Abstract
The mechanism behind the reduction in shortening velocity in skeletal muscle fatigue is unclear. In the present study we have measured the maximum shortening velocity (V0) with slack tests during fatigue produced by repeated, 350 ms tetani in intact, single muscle fibres from the mouse. We have focused on two possible mechanisms behind the reduction in V0: reduced tetanic Ca2+ and accumulation of ADP.
During fatigue V0 initially declined slowly, reaching 90 % of the control after about forty tetani. The rate of decline then increased and V0 fell to 70 % of the control in an additional twenty tetani. The reduction in isometric force followed a similar pattern.
Exposing unfatigued fibres to 10 μM dantrolene, which reduces tetanic Ca2+, lowered force by about 35 % but had no effect on V0.
In order to see if ADP might increase rapidly during ongoing contractions, we used a protocol with a tetanus of longer duration bracketed by standard-duration tetani. V0 in these three tetani were not significantly different in control, whereas V0 was markedly lower in the longer tetanus during fatigue and in unfatigued fibres where the creatine kinase reaction was inhibited by 10 μM dinitrofluorobenzene.
We conclude that the reduction in V0 during fatigue is mainly due to a transient accumulation of ADP, which develops during contractions in fibres with impaired phosphocreatine energy buffering.
The shortening velocity generally becomes reduced in skeletal muscle during a period of intense activity. This slowing is one component of the decline in performance known as skeletal muscle fatigue (for recent reviews, see Fitts, 1994; Allen, Lännergren & Westerblad, 1995). The cellular mechanisms behind this decrease in shortening velocity have received relatively little attention, especially in mammalian muscle. Acidification due to lactic acid accumulation is probably the dominant factor behind the slowing in fatigued frog muscle fibres (Edman & Mattiazzi, 1981). Recent studies on mammalian muscle have shown that while acidification reduces the shortening speed at low temperatures, it has little or no effect at temperatures of 30°C or higher (Pate, Bhimani, Franks-Skiba & Cooke, 1995; Westerblad, Bruton & Lännergren, 1997). Thus some factor(s) other than acidification must be the cause of the slowing observed in mammalian muscle studied at physiological temperature (e.g. Hatcher & Luff, 1987). Another metabolite that might reduce the shortening speed is ADP (e.g. Cooke & Pate, 1985) and some data indicate that during fatigue the free myoplasmic [ADP] ([ADP]i) may reach a level where it significantly inhibits shortening (Westerblad & Lännergren, 1995).
In the present study we have measured the maximum shortening velocity (V0) during fatigue produced by repeated short tetani in intact, single fibres from a mouse toe muscle. The experiments were performed at room temperature, where acidification has a depressant effect on shortening velocity (Westerblad et al. 1997), but the fibres under study fatigue were without significant acidification (Westerblad & Allen, 1992a) and hence a reduction in V0 cannot be due to decreased pH. We observed a substantial decline in V0 during fatigue, and the rate of decline increased markedly during the final phase of fatiguing stimulation. In this phase of fatigue there is also a rapid decline in isometric force, which has been ascribed to impaired sarcoplasmic reticulum (SR) Ca2+ release due to phosphocreatine depletion and altered energy status (Westerblad & Allen, 1992b; for review see Allen et al. 1995). We then hypothesized that the rapid decline in V0 was either caused by the concurrent decline in free myoplasmic [Ca2+] ([Ca2+]i) or by an increase in [ADP]i due to inadequate energy buffering in the absence of phosphocreatine. Dantrolene, which inhibits SR Ca2+release (Ellis & Bryant, 1972; Parness & Palnitkar, 1995), was used to test the effect of reduced tetanic [Ca2+]i and was found not to affect V0. In the absence of phosphocreatine, [ADP]i might increase rapidly during contraction and then rapidly decline when the contraction is over (Funk, Clark & Connett, 1989; Sahlin, 1992; Westerblad & Lännergren, 1995). The possible role of ADP accumulation was tested by comparing V0 during the standard short tetani and during tetani with increased duration (Westerblad & Lännergren, 1995). The results show that an increase in the tetanus duration in fatigue resulted in a marked decrease in V0 which was rapidly reversed. Similar results were obtained with inhibition of the creatine kinase reaction in rested fibres. Thus these results suggest that the reduction in shortening speed was mainly due to ADP accumulation.
METHODS
Fibre dissection, mounting and stimulation
Male mice were killed by rapid neck disarticulation and the flexor brevis muscles of the hindlimb were removed. Single fibres were then dissected from these muscle using a technique that is described in detail in Lännergren & Westerblad (1987). The isolated fibre was mounted between an Akers 801 force transducer and the moveable arm of a galvanometer (G120DT; General Scanning, Watertown, MA, USA). The position of the galvanometer arm and the signal from the force transducer were stored in a personal computer and measurements of the force and shortening velocity were made from data stored in the computer. The length of the fibre was adjusted to the length giving maximum tetanic force (L0), which occurs at a sarcomere length of 2.3-2.4 μm (Westerblad et al. 1997).
The fibre was stimulated with brief current pulses delivered via platinum plate electrodes lying parallel to the long axis of the fibre. The amplitude of the current pulses was adjusted to about 120 % of the contraction threshold. The tetanic stimulation frequency was set so that fibres produced close to maximum tetanic force under control conditions. This was achieved with 70 or 100 Hz stimulation; 100 Hz stimulation was used when the force at 100 Hz was markedly larger than that at 70 Hz. The tetanus duration was 350 ms. Fatigue was produced by initially giving tetani at 4 s intervals and the tetanic interval was reduced after 2 min to 3 s and again after an additional 2 min to 2.5 s. Fatiguing stimulation was continued until the maximum force during tetani was reduced to about 50 % of the original.
Solutions
During the experiment the fibre was superfused with a Tyrode solution of the following composition (mM): NaCl, 121; KCl, 5.0; CaCl2, 1.8; MgCl2, 0.5; NaH2PO4, 0.4; NaHCO3, 24.0; glucose, 5.5. This solution was bubbled with 5 % CO2- 95 % O2. Fetal calf serum (about 0.2 %) was added to the solution to help maintain the viability of the fibres (Lännergren & Westerblad, 1987). In some experiments 10 μM dantrolene (Sigma) was added to the solution to inhibit SR Ca2+ release. In other experiments 10 μM dinitrofluorobenzene (DNFB; Sigma) was added to inhibit the creatine kinase reaction (PCr + ADP + H+ ⇌ Cr + ATP) (Tombes, Brokaw & Shapiro, 1987). Experiments were performed at room temperature (22-24°C).
Shortening velocity measurements
The maximum shortening velocity (V0) was obtained with slack tests (Edman, 1979). V0 under control conditions and after addition of dantrolene was obtained by performing rapid releases of at least four different amplitudes in successive tetani given at 1 min intervals and measuring the time required to take up the slack. The releases were given 200 ms after the start of tetanic contractions. To get an objective measure of the starting point of force redevelopment, we fitted a single exponential function to the initial 40 ms of force redevelopment (starting at a point where force was clearly above the baseline) and the time to take up the slack was obtained by extrapolation to zero force. The release amplitudes were plotted against the time to take up the slack obtained from the curve fits. Linear regression was used to draw a straight line through the data points and V0 was obtained by dividing the slope of this line with L0. Changes in V0 during fatigue and in tetani of different durations (see below) are expressed as a percentage of V0 obtained from repeated releases under control conditions.
The largest releases used were less than 20 % of L0. Larger releases were not used because data points obtained from these deviated from a straight line in that relatively longer times were required to take up the slack. Thus there seemed to be a biphasic relation with lower shortening velocity at large release steps, which is consistent with results from skinned fibres (Moss, 1986).
During fatiguing stimulation there is not a steady-state situation and some reduction in V0 might be expected for each fatiguing tetanus. This means that a series of releases cannot be used to establish V0 during fatigue, because a reduction in the shortening velocity during the series will affect the result. We therefore used single releases with an amplitude which was kept constant throughout the fatigue run. These releases were given during tetanus 5, 10 and 20 of the fatigue run and thereafter at an interval of about ten tetani. Recovery of the contractile performance was followed by producing tetani at 1, 2, 5, 10 and 20 min after the end of fatiguing stimulation. V0 at various stages of fatigue and recovery was established from the slope of a line between the measured data point and the intercept on the shortening axis obtained under control conditions (see Fig. 1C). This intercept (i.e. the total series compliance; Edman, 1979) was assumed to remain constant throughout individual experiments.
Figure 1. The maximum shortening velocity (V0) declines rapidly towards the end of fatigue runs.
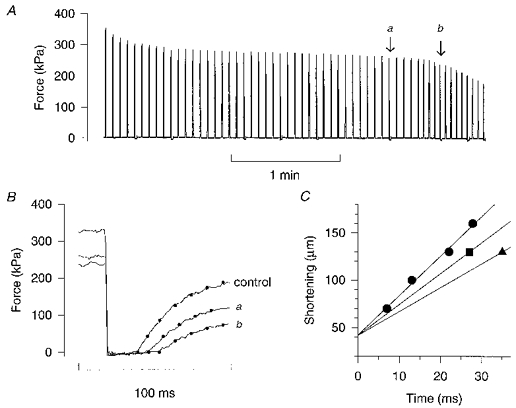
A, continuous force record from a fatigue run of a single mouse muscle fibre. Each spike represents a 100 Hz, 350 ms tetanus. Small deflections below the zero force level indicate tetani where the shortening step was produced; the amplitude of the shortening step was 130 μm and L0 was 768 μm. B, force responses to the rapid shortening step performed before fatiguing stimulation (control) and during fatigue at the tetani marked in A, i.e. at the end of phase 2 (a) and during phase 3 (b). In order to get an objective measure of the starting point of force redevelopment, a single exponential function was fitted to the each force record (dots). C, a plot of the amplitude of shortening steps vs. the times to take up the slack. Releases of four amplitudes were produced in control (•) and linear regression was used to get the slope and the intercept on the shortening axis, which was considered to be constant when V0 during fatigue was estimated. ▪ and ▴ show measurements from tetanus a and b, respectively.
To check for rapidly developing and disappearing changes in V0, we used a protocol previously used in a similar study on fibres from Xenopus frogs (Westerblad & Lännergren, 1995). This protocol consists of a 400 ms tetanus, followed after 2.6 s by a 1400 ms tetanus and finally, after an additional 4 s pause, by a 400 ms tetanus; rapid releases were given after 200, 1200 and 200 ms, respectively. Values of V0 in these contractions were obtained in the same manner as described above for V0 during fatigue. This series of three test tetani, which will be referred to as short-long-short tetani, was given in control, during fatiguing stimulation starting every tenth tetanus, and after 10 min exposure to 10 μM DNFB (Westerblad & Lännergren, 1995).
Statistics
Values are presented as means ± s.e.m. or as a range. Statistical significance was determined with one-way repeated measures ANOVA followed by Dunnett's test for multiple comparisons vs. control or by the Tukey test for pairwise multiple comparisons (SigmaStat, Jandel, San Rafael, CA, USA). The significance level was set at 0.05 throughout.
RESULTS
V0 in control and during fatigue
V0 under control conditions was 7.89 ± 0.35 L0 s−1 (n = 18, range 5.6-10.5 L0 s−1). The resting length, L0, of the fibres was 0.74 ± 0.02 mm and the total series compliance amounted to 5.0 ± 0.3 % of L0.
Figure 1 shows original records from a representative fatigue run. The continuous force record in A displays the typical pattern of force decline (Westerblad & Allen, 1991) with an initial rapid force decline (phase 1) followed by a period of almost stable force production (phase 2), and finally a rapid force decline (phase 3). Figure 1B shows the force response to rapid shortening steps in control, at the end of phase 2 (a; tetanus 40), and during phase 3 (b; tetanus 48). It can be seen that the time to take up the slack was increased at the end of phase 2 and this increase was approximately doubled in the shortening step given during phase 3. Thus the rate of V0 decline was markedly accelerated when the fibre entered phase 3; when plotting the amplitude of the shortening step against the time to take up the slack (Fig. 1C), V0 was found to be reduced by 20 % at the end of phase 2 and by 40 % during phase 3.
Figure 2A shows mean data (n = 7) of tetanic force and V0 during fatigue. The pattern of decline in force and V0 was rather similar, although the reduction in V0 was generally smaller than that of force. Thus both fell rapidly during phase 3, which might indicate a common underlying mechanism. Recovery was followed in four fibres and mean data are plotted in Fig. 2B. It can be seen that V0 was not significantly different from the control even in the tetanus given 1 min after the end of fatiguing stimulation, whereas force was still significantly reduced after 20 min. Moreover, between 10 and 20 min of recovery, V0 showed a tendency (not significant) to increase above the control value.
Figure 2. The decline in relative force and V0 follow a similar pattern during fatigue.
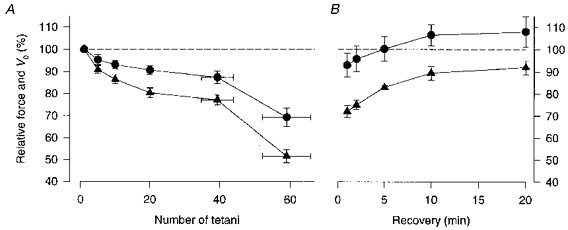
Mean data (± s.e.m.) of relative force (▴) and V0 (•) during fatigue (A; n = 7) and recovery (B; n = 4). Force and V0 in control are set to 100 %; for clarity the 100 % level is indicated by the dashed lines. The position on the x-axis of the last two data points in A represents the mean number of tetani (± s.e.m.) required to reach the end of phase 2 and to reduce force to about 50 % of the control, respectively.
Tetanic [Ca2+]i and V0
The rapid force decline during phase 3 has been shown to be due to declining tetanic [Ca2+]i (e.g. Westerblad & Allen, 1991). It is possible that the rapid decline in V0 during the same period is also caused by declining [Ca2+]i. To test this hypothesis, we exposed unfatigued fibres to dantrolene, which reduces SR Ca2+ release. Control experiments, in which [Ca2+]i was measured with indo-1, showed that the decrease in tetanic force with dantrolene could be explained by reduced tetanic [Ca2+]i (H. Westerblad, unpublished observation). Records from one experiment with dantrolene are shown in Fig. 3A. In this example the tetanic force was reduced by about 25 %, but the time to take up the slack after the shortening step was not changed. When the amplitudes of shortening steps are plotted vs. times to take up the slack in control and with dantrolene it becomes clear that V0 was not markedly altered by reduced [Ca2+]i (Fig. 3B). Mean data from four fibres exposed to dantrolene show a decline in tetanic force to 63.5 ± 4.1 %, which would represent a decline in tetanic [Ca2+]i similar to that seen when force is reduced to about 50 % in fatigue, taking into account the reduction in force at saturating [Ca2+]i and the reduced myofibrillar Ca2+ sensitivity in fatigue (Westerblad & Allen, 1991). V0, on the other hand, was not significantly altered by dantrolene (96.8 ± 2.9 % of the control).
Figure 3. Dantrolene depresses force but has no effect on V0.
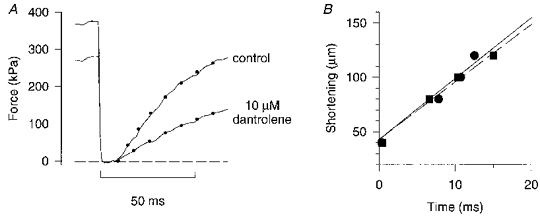
A, force responses to a 100 μm release (L0 = 740 μm) in control and after 7 min in 10 μM dantrolene. Dots represent mono-exponential curve fits to the force redevelopment. Horizontal dashed line indicates zero force. B, a plot of the shortening amplitude against time to take up the slack in control (•) and during exposure to dantrolene (▪). V0, obtained with linear regression, was 7.64 L0 s−1 in control (continuous line) and 7.18 L0 s−1 with dantrolene (dashed line).
The rate of force redevelopment after a shortening step may be used to get an estimate of the rate of cross-bridge cycling under isometric conditions (e.g. Brenner, 1988). The rates of force redevelopment measured for the smallest shortening step (40 μm), which gave the largest force redevelopment and hence the most accurate measurement of the rate constant, were 55.1 ± 3.5 s−1 in control and 58.5 ± 5.6 s−1 with dantrolene. Thus, a reduction in [Ca2+]i with dantrolene did not significantly affect the rate of force redevelopment.
Short-long-short tetani during fatigue and after inhibition of creatine kinase
Original records from test series with 400, 1400 and 400 ms tetani produced in control and during fatigue at the end of phase 2 are shown in Fig. 4. The times required to take up the slack in the three tetani were virtually identical in control (Fig. 4B). At the end of phase 2 (Fig. 4D), the times to take up the slack were generally longer and there was a clear difference between the three tetani: the shortest time required was during the first short tetanus (trace fitted with continuous line), the time required was markedly longer during the long tetanus (trace fitted by dots), and during the second short tetanus the redevelopment of force lay between the other two (trace fitted by dashed line). Thus, compared with control there was a reduction in V0 during the first tetanus and a substantial further slowing during the long tetanus which had partially recovered during the second short tetanus.
Figure 4. Increasing the tetanus duration delays force redevelopment during fatigue.
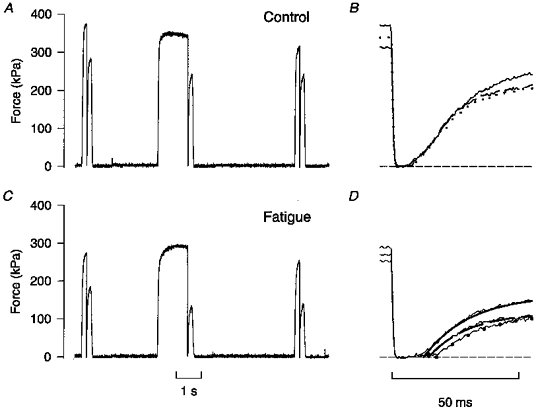
Test series consisting of a 400, a 1400, and a second 400 ms tetanus produced under control conditions (A) and during fatigue at the end of phase 2 (C). B, superimposed records of force redevelopment in the three control tetani shown in A: continuous trace, first short tetanus; dotted trace, long tetanus; discontinuous trace, second short tetanus. D, records of force redevelopment from C. Superimposed continuous and dashed lines and dots show mono-exponential curve fits for, respectively, the first and second short tetani and the long tetanus, as in B. Horizontal dashed lines in B and D represent zero force.
Figure 5A shows mean data during fatigue and recovery from four fibres. V0 was not significantly different in the three tetani under control conditions. In these experiments fibres entered phase 3 between tetanus 33 and 40, which is somewhat earlier than during standard fatigue runs. This is probably due to the more demanding protocol used, with every tenth tetanus having a duration of 1400 ms and with shortening steps produced in three out of every ten tetani because cross-bridge cycling, and hence energy consumption, is faster during shortening than under isometric conditions (e.g. Potma & Stienen, 1996). V0 measurements at the end of phase 2 (the test series starting at tetanus 30; ▴ in Fig. 5A) showed a reduction to about 80 % of the control during the first tetanus and a marked additional decrease during the long tetanus, which then partially recovered during the second short tetanus. Test series starting at tetanus 10 showed no significant difference in V0 during the three tetani; in the series starting at tetanus 20, V0 was significantly lower during the long tetanus than during the first short tetanus, albeit the difference was smaller than at the end of phase 2 (data not shown). During phase 3 (series starting at tetanus 40), the force redevelopment was rather small during the long tetanus, which made measurements of the time to take up the slack less accurate. Nevertheless, the picture that emerged was similar to that at the end of phase 2 with a marked slowing during the long tetanus which was partially reversed during the second short tetanus (data not shown). Figure 5A also shows data from test series produced 1 min after the end of fatiguing stimulation (▾ — ▾). At this time a marked general recovery of V0 had occurred and there were no significant differences between V0 measurements in the three tetani.
Figure 5. V0 declines with increasing tetanus duration in fatigue and with inhibition of creatine kinase.
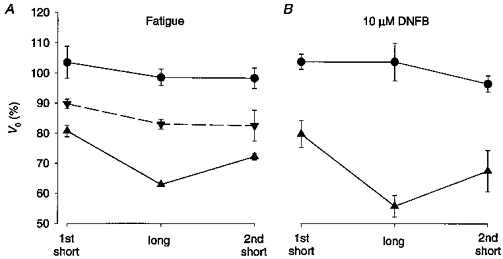
Mean V0 data (±s.e.m.) recorded during a short tetanus followed by a long tetanus and then a second short tetanus. A, data from four experiments with fatiguing stimulation: •, control; ▴, end of phase 2; ▾, 1 min after the end of fatiguing stimulation. B, data from four experiments where the creatine kinase reaction was inhibited by 10 μM dinitrofluorobenzene (DNFB): •, control; ▾, after 10 min in DNFB. 100 % represents V0 obtained from releases of various amplitudes performed in each fibre under control conditions (see Methods).
The creatine kinase reaction was inhibited by 10 μM DNFB in four fibres. The results from these experiments were similar to those obtained during fatigue. Original records from one experiment are shown in Fig. 6: in control, force redevelopment occurred at very similar times in the three tetani; after 10 min in DNFB, the time to take up the slack was markedly prolonged during the long tetanus (dots) compared with the two short tetani. Mean data are plotted in Fig. 5B and show a pattern similar to that obtained during fatigue (Fig. 5A). Under control conditions V0 was not significantly different during the three tetani, whereas V0 was significantly lower during the long tetanus after 10 min in DNFB and this reduction was partially reversed during the second short tetanus.
Figure 6. Inhibition of the creatine kinase reaction delays force redevelopment in tetani with increased duration.
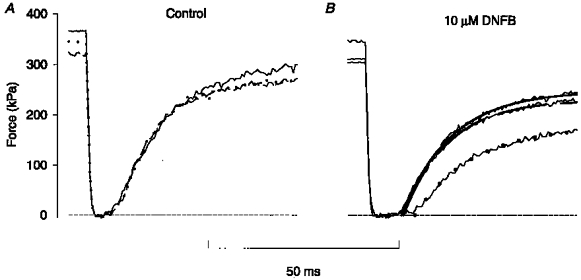
A, force records from releases produced during a short tetanus, followed by a long tetanus and then a second short tetanus under control conditions. Continuous trace, first short (400 ms) tetanus; dotted trace, long (1400 ms) tetanus; discontinuous trace, second short tetanus. B, force records from releases produced in the same fibre as in A after 10 min exposure to 10 μM DNFB. Mono-exponential curves were fitted to the force redevelopment; meaning of lines as in A. Horizontal dashed lines indicate zero force.
DISCUSSION
Several previous studies have looked at changes in shortening velocity in mammalian muscle during fatigue, but to our knowledge this is the first study directly aimed at revealing the cellular mechanisms behind these changes. During fatigue, when tetanic force was reduced to about 50 % of the control, V0 was reduced to about 70 %. This is in reasonable agreement with results from studies on fast-twitch mammalian muscle employing a fatiguing protocol similar to that used in the present study (e.g. Hatcher & Luff, 1987; Barclay, 1996). However, it should be noted that there is no obvious reason why the decline in isometric force and V0 during fatigue should follow a similar pattern in different studies, because the mechanisms underlying the decline in force and V0 are different (see Fig. 4 of Allen et al. 1995). Thus depending on, for instance, the fatiguing stimulation protocol and the fibre type composition of the muscle used, different mechanisms may dominate and the relation between the decline in force and V0 may alter.
During fatigue there are several metabolic changes that are known to affect cross-bridge function. For example, inorganic phosphate ions (Pi) will increase during fatigue mainly due to a breakdown of phosphocreatine. While accumulation of Pi is probably important for the reduction in isometric force, its effect on V0 is insignificant (e.g. Cooke & Pate, 1985; Metzger, 1996). An acidification occurs in many types of fatigue, mainly due to an accumulation of lactic acid. Acidification reduces V0 at the temperature used in the present study (Westerblad et al. 1997), but this will not be an important factor under the present experimental conditions because the fibres fatigue without significant acidification (Westerblad & Allen, 1992a). This lack of acidification has been attributed to a very effective outward co-transport of lactate and hydrogen ions (Westerblad & Allen, 1992a). Phosphorylation of the regulatory myosin light chain might occur during repeated activation of skeletal muscle and this has been shown to increase force production at subsaturating [Ca2+] (e.g. Persechini, Stull & Cooke, 1985). However, phosphorylation of this protein does not seem to have any significant effect on V0 (Butler, Siegman, Mooers & Barsotti, 1983; Persechini et al. 1985). Thus, none of these factors would be responsible for the decline in V0 in the present study and we have focused on two other possible factors: a reduction in tetanic [Ca2+]i and an increase in [ADP]i, and these will be discussed in turn.
[Ca2+]i and cross-bridge kinetics
During phase 3, both tetanic force and V0 fell rapidly. The decline in isometric force at this stage is due to a reduction in tetanic [Ca2+]i (e.g. Westerblad & Allen, 1991), which might suggest that the decrease in V0 is also due to declining [Ca2+]i. Experiments on skinned fibres show conflicting results concerning the effect of reduced Ca2+ on maximum shortening velocity, with both a depressant effect (e.g. Moss, 1982; Julian, Rome, Stephenson & Striz, 1986) and no effect (e.g. Podolsky & Teichholz, 1970) being reported. Moreover, exposing intact frog muscle fibres to dantrolene, which reduces SR Ca2+ release, has no effect on V0 (Edman, 1979). One possible explanation for the disparity in these results has been provided (Moss, 1986): with releases of up to about 80 nm per half-sarcomere the unloaded shortening velocity is fast and Ca2+ insensitive, whereas with longer releases the shortening velocity remains high at saturating [Ca2+] but declines in subsaturating [Ca2+]. In the present study we found no effect of dantrolene on the time to take up the slack after rapid releases. Our measurements of V0 were made from shortening steps with an amplitude of up to 15 % of L0. With releases of larger amplitude, data points deviated from a straight line in that relatively longer times were required to take up the slack. This means that we would have measured the high-velocity, Ca2+-insensitive phase of unloaded shortening described in skinned fibres (Moss, 1986).
The rate of force redevelopment (ktr) after a release and rapid re-extension has been proposed to reflect the rate of cross-bridge cycling under isometric conditions (e.g. Brenner, 1988). ktr has been found to fall with declining Ca2+ activation, which has led to the suggestion that Ca2+, besides acting on the thin filament regulatory proteins, also directly controls cross-bridge turnover kinetics. However, an alternative explanation was proposed by Millar & Homsher (1990). They activated caged Pi during contractions of skinned fibres and measured the rate of force decline due to the step increase in Pi (kPi), which also would reflect the rate of cross-bridge cycling under isometric conditions. kPi was found to be higher than ktr and it was not Ca2+ sensitive (Millar & Homsher, 1990). The difference between kPi and ktr can be explained as follows. In the experiments with shortening followed by re-extension, all cross-bridges will detach and tropomyosin will return to its inhibitory position on the actin filament. ktr will then reflect both the rate of tropomyosin displacement, which is Ca2+ sensitive, and the rate of cross-bridge cycling. In the experiments with caged Pi, on the other hand, some cross-bridges are always attached, preventing movement of tropomyosin, and kPi will only reflect the rate of cross-bridge cycling, which then would be Ca2+ insensitive. Our results showed that reducing tetanic [Ca2+]i with dantrolene has no effect on the rate of force redevelopment after a shortening step. One important difference between our experiments and those where ktr was measured according to Brenner (1988) is that we did not use any re-extension step. During shortening at zero load, a significant fraction of the cross-bridges will be attached at any point in time and the re-extension step is supposed to detach these cross-bridges. In our experiments, which did not involve re-extension, the cross-bridges remaining attached during rapid shortening might prevent tropomyosin movement. Thus, the rate of force redevelopment measured in our experiments would not include the rate tropomyosin displacement from the active sites on the actin filament, which is presumed to be Ca2+ sensitive. Our results then support the idea that the actual rate of cross-bridge cycling under isometric conditions is not Ca2+ sensitive.
V0 and [ADP]i
Our results indicate that the reduction in V0 observed during fatigue is mainly due to increased [ADP]i, which in skinned fibre experiments has been shown to have a large depressive effect on V0 (Cooke & Pate, 1985; Metzger, 1996). This conclusion is to some extent based on the exclusion of other possible mechanisms (see above). However, more direct evidence comes from the present findings that V0 is markedly reduced when the tetanus duration is increased, either during fatigue or with inhibition of the creatine kinase reaction. This reduction in V0 shows a large recovery during a 4 s rest period. These results agree with previous findings in muscle fibres from Xenopus (Westerblad & Lännergren, 1995). A model can be envisaged where ADP transiently accumulates during contraction in fibres with impaired phosphocreatine energy buffering (Funk et al. 1989; Sahlin, 1992). In line with this, several groups have shown a large increase in inosine monophosphate (IMP) and a corresponding reduction in adenine nucleotides during fatigue (e.g. Meyer & Terjung, 1979; Jansson, Dudley, Norman & Tesch, 1987; Sahlin, Gorski & Edström, 1990). IMP formation occurs via the myokinase reaction (2ADP ⇄ ATP + AMP) followed by the AMP deaminase reaction (AMP + H2O → IMP + NH3) and the flux in this pathway would not be large if ADP remained at a low level throughout fatigue. In this context, it is worth noting that an increase in IMP, as such, does not affect the shortening velocity of skinned muscle fibres (Myburgh & Cooke, 1997). Additionally, if there are transient increases in ADP as suggested above, transient increases in AMP might also occur. However, since AMP does not directly participate in the cross-bridge cycle, its effect V0 is likely to be much smaller than that of ADP.
The magnitude of proposed [ADP]i changes can be estimated on the assumption that ADP acts as a competitive inhibitor for V0 with an inhibition constant of 250 μM and a Michaelis constant of 150 μM (Cooke & Pate, 1985). If [ATP]i is 6 mM and [ADP]i is negligible at rest (Leijendekker & Elzinga, 1990) and the sum of [ATP]i and [ADP]i remains constant during fatigue, [ADP]i would be 1.0 mM at the end of phase 2 (mean V0 reduced to 87 % of the control) and 2.5 mM at the end of fatiguing stimulation (V0 equals 69 % of the control). A more realistic model for the estimate at the end of fatiguing stimulation includes the myokinase reaction and the subsequent deamination of AMP to IMP. It has previously been estimated that 2.3 mM IMP is formed during the present type of fatigue (Westerblad & Allen, 1992b). This means that the sum of [ATP]i and [ADP]i would be reduced to 3.7 mM at the end of fatiguing stimulation and the reduction in V0 to 69 % then gives an [ADP]i of 1.5 mM. With this model, [ATP]i during fatigue would be 2.2 mM, which is in reasonable agreement with a previous estimate (1.74 mM) based on measurements of the free myoplasmic [Mg2+] (Westerblad & Allen, 1992b). Thus, rather similar results are obtained with two completely different methods.
Our estimated changes in [ADP]i are markedly larger than those obtained by direct measurements of metabolites during fatigue (for review, see Vøllestad & Sejersted, 1988) and the directly measured increase in ADP should not have any significant impact on V0 (Chase & Kushmerick, 1995). There are several explanations for the difference between measured values and our estimates made from changes in V0. For instance, measurements of metabolites performed with NMR or with biochemical techniques in isolated muscles or muscle biopsies generally represent a mean value from many muscle fibres. It seems likely that depending on the fatigue resistance and history of activation, some fibres in a muscle might be severely fatigued, displaying large metabolic changes, whereas other fibres might not be significantly fatigued. In line with this, when individual fibres are analysed in biopsy samples obtained from fatigued human muscle, some fibres have a ATP content that is markedly lower than the mean value (Söderlund & Hultman, 1990). Furthermore, the time resolution of methods available for measuring metabolites might not be good enough to catch the rapid changes in ADP that we propose; the large increases in IMP measured during fatigue clearly indicate that ADP has been substantially elevated at some stage (see above). Finally, when the phosphocreatine store is depleted, there might be gradients of ATP and ADP with a lower ATP/ADP ratio close to sites of rapid energy turnover (Meyer, Sweeney & Kushmerick, 1984). If such gradients exist, they will remain undetected with present methods of measuring metabolites.
Acknowledgments
The study was supported by the Swedish Medical Research Council (project no. 10842), the Swedish National Centre for Sports Research, and funds at the Karolinska Institutet.
References
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Experimental Physiology. 1995;80:497–527. doi: 10.1113/expphysiol.1995.sp003864. [DOI] [PubMed] [Google Scholar]
- Barclay CJ. Mechanical efficiency and fatigue of fast and slow muscles of the mouse. The Journal of Physiology. 1996;497:781–794. doi: 10.1113/jphysiol.1996.sp021809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Effects of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: Implications for regulation of muscle contraction. Proceedings of the National Academy of Sciences of the USA. 1988;85:3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TM, Siegman MJ, Mooers SU, Barsotti RJ. Myosin light chain phosphorylation does not modulate cross-bridge cycling rate in mouse skeletal muscle. Science. 1983;220:1167–1169. doi: 10.1126/science.6857239. [DOI] [PubMed] [Google Scholar]
- Chase PB, Kushmerick MJ. Effect of physiological ADP concentrations on contraction of single skinned fibers from rabbit fast and slow muscles. American Journal of Physiology. 1995;268:C480–489. doi: 10.1152/ajpcell.1995.268.2.C480. [DOI] [PubMed] [Google Scholar]
- Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophysical Journal. 1985;48:789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate skeletal muscle. The Journal of Physiology. 1979;291:143–160. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Mattiazzi AR. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. Journal of Muscle Research and Cell Motility. 1981;2:321–334. doi: 10.1007/BF00713270. [DOI] [PubMed] [Google Scholar]
- Ellis KO, Bryant SH. Excitation-contraction uncoupling in skeletal muscle by dantrolene sodium. Naunyn-Schmiedeberg's Archives of Pharmacology. 1972;274:107–109. doi: 10.1007/BF00501011. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiological Reviews. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Funk C, Clark A, Connett RJ. How phosphocreatine buffers cyclic changes in ATP demand in working muscle. Advances in Experimental Medicine and Biology. 1989;248:687–692. doi: 10.1007/978-1-4684-5643-1_76. [DOI] [PubMed] [Google Scholar]
- Hatcher DD, Luff AR. Force-velocity properties of fatigue-resistant units in cat fast-twitch muscle after fatigue. Journal of Applied Physiology. 1987;63:1511–1518. doi: 10.1152/jappl.1987.63.4.1511. [DOI] [PubMed] [Google Scholar]
- Jansson E, Dudley GA, Norman B, Tesch P. ATP and IMP in single human muscle fibres after high intensity exercise. Clinical Physiology. 1987;7:337–345. doi: 10.1111/j.1475-097x.1987.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Julian FJ, Rome LC, Stephenson DG, Striz S. The influence of free calcium on the maximum speed of shortening in skinned frog muscle fibres. The Journal of Physiology. 1986;380:257–273. doi: 10.1113/jphysiol.1986.sp016284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. The Journal of Physiology. 1987;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijendekker WJ, Elzinga G. Metabolic recovery of mouse extensor digitorum longus and soleus muscle. Pflügers Archiv. 1990;416:22–27. doi: 10.1007/BF00370217. [DOI] [PubMed] [Google Scholar]
- Metzger JM. Effects of phosphate and ADP on shortening velocity during maximal and submaximal calcium activation of the thin filament in skeletal muscle fibers. Biophysical Journal. 1996;70:409–417. doi: 10.1016/S0006-3495(96)79584-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the ‘phosphocreatine shuttle’. American Journal of Physiology. 1984;246:C365–377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Terjung RL. Differences in ammonia and adenylate metabolism in contracting fast and slow muscle. American Journal of Physiology. 1979;237:C111–118. doi: 10.1152/ajpcell.1979.237.3.C111. [DOI] [PubMed] [Google Scholar]
- Millar NC, Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. Journal of Biological Chemistry. 1990;265:20234–20240. [PubMed] [Google Scholar]
- Moss RL. The effect of calcium on the maximum velocity of shortening in skinned skeletal muscle fibres of the rabbit. Journal of Muscle Research and Cell Motility. 1982;3:295–311. doi: 10.1007/BF00713039. [DOI] [PubMed] [Google Scholar]
- Moss RL. Effects on shortening velocity of rabbit skeletal muscle due to variations in the level of thin filament activation. The Journal of Physiology. 1986;377:487–505. doi: 10.1113/jphysiol.1986.sp016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myburgh KH, Cooke R. Response of compressed skinned skeletal muscle fibers to conditions that simulate fatigue. Journal of Applied Physiology. 1997;82:1297–1304. doi: 10.1152/jappl.1997.82.4.1297. [DOI] [PubMed] [Google Scholar]
- Parness J, Palnitkar SS. Identification of dantrolene binding sites in porcine skeletal muscle sarcoplasmic reticulum. Journal of Biological Chemistry. 1995;270:18465–18472. doi: 10.1074/jbc.270.31.18465. [DOI] [PubMed] [Google Scholar]
- Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. The Journal of Physiology. 1995;486:689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persechini A, Stull JT, Cooke R. The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal muscle fibers. Journal of Biological Chemistry. 1985;260:7951–7954. [PubMed] [Google Scholar]
- Podolsky RJ, Teichholz LE. The relation between calcium and contraction kinetics in skinned muscle fibres. The Journal of Physiology. 1970;211:19–35. doi: 10.1113/jphysiol.1970.sp009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potma EJ, Stienen GJM. Increase in ATP consumption during shortening in skinned fibres from rabbit psoas muscle: effects of inorganic phosphate. The Journal of Physiology. 1996;496:1–12. doi: 10.1113/jphysiol.1996.sp021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K. Metabolic factors in fatigue. Sports Medicine. 1992;13:99–107. doi: 10.2165/00007256-199213020-00005. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Gorski J, Edström L. Influence of ATP turnover and metabolite changes on IMP formation and glycolysis in rat skeletal muscle. American Journal of Physiology. 1990;259:C409–412. doi: 10.1152/ajpcell.1990.259.3.C409. [DOI] [PubMed] [Google Scholar]
- Söderlund K, Hultman E. ATP content in single fibres from human skeletal muscle after electrical stimulation and during recovery. Acta Physiologica Scandinavica. 1990;139:459–466. doi: 10.1111/j.1748-1716.1990.tb08947.x. [DOI] [PubMed] [Google Scholar]
- Tombes RM, Brokaw CJ, Shapiro BM. Creatine kinase-dependent energy transport in sea urchin spermatozoa. Biophysical Journal. 1987;52:75–86. doi: 10.1016/S0006-3495(87)83190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vøllestad NK, Sejersted OM. Biochemical correlates of fatigue. European Journal of Applied Physiology. 1988;57:336–347. doi: 10.1007/BF00635993. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. Journal of General Physiology. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. The Journal of Physiology. 1992a;449:49–71. doi: 10.1113/jphysiol.1992.sp019074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. The Journal of Physiology. 1992b;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. The Journal of Physiology. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J. Reduced maximum shortening velocity in the absence of phosphocreatine observed in intact fibres of Xenopus skeletal muscle. The Journal of Physiology. 1995;482:383–390. doi: 10.1113/jphysiol.1995.sp020525. [DOI] [PMC free article] [PubMed] [Google Scholar]


