Abstract
The effects of Cs+ on the action potential, post-train after-hyperpolarization (AHP), Ca2+-dependent post-spike depolarizing after-potential (DAP) and phasic firing were examined during intracellular recordings from magnocellular neurosecretory cells (MNCs) in superfused rat hypothalamic explants.
Extracellular Cs+ reversibly inhibited (IC50, ≈1 mM) DAPs, and associated after-discharges, that followed brief spike trains in each of sixteen cells tested. Although bath application of Cs+ also provoked a small reversible depolarization, inhibition of the DAP was retained when membrane voltage was kept constant by current injection.
Application of Cs+ had no significant effects on spike duration (n = 8), frequency-dependent spike broadening (n = 8), spike hyperpolarizing after-potentials (n = 14), or the amplitude of the isolated AHP (n = 7). Caesium-evoked inhibition of the DAP, therefore, does not result from diminished spike-evoked Ca2+ influx, and may reflect direct blockade of the conductance underlying the DAP.
Inhibition of the DAP was associated with an enhancement of the amplitude and duration of the AHP, indicating that the currents underlying the AHP and the DAP overlap in time following a train of action potentials, and that the relative magnitude of these currents is an important factor in determining the shape and time course of post-train after-potentials.
Bath application of Cs+ reversibly abolished phasic firing in each of seven cells tested. This effect was reversible and persisted at all subthreshold voltages tested. These results indicate that the current underlying the DAP is necessary for the genesis of plateau potentials and phasic firing in MNCs.
Hypothalamic magnocellular neurosecretory cells (MNCs) are responsible for the release of either vasopressin (VP; the antidiuretic hormone) or oxytocin (OT) into the blood (Poulain & Wakerley, 1982). Following synthesis in MNC somata, the peptides are packaged in vesicles and transported to axon terminals in the neurohypophysis where exocytosis is triggered by the arrival of action potentials (Dreifuss, Kalnins, Kelly & Ruf, 1971; Brownstein, Russell & Gainer, 1980). In both MNC types, action potentials regulating secretion are initiated at the soma, as a result of interactions between afferent synaptic signals and intrinsic membrane properties (Renaud & Bourque, 1991; Armstrong, 1995).
In the rat, VP-MNCs respond to hyperosmolality (Brimble & Dyball, 1977; Wakerley, Poulain & Brown, 1978) and hypovolaemia (Harris, Dreifuss & Legros, 1975) by increasing their firing rate and by adopting phasic firing, a pattern comprising alternating periods of activity (7-15 Hz) and silence lasting tens of seconds each. Studies using the neurohypophysis in vitro have shown that during repetitive stimulation with a fixed number of pulses VP release increases with frequency (Dreifuss et al. 1971; Bicknell, 1988). At physiologically relevant frequencies, however, VP release is effectively maximized by stimulation patterns mimicking phasic firing (Dutton & Dyball, 1979; Bicknell & Leng, 1981), presumably due to the reversal of secretory fatigue during periods of quiescence (Bicknell, Brown, Chapman, Hancock & Leng, 1984; Bicknell, 1988).
Action potentials in rat VP-MNCs are followed by a slow (2-3 s) depolarizing after-potential (DAP; Cobbett, Smithson & Hatton, 1986; Armstrong, Smith & Tian, 1994; Armstrong, 1995). It has been suggested that DAPs following consecutive action potentials summate temporally into a depolarizing plateau to initiate individual phasic bursts (Andrew & Dudek, 1983, 1984a). Firing during phasic bursts appears to be sustained by this plateau, and the eventual termination of each burst is associated with a repolarization of the plateau potential. Repolarization of the plateau potential at the end of phasic bursts has been proposed to result from an activity-dependent inactivation of the conductance underlying the DAP (GDAP; Andrew & Dudek, 1984a), and/or from the activation of a Ca2+-dependent K+ conductance (Andrew & Dudek, 1984b; Bourque, Randle & Renaud, 1985). While these observations suggest that the DAP may play an important role in the generation of phasic firing, direct evidence for this hypothesis has not been obtained.
Although the ionic basis of the current mediated by GDAP remains unknown, circumstantial evidence suggests that the DAP might be mediated by a decrease in K+ current (e.g. Andrew, 1987; Li & Hatton, 1997b) or via the activation of a non-selective cation current (Bourque, 1986). Interestingly, recent studies have revealed that K+ (e.g. Coggan, Purnyn, Knoper & Kreulen, 1994) and cation-permeable conductances (e.g. Reichling & Levine, 1997) can be blocked by the application of extracellular Cs+, suggesting that this monovalent cation might be useful as a probe to investigate the functional role of the DAP in hypothalamic MNCs. In this study, therefore, we examined the effects of extracellular Cs+ on spike after-potentials and phasic firing in MNCs recorded from the supraoptic nucleus in superfused explants of rat hypothalamus. Parts of the results obtained have been published as an abstract (Ghamari-Langroudi & Bourque, 1997).
METHODS
Preparation of superfused explants
Hypothalamic explants were prepared as described previously (Bourque, 1988b). Briefly, male Long-Evans rats (150-300 g) were briefly (5-10 s) restrained in a plastic cone and killed by decapitation using a small rodent guillotine (model 51330; Stoelting Company, Wood Dale, IL, USA). This tissue harvesting protocol has been approved by the McGill University Animal Care Committee. The brain was then rapidly removed from the cranial vault. A block of tissue (∼8 × 8 × 2 mm) comprising the basal hypothalamus was excised using razor blades and pinned, ventral side up, to the Sylgard base of a temperature-controlled (33-35°C) superfusion chamber. Within 2-3 min of decapitation, explants were being superfused (0.5-1 ml min−1) with an oxygenated (95 % O2-5 % CO2) artificial cerebrospinal fluid (ACSF; see below) delivered via a Tygon tube placed over the medial tuberal region. The arachnoid membranes covering the ventral surface of the supraoptic nucleus were removed using fine forceps and a cotton wick was placed at the rostral tip of the explant to facilitate drainage of ACSF.
Solutions and drugs
The ACSF (pH 7.4; 295 ± 1 mosmol kg−1) was composed of (mM): NaCl, 121; MgCl2, 1.3; KCl, 3; NaHCO3, 26; glucose, 10; CaCl2, 2.5 (all from Fisher Scientific Company, Pittsburgh, PA, USA). The effects of Cs+ were examined by addition of a known amount of CsCl into the solution perfusing the tissue or, in some instances, by isomolar replacement of NaCl. Both methods were effective at inhibiting the DAP and provoking membrane depolarization, indicating that the effects observed were not due to changes in osmolality or in extracellular chloride concentration.
Electrophysiology
Intracellular recordings were obtained using sharp micropipettes prepared from glass capillary tubes (1.2 mm o.d.; A. M. Systems Inc., Everett, WA, USA) pulled on a P87 Flaming-Brown puller (Sutter Instruments Co., Novato, CA, USA). Pipettes were filled with 2 M potassium acetate, yielding a DC resistance of 70- 150 MΩ relative to a Ag-AgCl wire electrode immersed in ACSF. Recordings of membrane voltage were obtained through an Axoclamp 2A amplifier (Axon Instruments Inc.). Signals acquired during each experiment were displayed on a chart recorder and digitized (44 kHz; Neurodata Instruments Co., Delaware Water Gap, PA, USA) for storage onto videotape. Current pulses were delivered through an external pulse generator, or via a Labmaster interface driven by pCLAMP software (Axon Instruments Inc.) running on an AT-compatible computer.
Throughout the paper, data are expressed as the mean ± standard error of the mean (± s.e.m.). Differences between mean values recorded under control and test conditions were evaluated using Student's paired t test and were considered significant when P < 0.05.
RESULTS
The data presented below were obtained during intracellular recordings made from fifty supraoptic nucleus neurones impaled with sharp microelectrodes in superfused explants of rat hypothalamus. These cells had resting membrane potentials more negative than −50 mV, input resistances exceeding 120 MΩ, and fired action potentials with amplitudes greater than 60 mV when measured from baseline. Each of these cells also displayed frequency-dependent spike broadening (Andrew & Dudek, 1985; Bourque & Renaud, 1985b) and transient outward rectification (Bourque, 1988b) when examined from initial membrane potentials below −75 mV. These combined characteristics have been shown to be specific to magnocellular neurosecretory neurones, but not to neighbouring non-neuroendocrine cells, during intracellular recordings in vitro (Renaud & Bourque, 1991) and in vivo (Bourque & Renaud, 1991; Dyball, Tasker, Wuarin & Dudek, 1991).
Protocol for eliciting reproducible DAPs from MNCs
Previous experiments have shown that the amplitude of DAPs following trains of action potentials is strongly affected by the number of spikes in the train (Andrew & Dudek, 1984b). In the present experiments, therefore, DAPs were evoked by trains of a constant number of action potentials, and each train was elicited by a single current pulse (50-150 ms). When the voltage at the peak of the evoked DAP exceeded spike threshold (−50 mV; Armstrong et al. 1994), an after-discharge comprising one or more action potentials resulted from the establishment of a plateau potential. Since the amplitude of a DAP can be attenuated by preceding activity (Andrew & Dudek, 1984a), trains were evoked at a low frequency (< 0.1 Hz) and spontaneous firing, when present, was prevented by injecting a small amount of continuous negative current (usually 10-90 pA).
Effects of Cs+ on DAPs and membrane potential
Using the protocol described above, post-train DAPs of consistent amplitude and time course could be recorded for prolonged periods of time (up to 2 h), whether or not they were associated with the production of an after-discharge. In individual cells, the presence or absence of an after-discharge was determined by the membrane potential which was set by DC current injection. In each of seven cells featuring an after-discharge, bath application of Cs+ (3-6 mM) first eliminated the firing associated with the plateau potential and then reduced the amplitude of the post-train DAP (Fig. 1). Upon washout of Cs+, post-train DAPs recovered first, followed by the reappearance of after-discharges. In each of nine additional cells, where the post-train DAP was not associated with an after-discharge, bath application of Cs+ (3-6 mM) caused a gradual and reversible decrease in the amplitude of the DAP. In addition to these effects on DAPs and after-discharges, bath application of Cs+ provoked a reversible membrane depolarization (2−7 mV) whose time course was comparable to the inhibitory effects of Cs+ on the DAP (e.g. Fig. 1A). This response was not due to a presynaptic effect since reversible, and reproducible, Cs+-evoked depolarizations were also seen in each of three cells tested in the presence of 0.5 μM tetrodotoxin (TTX) to block action-potential-dependent synaptic transmission (data not shown).
Figure 1. Effects of extracellular Cs+ on post-train DAPs.
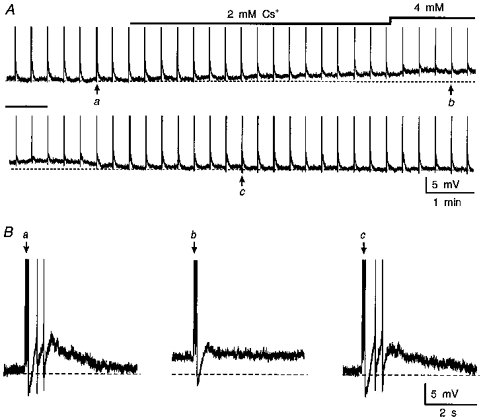
A, chart recording from a supraoptic neurone impaled in a superfused hypothalamic explant. Individual vertical deflections are spike trains (full amplitude not shown) comprising 3 action potentials each, evoked by a brief current pulse (0.2 nA, 50 ms). Each train is followed by a slowly decaying DAP. Note that consecutive application of 2 mM (lower bar) and 4 mM (upper bar) Cs+ provokes an increasing depolarization (dashed line; −59 mV) associated with progressive inhibition of DAPs. The traces in B are excerpts of the recording shown in A (lettered arrows) showing the DAPs recorded before (a), in the presence of 4 mM Cs+ (b), and following washout (c).
Previous studies have shown that the amplitude of the post-train DAP is strongly affected by the membrane potential from which it is evoked (Vinitial). For example, no DAP can be evoked by a train of action potentials triggered at a Vinitial negative to −80 mV. DAPs become apparent when evoked from a Vinitial of about −75 mV, and their amplitude increases with voltage up to ∼10 mV below action potential threshold (Bourque, 1986; Andrew, 1987). Further depolarization, however, causes a decrease in DAP amplitude, presumably because of the increasing sustained outward rectification that develops at these voltages (Bourque 1988b; Stern & Armstrong, 1995, 1996, 1997). It is possible, therefore, that the reduction of DAP amplitude caused by Cs+ in the above experiments might have occurred as an indirect consequence of membrane depolarization. In order to resolve this issue, we examined the effects of Cs+ while maintaining the Vinitial constant by means of DC current injection. In each of seventeen cells tested in this way, bath application of Cs+ (3-6 mM) reversibly reduced the amplitude of post-train DAPs, as well as any associated after-discharge (n = 9 cells; Fig. 2). Furthermore, when the inhibitory effects of Cs+ were compared on post-train DAPs evoked from a variety of Vinitial values, an equivalent reduction of DAP amplitude was observed at all of the potentials tested. Figure 3 shows that the inhibitory effect of Cs+ on the amplitude of post-train DAPs was dose dependent, with half-maximal inhibition (IC50) occurring at a concentration of about 1 mM.
Figure 2. Cs+-mediated inhibition of the DAP is not voltage dependent.
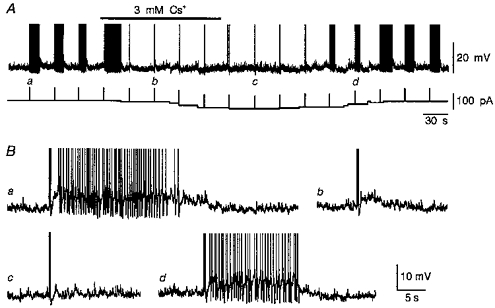
A, chart recording of membrane voltage (upper trace) showing the effects of 3 mM Cs+ (bar) on DAPs following trains of 4 action potentials triggered by depolarizing current pulses (upward deflections in lower trace). In this experiment a variable amount of inward current (lower trace) was injected into the cell to maintain a constant initial potential (−57 mV) during exposure to Cs+. The traces in B are excerpts of the recording shown in A. Note that compared with control (a), addition of Cs+ first inhibited the after-discharge (b) and then caused a further decrease in the amplitude of the DAP (c). The effects were reversible upon washout of Cs+ (d). Full spike amplitude not shown.
Figure 3. Inhibition of DAPs by Cs+ is dose dependent.
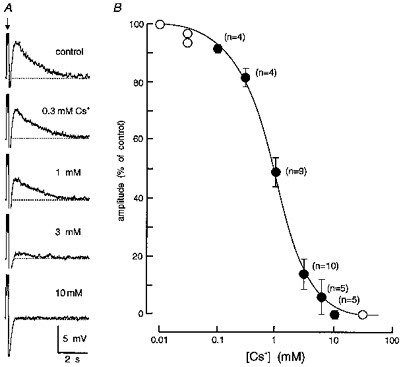
A, excerpts of an intracellular recording obtained from an MNC in which DAP amplitude was examined under control conditions and in the presence of different concentrations of Cs+ (as indicated). The traces are aligned vertically along the time at which a train of 3 spikes (clipped amplitude) was evoked (arrow) by a current pulse to elicit a post-train DAP (dashed line, −62 mV). The full amplitude of the AHP is not shown, except for the trace recorded in 10 mM Cs+. B, the graph plots the relative amplitude of DAPs (% of control) recorded from MNCs during bath application of different concentrations of Cs+. Note that half-maximal inhibition occurred near 1 mM. Open symbols plot data obtained from single cells whereas filled symbols show means ± s.e.m. The number of cells from which the mean was calculated is shown in parentheses beside each point.
Effects of Cs+ on Ca2+-dependent potentials
The DAP has been shown to be dependent on Ca2+ influx for its activation (Li, Decavel & Hatton, 1995; Li & Hatton, 1997a). The inhibitory effect of Cs+ on DAPs, therefore, could be due to a decrease in Ca2+ influx during the train of action potentials. To determine if this was the case, we examined the effects of external Cs+ on other electrophysiological characteristics known to be Ca2+ dependent. Previous studies in MNCs have shown that reducing the concentration of extracellular Ca2+, or addition of Ca2+ channel antagonists, decreases action potential duration (Bourque & Renaud, 1985a), frequency-dependent spike broadening (Bourque & Renaud, 1985b), the amplitude of the post-spike hyperpolarizing after-potential (HAP; Bourque et al. 1985) and the magnitude of the post-train after-hyperpolarization (AHP; Andrew & Dudek, 1984b; Bourque et al. 1985; Andrew, 1987). To examine the effects of Cs+ on spike duration and HAPs, action potentials were evoked at a slow frequency (< 0.1 Hz), from a constant Vinitial, to avoid the complicating effects of frequency-dependent spike broadening (Andrew & Dudek, 1985; Bourque & Renaud, 1985b) and changes in membrane potential (Bourque, 1988a). The effects of Cs+ on spike broadening were assessed by comparing the relative increases in spike duration observed between the first and last action potentials in spike trains evoked in the presence and absence of Cs+. In these experiments, trains comprising constant numbers of spikes (4-13) were evoked by current pulses delivered at a frequency < 0.1 Hz, from a constant Vinitial. As illustrated in Fig. 4(A-C), bath application of 3-6 mM Cs+ had no effect on spike duration (1.14 ± 0.08 ms in control vs. 1.18 ± 0.09 ms in Cs+; P = 0.13; n = 8), spike broadening (154 ± 11 % in control vs. 162 ± 15 % in Cs+; P = 0.082; n = 8), or HAP amplitude (9.2 ± 0.4 mV in control vs. 8.9 ± 0.4 mV in Cs+; P = 0.16; n = 14). High frequency trains of action potentials were used to examine the effects of Cs+ on the Ca2+-dependent AHP in the absence of overlapping effects on the DAP; trains of ten or more spikes fired at frequencies ≥ 50 Hz are followed by a prominent AHP without the expression of a subsequent DAP (Andrew & Dudek, 1984b), presumably due to an activity-dependent inactivation of the DAP (Andrew & Dudek, 1984a). As illustrated in Fig. 4D, bath application of 3-6 mM Cs+ failed to affect the amplitude of the isolated AHP (9.2 ± 0.8 mV in control vs. 8.9 ± 0.8 mV in Cs+; P = 0.17; n = 7). Since none of the Ca2+-dependent characteristics examined in MNCs were reduced by external Cs+, it is unlikely that the effects of Cs+ on the DAP were mediated through an action on spike-evoked Ca2+ influx and accumulation.
Figure 4. Effects of Cs+ on other Ca2+-dependent parameters.
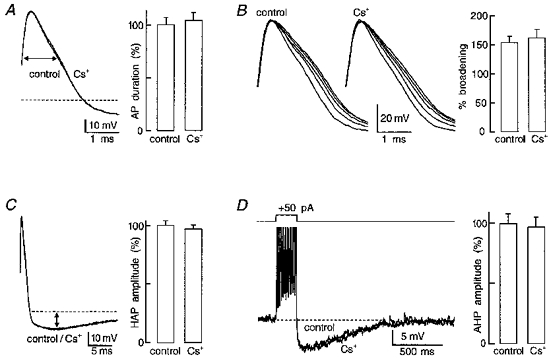
Each panel shows voltage traces recorded in the presence and absence of 3 mM Cs+ (all are superimposed except in B where only the spikes evoked by a single train are superimposed) to illustrate effects on: A, action potential (AP) duration measured at half-amplitude (e.g. horizontal arrow), relative to baseline (dashed line); B, AP broadening, measured as the percentage change in duration observed between the first and the last AP evoked by a train; C, HAP amplitude (e.g. vertical arrow); and D, AHP amplitude relative to baseline. All action potentials (A and C) were evoked from a constant initial membrane potential and a silent interval of at least 20 s occurred before each test. The spike trains in B and D (clipped amplitude) were evoked by a depolarizing current pulse applied every 20 s and the initial membrane potential was maintained constant by DC current injection. The right panels are bar histograms showing the effects of 3-6 mM Cs+ on mean (+ s.e.m.) AP duration (A; % of control; n = 8), AP broadening (B; % broadening; n = 8) HAP amplitude (C; % of control; n = 14) and AHP amplitude (D; % of control; n = 7). Note that none of the parameters were significantly affected (i.e. P > 0.05; see text for absolute values).
Time overlap between the AHP and the DAP
In previous studies application of apamin, a selective blocker of the Ca2+-dependent K+ current underlying the AHP (IAHP) in MNCs, resulted in an increase in the amplitude of DAPs evoked by brief trains of action potentials, suggesting a temporal overlap between the post-train after-current mediating the DAP (IDAP) and IAHP (Bourque & Brown, 1987; Armstrong et al. 1994; Kirkpatrick & Bourque, 1996). Selective inhibition of IDAP with Cs+, therefore, might be expected to increase the relative magnitude of the AHP. The effects of Cs+ on after-potentials evoked by low frequency trains were therefore examined in eleven cells maintained at a constant Vinitial. As illustrated in Fig. 5, application of Cs+ (3-6 mM) decreased the mean (± s.e.m.) amplitude of DAPs from 4.5 ± 0.6 to 0.9 ± 0.3 mV (P = 0.00013). In the same cells, mean AHP amplitude increased from 4.3 ± 0.8 to 5.5 ± 0.8 mV (P = 0.02) and AHP duration was enhanced from 190 ± 36 ms in control to 250 ± 36 ms (P = 0.0007) in the presence of Cs+. These results indicate that IAHP and IDAP partly overlap in time following a brief train of action potentials, and that the shape of the post-train after-potential can be regulated by the modulation of IDAP.
Figure 5. Blockade of the DAP enhances the overlapping AHP.
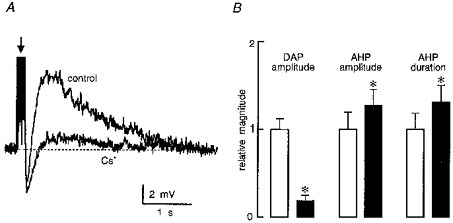
A, superimposed traces showing post-train (arrow; spike amplitude not shown) after-potentials comprising AHP (below the dashed line) and DAP (above the dashed line) components, recorded in the absence (control) and presence (Cs+) of 3 mM extracellular Cs+. B, bar histograms illustrating mean (+ s.e.m.; n = 11) relative changes in DAP amplitude, AHP amplitude and AHP duration, during application of 3-6 mM Cs+. □, control; ▪, Cs+. Note that * indicates P < 0.05 (see text for absolute values).
Effects of Cs+ on phasic firing
Previous studies have shown that each episode of activity during phasic firing arises as a consequence of the depolarizing effect of a plateau potential (Andrew & Dudek, 1983, 1984a). While the latter has been proposed to arise from the summation of DAPs following consecutive action potentials, evidence for the involvement of DAPs in generating the plateau potential, or phasic bursting activity, has not been obtained. We, therefore, examined the effects of Cs+ on MNCs displaying spontaneous phasic firing. For the purposes of this analysis, phasic activity was defined as a firing pattern consisting of alternating periods of uninterrupted firing (> 2 Hz), lasting at least 10 s, separated by well-defined silent intervals (< 0.2 Hz) lasting at least 10 s. In each of seven cells tested, bath application of 3-10 mM Cs+ reversibly depolarized the cells and provoked continuous firing (Fig. 6). Under such conditions, maintained injection of hyperpolarizing current could not restore phasic activity, at any membrane potential (Fig. 6). In two cells, application of 1-2 mM Cs+ produced a weaker depolarization and an increase in burst duration, but did not prevent the generation of phasic bursts. Increasing the concentration of Cs+ to 3-5 mM, however, promptly abolished the pattern in these cells, suggesting that profound blockade of the conductance underlying the DAP is required to abolish phasic firing. As illustrated in Fig. 7, the absence of phasic firing in the presence of Cs+ was associated with the absence of a DAP following single spikes and of their apparent summation into a plateau potential.
Figure 6. Effects of Cs+ on phasic firing.
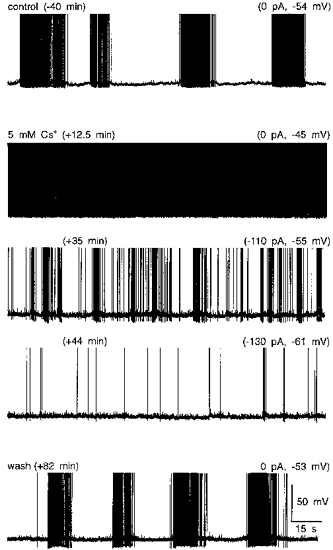
Traces shown are excerpts of an intracellular voltage recording from a supraoptic MNC obtained under control conditions (top), in the presence of 5 mM Cs+ (3 middle panels), and following return to ACSF (wash; bottom). The values shown above and to the left of each trace indicate the time at which the corresponding excerpts begin, relative to the onset of the application of Cs+. The values above and to the right of each trace indicate the holding current, relative to control, as well as the membrane voltage observed between action potentials. This cell showed spontaneous phasic firing with no current injected under control conditions. Bath application of Cs+ caused a depolarization associated with the appearance of continuous firing (0 pA). Sustained hyperpolarization by DC current injection (-110 pA) reduced the rate at which the cell fired continuously, or provoked slow irregular firing (e.g. -130 pA), but could not restore phasic firing. Further hyperpolarization of this cell abolished spontaneous firing (+60 min, -270 pA, −65 mV; not shown).
Figure 7. Cs+ blocks DAPs following single spikes.
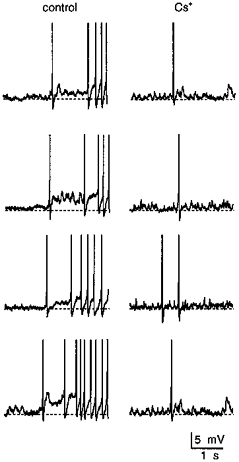
The traces shown are brief excerpts of the recording obtained from the cell shown in Fig. 6. The left panels show examples of the initial action potentials associated with the onset of 4 spontaneous phasic bursts recorded under control conditions. The right panels show isolated spikes, and spike doublets, recorded from the same cell while phasic activity was abolished by the application of 5 mM Cs+. Note that the loss of phasic firing is associated with the absence of DAPs following single action potentials, and of their summation during consecutive spikes.
DISCUSSION
The results of this study identify Cs+ as a blocker of the DAP that follows action potentials in MNCs of the rat supraoptic nucleus. Blockade of the DAP by external Cs+ was dose dependent (IC50, ∼1 mM), and reversed completely upon washout.
Caesium is a direct blocker of the conductance underlying DAPs
The generation of a DAP has been shown to be dependent on the influx of Ca2+ during the preceding train of action potentials (Li et al. 1995; Li & Hatton, 1997a). The inhibition of DAPs by Cs+, therefore, might be accomplished: (i) through a reduction of Ca2+ influx and/or its intracellular accumulation during action potentials, (ii) through an effect on the Ca2+-dependent modulation of the channels responsible for the generation of DAPs, or (iii) through direct blockade of these channels. Since the shoulder on the repolarizing phase of action potentials reflects Ca2+ influx in MNCs (Bourque & Renaud, 1985a, b), we examined whether action potential width, or frequency-dependent broadening, were reduced by external Cs+. The data in Fig. 4(A and B) show that this was not the case. In addition, bath application of Cs+ did not affect the amplitude of HAPs following single action potentials (Fig. 4C), or that of isolated AHPs evoked by high frequency spike trains (Fig. 4D), both of which are attenuated under conditions reducing Ca2+ influx (Bourque et al. 1985) or intracellular Ca2+ accumulation (Andrew & Dudek, 1984b) in MNCs. The effects of Cs+ on the DAP, therefore, were presumably not due to an inhibition of Ca2+ influx and/or accumulation during the train. Although no information is currently available concerning a possible sensitivity of DAPs to intracellular Cs+, intracellular injection of Cs+ in MNCs has previously been shown to inhibit the Ca2+-activated K+ current underlying the AHP (Bourque, Brown & Renaud, 1986). Since the AHP was not reduced by bath application of Cs+ (Fig. 4D), it is unlikely that Cs+ leaked into the cytoplasm. The inhibition of DAPs during bath application of Cs+, therefore, was presumably not due to an action of Cs+ at an intracellular site. We conclude, therefore, that Cs+ blocks DAPs through a direct interaction with the channels responsible for their production. Further analysis will be required to investigate the basis for this block.
Ionic basis of the DAP in supraoptic neurones
Although previous studies have examined various biophysical and pharmacological aspects of the DAP in MNCs (Andrew & Dudek, 1983, 1984a, b; Bourque, 1986; Andrew, 1987; Li et al. 1995; Li & Hatton, 1997a), the nature of GDAP remains unclear. Ionic mechanisms that have been proposed to underlie the inward current responsible for the production of DAPs include the activation of a non-selective cation conductance (Bourque, 1986) and the reduction of a resting K+ current (Andrew, 1987; Li & Hatton, 1997b).
In principle, the nature of the conductance could be partly resolved biophysically by determining whether membrane resistance increases (i.e. from suppression of K+ conductance) or decreases (i.e. from the activation of cation channels) during the DAP. In previous current-clamp studies, electrotonic potential (ETP) responses to small hyperpolarizing current pulses were found to increase in amplitude during the DAP (Bourque, 1986; Andrew, 1987), suggesting that membrane resistance may increase during the after-potential. This interpretation is questionable, however, because an increase in ETP amplitude should be expected regardless of the conductance change, given that the amplitude of the underlying current displays negative slope between -80 and −50 mV (Bourque, 1986). Instantaneous current-voltage analysis in MNCs, therefore, may be required to determine the reversal potential of IDAP, and to establish whether the generation of this current is associated with an increase or a decrease in membrane conductance.
The data presented here establish sensitivity to Cs+ as a new pharmacological criterion to be satisfied in the identification of IDAP. In this regard, it is interesting to note that extracellularly applied Cs+ is known to block a variety of K+ and non-selective cation permeable conductances in other types of cells. For example, millimolar concentrations of extracellular Cs+ have been reported to block the M-type K+ current in prevertebral neurones (Coggan et al. 1994). If DAPs in MNCs were due to the transient suppression of a K+ conductance, then inhibition of such a conductance could explain why, in our studies, blockade of the DAP by Cs+ was accompanied by membrane depolarization (e.g. Fig. 1). Another well-known action of externally applied Cs+ is its blocking effect on the hyperpolarization-activated cation current IH (e.g. McCormick & Pape, 1990). The presence of a Cs+-sensitive IH in MNCs has already been reported from voltage-clamp studies on hypothalamic slices obtained from guinea-pig (Erickson, Ronnekliev & Kelly, 1993). Current-clamp analysis of MNCs in rat hypothalamic explants also suggests the presence of IH in this species (Stern & Armstrong, 1997). The voltage dependency of IH, however, is opposite to that of IDAP. Moreover, inhibition of steady-state IH would be expected to cause hyperpolarization upon application of Cs+, rather than depolarization. Finally, as in the guinea-pig (Erickson et al. 1993), many of the MNCs recorded in rat supraoptic nucleus show no evidence for the presence of IH, as determined by the absence of a depolarizing sag during 500-2000 ms hyperpolarizing pulses applied between -70 and −100 mV (M. Ghamari-Langroudi & C. W. Bourque, unpublished observations). It seems unlikely, therefore, that IH is involved in the generation of the DAP, or that blockade of IH is responsible for Cs+-evoked depolarizations. Interestingly, a recent study in neurones of the dorsal root ganglion (Reichling & Levine, 1997) has revealed the existence of a Cs+-sensitive, Ca2+-dependent, cation channel exhibiting a voltage dependency similar to that of IDAP in MNCs (Bourque, 1986). While the properties of this conductance are compatible with those of DAPs, inhibition of this conductance alone could not explain the depolarization seen during bath application of Cs+. Thus, if a Cs+-sensitive, non-selective cation conductance is involved in the genesis of the DAP, an alternative mechanism will have to be identified in order to explain the depolarizing effects of Cs+. Further experiments will be required to determine the nature of the Cs+-evoked depolarization in MNCs.
DAPs play a role in phasic firing
Although previous experiments have suggested a role for DAPs in the generation of phasic firing in MNCs, direct evidence for this hypothesis had not been obtained. As shown in Fig. 6, bath application of a concentration of Cs+ sufficient to reduce DAPs by more than 80 % (i.e. ≥ 3 mM) eliminated phasic firing over the entire range of membrane potentials in which the cells could be seen to fire spontaneous action potentials (i.e. > −63 mV). This behaviour is in contrast with that observed in the absence of Cs+, where progressive hyperpolarization over the same range of potentials does not prevent the expression of phasic activity, but simply causes a decrease in burst duration (Andrew & Dudek, 1983, 1984a; Randle, Bourque & Renaud, 1985; Armstrong et al. 1994). These observations provide direct evidence for a role of IDAP in the generation of phasic firing.
Modulation of DAPs provides a mechanism for the regulation of phasic firing
During physiological activation, the proportion of VP-releasing MNCs firing phasically increases, optimizing the secretory response of the neurohypophysis. Although this response is believed to arise as a result of interactions between the intrinsic and synaptic properties of MNCs (Renaud & Bourque, 1991), the cellular basis for these changes is unknown. The results of our study imply that neurotransmitter regulation of post-spike DAPs is a mechanism by which the expression of phasic firing could be regulated by central synaptic afferents to hypothalamic MNCs. Since the currents underlying the AHP and the DAP partly overlap in time following a train of action potentials (e.g. Fig. 5), the magnitude of the post-train DAP could potentially be regulated via the modulation of either IAHP or IDAP. Evidence supporting the possibility that modulation of IAHP or IDAP might regulate the expression of phasic firing has been obtained from previous pharmacological experiments on MNCs. Thus, selective blockade of IAHP with apamin has been shown to enhance DAP amplitude (Bourque & Brown, 1987; Armstrong et al. 1994) and to increase intraburst firing rate during spontaneous phasic firing (Kirkpatrick & Bourque, 1996). Similarly, inhibition of IAHP by the peptide transmitter neurotensin has been reported to enhance DAP amplitude and to promote the generation of post-train after-discharges (Kirkpatrick & Bourque, 1995). Previous studies have also indicated that putative neurotransmitters might regulate phasic firing through a specific action on IDAP. For example histamine, acting at an H1 receptor, increases phasic burst duration and intraburst firing rate (Armstrong & Sladek, 1985) in association with a selective enhancement of the DAP (Smith & Armstrong, 1993). Conversely, a recent study has revealed that inhibition of the DAP by the neuropeptide galanin blocks phasic firing (Papas & Bourque, 1997). The existence of neurotransmitter systems regulating the magnitude of the post-train DAP may therefore provide the central nervous system with a powerful mechanism for the afferent control of patterned activity in MNCs.
Acknowledgments
We thank E. L. Seifert and Y. Chakfe for their helpful comments during the preparation of this manuscript. Our work was supported by an operating grant from the Medical Research Council (MRC) of Canada to C. W. B., and by MRC Studentship and Senior Scientist Awards to M. G. -L. and C. W. B., respectively.
References
- Andrew RD. Endogenous bursting by rat supraoptic neuroendocrine cells is calcium dependent. The Journal of Physiology. 1987;384:451–465. doi: 10.1113/jphysiol.1987.sp016463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- Andrew RD, Dudek FE. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. Journal of Neurophysiology. 1984a;51:552–566. doi: 10.1152/jn.1984.51.3.552. [DOI] [PubMed] [Google Scholar]
- Andrew RD, Dudek FE. Intrinsic inhibition in magnocellular neuroendocrine cells of rat hypothalamus. The Journal of Physiology. 1984b;353:171–185. doi: 10.1113/jphysiol.1984.sp015330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew RD, Dudek FE. Spike broadening in magnocellular neuroendocrine cells of rat hypothalamic slices. Brain Research. 1985;334:176–179. doi: 10.1016/0006-8993(85)90583-9. 10.1016/0006-8993(85)90583-9. [DOI] [PubMed] [Google Scholar]
- Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Progress in Neurobiology. 1995;47:291–339. 10.1016/0301-0082(95)00025-9. [PubMed] [Google Scholar]
- Armstrong WE, Sladek CE. Evidence for excitatory actions of histamine on supraoptic neurons in vitro: mediation by an H1-type receptor. Neuroscience. 1985;16:307–322. doi: 10.1016/0306-4522(85)90004-1. 10.1016/0306-4522(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Smith BN, Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurones in vitro. Journal of Physiology. 1994;475:115–128. doi: 10.1113/jphysiol.1994.sp020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell RJ. Optimizing release from peptide hormone secretory nerve terminals. Journal of Experimental Biology. 1988;139:51–65. doi: 10.1242/jeb.139.1.51. [DOI] [PubMed] [Google Scholar]
- Bicknell RJ, Brown D, Chapman C, Hancock PD, Leng G. Reversible fatigue of stimulus-secretion coupling in the rat neurohypophysis. The Journal of Physiology. 1984;348:601–613. doi: 10.1113/jphysiol.1984.sp015128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell RJ, Leng G. Relative efficiency of neural firing patterns for vasopressin release in vitro. Neuroendocrinology. 1981;33:295–299. doi: 10.1159/000123248. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory cells. Neuroscience Letters. 1986;70:204–209. doi: 10.1016/0304-3940(86)90464-7. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Contribution of the transient outward current to spike repolarization in rat supraoptic neurosecretory cells. Canadian Journal of Physiology and Pharmacology. 1988a;66:7. [Google Scholar]
- Bourque CW. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. The Journal of Physiology. 1988b;97:331–347. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Brown DA. Apamin and d-tubocurarine block the after-hyperpolarization of rat supraoptic neurosecretory neurons. Neuroscience Letters. 1987;82:185–190. doi: 10.1016/0304-3940(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Brown DA, Renaud LP. Barium ions induce prolonged plateau depolarizations in neurosecretory neurones of the adult rat supraoptic nucleus. The Journal of Physiology. 1986;375:573–586. doi: 10.1113/jphysiol.1986.sp016134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Randle JCR, Renaud LP. Calcium-dependent potassium conductance in rat supraoptic nucleus neurosecretory neurons. Journal of Neurophysiology. 1985;54:1375–1382. doi: 10.1152/jn.1985.54.6.1375. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Renaud LP. Calcium-dependent action potentials in rat supraoptic neurosecretory neurones recorded in vitro. The Journal of Physiology. 1985a;363:419–428. doi: 10.1113/jphysiol.1985.sp015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Renaud LP. Activity dependence of action potential duration in rat supraoptic neurosecretory neurones recorded in vitro. Journal of Physiology. 1985b;363:429–439. doi: 10.1113/jphysiol.1985.sp015720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Renaud LP. Membrane properties of rat magnocellular neuroendocrine cells in vivo. Brain Research. 1991;540:349–352. doi: 10.1016/0006-8993(91)90535-4. [DOI] [PubMed] [Google Scholar]
- Brimble MJ, Dyball REJ. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. The Journal of Physiology. 1977;271:253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Cobbett P, Smithson KG, Hatton GI. Immunoreactivity to vasopressin- but not oxytocin-associated neurophysin antiserum in phasic neurons of rat hypothalamic paraventricular nucleus. Brain Research. 1986;362:7–16. doi: 10.1016/0006-8993(86)91392-2. [DOI] [PubMed] [Google Scholar]
- Coggan JS, Purnyn SL, Knoper SR, Kreulen DL. Muscarinic inhibition of two potassium currents in guinea-pig prevertebral neurons: differentiation by extracellular cesium. Neuroscience. 1994;59:349–361. doi: 10.1016/0306-4522(94)90601-7. [DOI] [PubMed] [Google Scholar]
- Dreifuss JJ, Kalnins I, Kelly JS, Ruf KB. Action potentials and release of neurohypophysial hormones in vitro. Journal of Physiology. 1971;215:805–817. doi: 10.1113/jphysiol.1971.sp009499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton DA, Dyball REJ. Phasic firing enhances vasopressin release from the rat neurohypophysis. The Journal of Physiology. 1979;290:433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball REJ, Tasker J-G, Wuarin J-P, Dudek FE. In vivo intracellular recording of neurons in the supraoptic nucleus of the rat hypothalamus. Journal of Neuroendocrinology. 1991;3:383–386. doi: 10.1111/j.1365-2826.1991.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Erickson KR, Ronnekliev OK, Kelly MJ. Electrophysiology of guinea-pig supraoptic neurones: role of a hyperpolarization-activated cation current in phasic firing. The Journal of Physiology. 1993;460:407–425. doi: 10.1113/jphysiol.1993.sp019478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Cesium blocks depolarizing afterpotentials and phasic activity in rat supraoptic neurons. Society for Neuroscience Abstracts. 1997;22:419. [Google Scholar]
- Harris MC, Dreifuss JJ, Legros JJ. Excitation of phasically-firing supraoptic neurones during vasopressin release. Nature. 1975;258:80–82. doi: 10.1038/258080b0. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Bourque CW. Effects of neurotensin on rat supraoptic neurones in vitro. Journal of Physiology. 1995;482:373–381. doi: 10.1113/jphysiol.1995.sp020524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick K, Bourque CW. Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. Journal of Physiology. 1996;494:389–398. doi: 10.1113/jphysiol.1996.sp021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Decavel C, Hatton GI. Calbindin-D28k: role in determining intrinsically generated firing patterns in rat supraoptic neurones. The Journal of Physiology. 1995;488:601–608. doi: 10.1113/jphysiol.1995.sp020993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Ca2+ release from internal stores: role in generating depolarizing after-potentials in rat supraoptic neurones. The Journal of Physiology. 1997a;498:339–350. doi: 10.1113/jphysiol.1997.sp021862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Reduced outward K+ conductances generate depolarizing after-potentials in rat supraoptic nucleus neurones. The Journal of Physiology. 1997b;505:95–106. doi: 10.1111/j.1469-7793.1997.095bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay cells. The Journal of Physiology. 1990;431:319–342. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas S, Bourque CW. Galanin inhibits continuous and phasic firing in rat hypothalamic magnocellular neurosecretory cells. Journal of Neuroscience. 1997;17:6048–6056. doi: 10.1523/JNEUROSCI.17-16-06048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Randle JCR, Bourque CW, Renaud LP. α1-Adrenergic receptor activation depolarizes rat supraoptic neurosecretory neurons in vitro. American Journal of Physiology. 1985;251:R569–574. doi: 10.1152/ajpregu.1986.251.3.R569. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Heat transduction in rat sensory neurons by calcium-dependent activation of a cation channel. Proceedings of the National Academy of Sciences of the USA. 1997;94:7006–7011. doi: 10.1073/pnas.94.13.7006. 10.1073/pnas.94.13.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Progress in Neurobiology. 1991;36:131–169. doi: 10.1016/0301-0082(91)90020-2. 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- Smith BN, Armstrong WE. Histamine enhances the depolarizing afterpotential of immunohistochemically identified vasopressin neurons in the rat supraoptic nucleus via H1-receptor activation. Neuroscience. 1993;53:855–864. doi: 10.1016/0306-4522(93)90630-x. 10.1016/0306-4522(93)90630-X. [DOI] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Electrophysiological differences between oxytocin and vasopressin neurons recorded from female rats in vitro. The Journal of Physiology. 1995;488:701–708. doi: 10.1113/jphysiol.1995.sp021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic oxytocin and vasopressin neurons during lactation. Journal of Neuroscience. 1996;16:4861–4871. doi: 10.1523/JNEUROSCI.16-16-04861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Sustained outward rectification of oxytocinergic neurones in the rat supraoptic nucleus: ionic dependence and pharmacology. The Journal of Physiology. 1997;500:497–508. doi: 10.1113/jphysiol.1997.sp022036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakerley JB, Poulain DA, Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Research. 1978;148:425–440. doi: 10.1016/0006-8993(78)90730-8. 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]


