Abstract
Many studies of the α7 subunit of the neuronal nicotinic acetylcholine receptor (nAChR) family have demonstrated that this α-bungarotoxin (α-BgTx)-binding neuronal receptor can participate in ACh-gated channels. Heterologous expression studies reveal that α7 subunits form homomeric channels of unusually high Ca2+ permeability. However, the physiological role of the α7 subunit in native neuronal nAChR channels is less clear.
We present evidence that the α7 subunit contributes to the function of at least three subtypes of native nAChR expressed by embryonic chick sympathetic neurones. These subtypes are functionally distinct from heterologously expressed homomeric α7 nAChRs as well as homomeric-like currents described in studies of hippocampal and parasympathetic neurones.
The proposed nAChRs differ from one another and from homomeric α7 nAChRs in their sensitivity to block by α7 subunit-specific antagonists: α-BgTx and methyllycaconitine (MLA). While MLA blocks 60 % of the macroscopic ACh response, α-BgTx inhibits a small component of the macroscopic current described by slow-on and slow-off kinetics.
Functional deletion of the α7 subunit by antisense oligonucleotide treatment eliminates the susceptibility of the nAChRs to block by both MLA and α-BgTx.
Single channel recordings combined with pharmacological and antisense-mediated ‘deletion’ techniques reveal that α-BgTx-sensitive α7-containing nAChRs have a small unitary conductance (18 pS), brief open time kinetics and relatively low open probability (Po). MLA-sensitive α7 nAChRs are characterized by a conductance of ≈35 pS, intermediate burst duration, and a relatively high Po.
The third nAChR subtype deleted by α7 antisense treatment is characterized by a unitary conductance of 50 pS and prolonged opening duration.
We propose that these three populations of native α7-containing nAChRs are distinct heteromeric complexes that include other α and/or β nAChR subunits.
Neurotransmission at interneuronal synapses is mediated in part by activation of ligand-gated ion channels. In general, such channels are composed of one or more subunit types encoded by a family of receptor subunit genes. The diversity of subunit expression correlates with the diversity of receptor channels detected in individual neurones. Although considerable effort has elucidated key molecular, biochemical and biophysical characteristics of numerous neuronal ligand-gated receptors, the composition of native channels at neuronal synapses is still largely unknown.
The neuronal nicotinic acetylcholine receptors (nAChRs) constitute a subfamily of ligand-gated ion channels that are widely distributed in both the peripheral and central nervous system (PNS and CNS, respectively). To date, eleven genes of this family have been cloned. These genes share a number of structural and functional features, and encode α- and β-subunits (eight α-subunits: α2-α9; three β-subunits: β2-β4; for review see Sargent, 1993; McGehee & Role, 1995). Correspondingly, a vast array of receptor subtypes have been detected and characterized in specific neuronal systems (for reviews see Sargent, 1993; McGehee & Role, 1995).
Neuronal nAChRs were initially identified as two major groups in the brain, high affinity nicotine binding sites and α-bungarotoxin (α-BgTx) binding sites. Despite the wide distribution of α-BgTx binding sites throughout the vertebrate nervous system, the physiological role of α-BgTx binding nAChRs remained elusive (Clarke, Schwartz, Paul & Pert, 1985; Clarke, 1992; Keyser et al. 1993; Lindstrom, Anand, Peng, Gerzanich, Wang & Li, 1995). The isolation of the α7 nAChR subunit gene and the demonstration that expression of the α7 subunit in heterologous systems yields α-BgTx-blockable nAChR channels transformed the nAChR field (Couturier et al. 1990). α7 homomeric channels, when gated by agonist, mediate rapidly activating and desensitizing currents with strong inward rectification at hyperpolarized membrane potentials. nAChRs composed solely of α7 are blocked by α-BgTx at nanomolar concentrations (Couturier et al. 1990; Bertrand, Bertrand & Ballivet, 1992), and are highly permeable to calcium (Seguela, Wadiche, Dineley-Miller, Dani & Patrick, 1993).
Several recent reports describe similarly rapid, α-BgTx-sensitive nicotine-gated currents in specific populations of both CNS and PNS neurones, most notably rat hippocampal neurones (Alkondon & Albuquerque, 1993; Castro & Albuquerque, 1995; Alkondon, Pereira, Barbosa & Albuquerque, 1997a), olfactory bulb (Alkondon & Albuquerque, 1994) and parasympathetic ganglion neurones (Vijayaraghavan, Pugh, Zhang, Rathouz & Berg, 1992; Zhang, Vijayaraghavan & Berg, 1994). The biophysical and pharmacological characteristics of these native nAChRs are comparable to those of the α7 homomeric nAChRs, supporting the contention that these neurones express α7 homomeric channels.
Despite the demonstration of these ‘α7 homomeric-like’ currents, many neurones express high levels of both α7 mRNA and protein, and yet α-BgTx-sensitive currents are not detected, even with the use of rapid drug delivery systems. The broad expression of α7 mRNA and protein throughout the CNS and PNS, compared with the relative paucity of reports of α7 homomeric-like currents, may reflect the participation of α7 in functionally distinct nAChR complexes. Indeed, studies by Lindstrom and colleagues suggest that chick retinal ganglion neurones express heteromeric channels of α7 and α8 that may include other α and/or β nAChR subunits (Britto, Hamassaki-Britto, Ferro, Keyser, Karten & Lindstrom, 1992; Anand, Peng, Ballesta & Lindstrom, 1993a; Anand, Peng & Lindstrom, 1993b). Biochemical studies also support the possibility of α7 heteromeric nAChRs as α7 protein can be immunoprecipitated with other subunits, although the identity of these subunits remains to be determined (Pugh, Corriveau, Conroy & Berg, 1995). Furthermore, recent results demonstrate α7 can form heteromeric complexes with α5 and β2 or β4 (G. Crabtree, J. Ramirez & L. W. Role, unpublished results).
In this study we examine the contribution of α7 subunits to ACh-induced currents in embryonic chick sympathetic neurones. These neurones express the α7 gene and α7 protein as indicated from Northern blot, in situ, immunohistochemical and immunoprecipitation studies (Devay, Qu & Role, 1994; Pugh et al. 1995). Furthermore, the levels of expression of α7 mRNA and protein are developmentally regulated by chronic depolarization, presynaptic input and target interaction (Margiotta & Gurantz, 1989; Jacob, 1991; Devay et al. 1994; De Koninck & Cooper, 1995). Despite the prominent expression of α7 and the use of rapid perfusion techniques, α7 homomeric channels are not detected in rat or chick sympathetic neurones (De Koninck & Cooper, 1995; authors’ unpublished results). This study demonstrates that ACh elicits currents with distinct kinetics and pharmacological properties that are blocked by α-BgTx or methyllycaconitine (MLA) in chick sympathetic neurones. The α-BgTx-sensitive component of the macroscopic currents is characterized by both slow-on and slow-off kinetics, and by an 18 pS unitary conductance. The MLA-sensitive component is a relatively rapid current (though considerably slower than that of α7 homomeric channels) and is mediated by a 35 pS, intermediate burst duration channel. Confirmation of these results by antisense oligonucleotide-mediated deletion of α7 also reveals that the α7 subunit is present in a 50 pS conductance channel distinguished by prolonged channel opening kinetics.
METHODS
Tissue culture
Ten- to twelve-day-old chicken embryos were removed from the egg and rapidly decapitated without anaesthesia. In vitro preparations of chick sympathetic neurones were obtained by removal of the lumbar sympathetic chain ganglia, followed by dispersion of the ganglia to individual cells after trypsin treatment (100 μg ml−1; 20-30 min). The resultant cell suspension was plated under conditions that suppress the proliferation of non-neuronal cells. The culture medium was Eagle's minimal essential medium (MEM) supplemented with 10 % heat-inactivated horse serum, 5 % chick embryo extract, 500 μg ml−1 penicillin, 50 μg ml−1 streptomycin, 2.4 mM glutamine and 0.5 μg ml−1 2.5 S nerve growth factor. Cells were plated at a density of 0.75 chain per 1.5 ml media in 35 mm polyornithine-coated tissue culture dishes.
Antisense oligonucleotide treatment
Prior to antisense oligonucleotide treatment, surface nAChRs were functionally blocked by covalent binding with bromo-acetylcholine bromide (BAC). After 3 days in culture, the cells were treated with 2 mM dithiothreitol (DTT, pH 8.0) for 5 min, 30 μM BAC (pH 7.2) for 10 min and 1 mM 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) (pH 7.2) for 5 min. The cultures were flushed with control medium between each of these treatments to ensure removal of the reagents. Following a final rinse with control medium, neurones were incubated in culture medium supplemented with antisense oligonucleotides (20 μM; see Yu, Brussaard, Yang, Listerud & Role, 1993) which was subsequently replenished daily. nAChR properties were assayed using macroscopic and single channel recording after 2-3 days of oligonucleotide treatment. The antisense oligonucleotides used were unmodified (d-oligo) 15mers whose sequences corresponded to the region around the translation initiation site of the targeted subunit. Our criteria for choosing the precise sequence to be targeted required that the corresponding antisense sequence (1) did not include more than three possible ‘within sequence’ base pairs (to decrease the probability of oligonucleotide dimerization and/or hairpin structures), (2) would not form stable oligonucleotide dimers and (3) included at least three bases within the fifteen that would mismatch with any region of all other known sequences listed in the current (chick) gene bank. Each experiment tested a variety of control oligonucleotides as well as ‘BAC treatment’ and ‘no treatment’ controls. Control oligonucleotide sequences were typically three base mismatches as well as scrambled versions of the antisense sequence. This study tested two different α7 antisense (AS) sequences, two corresponding ‘mismatch’ (MM) controls and one scrambled sequence, although most studies employed the following α7 AS and α7 AS mismatch sequences: α7 AS, GCA TCA GCG CCC GGA; α7 AS mismatch, GCA ACA GAG CCA GGA. Results obtained with the various antisense sequences were indistinguishable and are distinct from results obtained from mismatch sequences.
Patch clamp
Tight seal whole-cell recording and single channel recording employed the patch clamp technique at room temperature (25°C). Patch electrodes were obtained by two-stage pull on List L/M-3P-A vertical electrode puller. Plates of neurones in vitro were rinsed free of culture media with L-15 (Gibco) solution and subsequently incubated in L-15 throughout the recording experiments. Standard internal solution for whole-cell recording contained (mM): 150 KCl, 2 MgCl2, 10 Hepes, and 1 EGTA at pH 7.2 supplemented with 5 mM Mg-ATP and 0.3 mM GTP. CsCH3SO4-high EGTA solution contained (mM): 140 CsCH3SO4, 10 KCl, 2 MgCl2, 10 Hepes and 10 EGTA at pH 7.2 supplemented with 5 ATP and 0.3 GTP. The external and electrode solution in cell-attached single channel recordings experiments contained (mM): 140 NaCl, 3 KCl, 1 MgCl2, 1 CaCl2, and 10 Hepes at pH 7.2. ACh was included in the external solution. For experiments using α-BgTx (Molecular Probes) or MLA, the drugs were dissolved in L15 and external solution and added to the recording chamber for at least 10 min prior to assay. All reagents were purchased from Sigma unless otherwise stated.
Data analysis
Instruments and software
Data were collected with an Axopatch 200A amplifier from Axon Instruments, stored for subsequent analysis with a VR-10B Digital Data Recorder from Instrutech Corporation and recorded on a Sony VCR. Data were filtered with an eight-pole low-pass filter (Frequency Devices) and digitized with Fetchex (pCLAMP 6.0 software package; Axon Instruments). For single channel and macroscopic analyses Fetchan, pStat (pCLAMP 6.0; Axon Instruments) and Microcal Origin software were used. Graphic representation was performed using Microcal Software's Origin Technical Graphic Program.
Whole-cell current analysis
Current traces were digitized and the peaks of the response were measured. The peak amplitudes of the currents (Ipeak) evoked under the same conditions (i.e. same agonist, same concentration, same experiment) were averaged. To address the variability in the magnitude of the ACh-evoked responses between different experiments, each experiment began and ended with assessment of the average peak response evoked by 500 μM ACh. Comparison of data from different sets of experiments involved normalization of current amplitudes to these ‘maximal’ responses.
The time course of agonist-evoked macroscopic currents was assessed from measurement of the time at which the current has declined to 50 % (t½) of the peak response (t = 0 at peak response). This ‘t½’ was used as an indicator for the rate of decay of the macroscopic currents. When nAChRs were activated by low concentrations of agonist, currents typically decayed with a mono-exponential time course to a steady-state level. Under these conditions, the decay phase of the currents was fitted and the time constant of the exponential decay (τ) was determined.
Dose-response curves for the peak response or the decay time constant were generated by plotting the amplitude of the current or the decay time constant against the concentration of the agonist or antagonist. A single sigmoidal curve described by the following equation typically provided a good fit to the data:
where k is the EC50 for agonist or IC50 for antagonist, x being the concentration and n the Hill coefficient, A1 being the basal activity when no agonist or antagonist is used and A2 the maximal response value. When biphasic dose-response curves were obtained, the curve was fitted with:
Comparisons of peak amplitude and t½ of whole-cell currents required both parametric statistical analyses (i.e. data did not typically conform to a Gaussian distribution) so are presented as box plots (e.g. Fig. 2C). Each box plot represents all data collected under the same experimental conditions and is marked with a vertical line, three horizontal lines and five symbols that designate the following parameters: the bottom symbol is the minimum value of the data group (the 0th percentile). The second symbol from the bottom indicates the 1st percentile. The bottom of the vertical line marks the 5th percentile. The bottom of the box marks the 25th percentile, the middle line marks the 50th percentile (median), the top of box marks the 75th percentile, and the top of the vertical line marks the 95th percentile. The symbol in the middle marks the mean of the data. The two symbols above the vertical line mark the 99th and 100th percentiles. Note that the 0th to 5th percentile symbols as well as the 95th to 100th percentile symbols often overlap due to sample size.
Figure 2. α-BgTx inhibition of ACh-evoked currents was direct and independent of Ca2+-induced Cl− conductance.
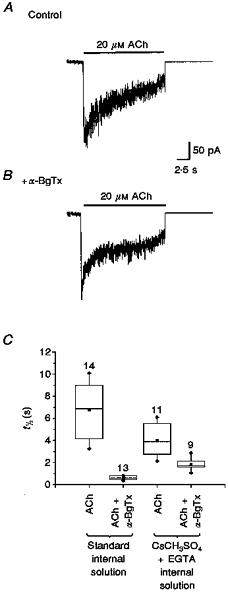
ACh (20 μM)-evoked macroscopic currents in the presence or absence of α-BgTx (500 nM) were recorded with CsCH3SO4+ high EGTA internal solution (see Methods). The recorded currents were digitized, averaged and analysed. A, averaged currents evoked by ACh (20 μM) in neurones using CsCH3SO4 internal solution. B, averaged currents evoked by ACh +α-BgTx under the same conditions as in A. The time course of the current decay was faster than that recorded with standard internal solution. C, the t½ values of the decay phase of the currents were analysed with non-parametric methods and presented as box plots (see Methods). Comparison of t½ of all ACh (20 μM)-evoked currents in the absence or presence of α-BgTx recorded under control conditions in standard internal solution or in CsCH3SO4+ EGTA internal solution revealed significant (P≤ 0.001) inhibition of ACh-evoked currents by α-BgTx whether or not Ca2+-activated Cl− conductance was blocked. The number of neurones recorded are noted above each box plot.
Single channel analysis
Single channel records were typically digitized at 10 kHz and filtered at 1-4 kHz prior to analysis. Following a determination of the zero current baseline and detection threshold, records were screened manually with computer-assisted event detection. Events were detected as a deviation from baseline of greater than twice the threshold value. Events were analysed to generate an ‘all points’ amplitude histogram using a sampling rate of 10 kHz. The resultant histograms revealed multiple peaks, each of which were well-fitted by Gaussian distributions, as previously reported (Moss, Schuetze & Role, 1989; Simmons, Schuetze & Role, 1990; Moss & Role, 1993). The average amplitude of each peak of the all point histogram was then plotted versus the holding potential to obtain the slope conductance of the channel. Each slope conductance determination was generated from the data of an individual patch unless otherwise stated.
Open probability (Po) and dwell time plots were generated from the list determined with the Fetchan program of pCLAMP 6.0. The amplitude and duration (dwell time) of each open event were measured and the events were grouped according to amplitude. Dwell times for each amplitude level were then pooled to generate the dwell time plot. The plots were then fitted with the sum of one or two exponential components for calculation of open time constants (τ1 and τ2). Po was calculated as:
where to is the total open time for the level specified, ti is the time interval over which Po is measured, and N is the number of channels in the patch (defined as 1 since open frequency is low). Scatter plots are generated by plotting all events (channel openings) as a function of time elapsed.
Protein immunoblot
Culture dishes were washed in phosphate-buffered saline (PBS) and neurones were collected in 1 % Triton X-100 lysis buffer. Samples were assayed for total protein (BioRad Protein Assay Kit) to ensure equivalent loading. The samples were fractionated on a 10 % SDS-PAGE denaturing gel and transferred to polyvinylidene fluoride membrane. The α7 protein was assayed with antisera to an α7 fusion protein (1:1000 dilution). The α7 antisera were produced in rabbit against a bacterially expressed fusion protein containing part of the intracellular loop of the α7 subunit (amino acids 332-387; see McGehee, Heath, Gelber, Devay & Role, 1995).
RESULTS
nAChRs expressed in neurones are sensitive to α-BgTx in a dose-dependent manner
Previous studies of sympathetic neurones suggested the participation of α7 subunits in a small fraction of the current carried by nAChR channels of embryonic chick sympathetic neurones (Listerud, Brussaard, Devay, Colman & Role, 1991; Yu et al. 1993). As the α7 subunit is the only known α-BgTx-binding subunit expressed by these neurones and the level of α-BgTx binding sites is quite high (Listerud et al. 1991; Devay et al. 1994), the apparently small contribution of α-BgTx-sensitive nAChRs to ACh-elicited currents was puzzling. To investigate the role of α7 subunits in native nAChR channels, we first examined the α-BgTx-sensitive currents in these neurones in detail.
Embryonic chick sympathetic neurones, maintained in vitro for 4-6 days, were incubated with α-BgTx for 15 min prior to assay of toxin sensitivity. As in previous studies (Listerud et al. 1991; Yu et al. 1993), assay of the concentration dependence of ACh-evoked macroscopic current amplitude revealed maximal responses at ≥ 500 μM ACh. The amplitude of such currents was not significantly altered by pre-treatment with α-BgTx at concentrations of 0.1-0.5 μM (e.g. control vs.α-BgTx: Ipeak, 992 ± 41 vs. 1024 ± 211 pA; n = 16 neurones; Fig. 1A). Examination of the decay phase of the currents in the continued presence of ACh, however, revealed a distinct α-BgTx-sensitive component of the nAChR-mediated current (e.g. control vs.α-BgTx: t½, 0.46 ± 0.20 vs. 0.36 ± 0.1 s).
Figure 1. α-BgTx blocked ACh-evoked currents.
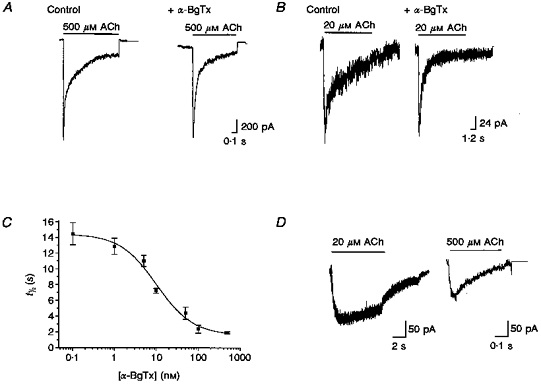
The sensitivity of ACh-evoked currents to α-BgTx was examined by incubating the neurones in the absence (control) or presence of 500 nM α-BgTx for ≥ 15 min before ACh application. A, when macroscopic currents were evoked by high concentrations of ACh (here 500 μM), α-BgTx had little effect on current amplitude. B, the net current elicited by lower concentrations of ACh (here 20 μM) was significantly reduced by α-BgTx, primarily due to a decrease in a later, slow component with little change in peak amplitude. Thus the time course of the current was significantly faster. C, plot of the decay time constant of macroscopic currents elicited by ACh (20 μM) as a function of α-BgTx concentration. The concentration-dependent inhibition by α-BgTx was well fitted by a sigmoidal curve indicating an IC50 of 10.05 nM and an apparent Hill coefficient of 0.995. Each point on this curve was derived from the τ values of single exponential fits of three to nine ACh-evoked currents in the presence of the indicated concentration of α-BgTx. Error bars indicate s.e.m.D,α-BgTx-sensitive component currents. The amplitude and time course of the α-BgTx-sensitive component of ACh-evoked current were obtained by pointwise subtraction of averaged currents recorded in the presence and absence of α-BgTx (see text). ACh-evoked currents were averaged by digitization and alignment at the onset of ACh application (control, n = 7; +α-BgTx, n = 6). The subtracted current revealed that the α-BgTx-sensitive component of the currents evoked by 20 μM ACh had a protracted time course with little desensitization over the 10 s period examined.
The α-BgTx-sensitive component was most evident from analysis of responses to relatively low concentrations of agonist. Although currents evoked by 20 μM ACh were similar in amplitude whether or not neurones were treated with α-BgTx (e.g. control vs.α-BgTx: Ipeak, 214 ± 79 vs. 217 ± 101 pA; n = 39; Fig. 1B), the time courses of these currents were clearly altered. In the presence of α-BgTx, macroscopic currents evoked by 20 μM ACh were characterized by a 5- to 10-fold faster rate of decay (control vs.α-BgTx: t½, 6.77 ± 0.65 vs. 0.89 ± 0.27 s; Fig. 1B and C). The time courses of the decay phase of the ACh-evoked macroscopic currents were well fitted by a single exponential (see Methods). Examination of the kinetics of the macroscopic currents in neurones pre-treated with a range of α-BgTx concentrations indicated an IC50 of ∼10 nM for α-BgTx, with an apparent Hill coefficient of ∼1.0 (Fig. 1C). Thus, the effect of α-BgTx on macroscopic currents elicited by ACh in sympathetic neurones was significantly different from those reported for α7 homomeric channels expressed in Xenopus oocytes where the α-BgTx IC50 was 10-fold smaller with an apparent Hill coefficient of 6 or larger (Couturier et al. 1990). Thus, both the time course and antagonist sensitivity of native α-BgTx-blockable nAChRs in these neurones are distinct from those of heterologously expressed α7 homomeric channels.
Further resolution on the functional profile of the α-BgTx-sensitive ACh-evoked current in sympathetic neurones was provided by assessment of ‘subtracted currents’, obtained by the pointwise subtraction of currents evoked in the presence of α-BgTx (I(t)+α-BgTx) from those elicited under control conditions (I(t)control; Fig. 1D). Subtraction analysis of the α-BgTx-sensitive component revealed that it had a protracted time course and accounted for a relatively small fraction of the total ACh-elicited current (Fig. 1D). Both the time to reach peak (t0-peak) and the desensitization rate are slower than that of the α-BgTx-insensitive component. At 20 μM ACh, the t0-peak of the α-BgTx-sensitive component was typically ≥ 2 s, whereas the t0-peak of the α-BgTx-insensitive component was typically 200-400 ms. Currents evoked by high concentrations of ACh (500 μM) revealed similar differences in the rise time of the α-BgTx-sensitive vs. -insensitive components (e.g. +α-BgTx vs.α-BgTx-insensitive components: t0-peak, 79 vs. 19 ms; t½, 270 vs. 65 ms; Fig. 1D). The peak amplitude of the subtracted currents indicated that the α-BgTx-sensitive component was activated at relatively low concentrations of ACh; increasing concentrations of ACh did not substantially increase the amplitude of this component (e.g. at 20 vs. 500 μM ACh: average Ipeak, 154 vs. 195 pA).
α-BgTx sensitivity is not due to involvement of Ca2+-induced Cl− currents
Since α7 homomeric channels are highly permeable to Ca2+, one possibility is that ACh-activated Ca2+ entry through α-BgTx-sensitive channels indirectly leads to activation of Ca2+-activated Cl− currents. To test this possibility, we measured the α-BgTx sensitivity of the ACh currents using CsCH3SO4 -high EGTA as intracellular recording solution. With this internal solution, Cl− anions are replaced by the non-permeable CH3SO3− anions and Ca2+ is chelated. The currents recorded in response to 20 μM ACh with CsCH3SO3+ EGTA internal solution are faster than those recorded with standard internal solution, but a slow, α-BgTx-sensitive component of the total elicited current is still evident (Fig. 2), indicating that the α-BgTx-sensitive component of the ACh-evoked current is unlikely to be due to Ca2+-induced Cl− current. The change of kinetics may arise from modulation of nAChR open probability by internal calcium (J. Ramirez-Latorre, G. Crabtree and L. W. Role, unpublished results) or other calcium-dependent mechanisms (Hopfield, Tank, Greengard & Huganir, 1988; Hoffman, Ravindran & Huganir, 1994).
ACh-evoked responses are partially blocked by MLA
The α-BgTx sensitivity of ACh-evoked macroscopic currents suggests that α7 subunits participate in a subset of the native nAChRs expressed by the sympathetic neurones. To test this hypothesis further, we examined the effects of MLA, a neurotoxin that binds specifically to brain α-BgTx binding sites (but not to other nAChRs) with nanomolar affinity (Ward, Cockcroft, Lunt, Smillie & Wonnacott, 1990; Alkondon & Albuquerque, 1993; Wilkie, Hutson, Sullivan, & Wonnacott, 1996). MLA blocks ACh-evoked currents that are sensitive to α-BgTx in hippocampal neurones (Alkondon et al. 1992; Alkondon et al. 1997a; Alkondon, Pereira, Cortes, Maelicke & Albuquerque, 1997b) and currents carried by α7 homomeric channels expressed in Xenopus oocytes (Palma, Bertrand, Binzoni & Bertrand, 1996). In view of the cumulative data implicating MLA as an α7 specific antagonist, examination of the nAChR channel profile in the absence and presence of MLA was expected to provide additional insight into the subunit composition of native channels.
MLA (10 nM) decreased the peak amplitude of currents evoked by ACh; the extent of inhibition by MLA increased at high concentrations of agonist (e.g. control vs. MLA: Ipeak at 20 μM ACh, 217 ± 79 vs. 128 ± 5.8 pA, a 40 % decrease compared with Ipeak at 500 μM ACh, 998 ± 41 vs. 388 ± 98 pA, ∼60 % decrease; Fig. 3A and B). Unlike α-BgTx, the effect of MLA was largely confined to altering the peak of ACh-evoked currents, with similar, albeit less profound, changes in the macroscopic current time course (Fig. 3A and B).
Figure 3. MLA blocked a component of the macroscopic currents evoked by ACh.
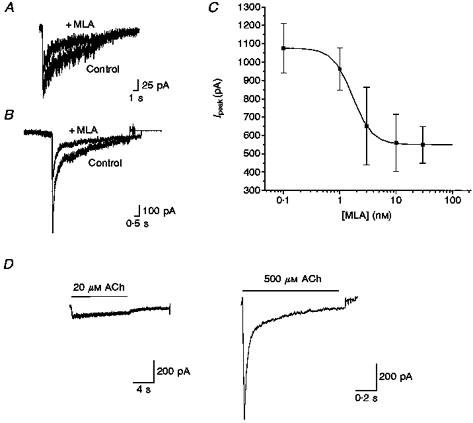
The susceptibility of ACh-elicited currents to block by MLA was examined by exposure of neurones to 10 nM MLA. A, representative recording of macroscopic currents evoked by ACh (20 μM) in the absence (Control) and presence of MLA (10 nM). MLA reduced the peak amplitude of the current by ≈40 %. B, representative recording of macroscopic currents evoked by ACh (500 μM) in the absence and presence of MLA (10 nM). MLA reduced the peak amplitude of the current by ≈60 %. C, concentration dependence of the effect of MLA on the amplitude of ACh (500 μM)-evoked currents. The dose-response curve for inhibition by MLA inhibition is fitted by a sigmoidal curve indicating an IC50 of 1.69 nM and an apparent Hill coefficient of 2.49. Error bars indicate s.e.m.D, the stimulus-averaged MLA-sensitive component of ACh-elicited macroscopic currents was determined by subtraction analysis. The MLA-sensitive components of macroscopic currents evoked by either 20 or 500 μM ACh are strikingly different from the α-BgTx-sensitive components; MLA selectively blocks a rapidly activating and inactivating component, thereby decreasing peak amplitude of the macroscopic current (control, n = 6; + MLA, n = 4).
Examination of the dose dependence of inhibition of ACh-evoked macroscopic responses by MLA revealed an IC50 of ∼1.7 nM and an apparent Hill coefficient of ≥ 2. Inhibition was maximal with 10 nM MLA; 30 nM MLA had no additional effects (Fig. 3C). The potent inhibition of nAChR-mediated currents in sympathetic neurones by MLA is consistent with previous demonstration of the toxin's high affinity for α7 homomeric receptors in heterologous systems and for α-BgTx-sensitive currents in hippocampal neurones (Alkondon et al. 1992; Palma et al. 1996; Alkondon et al. 1997a, b).
The MLA-sensitive current, unlike the α-BgTx-sensitive component, was most evident in macroscopic currents elicited by relatively high concentrations of ACh. Examination of the MLA-sensitive component by subtraction analysis (IACh - IACh + MLA, where IACh is the ACh-evoked current and IACh+MLA is IACh in the presence of MLA) revealed significant increases in the amplitude of this component with higher concentrations of ACh (e.g. at 20 vs. 500 μM ACh: average Ipeak,MLA, 154 vs. 1298 pA; Fig. 3D). The time course of the MLA-sensitive component is considerably faster than that of the α-BgTx-sensitive component (20 vs. 500 μM ACh, + MLA: t0-peak, 317 vs. 18 ms; t½, 9.46 s vs. 31 ms; +α-BgTx: t0-peak, > 2 s vs. 79 ms; t½, > 10 s vs. 270 ms). Based on these observations, we propose that MLA- and α-BgTx-sensitive channels in sympathetic neurones represent distinct molecular entities (see Discussion).
Treatment with α7-specific antisense oligonucleotides revealed that both α-BgTx- and MLA-sensitive nAChRs require expression of the α7 subunit
The sensitivity of ACh-evoked currents to α-BgTx and to MLA is consistent with the participation of the α7 subunit in a subset of nAChR channels expressed by chicken sympathetic neurones. The potency of these agents in inhibiting distinct components of the ACh-evoked currents is consistent with previous demonstrations of the specificity of both of these toxins for α7-containing nAChRs. Nevertheless, as previous studies demonstrate α-BgTx and MLA inhibition of rapidly activating and desensitizing, α7 homomeric-like currents, we sought further confirmation that these novel α-BgTx- and MLA-sensitive components of nAChR currents in sympathetic neurones arise from α7-containing nAChRs. We compared the properties of ACh-elicited currents in sympathetic neurones under control conditions with those with depressed levels of α7 using antisense oligonucleotide-mediated deletion (Yu et al. 1993; Brussaard, Yang, Doyle, Huck & Role, 1994).
For these studies, neurones were maintained in vitro for 2 days and then treated with BAC, conjugated to surface nAChRs by oxidation/reduction to eliminate the contribution of pre-existing surface nAChRs to currents elicited by subsequent application of agonists (Listerud et al. 1991; Brussaard et al. 1994). Neurones were then incubated with either α7-specific antisense or one of the control oligonucleotides for 3 days prior to assay of newly expressed nAChR channels (see Methods and Discussion of accompanying paper, Yu & Role, 1998). Because of the rapid turnover of nAChRs in sympathetic neurones as well as the rapid uptake of oligonucleotide, ≤ 20 % of pre-existent nAChR channels remain on the cell surface at the time of assay (Yu et al. 1993). Treatment with α7-specific antisense oligonucleotides resulted in reduction of α7 subunit protein by > 50 % as indicated by Western blot analysis and control (mismatch) oligonucleotides were without effect (Fig. 4A; also see McGehee et al. 1995).
Figure 4. Funtional deletion of α7 abolished the sensitivity of the ACh-evoked currents to α-BgTx and MLA.
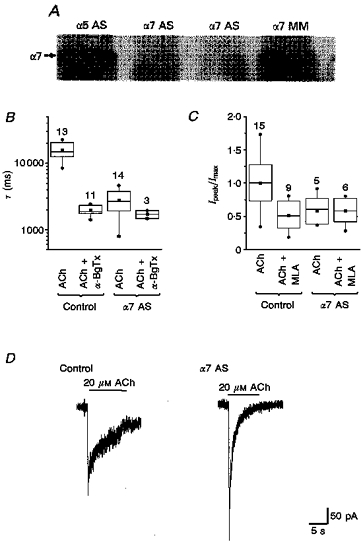
The ACh-elicited macroscopic currents and the effects of α-BgTx and MLA were compared in untreated neurones, neurones treated with control oligonucleotides, and neurones treated with α7-specific antisense oligonucleotides. A, immunoblot of total protein from neurons treated with different oligonucleotides, probed with an α7 antiserum. Arrow indicates the extent of migration for a 50 kDa protein, which is the predicted size of α7 protein. The neurones were treated with α5 antisense (α5 AS), α7 antisense (α7 AS) and α7 mismatch (α7 MM) oligonucleotides. There was marked reduction in the protein level of the α7 subunit in neurones treated with α7 antisense oligonucleotides compared with those treated with α5 antisense oligonucleotide and α7 mismatch oligonucleotide. B, functional deletion of α7 removes the α-BgTx-sensitive ‘slow’ currents. The decay time constant (τ) of ACh (20 μM)-evoked current was determined from single exponential fits to the elicited currents. Comparison of τ for ACh-elicited currents in control and α7 antisense oligonucleotide-treated neurones in the absence and presence of α-BgTx revealed that functional deletion of α7 removes the α-BgTx-sensitive component (P≥ 0.25, α7 deletion vs.α7 deletion +α-BgTx; P≤ 0.001, control vs. control +α-BgTx). The numbers of neurones for each condition are indicated above each box plot. C, functional deletion of α7 removed the MLA-sensitive component of ACh-elicited currents. The amplitudes of ACh (500 μM)-evoked currents are normalized to the peak responses recorded under control conditions (i.e. neurones treated with control oligonucleotides, which were equivalent to those elicited in untreated neurones). Comparison of the amplitudes of ACh-elicited currents using non-parametric analyses and presented as box plots revealed that treatment with α7-specific antisense oligonucleotides removed MLA-sensitive components. One-way ANOVA revealed that MLA no longer blocks macroscopic currents following α7 deletion (P≤ 0.996, α7 AS vs.α7 AS + MLA). D, comparison of macroscopic currents elicited by 20 μM ACh in control vs.α7-antisense oligonucleotide-treated neurones revealed that low concentrations of ACh typically evoked larger and faster macroscopic currents in α7 minus neurones.
Apparently, a complete elimination of subunit protein from the neurones treated with subunit-specific antisense oligonucleotide is not typically obtained. The partial elimination of α7 protein shown here may reflect subunit protein covalently modified by the BAC treatment and/or translated before oligonucleotide uptake. On the other hand, it is also possible that α7 protein is still translated following antisense treatment, albeit at a markedly reduced level. The antisense oligonucleotides are presumed to block protein translation by forming a double-stranded structure with mRNA, which may or may not reduce the mRNA level. In fact, studies employing Northern blot, RNAse protection or reverse transcriptase-polymerase chain reaction (RT-PCR) techniques have failed to detect significant changes in mRNA level in cells treated with antisense oligonucleotides despite substantial decreases in the level of functional protein (see Yu et al. 1993 and references therein).
Macroscopic currents elicited by ACh in α7 antisense-treated neurones were insensitive to both α-BgTx and MLA. Thus, neither the amplitude nor the time course of ACh-evoked currents was altered by either α-BgTx or MLA in α7 minus neurones (Fig. 4B and C). These results suggest that α7-specific antisense treatment effectively decreased the level of surface nAChR channels that included α7 subunits. Furthermore, these findings are consistent with the proposed selectivity of both MLA and α-BgTx for α7-containing receptors and confirms our proposal that α7 participates in the nAChR channels normally expressed by embryonic sympathetic neurones.
Analysis of macroscopic currents evoked by ACh (20-500 μM) in α7 minus neurones revealed a fast time course, reminiscent of the α-BgTx-insensitive component of ACh-elicited currents in control neurones (e.g. compare Fig. 1B with Fig. 4D). The t½ of α7 minus neurones was 1.37 ± 0.365 s, whereas the t½ of currents of neurones incubated with mismatched or scrambled sequences of the α7 antisense oligonucleotides was equivalent to neurones subjected only to the oxidation/reduction treatment and to untreated neurones. Thus, the ACh-evoked currents elicited in α7 minus neurones lacked a component characterized by slow kinetics.
The larger average value of the peak of ACh-evoked currents in α7 minus neurones compared with control in response to 20 μM ACh was not expected (Ipeak, 403 ± 51.7 vs. 215 ± 75 pA, respectively; Fig. 4D). A possible explanation for the increased amplitude currents evoked by low agonist concentration (≤ 20 μM) following α7 deletion is that these nAChRs include enhanced synthesis and/or assembly of other non-α7-containing nAChR complexes after removal of α7 subunits (see accompanying paper, Yu & Role, 1998).
Distinct effects of MLA, α-BgTx and α7 antisense treatment suggest three populations of α7-containing nAChR channels
The studies presented above reveal that ACh-elicited currents in sympathetic neurones are mediated, in part, by nAChRs that require the expression of α7 and which are potently blocked by antagonists previously documented as α7 specific (Couturier et al. 1990; Ward et al. 1990; Seguela et al. 1993; Alkondon & Albuquerque, 1993; Palma et al. 1996; Alkondon et al. 1997a, b), supporting our proposal that sympathetic neurones express α7-containing nAChRs. However, both the pharmacological and electrophysiological profile of the putative α7 nAChRs are distinct from one another and unlike previously described α7 homomeric- and heteromeric-like channels. To investigate further the contribution of α7 to these novel αBgTx- and MLA-sensitive nAChRs, we examined the properties of ACh-elicited single channel currents.
As our previous analyses of the nAChRs expressed by embryonic sympathetic neurones revealed substantial changes in the channel profile during development (Moss & Role, 1989; Listerud et al. 1991), we sought conditions that would permit analysis of all of the subtypes previously detected. Although the relative contribution of each conductance state varies from patch to patch, records obtained from neurones between the extremes of embryonic stages previously described include each subtype; the typical profile relative to Po is: 65 pS << 18 pS ≤ 30 pS ≪ 50 pS (long) < 50 pS (short) ≤ 15 pS ≥ 35 pS (by previous nomenclature XL << S17 ≤ M10 ≪L17 < L10 ≤ S10 ≥ M17; see also Appendix of accompanying paper, Yu & Role, 1998).
The properties of α7 nAChR channels were first examined in MLA- or α-BgTx-treated neurones. Ten nanomolar MLA significantly decreased the frequency of 35 pS openings (Fig. 5A). MLA appeared to selectively decrease the probability of opening of the 35 pS (M17) channels (Fig. 5B;n = 8) without altering the open time kinetics of these channels (data not shown).
Figure 5. MLA blocked the 35 pS AChR channels.
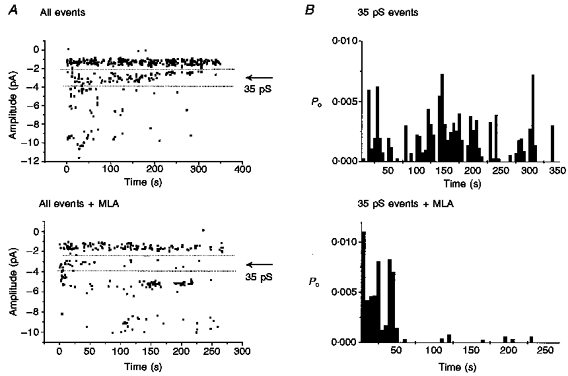
Cell-attached single channel opening elicited by ACh (2.5 μM) with or without MLA (10 nM) in the pipette solution. Inclusion of MLA in the patch pipette significantly reduces the number of events detected in the 35 pS conductance range in eight separate experiments. A, scatter plot of all openings recorded in a representative cell-attached patch over the duration of 3-5 min. Each point represents an individual event at the indicated amplitude. Openings within the range of 35 pS channels are delimited by the dotted lines ( ± 1 pA). In the presence of MLA, the opening frequency of these channels was markedly reduced. Note that these plots are individual patch recordings and, as such, the relative activity levels of other classes may not be representative. Determination of the pharmacology of single channel currents in cell-attached patches requires comparison of many such records under ACh alone vs. ACh + MLA conditions. These records are representative of the effect of MLA in eight experiments. B, plot of the open probability of the 35 pS channel as a function of time. In this and all other experiments testing MLA, the Po of the 35 pS channel decreased in the presence of MLA.
A contribution of α7 to the 18 pS (S17) class is suggested by examination of the profile of channels in the presence and absence of α-BgTx (Fig. 6; n = 5). α-BgTx treatment dramatically decreased the frequency of openings of the 18 pS channel. It should be noted that the overall Po for the 18 pS subtype is typically low under our control conditions. In other studies (see accompanying paper, Yu & Role, 1998) we found that the Po of the 18 pS class channels was substantially increased in neurones treated with α5 antisense oligonucleotide, allowing us to further test our hypothesis that α-BgTx selectively blocks the 18 pS/S17 channel subtype (Fig. 6). Indeed, α-BgTx decreases the Po of the 18 pS channel in α5 antisense-treated as well as in control neurones, supporting our proposal that the 18 pS/S17 channel underlies the α-BgTx-sensitive nAChR-mediated currents.
Figure 6. α-BgTx blocks the 18 pS nAChR channels.
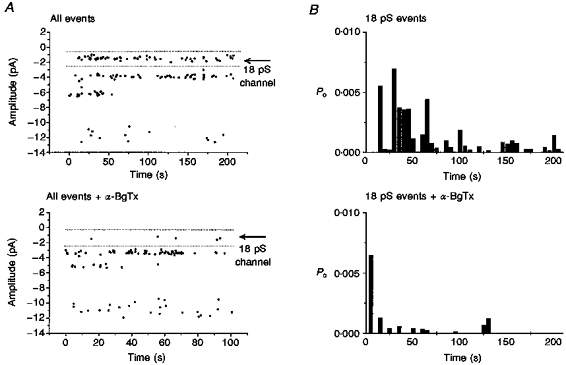
Cell-attached single channel opening elicited by ACh (2.5 μM) with or without α-BgTx (500 μM) included in the patch pipette. The neurones have been incubated with α-BgTx for ≥ 15 min prior to recording. The opening probability of the 18 pS channel is significantly reduced when α-BgTx is included in the patch pipette (n = 7). The records shown here were obtained from ‘α5 minus’ neurones as the contribution of the 18 pS channels is significantly increased under these conditions (see accompanying paper, Yu & Role, 1998). Identical results were obtained from recordings of control neurones. A, sample scatter plots of all channel openings in a typical patch recording with ACh ± α-BgTx in the patch pipette. Each point represents an individual opening at the amplitude indicated on the y axis; openings of the 18 pS conductance range are delineated by dotted lines. With α-BgTx in the pipette, the number of openings of this class of channels is significantly reduced. B, open probability of the 18 pS channel is plotted as a function of time. The Po of the 18 pS channel is greatly reduced in the presence of α-BgTx.
Analysis of single channel recordings from neurones treated with α7 antisense oligonucleotides indicates that a subset of the 50 pS channels also include the α7 subunit. In ∼60 % of the experiments (ten of sixteen patches) channels that resembled the 50 pS class in control neurones were detected (γ3 = 47.0 ± 5.81 pS, all samples; Fig. 7). In the remaining patches (n = 6), the 50 pS channel class was not detected. One interpretation of these results is that the α7 subunit participates in a subpopulation of the 50 pS channels that are normally expressed by sympathetic neurones. In fact, embryonic sympathetic neurones do express two kinetically distinct 50 pS channel subtypes (brief opening events, τ≪ 3 ms; long open time events, τ≪ 20 ms). The relative contribution of the ‘brief’vs.‘long’ openings at 50 pS changes during embryonic development (previously designated L10 and L17, respectively; Moss et al. 1989; Moss & Role, 1993). At the intermediate development stages examined in the current study, both classes were detected. An alternative interpretation of the observed variability in detecting 50 pS channels in patches from α7 antisense-treated neurones is that the antisense treatment is not reliably affecting the level of α7 expression and, hence, the extent to which α7-containing nAChRs are expressed. The latter interpretation is unlikely as all α7 antisense-treated neurones are insensitive to α7-specific antagonists (see above).
Figure 7. α7 deletion altered the single channel profiles of the nAChRs.

Single channel recording conducted in the cell-attached configuration (ACh, 2.5 μM) in control neurones (control oligonucleotide treated, identical to untreated neurones) and in neurones treated with α7-antisense oligonucleotides indicates that the 18 pS/α-BgTx-sensitive, 35 pS/MLA-sensitive and the 50 pS/long duration channels are absent in the α7 minus neurones. A, single channel records from patches in control neurones. A subset of the channel classes detected in all such experiments are prominent in this recording (15, 35 and 50 pS short and long durations). The opening of the 18 and 65 pS classes that are also observed but at lower open frequency in most records are indicated by dotted lines. The slope conductance plot displays the conductances detected in this (continuous lines) and other (dotted lines) control patches. B, single channel profiles from 60 % recordings obtained from α7 minus neurones include openings of three major conductance channels (15, 35 and 50 pS). The 18 pS channel was absent in all α7 minus neurones. Analysis of the pharmacology and open time kinetics of the channels of 35 and 50 pS conductances further revealed that (a) long burst, 50 pS openings were absent in α7 minus neurones (see Fig. 8) and (b) that 35 pS, MLA-sensitive channels were deleted. The 35 pS conductance channels detected following α7 deletion are likely to be a novel class (Fig. 8). C, single channel recordings from 40 % of the patches from α7 minus neurones revealed that all 50 pS channel openings were absent. In addition, a novel channel class of 60 pS conductance was detected.
To distinguish between these possibilities, we examined the single channel kinetics of the 50 pS channels in patches from control neurones and compared their properties with those of the 50 pS channel openings that remained following α7 antisense oligonucleotide treatment. This analysis revealed that whereas under control conditions we detected both ‘brief’ and ‘long’ open time kinetics (τ1, 1.57 ± 1.049 ms; τ2, 22.01 ± 13 ms), following α7 ‘deletion’ the 50 pS channels were either eliminated (40 % of the patches) or included only the ‘brief’ channel openings (τ, 2.4 ± 1.4 ms; Fig. 8A and B). This analysis supports our proposal that the 50 pS channels normally expressed by sympathetic neurones include two distinct subtypes, one with relatively brief open time that does not include α7 and another, long burst duration channel that includes the α7 subunit as it is not expressed by α7 minus neurones. Comparison of the properties of ACh-gated channels in control vs.α7 antisense-treated neurones confirmed the proposed inclusion of α7 in the α-BgTx-sensitive (18 pS/S17 type) channels, as this subtype was not detected in patches from α7 minus neurones. The nAChR subtypes that were typically detected in α7 minus neurones included a 15 pS channel (Fig. 7B and C) with kinetics comparable to that of the 15 pS channels in control neurones, a channel with conductance of 36 ± 5.34 pS and, in a subset of recordings, a channel of ∼60 pS. Further analyses of the 36 and 60 pS channel in α7 minus neurones indicate that both of these classes are kinetically and/or pharmacologically distinct from either the 35 or 65 pS channels detected in control sympathetic neurones. In fact, consideration of the conductance profile of nAChR channels detected in α7 minus neurones underscores the importance of determining the agonist and antagonist pharmacology, as well as the kinetics and conductance of agonist-elicited currents when attempting to dissect receptor composition by antisense-mediated deletion. Thus the 36 pS conductance channel detected in α7 minus neurones, where ACh-elicited currents are insensitive to both α-BgTx and MLA, could not represent a MLA- (or α-BgTx-) sensitive nAChR channel. Indeed, examination of the open kinetics of these channels revealed burst characteristics distinct from those of the 35 pS channel expressed under control conditions, suggesting that they were likely to be ‘mutant channels’ expressed as a result of α7 deletion (control vs.α7 minus neurones: τ1, 1.06 ± 0.32 vs. 1.51 ± 0.30 ms; τ2, 7.07 ± 2.22 vs. 10.97 ± 2.53 ms; Fig. 8C and D).
Figure 8. α7 deletion also removed the long τ 50 pS and native 35 pS channels.
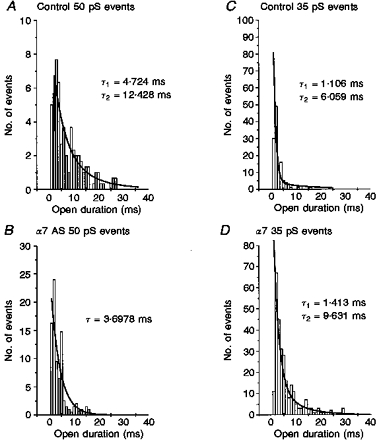
The open time kinetics of individual conductance classes were analysed from single channel records obtained from the control (mismatch or sham treatments) and α7 minus neurones. The distribution of burst duration for each conductance was best fitted by one or the sum of two exponentials, as noted, thus indicating one or more open time constant (τopen). A, burst duration distribution for all openings at 50 pS under control conditions (control oligonucleotide treatment) are best fitted by the sum of two exponential curves, indicating fast and slow open time constants of τfast = 4.7 ms and τslow = 12.4 ms, respectively. B, burst duration distribution of all openings at 50 pS detected in neurones treated with α7 antisense oligonucleotides. The open duration histogram is best fitted by a single exponential with τ = 3.7 ms, equivalent to the fast τ 50 pS opening in control neurones. Thus the long open duration 50 pS channels were deleted by α7 antisense treatment. C and D, comparison of the burst duration distribution of all 35 pS channel openings in control vs.α7 minus neurones. The 35 pS conductance channels recorded in α7 minus neurones differed from native 35 pS channel openings in their open kinetics. While the native 35 pS channels include two open states with τ values of 1 and 6 ms (1.06 ± 0.32 ms and 7.07 ± 2.22 ms, all control data), the 35 pS channels in α7 minus neurones are described by τopen of 1.4 and 9.6 ms (1.51 ± 0.289 and 10.97 ± 2.53 ms, all α7 minus data; n = 6). This analysis suggests that the native 35 pS channel is absent in α7 minus neurones (see Discussion and Appendix to accompanying paper, Yu & Role, 1998).
DISCUSSION
The principal conclusion of this study is that chick sympathetic ganglion neurones express α7-containing nAChRs that are distinct from homomeric α7 nAChRs. We propose that the α7 nAChRs expressed by sympathetic neurones are heteromeric subunit complexes which underlie three distinct nAChR channel subtypes.
Our conclusion that sympathetic neurones normally express α7-containing nAChRs and that such α7 nAChRs are non-homomeric is based on both current and previous studies. The expression of α7 mRNA and protein in chick sympathetic neurones has been extensively documented (see reviews by Sargent, 1993; McGehee & Role, 1995). The participation of the α7 subunit in a subset of channels expressed by these sympathetic neurones is demonstrated by inhibition of specific components of the ACh-elicited currents with α7-specific antagonists, such as α-BgTx and MLA, and by the ‘functional deletion’ of α7 subunits from the neurones by treatment with α7-specific (but not by several control) antisense oligonucleotides. Although all three approaches indicate that α7 participates in native nAChR channels, neither the pharmacological nor the biophysical properties of the α7 nAChR-mediated currents in sympathetic neurones conform to the profile of α7 homomeric nAChR channels. Thus, although components of the currents are inhibited by α-BgTx or MLA, the apparent affinity and/or Hill coefficients that describe this block are not equivalent to those of homomeric α7 nAChRs. Likewise, the time courses of the proposed α7 nAChR-mediated currents are 10- to 100-fold slower than homomeric α7 AChRs. Finally, neither the unitary conductance nor the kinetics of the α7-nAChR single channel currents in sympathetic neurones match reported values for α7 homomeric channels (Galzi, Devillers-Thiery, Hussy, Bertrand, Changeaux & Bertrand, 1992; Bertrand et al. 1992). Although we favour the hypothesis that heteromeric α7 nAChRs underlie the currents described, we cannot rule out the possibility that post-translational modification of homomeric α7 nAChRs could contribute to the properties of the ACh-elicited currents described. Resolution of this issue requires biochemical and physiological evidence that heteromeric complexes of α7 and other subunits can assemble to form functional nAChRs. In this regard, initial characterization of ACh-elicited currents from Xenopus oocytes co-expressing α7 and α5 suggests, at least, that such heteromeric complexes can form (G. Crabtree, J. Ramirez-Latorre & L. W. Role, unpublished results).
Our final proposal, which is admittedly speculative, is that the heteromeric α7-containing nAChR channels expressed by sympathetic neurones include α3, α5, β4 and/or other as yet unidentified subunit(s) expressed by sympathetic neurones. This contention is based on the findings presented here and in the accompanying paper (Yu & Role, 1998), as well as on previous biochemical and electrophysiological studies from many laboratories (see accompanying paper and references therein). A more detailed discussion of this proposal is presented in the Appendix to the accompanying paper.
Our studies demonstrate that a subpopulation of the nAChRs are sensitive to α-BgTx. The effect of α-BgTx is unlikely to be due to contamination by neuronal bungarotoxin (n-BgTx; also called 3.1, or kappa toxin) as we have previously demonstrated that n-BgTx, unlike α-BgTx, inhibits all of the nAChR subtypes previously detected in these neurones (Moss & Role, 1993). Furthermore, although 10 nM n-BgTx obliterates the macroscopic currents evoked by 500 μM ACh, α-BgTx at a concentration of up to 10 μM has little detectable effect on the magnitude of these currents (Listerud et al. 1991). The lack of effect of α-BgTx following the treatment of neurones with α7-specific antisense oligonucleotides provides further support that the α-BgTx-blockable component of ACh-evoked currents requires α7 expression and that a n-BgTx contaminant does not account for the observed effects of α-BgTx.
We also found that a subpopulation of nAChRs expressed by sympathetic neurones was blocked by nanomolar concentrations of MLA. The potency of MLA-inhibition of sympathetic neuronal nAChRs is comparable to that for the α7 ‘homomeric-like’ current in rat hippocampal and olfactory bulb neurones (Alkondon & Albuquerque, 1993; Alkondon & Albuquerque, 1994). However, MLA inhibition of the native nAChRs is > 40-fold less potent than MLA inhibition of α7 homomeric channels expressed in Xenopus oocytes (IC50, 25 pM; Palma et al. 1996). Although micromolar concentrations of MLA can apparently block α3β2 and α4β2 nAChR channels (Drasdo, Caulfield, Bertrand, Bertrand & Wonnacott, 1992), the sensitivity of sympathetic nAChRs to MLA, as well as the loss of MLA sensitivity with functional deletion of the α7 subunit is consistent with an α7 subunit-dependent block. Finally, the possibility that MLA or α-BgTx interact with α8 or α9 is unlikely as neither subunit is known to be expressed by chick sympathetic neurones (P. Devay, X. Yang & L. W. Role, unpublished results).
Our proposal that the α7 nAChRs that comprise the α-BgTx- and MLA-sensitive currents are distinct molecular entities is supported by both macroscopic and single channel analyses. Examination of the differences between the αBgTx- and MLA-sensitive components of the macroscopic currents reveals that the α-BgTx-sensitive α7 nAChRs constitute a relatively small portion of the total current activated by ACh, with the largest contribution evident at relatively low ACh concentrations. Thus, αBgTx-sensitive α7 nAChRs account for ∼50 % of the total current at low agonist concentration, whereas at higher concentrations of ACh, they constitute < 10 % of the total currents. The MLA-sensitive α7 nAChRs, in contrast, conduct < 40 % of the total current elicited by low concentrations of ACh, but account for up to 60 % of the total current by higher ACh concentrations. Difference in macroscopic current time course further distinguishes the α-BgTx- vs. MLA-sensitive components; the MLA-sensitive component of ACh-elicited macroscopic currents is relatively fast in onset and decay, whereas the α-BgTx-sensitive component rises slowly and desensitizes very slowly, if at all. The final distinction between the α-BgTx- and MLA-sensitive α7 nAChRs is provided by analysis of the unitary currents that underlie these components. α-BgTx specifically blocks a low Po subtype with a conductance of 18 pS and brief open kinetics; MLA-sensitive α7 AChRs have a considerably higher opening probability, a conductance of 35 pS, and open channel kinetics of ∼1 and 7 ms.
A third population of α7 nAChRs is identified using α7-specific antisense ‘deletion’. These receptors are characterized by a unitary conductance of 50 pS with long open duration as this type of channel is removed following antisense oligonucleotide treatment. The 50 pS α7-containing channels are less abundant than the 50 pS ‘brief’ open events (τ, ∼3 ms) as ∼40 % of the patches are devoid of 50 pS channels following α7 deletion.
Interestingly, each of the proposed α7 nAChRs are biophysically similar to the nAChRs detected in sympathetic neurones only during later developmental stages. Previous studies demonstrated that the nAChRs expressed later in embryonic development include larger conductance and longer open duration channels (designated S17, M17, L17; Moss et al. 1989; Moss & Role, 1993) which are strikingly similar to the nAChR subtypes that we characterize and propose as the α7-containing channels in the current study. The developmental changes in the abundance of S17, M17, L17 and XL subtypes correlated with increased expression of α7 is consistent with increased assembly of α7-containing nAChR channels. In sum, these data support the proposal that sympathetic neurones normally express α7-containing heteromeric nAChRs and further suggest that these α7 nAChR subtypes correspond to at least three of the four channel classes previously demonstrated to be upregulated during embryonic development and synaptogenesis.
References
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Presence of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat olfactory bulb neurons. Neuroscience Letters. 1994;176:152–156. doi: 10.1016/0304-3940(94)90070-1. 10.1016/0304-3940(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons define methyllycaconitine as a potent and specific receptor antagonist. Molecular Pharmacology. 1992;41:802–808. [PubMed] [Google Scholar]
- Alkondon M, Pereira EFR, Barbosa CTF, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates GABA release from CA1 neurons of rat hippocampal slices. Journal of Pharmacology and Experimental Therapeutics. 1997a;283:1396–1411. [PubMed] [Google Scholar]
- Alkondon M, Pereira EFR, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha 7 nicotinic acetylcholine receptors in the rat brain neurons. European Journal of Neuroscience. 1997b;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Anand R, Peng X, Ballesta JJ, Lindstrom J. Pharmacological characterization of alpha-bungarotoxin-sensitive acetylcholine receptors immunoisolated from chick retina: contrasting properties of alpha 7 and alpha 8 subunit-containing subtypes. Molecular Pharmacology. 1993a;44:1046–1050. [PubMed] [Google Scholar]
- Anand R, Peng X, Lindstrom J. Homomeric and native alpha 7 acetylcholine receptors exhibit remarkably similar but non-identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Letters. 1993b;327:241–246. doi: 10.1016/0014-5793(93)80177-v. 10.1016/0014-5793(93)80177-V. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Bertrand S, Ballivet M. Pharmacological properties of the homomeric alpha 7 receptor. Neuroscience Letters. 1992;146:87–90. doi: 10.1016/0304-3940(92)90179-b. [DOI] [PubMed] [Google Scholar]
- Britto LR, Hamassaki-Britto DE, Ferro ES, Keyser KT, Karten HJ, Lindstrom JM. Neurons of the chick brain and retina expressing both alpha-bungarotoxin-sensitive and alpha-bungarotoxin-insensitive nicotinic acetylcholine receptors: an immunohistochemical analysis. Brain Research. 1992;590:193–200. doi: 10.1016/0006-8993(92)91095-v. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Yang X, Doyle JP, Huck S, Role LW. Developmental regulation of multiple nicotinic AChR channel subtypes in embryonic chick habenula neurones: contributions of both the alpha 2 and alpha 4 subunit genes. Pflügers Archiv. 1994;429:27–43. doi: 10.1007/BF02584027. [DOI] [PubMed] [Google Scholar]
- Castro NG, Albuquerque EX. alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophysical Journal. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB. The fall and rise of neuronal alpha-bungarotoxin binding proteins. Trends in Pharmacological Sciences. 1992;13:407–413. doi: 10.1016/0165-6147(92)90125-p. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Schwartz RD, Paul SM, Pert A. Nicotine binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. Journal of Neuroscience. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- De Koninck P, Cooper E. Differential regulation of neuronal nicotinic ACh receptor subunit genes in cultured neonatal rat sympathetic neurons: specific induction of alpha 7 by membrane depolarization through a Ca2+/calmodulin-dependent kinase pathway. Journal of Neuroscience. 1995;15:7966–7978. doi: 10.1523/JNEUROSCI.15-12-07966.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devay P, Qu X, Role L. Regulation of nAChR subunit gene expression relative to the development of pre- and postsynaptic projections of embryonic chick sympathetic neurons. Developmental Biology. 1994;162:56–70. doi: 10.1006/dbio.1994.1066. [DOI] [PubMed] [Google Scholar]
- Drasdo A, Caulfield M, Bertrand D, Bertrand S, Wonnacott S. Methyllycaconitine: a novel nicotinic antagonist. Molecular and Cellular Neurosciences. 1992;3:237–243. doi: 10.1016/1044-7431(92)90043-2. [DOI] [PubMed] [Google Scholar]
- Galzi JL, Devillers-Thiery A, Hussy N, Bertrand S, Changeux JP, Bertrand D. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992;359:500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- Hoffman PW, Ravindran A, Huganir RL. Role of phosphorylation in desensitization of acetylcholine receptors expressed in Xenopus oocytes. Journal of Neuroscience. 1994;14:4185–4195. doi: 10.1523/JNEUROSCI.14-07-04185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JF, Tank DW, Greengard P, Huganir RL. Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature. 1988;336:677–680. doi: 10.1038/336677a0. [DOI] [PubMed] [Google Scholar]
- Jacob MH. Acetylcholine receptor expression in developing chick ciliary ganglion neurons. Journal of Neuroscience. 1991;11:1701–1712. doi: 10.1523/JNEUROSCI.11-06-01701.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser KT, Britto LR, Schoepfer R, Whiting P, Cooper J, Conroy W, Brozozowska-Prechtl A, Karten HJ, Lindstrom J. Three subtypes of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors are expressed in chick retina. Journal of Neuroscience. 1993;13:442–454. doi: 10.1523/JNEUROSCI.13-02-00442.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J, Anand R, Peng X, Gerzanich V, Wang F, Li Y. Neuronal nicotinic receptor subtypes. Annals of the New York Academy of Sciences. 1995;757:100–116. doi: 10.1111/j.1749-6632.1995.tb17467.x. [DOI] [PubMed] [Google Scholar]
- Listerud M, Brussaard AB, Devay P, Colman DR, Role LW. Functional contribution of neuronal AChR subunits revealed by antisense oligonucleotides. Science. 1991;254:1518–1521. doi: 10.1126/science.1720573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Review of Physiology. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- Margiotta JF, Gurantz D. Changes in the number, function, and regulation of nicotinic acetylcholine receptors during neuronal development. Developmental Biology. 1989;135:326–339. doi: 10.1016/0012-1606(89)90183-8. [DOI] [PubMed] [Google Scholar]
- Moss BL, Role LW. Enhanced ACh sensitivity is accompanied by changes in ACh receptor channel properties and segregation of ACh receptor subtypes on sympathetic neurons during innervation in vivo. Journal of Neuroscience. 1993;13:13–28. doi: 10.1523/JNEUROSCI.13-01-00013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss BL, Schuetze SM, Role LW. Functional properties and developmental regulation of nicotinic acetylcholine receptors on embryonic chicken sympathetic neurons. Neuron. 1989;3:597–607. doi: 10.1016/0896-6273(89)90270-5. [DOI] [PubMed] [Google Scholar]
- Palma E, Bertrand S, Binzoni T, Bertrand D. Neuronal nicotinic α7 receptor expressed in Xenopus oocytes presents five putative binding sites for methyllycaconitine. The Journal of Physiology. 1996;491:151–162. doi: 10.1113/jphysiol.1996.sp021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh PC, Corriveau RA, Conroy WG, Berg DK. Novel subpopulation of neuronal acetylcholine receptors among those binding alpha-bungarotoxin. Molecular Pharmacology. 1995;47:717–725. [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annual Review of Neuroscience. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. Journal of Neuroscience. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons LK, Schuetze SM, Role LW. Substance P modulates single-channel properties of neuronal nicotinic acetylcholine receptors. Neuron. 1990;4:393–403. doi: 10.1016/0896-6273(90)90051-g. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Pugh PC, Zhang ZW, Rathouz MM, Berg DK. Nicotinic receptors that bind alpha-bungarotoxin on neurons raise intracellular free Ca2+ Neuron. 1992;8:353–362. doi: 10.1016/0896-6273(92)90301-s. [DOI] [PubMed] [Google Scholar]
- Ward JM, Cockcroft VB, Lunt GG, Smillie FS, Wonnacott S. Methyllycaconitine: a selective probe for neuronal alpha-bungarotoxin binding sites. FEBS Letter. 1990;270:45–48. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- Wilkie GI, Hutson P, Sullivan JP, Wonnacott S. Pharmacological characterization of a nicotinic autoreceptor in rat hippocampal synaptosomes. Neurochemistry Research. 1996;21:1141–1148. doi: 10.1007/BF02532425. [DOI] [PubMed] [Google Scholar]
- Yu C, Brussaard AB, Yang X, Listerud M, Role LW. Uptake of antisense oligonucleotides and functional block of acetylcholine receptor subunit gene expression in primary embryonic neurons. Developmental Genetics. 1993;14:296–304. doi: 10.1002/dvg.1020140407. [DOI] [PubMed] [Google Scholar]
- Yu CR, Role LW. Functional contribution of the α5 subunit to neuronal nicotinic channels expressed by chick sympathetic ganglion neurones. The Journal of Physiology. 1998;509:667–681. doi: 10.1111/j.1469-7793.1998.667bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Vijayaraghavan S, Berg DK. Neuronal acetylcholine receptors that bind alpha-bungarotoxin with high affinity function as ligand-gated ion channels. Neuron. 1994;12:167–177. doi: 10.1016/0896-6273(94)90161-9. [DOI] [PubMed] [Google Scholar]


