muscle fibres (Fig. 10) these results indicate that the number of muscle fibres per motoneurone (i.e. the innervation ratio) is directly related to axon potential amplitude and, therefore, axon size. A similar strong correlation returned in muscles innervated by ∼25 % of their normal complement of motor units after partial denervation (Fig. 9C) or MG nerve crush (Fig. 9E). The slopes ( ± s.e.) of the regression lines were similar: 0.12 ± 0.02 (r = 0.60), 0.15 ± 0.03 (r = 0.83) and 0.19 ± 0.06 (r = 0.53) for normal, partially denervated and reinnervated muscles after nerve crush, respectively. The slopes are significantly different from zero, and not different from each other (P < 0.05).
Figure 9. Relationship between axon potential amplitude and twitch contraction time with unit tetanic force.
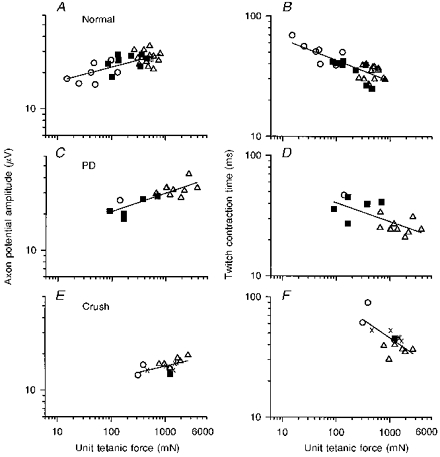
Axon potential amplitude and twitch contraction time plotted as a function of tetanic force in motor units from normal (A and B), partially denervated muscle innervated by 25 % of its motor units (C and D), and muscles reinnervated after nerve crush by 25 % of its motor units (E and F). ○, S units; ▪, FR units; ▵, FI and FF units;, unclassifiable units. The slopes ( ± s.e.) of the regression lines for axon potential amplitude and tetanic force (0.12 ± 0.02, 0.15 ± 0.03 and 0.19 ± 0.06; r = 0.60, 0.83 and 0.53, respectively) are all significantly different from zero (P < 0.01-0.05) and not different from each other. The negative slopes of the regression lines for contraction time and tetanic force (0.14 ± 0.02, 0.16 ± 0.05 and 0.28 ± 0.07; r = 0.66, 0.68 and 0.70, respectively) are also significantly different from zero. Slopes of B and D are not different from each other; however, the slope in F is significantly steeper.
Figure 10. Ranges in cross-sectional area (CSA) of each muscle fibre type in normal and reinnervated muscles.
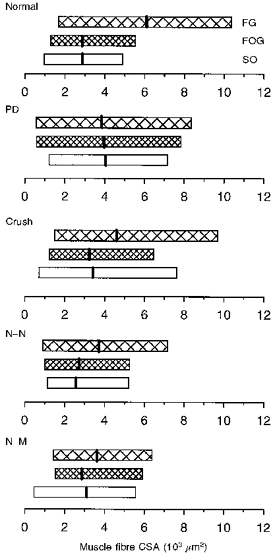
Ranges in CSA of each muscle fibre type in normal, partially denervated (PD) and reinnervated muscles after nerve crush or nerve transection with N-N or N-M sutures. All experimental muscles were reinnervated by ≈25 % of their normal complement of motor units. Mean ( ± s.e.m.) CSAs (vertical bars) for FG (fast glycolytic), FOG (fast oxidative glycolytic) and SO (slow oxidative) fibres, respectively, are (103μm2): 6.2 ± 0.1, 2.8 ± 0.07 and 2.8 ± 0.06 (Normal); 4.6 ± 0.06, 3.2 ± 0.05 and 3.3 ± 0.08 (Crush); 3.5 ± 0.14, 2.7 ± 1.1 and 2.5 ± 0.07 (N-N); 3.6 ± 0.04, 2.8 ± 0.05 and 3.0 ± 0.04 (N-M).
The inverse relationship between twitch contraction time and motor unit tetanic force in normal MG muscles (Fig. 9B) was also re-established in partially denervated (Fig. 9D) and reinnervated muscles (Fig. 9F) after nerve crush. The slopes ( ± s.e.) of the regression lines were: -0.14 ± 0.03 (r = 0.66), -0.16 ± 0.05 (r = 0.68) and -0.28 ± 0.07 (r = 0.70) for normal, partially denervated and reinnervated muscles after nerve crush, respectively. All slopes are significantly different from zero (P < 0.05). Following nerve crush, the size of the reinnervated S unit increased proportionately more than the FF motor units (▵) as shown by the larger shift of the S motor units (○) along the x-axis in Fig. 9E. This greater enlargement of S motor units accounts for the steeper relationship between twitch contraction and motor unit tetanic force in reinnervated muscle after nerve crush compared to normal (cf. Fig. 9B and F).
Taken together, these results show that intact motoneurones, in partially denervated muscles, and crushed regenerating axons branch and innervate muscle fibres in a size-dependent manner even when they reinnervate many more muscle fibres than normal.
N-N and N-M sutures
S units increased their force-generating capacity by a greater extent than F motor units in muscles reinnervated by ∼25 % of their normal complement of axons after nerve section and repair (N-N and N-M) (Fig. 7; see also Foehring, Sypert & Munson, 1986a; Rafuse & Gordon, 1996a). The smaller increase in force of F motor units can partly be explained by the decrease in size of the largest FG muscle fibres (Fig. 10). As shown in Fig. 10, the CSAs of the SO, FOG and FG fibres were very similar in reinnervated muscles (N-N and N-M), in contrast to normal where FG fibres were usually larger. However, the increase in force of the S units must be attributable to a relatively larger increase in innervation ratio since the size of the SO fibres did not change significantly following reinnervation (Fig. 10).
The normal correlation between axon potential amplitude and motor unit tetanic force was also observed in reinnervated muscles, regardless of the type of surgical repair (i.e. N-N or N-M), provided the muscle was reinnervated by > 50 % of its motor units (Fig. 11A and C). If < 50 % of the normal complement of motor units reinnervated the MG muscle, the correlation between axon potential amplitude and motor unit force was re-established after N-N (Fig. 11B), but not after N-M suture (Fig. 11D). Motor unit force reflects innervation ratio when changes in muscle fibre CSA are taken into account. It is likely that the apparent loss of the relationship between axon size and motor unit force after N-M suture is due to both the reduced forces developed by the FF motor units, which contain smaller than normal FG muscle fibres, and the higher forces developed by the S motor units. The restricted range in motor unit force in muscles with N-M sutures may obscure the correlation between axon size and innervation ratio which is evident in the partially denervated muscles and the muscle reinnervated after crush injury or N-N suture.
Figure 11. Size relationship between axon potential amplitude and unit tetanic force when reinnervated by few or many motoneurones.
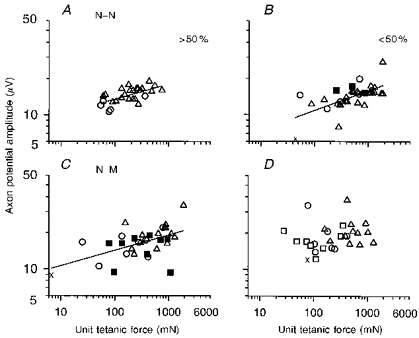
Axon potential amplitude plotted as a function of tetanic force in motor units from muscles reinnervated by > 50 % and < 50 % of their normal complement of motor units after N-N (A and B, respectively) or N-M sutures (C and D, respectively). Unit types denoted by same symbols as in Fig. 9. Muscles in A, B, C and D were reinnervated by 88, 32, 88 and 31 % of their motor units, respectively. The slopes ( ± s.e.) of the regression lines in A-C(0.13 ± 0.03, 0.13 ± 0.03 and 0.12 ± 0.02; r = 0.63, 0.58 and 0.67, respectively) are all significantly different from zero (P < 0.01-0.05) and not different from each other or from normal (Fig. 9A). The regression line fitted to the values in D is not significantly different from zero (P < 0.05) and therefore has not been drawn.
CSA of muscle unit fibres
In contrast to normal MG muscles, the muscle fibre size within a single glycogen-depleted FF motor unit spanned the same range as all the FG muscle fibres measured in the reinnervated muscle. Figure 12B and C schematizes the overlap in FG fibre size within single glycogen-depleted reinnervated FF motor units (□) with the size of non-depleted FG fibres measured in the same muscle area (τ). These results are compared with normal FF motor units in control MG muscles (Fig. 12A). Two examples of the smaller SO muscle fibres in S motor units in normal muscles are also shown for comparison. The range in CSA of reinnervated FG fibres, randomly sampled from many motor units (non-depleted fibres), was the same as the mean and range of the corresponding FG fibres in normal control MG muscles. The mean CSA of reinnervated FG muscle fibres belonging to a single FF motor unit (depleted fibres) was the same as in normal FF motor units but the range was obviously larger. The overlap in the ranges of depleted and non-depleted muscle fibres in reinnervated muscles is consistent with an incomplete neural determination of muscle fibre size (see Discussion).
Figure 12. Ranges in cross-sectional area (CSA) from single motor units in normal and reinnervated muscles.
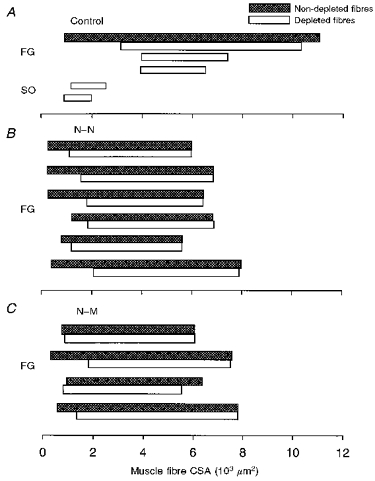
Ranges in fibre CSA from glycogen-depleted muscle unit fibres (□) and non-depleted fibres of the same histochemical types (τ) for 5 normal (3 FF units; 2 S units) and 10 reinnervated motor units (6 N-N; 4 N-M). FG, fast-glycolytic; SO, slow oxidative.
DISCUSSION
Using surgical manipulations to force axons to reinnervate many ‘foreign’ muscle fibres the results of this study (1) support the hypothesis that adult muscle properties are not fully converted by novel nerve innervation and (2) provide further evidence that a size-dependent branching of regenerating axons restores the normal relationship between axon size and motor unit force by specifying innervation ratio (number of muscle fibres per motoneurone) and not muscle fibre CSA.
Incomplete conversion of reinnervated motor unit muscle fibres
Contractile speed: dissociation of contraction time and ‘sag’
The majority of motor units in all experimental protocols were normally classified as either S, FR, FI or FF. These results support the view that muscle fibre contractile properties are, at least in part, regulated by some property of the innervating motoneurone. However, they also indicate that this respecification is incomplete. Normally, all motor units that do not ‘sag’ during unfused tenani are slow contracting and resistant to fatigue. In reinnervated muscles this clear distinction is often less clear. For example, several reinnervated motor units that did not ‘sag’ had atypically fast contraction times and/or were atypically fatigable (Figs 1 and 7; Table 1). Using the same criterion Dum et al. (1985) classified all motor units in cross-reinnervated soleus muscles as S-type motor units (i.e. did not sag) despite the fact that the muscle was reinnervated by fast motoneurones. Other studies (Gordon et al. 1986, 1988) found that some motor units in the cross- reinnervated soleus muscles exhibited ‘sag’ and a few fatigued. Once again, the ‘sag’ behaviour was dissociated from contractile speed in the majority of motor units (Gordon et al. 1986, 1988). Therefore the discrepancies revealed in the classification of reinnervated motor units strongly suggest that reinnervated muscle fibres continue to express some of their original phenotypes despite the novel innervation.
The occurrence of ‘sag’ has been described as a subtle change in the fibre active state (Burke et al. 1973) which appears to decrease the period of effective force generation of muscle fibres during the time course of the unfused tetanic contractions (Burke, Rudomin & Zajac, 1976). The different sag responses of F and S motor units may be due to differences in the sarcoplasmic reticulum between the two motor unit types (Burke et al. 1976). Fast muscle fibres have a greater volume of sarcoplasmic reticulum and contain fast isoforms of the Ca2+ release channels and Ca2+-ATPases (reviewed by Pette & Vrbova, 1992). Together, these proteins favour the fast release and uptake of Ca2+ during each action potential and may account for the characteristic sag of unfused tetanic contractions in F motor units. The ‘inappropriate’ sag responses observed by some motor units in reinnervated muscles may represent incomplete conversion of sarcoplasmic reticulum proteins by the innervating motoneurone.
The degree to which regenerating axons convert the fast or slow isoforms of regulatory and contractile proteins in reinnervated muscles is not certain. Several possible combinations of myosin heavy and light chain isoforms, regulatory proteins, ryanodine (Ca2+ release channels) and Ca2+-ATPase (Pette & Staron, 1990) are likely to account for the normal 2- to 3-fold range in contraction times in each motor unit type, even in normal muscles (Fig. 1). Co-expression of different myosin heavy and light chain isoforms in single reinnervated muscle fibres has been detected using immunohistochemistry, but not with traditional histochemical methods (Gauthier, Burke, Lowey, & Hobbs, 1983; Gillespie et al. 1987). Thus, although conversion occurs, there is good evidence for continued expression of some of the muscle fibres’ original protein isoforms.
Increased susceptibility to fatigue
We previously suggested that the significantly higher proportions of FI motor units in reinnervated muscles could be explained by the inclusion of a heterogeneous group of muscle fibres in each motor unit that retain their former oxidative/glycolytic enzyme potential to some extent despite the novel innervation (Gordon & Pattullo, 1993). Inclusion of fatigable and non-fatigable muscle fibres in the same motor unit after reinnervation would result in a motor unit with intermediate fatigue characteristics. The present study of enlarged motor units after partial denervation and nerve lesions provides a stringent test of this idea. As predicted, after partial denervation the normally small proportion of FI motor units in the MG muscle increased slightly because each motor unit now contained original and ‘foreign’ muscle fibres (Table 2; 3-5 months). Also, in agreement with this prediction, the number of FI units was greater when the nerve was transected and repaired by N-N or N-M sutures because the reinnervated motor units in these muscles contained only ‘foreign’ muscle fibres (Fig. 5; Table 2; 3-5 months). Therefore, the critical factor in determining the relative number of FI units 3-5 months after denervation appears to be the relative proportion of ‘foreign’ muscle fibres within each motor unit. In a previous study (Rafuse & Gordon, 1996a, b) we showed that regenerating cat MG motor axons can form enlarged motor unit territories only when they grow along the distal endoneurial sheath (i.e. after N-N suture, but not after N-M suture). The distal endoneurial nerve sheath appears to facilitate proximal axon branching, which in turn establishes motor unit territory size. Consequently, when few axons reinnervate the muscle the regenerating motor axons can form enlarged motor units after N-N, but not after N-M suture (Rafuse & Gordon, 1996a, b). Interestingly, the proportion of FI motor units was not significantly greater in muscles reinnervated after N-N suture compared with N-M suture, even though each motor unit was substantially larger in the former condition. These results indicate that it was the high proportion of ‘foreign’ muscle fibres within each reinnervated motor unit, rather than the absolute number of muscle fibres per motoneurone, that accounted for the high number of FI units in the reinnervated motor unit population 3-5 months after denervation.
Although there was a trend for there to be more FI units in partially denervated muscles, and reinnervated muscles after 9-14 months, the number of FI units was not statistically different from normal. This observation suggests that modification of fatigue resistance following reinnervation is a relatively slow process. Fatigue resistance is normally positively correlated with oxidative capacity, as indicated by the level of succinate dehydrogenase (SDH) activity in isolated motor units that vary in fatigability (Kugelberg & Lindegren, 1979). Consistent with incomplete conversion of muscle fibre metabolic enzymes by the reinnervating motor axon, the level of SDH activity is more divergent between fibres of a single reinnervated motor unit compared with normal motor units (Unguez et al. 1995). Biochemical evidence for higher than normal variance of oxidative and glycolytic enzymes in reinnervated rat motor units (Sesodia, Gordon & Nemeth, 1993) also provides further support for incomplete conversion of reinnervated muscle fibre properties.
Innervation ratio re-establishes the size principle
The range in muscle fibre CSA in reinnervated motor units was larger than normal, as if reinnervated muscle fibres recovered their former size irrespective of their neural supply. This is consistent with previous findings in reinnervated rat tibialis anterior (TA) muscle (Totosy de Zepetnek et al. 1992). Even in normal motor units, there is a 2.5- to 7.5-fold range in cat MG (Fig. 11A), cat TA (Bodine et al. 1987) and rat TA (Totosy de Zepetnek et al. 1992) muscles. Because it may be assumed that all muscle fibres within a single motor unit are subjected to the same neural influence, the normal variability in fibre CSA suggests that fibre size is not entirely neurally regulated (see also Totosy de Zepetnek et al. 1992). Some of the size variation is due to the fact that CSA varies along the length of each muscle fibre (Ounjian et al. 1991). In addition, there are regional differences in muscle fibre CSA along the transverse axis of the muscle (Pullin, 1977; Rafuse & Gordon, 1996b). Superficial muscle fibres tend to be larger than fibres located closer to the bone. This size variation has been attributed to intrinsic differences between muscle fibres, which are established during development (Butler, Cosmos & Brierly, 1982; Miller & Stockdale, 1987), or to differential loading conditions of muscle fibres in different regions of the muscles (Gordon & Pattullo, 1993). Neither developmentally intrinsic muscle fibre differences or differential loading conditions are under neural control.
Following reinnervation, glycogen-depleted FF motor units included FG fibres of all sizes within the entire range of the non-depleted, non-unit FG muscle fibres (Fig. 12). There was also extensive overlap in size of muscle fibres of different types in reinnervated muscles after N-N or N-M suture (Fig. 10). The abnormally wide range in muscle fibre size within a single reinnervated motor unit is also consistent with the idea that novel reinnervation does not respecify muscle fibre properties very well. With respect to fibre size, either mechanical loading or intrinsic developmental factors of muscle fibres may set the range in which muscle fibre properties can be modulated by the innervating motoneurone. The wide range of motor unit fibre size in reinnervated MG muscles is consistent with our previous findings in reinnervated rat TA muscles (Totosy de Zepetnek et al. 1992). A similar preset adaptive range was suggested for all muscle properties to explain incomplete fast to slow conversion of chronically stimulated rat muscles (Gundersen, Leberer, Lomo, Pette & Staron, 1988; Ausoni, Gorza, Schiaffino, Gundersen & Lomo, 1990).
The overall range in muscle fibre CSA is substantially less in the N-N and N-M experiments than in the control animals (Fig. 12). This smaller range is due to a decrease in size of the largest FG fibres. In a previous study (Rafuse & Gordon, 1996a), we proposed that, after MG nerve transection, axon potential conduction delays below the suture line cause asynchrony of the MG muscle contraction with its synergist muscles (see Discussion in Rafuse & Gordon, 1996a, for details). Consequently, during hindlimb extension the MG muscle fibres contract in an already shortened position and against a smaller load. Increased mechanical loading causes fibre hypertrophy, whereas contractions in an already shortened position lead to muscle fibre atrophy (Edgerton, 1978). This atrophy appears to be most detrimental to the FG fibres following reinnervation.
Normally, muscle fibre CSA and innervation ratio are significant cofactors in determining differences in motor unit force. However, because regenerating axons do not appear to respecify muscle fibre CSA, the mean CSAs between motor units in reinnervated muscles become very similar (Fig. 12). As a result, innervation ratio becomes the major factor determining the range in motor unit force (see also Totosy de Zepetnek et al. 1992). The return of the normal relationships between motor unit force, innervation ratio and axon size after reinnervation (Figs 9 and 11; see also Totosy de Zepetnek et al. 1992; Rafuse & Gordon, 1996a), without respecification of muscle fibre CSA, strongly suggests that muscle fibre size respecification is not the basis for rematching axon size and motor unit force after nerve injuries. Even when the number of motor units is substantially reduced, motor unit contractile speed, force and axon size are directly correlated as observed in normal muscles. Because motor unit force varies directly with innervation ratio and with electrophysiological measures of axon size, these results demonstrate that the number of muscle fibres reinnervated by each regenerating axon is directly related to the parent motoneurone size. These findings provide support for the view that axon size, or properties associated with size, govern the number of muscle fibres that the regenerating axons will reinnervate (see Totosy de Zepetnek et al. 1992).
The loss of the correlation between axon potential amplitude in reinnervated muscles after N-M suture, when motor unit number was < 50 % that of normal, can be accounted for by the increased force of the S motor units and the incomplete recovery of FG muscle fibre size. The findings that F motor units were, nevertheless, more forceful than S motor units suggests that these compounding factors obscure the normal relationship between innervation ratio and axon size.
Conclusions
We have demonstrated that inclusion of many ‘foreign’ muscle fibres into reinnervated motor units obscures the normal differences between motor unit types. The increase in number of UC units, the loss of the normal bimodal distribution of motor unit fatigue index, the greater overlap of force generated by S, FR, FI and FF motor units, and the overlap in size of their corresponding muscle fibre types can be explained by a limited conversion of muscle properties by reinnervating motor axons. Nevertheless, motoneurones reinnervate muscle fibres according to their size (i.e. larger axons reinnervate more fibres than smaller axons) to restore the normal size relationship between axon size and muscle fibre contractile properties. Consequently, the normal order of recruitment is maintained and motor units are still progressively recruited in order of size and force according to the size principle (Cope et al. 1991).
Acknowledgments
We are grateful for the much appreciated support of the Medical Research Council and Muscular Dystrophy Association of Canada for the current work. We also thank the Alberta Heritage Foundation for Medical Research for their personal support of V. R. as a graduate student and T. G. as a Heritage Scientist. N. Tyreman and S. Erdebil provided valuable technical assistance and Dr Roger Enoka kindly provided constructive criticism of the manuscript. The work forms part requirement for V. R.‘s PhD thesis. Dr John Munson served as the external examiner and we are grateful for his helpful comments.
References
- Ausoni S, Gorza L, Schiaffino S, Gundersen K, Lomo T. Expression of myosin heavy chain isoforms in stimulated fast and slow rat muscles. Journal of Neuroscience. 1990;10:153–160. doi: 10.1523/JNEUROSCI.10-01-00153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine SC, Roy RR, Eldred E, Edgerton VR. Maximal force as a function of anatomical features of motor units in the cat tibialis anterior. Journal of Neurophysiology. 1987;57:1730–1745. doi: 10.1152/jn.1987.57.6.1730. [DOI] [PubMed] [Google Scholar]
- Boyd IA, Davey MR. Composition of Peripheral Nerves. London: Livingston; 1968. [Google Scholar]
- Brooke MH, Kaiser KK. Three ‘myosin adenosine triphosphatase’ systems: the nature of their pH lability and sulfhydryl dependence. Journal of Histochemistry and Cytochemistry. 1970;19:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. The Journal of Physiology. 1960;150:399–416. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. The Journal of Physiology. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Cosmos E, Brierly J. Differentiation of muscle fibre types in aneurogenic brachial muscles of the chick embyro. Journal of Experimental Zoology. 1982;224:65–80. doi: 10.1002/jez.1402240108. [DOI] [PubMed] [Google Scholar]
- Cope TC, Webb CB, Botterman BR. Control of motor unit tension by rat modulation during sustained contractions in reinnervated cat muscle. Journal of Neurophysiology. 1991;65:648–656. doi: 10.1152/jn.1991.65.3.648. [DOI] [PubMed] [Google Scholar]
- Dum RP, O'Donovan MJ, Toop J, Tsairis P, Pinter MJ, Burke RE. Cross-reinnervated motor units in cat muscle. II. Soleus reinnervated by flexor digitorum longus muscles. Journal of Neurophysiology. 1985;54:837–851. doi: 10.1152/jn.1985.54.4.837. [DOI] [PubMed] [Google Scholar]
- Edgerton VR. Mammalian muscle fiber types and their adaptability. American Zoologist. 1978;18:113–125. [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. I. Long-term reinnervation. Journal of Neurophysiology. 1986a;55:931–946. doi: 10.1152/jn.1986.55.5.931. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. II. Axotomized motoneurons and time course of recovery. Journal of Neurophysiology. 1986b;55:947–965. doi: 10.1152/jn.1986.55.5.947. [DOI] [PubMed] [Google Scholar]
- Fu SU, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. Journal of Neuroscience. 1995;15:3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier GF, Burke RE, Lowey S, Hobbs AW. Myosin isoforms in normal and cross-reinnervated cat skeletal muscle fibers. Journal of Cell Biology. 1983;97:756–771. doi: 10.1083/jcb.97.3.756. 10.1083/jcb.97.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie MJ, Gordon T, Murphy PR. Motor units and histochemistry in rat lateral gastrocnemius and soleus muscles: evidence for dissociation of physiological and histochemical properties after reinnervation. Journal of Neurophysiology. 1987;57:921–937. doi: 10.1152/jn.1987.57.4.921. [DOI] [PubMed] [Google Scholar]
- Gordon T, Pattullo MC. Plasticity of muscle fiber and motor unit types. Exercise and Sports Sciences Reviews. 1993;21:331–362. [PubMed] [Google Scholar]
- Gordon T, Stein RB. Reorganization of motor-unit properties in reinnervated muscles of the cat. Journal of Neurophysiology. 1982a;48:1175–1190. doi: 10.1152/jn.1982.48.5.1175. [DOI] [PubMed] [Google Scholar]
- Gordon T, Stein RB. Time course and extent of recovery in reinnervated motor units of cat triceps surae muscles. The Journal of Physiology. 1982b;323:307–323. doi: 10.1113/jphysiol.1982.sp014074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Stein RB, Thomas CK. Organization of motor units following cross-reinnervation of antagonist muscles in the cat hindlimb. The Journal of Physiology. 1986;374:443–456. doi: 10.1113/jphysiol.1986.sp016090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Thomas CK, Stein RB, Eredebil S. Comparison of physiological and histochemical properties of motor units after cross-reinnervation of antagonistic muscles in the cat hindlimb. Journal of Neurophysiology. 1988;60:365–378. doi: 10.1152/jn.1988.60.1.365. [DOI] [PubMed] [Google Scholar]
- Gunderson K, Leberer E, Lomo T, Pette D, Staron RS. Fibre types, calcium-sequestering proteins and metabolic enzymes in denervated and chronically stimulated muscles of the rat. The Journal of Physiology. 1988;398:177–189. doi: 10.1113/jphysiol.1988.sp017037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L, Samaha FJ. Procedure for the histochemical demonstration of actomyosin ATPase. Experimental Neurology. 1970;28:365–367. 10.1016/0014-4886(70)90244-X. [PubMed] [Google Scholar]
- Jansen J K S, Fladby T. The perinatal reorganization of the innervation of skeletal muscle in mammals. Progress in Neurobiology. 1990;34:39–90. doi: 10.1016/0301-0082(90)90025-c. 10.1016/0301-0082(90)90025-C. [DOI] [PubMed] [Google Scholar]
- Käser L, Müntener M. Delayed muscle fiber transformation after foreign-reinnervation of excessive muscle tissue. Anatomical Record. 1989;223:347–355. doi: 10.1002/ar.1092230314. [DOI] [PubMed] [Google Scholar]
- Kernell D. Organized variability in the neuromuscular system: a survey of task-related adaptations. Archives Italiennes de Biologie. 1992;130:19–66. [PubMed] [Google Scholar]
- Kugelberg E, Edstrom L, Abbruzzese M. Mapping of motor units in experimentally reinnervated rat muscles. Journal of Neurology, Neurosurgery and Psychiatry. 1970;33:319–329. doi: 10.1136/jnnp.33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E, Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. The Journal of Physiology. 1979;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- Miller JB, Stockdale FE. What muscle cells know that nerves don't tell them. Trends in Neuroscience. 1987;10:325–329. 10.1016/0166-2236(87)90089-0. [Google Scholar]
- Ounjian M, Roy RR, Eldred E, Garfinkel A, Payne JR, Armstrong A, Toga AW, Edgerton VR. Physiological and developmental implications of motor unit anatomy. Journal of Neurobiology. 1991;22:547–559. doi: 10.1002/neu.480220510. [DOI] [PubMed] [Google Scholar]
- Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE. Metabolic profiles of three fibre types of skeletal muscles in guinea pigs and rabbits. Biochemistry. 1972;11:2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Reviews of Physiology, Biochemistry and Pharmacology. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbova G. Adaptation of mammalian skeletal muscle fibers to chronic electrical stimulation. Reviews of Physiology, Biochemistry and Pharmacology. 1992;120:115–194. doi: 10.1007/BFb0036123. [DOI] [PubMed] [Google Scholar]
- Pullen AH. The distribution and relative sizes of three histochemical fibre types in the rat tibialis anterior muscle. Journal of Anatomy. 1977;123:1–19. [PMC free article] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T. Self-reinnervated cat medial gastrocnemius muscles. I. Comparison of the capacity of regenerating nerves to form enlarged motor units after extensive peripheral nerve injuries. Journal of Neurophysiology. 1996a;75:268–281. doi: 10.1152/jn.1996.75.1.268. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T. Self-reinnervated cat medial gastrocnemius muscles. II. Analysis of the mechanism and significance of fiber type-grouping in reinnervated muscles. Journal of Neurophysiology. 1996b;75:282–297. doi: 10.1152/jn.1996.75.1.282. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T, Orozco R. Proportional enlargement of motor units after partial denervation of cat triceps surae muscles. Journal of Neurophysiology. 1992;68:1261–1276. doi: 10.1152/jn.1992.68.4.1261. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Milner LD, Landmesser LT. Selective reinnervation of fast and slow muscle regions during early chick neuromuscular development. Journal of Neuroscience. 1996;16:6864–6877. doi: 10.1523/JNEUROSCI.16-21-06864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesodia S, Gordon T, Nemeth P. Increased metabolic enzyme heterogeneity of muscle unit fibers after reinnervation. Society for Neuroscience Abstracts. 1993;19:65.4. [Google Scholar]
- Totosy de Zepetnek JE, Zung HV, Erdebil S, Gordon T. Innervation ratio is an important determinant of force in normal and reinnervated rat tibialis anterior muscles. Journal of Neurophysiology. 1992;67:1385–1403. doi: 10.1152/jn.1992.67.5.1385. [DOI] [PubMed] [Google Scholar]
- Unguez GA, Roy RR, Pierotti DJ, Bodine-Fowler S, Edgerton VR. Further evidence for incomplete control of muscle properties in cat tibialis anterior motor units. American Journal of Physiology. 1995;268:C527–534. doi: 10.1152/ajpcell.1995.268.2.C527. [DOI] [PubMed] [Google Scholar]
- Vrbova G, Gordon T, Jones R. Nerve-Muscle Interaction. 2. London: Chapman & Hall; 1995. [Google Scholar]


