Abstract
The ability of the primary motor cortex (M1) to modulate motor responses in ipsilateral hand muscles seems to be important for normal motor control and potentially also for recovery after brain lesions. It is not clear which pathways mediate this ipsilateral modulation. Transcallosal connections have been proposed, but are known to be sparse between cortical hand motor representations in primates. The present study was performed to determine whether descending ipsilateral modulation of motor responses might also be mediated below the cortical level in humans.
A paired-pulse protocol was used, in which motor-evoked potentials (MEPs) were produced by cortical transcranial magnetic stimulation (cTMS) or by electrical stimulation of the pyramidal tract at the level of the pyramidal decussation (pdTES), in both preactivated and relaxed hand muscles. Paired stimuli were applied at various interstimulus intervals (ISIs) between 2 and 100 ms. The conditioning stimulus (CS) was always magnetic, and delivered to the M1 ipsilateral to the target hand, prior to the test stimulus (TS). The magnetic TS was delivered to the M1 contralateral to the target hand; the electrical TS was applied through electrodes placed over the mastoid process bilaterally. Further experiments included cortical electrical stimulation and H-reflexes. The MEP amplitudes were averaged separately for each ISI and the control condition (no CS), and expressed as a percentage of the unconditioned response.
Conditioning stimulation of the ipsilateral M1 resulted in significant inhibition of magnetically evoked MEPs, and also of MEPs produced by pdTES. Inhibition occurred at ISIs between 6 and 50 ms, and was observed in preactivated and relaxed muscles. Higher CS intensities caused greater inhibition of both cTMS- and pdTES-evoked MEPs.
While the conditioning effects on magnetically evoked muscle responses could be explained by a transcallosal mechanism, the effects on pdTES-evoked MEPs cannot, because they are elicited subcortically and are therefore not susceptible to inhibitory mechanisms transmitted at the cortico-cortical level.
In conclusion, the present results provide novel evidence that the inhibitory influence of the human M1 on ipsilateral hand muscles is to a significant extent mediated below the cortical level, and not only through cortico-cortical transcallosal connections. They point to a concept of inhibitory interaction between the two primary motor cortices that is relayed at multiple levels along the neuroaxis, thus perhaps providing a structurally redundant system which may become important in case of lesions.
The primary motor cortex (M1) is capable of exerting control over motor responses not only in the contralateral arm, but also in the ipsilateral arm (Colebatch & Gandevia, 1989; Jones, Donaldson & Parkin, 1989; Wassermann, Fuhr, Cohen & Hallett, 1991; Chiappa, Cros, Kiers, Triggs, Clouston & Fang, 1995). In humans, this descending ipsilateral motor control seems to be functionally relevant under physiological as well as pathophysiological conditions. It has been suggested, for example, that after a unilateral cerebral stroke the unaffected M1 ipsilateral to the paralytic limb may take over functions for the damaged M1 (Frackowiak, Weiller & Chollet, 1991; Miller-Fisher, 1992). Further, it has been speculated that a decrease in inhibition between the M1 of both hemispheres may cause mirror movements (Schott & Wyke, 1981). It is not clear, however, where along the neuroaxis this descending modulatory influence of the M1 on muscles of the ipsilateral limb is relayed. A transcallosal route has been proposed (Ferbert, Priori, Rothwell, Day, Colebatch & Marsden, 1992; Meyer, Roricht, Graefin von Einsiedel, Kruggel & Weindl, 1995; Boroojerdi, Diefenbach & Ferbert, 1996), but transcallosal connections between cortical hand motor representations in primates are sparse (Gould, Cusick, Pons & Kaas, 1986; Rouiller, Babalian, Kazennikov, Moret, Yu & Wiesendanger, 1994) and, from an anatomical point of view, various other pathways such as the reticulospinal tract (Nathan, Smith & Deacon, 1996) or spinal interneuron circuits (Shahani & Young, 1971; Roby-Brami & Bussel, 1987; Burke, Gracies, Mazevet, Meunier & Pierrot-Deseilligny, 1992; Gracies, Meunier & Pierrot-Deseilligny, 1994; Mazevet, Pierrot-Deseilligny & Rothwell, 1996) could also mediate ipsilateral effects, because they are, at least in part, bilaterally organized. Therefore, we hypothesized that the descending modulatory influence of the human M1 on muscles of the ipsilateral limb may be relayed not only through the corpus callosum but also through subcortical routes.
Cortical transcranial magnetic stimulation (cTMS) in humans has shown that the influence of the M1 on ipsilateral limb muscle activation is mostly inhibitory (Ferbert et al. 1992; Meyer et al. 1995; Brown, Ridding, Werhahn, Rothwell & Marsden, 1996). A conditioning stimulus (CS) applied over the M1 of one hemisphere induces an amplitude decrease of motor-evoked potentials (MEPs) elicited by a subsequent test stimulus (TS) over the homologous M1 of the other hemisphere (Ferbert et al. 1992). It was proposed that this particular effect might be mediated through a cortico-cortical route via the corpus callosum. If this effect is entirely transmitted through the corpus callosum, then MEPs elicited by non-cortical, direct stimulation of the pyramidal tract should not be affected by a conditioning shock applied over the homologous M1, since this type of stimulation would bypass modulatory cortico-cortical influences. Cortical TMS (cTMS) is thought to stimulate pyramidal cells largely trans-synaptically, thereby producing predominantly indirect corticospinal volleys (I-waves). These are subject to modulations of cortical excitability, including possible cortico-cortical inhibitory interactions via the corpus callosum. In contrast, transcranial electrical stimulation at the level of the pyramidal decussation (pdTES) can produce corticospinal volleys that are clearly not modulated by different levels in cortical excitability (Ugawa, Rothwell, Day, Thompson & Marsden, 1991). Cortical transcranial electrical stimulation (cTES) can produce both D-waves and I-waves, depending on the stimulus intensities applied (Rothwell, Burke, Hicks, Stephen, Woodforth & Crawford, 1994). A systematic combination of magnetic and electrical stimuli can therefore be used to differentiate where modulatory effects may be relayed (cortical or subcortical).
In the present study, we investigated the effects of magnetic cortical conditioning stimulation, applied to the M1 ipsilateral to a target muscle in the upper limb, on MEPs elicited by cTMS and pdTES, in both relaxed and preactivated muscles; further experiments included cTES and monosynaptic H-reflexes.
METHODS
Subjects
We studied sixteen normal volunteers (11 men and 5 women) whose mean (± s.d.) age was 36.2 ± 14.2 years. Fourteen subjects were right handed, according to the Edinburgh handedness inventory, and two were ambidextrous. The study protocol was approved by the Institutional Review Board, and all subjects gave their written informed consent for the study.
Stimulation technique
All stimulation parameters, as well as motor threshold values, and unconditioned control amplitudes are listed in Table 1 (relaxed target muscles) and Table 2 (preactivated target muscles). All values are presented as means ± 1 s.d.
Table 1.
Experimental series 1 with muscles at rest: stimulation parameters, motor thresholds and control values
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | ||||
|---|---|---|---|---|---|---|---|
| Mode of stimulation CS: | cTMS | cTMS | cTMS | cTMS | cTMS | cTMS | cTMS |
| Mode of stimulation TS: | cTMS | cTMS | pdTES | cTMS | cTES | cTMS | H-reflex |
| Test stimulation | |||||||
| Motor threshold (right FDI) (% stimulator output) | 68 ± 10 | 69 ± 9 | 60 ± 14 | 63 ± 12 | 44 ± 7 | 64 ± 13 | 5.76 ± 2.39 mA |
| TS intensity (% MT) | 124 ± 10 | 123 ± 13 | 120 ± 0 | 131 ± 12 | 122 ± 4 | 135 ± 4 | 113 ± 4 |
| (504 ± 127) * | (404 ± 52) * | ||||||
| Control response amplitude (no CS) (mV) | 1.23 ± 0.89 | 1.88 ± 0.39 | 0.67 ± 0.22 | 1.64 ± 0.89 | 1.27 ± 0.35 | 0.75 ± 0.19 | 0.43 ± 0.19 |
| Conditioning stimulation | |||||||
| Motor threshold (left FDI) (% stimulator output) | 69 ± 9 | 66 ± 5 | 66 ± 5 | 63 ± 6 | 63 ± 6 | 65 ± 12 | 65 ± 12 |
| CS intensity (%MT) | 127 ± 14 | 143 ± 6 | 143 ± 6 | 142 ± 16 | 142 ± 16 | 143 ± 16 | 143 ± 16 |
| CS-induced MEP amplitude (mV) | 1.60 ± 0.87 | 2.04 ± 0.57 | 2.35 ± 0.72 | 2.20 ± 1.12 | 2.56 ± 0.96 | 1.18 ± 0.68 | 1.33 ± 0.74 |
| Interstimulus intervals | 2, 5, 10, 20, 30, 40, 50, 70, 100 | 10, 20 | 10, 20, 40, 70, 100 | 10, 40, 100 | |||
Values are means ± S.D.
Absolute output of electrical stimulator in volts.
Table 2.
Experimental series 2 with preactivated muscles: stimulation parameters, motor thresholds and control values
| Randomized presentation of magnetic and electrical test stimuli | ||
|---|---|---|
| Mode of stimulation CS: | cTMS | cTMS |
| Mode of stimulation TS: | cTMS | pdTES |
| Test stimulation | ||
| Active motor threshold (aMT) | 51 ± 6 | 42 ± 9 |
| Relaxed motor threshold (rMT) (right FDI) (% stimulator output) | 58 ± 6 | 49 ± 8 |
| TS intensity | ||
| (% aMT) | 120 ± 7 | 116 ± 7 |
| (% rMT) | 105 ± 5 | 99 ± 8 |
| (366 ± 83) * | ||
| Control response amplitude (no CS) (mV) | 2.46 ± 0.77 | 1.51 ± 1.08 |
| Conditioning stimulation | ||
| Active motor threshold (aMT) | 44 ± 8 | 44 ± 8 |
| Relaxed motor threshold (rMT) (left FDI) (% stimulator output) | 52 ± 6 | 52 ± 6 |
| CS intensity | ||
| (% aMT) | 137 ± 8 | 137 ± 8 |
| (% rMT) | 116 ± 14 | 116 ± 14 |
| CS-induced MEP amplitude (mV) | 2.81 ± 0.65 | 3.06 ± 0.62 |
| Interstimulus intervals (ms) | 6, 7, 10, 20 | 10, 20 |
Values are means ±s.d.
Absolute output of electrical stimulator in volts.
Magnetic stimulation
For paired-pulse cTMS, we used two magnetic stimulators (Cadwell Laboratories Inc., Kennewick, WA, USA) with figure of 8-shaped coils, the characteristics of which are described elsewhere (Cohen et al. 1990). Each loop of the coils measured 4.5 cm in diameter. With each coil, the intersection of the loops was placed perpendicular to the expected orientation of the central sulcus of the respective hemisphere, tangentially to the scalp surface, and then adjusted until an MEP of maximal amplitude was produced in the relaxed target muscle, at a given stimulus intensity. At the optimal orientation, the coil handle usually pointed posteriorly with an angle of 45-80 deg to the sagittal axis. Stimulus intensity was expressed as a percentage of the motor threshold of the target muscle. Motor threshold was defined as the minimal output of the stimulator capable of inducing five MEPs in the target muscle with an amplitude of ≥ 50 μV in ten trials with single stimuli applied to the optimal scalp position for activation of this muscle. This was done at rest (the relaxed motor threshold, rMT) or with the target muscle preactivated at about 5 % maximum (the active motor threshold, aMT; cf. Ferbert et al. 1992). For conditioning and test stimulation, we used intensities that were likely to produce test responses comparable to the ones reported by Ferbert et al. (1992). In relaxed muscles, we aimed at inducing stable unconditioned test MEPs of approximately 1.5 mV peak-to-peak amplitude with cTMS and cTES, and in preactivated muscle MEPs of approximately 2.5 mV peak-to-peak amplitude (only cTMS). For the CS, the intensity chosen was slightly higher, since the inhibitory effects are more pronounced as the CS intensity increases (Ferbert et al. 1992). With electrical stimulation at the level of the foramen magnum, the maximal MEP amplitudes that could be obtained at a tolerable level of discomfort and with no significant background EMG activity were of the order of 0.5-1.0 mV in relaxed muscles, and approximately 1.5 mV in preactivated muscles.
Electrical stimulation
Brainstem and cortical TES
A Digitimer D180 electrical stimulator (maximal output, 750 V; time constant, 100 μs; Digitimer, Welwyn, Garden City, UK) was used for pdTES and cTES. For pdTES, the electrodes were placed over the mastoid process bilaterally, approximately 5 cm lateral to the inion (Ugawa et al. 1991), and the polarity was chosen so that the response was larger in the right than in the left target muscle. Stimulus intensity was then increased stepwise until stable MEP responses could be elicited in at least five subsequent trials. This intensity was considered to be the motor threshold. The slightly different approach in pdTES, compared with cTMS and cTES, was chosen in order to minimize the number of stimuli applied, since pdTES is generally associated with more discomfort owing to neck muscle contraction. For cTES, the anode (cup-shaped gold electrode) was placed over the M1 contralateral to the target muscle (7 cm lateral to the Cz position of the international 10/20 system of electrode placement), and the cathode over position Fz. Thresholds and stimulus intensities were determined as described for cTMS.
H-reflex
The stimulator module of a Dantec Counterpoint electromyograph (Dantec Medical A/S, Skovlunde, Denmark) was used to apply a single-pulse stimulus to the median nerve at the elbow for eliciting an H-reflex in the flexor carpi radialis (FCR) muscle.
Recording technique and data pre-processing
Bipolar EMG was recorded from the first dorsal interosseous (FDI) or FCR muscle (H-reflex experiment) bilaterally. For FDI recordings, the active electrode was placed over the muscle belly and the reference electrode over the radial styloid process (approximately 5 cm interelectrode distance). For FCR recordings, the active electrode was placed over the muscle belly, approximately 6 cm distal to the elbow, and the reference electrode was positioned 5 cm distal and radial to the active one. The EMG was sampled at 5 kHz, band-pass filtered from 5 Hz to 1.5 kHz (Dantec Counterpoint electromyograph), and stored in a personal computer (IBM compatible) for off-line analysis.
To assess the conditioning effects, peak-to-peak amplitudes were measured, and averages across trials were computed for each experiment and normalized as described below. In addition, the MEP latencies were determined for cTMS, pdTES and cTES experiments.
Experimental protocol
Two series of experiments were carried out. In the first series (Table 1), the effect of conditioning stimulation on responses elicited by cTMS, pdTES or cTES, or on H-reflexes was explored in relaxed muscles. In the second series (Table 2), the conditioning effect on responses evoked by cTMS and pdTES test stimuli was studied in preactivated muscles.
For all experiments, subjects were seated comfortably in an armchair with both arms resting on a pillow. The CS was always magnetic (cTMS) and always applied to the right M1, ipsilateral to the relaxed target muscle. The interstimulus intervals (ISIs) between CS and TS were presented in a random fashion, interleaved with control trials (without CS). In experimental series 1 magnetic and electrical test stimuli were presented blockwise within the same session; in series 2 cTMS and pdTES stimuli were presented randomly within the same run of trials. Accurate triggering of CS and TS was achieved with a personal computer and a custom-made software module based on ASYST (version 4.0, Keithley Instruments, Inc., Cleveland, OH, USA). The pairs of stimuli were applied once every 4-10 s for magnetic and electrical transcranial stimulation, and every 10-20 s for the H-reflex experiments. Short breaks between stimulations were allowed as necessary to assure that the subject stayed alert and relaxed (series 1) or could maintain the required preactivation (series 2).
Series 1 (conditioning effects on MEPs in relaxed muscles)
Experiment 1 (cTMS)
Nine subjects participated in experiment 1. The TS was magnetic and applied to the left M1; the CS was also magnetic and applied to the right M1. ISIs between CS and TS were 2, 5, 10, 20, 30, 40, 50, 70 and 100 ms. EMG was recorded from FDI bilaterally (right FDI was the target muscle). Ten MEP peak-to-peak amplitudes per ISI were averaged, and for each ISI the mean MEP amplitude was expressed as a percentage of unconditioned control (100 %).
Experiment 2 (pdTES)
Nine subjects participated in experiment 2. Five subjects could not tolerate pdTES, or could not relax sufficiently during the pre-stimulus period. The final analysis was therefore based on four subjects. The ISIs were 10 and 20 ms. EMG was recorded from FDI bilaterally (right FDI was the target muscle). Five MEP peak-to-peak amplitudes per ISI were averaged. Experiment 1 was repeated for the ISIs of 10 and 20 ms within the same session.
Experiment 3 (cTES)
Six subjects participated in experiment 3. One subject had to be excluded because of failure to relax. The ISIs were 10, 20, 40, 70 and 100 ms. EMG was recorded from FDI bilaterally (right FDI was the target muscle). Five MEP peak-to-peak amplitudes per ISI were averaged. Experiment 1 was repeated for the ISIs of 10, 20, 40, 70 and 100 ms within the same session.
Experiment 4 (H-reflex)
Five subjects participated in experiment 4. In one subject, a stable H-reflex could not be obtained. To elicit an H-reflex in the FCR muscle, the right median nerve was stimulated at the elbow using a standard bar electrode. Stimulus duration was 0.7 ms. The ISIs were 10, 40 and 100 ms. EMG was recorded from FCR bilaterally (right FCR was the target muscle). Twenty H-reflex peak-to-peak amplitudes per ISI were averaged. The FCR muscle was selected because it was not possible to elicit a stable H-reflex in the FDI in a sufficient number of subjects. To ensure that the FCR behaviour was comparable with the behaviour of the FDI in the magnetic-magnetic paired-pulse protocol, we repeated experiment 1 for the ISIs of 10, 40 and 100 ms using the FCR as the target muscle in this session.
Series 2 (conditioning effects on MEPs in preactivated muscles)
Test stimulation: cTMS (cf. series 1, experiment 1) and pdTES (cf. series 1, experiment 2) randomized
Eight subjects participated in series 2. Two subjects could not tolerate pdTES and therefore could not complete the study. In four subjects, we focused on the randomized presentation of cTMS and pdTES test stimuli. For this part, the ISIs were 6, 7, 10 and 20 ms for cTMS, and 10 and 20 ms for pdTES. A direct comparison of ISIs of 6 and 7 ms with cTMS and 10 ms with pdTES was desired in order to match the response latencies when taking into account the differences in central conduction time for the two stimulus modalities (pdTES 3-4 ms shorter than cTMS). In another subgroup of four subjects, we assessed the effects of different stimulus intensities on pdTES-evoked responses. This was done for ISIs of 10 ms, since inhibition was generally most pronounced at that latency. EMG was recorded from preactivated FDI bilaterally (right FDI was the target muscle). Five MEP peak-to-peak amplitudes per ISI were averaged for pdTES, ten for cTMS. The different number of trials averaged for pdTES and cTMS was associated with comparable variances across conditions (s.d., 0.67 mV for all cTMS-evoked responses pooled, 0.61 for all pdTES test shock responses pooled).
Statistical analyses
All statistical tests were computed on normalized (percentage) data to account for intersubject variability of absolute MEP or H-reflex amplitudes. Effects were considered significant if P < 0.05 after appropriate correction for multiple comparisons.
A factorial one-way ANOVA (main effect for INTERVAL) and Bonferroni-corrected post hoc comparisons were used to determine significant inhibition of the TS-evoked amplitudes (MEP, H-reflex) for the different ISIs in experimental series 1 and 2.
Linear regression analysis was used in series 2 to determine the effect of changing the CS intensity on the amount of inhibition of the test response elicited by pdTES.
RESULTS
Series 1 (experiments in relaxed muscles)
Effects of ipsilateral conditioning stimulation on cTMS-evoked responses in the relaxed FDI (experiment 1)
Conditioning of cTMS-evoked test MEPs in the relaxed right FDI resulted in significant inhibition (P < 0.0001; ANOVA main effect for INTERVAL) at ISIs of 10, 20, 30, 40 and 50 ms (P < 0.05,post hoc analysis). Maximal inhibition was observed at the ISI of 10 ms (MEP reduced to 37.7 ± 16.2 % of the unconditioned MEP). There was a trend toward facilitation of the MEP at the ISI of 2 ms, which, however, was very variable across subjects, and did not reach significance in the group statistics (P = 0.13,post hoc analysis). The amplitudes of the unconditioned MEPs are given in Table 1. Figure 1 shows the magnitude of inhibition as a function of the ISI. The mean latency was 23 ± 2 ms for the unconditioned MEP, and did not change significantly across ISIs when the CS was applied.
Figure 1. Inhibition of MEPs produced by cTMS in the relaxed FDI muscle (series 1, experiment 1).
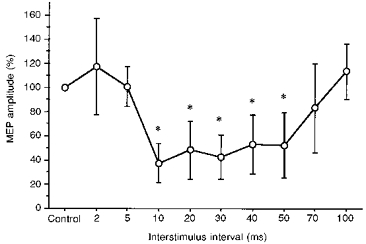
CS, cTMS; TS, cTMS. Error bars indicate ± 1 s.d. Asterisks indicate significant MEP inhibition compared with the unconditioned control MEP (Control, 100 %; P < 0.05, ANOVA; post hoc analysis).
Effects of ipsilateral conditioning stimulation on MEPs evoked by pdTES in the relaxed FDI (experiment 2)
Conditioning of test MEPs evoked in the relaxed FDI by pdTES resulted in significant inhibition (P < 0.0002; ANOVA main effect for INTERVAL) at ISIs of 10 ms (39.0 ± 16.1 % of control) and 20 ms (38.3 ± 11.9 % of control) (P < 0.05,post hoc analysis). The amplitudes of the unconditioned MEPs are given in Table 1. Figure 2 shows an example of a pdTES-evoked MEP with and without CS, and the magnitude of inhibition as a function of the ISI for MEPs evoked by pdTES and cTMS.
Figure 2. Inhibition of MEPs produced by pdTES in the relaxed FDI muscle (series 1, experiment 2).
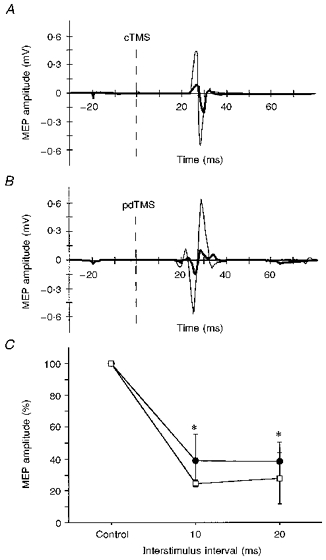
CS, cTMS; TS, pdTES or cTMS. A, example of an unconditioned (thin line) and conditioned (thick continuous line) MEP evoked by cTMS in the relaxed right FDI (single trial; ISI for conditioned response, 20 ms). The dashed vertical line indicates the time of the magnetic test stimulus. B, example of an unconditioned (thin line) and conditioned (thick continuous line) MEP evoked by pdTES in the relaxed right FDI (single trial, same subject as in A; ISI, 20 ms). The dashed vertical line indicates the time of the electrical test stimulus. C, intra-session comparison of ipsilateral inhibition of MEPs evoked by pdTES (•) or cTMS (□) in 4 subjects. Asterisks indicate significant MEP inhibition compared with the unconditioned control MEP (CTR, 100 %) (P < 0.05, ANOVA; post hoc analysis). Error bars indicate ± 1 s.d.
The latencies of MEPs evoked by pdTES were 4 ± 1 ms shorter than the latencies of the MEPs evoked by cTMS in the same experimental session (pdTES-TS, 20 ± 2 ms; cTMS-TS, 24 ± 3 ms), which is consistent with direct stimulation of the pyramidal tract at the level of the pyramidal decussation (Rothwell et al. 1994). However, the waveform of the MEPs evoked by pdTES in relaxed muscles was sometimes polyphasic, as shown in Fig. 2, rendering it possible that at the relatively high stimulus intensities used (504 ± 127 V) it was not exclusively fast conducting, large diameter corticospinal tract fibres that were stimulated. For this reason, experimental series 2 was carried out on preactivated muscles and with lower stimulus intensities (see below).
Effects of ipsilateral conditioning stimulation on MEPs evoked by cTES in the relaxed FDI (experiment 3)
Conditioning of test MEPs evoked by anodal cTES also resulted in significant inhibition (P < 0.0014; ANOVA main effect for INTERVAL) at ISIs of 10, 20 and 40 ms (P < 0.05,post hoc analysis). Maximal inhibition was observed at the ISI of 10 ms. The amount of inhibition for each ISI was similar to the effects of conditioning a cTMS-evoked MEP in the same session. The amplitudes of the unconditioned MEPs are given in Table 1.
Effects of ipsilateral conditioning stimulation on H-reflexes in the relaxed FCR (experiment 4)
Conditioning stimulation over the ipsilateral M1 had no significant effect on the amplitude of the FCR H-reflex. The inhibition pattern with cTMS-evoked test MEPs in the FCR muscle was comparable to the patterns in experiment 1 with the FDI muscle, with significant inhibition at ISIs of 10 and 40 ms (P < 0.0001; ANOVA, main effect for INTERVAL). The amplitudes of the unconditioned H-reflex responses are given in Table 1.
Series 2 (experiments in preactivated muscles)
Effects of conditioning stimulation on pdTES- and cTMS-evoked responses in the preactivated FDI
With preactivation of the FDI bilaterally and randomized presentation of magnetic and electrical test stimuli, the MEP amplitude was significantly reduced for both cTMS (P < 0.0001; ANOVA main effect for INTERVAL) and pdTES (P = 0.0026). The results are illustrated in Fig. 3.
Figure 3. Inhibition of MEPs produced by pdTES and cTMS in the preactivated FDI muscle (series 2).
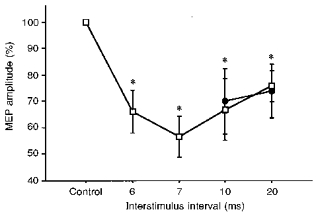
CS, cTMS; TS, pdTES. Intra-session comparison of ipsilateral inhibition of MEPs evoked by pdTES (•) or cTMS (□) in 4 subjects. Electrical and magnetic test stimuli were presented randomly. Both FDI muscles were preactivated. For both modalities, the ISIs of 10 and 20 ms were studied as in the relaxed muscle. For cTMS, 6 and 7 ms ISIs were added to provide ISIs that are directly comparable to the 10 ms ISI for pdTES (with respect to arrival of the corticospinal tract volley at the spinal cord segment). Asterisks indicate significant MEP inhibition compared with the unconditioned control MEP (CTR, 100 %) (P < 0.05, ANOVA; post hoc analysis). Error bars indicate ± 1 s.d.
The conditioning shock intensity in series 2 was 136 ± 8 % of the active motor threshold (aMT), corresponding to 113 ± 14 % of the MT in the relaxed left FDI (rMT). In series 1, we had used 143 ± 6 % of rMT. The intensity of the conditioning shock was therefore considerably lower in series 2, but still sufficient to cause significant inhibition. With the lower conditioning shock intensities of series 2, the amount of inhibition was smaller than in series 1. Responses evoked by cTMS were reduced to 66 ± 8 % (6 ms ISI), 57 ± 8 % (7 ms ISI), 67 ± 12 % (10 ms ISI) and 76 ± 6 % (20 ms ISI) of control MEP size; responses elicited by pdTES were reduced to 70 ± 13 % and 74 ± 10 % at ISIs of 10 and 20 ms, respectively (all P < 0.05, post hoc Scheffé test).
The configuration of the pdTES-evoked MEPs in preactivated FDI muscles was biphasic (Fig. 4) and therefore likely to represent activity of the fast conducting corticospinal fibres. The latency difference between the cTMS- and pdTES-evoked responses was 4 ± 1 ms (as in series 1), and did not change systematically when conditioning stimulation was applied (Fig. 4B).
Figure 4. Inhibition of MEPs produced by pdTES in the preactivated FDI muscle: examples.
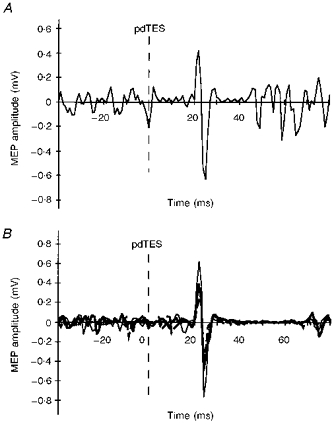
CS, cTMS; TS, pdTES. A, example of an unconditioned MEP evoked by pdTES in the preactivated right FDI (one subject; single trial). B, overlay of an unconditioned MEP (thin line) and 2 conditioned MEPs (bold; dashed line, ISI 10 ms; continuous line, ISI 20 ms) evoked by pdTES in the preactivated right FDI (one subject; each waveform represents an average of 5 trials). The dashed vertical line indicates the time of the electrical test stimulus. Note the simple, biphasic configuration of the MEPs in A and B, which was preserved also in the conditioned responses.
Effects of different stimulation intensities
Decreasing the CS intensity from 142 to 131 % of aMT (corresponding to 121 and 111 % of rMT) reduced the amount of inhibition from 47 to 41 % in one subject. This was reproduced in two other subjects where decreasing the CS intensity from 133 to 111 % of aMT (corresponding to 112 and 93 % of rMT) reduced the amount of inhibition from 24 to 14 %, or decreasing the CS intensity from 140 to 102 % of aMT (corresponding to 119 and 90 % of rMT) reduced the amount of inhibition from 23 to 0 %. Regression analysis showed a significant positive correlation between conditioning stimulus intensity and amount of inhibition of the pdTES-evoked MEP (P < 0.05). Similarly, in another subject increasing the (pdTES) test stimulus intensity from 108 to 122 % of aMT (corresponding to 89 and 100 % of the rMT) reduced the amount of inhibition from 19 to 15 %. This behaviour of the conditioning effect on pdTES responses was similar to the behaviour reported previously (Ferbert et al. 1992) with respect to MEPs evoked by cTMS.
DISCUSSION
The present results provide novel evidence that the inhibitory influence of the human M1 on ipsilateral hand muscles is to a significant extent mediated below the cortical level, and not only through cortico-cortical transcallosal connections.
Ipsilateral inhibition of cortically evoked muscle responses
Conditioning stimulation over the M1 caused pronounced inhibition of MEPs in the FDI and FCR muscles of the ipsilateral arm evoked by a magnetic test stimulus to the contralateral M1. Inhibition occurred when the conditioning stimulus was given between 6 and 50 ms before the test stimulus.
A transcallosal route has been proposed for the transmission of inhibitory interactions between the bilateral M1 hand representations (Wassermann et al. 1991; Ferbert et al. 1992; Meyer et al. 1995; Boroojerdi et al. 1996). In non-human primates, however, the transcallosal connections between motor representations of distal arm muscles are sparse, compared with the numerous connections between representations of proximal muscles (Pandya, Karol & Heilbronn, 1971; Pandya & Vignolo, 1971; Gould et al. 1986; Rouiller et al. 1994). It is not known whether the relatively few interhemispheric connections between distal limb motor representations transmit predominantly facilitatory or inhibitory commands (Matsunami & Hamada, 1984; Jones, 1993). In humans, behavioural experiments in patients with callosotomy indicated that the corpus callosum plays an important role in sensory and high-level cognitive integration (Seymour, Reuter-Lorenz & Gazzaniga, 1994; Lassonde, Sauerwein & Lepore, 1995), but that, with respect to motor performance in particular, there is little evidence for the transfer of explicit motor commands (Geffen, Jones & Geffen, 1994; Sauerwein & Lassonde, 1994). It is also important to realize that mirror movements are not the predominant feature of the ‘split-brain’ syndrome in patients with lesions of the corpus callosum (Seymour et al. 1994; Sauerwein & Lassonde, 1997), rendering it unlikely that inhibitory interactions between the output of the two primary motor cortices are solely relayed through the corpus callosum. On the other hand, there is anatomical and physiological evidence for ipsilateral projections to the spinal cord and for crossed spinal interneuron circuits, which could also mediate descending modulatory effects from the M1 upon ipsilateral limb muscles (Shahani & Young, 1971; Roby-Brami & Bussel, 1987; Delwaide & Pepin, 1991; Burke et al. 1992; Gracies et al. 1994; Mazevet et al. 1996; Nathan et al. 1996).
If the inhibitory interaction between the right and left M1 were exclusively transmitted through the corpus callosum, that is, caused by a cortico-cortically mediated decrease in the excitability of the M1 contralateral to the target muscle, then MEPs that are elicited by stimulation at subcortical levels (pdTES) should be unaffected. The present findings demonstrate that under various experimental conditions MEPs which are not evoked at the cortical level can also be modulated by conditioning stimuli applied to the ipsilateral M1. A first hint was that MEPs induced by cTES were inhibited (series 1). It has been suggested that anodal cTES can produce MEPs by stimulation of pyramidal cell axons directly (D-waves) rather than by trans-synaptical intracortical stimulation indirectly (I-waves) (Day, Thompson, Dick, Nakashima & Marsden, 1987; Rothwell et al. 1994). The stimulus intensities used for cTES in series 1 (on average >400 V) were 20-30 % above threshold and, therefore, likely to stimulate the pyramidal cell axons distal of the axon hillock evoking so-called D2-waves and possibly some D3-waves (Rothwell et al. 1994). However, at these relatively high stimulus intensities some low-threshold D1-waves and I-waves may have contributed to the EMG responses as well. The latter can be affected by cortico-cortical transcallosal inhibition mechanisms. Therefore, the cTES results alone were not conclusive, and it was necessary to study the effects of conditioning stimulation on MEPs that were elicited unequivocally below the cortical level, that is, at the level of the brainstem by pdTES.
Ipsilateral inhibition of muscle responses elicited at the level of the brainstem
The MEPs that are least likely to be subject to changes in cortical excitability and thus least likely to be sensitive to transcallosal cortico-cortical inhibition are those evoked by electrical stimulation of the pyramidal tract at the level of the brainstem (pyramidal decussation; cf. D3-waves) (Ugawa et al. 1991; Rothwell et al. 1994). In experiment 2 of series 1, pdTES-evoked MEPs were studied in relaxed FDI muscles. These responses were significantly inhibited by conditioning stimulation, similar to the cTMS-evoked responses (experiment 1 of series 1), but the inhibition effect was slightly less pronounced. This added further support to the interpretation that ipsilateral inhibitory modulation of motor responses can be mediated, or at least significantly supplemented, at the subcortical level.
A potential problem with pdTES in relaxed muscles is that very high stimulus intensities (504 ± 127 V) are necessary to elicit stable MEPs. High intensities of brain stimulation can produce more than one descending volley in the pyramidal tract, and it cannot be excluded that the reticulospinal or vestibulospinal tracts are also engaged in the production of responses thus elicited. One could speculate that inhibition directed to some of the segmental interneurons which mediate motoneuron responses to such stimuli could be in part responsible for the inhibition seen in experiment 2 (series 1). The polyphasic waveforms of some of the MEPs produced in relaxed muscles by high intensity pdTES would be consistent with this concern. To address this problem, we performed the second series of experiments with pdTES on preactivated FDI muscles. This approach allowed us to produce clean biphasic responses at substantially lower stimulation intensities compared with series 1.
Significant inhibition of pdTES-evoked responses also occurred under preactivation conditions, at both ISIs tested (10 and 20 ms). The stimulus intensities for TS and CS were substantially lower than in the experiments on relaxed muscles. The configuration of the pdTES-evoked MEPs in the preactivated FDI was simple and biphasic, not only in the control condition (no CS) but also when preceded by a conditioning shock and partially inhibited. There was no evidence that only certain parts of the MEP were inhibited. Altogether, the inhibition induced in pdTES-evoked responses under preactivation conditions is hard to explain by the action of pathways other than the large diameter corticospinal tract with oligosynaptic inputs to motoneurons. In this series, presentation of cortical magnetic and brainstem electrical test responses was also randomized, so that it was not possible to predict the type of stimulus to come. This rules out non-specific effects related to anticipation of different amounts of discomfort associated with the two types of stimulation. Finally, in a similar fashion to previous results on inhibition of cTMS-evoked MEPs (Ferbert et al. 1992), the conditioning shock intensity was positively correlated with the amount of inhibition of pdTES responses. This supports further the possibility that the conditioning effects on cortically and subcortically evoked MEPs were similar phenomena and that they can be interpreted in a comparative way.
The H-reflex experiment in series 1 reproduced the observation by Ferbert et al. (1992) that postsynaptic inhibition of the α-motoneuron pool cannot explain the results. In addition, the absence of inhibition of the H-reflex in our study may serve as a negative control, indicating that there was no technical peculiarity to our experimental set-up that would have caused a generally less specific inhibition pattern than reported previously.
Taking all observations of the present study together, there is little doubt that ipsilateral inhibition is at least in part mediated at the subcortical level, similar to what has been reported for parts of the silent period (Fuhr, Agostino & Hallett, 1991; Inghilleri, Berardelli, Cruccu & Manfredi, 1993). The tendency for pdTES responses to be somewhat less susceptible to conditioning stimulation (Figs 2 and 3) could be indicative of a combined cortical (transcallosal) and subcortical mediation of ipsilateral inhibition in the normal brain. Physiologically, this interpretation is attractive because it offers a concept of inhibitory interaction that includes considerably higher redundancy than a concept which relies exclusively on the corpus callosum. A subcortical component also offers an explanation for the frequent absence of pathological mirror movements in patients with lesions of the corpus callosum, which is rather difficult to explain if one assumes that ipsilateral inhibition is exclusively transcallosally relayed (see above, Seymour et al. 1994; Sauerwein & Lassonde, 1997).
TMS studies in patients with callosal lesions
Rothwell et al. (1991) studied a 19-year-old woman with mirror movements and agenesis of the corpus callosum. In this patient, ipsilateral inhibition, demonstrated with the same protocol as in our experiment 1, was absent. This and the presence of mirror movements was interpreted as evidence for a transcallosal route of this effect in normal subjects. However, this patient had a symptomatic cervical meningomyelocele as well, so that these findings are, in the light of the present results, equally consistent with transmission of ipsilateral inhibition at the level of the cervical spinal cord. Boroojerdi et al. (1996) used the same protocol in patients with subcortical and cortical strokes. Inhibition was not significantly different in the two patient groups, with MEP amplitude reductions to 20-67 % in the subcortical group, and more variable MEP amplitude reductions to 5-80 % in the group with cortical lesions. The authors interpreted the preserved inhibition in the subcortical stroke group as evidence for a transcallosal route, but could not explain why inhibition was also present in patients with cortical lesions. Our results offer an explanation for these findings, namely, the existence of multiple routes that relay ipsilateral inhibition, with a considerable portion of them being subcortical. These would still be efficient in patients with cortical lesions. Meyer et al. (1995) reported that in patients with radiological abnormalities of the corpus callosum, the ipsilateral silent period (iSP) was absent or altered. The authors concluded that the iSP (as another example of descending ipsilateral inhibition) was mediated via the corpus callosum. However, it is not known whether in patients with chronic malformations of the corpus callosum (7 of 10 had congenital lesions) the abnormalities are restricted to that structure. It cannot be ruled out that other candidate pathways for bilateral motor integration are altered as well. In fact, in the three patients of that study who had acquired lesions of the corpus callosum, the iSP was actually present, although with delayed onset latency. This would support the caveat that the pattern of abnormalities in patients with congenital lesions of the corpus callosum might be more complex and involve other structures as well. It is also noteworthy that the occurrence of an iSP was not dependent on a normal volume of the corpus callosum in that study (Meyer et al. 1995).
Mechanisms
We can only speculate on underlying mechanisms and on candidate systems that may be the anatomical substrate of ipsilateral inhibition. Particularly interesting are spinal interneuron circuits, a precise physiological and anatomical identification of which was beyond the scope of the present series of experiments.
Inhibitory modulation of cortically generated motor responses at a subcortical level has been described for the SP (Fuhr et al. 1991; Inghilleri et al. 1993; Ziemann, Netz, Szelenyi & Homberg, 1993). It is therefore tempting to speculate that combining cortical and subcortical circuits to serve inhibitory fine tuning of the M1 outflow might be a more general principle in the motor system. Candidate systems include those that utilize uncrossed monosynaptic corticospinal pathways (8-10 % of the human pyramidal tract fibres; Yakolev & Rakic, 1966) and those that cross twice, once at the level of the pyramidal decussation and once more at the level of the spinal cord (Delwaide & Pepin, 1991) (e.g. related to reciprocal inhibition or polysynaptic interneuron circuits). Ipsilateral inhibition following a conditioning shock to the M1 could occur as a result of after-hyperpolarization (AHP) or recurrent (Renshaw) inhibition of the motoneuron pool. Since conditioning stimulation was not associated with ipsilateral MEPs this explanation seems unlikely. As already demonstrated by Ferbert et al. (1992) and reproduced in the present study, there is no evidence for postsynaptic inhibition of the α-motoneuron pool in this protocol (absence of H-reflex inhibition), at least with the muscles at rest. Thus, inhibition via Renshaw cells, Ia-inhibitory interneurons or Ib-inhibitory interneurons, or presynaptic inhibition of Ia afferents is unlikely under these circumstances. Another possibility is that the ipsilateral inhibitory effects are caused by disfacilitation of an excitatory drive onto the α-motoneuron. In order to explain the inhibition of pdTES-evoked MEPs in relaxed and preactivated muscles, this excitatory drive should be generated in the caudal brainstem or the spinal cord, for example by excitatory spinal interneuron pathways such as flexor reflex neurons (Shahani & Young, 1971; Roby-Brami & Bussel, 1987; Floeter, Gerloff & Hallett, 1996) or C3-C4 propriospinal interneurons (Alstermark & Kummel, 1990; Burke et al. 1992; Gracies et al. 1994; Mazevet et al. 1996). The physiological significance of these interneurons in humans, however, is largely unclear. In preactivated muscles, a conditioning stimulus may also remove some of the normal tonic voluntary facilitation of the ipsilateral motoneuron pool and this may contribute to the ipsilateral inhibition seen in series 2. The precise identification of the subcortical system (or systems) relaying ipsilateral inhibition remains to be investigated.
In conclusion, the present data point to a concept of inhibitory interaction between the two primary motor cortices which is relayed at multiple levels along the neuroaxis, thus perhaps providing a structurally redundant system which may become important in case of lesions.
Acknowledgments
The authors wish to thank Drs E. Wassermann and U. Ziemann for discussion of the results and comments on the manuscript, and Ms B. J. Hessie for skilful editing. Dr C. Gerloff was supported by the Deutsche Forschungsgemeinschaft (grant Ge 844/1-1).
References
- Alstermark B, Kummel H. Transneuronal transport of wheat germ agglutinin conjugated horseradish peroxidase into last order spinal interneurones projecting to acromio- and spinodeltoideus motoneurones in the cat. 1. Location of labelled interneurones and influence of synaptic activity on the transneuronal transport. Experimental Brain Research. 1990;80:83–95. doi: 10.1007/BF00228850. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. Journal of the Neurological Sciences. 1996;144:160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- Brown P, Ridding MC, Werhahn KJ, Rothwell JC, Marsden CD. Abnormalities of the balance between inhibition and excitation in the motor cortex of patients with cortical myoclonus. Brain. 1996;119:309–317. doi: 10.1093/brain/119.1.309. [DOI] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E. Convergence of descending and various peripheral inputs onto common propriospinal-like neurones in man. The Journal of Physiology. 1992;449:655–671. doi: 10.1113/jphysiol.1992.sp019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappa KH, Cros D, Kiers L, Triggs W, Clouston P, Fang J. Crossed inhibition in the human motor system. Journal of Clinical Neurophysiology. 1995;12:82–96. [PubMed] [Google Scholar]
- Cohen LG, Roth BJ, Nilsson J, Dang N, Panizza M, Bandinelli S, Friauf W, Hallett M. Effects of coil design on delivery of focal magnetic stimulation. Technical considerations. Electroencephalography and Clinical Neurophysiology. 1990;75:350–357. doi: 10.1016/0013-4694(90)90113-x. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112:749–763. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- Day BL, Thompson PD, Dick JP, Nakashima K, Marsden CD. Different sites of action of electrical and magnetic stimulation of the human brain. Neuroscience Letters. 1987;75:101–106. doi: 10.1016/0304-3940(87)90083-8. 10.1016/0304-3940(87)90083-8. [DOI] [PubMed] [Google Scholar]
- Delwaide PJ, Pepin JL. The influence of contralateral primary afferents on Ia inhibitory interneurones in humans. The Journal of Physiology. 1991;439:161–179. doi: 10.1113/jphysiol.1991.sp018662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. The Journal of Physiology. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter MK, Gerloff C, Hallett M. Upper extremity flexor reflexes. Muscle and Nerve. 1996;19:1206. doi: 10.1002/(sici)1097-4598(199805)21:5<591::aid-mus5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ, Weiller C, Chollet F. The functional anatomy of recovery from brain injury. Ciba Foundation Symposium. 1991;163:235–244. doi: 10.1002/9780470514184.ch14. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalography and Clinical Neurophysiology. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. 10.1016/0168-5597(91)90011-L. [DOI] [PubMed] [Google Scholar]
- Geffen GM, Jones DL, Geffen LB. Interhemispheric control of manual motor activity. Behavioural Brain Research. 1994;64:131–140. doi: 10.1016/0166-4328(94)90125-2. 10.1016/0166-4328(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Gould HJ, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. Journal of Comparative Neurology. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- Gracies JM, Meunier S, Pierrot-Deseilligny E. Evidence for corticospinal excitation of presumed propriospinal neurones in man. The Journal of Physiology. 1994;475:509–518. doi: 10.1113/jphysiol.1994.sp020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. The Journal of Physiology. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cerebral Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- Jones RD, Donaldson IM, Parkin PJ. Impairment and recovery of ipsilateral sensory-motor function following unilateral cerebral infarction. Brain. 1989;112:113–132. doi: 10.1093/brain/112.1.113. [DOI] [PubMed] [Google Scholar]
- Lassonde M, Sauerwein HC, Lepore F. Extent and limits of callosal plasticity: presence of disconnection symptoms in callosal agenesis. Neuropsychologia. 1995;33:989–1007. doi: 10.1016/0028-3932(95)00034-z. 10.1016/0028-3932(95)00034-Z. [DOI] [PubMed] [Google Scholar]
- Matsunami K, Hamada I. Effects of stimulation of corpus callosum on precentral neuron activity in the awake monkey. Journal of Neurophysiology. 1984;52:676–691. doi: 10.1152/jn.1984.52.4.676. [DOI] [PubMed] [Google Scholar]
- Mazevet D, Pierrot-Deseilligny E, Rothwell JC. A propriospinal-like contribution to electromyographic responses evoked in wrist extensor muscles by transcranial stimulation of the motor cortex in man. Experimental Brain Research. 1996;109:495–499. doi: 10.1007/BF00229634. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Graefin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Miller-Fisher C. Concerning the mechanism of recovery in stroke hemiplegia. Canadian Journal of the Neurological Sciences. 1992;19:57–63. [PubMed] [Google Scholar]
- Nathan PW, Smith M, Deacon P. Vestibulospinal, reticulospinal and descending propriospinal nerve fibres in man. Brain. 1996;119:1809–1833. doi: 10.1093/brain/119.6.1809. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Karol EA, Heilbronn D. The topographical distribution of interhemispheric projections in the corpus callosum of the rhesus monkey. Brain Research. 1971;32:31–43. doi: 10.1016/0006-8993(71)90153-3. 10.1016/0006-8993(71)90153-3. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Vignolo LA. Intra- and interhemispheric projections of the precentral, premotor and arcuate areas in the rhesus monkey. Brain Research. 1971;26:217–233. 10.1016/0006-8993(71)90215-0. [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Long-latency spinal reflex in man after flexor reflex afferent stimulation. Brain. 1987;110:707–725. doi: 10.1093/brain/110.3.707. [DOI] [PubMed] [Google Scholar]
- Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. The Journal of Physiology. 1994;481:243–250. doi: 10.1113/jphysiol.1994.sp020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell J, Colebatch J, Britton TC, Priori A, Thompson PD, Day BL, Marsden CD. Physiological studies in a patient with mirror movements and agenesis of the corpus callosum. The Journal of Physiology. 1991;438:34. P. [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Experimental Brain Research. 1994;102:227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Sauerwein HC, Lassonde M. Cognitive and sensori-motor functioning in the absence of the corpus callosum: neuropsychological studies in callosal agenesis and callosotomized patients. Behavioral Brain Research. 1994;64:229–240. doi: 10.1016/0166-4328(94)90135-x. 10.1016/0166-4328(94)90135-X. [DOI] [PubMed] [Google Scholar]
- Sauerwein HC, Lassonde M. Neuropsychological alterations after split-brain surgery. Journal of Neurosurgical Sciences. 1997;41:59–66. [PubMed] [Google Scholar]
- Schott GD, Wyke MA. Congenital mirror movements. Journal of Neurology, Neurosurgery, and Psychiatry. 1981;44:586–599. doi: 10.1136/jnnp.44.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour SE, Reuter-Lorenz PA, Gazzaniga MS. The disconnection syndrome. Basic findings reaffirmed. Brain. 1994;117:105–115. doi: 10.1093/brain/117.1.105. [DOI] [PubMed] [Google Scholar]
- Shahani BT, Young RR. Human flexor reflexes. Journal of Neurology, Neurosurgery, and Psychiatry. 1971;34:616–627. doi: 10.1136/jnnp.34.5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Annals of Neurology. 1991;29:418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Fuhr P, Cohen LG, Hallett M. Effects of transcranial magnetic stimulation on ipsilateral muscles. Neurology. 1991;41:1795–1799. doi: 10.1212/wnl.41.11.1795. [DOI] [PubMed] [Google Scholar]
- Yakolev PI, Rakic P. Patterns of decussation of bulbar pyramids and distribution of pyramidal tracts on two sides of the spinal cord. Transcripts of the American Neurological Association. 1966;91:366–367. [Google Scholar]
- Ziemann U, Netz J, Szelenyi A, Homberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neuroscience Letters. 1993;156:167–171. doi: 10.1016/0304-3940(93)90464-v. 10.1016/0304-3940(93)90464-V. [DOI] [PubMed] [Google Scholar]


