Abstract
The influence of prolonged exhaustive exercise on mitochondrial oxidative function was investigated in ten men.
Muscle biopsies were taken before and after exercise and mitochondrial respiration investigated in fibre bundles made permeable by pretreatment with saponin.
After exercise, respiration in the absence of ADP increased by 18 % (P < 0.01), but respiration at suboptimal ADP concentration (0.1 mM) and maximal ADP-stimulated respiration (1 mM ADP) remained unchanged.
In the presence of creatine (20 mM), mitochondrial affinity for ADP increased markedly and respiration at suboptimal ADP concentration (0.1 mM) was similar (pre-exercise) or higher (post-exercise; P < 0.05) than with 1 mM ADP alone. The increase in respiratory rate with creatine was correlated to the relative type I fibre area (r = 0.84). Creatine-stimulated respiration increased after prolonged exercise (P < 0.01).
The respiratory control index (6.8 ± 0.4, mean ± s.e.m.) and the ratio between respiration at 0.1 and 1 mM ADP (ADP sensitivity index, 0.63 ± 0.03) were not changed after exercise. The sensitivity index was negatively correlated to the relative type I fibre area (r = −0.86).
The influence of exercise on muscle oxidative function has for the first time been investigated with the skinned-fibre technique. It is concluded that maximal mitochondrial oxidative power is intact or improved after prolonged exercise, while uncoupled respiration is increased. The latter finding may contribute to the elevated post-exercise oxygen consumption. The finding that the sensitivity of mitochondrial respiration for ADP and creatine are related to fibre-type composition indicates intrinsic differences in the control of mitochondrial respiration between fibres.
Mitochondrial respiration is of paramount importance for muscle energetics during sustained exercise. However, exercise is associated with perturbations of the intracellular milieu, which may alter mitochondrial function. Experiments on isolated rat cardiac muscle mitochondria show that incubation of isolated mitochondria under conditions known to exist in skeletal muscle during exercise (increased concentrations of Ca2+, lactate and phosphate, and elevated temperature) impairs mitochondrial oxidative phosphorylation (Mukherjee, Wong, Templeton, Buja & Willerson, 1979). During prolonged exercise the muscle cell is exposed to elevated Ca2+, phosphate and temperature for a long period of time with a potential risk of mitochondrial dysfunction. Production of reactive oxygen species (ROS) is another factor which may affect mitochondrial function. The increased oxygen consumption and metabolic stress during prolonged exercise may result in increased production of ROS and hence mitochondrial damage (Sjödin, Hellsten-Westing & Apple, 1990).
Several studies in rat have demonstrated that prolonged exhaustive exercise impairs mitochondrial respiration (Dohm, Huston, Askew, Weiser, 1972; Dohm, Huston, Askew & Fleshwood, 1973; Dohm, Barakat, Stephenson, Pennington & Tapscott, 1976; Barakat, Kasperek, Dohm, Tapsott & Snider, 1982). However, in other studies in both rat (Terjung, Baldwin, Mole, Klinkerfuss & Holloszy, 1972) and horse (Chen & Gollnick, 1994), mitochondrial function was unaffected by prolonged exercise to exhaustion. The reasons for these disparate findings are unclear. The acute effects of prolonged exercise on oxidative function in human skeletal muscle have been studied in only a few experiments. Muscle mitochondria morphology and oxidative enzyme activities have been reported to be unaffected by prolonged running to exhaustion (Cooper, Jones, Edwards, Corbucci, Montanari & Trevisani, 1986; Kayar, Hoppeler, Howald, Claassen & Oberholzer, 1986). Tonkonogi, Harris & Sahlin (1997) found that activity of muscle citrate synthase, which is a marker enzyme for the citric acid cycle, was increased after cycling to exhaustion at 75 % of peak O2 uptake (V̇O2,peak), whereas activities of marker enzymes for fatty acid oxidation (β-hydroxyacyl-CoA dehydrogenase) and glycolysis (phosphofructokinase) were unaffected. However, measurements of the activity of oxidative enzymes and mitochondrial morphology will give only limited information about integrated mitochondrial function in vivo. To our knowledge, the immediate effects of prolonged exercise on mitochondrial respiration in human skeletal muscle have only been studied by Madsen, Ertbjerg, Djurhuus & Pedersen (1996). The authors found that the respiratory control index (RCI) in isolated mitochondria was elevated immediately after termination of exercise, whereas State III and State IV respiration were not significantly changed.
A potential problem with the study of isolated mitochondria is that the structure and function of mitochondria may be affected during the isolation procedure. An alternative technique is to measure mitochondrial respiration in situ by using muscle fibres with the sarcolemma made permeable with saponin (Veksler, Kuznetsov, Sharov, Kapelko & Saks, 1987; Saks et al. 1995). Saponin is a plant extract which makes the sarcolemma permeable by destroying cholesterol-containing membranes. The structure and function of mitochondria remain intact after treatment with saponin (Veksler et al. 1987). With this technique mitochondria may be studied inside the fibres as if they were isolated. Studies of mitochondrial respiration in saponin-skinned muscle fibres from rat have demonstrated striking differences between the kinetics of ADP control of respiration in muscles with slow- and fast-twitch fibres (Veksler et al. 1995; Kuznetsov et al. 1996). The presence of creatine markedly increased mitochondrial ADP sensitivity in slow-twitch muscles, whereas that of fast-twitch muscles was unaffected (Saks et al. 1995; Veksler et al. 1995). This type of control was not observed in isolated mitochondria (Saks et al. 1995), and it was argued that mitochondrial structure and function were changed during the process of isolation. The requirement for large muscle samples has limited research on mitochondrial function in humans. An advantage of using the saponin skinning technique is that the minimum sample size is much less (about 5 mg) than when isolated mitochondria are used (about 100 mg). The skinned-fibre technique has been used for studies of mitochondrial respiration in human skeletal muscle (Letellier, Malgat, Coquet, Moretto, Parrot-Rouland & Mazat, 1992; Kunz et al. 1993; Nemirovskaya, Shenkman, Nekrasov, Kuznetsov & Saks, 1993), but the acute effects of exercise have so far not been studied.
The purpose of the present study was to investigate the influence of prolonged dynamic exercise to exhaustion on mitochondrial oxidative function in human muscle using the saponin-skinned muscle fibre technique.
METHODS
Subjects
Ten healthy men whose mean (range) age, height and weight were, respectively, 27 (19-34) years, 183 (173-194) cm and 80 (69-90) kg participated in the study. Their peak O2 uptake (V̇O2,peak), estimated as described below, was 52.1 (35.8-68.9) ml min−1 (kg body mass)−1. Mean fibre-type composition was 58.9 (45.7-74.5) % type I fibres, 27.2 (19.5-35.4) % type II A fibres and 12.4 (5.4-20.2) % type II B fibres. The experimental procedures and the possible risks and discomforts involved were explained to the participants before obtaining their written consent. The study was approved by the Ethical Committee of the Karolinska Institute, Stockholm, Sweden.
Experiments
Pre-tests
At least 2 days prior to the main experiment the subjects performed an ergometer test for estimation of V̇O2,peak. They cycled at three or more submaximal work rates (4 min each) and at a supramaximal work rate until exhaustion. Heart rate and V̇O2 were recorded continuously and V̇O2,peak was estimated by the levelling-off criterion.
Experimental protocol
The main experiment was performed in the morning. Subjects were instructed to abstain from heavy physical activity and alcohol the day before the experiment. They were also instructed to eat a light breakfast with no coffee or tea on the morning of the experiment. A Monark cycle 818E cycle ergometer was used in the experiments. After warm-up (cycling for 5 min at 40 % of their estimated V̇O2,peak), subjects cycled (60 r.p.m.) at a constant work rate estimated to correspond to 75 % of the predetermined V̇O2,peak until voluntary fatigue. Fatigue was defined as the point at which the subjects were unable to continue the exercise at the predetermined intensity, despite verbal encouragement. The subjects were allowed to drink water during the exercise. The expired air was analysed at rest, every 20 min and at fatigue with an open-circuit system (Medical Graphics Corp. CPX system, St Louis, MO, USA). Heart rate was registered continuously with a Polar sport tester 3000 (Polar Electro, Kempele, Finland). Ratings of perceived exertion for the legs and the breathing were made according to Borg (1970).
Blood samples
Before exercise a short indwelling catheter was placed in an antecubital vein. Blood samples were collected into heparinized syringes at rest and every 20 min during exercise. An aliquot of blood was deproteinized with perchloric acid (0.75 M), neutralized with KHCO3 (2.2 M) and analysed for lactate and glucose by NAD(P)H-coupled enzymatic techniques based on fluorometric detection. Plasma ammonia concentration (NH3 + NH4+) was determined with an automatic analyser (Ammonia Checker, Biomen Ltd, UK).
Preparation of saponin-skinned muscle fibres
Muscle biopsies were taken with the needle-biopsy technique from the lateral aspect of the quadriceps femoris muscle of both legs. The biopsies were taken at a depth of 2-3 cm at about one-third of the distance from the upper margin of the patella to the anterior superior iliac spine. After local skin anaesthesia (1-2 ml of 20 mg ml−1 carbocain; Astra, Södertälje, Sweden) incisions through the skin and the muscle fascia were made (one on each leg) while subjects rested in the supine position. Biopsies were taken at rest and immediately after termination of exercise (randomized between left and right leg). Biopsies were divided into two portions. One portion of each muscle sample (10-15 mg) was rapidly frozen in liquid nitrogen and stored at -70°C until determination of muscle enzyme activities (citrate synthase, β-hydroxyacyl-CoA dehydrogenase and phosphofructokinase). Results on muscle enzyme activities have been published earlier (Tonkonogi et al. 1997). The second part was placed in an ice-cold medium consisting of (mmol l−1): 100 sucrose, 100 KCl, 50 Tris-HCl, 1 KH2PO4, 0.1 EGTA and 0.2 % BSA (bovine serum albumin), pH 7.40. By means of sharp-ended needles and forceps, four to five muscle-fibre bundles were excised from the sample and the muscle fibres were separated from each other, leaving only small areas of contact. The fibre bundles were transferred to 1.5 ml of the above medium containing 50 μg ml−1 of saponin to selectively destroy the integrity of the sarcolemma. After 30 min of incubation with mild stirring, fibre bundles were transferred to 1.5 ml of cooled (0°C) medium consisting of (mmol l−1): 225 mannitol, 75 sucrose, 10 Tris and 0.1 EDTA, pH 7.40, and washed for 5 min with gentle stirring; this washing procedure was repeated three times to completely remove saponin and metabolites. All procedures were carried out at 4°C. The fibre bundles were kept on ice until determination of respiratory activity.
Measurement of respiration
Mitochondrial oxygen consumption was measured polarographically using a Clark-type electrode (Hansatech DW1) in a water-jacketed glass chamber maintained at 25°C and equipped with magnetic stirring. The O2 tension within the reaction chamber and the rate of oxygen utilization were displayed on a connected recorder. The measurements were carried out in 0.3 ml aliquots of a reaction medium containing (mmol l−1): 225 mannitol, 75 sucrose, 10 Tris, 10 KCl, 10 K2HPO4, 0.1 EDTA, 2 MgCl2, 5 pyruvate, 2 L-malate, and 100 mg ml−1 BSA, pH 7.50. The reaction medium was saturated with air prior to measurements. The solubility of oxygen in the medium was considered to be equal to 237.5 μmol l−1.
Respiration was initiated by placing the fibre bundle into the reaction medium containing respiratory substrate (pyruvate + malate) but not ADP. Respiration was measured after addition of 0.1 mM ADP (submaximal ADP-stimulated respiration), and after further addition of ADP (final concentration, 1 mM). In a second fibre bundle the effect of creatine on ADP kinetic parameters was investigated. Respiration was measured first without ADP, in the presence of 0.1 mM ADP and then after addition of creatine (20 mM), while maintaining isosmolar conditions. Normally, a third fibre bundle was used to repeat the measurement of ADP-stimulated respiration (0, 0.1 and 1 mM ADP). When applicable the statistical treatment of data was based on the mean of these duplicate measurements. After measurement, the fibre bundles were removed, quick-frozen in liquid nitrogen, freeze-dried and weighed. The dry weight was converted to wet weight by assuming a water content of 77 %. Respiration rates were expressed as mmol O2 min−1 (kg wet wt)−1.
The compositions of the solutions were somewhat different from those used previously in studies on saponin-skinned human muscle fibres (Kunz et al. 1993). Therefore, in a pre-experiment we evaluated the optimal duration of saponin treatment. The rates of respiration in the absence and presence of ADP in relation to the incubation time with saponin are shown in Fig. 1. The data demonstrate that 30 min of saponin treatment is suitable for obtaining optimal values of maximal ADP-stimulated respiration and RCI, in agreement with previous results (Kunz et al. 1993).
Figure 1. Effect of duration of saponin treatment on maximal ADP-stimulated respiration and respiration in the absence of ADP in human skeletal muscle fibres.
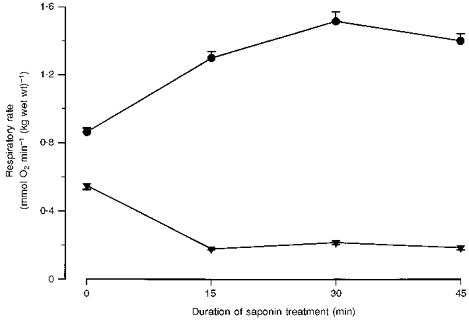
Values are means ± s.e.m. of 4 (0, 15 and 45 min) or 8 (30 min) fibre bundles. •, 1 mM ADP; ▾, 0 mM ADP.
In order to evaluate the loss of mitochondria during the preparative steps, the activity of a mitochondrial enzyme (citrate synthase) was measured in the saponin and washing solutions with a technique described previously (Tonkonogi et al. 1997). Less than 4 % of total muscle citrate synthase was liberated into the media, indicating that the majority of the mitochondria were intact and remained in the fibre bundles for measurements of mitochondrial respiration. The results have not been corrected for the loss of citrate synthase from the fibres. The fibre bundles were visually examined after weighing. One fibre bundle contained a large proportion of connective tissue and data from this fibre bundle were excluded.
The stability of the skinned fibres was assessed from measurements of mitochondrial respiration before and after 2.5 h storage on ice (n = 7). On average, maximal ADP-stimulated respiration increased by 0.4 %, but the change was not statistically significant. The methodological variation, expressed as c.v., calculated from consecutive measurements of maximal and submaximal ADP-stimulated respiration and respiration in the absence of ADP on the two fibre bundles from the same biopsy, was 10.9, 7.9 and 18.0 %, respectively.
Fibre-type composition
One part of each biopsy sample obtained at rest was used for determination of fibre-type composition. Samples were frozen in isopentane cooled in liquid nitrogen and stored at -70°C. Serial transverse sections were cut (10 μm thick) with a cryostat (Leica Jung Frigocut 2800 E) at -20°C and mounted on coverslips. The sections were stained for myofibrillar ATPase activity at pH 9.4 after preincubation at pH 10.3, 4.6 and 4.3 (Brooke & Kaiser, 1970). A computerized image analyser system (TEMA, Bio-Rad, Scan Beam/AS, Hadsund, Denmark) was used to quantify the areas and to calculate fibre-type composition after visual discrimination of fibre types.
All chemicals were purchased from Sigma or Merck.
Data analysis
Data given in the text are presented as means ± s.e.m. Bivariate correlation coefficients were computed on the data, and Fisher's test was used to determine their statistical significance. Differences between means were tested for statistical significance with Student's paired t test. Statistical significance was set at P < 0.05. The coefficient of variation (c.v.) was determined from:
where d denotes the difference between two duplicate determinations of the same sample, x¯ is the mean value of each pair, and n the number of duplicate analyses.
RESULTS
Time to exhaustion was 71 ± 6 min (range, 48-110 min). Oxygen uptake increased from 0.35 ± 0.02 l min−1 at rest to 2.94 ± 0.16 l min−1 after 20 min of exercise, and 3.10 ± 0.18 l min−1 at exhaustion (P < 0.01vs. 20 min value). These exercise values correspond to 71.1 ± 1.3 and 74.8 ± 1.1 % of V̇O2,peak, respectively. Heart rate, having increased at the onset of exercise, showed a further upward drift during the cycling. After 20 min of exercise, heart rate averaged 86 % of peak heart rate (as measured during the pre-test), and at fatigue it was 93 %. Blood lactate concentration increased from 0.9 ± 0.1 mmol l−1 at rest to 3.1 ± 0.4 mmol l−1 at the end of the exercise (P < 0.001). The concentration of blood glucose was 4.3 ± 0.2 and 4.5 ± 0.4 mmol l−1 before and after exercise, respectively (n.s.). Plasma ammonia concentration increased from 38 ± 4 μmol l−1 at rest to 104 ± 13 μmol l−1 at fatigue (P < 0.001).
Prior to exercise the respiratory rate in the fibre bundles was 0.21 ± 0.01 mmol O2 min−1 (kg wet wt)−1 in the absence of ADP, and increased to 0.94 ± 0.04 and 1.38 ± 0.07 mmol O2 min−1 (kg wet wt)−1 at 0.1 and 1 mM ADP, respectively. Further additions of ADP (final concentration, 2 mM) gave no further increase in respiration (data not shown), indicating that near-maximal respiration was reached at 1 mM ADP. Addition of 20 mM creatine at 0.1 mM ADP increased the respiratory rate to that at 1 mM ADP (Fig. 2). The creatine-mediated increase in respiratory rate showed a significant correlation to relative type I fibre area (Fig. 3). The respiratory rates measured at 0.1 mM (submaximal respiration) and 1 mM ADP (maximal respiration) were not significantly different at fatigue compared with pre-exercise (Fig. 2). However, respiration in the absence of added ADP was 18 % greater after exhaustive cycling (Fig. 2). The increase was not correlated to exercise duration or subject's training status (expressed as muscle citrate synthase activity, V̇O2,peak, or muscle fibre-type composition). The mitochondrial respiration rate at 0.1 mM ADP in the presence of creatine increased significantly after exercise compared with that at rest (Fig. 2). The respiratory control indices, defined as the ratio of maximal ADP-stimulated respiration to respiration in the absence of ADP, were 6.8 ± 0.4 and 5.8 ± 0.3 before and after exercise, respectively (n.s.).
Figure 2. Mitochondrial respiratory rate before and after prolonged exhaustive exercise.
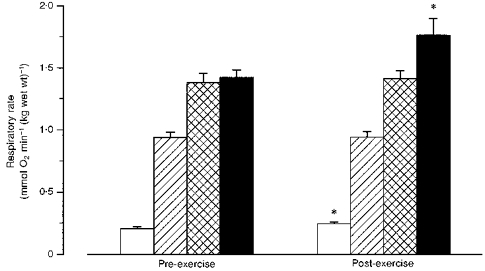
Values are means ± s.e.m. □, 0 mM ADP;  , 0.1 mM ADP;
, 0.1 mM ADP;  , 1 mM ADP; ▪, 0.1 mM ADP + 20 mM creatine. * Significantly different from pre-exercise (P < 0.01,n = 10).
, 1 mM ADP; ▪, 0.1 mM ADP + 20 mM creatine. * Significantly different from pre-exercise (P < 0.01,n = 10).
Figure 3. Creatine-induced increase in respiration in relation to relative type I fibre area.
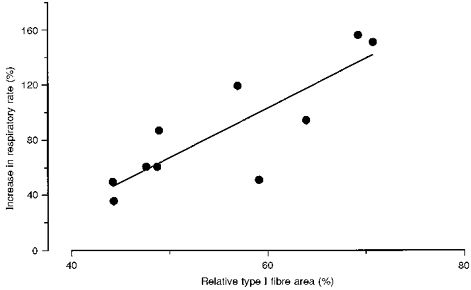
Creatine-induced increase in respiration is expressed as: 100 × [(R: 0.1 mM ADP + 20 mM creatine) - (R: 0.1 mM ADP)]/(R: 0.1 mM ADP),where R denotes respiration. Values are the means of pre- and post-exercise samples (r = 0.84; P < 0.01,n = 10; y = 3.6x - 113).
The ratio between respiration at 0.1 and 1 mM ADP (ADP sensitivity index) was 0.63 ± 0.03 before and 0.60 ± 0.03 after exercise. Assuming Michaelis-Menten kinetics it can be calculated that half-maximal mitochondrial respiration was reached at 63 ± 7 and 71 ± 9 μM ADP before and after exercise, respectively (n.s.). ADP sensitivity index was negatively correlated to relative type I fibre area (Fig. 4) and to the whole-muscle citrate synthase activity (r = -0.69; P < 0.05).
Figure 4. Relationship between ADP sensitivity index and relative type I fibre area.
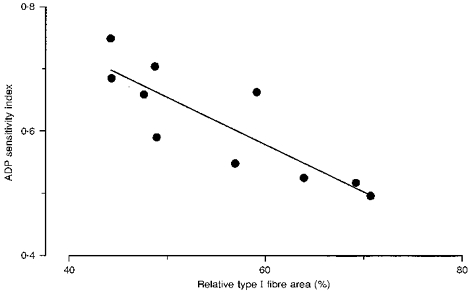
ADP sensitivity index is defined as the ratio of respiration at 0.1 and 1 mM ADP. Values are the means of pre- and post-exercise samples (r = -0.86; P < 0.01,n = 10; y = -0.008x + 1.04).
DISCUSSION
The principal finding of the present study is that maximal ADP-stimulated mitochondrial respiration remained unchanged after prolonged exhaustive exercise. Our data on saponin-skinned human muscle fibres confirm earlier observations on isolated human skeletal muscle mitochondria (Madsen et al. 1996). An interesting new finding was that creatine-stimulated respiration increased after exercise and reached higher values than with ADP alone. The concentration of creatine (20 mM) which was used during the measurements is similar to the muscle concentration in vivo during this type of exercise (Sahlin, Söderlund, Tonkonogi & Hirakoba, 1997), and implies that maximal muscle oxidative rate is increased after prolonged exercise. The mechanism for this is unclear.
An important further finding was that mitochondrial respiration in the absence of ADP (uncoupled respiration) increased after exercise. In a study of isolated mitochondria, uncoupled respiration (vs. that at rest) was unchanged immediately after prolonged exercise but increased after 30-60 min of recovery (Madsen et al. 1996). Uncoupled respiration is due to back-leakage of protons through the inner membrane of the mitochondria (Brand, Chien, Ainscow, Rolfe & Porter, 1994). The reason for the increased uncoupled respiration is not known, but it may be a consequence of increased permeability of the inner mitochondrial membrane to H+, caused either by damage or by altered composition/structure of the membrane. The role of mitochondrial proton leak in energy metabolism in intact muscle has been discussed recently (Brand et al. 1994; Rolfe & Brand, 1996). Proton leak was shown to account for around one-half of the resting respiration rate of perfused rat skeletal muscle (Rolfe & Brand, 1996), and constitutes a significant proportion of the animal's basal metabolic rate. It has previously been shown that oxygen consumption remains elevated up to 12 h after exercise (Maehlum, Grandmontagne, Newsholme & Sejersted, 1986). The increased uncoupled respiration may be one factor which contributes to the slow component of excess post-exercise oxygen consumption.
Consistent with previous studies we observed an upward drift in V̇O2 during exercise. The increase in V̇O2 is much larger than can be explained by the observed increase in uncoupled respiration and must, therefore, largely be explained by other factors such as decreased chemical efficiency in the energy transfer (P/O ratio) or decreased mechanical efficiency (altered recruitment pattern of fibres and muscles) during exercise.
ADP sensitivity index was negatively correlated to the proportion of type I fibre area in the subjects. The physiological consequence is that subjects with a large type I proportion would require a larger increase in ADP to obtain a certain proportion of maximal muscle V̇O2. This finding seems to be paradoxical, since increases in ADP are also associated with other metabolic changes such as decrease in phosphocreatine and increased rate of glycolysis, which are known to be less pronounced in type I fibres. However, the paradox might be explained by creatine-stimulated respiration, which is more pronounced in type I fibres (see below). Several other reports also suggest that the sensitivity of mitochondrial respiration to ADP is greater in type II fibres than in type I fibres. Studies of perfused cat skeletal muscle have shown that, despite similar basal O2 consumption rate, cytoplasmic free-ADP concentration, calculated from data obtained with phosphorus magnetic resonance technique, is severalfold higher in slow- compared with fast-twitch muscle (Meyer, Brown & Kushmerick, 1985). Based on the metabolic response during stimulation and during recovery, Kushmerick, Meyer & Brown (1992) concluded that the control of cellular respiration is different in fast- and slow-twitch skeletal muscle. Veksler et al. (1995) and Kuznetsov et al. (1996) reported mitochondrial apparent Km for ADP to be more than 30-fold higher in mouse and rat soleus (slow-twitch) skinned fibres than in gastrocnemius (fast-twitch) skinned fibres. Our results are compatible with these reports and indicate that fibre-type-specific control of mitochondrial respiration is also present in human skeletal muscle. In a previous study on isolated human skeletal muscle mitochondria, we found that mitochondrial affinity for ADP was inversely correlated to both muscle and whole-body aerobic capacity (Tonkonogi & Sahlin, 1997). On the basis of the results in the current study we can suggest that this phenomenon is due to the relatively large portion of mitochondria originating from type I fibres which were present in the mitochondrial suspension derived from the subjects with high aerobic capacity.
The stimulation of respiration with creatine has been suggested to be a new type of control mechanism (Veksler et al. 1995). An increase in ADP would, through the creatine kinase equilibrium, result in a breakdown of phosphocreatine to creatine. The increase in creatine would then amplify the ADP signal in mitochondrial respiratory control. It has been reported that, in rodents, mitochondrial affinity for ADP increased dramatically in the presence of 20 mM creatine. The effect was observed only in skinned fibres of cardiac and slow-twitch skeletal muscle (Saks et al. 1995; Veksler et al. 1995; Kuznetsov et al. 1996). Creatine had no effect on ADP affinity in fast-twitch muscle or isolated mitochondria (Saks et al. 1995; Veksler et al. 1995; Kuznetsov et al. 1996). In the present study, addition of creatine increased the mitochondrial respiratory rate with a suboptimal ADP concentration up to, or even above, the maximal ADP-stimulated respiration. The creatine-mediated increase in respiratory rate was positively related to the relative type I fibre area, which indicates that, even in human skeletal muscle, the creatine-dependent control mechanism of cellular respiration is related to high oxidative muscle fibres. The modulation of mitochondrial sensitivity for ADP by creatine may provide an advantage, in that the fluctuations in cytosolic free-ADP concentration (and as a consequence ΔG (change in free energy) of the ATP hydrolysis) are reduced. This may be of physiological importance, in that metabolic perturbations are reduced and muscle fatigue delayed.
An alternative explanation for the creatine-stimulated respiration at suboptimal ADP concentration could be that the diffusion of ADP within the fibre is restricted (Korge, 1995). With this scenario, ADP concentration in the vicinity of the mitochondria is lower than in the medium. Addition of creatine would, through the creatine-creatine phosphate shuttle, reduce intracellular ADP gradients and result in increased mitochondrial ADP concentration and, as a consequence, increased cellular respiration. This could also explain why ADP sensitivity in skinned fibre preparations is much lower than in isolated mitochondria (see below). However, a number of findings speak against this hypothesis. Firstly, the creatine effect is abolished when the medium contains 0.125 M KCl (which dissociates mitochondrial creatine kinase from the inner membrane) and after hyposmotic treatment (rupture of the outer mitochondrial membrane) despite unchanged maximal respiration (Saks et al. 1995). Secondly, after removal of myosin thick filaments with 0.8 M KCl (which should reduce at least part of the restriction to ADP diffusion) apparent Km for ADP remains high (Saks et al. 1995). Thirdly, the creatine effect is pronounced in cardiomyocytes and soleus but absent in fast-twitch fibres, despite the fact that fibre diameter is larger in the latter fibre type (Kuznetsov et al. 1996). After modelling the creatine phosphate shuttle, diffusion limitation was not considered to be important in cardiomyocytes (Meyer, Sweeney & Kushmerick, 1984). Although these experiments refute the explanation based on limitation of ADP diffusion, further experiments are necessary to elucidate the mechanism by which creatine stimulates respiration.
A large number of studies have shown that dietary creatine supplementation results in increased muscle concentrations of creatine and phosphocreatine. It was recently demonstrated that the rate of phosphocreatine resynthesis after exercise increased when the muscle creatine store was elevated (Greenhaff, Bodin, Söderlund & Hultman, 1994). The post-exercise rate of phosphocreatine resynthesis was unchanged during the first period (0-60 s) but higher during 1-2 min of recovery. The findings of the present study, that creatine primarily stimulates respiration at suboptimal concentrations of ADP, are consistent with these data in vivo.
In the present study of saponin-skinned muscle fibres, half-maximal respiration was reached at 60-70 μM ADP, which is much higher than previously reported in isolated mitochondria (Tonkonogi & Sahlin, 1997). This discrepancy has been documented previously for cardiac and soleus muscle in rodent (Veksler et al. 1995) and was ascribed to altered structure of the mitochondrial membrane obtained during the isolation procedure (Saks et al. 1995; Kuznetsov et al. 1996). If the high mitochondrial affinity for ADP, previously observed in isolated mitochondria, is correct it should, even in quiescent skeletal muscle, correspond to an unreasonably high rate of oxidative phosphorylation. Assuming Michaelis-Menten kinetics and taking muscle free-ADP concentration to be 6.7-20 μM (Radda, 1986; Sahlin et al. 1997) and mitochondrial Km for ADP to be 11-20 μM (Chance & Williams, 1955; Saks et al. 1995; Tonkonogi & Sahlin, 1997), it can be calculated that muscle respiration at rest should be 25-65 % of maximal respiration. The low Km value observed in isolated mitochondria cannot, therefore, correspond to that in vivo unless other control mechanisms exist. The higher Km value observed with saponin-skinned muscle fibres is more compatible with the oxygen utilization and ADP concentration of resting muscle.
Maximal ADP-stimulated oxygen utilization in saponin-skinned human muscle fibres was similar to that previously measured in isolated human skeletal muscle mitochondria and muscle homogenates as well as in vivo during recovery after exercise (Ivy, Withers, Van Handel, Elger & Costill, 1980; McCully et al. 1994; Tonkonogi & Sahlin, 1997). However, extrapolation of measured rate of O2 uptake in skinned fibres to an assumed muscle temperature of 38°C gives a value which is 2.5-5 times lower than the measured oxygen utilization during exercise. The discrepancy has been discussed before (Tonkonogi & Sahlin, 1997), and activation of mitochondrial respiration by some chemical or physical changes associated with exercise was suggested to be the most plausible explanation, rather than mitochondrial damage during the isolation procedure. The results of the present study support this hypothesis. However, further studies are required to clarify the mechanism of exercise-related activation of mitochondrial function.
In summary, after prolonged exercise maximal ADP-stimulated respiration was unchanged whereas creatine-stimulated respiration increased and reached higher values than with ADP alone. The creatine-mediated increase in respiratory rate was more pronounced in samples with large relative type I fibre area. Uncoupled respiration increased after exercise and may contribute to excess post-exercise oxygen consumption. Mitochondrial sensitivity for ADP was negatively correlated to relative type I fibre area and muscle citrate synthase activity. These data indicate intrinsic differences in the control of cellular respiration in slow- and fast-twitch fibres in human skeletal muscle.
Acknowledgments
The present study was supported by grants from Swedish National Centre for Research in Sports and from the Otto Swärds Foundation. We are grateful for the expert help from Professor V. Saks, P. Sikk and T. Kaambre with the skinned muscle fibre technique.
References
- Barakat HA, Kasperek GJ, Dohm GL, Tapscott EB, Snider RB. Fatty acid oxidation by liver and muscle preparations of exhaustively exercised rats. Biochemical Journal. 1982;208:419–424. doi: 10.1042/bj2080419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scandinavian Journal of Rehabilitation Medicine. 1970;2:92–98. [PubMed] [Google Scholar]
- Brand MD, Chien L-F, Ainscow EK, Rolfe DFS, Porter RK. The causes and functions of mitochondrial proton leak. Biochimica et Biophysica Acta. 1994;1187:132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fibre types: How many and what kind? Archives of Neurology. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. 1. Kinetics of oxygen utilization. Journal of Biological Chemistry. 1955;217:383–393. [PubMed] [Google Scholar]
- Chen J, Gollnick PD. Effect of exercise on hexokinase distribution and mitochondrial respiration in skeletal muscle. Pflügers Archiv. 1994;427:257–263. doi: 10.1007/BF00374532. [DOI] [PubMed] [Google Scholar]
- Cooper MB, Jones DA, Edwards RH, Corbucci GC, Montanari G, Trevisani C. The effect of marathon running on carnitine metabolism and on some aspects of muscle mitochondrial activities and antioxidant mechanisms. Journal of Sports Sciences. 1986;4:79–87. doi: 10.1080/02640418608732103. [DOI] [PubMed] [Google Scholar]
- Dohm GL, Barakat H, Stephenson TP, Pennington SN, Tapscott EB. Changes in muscle mitochondrial lipid composition resulting from training and exhaustive exercise. Life Science. 1976;17:1075–1080. doi: 10.1016/0024-3205(75)90327-6. [DOI] [PubMed] [Google Scholar]
- Dohm GL, Huston RL, Askew EW, Fleshwood HL. Effects of exercise, training, and diet on muscle citric acid cycle enzyme activity. Canadian Journal of Biochemistry. 1973;51:849–854. doi: 10.1139/o73-105. [DOI] [PubMed] [Google Scholar]
- Dohm GL, Huston RL, Askew EW, Weiser PC. Effects of exercise on activity of heart and muscle mitochondria. American Journal of Physiology. 1972;223:783–787. doi: 10.1152/ajplegacy.1972.223.4.783. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Bodin K, Söderlund K, Hultman E. Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. American Journal of Physiology. 1994;266:E725–730. doi: 10.1152/ajpendo.1994.266.5.E725. [DOI] [PubMed] [Google Scholar]
- Ivy JL, Withers RT, Van Handel PJ, Elger DH, Costill DL. Muscle respiratory capacity and fiber type as determination of the lactate threshold. Journal of Applied Physiology. 1980;48:523–527. doi: 10.1152/jappl.1980.48.3.523. [DOI] [PubMed] [Google Scholar]
- Kayar SR, Hoppeler H, Howald H, Claassen H, Oberholzer F. Acute effects of endurance exercise on mitochondrial distribution and skeletal muscle morphology. European Journal of Applied Physiology. 1986;54:578–584. doi: 10.1007/BF00943344. [DOI] [PubMed] [Google Scholar]
- Korge P. Factors limiting adenosine triphosphate function during high intensity exercise. Sports Medicine. 1995;20:215–255. doi: 10.2165/00007256-199520040-00002. [DOI] [PubMed] [Google Scholar]
- Kunz WS, Kuznetsov AV, Shulze W, Eichorn K, Schild L, Striggow F, Bohnensack R, Neuhof S, Grashoff H, Neumann HW, Gellerich FN. Functional characterization of mitochondrial oxidative phosphorylation in saponin-skinned human muscle fibers. Biochimica et Biophysica Acta. 1993;1144:46–53. doi: 10.1016/0005-2728(93)90029-f. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Meyer RA, Brown TR. Regulation of oxygen consumption in fast- and slow-twitch muscle. American Journal of Physiology. 1992;263:C598–606. doi: 10.1152/ajpcell.1992.263.3.C598. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Tiivel T, Sikk P, Kaambre T, Kay L, Daneshrad Z, Rossi A, Kadaja L, Peet N, Seppet E, Saks A. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. European Journal of Biochemistry. 1996;241:909–915. doi: 10.1111/j.1432-1033.1996.00909.x. [DOI] [PubMed] [Google Scholar]
- Letellier T, Malgat M, Coquet M, Moretto B, Parrot-Rouland F, Mazat JP. Mitochondrial myopathy studies on permeabilized muscle fibers. Pediatric Research. 1992;32:17–22. doi: 10.1203/00006450-199207000-00004. [DOI] [PubMed] [Google Scholar]
- McCully KK, Iotti S, Kendrick K, Wang Z, Posner JD, Leigh JJ, Chance B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. Journal of Applied Physiology. 1994;77:5–10. doi: 10.1152/jappl.1994.77.1.5. [DOI] [PubMed] [Google Scholar]
- Madsen K, Ertbjerg P, Djurhuus MS, Pedersen PK. Calcium content and respiratory control index of skeletal muscle mitochondria during exercise and recovery. American Journal of Physiology. 1996;271:E1044–1050. doi: 10.1152/ajpendo.1996.271.6.E1044. [DOI] [PubMed] [Google Scholar]
- Maehlum S, Grandmontagne M, Newsholme EA, Sejersted OM. Magnitude and duration of excess post-exercise oxygen consumption in healthy young subjects. Metabolism. 1986;35:425–429. doi: 10.1016/0026-0495(86)90132-0. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Brown TR, Kushmerick MJ. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. American Journal of Physiology. 1985;248:C279–287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the “phosphocreatine shuttle”. American The Journal of Physiology. 1984;246:C365–377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Wong TM, Templeton G, Buja LM, Willerson JT. Influence of volume dilution, lactate, phosphate, and calcium on mitochondrial functions. American Journal of Physiology. 1979;237:H224–238. doi: 10.1152/ajpheart.1979.237.2.H224. [DOI] [PubMed] [Google Scholar]
- Nemirovskaya TL, Shenkman BS, Nekrasov AN, Kuznetsov AV, Saks VA. Effects of physical training on the structure and metabolism of skeletal muscles in athletes. Biokhimiya. 1993;58:471–479. [PubMed] [Google Scholar]
- Radda GK. Control of bioenergetics: from cells to man by phosphorus nuclear-magnetic-resonance spectroscopy. Biochemical Society Transactions. 1986;14:517–525. doi: 10.1042/bst0140517. [DOI] [PubMed] [Google Scholar]
- Rolfe DFS, Brand MD. Contribution of mitochondrial proton leak to skeletal muscle respiration and to standard metabolic rate. American Journal of Physiology. 1996;271:C1380–1389. doi: 10.1152/ajpcell.1996.271.4.C1380. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Söderlund K, Tonkonogi M, Hirakoba K. Phosphocreatine content in single fibers of human muscle after sustained submaximal exercise. American Journal of Physiology. 1997;273:C172–178. doi: 10.1152/ajpcell.1997.273.1.C172. [DOI] [PubMed] [Google Scholar]
- Saks VA, Kuznetsov AV, Khuchua ZA, Vasilyeva EV, Belikova JO, Kesvatera T, Tiivel T. Control of cellular respiration in vivo by mitochondrial outer membrane and by creatine kinase. A new speculative hypothesis: possible involvement of mitochondrial-cytoskeleton interactions. Journal of Molecular and Cellular Cardiology. 1995;27:625–645. doi: 10.1016/s0022-2828(08)80056-9. [DOI] [PubMed] [Google Scholar]
- Sjödin B, Hellsten-Westing Y, Apple FS. Biochemical mechanisms for oxygen free radical formation during exercise. Sports Medicine. 1990;10:236–254. doi: 10.2165/00007256-199010040-00003. [DOI] [PubMed] [Google Scholar]
- Terjung RL, Baldwin KM, Mole PA, Klinkerfuss GH, Holloszy JO. Effect of running to exhaustion on skeletal muscle mitochondria: a biochemical study. American Journal of Physiology. 1972;223:549–554. doi: 10.1152/ajplegacy.1972.223.3.549. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Harris B, Sahlin K. Increased activity of citrate synthase in human skeletal muscle after a single bout of prolonged exercise. Acta Physiologica Scandinavica. 1997;161:435–436. doi: 10.1046/j.1365-201X.1997.00233.x. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Sahlin K. Rate of oxidative phosphorylation in isolated mitochondria from human skeletal muscle: effect of training status. Acta Physiologica Scandinavica. 1997;161:345–353. doi: 10.1046/j.1365-201X.1997.00222.x. [DOI] [PubMed] [Google Scholar]
- Veksler VI, Kuznetsov AV, Anflous K, Mateo P, van Deursen J, Wieringa B, Ventura-Clapier R. Muscle creatine kinase-deficient mice II. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. Journal of Biological Chemistry. 1995;270:19921–19929. doi: 10.1074/jbc.270.34.19921. [DOI] [PubMed] [Google Scholar]
- Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment using saponin-skinned fibers. Biochimica et Biophysica Acta. 1987;892:191–196. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]


