Abstract
Glucose-induced insulin release from single islets of Langerhans is pulsatile. We have investigated the correlation between changes in cytosolic free calcium concentration ([Ca2+]i) and oscillatory insulin secretion from single mouse islets, in particular examining the basis for differences in secretory responses to intermediate and high glucose concentrations. Insulin release was monitored in real time through the amperometric detection of the surrogate insulin marker 5-hydroxytryptamine (5-HT) via carbon fibre microelectrodes. The [Ca2+]i was simultaneously recorded by whole-islet fura-2 microfluorometry.
In 82 % of the experiments, exposure to 11 mM glucose evoked regular high-frequency (average, 3.4 min−1) synchronous oscillations in amperometric current and [Ca2+]i. In the remaining experiments (18 %), 11 mM glucose induced an oscillatory pattern consisting of high-frequency [Ca2+]i oscillations that were superimposed on low-frequency (average, 0.32 min−1) [Ca2+]i waves. Intermittent high-frequency [Ca2+]i oscillations gave rise to a similar pattern of pulsatile 5-HT release.
Raising the glucose concentration from 11 to 20 mM increased the duration of the steady-state [Ca2+]i oscillations without increasing their amplitude. In contrast, both the duration and amplitude of the associated 5-HT transients were increased by glucose stimulation. The amount of 5-HT released per secretion cycle was linearly related to the duration of the underlying [Ca2+]i oscillations in both 11 and 20 mM glucose. The slopes of the straight lines were identical, indicating that there is no significant difference between the ability of calcium oscillations to elicit 5-HT/insulin release in 11 and 20 mM glucose.
In situ 5-HT microamperometry has the potential to resolve the high-frequency oscillatory component of the second phase of glucose-induced insulin secretion. This component appears to reflect primarily the duration of the underlying [Ca2+]i oscillations, suggesting that glucose metabolism and/or access to glucose metabolites is not rate limiting to fast pulsatile insulin release.
Impairment of glucose-induced insulin secretion from the β-cells of the islets of Langerhans may either trigger the development of non-insulin-dependent diabetes mellitus (NIDDM) or compound peripheral insulin resistance leading, in turn, to the onset of this pathology (Porte & Kahn, 1995). Classically, insulin release has been monitored from single islets by periodically sampling the perifusate and later assaying the hormone, using sensitive but laborious procedures that have limited the time resolution of the analysis: radioimmunoassay (RIA) -10 s resolution (Rosario, Atwater & Scott, 1986; Gilon, Shepherd & Henquin, 1993) and enzyme-linked immunosorbent assay (ELISA) -3 s resolution (Bergsten & Hellman, 1993). More recently, however, the development of ultra-sensitive microvoltammetric and microamperometric methods (Wightman et al. 1991; Chow, von Ruden & Neher, 1992; Alvarez de Toledo, Fernandez-Chacon & Fernandez, 1993) has allowed the real time analysis (millisecond resolution) of exocytotic secretion from single pancreatic β-cells (Smith, Duchen & Ashcroft, 1995; Huang, Shen, Atkinson & Kennedy, 1995; Zhou & Misler, 1996) and single intact islets (Barbosa, Silva, Tomé, Stamford, Santos & Rosário, 1996b). In some of the latter studies, insulin release was monitored following preloading of the insulin-containing granules with the electroactive amine 5-hydroxytryptamine (5-HT; cf. Smith et al. 1995). Exogenously applied 5-HT is indeed readily transported across the β-cell membrane, accumulated in insulin granules and released in response to appropriate stimuli (Gylfe, 1978), thus behaving as an effective label.
Glucose-stimulated islets exhibit a pattern of bursting electrical activity and synchronous [Ca2+]i oscillations (Santos, Rosário, Nadal, Garcia-Sancho, Soria & Valdeolmillos, 1991), which appear to drive pulsatile insulin release (Rosario et al. 1986; Gilon et al. 1993; Barbosa et al. 1996b). By performing simultaneous measurements of intra-islet 5-HT release and [Ca2+]i we have now studied the correlation between oscillatory calcium levels and pulsatile insulin secretion, and investigated whether the graded secretory response to glucose in the intermediate-high concentration range can be explained entirely by the characteristics of the [Ca2+]i oscillations.
METHODS
Islet preparation and handling
Three- to six-month-old albino mice were killed by a blow to the head, followed by cervical dislocation. The islets were then isolated by collagenase (Type P, Boehringer Mannheim) digestion of the pancreas and maintained in culture (5 % CO2 and 95 % air-humidified atmosphere) for 24 h prior to the experiments, as reported in detail (Silva, Rosário & Santos, 1994; Salgado, Silva, Santos & Rosário, 1996). The islets were cultured in RPMI 1640-based medium containing 11 mM glucose (first 5-7 h) and 5.5 mM glucose + 1 mM 5-HT (remaining time). Loading with the fluorescent Ca2+ indicator fura-2 was carried out in 5-HT-free culture medium as described (Silva et al. 1994). Briefly, groups of four to eight islets were typically incubated in this medium with 4 μM fura-2 AM for 45 min at 37°C. Fura-2/5-HT-loaded islets were transferred to a fast perifusion chamber placed on the stage of an inverted epifluorescence microscope. Stable islet attachment to the poly-L-lysine-coated glass chamber bottom was achieved in less than 15 min under stationary conditions. Islets were then perifused with the following salt solution (mM): 120 NaCl, 5 KCl, 25 NaHCO3, 2.56 CaCl2, 1.1 MgCl2 and glucose (concentration as required), and constantly gassed with 95 % O2 and 5 % CO2 at pH 7.4. Complete solution exchange was achieved by a stop-cock valve in less than 5 s at a flow rate of 1.5-2 ml min−1. The temperature in the chamber was 37°C.
Microfluorometry
The [Ca2+]i was recorded from single islets using a dual-excitation microfluorescence system as described in detail (Silva et al. 1994; Salgado et al. 1996). Briefly, fura-2 was excited at 340 and 380 nm via two monochromators and the fluorescence was detected by a photomultiplier after passing through a band-pass interference filter (40 nm band width) centred at 510 nm. The data were acquired at 10 Hz by computer.
Microamperometry
The release of pre-loaded 5-HT was monitored amperometrically using glass-encased carbon fibre microelectrodes, as described for single β-cells (Smith et al. 1995; Zhou & Misler, 1996) and islets (Barbosa et al. 1996b). The microelectrodes were made essentially as described (Stamford, Palij, Davidson, Jorm & Phillips, 1995). Briefly, single carbon fibres (Courtaulds Ltd, UK) were inserted into borosilicate glass capillaries (1.16 mm i.d. × 2.0 mm o.d.; Clark Electromedical Instruments, UK). The capillaries were then pulled on a horizontal puller (P-77, Sutter Instrument Company, CA, USA), after which the protruding carbon fibres were cut to the required size (30-50 μm) and bevelled at 30 deg on a grinder (EG-4, Narishige, Japan). Electrical contact between the carbon fibre and the wire was provided by electrically conductive paint (RS, UK).
The protruding carbon fibre was gently inserted into the islet tissue (diameter, ∼200-300 μm), such that the active surface of the microelectrode remained in the islet outermost region. The amperometric currents were measured using a nano-amperometer (Tacussel/Radiometer AMU 130, France), with the working electrode held at a potential of +0.55 V vs. Ag-AgCl. Amperometric current was low-pass filtered (cut-off frequency, 100 Hz), amplified × 10 and stored on digital tape via a DAT recorder. The data were transferred off-line at 1 kHz to a computer and subsequently refiltered via a fast Fourier transform (FFT) smoothing routine provided by commercially available software (Microcal Origin), in order to eliminate 50 Hz mains interference.
The responsiveness of the recording electrodes was assessed by flow injection analysis prior to the experiments. Briefly, each electrode was subjected to repetitive 30 s pulses of 10 μM 5-HT at a frequency of approximately 1 pulse min−1. There was an appreciable (< 61 %) decay in peak current amplitude, which stabilized in less than 5 min so that subsequent 5-HT exposures did not cause signal loss. This decay originates from the adsorption of 5-HT and its oxidation products at the active surface of the electrode (Jackson, Dietz & Wightman, 1995). Electrode poisoning by 5-HT released in situ is likely to account, at least in part, for the pronounced decay in amperometric current that was observed immediately after inserting the microelectrode in the islet (adsorption of insulin (Barbosa, Rosário, Brett & Oliveira-Brett, 1996a) and other intra-islet substances may also contribute to this decay). Here electrode stabilization was essentially reached in less than 15 min. Thus current recordings were only initiated after 20-25 min had elapsed from the moment of insertion.
We have attempted to record amperometric responses from control, 5-HT-free islets. No detectable signals could be obtained in response to 11-20 mM glucose or 30 mM KCl in the perifusion medium (3 experiments, not shown).
Integral charge was determined as the time integral of the amperometric current, after subtraction of the respective background area (delimited by a straight line linking the beginning and end of the integrated signal). Integration was carried out using commercially available software (Microcal Origin).
Intracellular recording of membrane potential
The membrane potential was recorded from microdissected mouse islets using a high-impedance amplifier and high-resistance (150-200 MΩ) glass microelectrodes, essentially as previously reported (Rosário, Barbosa, Antunes, Silva, Abrunhosa & Santos, 1993). Briefly, microdissected islets were pinned to the plastic bottom of a fast perifusion chamber through which salt solution (composition as above) flowed at a rate of ca 2 ml min−1 at 37°C.
Materials
Fura-2 AM was from Molecular Probes. Antibiotics and RPMI 1640 were from Biological Industries (Beth Haemeck, Israel). 5-Hydroxytryptamine hydrochloride and all other chemicals were from Sigma.
RESULTS
We recorded [Ca2+]i from single 5-HT-loaded mouse islets using the fluorescent Ca2+ indicator fura-2 (Silva et al. 1994). The typical effect of raising glucose concentration from 3 to 11 mM on this signal was triphasic: there was (1) an initial [Ca2+]i depression arising from the stimulation of Ca2+ buffering mechanisms (Gylfe, 1988); (2) a rapid increase; and (3) an oscillatory pattern superimposed on an elevated plateau (Fig. 1A, upper trace). The frequency of these oscillations averaged 3.4 ± 1.0 min−1 (mean ± s.d., n = 31 islets; range 1.3-5.6 min−1). We and others have previously demonstrated that the [Ca2+]i follows closely the dynamics of glucose-evoked electrical activity, the initial peak rise relating to a prolonged burst of calcium action potentials and the oscillations to a pattern of membrane potential waves, with spikes firing from the depolarized plateau phases (Santos et al. 1991; Worley, McIntyre, Spencer, Mertz, Roe & Dukes, 1994). Thus each high-frequency [Ca2+]i oscillation was primarily the result of fast Ca2+ influx, mediated by the activation of voltage-sensitive Ca2+ channels.
Figure 1. Simultaneous recording of [Ca2+]i and 5-HT/insulin release from single pancreatic islets.
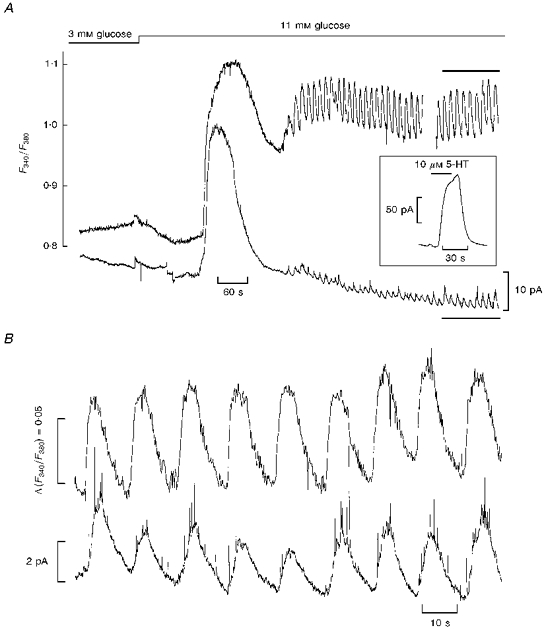
A, upper trace: microfluorometric recording of [Ca2+]i from a single fura-2/5-HT-loaded islet, as given by the ratio of fura-2 fluorescence at 340 and 380 nm (F340/F380). The gap represents a period where the [Ca2+]i was not recorded. Lower trace: continuous recording of amperometric current from the same islet through a carbon fibre microelectrode. Glucose concentration was stepped from 3 to 11 mM as indicated. Inset: effect of applying a step pulse (bar) of a 5-HT-containing solution on the amperometric current recorded in situ. B, expanded representation of steady-state [Ca2+]i and amperometric current in 11 mM glucose (from the experiment depicted in A, portion depicted by bars).
Figure 1A (lower trace) shows a typical in situ amperometric recording of 5-HT using a carbon fibre microelectrode. Raising glucose concentration from 3 to 11 mM evoked a sharp transient increase in amperometric current. While in some experiments the time course of this change was virtually identical to that of the ascending phase of the simultaneous [Ca2+]i rise, in other experiments (an example of which is depicted in Fig. 1A) the amperometric response either preceded or lagged behind the [Ca2+]i rise for a variable period of a few seconds (-8 s in the experiment of Fig. 1A, meaning that the amperometric response preceded the [Ca2+]i rise by 8 s in this particular case; range from -8 to +10 s, n = 7 experiments). Regular fluctuations in amperometric current were then observed alongside the [Ca2+]i oscillations, indicating that 5-HT/insulin was released in a pulsatile fashion. The expanded traces in Fig. 1B show that the 5-HT fluctuations were synchronous with the [Ca2+]i oscillations, reinforcing the view that pulsatile insulin release is controlled by [Ca2+]i (Gilon et al. 1993). Interestingly, fast current spikes were frequently seen in the amperometric recordings (Fig. 1B). The characteristics of these spikes (e.g. integral charge per spike and peak amplitude, described in Barbosa et al. 1996b) were similar to those reported for single stimulated β-cells (Smith et al. 1995), suggesting the contribution of discrete exocytotic events resulting from the fusion of individual secretory granules with the plasma membrane of β-cells lying in close contact with the microelectrode.
We measured the lag time (time from glucose application to the initiation of the 5-HT response, i.e. latency) and the duration of the initial 5-HT transient (time from half-rise to half-decay): 161 ± 33 and 52 ± 18 s (mean ± s.d., n = 7 islets), respectively. In addition, we calculated the time integral of the current response (i.e. the total charge generated by 5-HT oxidation at the electrode surface) both for the initial 5-HT transient (R1) and for an equivalent segment of steady-state 5-HT oscillatory activity (R2). Integration times ranged from 2 to 3.3 min depending on the duration of the initial 5-HT transient. This analysis yielded R1/R2 values in the range 4.7-13.2 (11.2 for the experiment depicted in Fig. 1; 9.5 ± 3.3, mean ± s.d., n = 7 islets). These values provide an estimate of the ratio of the secretory rates over the first and second phases of glucose-induced insulin secretion (see Discussion).
Raising the glucose concentration from 11 to 20 mM increased the duration of the steady-state [Ca2+]i oscillations without increasing their amplitude (Fig. 2A, upper trace; average amplitude data from several similar experiments in Fig. 2D). The increased duration of the [Ca2+]i waves mirrors the effect of glucose on the electrical activity (Santos et al. 1991). In contrast, both the duration and amplitude of the associated 5-HT transients were enhanced by glucose stimulation (Fig. 2A, lower trace). We calculated the time integral of the current response for each secretory cycle at steady state for both 11 and 20 mM glucose. This parameter reflects the transient accumulation of 5-HT in the islet interstitial space and can therefore be taken as a lower limit for the actual amount of 5-HT/insulin secreted per cycle. The average charge per oscillation in 11 and 20 mM glucose was 9.0 and 33.6 pC, respectively (Fig. 2B). The average oscillatory frequency at the steady state of 11 and 20 mM glucose was 2.1 and 1.2 min−1, respectively (Fig. 2B). Thus the overall release rate (taken as the product of the charge per oscillation and the oscillatory frequency) can be estimated to increase from > 19 pC min−1 in 11 mM glucose to > 40 pC min−1 in 20 mM glucose, a positive increment of at least 111 %. This is comparable to published single islet data using RIA and ELISA assays for insulin secretion (Scott, Atwater & Rojas, 1981; Bergsten & Hellman, 1993).
Figure 2. Modulation of intracellular calcium burst dynamics and pulsatile 5-HT/insulin release by glucose.
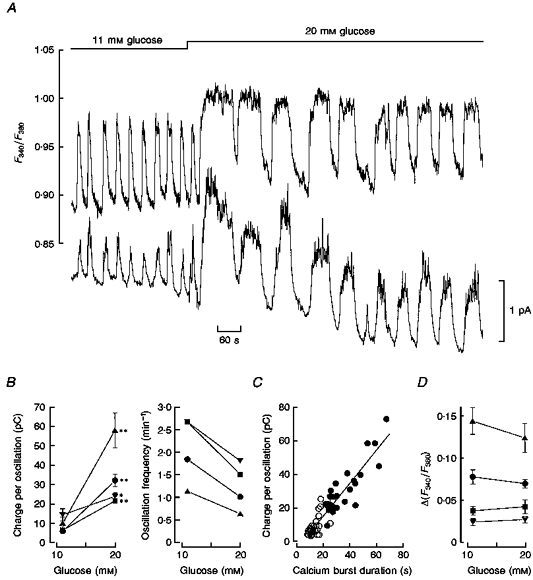
A, upper trace: continuous recording of [Ca2+]i (F340/F380 fluorescence ratio) from a single fura-2/5-HT-loaded islet; lower trace: continuous recording of 5-HT/insulin release (amperometric current) from the same islet. Glucose concentration was stepped from 11 to 20 mM (bar). B, analysis of the glucose dependence of pulsatile 5-HT/insulin release, in terms of the time integral of the current response for each cycle (in pC, left) and oscillation frequency (right). Both parameters were assessed at the steady state of 11 and 20 mM glucose (data from four experiments including that depicted in A, represented by circles). Each point represents the mean value ± s.e.m. (n = 6 cycles). Statistical significance of differences was assessed by Student's t test (* P < 0.05; ** P < 0.001). C, correlation between the integrated current response per cycle and the duration (time from half-rise to half-decay) of the underlying [Ca2+]i oscillation. Both parameters were assessed at the steady state of 11 and 20 mM glucose. Pooled non-normalized data from four experiments (open and filled symbols for 11 and 20 mM glucose, respectively). The straight line was drawn by least squares fitting to the whole data (slope, 0.97 pC s−1; correlation coefficient, 0.93). D, maximal amplitude of the [Ca2+]i oscillations as a function of glucose concentration. Δ (F340 /F380) was assessed at the steady state of 11 and 20 mM glucose (data from the same experiments used to generate the data in B). Each point represents the mean value ± s.e.m., for n = 6 cycles (the differences within each experiment were not statistically significant by Student's t test, P > 0.05).
At the steady state the amount of 5-HT released per secretion cycle is linearly related to the duration of the underlying calcium oscillation in the range 5-70 s (Fig. 2C), indicating that the readily releasable pool of insulin granules shows no sign of depletion within each cycle. It is noteworthy that the slopes of the straight lines relating charge per oscillation to calcium burst duration in 11 mM glucose (open symbols in Fig. 2C) and 20 mM glucose (filled symbols in Fig. 2C) were 0.93 and 0.95 pC s−1, respectively (correlation coefficients: 0.69 and 0.87). This suggests that there is no significant difference between the ability of calcium oscillations to elicit 5-HT/insulin release in 11 and 20 mM glucose.
The above analysis is representative of experiments where the [Ca2+]i oscillated at a regular pace in the presence of 11 mM glucose. In other experiments (18 %, i.e. 7 of 38), however, groups of high-frequency (fast) [Ca2+]i oscillations appeared superimposed on low-frequency (slow) [Ca2+]i waves (Fig. 3A, upper trace), as reported previously (Valdeolmillos, Santos, Contreras, Soria & Rosario, 1989; Bergsten, 1995). The frequency of these waves averaged 0.32 ± 0.16 min−1 (± s.d., n = 7; range 0.18-0.55 min−1). This complex oscillatory mode resembles the intermittent bursting pattern of electrical activity previously reported by Henquin, Meissner & Schmeer (1982), which we also found in some intracellular recording experiments (Fig. 3B). (The low-frequency waves of electrical activity are apparent from the estimation of average spike frequency, which is plotted against time underneath the membrane potential record in Fig. 3B. Analysis of this plot revealed a secondary oscillation frequency of 0.25 min−1 (0.36 ± 0.11 min−1, mean ± s.d., n = 5 similar experiments).) Figure 3A (middle trace) shows that fast [Ca2+]i oscillations triggered 5-HT release transients, the magnitude of which tended to rise towards the peak of each slow wave and to vanish at the respective troughs. Consequently, there was a slow regular variation in global 5-HT/insulin release (Fig. 3A, lower trace) bearing some resemblance to the previously reported slow pattern of pulsatile insulin release (Longo et al. 1991; Bergsten & Hellman, 1993).
Figure 3. Low-frequency variation in global 5-HT/insulin release and electrical activity.
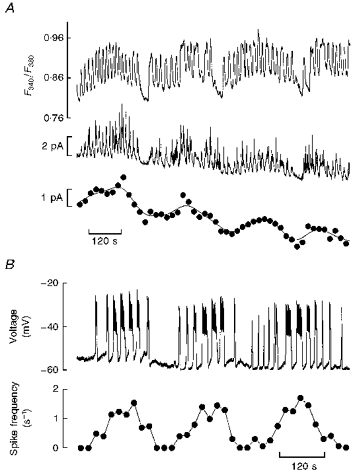
A, simultaneous [Ca2+]i (uppermost trace) and amperometric current recording (middle trace) from an islet displaying periodic low-frequency oscillations (11 mM glucose throughout). The lowest trace is a re-plot of the continuous amperometric recording, using 20 s averaging periods. The line through the points was generated by fast Fourier transform smoothing of the original amperometric data. Note the appearance of a low-frequency pattern of global 5-HT/insulin release. B, trace: continuous intracellular recording of membrane potential from a microdissected islet depicting an intermittent bursting pattern of electrical activity (11 mM glucose throughout); plot: average spike frequency vs. time for the experiment depicted in the upper trace. Spike frequency was calculated at 20 s intervals. The plot reveals a secondary oscillation frequency of 0.25 min−1. Representative of five similar experiments.
DISCUSSION
Islet insulin output follows a biphasic pattern in response to glucose, regardless of the animal species. In isolated mouse islets, the initial secretory response (first phase) has lag times in the range 2-3 min, lasts typically less than 3.5 min (approximately 1 min in single islets (Zaitsev, Efendic, Arkhammar, Bertorello & Berggren, 1995)) and is consistently larger than the insulin secretory rate measured at a later stage (second phase) (Bozem, Nenquin & Henquin, 1987; Zaitsev et al. 1995; Zawalich & Zawalich, 1996). The initial 5-HT release response that we have obtained following islet stimulation with 11 mM glucose seems to be representative of the first phase of glucose-induced insulin secretion, inasmuch as both events have comparable lag times and durations (for 5-HT release approximately 2.7 and 0.9 min, respectively). In contrast, the oscillatory 5-HT output that we have recorded at the steady state of glucose stimulation appears to underestimate the secretion second phase: the ratio of the insulin secretory rates measured over the first and second phases is typically in the range 2-5 (Bozem et al. 1987; Zawalich & Zawalich, 1996), i.e. much lower than the R1/R2 ratio (average 9.5), which we have computed from the 5-HT experiments. This is consistent with the possibility that the secretion second phase might have components with various frequency characteristics (e.g. high-frequency secretory events associated with bursting electrical activity and slower, as yet unidentified events), with the the slower components remaining underestimated by our system. Indeed, our 5-HT detection system may be viewed as a high-pass filter, allowing the monitoring of high-frequency pulsatile events but limiting the detection of putative slow secretory components (Barbosa et al. 1996b). One of the latter components may be the low-frequency insulin oscillations (Longo et al. 1991; Bergsten & Hellman, 1993) that have been hypothesized by some authors (Longo et al. 1991) to arise from oscillations in glucose metabolism. It is also noteworthy that inter-burst [Ca2+]i is consistently higher than in the resting state (Fig. 1A). These increased Ca2+ levels may support a steady, non-oscillatory insulin output that may account for part of the secretion second phase.
When measured at the whole-islet level, the fluorescence and amperometric current signals are likely to arise from different islet regions, as previously suggested (Barbosa et al. 1996b). The extent of cell-to-cell coupling within the islet increases with glucose concentration (Meda, Perrelet & Orci, 1979; Kohen, Kohen & Rabinovitch, 1983; Eddlestone, Gonçalves, Bangham & Rojas, 1984) and it is therefore plausible that there may be phase lags between both signals during a transition from low to high glucose. This was indeed observed in several experiments (e.g. that depicted in Fig. 1A). In contrast, the spontaneous [Ca2+]i oscillations recorded at the steady-state of 11 or 20 mM glucose were synchronous with the 5-HT fluctuations. This reinforces the view that native β-cells are tightly coupled in the presence of stimulatory glucose levels (Santos et al. 1991) and indicates that the observed calcium signal is representive of the signalling properties of β-cells contributing to the 5-HT fluctuations.
We note that the maximal amplitude of the [Ca2+]i oscillations did not change with glucose concentration in the range 11-20 mM (Fig. 2D), implying that there was no correlation between the amount of 5-HT released per secretion cycle and this amplitude (cf. Fig. 2B and D). This probably reflects the fact that instantaneous spike frequency (i.e. the frequency of action potentials at the onset of a burst of electrical activity) does not increase with glucose concentration (R. M. Santos & L. M. Rosário, unpublished observations), in contrast with average spike frequency, which rises sigmoidally with glucose concentration mainly as a consequence of a dose-dependent increase in burst duration (Scott et al. 1981).
We found that the amount of 5-HT released per secretion cycle increased with the duration of the underlying [Ca2+]i oscillation. This is expected because the latter reflects active spiking (Santos et al. 1991) and this, in turn, supports exocytosis in β-cells (Ämmälä, Eliasson, Bokvist, Larsson, Ashcroft & Rorsman, 1993). Moreover, the extent of the Ca2+-dependent reactions involved in exocytosis is likely to increase with the duration of the calcium signal. More surprising, however, was the observation that there was seemingly no difference between the effectiveness of calcium oscillations to elicit 5-HT/insulin release at the steady-state of 11 and 20 mM glucose. Indeed, there is growing evidence that glucose metabolism exerts stimulatory actions on β-cells beyond depolarization and the associated [Ca2+]i rises, suggesting that Ca2+-induced exocytosis is dependent upon the availability of one or several metabolites (e.g. cytoplasmic ATP) (Gembal, Detimary, Gilon, Gao & Henquin, 1993). Two possibilities may be envisaged to explain the apparent discrepancy between the latter studies and our data. First, cytoplasmic levels of the putative metabolite may already be saturated at 11 mM glucose. Although this seems to be essentially the case for ATP, it should be born in mind that the cytoplasmic ATP/ADP ratio increases somewhat between 11 and 20 mM glucose (Detimary, Jonas & Henquin, 1995) and that the phosphorylation potential of the β-cell appears to be the relevant factor for ‘membrane potential-independent’ exocytosis (Gembal et al. 1993). Secondly, the slow component of secretion that may escape our detection procedure may be more critically dependent on regulation by glucose metabolites than the high-frequency pulsatile component. It is noteworthy that whereas the mobilization of granules from the reserve pool into the readily releasable pool is an ATP-dependent process, the exocytotic process itself appears largely ATP independent (Parsons, Coorssen, Horstmann & Almers, 1995; Rorsman, 1997). It therefore seems possible that the processes investigated in this study (high-frequency pulsatile insulin release) reflect principally the distal processes that are insensitive to changes in cytoplasmic purine nucleotide levels.
In addition to fast regular oscillations, we have also demonstrated the appearance of a secondary oscillator in 18 % of the experiments, leading to regular low-frequency variations in 5-HT/insulin release that can be detected when 5-HT release is integrated over 20 s bins. The frequency of the latter (average, 0.32 min−1) is similar to that of the intermittent bursting pattern of electrical activity (average, 0.36 min−1), which we have found in separate intracellular recording experiments and which resembles the irregular bursting pattern previously reported by other authors (Henquin et al. 1982). This strongly suggests that intermittent bursting is the immediate cause of the slow variations in global 5-HT/insulin reported in this study, although it should be emphasized that the ultimate nature of the secondary oscillator remains unknown. One possibility is that oscillatory glycolysis (Longo et al. 1991) might cause regular variations in the activity of ATP-dependent K+ channels (Dryselius, Lund, Gylfe & Hellman, 1994) and/or in the phosphorylation state of voltage-sensitive Ca2+ channels, leading to intermittent bursting. Alternatively, intermittent bursting may be looked upon as being reminiscent of the situation found in vivo, where this bursting mode might become preponderant owing to the regulation of β-cell ion channels by the intrapancreatic hormonal and nervous systems. Significantly, slow insulin pulsatility has been reported to occur at various levels of complexity, ranging from single isolated islets to the intact organism (i.e. circulating plasma) (Bergsten, 1995; Stagner, 1991). Moreover, the frequency of these oscillations seems to decrease as a function of the level of complexity (Bergsten, 1995) and, when measured from single islets or even batches of isolated islets, becomes notably similar to that of the slow 5-HT/insulin oscillatory pattern reported in our study. It is therefore plausible that this pattern might account, at least in part, for the slow variations of insulin output in vivo.
Acknowledgments
We thank the Calouste Gulbenkian Foundation, JNICT-Praxis XXI, the British Council and EEC Science Programme for financial support.
References
- Alvarezde Toledo G, Fernandez-Chacon R, Fernandez JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. doi: 10.1038/363554a0. 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Ämmälä C, Eliasson L, Bokvist K, Larsson O, Ashcroft FM, Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic β-cells. The Journal of Physiology. 1993;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa RM, Rosário LM, Brett CMA, Oliveira-Brett AM. Electrochemical studies of zinc in zinc-insulin solution. Analyst. 1996a;121:1789–1793. doi: 10.1039/an9962101789. [DOI] [PubMed] [Google Scholar]
- Barbosa RM, Silva AM, Tomé AR, Stamford JA, Santos RM, Rosário LM. Real time electrochemical detection of 5-HT/insulin secretion from single pancreatic islets: effect of glucose and K+ depolarization. Biochemical and Biophysical Research Communications. 1996b;228:100–104. doi: 10.1006/bbrc.1996.1622. [DOI] [PubMed] [Google Scholar]
- Bergsten P. Slow and fast oscillations of cytoplasmic Ca2+ in pancreatic islets correspond to pulsatile insulin release. American Journal of Physiology. 1995;268:E282–287. doi: 10.1152/ajpendo.1995.268.2.E282. [DOI] [PubMed] [Google Scholar]
- Bergsten P, Hellman B. Glucose-induced amplitude regulation of pulsatile insulin secretion from individual pancreatic islets. Diabetes. 1993;42:670–674. doi: 10.2337/diab.42.5.670. [DOI] [PubMed] [Google Scholar]
- Bozem M, Nenquin M, Henquin J-C. The ionic, electrical, and secretory effects of protein kinase C activation in mouse pancreatic β-cells: studies with a phorbol ester. Endocrinology. 1987;121:1025–1033. doi: 10.1210/endo-121-3-1025. [DOI] [PubMed] [Google Scholar]
- Chow RH, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Detimary P, Jonas J-C, Henquin J-C. Possible links between glucose-induced changes in the energy state of pancreatic β-cells and insulin release. Journal of Clinical Investigation. 1995;96:1738–1745. doi: 10.1172/JCI118219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryselius S, Lund PE, Gylfe E, Hellman B. Variations in ATP-sensitive K+ channel activity provide evidence for inherent metabolic oscillations in pancreatic β-cells. Biochemical and Biophysical Research Communications. 1994;205:880–885. doi: 10.1006/bbrc.1994.2746. 10.1006/bbrc.1994.2746. [DOI] [PubMed] [Google Scholar]
- Eddlestone GT, Gonçalves A, Bangham JA, Rojas E. Electrical coupling between cells in islets of Langerhans from mouse. Journal of Membrane Biology. 1984;77:1–14. doi: 10.1007/BF01871095. [DOI] [PubMed] [Google Scholar]
- Gembal M, Detimary P, Gilon P, Gao Z-Y, Henquin J-C. Mechanisms by which glucose can control insulin release independently from its action on adenosine triphosphate-sensitive K+ channels in mouse β-cells. Journal of Clinical Investigation. 1993;91:871–880. doi: 10.1172/JCI116308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilon P, Shepherd RM, Henquin J-C. Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidenced in single pancreatic islets. Journal of Biological Chemistry. 1993;268:22265–22268. [PubMed] [Google Scholar]
- Gylfe E. Association between 5-hydroxytryptamine release and insulin secretion. Journal of Endocrinology. 1978;78:239–248. doi: 10.1677/joe.0.0780239. [DOI] [PubMed] [Google Scholar]
- Gylfe E. Nutrient secretagogues induce bimodal early changes in cytoplasmic calcium of insulin-releasing ob/ob mouse β-cells. Journal of Biological Chemistry. 1988;263:13750–13754. [PubMed] [Google Scholar]
- Henquin J-C, Meissner HP, Schmeer W. Cyclic variations of glucose-induced electrical activity in pancreatic β-cells. Pflügers Archiv. 1982;393:322–327. doi: 10.1007/BF00581418. [DOI] [PubMed] [Google Scholar]
- Huang L, Shen H, Atkinson MA, Kennedy RT. Detection of exocytosis at individual pancreatic β-cells by amperometry at a chemically modified microelectrode. Proceedings of the National Academy of Sciences of the USA. 1995;92:9608–9612. doi: 10.1073/pnas.92.21.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BP, Dietz SM, Wightman RM. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Analytical Chemistry. 1995;67:1115–1120. doi: 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- Kohen E, Kohen C, Rabinovitch A. Cell-to-cell communication in rat pancreatic islet monolayer cultures is modulated by agents affecting islet-cell secretory activity. Diabetes. 1983;32:95–98. doi: 10.2337/diab.32.1.95. [DOI] [PubMed] [Google Scholar]
- Longo EA, Tornheim K, Deeney JT, Varnum BA, Tillotson D, Prentki M, Corkey BE. Oscillations in cytosolic free Ca2+, oxygen consumption, and insulin secretion in glucose-stimulated rat pancreatic islets. Journal of Biological Chemistry. 1991;266:9314–9319. [PubMed] [Google Scholar]
- Meda P, Perrelet A, Orci L. Increase of gap junctions between pancreatic β-cells during stimulation of insulin secretion. Journal of Cell Biology. 1979;82:441–448. doi: 10.1083/jcb.82.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Coorssen JR, Horstmann H, Almers W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Porte D, Jr, Kahn SE. The key role of islet dysfunction in type II diabetes mellitus. Clinical Investigations in Medicine. 1995;18:247–254. [PubMed] [Google Scholar]
- Rorsman P. The pancreatic β-cell as a fuel sensor: an electrophysiologist's viewpoint. Diabetologia. 1997;40:487–495. doi: 10.1007/s001250050706. [DOI] [PubMed] [Google Scholar]
- Rosario LM, Atwater I, Scott AM. Pulsatile insulin release and electrical activity from single ob/ob mouse islets of Langerhans. Advances in Experimental Medicine and Biology. 1986;211:413–425. doi: 10.1007/978-1-4684-5314-0_40. [DOI] [PubMed] [Google Scholar]
- Rosário LM, Barbosa RM, Antunes CM, Silva AM, Abrunhosa AJ, Santos RM. Bursting electrical activity in pancreatic β-cells: evidence that the channel underlying the burst is sensitive to Ca2+ influx through L-type Ca2+ channels. Pflügers Archiv. 1993;424:439–447. doi: 10.1007/BF00374906. [DOI] [PubMed] [Google Scholar]
- Salgado AP, Silva AM, Santos RM, Rosário LM. Multiphasic action of glucose and α-ketoisocaproic acid on cytosolic pH of pancreatic β-cells. Evidence for an acidification pathway linked to the stimulation of Ca2+ influx. Journal of Biological Chemistry. 1996;271:8738–8746. doi: 10.1074/jbc.271.15.8738. [DOI] [PubMed] [Google Scholar]
- Santos RM, Rosário LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflügers Archiv. 1991;418:417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- Scott AM, Atwater I, Rojas E. A method for the simultaneous measurement of insulin release and β-cell membrane potential in single mouse islets of Langerhans. Diabetologia. 1981;21:470–475. doi: 10.1007/BF00257788. [DOI] [PubMed] [Google Scholar]
- Silva AM, Rosário LM, Santos RM. Background Ca2+ influx mediated by a dihydropyridine- and voltage-insensitive channel in pancreatic β-cells. Modulation by Ni2+, diphenylamine-2-carboxylate, and glucose metabolism. Journal of Biological Chemistry. 1994;269:17095–17103. [PubMed] [Google Scholar]
- Smith PA, Duchen MR, Ashcroft FM. A fluorimetric and amperometric study of calcium and secretion in isolated mouse pancreatic β-cells. Pflügers Archiv. 1995;430:808–818. doi: 10.1007/BF00386180. [DOI] [PubMed] [Google Scholar]
- Stagner JI. Pulsatile secretion from the endocrine pancreas. Metabolic, hormonal, and neural modulation. In: Samols E, editor. The Endocrine Pancreas. New York: Raven Press; 1991. pp. 283–302. [Google Scholar]
- Stamford JA, Palij P, Davidson C, Jorm CM, Phillips PEM. Fast cyclic voltammetry in brain slices. In: Boulton A, Baker G, Adams RN, editors. Neuromethods Voltammetric Methods in Brain Systems. Vol. 27. Totawa, NJ, USA: Humana Press Inc.; 1995. pp. 81–116. [Google Scholar]
- Valdeolmillos M, Santos RM, Contreras D, Soria B, Rosario LM. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of Langerhans. FEBS Letters. 1989;259:19–23. doi: 10.1016/0014-5793(89)81484-x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proceedings of the National Academy of Sciences of the USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley JF, McIntyre MS, Spencer B, Mertz RJ, Roe MW, Dukes ID. Endoplasmic reticulum calcium store regulates membrane potential in mouse islet β-cells. Journal of Biological Chemistry. 1994;269:14359–14362. [PubMed] [Google Scholar]
- Zaitsev SV, Efendic S, Arkhammar P, Bertorello AM, Berggren P-O. Dissociation between changes in cytoplasmic free Ca2+ concentration and insulin secretion as evidenced from measurements in mouse single pancreatic islets. Proceedings of the National Academy of Sciences of the USA. 1995;92:9712–9716. doi: 10.1073/pnas.92.21.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawalich WS, Zawalich KC. Regulation of insulin secretion by phospholipase C. American Journal of Physiology. 1996;271:E409–416. doi: 10.1152/ajpendo.1996.271.3.E409. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Misler S. Amperometric detection of quantal secretion from patch-clamped rat pancreatic β-cells. Journal of Biological Chemistry. 1996;271:270–277. doi: 10.1074/jbc.271.1.270. [DOI] [PubMed] [Google Scholar]


