Abstract
Adenosine has been suggested to be the mediator of a metabolic feedback mechanism which transfers acute changes in the tubular load into opposite changes in renal blood flow (RBF). The goal of the present experiments was to assess the importance of endogenously formed adenosine as a ‘homeostatic metabolite’ during short-term changes in metabolic demand.
In nine chronically instrumented conscious foxhounds, both the direct effects of adenosine injections (10, 30 and 100 nmol) into the renal artery and the temporal changes of RBF after short renal artery occlusions (15, 30 and 60 s duration), the most widely used experimental model to study the metabolic feedback mechanism in vivo, were studied.
Intrarenal bolus injections of adenosine (10, 30 and 100 nmol) induced dose-dependent decreases of RBF (RBF: −34 ± 5, −59 ± 4 and −74 ± 4 %, respectively). This vasoconstrictor effect of adenosine was significantly larger (RBF: −51 ± 4, −68 ± 4 and −83 ± 3 %, respectively) when the dogs received a low salt diet.
The post-occlusive responses were characterized by a transient hyperaemia with no detectable drop of RBF below the preocclusion level. The post-occlusive responses were affected neither by changes in local angiotensin II levels, nor by intrarenal infusions of hypertonic NaCl or blockade of A1 adenosine receptors.
When intrarenal adenosine levels were elevated by infusion of the adenosine uptake inhibitor dipyridamole, a transient, although weak, post-occlusive vasoconstriction was detected.
In summary, the present data demonstrate that adenosine acts as a potent renal vasoconstrictor in the conscious dog. The endogenous production of adenosine during short-lasting occlusions of the renal artery, however, appears to be too small to induce a post-occlusive vasoconstrictor response of RBF. These results suggest that a metabolic feedback with adenosine as ‘homeostatic metabolite’ is of minor importance in the short-term regulation of RBF in the conscious, unstressed animal.
A general principle of local circulatory control is a vasodilatation of precapillary arterioles during periods of elevated metabolic activity. This response can be regarded as an adaptive mechanism in most organs, which ensures an adequate supply of energy depending on the local demands. In the kidney, however, such a mechanism would lead to a positive feedback, since any selective preglomerular vasodilatation will further increase the glomerular filtration rate, and thus subsequently the tubular load and energy consumption (Thurau, 1964). Therefore, it was postulated that in the kidney a metabolic vasoconstrictor system operates which transfers an increase in metabolism into a decrease in blood flow (Osswald, Schmitz & Kemper, 1977; Osswald, Hermes & Nabakowski, 1982).
This concept of a negative metabolic feedback control of renal blood flow (RBF) was supported by the observation of a transient reduction of RBF after a short-lasting occlusion of the renal artery in anaesthetized rats, cats and dogs (Osswald et al. 1977; Spielman & Osswald, 1978, 1979). Since ischaemia rapidly reduces the ATP/ADP ratio and increases interstitial adenosine concentrations (Osswald et al. 1977; Miller, Thomas, Berne & Rubio, 1978), the transient vasoconstriction, reaching a maximum after 10-60 s following the reopening of the occlusion, was attributed to the formation of adenosine. Accordingly, the post-occlusive vasoconstriction was blunted by the adenosine antagonist theophylline (Osswald et al. 1977), and acute intrarenal infusions of adenosine caused transient reductions of RBF (Osswald, Schmitz & Heidenreich, 1975; Hall, Granger & Hester, 1985; Pflueger, Schenk & Osswald, 1995). Collectively, these data suggest that in the kidney, as in other regional circulations, adenosine acts as a ‘homeostatic metabolite’ (Newby, 1984; Schrader, 1990; Le Hir & Kaissling, 1993), regulating local blood flow in response to short-term variations in metabolic work.
This simple picture is considerably complicated by the fact that the effects of adenosine are modulated by a large number of other factors (for reviews, see Spielman & Thompson, 1982; Osswald, 1983). Of major importance appear to be interactions of adenosine with the prostaglandin system. Blockade of prostaglandin synthesis by meclofenamate or indomethacin markedly potentiated the post-occlusive vasoconstriction in anaesthetized cats (Spielman & Osswald, 1978). Moreover, a number of reports suggest a strong influence of the renin-angiotensin system on the renal haemodynamic effects of adenosine. For example, the preglomerular vasoconstrictor effect of adenosine was almost entirely blocked when the formation of angiotensin II was prevented by angiotensin converting enzyme inhibition in anaesthetized dogs (Spielman & Osswald, 1979; Hall et al. 1985). Similarly, the post-occlusive reduction in RBF was attenuated by the simultaneous infusion of the angiotensin II receptor antagonist saralasin (Spielman & Osswald, 1979). A recent combined in vivo and in vitro study using in situ micropuncture and isolated vessels indicates that adenosine and angiotensin II interact in a complex synergistic manner to produce afferent arteriolar vasoconstriction (Weihprecht, Lorenz, Briggs & Schnermann, 1994).
Therefore, the present studies were carried out to investigate the renal haemodynamic effects of exogenous and endogenous adenosine under physiological conditions in the conscious dog, which has low basal production rates of adenosine, angiotensin II and prostaglandins (Terragno, Terragno & McGiff, 1977; Kost & Jackson, 1991; Ehmke, Persson, Hackenthal, Schweer, Seyberth & Kirchheim, 1993). The rationale of our experiments and the targets of the pharmacological interventions are summarized in Fig. 1. The direct effect of exogenous adenosine on RBF and its modulation by the renin-angiotensin system were studied by bolus injections of adenosine into the renal artery in dogs fed a normal salt or a low salt diet. In order to assess the importance of endogenously formed adenosine as a ‘homeostatic metabolite’ during short-term changes in metabolic demands, post-occlusive responses of RBF were investigated under control conditions, and during elevated angiotensin II levels induced either by intrarenal infusions of angiotensin II or by salt restriction for at least 10 days. Finally, in a third series of experiments the effects of modulations of the local adenosine system on the post-occlusive response were investigated. The local concentrations of adenosine were increased either by the nucleoside transport inhibitor dipyridamole (Arend, Thompson & Spielman, 1985) or by an intrarenal infusion of hypertonic NaCl (Osswald, Nabakowski & Hermes 1980; Urbaitis, 1984), or the effects of adenosine were blocked by intrarenal infusions of the A1 adenosine receptor antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX). These experiments were done in animals receiving a low salt diet which has been shown previously to greatly augment the renal vasoconstrictor responses to exogenous adenosine in anaesthetized rats (Osswald et al. 1975).
Figure 1. Illustration of the components of the renal adenosine system that were experimentally altered in the present study.
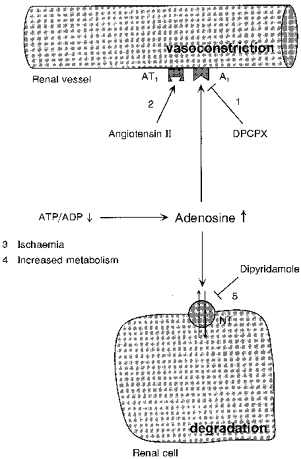
Activation of smooth muscle A1 adenosine receptors (site 1) elicits a vasoconstrictor response of the afferent arteriole which is synergistically modulated by angiotensin II via AT1 receptors (site 2). Renal interstitial adenosine levels are elevated during an increased ATP/ADP breakdown (sites 3 and 4). A major pathway for the removal of adenosine from the interstitial space occurs via the nucleoside transporter (NT; site 5). A1 adenosine receptors are blocked by the specific receptor antagonist DPCPX. The nucleoside transporter is inhibited by dipyridamole.
METHODS
All data were derived from twenty-three experiments on nine chronically instrumented conscious foxhounds of either sex weighing 27-43 kg. Each dog received two diets with different amounts of sodium. During the normal sodium diet (SSniff Spezialdiäten GmbH, Soest, Germany) the daily sodium intake was about 100 mequiv. Sodium reduction was achieved by a sodium-reduced diet (‘Limited diets canine; IVD, Nature's recipe pet foods’) for at least 10 days combined with intravenous infusions of 40 mg furosemide (Lasix, Hoechst AG, Germany) on the first 2 days of the sodium-restricted diet. During this period the daily sodium intake was < 30 mequiv. The dogs had free access to water and were kept under an artificial light-dark cycle (06.00-18.00 h light, 18.00-06.00 h dark). In one dog, a technical failure of the flow probe occurred during the normal sodium diet. Accordingly, experiments were made in a total of nine dogs on a low sodium diet and in a total of eight dogs on a normal sodium diet. All experiments and procedures were done in accordance with the national law for the care and use of research animals.
Surgical procedures
The dogs were surgically prepared under sterile conditions. After premedication with atropine (Atropin, 0.5 mg s.c.; Braun, Melsungen, Germany) and propionylpromazine (Combelen, 0.05 ml kg−1s.c.; Bayer, Leverkusen, Germany), anaesthesia was induced by pentobarbitone sodium (Nembutal, 20 mg kg−1i.v.; Sanofi, Libourne Cedex, France) and maintained with halothane (Fluothane, 0.8-1.0 %; Zeneca, Planckstadt, Germany) and N2O (0.5 l min−1). Through a left-flank incision polyurethane catheters were implanted into the abdominal aorta and the left renal artery. An inflatable cuff was placed around the renal artery proximal to the tip of the renal artery catheter. An ultrasound transit-time flow probe (6 mm diameter; Transonic Systems, Ithaca, NY, USA) was fixed around the left renal artery between the origin of the artery from the aorta and the cuff. The flow probe was carefully wrapped with dacron velour material (Protgraft, Braun-Dexon, Spangenberg, Germany) to prevent ingrowth of fatty tissue and enhance probe stabilization after healing. In addition, the flow probe was secured in place by slightly fixing the probe cable at the aortic adventitia. No surgery was made on the right kidney. The catheters and cuff leads were led subcutaneously to the dog's neck and brought through the skin. After surgery the dogs received a combination of benzathine benzylpenicillin and sulphatolamide (Tardomycel, 3 ml s.c.; Bayer, Leverkusen, Germany) every third day for 9 days. The catheters were flushed every second day with sterile saline solution and filled with a solution of heparin (1700 i.u. ml−1; Braun, Melsungen, Germany) and cephtazidim (Fortum, 16 mg ml−1; Glaxo, Bad Oldesloh, Germany). The dogs were allowed to recover from surgery for at least 10 days before being studied.
Measurements and blood sampling
Blood pressure was measured in the abdominal aorta and in the renal artery using Statham pressure transducers (P23Db) and Gould Pressure Processors. Heart rate (HR) was recorded instantaneously with a rate meter (Gould Pressure Processor). The flow probe signals were passed through a 10 Hz filter (Transonic). An analog recorder (Gould 2600) was used to display directly the measured variables (pulsatile and mean arterial blood pressures, RBF and HR). All data were sampled at 10 Hz and stored on-line (IBM PC 386) after analog-to-digital conversion with 10 Hz and with 1 Hz after calculation of a moving average.
To determine plasma renin activity, plasma samples (1 ml) were taken from the aortic catheter at the beginning of each experiment. The amount of angiotensin I formed was estimated by radioimmunoassay.
Experimental protocols
All experiments were carried out on trained, conscious dogs resting quietly on their right sides on a bench. The dogs were connected to the recording instruments via extension cables. The renal cuff could be inflated without distracting the dog's attention. The experiments started between 08.00 and 09.00 h, 16-20 h after the last feeding. Two experimental protocols were followed.
Adenosine dose-response curves
To investigate the direct effects of adenosine on RBF, bolus injections (1 ml each) of 10, 30 and 100 nmol of adenosine were given into the renal artery. The interval between two injections was 5 min. The experiments during the normal salt and during the low salt diet were carried out on the same dogs (n = 7). To test the efficacy of the A1 adenosine receptor antagonist DPCPX, during the low salt diet the protocol was conducted twice, before and after blockade of the A1 adenosine receptors (n = 6). DPCPX was infused intrarenally (10 μg kg−1 bolus plus 10 μg kg−1 min−1) for 30 min before the adenosine injections were repeated.
Post-occlusive responses of RBF
By inflating the vascular cuff the renal artery was completely occluded for 15, 30 or 60 s. A recovery time of 5 min was allowed between each occlusion. In dogs on a normal salt diet, the post-occlusive responses of RBF were investigated under control conditions (n = 8) and during an intrarenal infusion of angiotensin II (1 ng kg−1 min−1; n = 7). In dogs on a low salt diet, the post-occlusive responses of RBF were investigated under control conditions (n = 9) and during the following experimental alterations of the endogenous adenosine system: (1) during a blockade of A1 adenosine receptors by an intrarenal infusion of the specific antagonist DPCPX (10 μg kg−1 bolus plus 10 μg kg−1 min−1; n = 7); (2) during an elevation of endogenous adenosine levels by an intrarenal infusion of the nucleoside transport inhibitor dipyridamole (0.2 mg kg−1 bolus plus 10 μg kg−1 min−1; n = 4); and (3) during an intrarenal infusion of hypertonic NaCl (1 M; infusion rate, 2 ml min−1; n = 4). The infusion of NaCl was calculated to elevate renal arterial plasma sodium concentrations by about 15-20 mequiv l−1. To achieve steady-state conditions, 15 min were allowed after the start of all infusions before the occlusion experiments began. During each occlusion, the infusions were stopped. The order in which the protocols were done was different for each dog. At least 1 day of recovery was allowed after each experiment. After all experiments planned for a dog had been done, or if serious complications or technical failures occurred, the dog was killed by an overdose of sodium pentobarbitone i.v.
Data analysis
For the analysis of the adenosine effect on RBF, the maximum decrease in mean RBF after each injection was compared with the corresponding preinjection level and expressed as a percentage of change from baseline. For each adenosine injection the overall mean of the effects on RBF among the different dogs was calculated. Differences between the adenosine effects during salt loading and salt restriction and differences between the effects with and without DPCPX infusions were statistically evaluated by Student's paired t test. The effects of the different salt diets on the baseline levels of mean arterial blood pressure (MABP), HR, RBF and renal vascular resistance (RVR) were analysed by Student's unpaired t test. The values of plasma renin activity (PRA) varied greatly during salt restriction. Therefore, the effect of the low salt diet on PRA was analysed by the non-parametric Mann-Whitney U test.
The post-occlusive responses of RBF were analysed as previously described by Spielman & Osswald (1978), with the exception that mean RBF values were evaluated instead of systolic RBF values. Briefly, the first minimum value of RBF, which was usually observed 10-20 s after the release of the occluding cuff, was determined (see Fig. 6). The difference between this value and the corresponding pre-occlusion level of mean RBF was expressed as percentage change. For each duration of occlusion the overall mean of the post-occlusive response among all dogs was calculated. If one protocol was performed several times on a single dog, the mean value of all these responses was calculated first. Differences between the effects of the different experimental conditions on the post-occlusive minimum values of RBF were evaluated statistically by the Bonferroni test for repeated comparisons. To determine the effects of the angiotensin II, DPCPX, dipyridamole and 1 M NaCl infusions on MABP, HR, RBF and RVR, 10 min were allowed to reach a new steady state after the start of each infusion; then, for all variables, means were obtained over the next 5 min. For statistical evaluation, these means were compared with the preinfusion values by Student's paired t test.
Figure 6. Effects of complete occlusions of the renal artery for 60 s on RBF in a single, salt-depleted dog.
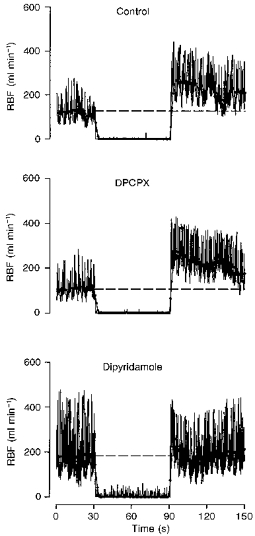
Illustrated are the responses during control conditions, during an intrarenal infusion of the A1 adenosine receptor antagonist DPCPX (10 μg kg−1 bolus plus 10 μg kg−1 min−1), and during an intrarenal infusion of the nucleoside transport inhibitor dipyridamole (0.2 mg kg−1 bolus plus 10 μg kg−1 min−1). The dashed lines indicate pre-occlusion levels of mean RBF.
All results are presented as means ± s.e.m. Differences at the 5 % level were considered statistically significant.
RESULTS
Adenosine dose-response curves
Figure 2 shows an original recording of the effects of intrarenal bolus injections of adenosine on MABP and RBF in a single dog on the normal salt diet. Adenosine elicited a marked renal vasoconstrictor response in the absence of changes in MABP, indicating a local response. The highest dose of adenosine (100 nmol) reduced RBF to almost zero. On average, in dogs on the normal salt diet, bolus injections of adenosine (10, 30 and 100 nmol) caused dose-dependent decreases in mean RBF (Fig. 3). After salt restriction the effects of the adenosine injections were significantly enhanced (Fig. 3). Salt restriction had no influence on baseline MABP, HR, RBF or RVR, but increased PRA approximately 3-fold (Table 1). Infusion of the A1 adenosine receptor antagonist DPCPX attenuated the effects of adenosine injections during the low salt diet by a factor of 3 (Fig. 4).
Figure 2. Effects of intrarenal injections of adenosine on arterial blood pressure (ABP) and renal blood flow (RBF) in a single dog.
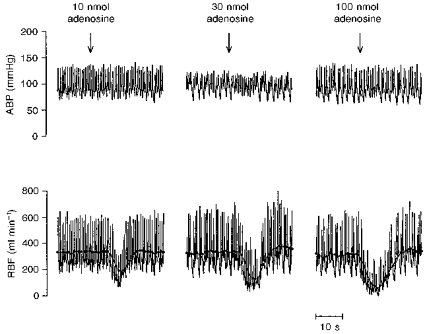
Adenosine was intrarenally injected at doses of 10, 30 and 100 nmol. In the lower traces, pulsatile and mean RBF (dots) are displayed.
Figure 3. Influence of different salt diets on the vasoconstrictor action of exogenous adenosine.
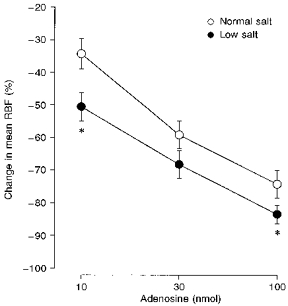
Depicted are the effects of intrarenal bolus injections of adenosine (10, 30 and 100 nmol) on mean RBF in 7 conscious dogs maintained on either a normal (○) or a low (•) sodium diet. * P < 0.05vs. normal sodium diet. Values represent means ± s.e.m.
Table 1.
Cardiovascular and hormonal effects of salt restriction
| Normal salt n = 8 | Low salt n = 9 | |
|---|---|---|
| MABP (mmHg) | 93 ± 3 | 87 ± 4 |
| HR (beats min−1) | 75 ± 5 | 69 ± 4 |
| RBF (ml min−1) | 239 ± 26 | 246 ± 25 |
| RVR (mmHg ml−1 min−1) | 0.39 ± 0.05 | 0.38 ± 0.04 |
| PRA ((ng AngI) ml−1 h−1) | 1.60 ± 0.13 | 4.54 ± 1.33 * |
Values are means ± s.e.m.
P < 0.05 vs. normal salt diet. MABP, mean arterial blood pressure; HR, heart rate; RBF, renal blood flow; RVR, renal vascular resistance; PRA, plasma renin activity; AngI, angiotensin I.
Figure 4. Blockade of adenosine-induced renal vasoconstriction by DPCPX.
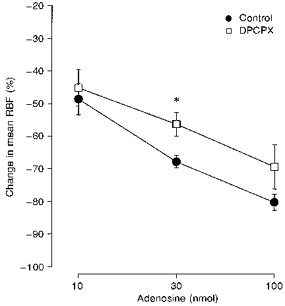
Depicted are the effects of intrarenal bolus injections of adenosine (10, 30 and 100 nmol) on mean RBF in 6 sodium-restricted conscious dogs without (•) or with infusion of the A1 adenosine receptor antagonist DPCPX (10 μg kg−1 bolus plus 10 μg kg−1 min−1; □). * P < 0.05vs. control conditions. Values represent means ± s.e.m.
Post-occlusive responses of RBF
Under control conditions, in most experiments no vasoconstrictor response of mean RBF was observed after an occlusion of the renal artery (Figs 5 and 6). On the contrary, the post-occlusive values of mean RBF, including the first minimum, demonstrated a vasodilator response which further increased with the duration of the occlusion (Fig. 5). During the normal salt diet the changes in mean RBF were +5 ± 5, +15 ± 4 and +41 ± 7 % after occlusions of 15, 30 and 60 s duration, respectively (Fig. 5). Neither an endogenous increase of angiotensin II levels by salt restriction nor an exogenous increase of angiotensin II by an intrarenal infusion of angiotensin II in dogs on a normal salt diet (Fig. 5) had any effect on the post-occlusive responses of mean RBF.
Figure 5. Effects of angiotensin II on the post-occlusive response of RBF.
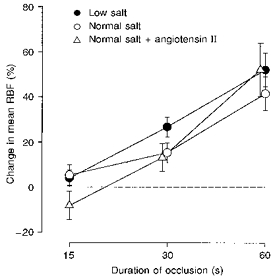
Different angiotensin II levels were achieved by either maintaining the dogs on a normal (○; n = 8) or a low (•; n = 9) sodium diet, or by an intrarenal infusion of angiotensin II (1 ng kg−1 min−1) in dogs on a normal sodium diet (▵; n = 7). Values represent means ± s.e.m. The dashed line indicates no change from pre-occlusion level of mean RBF.
To further examine the role of different endogenous adenosine levels, in salt-restricted dogs post-occlusive responses of RBF were investigated either during a blockade of A1 adenosine receptors by the specific receptor antagonist DPCPX, or during elevation of endogenous adenosine levels by either the uptake inhibitor dipyridamole or an intrarenal infusion of hypertonic NaCl. Original recordings of the post-occlusive responses in RBF obtained from the same dog during control conditions and during infusions of DPCPX or dipyridamole are depicted in Fig. 6. The averaged values obtained from all dogs are summarized in Fig. 7. While under control conditions and during DPCPX infusion no post-occlusive vasoconstrictor responses occurred, a transient vasoconstriction was observed during the infusion of dipyridamole which further increased with the duration of the occlusion (Fig. 7). In contrast to dipyridamole, a stimulation of the local formation of adenosine by an intrarenal infusion of hypertonic NaCl failed to elicit a post-occlusive vasoconstrictor response (Fig. 8).
Figure 7. Effects of alterations of the adenosine system on the post-occlusive responses of RBF.
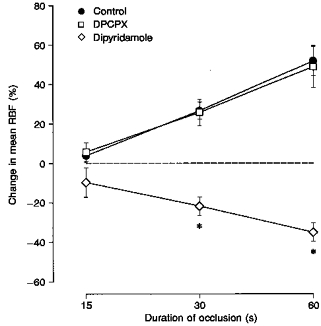
Shown are the responses during control conditions (•; n = 9), during an intrarenal infusion of DPCPX (10 μg kg−1 bolus plus 10 μg kg−1 min−1; □; n = 8), and during an intrarenal infusion of dipyridamole (0.2 mg kg−1 bolus plus 10 μg kg−1 min−1; ⋄; n = 4) in conscious, salt-depleted dogs. * P < 0.05vs. control conditions. Values represent means ± s.e.m. The dashed line indicates no change from pre-occlusion level of mean RBF.
Figure 8. Effects of intrarenal infusions of hypertonic NaCl on the post-occlusive responses of RBF.
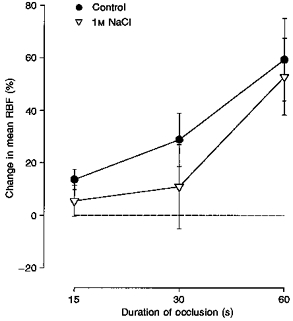
Illustrated are the responses before (•) and during infusion of 1 M NaCl into the renal artery (▿) in 4 conscious, salt-depleted dogs. Values represent means ± s.e.m. The dashed line indicates no change from pre-occlusion level of mean RBF.
The effects of the different intrarenal infusions on MABP, HR, mean RBF, and RVR are summarized in Table 2. Neither MABP nor HR were influenced by the various infusions. Angiotensin II, DPCPX, and hypertonic NaCl induced significant increases in RVR, whereas dipyridamole significantly reduced RVR. Correspondingly, RBF increased in response to dipyridamole and decreased during the infusions of angiotensin II and DPCPX. Quantitatively, however, the effects of DPCPX were much smaller than those of angiotensin II and dipyridamole.
Table 2.
Haemodynamic effects of the different experimental infusions
| n | Baseline | After 10 min infusion | |
|---|---|---|---|
| MABP (mmHg) | |||
| Angiotensin II | 7 | 97 ± 5 | 101 ± 5 |
| DPCPX | 7 | 90 ± 4 | 96 ± 5 |
| Dipyridamole | 4 | 95 ± 5 | 98 ± 4 |
| 1 M NaCl | 4 | 98 ± 6 | 105 ± 4 |
| HR (beats min−1) | |||
| Angiotensin II | 7 | 73 ± 5 | 74 ± 7 |
| DPCPX | 7 | 71 ± 6 | 71 ± 7 |
| Dipyridamole | 4 | 72 ± 8 | 83 ± 4 |
| 1 M NaCl | 4 | 70 ± 4 | 72 ± 7 |
| RBF (ml min−1) | |||
| Angiotensin II | 7 | 251 ± 33 | 170 ± 25 * |
| DPCPX | 7 | 239 ± 34 | 211 ± 30 * |
| Dipyridamole | 4 | 231 ± 41 | 306 ± 53 * |
| 1 M NaCl | 4 | 203 ± 32 | 196 ± 28 |
| RVR (mmHg ml−1 min−1) | |||
| Angiotensin II | 7 | 0.36 ± 0.04 | 0.54 ± 0.06 * |
| DPCPX | 7 | 0.40 ± 0.06 | 0.43 ± 0.05 * |
| Dipyridamole | 4 | 0.40 ± 0.07 | 0.30 ± 0.05 * |
| 1 M NaCl | 4 | 0.50 ± 0.06 | 0.55 ± 0.06 * |
Values are means ± s.e.m.
P < 0.05vs. baseline. MABP, mean arterial blood pressure; HR, heart rate; RBF, renal blood flow; RVR, renal vascular resistance.
DISCUSSION
Adenosine has been suggested to play an important role in the regulation of RBF as a mediator of the tubulo-glomerular feedback response (Osswald et al. 1982; Schnermann, Weihprecht, Lorenz & Briggs, 1991), which inversely links the NaCl concentration at the macula densa to RBF, and as the mediator of a metabolic feedback mechanism which transfers acute changes in the tubular load into opposite changes in RBF (Osswald et al. 1982; Spielman & Thompson, 1982). The present experiments addressed the physiological significance of this latter regulatory mechanism and its modulation by the renin- angiotensin system. The most widely used experimental model to study the metabolic feedback mechanism in vivo has been the post-occlusive RBF response. In contrast to other organs, which display a reactive hyperaemia following an interruption of blood flow, in the kidney a vasoconstriction was observed in response to short-term (less than 3 min) complete occlusions of the renal artery (Osswald et al. 1977; Spielman & Osswald, 1978, 1979; Pflueger et al. 1995). In anaesthetized animals, this duration of ischaemia is sufficient to significantly stimulate adenosine production in the kidney. In dogs, the level of adenosine in the urine was elevated 2-fold after an occlusion of the renal artery for 1 min (Miller et al. 1978). The tissue content of adenosine in rat kidneys after ischaemia of 30-60 s has been shown to be increased 3- to 6-fold (Osswald et al. 1977). Also, 5′ nucleotidase, the enzyme which catalyses the generation of adenosine, is activated by ischaemia (Le Hir & Kaissling, 1993). All previous experiments, however, which were able to demonstrate a post-occlusive vasoconstriction, were carried out in anaesthetized animals in which plasma adenosine levels are elevated about 2-fold (Kost & Jackson, 1991), while plasma renin and prostaglandin levels may be elevated by as much as 5- to 10-fold (Terragno et al. 1977) compared with conscious animals (Ehmke et al. 1993). Since the effects of adenosine may be largely dependent on the activity of the prostaglandin and renin-angiotensin system (Spielman & Osswald, 1978, 1979; Hall et al. 1985; Hall & Granger, 1986; Weihprecht et al. 1994), in the present study we investigated the effects of exogenous adenosine and of short-term complete occlusions of the renal artery on RBF in conscious dogs.
Should, according to the concept of the metabolic feedback mechanism, adenosine indeed contribute importantly to the regulation of RBF under physiological conditions, two assumptions must be fulfilled.
Firstly, exogenous adenosine should cause a reduction of RBF. In the present study, we observed marked renal vasoconstrictor effects of adenosine injected directly into the renal artery. Adenosine caused dose-dependent reductions of RBF (Fig. 3). In several experiments, a single dose of 100 nmol of adenosine was sufficient to cause a short-lasting complete interruption of RBF (Fig. 2). Furthermore, in accordance with observations from other in vivo and in vitro studies (Osswald et al. 1975; Spielman & Osswald, 1979; Weihprecht et al. 1994), the effects of adenosine on RBF were significantly enhanced when the renin-angiotensin system was stimulated by a low salt diet (Fig. 3). Interestingly, the renal vasculature was also highly sensitive to exogenous adenosine in dogs on a normal sodium intake which show very low plasma renin activities. Previous experiments in the split hydronephrotic kidney preparation (Dietrich, Endlich, Parekh & Steinhausen, 1991) and in anaesthetized dogs (Hall et al. 1985) suggested that the presence of angiotensin II may be a prerequisite for the vasoconstrictor action of adenosine. Thus, in the conscious animal even low levels of angiotensin II may suffice to allow a renal vasoconstriction by adenosine. On the other hand, experiments in isolated perfused kidneys (Rossi, Churchill, Jacobsen & Leahy, 1986; Barrett & Droppleman, 1993) and in isolated renal vessels (Weihprecht et al. 1994; Carmines & Inscho, 1994), suggested that independent activation of either adenosine or angiotensin II receptors can lead to renal vasoconstriction. Accordingly, in the present study adenosine may have exerted its vasoactive effect independent of angiotensin II. Taken together, these findings demonstrate that in the conscious dog adenosine has a vasoconstrictor effect which appears to be modulated by the activity of the renin-angiotensin system.
Secondly, changes in renal function, which increase the formation of adenosine by an increased ATP breakdown, should induce a vasoconstrictor response. None of the physiological interventions used in the present study elicited a post-occlusive vasoconstriction. In dogs with a normal sodium intake, the post-occlusive RBF was always higher than the baseline level before the occlusion (Fig. 5). Neither a stimulation of the endogenous angiotensin II production rate by a low salt diet nor a simultaneous intrarenal infusion of angiotensin II changed the post-occlusive responses of RBF (Fig. 5). Also, an infusion of hypertonic NaCl, which is associated with an increased ATP breakdown and the formation of adenine nucleotides (Osswald et al. 1980; Urbaitis, 1984), failed to alter the post-occlusive response of RBF (Fig. 8). These results suggest that in the conscious animal the endogenous production of adenosine during a short-lasting occlusion of the renal artery may be too small to induce a post-occlusive vasoconstrictor reaction of the renal circulation. Consistent with this conclusion are the results from the experiments with intrarenal infusions of the specific antagonist of the A1 adenosine receptor DPCPX, or of the nucleoside transport inhibitor dipyridamole (Figs 6 and 7). Whereas DPCPX did not influence the post-occlusive response of RBF, infusion of dipyridamole, which strongly increases interstitial adenosine concentrations, induced a transient post-occlusive vasoconstriction. An alternative explanation for the lack of a post-occlusive vasoconstrictor response of RBF in the conscious animal may be the action of a vasodilator substance which antagonizes the vasoconstrictor influence of adenosine. The complete occlusion of the renal artery induces large changes in metabolic demand but at the same time is a rather unphysiological stimulus. Thus, it is not unlikely that besides adenosine other agents are released. An augmented release of a vasodilator substance could also explain why, in the present study, intrarenal infusion of hypertonic saline failed to exert a consistent vasoconstrictor effect on basal RBF (Table 2). An earlier study in anaesthetized dogs reported a reduction of RBF by 54 % in response to an increase of the NaCl concentration by 30 mequiv l−1 (Gerber, Branch, Nies, Hollifield & Gerkens, 1979). It remains unclear, however, whether such a physiological adenosine-antagonizing substance actually exists within the kidney. Prostaglandins are unlikely candidates, since their synthesis is strongly stimulated by anaesthesia and acute surgical trauma (Terragno et al. 1977), and since an inhibition of prostaglandin synthesis augments the post-occlusive vasoconstrictor response of RBF in anaesthetized animals (Spielman & Osswald, 1978). Taken together, the results of the present experiments on the post-occlusive responses of RBF most probably suggest that in the conscious animal a transient occlusion of the renal artery does not stimulate the endogenous production of adenosine sufficiently to elicit a post-occlusive vasoconstrictor reaction of RBF.
In contrast to bolus injections or short-term infusions, sustained infusions of adenosine lasting > 5 min increase RBF (Spielman & Thompson, 1982; Hall et al. 1985; Hall & Granger, 1986). The increases in baseline RBF during dipyridamole infusion and the reduction of baseline RBF during the infusions of DPCPX in the present study (Table 2) are consistent with these earlier observations. The mechanism of this increase is still not fully understood. Since adenosine is a potent inhibitor of renin release, it has been suggested that this increase may be partly mediated by a suppression of endogenous angiotensin II. Accordingly, the vasodilatation during a sustained adenosine infusion was prevented when the circulating angiotensin II levels were experimentally kept constant in anaesthetized dogs (Hall et al. 1985). A similar, albeit more chronic experimental protocol, however, did not abolish the adenosine-induced vasodilatation in conscious dogs (Hall & Granger, 1986). The vasodilator effect may be partly mediated by A2 adenosine receptors located in renal medullary vessels (Agmon, Dinour & Brezis, 1993), but an increase in this vascular bed cannot quantitatively account for the observed effects on total RBF. The observation of a further increase of RBF immediately after the sustained infusion of adenosine has been stopped (Osswald, 1983; Hall et al. 1985) may also argue in favour of the activation of a secondary vasodilator system which antagonizes the vasoconstrictor effects of adenosine (Spielman & Thompson, 1982).
It should be noted that the present results do not exclude possible physiological roles of adenosine in RBF regulation other than mediating a metabolic feedback. The finding that blockade of A1 adenosine receptors by DPCPX induced significant, albeit small changes in resting RBF (Table 2) actually indicates a participation of intrarenally released adenosine in the normal control of RBF. Further studies are required to determine the precise nature of such mechanisms as well as their quantitative significance. Also, it remains to be shown why the blockade of A1 adenosine receptors, which usually mediate a vasoconstrictor response, resulted in a decrease of RBF.
In conclusion, the present study demonstrates that conscious dogs under physiological conditions do not show a vasoconstrictor response to transient interruptions of RBF. The apparent discrepancies with studies in anaesthetized animals may be due to a low production of adenosine, a modulator influence of an unknown vasodilator system, or a combined action of both mechanisms in the conscious animal. Therefore, the metabolic feedback which appears to participate in the control of RBF during anaesthesia (Spielman & Osswald, 1978, 1979) or in pathophysiological states like diabetes mellitus (Pflueger et al. 1995) does not appear to play an important role in the short-term regulation of RBF in the conscious, unstressed animal.
Acknowledgments
We thank I. Keller, L. Mahl and E. Röbel for expert technical assistance, and U. Hilgenfeldt for the determination of PRA. This study was supported by the German Research Foundation (Graduiertenkolleg ‘Experimentelle Nieren- und Kreislaufforschung’; Ki 151/5-3).
References
- Agmon Y, Dinour D, Brezis M. Disparate effects of adenosine A1- and A2-receptor agonists on intrarenal blood flow. American Journal of Physiology. 1993;265:F802–806. doi: 10.1152/ajprenal.1993.265.6.F802. [DOI] [PubMed] [Google Scholar]
- Arend LJ, Thompson CI, Spielman WS. Dipyridamole decreases glomerular filtration in the sodium-depleted dog. Circulation Research. 1985;56:242–251. doi: 10.1161/01.res.56.2.242. [DOI] [PubMed] [Google Scholar]
- Barrett RJ, Droppleman DA. Interactions of adenosine A1 receptor-mediated renal vasoconstriction with endogenous nitric oxide and ANG II. American Journal of Physiology. 1993;265:F651–659. doi: 10.1152/ajprenal.1993.265.5.F651. [DOI] [PubMed] [Google Scholar]
- Carmines P, Inscho EW. Renal arteriolar angiotensin responses during varied adenosine receptor activation. Hypertension. 1994;23(supp. I):I114–119. doi: 10.1161/01.hyp.23.1_suppl.i114. [DOI] [PubMed] [Google Scholar]
- Dietrich MS, Endlich K, Parekh N, Steinhausen M. Interaction between adenosine and angiotensin II in renal microcirculation. Microvascular Research. 1991;41:275–288. doi: 10.1016/0026-2862(91)90028-a. 10.1016/0026-2862(91)90028-A. [DOI] [PubMed] [Google Scholar]
- Ehmke H, Persson PB, Hackenthal E, Schweer H, Seyberth HW, Kirchheim HR. Is arterial pressure a determinant of renal prostaglandin release? American Journal of Physiology. 1993;264:R402–408. doi: 10.1152/ajpregu.1993.264.2.R402. [DOI] [PubMed] [Google Scholar]
- Gerber JG, Branch RA, Nies AS, Hollifield JW, Gerkens JF. Influence of hypertonic saline on canine renal blood flow and renin release. American Journal of Physiology. 1979;237:F441–446. doi: 10.1152/ajprenal.1979.237.6.F441. [DOI] [PubMed] [Google Scholar]
- Hall JE, Granger JP. Renal hemodynamics and arterial pressure during chronic intrarenal adenosine infusion in conscious dogs. American Journal of Physiology. 1986;250:F32–39. doi: 10.1152/ajprenal.1986.250.1.F32. [DOI] [PubMed] [Google Scholar]
- Hall JE, Granger JP, Hester RL. Interactions between adenosine and angiotensin II in controlling glomerular filtration. American Journal of Physiology. 1985;248:F340–346. doi: 10.1152/ajprenal.1985.248.3.F340. [DOI] [PubMed] [Google Scholar]
- Kost CK, Jr, Jackson EK. Effect of angiotensin II on plasma adenosine concentration in the rat. Journal of Cardiovascular Pharmacology. 1991;17:838–845. doi: 10.1097/00005344-199105000-00021. [DOI] [PubMed] [Google Scholar]
- Le Hir M, Kaissling B. Distribution and regulation of renal ecto-5′-nucleotidase: implications for physiological functions of adenosine. American Journal of Physiology. 1993;264:F377–387. doi: 10.1152/ajprenal.1993.264.3.F377. [DOI] [PubMed] [Google Scholar]
- Miller WL, Thomas RA, Berne RM, Rubio R. Adenosine production in the ischemic kidney. Circulation Research. 1978;43:390–397. doi: 10.1161/01.res.43.3.390. [DOI] [PubMed] [Google Scholar]
- Newby AC. Adenosine and the concept of retaliatory metabolites. Trends in Biochemical Sciences. 1984;9:42–44. 10.1016/0968-0004(84)90176-2. [Google Scholar]
- Osswald H. Adenosine and renal function. In: Berne RM, Rall TW, Rubio R, editors. Regulatory Function of Adenosine. Boston: Martinus Nijhoff Publishers; 1983. pp. 399–415. [Google Scholar]
- Osswald H, Hermes HH, Nabakowski G. Role of adenosine in signal transmission of tubuloglomerular feedback. Kidney International Supplement. 1982;12:S136–142. [PubMed] [Google Scholar]
- Osswald H, Nabakowski G, Hermes H. Adenosine as a possible mediator of metabolic control of glomerular filtration rate. International Journal of Biochemistry. 1980;12:263–267. doi: 10.1016/0020-711x(80)90082-8. 10.1016/0020-711X(80)90082-8. [DOI] [PubMed] [Google Scholar]
- Osswald H, Schmitz HJ, Heidenreich O. Adenosine response of the rat kidney after saline loading, sodium restriction and hemorrhagia. Pflügers Archiv. 1975;357:323–333. doi: 10.1007/BF00585986. [DOI] [PubMed] [Google Scholar]
- Osswald H, Schmitz HJ, Kemper R. Tissue content of adenosine, inosine and hypoxanthine in the rat kidney after ischemia and postischemic recirculation. Pflügers Archiv. 1977;371:45–49. doi: 10.1007/BF00580771. [DOI] [PubMed] [Google Scholar]
- Pflueger AC, Schenk F, Osswald H. Increased sensitivity of the renal vasculature to adenosine in streptozotocin-induced diabetes mellitus rats. American Journal of Physiology. 1995;269:F529–535. doi: 10.1152/ajprenal.1995.269.4.F529. [DOI] [PubMed] [Google Scholar]
- Rossi NF, Churchill PC, Jacobsen KA, Leahy AE. Further characterization of the renovascular effects of N6-cyclohexyladenosine in the isolated perfused rat kidney. Journal of Pharmacology and Experimental Therapeutics. 1986;240:911–915. [PMC free article] [PubMed] [Google Scholar]
- Schnermann J, Weihprecht H, Lorenz JN, Briggs JP. The afferent arteriole - the target for macula densa-generated signals. Kidney International Supplement. 1991;32:S74–77. [PubMed] [Google Scholar]
- Schrader J. Adenosine. A homeostatic metabolite in cardiac energy metabolism. Circulation. 1990;81:389–391. doi: 10.1161/01.cir.81.1.389. [DOI] [PubMed] [Google Scholar]
- Spielman WS, Osswald H. Characterization of the postocclusive response of renal blood flow in the cat. American Journal of Physiology. 1978;235:F286–290. doi: 10.1152/ajprenal.1978.235.4.F286. [DOI] [PubMed] [Google Scholar]
- Spielman WS, Osswald H. Blockade of postocclusive renal vasoconstriction by an angiotensin II antagonist: evidence for an angiotensin-adenosine interaction. American Journal of Physiology. 1979;237:F463–467. doi: 10.1152/ajprenal.1979.237.6.F463. [DOI] [PubMed] [Google Scholar]
- Spielman WS, Thompson CI. A proposed role for adenosine in the regulation of renal hemodynamics and renin release. American Journal of Physiology. 1982;242:F423–435. doi: 10.1152/ajprenal.1982.242.5.F423. [DOI] [PubMed] [Google Scholar]
- Terragno NA, Terragno DA, McGiff JC. Contribution of prostaglandins to the renal circulation in conscious, anesthetized, and laparotomized dogs. Circulation Research. 1977;40:590–595. doi: 10.1161/01.res.40.6.590. [DOI] [PubMed] [Google Scholar]
- Thurau K. Renal hemodynamics. American Journal of Medicine. 1964;36:689–719. doi: 10.1016/0002-9343(64)90181-0. [DOI] [PubMed] [Google Scholar]
- Urbaitis BK. Effect of ischemia and hypertonic saline loading on renal adenine nucleotides. Renal Physiology. 1984;7:22–31. doi: 10.1159/000172921. [DOI] [PubMed] [Google Scholar]
- Weihprecht H, Lorenz JN, Briggs JP, Schnermann J. Synergistic effects of angiotensin and adenosine in the renal microvasculature. American Journal of Physiology. 1994;266:F227–239. doi: 10.1152/ajprenal.1994.266.2.F227. [DOI] [PubMed] [Google Scholar]


