Abstract
The effects of 7 days of simulated space flight, achieved with the technique of ‘dry’ water immersion, on human triceps surae muscle function have been investigated.
The maximal voluntary contraction (MVC) was reduced by 33.8 % (P < 0.01) while the electrically evoked maximal tetanic contraction force (Po) decreased by 8.2 % (P > 0.05). This suggests that most of the force loss is due to a reduction in motor drive.
The decrease in Po was associated with a small increase in maximal rates of tension development (7.2 %). The twitch tension (Pt) was not significantly changed and the Pt:Po ratio was decreased by 8.7 % after immersion.
A standard fatigue test, consisting of sixty 1 s intermittent isometric contractions (50 impulses s−1) separated by 1 s rest decreased tetanic force to approximately 60 % of initial values, but force reduction was not significantly different before and after immersion: the fatigue index was 36.2 ± 5.4 % before and 38.6 ± 2.8 % after immersion (P > 0.05). Whilst there were similar changes in mechanical output between control and disused muscles, there were differences in the pattern of electrical activity.
It is well known that gravitational unloading during space flight, or in simulated models, such as hindlimb suspension of animals and bed rest of man, induce changes in the biochemical and physiological properties of limb skeletal muscle (Martin, Edgerton & Grindeland, 1988; Riley, Ilyina-Kakueva, Ellis, Bain, Slocum & Sedlak, 1990; Koryak, 1993, 1995a). In these conditions there is a reduced movement of muscles (hypokinesia) as well as a decrease in the force of contraction (hypodynamia).
The changes in contractile properties produced by disuse are complex. It is generally agreed that the twitch time-to-peak and its time to half-relaxation are shortened in disused slow muscles (MacDougall, Ward, Sale & Sutton, 1977), but there is no consensus with regard to fast muscles. No change in the twitch time course has been observed in fast muscles by some authors (Simard, Spector & Edgerton, 1982; St-Pierre & Gardiner, 1985), whereas slowing of the twitch time course has been reported by others (Witzmann, Kim & Fitts, 1982b;Koryak, 1995a). Reports of changes in the maximal rates of twitch development and relaxation of a tetanus have also been contradictory with respect to disused muscles. It has been observed that these parameters increase in slow muscles, whereas they do not change in fast muscles (Simard et al. 1982). The interesting finding that reduction of mechanical tension was not proportional to the reduction of muscle mass, fibre diameter and concentration of contractile proteins (St-Pierre & Gardiner, 1985) would suggest that changes in electrical activity might have contributed to the reduction in the contraction force in disused muscle (Booth, 1982).
Human muscle fatigue studies have been performed under a variety of experimental conditions and ‘fatigue’ has been defined as a ‘failure point’ at which the muscle is no longer able to maintain the required force or work output (Moritani, Nagata, De Vries & Muro, 1991), or as ‘an inability to maintain the required or expected force’ (Edwards, 1981). Despite numerous investigations, the aetiology of muscle fatigue remains unknown (Fitts, Courtright, Kim & Witzmann, 1982). Few easily measurable, objective, physiological indices of fatigue exist but maximally stimulated contractions serve as an index of muscle contractility and have been shown to be independent of central drive (Bigland-Ritchie, Jones & Woods, 1979).
Changes in the surface electromyogram (EMG) have been used extensively as indices of fatigue (Lindstrom, Kadefors & Petersen, 1977; Lindstrom & Magnusson, 1977), but the relationship between the EMG and fatigue remains unclear and it has been found that EMG changes often precede muscle fatigue (Lindstrom et al. 1977). The EMG recorded from a muscle during volitional activation has been described as the summation and interference of the motor unit action potentials from all the active motor units (De Luca, 1979), and the characteristics of the EMG depend on the firing patterns of the motor units as well as on the configuration of the motor unit action potentials (Lindstrom & Magnusson, 1977).
The work presented here is the first study to make quantitative measurements of the functional properties of the neuromuscular system in men exposed to long-term ‘dry’ water immersion, used to simulate microgravity (Shulzhenko & Vil-Villiams, 1976). The investigation was concerned with the mechanical responses of the triceps surae muscle, a postural anti-gravity muscle. Mechanical and electrical parameters were recorded during electrical stimulation of the muscle motor nerve to distinguish peripheral changes from those occurring centrally. Brief reports of the work have appeared elsewhere (Koryak, 1995a, 1998).
METHODS
Subjects
Experiments were performed on six healthy male volunteers. Their mean age, height and body mass were 22.7 ± 3.5 years, 1.76 ± 0.3 m and 66.4 ± 2.3 kg, respectively. All subjects were habitually active, had a lean body composition and were non-smokers. All were familiar with the procedures and gave their informed written consent. The study was approved by the Human Ethics Committee at the Institute of Biomedical Problems. Each subject served as his own control.
‘Dry’ water immersion
‘Dry’ water immersion was used to simulate microgravity as described by Shulzhenko & Vil-Villiams (1976). Each subject was positioned horizontally in a special bath on fabric film that separated him from the water. The water temperature was a constant 33.4°C and was maintained at this level throughout the 7 days of the experiment while the subjects were kept under medical observation. The functional properties of the neuromuscular system were evaluated before and after immersion.
Testing procedure and measurements
The experimental procedures and apparatus used to record the electrical and mechanical properties of the triceps surae were as described by Koryak (1992, 1995a). In brief, the subject was seated comfortably on a special chair in a standard position (at a knee joint angle between the tibia and the sole of the foot of 90 deg). The position of the seat was adjusted to the individual and then firmly secured. A rigid leg fixation ensured isometric conditions for the muscle contraction. The dynamometer was a steel ring with a saddle-shaped block attached to fit the Achilles tendon. The resting pressure between the sensor and the tendon was constant for all the subjects and was set at 5 kg.
The isometric twitch and tetanic contractions of the triceps surae muscle were induced by electrical stimulation of the tibial nerve using supramaximal rectangular pulses of 1 ms duration with a frequency of 150 impulses s−1 for the tetanic contractions (Koryak, 1994, 1995a). To stimulate the muscle, the active electrode (cathode, 1 cm in diameter) was located in the popliteal fossa and the anode (a 6 cm × 4 cm plate) was positioned on the lower third of the front of the thigh.
The maximal isometric peak twitch force (Pt) was measured from the tendon response (tendogram) of the triceps surae muscle in response to a single electrical stimulus applied to the tibial nerve. Time from stimulation to peak twitch (TPT), and the time from contraction peak to half-relaxation (½RT) were calculated from the tendogram.
The strength of the muscle contraction was determined with double stimulation, when the second impulse was generated at intervals of 3, 4, 5, 10, 20 and 50 ms (Koryak, 1992).
The maximal voluntary contraction (MVC), measured from the tendogram, was determined from the largest of three contractions of 3-4 s duration, separated by 3 min. Subjects were verbally encouraged and visual feedback was provided.
The maximal strength of a stimulated contraction (Po) was measured from the tendogram in response to stimulation of the tibial nerve at a frequency of 150 impulses s−1, as described by Koryak (1994, 1995a). The difference between Po and MVC, expressed as the percentage of the Po and referred to as the ‘force deficit’, was also calculated (Koryak, 1995a).
The rate of development of muscle tension was calculated as the time to reach 25, 50, 75 and 90 % of maximal tension from the voluntary contraction (Koryak, 1995a). Similarly, measurements were made of the rate of rise of the evoked contraction, in response to electrical stimulation of the nerve with a frequency of 150 impulses s−1 (Koryak, 1994, 1995a). The maximal rates of voluntary, twitch and tetanic force development (dP/dt) were obtained by differentiation of the analog signal.
At the end of the tests, after a rest of 4-5 min, the fatiguability of the muscle was evaluated. The contractile properties of the triceps surae muscle were studied during a standard series of sixty 1 s electrically evoked contractions (50 impulses s−1) separated by 1 s intervals, as described by Koryak, Polyakov, Potsepaev & Martyanov (1975). A frequency of 50 impulses s−1 was used because this is within the frequency range of muscle activation during the initial phase of strong voluntary contractions (Marsden, Meadows & Merton, 1971) and is the value that gives the maximal isometric tetanic contraction (Koryak, 1992).
Recording of muscle electrical activity (EMG) and surface action potential (SAP) was achieved with Ag-AgCl surface electrodes (8 mm diameter). The two recording electrodes were placed longitudinally over the soleus muscle belly, their centres 25 mm apart, with an inter-electrode resistance of less than 5 kΩ. The large grounding electrode (7.5 cm × 6.5 cm) was located on the proximal portion of the leg between the upper recording electrode and the stimulating electrodes. Recordings of the mechanical and electrical responses of the skeletal muscle during intermittent contractions were made during the first 1 s tetanus and then for a short period (about 0.2 s) at the end of each subsequent contraction. The EMG was analysed from the amplitude of the electrical responses (M-waves, peak-to-peak amplitude of SAP; Sica & McComas, 1971) as well as the amplitude, duration and area of the first phase of the SAP at the end of 1, 3, 5, 61 and 121 s of rhythmic muscle contraction. To determine the relative changes in contractile (C) and electrical (E) function the E:C ratio was calculated where E is the amplitude of the M-wave and C the mechanical response. The E:C ratio was calculated at the end of contractions at 1, 3, 5, 61 and 121 s during the electrical fatigue test. The fatiguability of the triceps surae muscle was calculated as the fatigue index, being the mean loss of force of the last five contractions, expressed as a percentage of the mean value of the first five contractions (Koryak et al. 1975).
Statistics
Conventional statistical methods were used for the calculation of means and standard errors. Differences between baseline (background) values of the subject and those post exposure were tested for significance by Student's paired t test. Values are given as means ± s.e.m. in the text. Significant differences between means were set at the P < 0.05 level.
RESULTS
Immersion and strength
The effects of 7-day dry immersion on the MVC are illustrated by Fig. 1A. A decrease in MVC was consistently observed in all subjects, the mean being 18.9 % (P < 0.01). Figure 1A also illustrates the effects of the experimental conditions on a tetanus of 0.3-0.5 s duration at 150 impulses s−1. The values of Po and Pt were not statistically significantly different from the control values. The force deficit increased significantly after immersion by a mean of 78.7 % (Fig. 1B, P < 0.01).
Figure 1. The effects of 7-day ‘dry’ immersion on contractile properties.
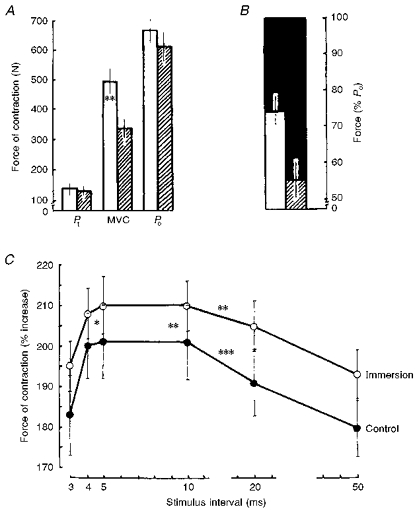
A, the maximal twitch response of force (Pt), maximal voluntary contraction (MVC) and maximal electrically evoked tetanic contraction (Po, recorded at 150 impulses s−1) in control (□) and disused muscle ( ). B, MVC force as % Po after 7-day immersion in control (□) and disused muscle (
). B, MVC force as % Po after 7-day immersion in control (□) and disused muscle ( ). ▪ indicates the force deficit, being the difference between Po and the MVC. C, mean value of the force of contraction of the triceps surae with doublet stimulation at different intervals between pulses. * P < 0.05; ** P < 0.01; *** P < 0.001.
). ▪ indicates the force deficit, being the difference between Po and the MVC. C, mean value of the force of contraction of the triceps surae with doublet stimulation at different intervals between pulses. * P < 0.05; ** P < 0.01; *** P < 0.001.
The mean changes in force of the triceps surae muscle contraction during doublet simulation, in which a second pulse was applied at various intervals, are presented graphically in Fig. 1C. The greatest force of contraction under these conditions was at an interval of between 4 and 10 ms and decreases or increases in the interval were accompanied by a considerable decline. The relative increase in force as a result of doublet stimulation was significantly greater after 7 days of immersion in comparison with the control value (P < 0.05-0.01).
Immersion and excitation-contraction (E-C) coupling
E-C coupling was examined by delivering a single supramaximal stimulus of short duration (1 ms) and studying the resulting response, both of the SAP and the twitch force. The results showed that soleus SAP was significantly altered by immersion (Table 1), the amplitude being decreased by 14.6 % (P < 0.05) and the duration prolonged by 18.8 % (P < 0.01). The area of the SAP decreased by 2.8 % after immersion. The concomitantly recorded mechanical twitch of the triceps surae revealed a significant decrease in the absolute values of dPt/dt and, when normalized (%Po), dPt/dt decreased by 5.3 % (P < 0.05). The ½RT was reduced by 5.3 %. The Pt:Po ratio was reduced by 8.7 %. These electromechanical changes suggest changes in both the electrical and mechanical properties of muscle as a result of immersion.
Table 1.
Twitch and surface action potential parameters before and after 7-day ‘dry’ water immersion
| Twitch | SAP, first phase | |||||||
|---|---|---|---|---|---|---|---|---|
| Pt (N) | TPT (ms) | ½RT (ms) | dPt/dt (%Pt ms−1) | Pt:Po | Amplitude (mV) | Duration (ms) | Area (mV ms) | |
| Control | 125.6 ± 13.7 | 118.2 ± 3.6 | 93.7 ± 3.3 | 2.06 ± 0.29 | 0.23 ± 0.02 | 4.6 ± 0.6 | 10.4 ± 1.0 | 21.6 ± 3.6 |
| Immersion | 139.3 ± 18.6 | 118.8 ± 3.4 | 88.7 ± 3.8 * | 1.77 ± 0.19 * | 0.21 ± 0.02 | 3.5 ± 0.5 * | 12.8 ± 0.7 ** | 10.2 ± 2.0 ** |
Values are means ± s.e.m. Pt, twitch contraction; TPT, time to peak twitch; ½RT, time to half-relaxation; dPt/dt, rate of force development; SAP, surface action potential
P < 0.05
P < 0.01, control compared with after immersion.
Immersion and speed of contraction
The decrease in the MVC (19 %) was associated with a significant slowing of the rate of tension development during a voluntary isometric contraction (Fig. 2A, left), by a decrease of dP/dt in absolute terms and a significant decrease of 9.6 % when normalized as the percentage MVC (Fig. 2A, right, P < 0.01).
Figure 2. Development of force of the triceps surae muscle expressed relative to the maximal force (left) and the maximal rate of rise of tension development (right).
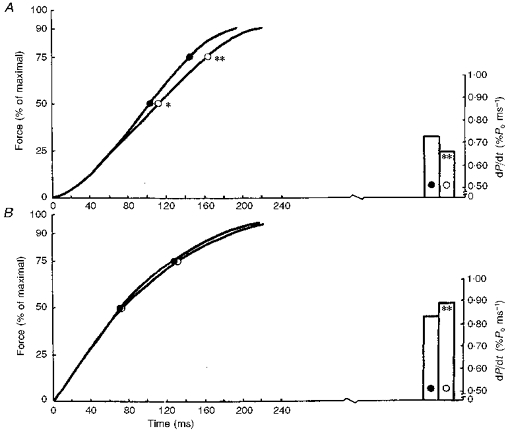
Average curves showing the development of force while executing explosive voluntary contraction (A) and as a result of electrical stimulation at 150 impulses s−1 (B). •, control; ○, after immersion. * P < 0.05; ** P < 0.01.
Analysis of the force-time curve of the electrically evoked contractions did not reveal significant differences (Fig. 2B, left) while the maximal dP/dt was slightly increased after immersion (Fig. 2B, right).
Immersion and fatiguability
The effects of 7-day dry immersion on the electrically evoked intermittent contractions stimulated at 50 impulses s−1 are illustrated in Fig. 3A. Tetanic force decreased gradually to about 60 % of its initial value. There were no significant differences between the measurements made before and after immersion: the fatigue indices were 36.2 ± 5.4 vs. 38.6 ± 2.8 %, respectively (P > 0.05). In addition to the loss of force, there were changes in the kinetics of contraction. The time course of the contractile activity of the muscle revealed a number of phases characterized by changes in the speed of contraction. There was an increase in speed compared with the initial value at 30-31 s (17-l8 %) with a subsequent relative slowing down at 60-61 s (8-9 %), followed by a slight rise at 90-91 s (11-12 %) with a slowing down in the last phase (3-7 %). High force isometric contractions interfere with intramuscular blood flow (Edwards, Hill & MacDonnell, 1972) and the different phases of the fatigue curve could be associated with alterations in blood flow as muscle force production changed. It is of particular interest that after disuse, there were no changes in the fatigue curves of the muscle.
Figure 3.
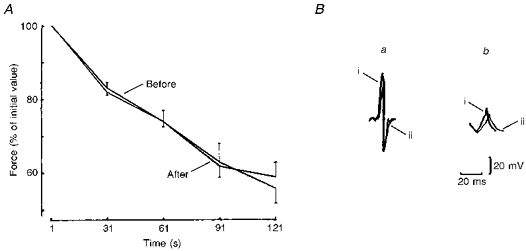
A, changes in force during 60 intermittent 1 s electrically evoked contractions, separated by 1 s intervals, in control conditions and after 7 days of dry water immersion. Values are expressed as the percentage of initial tetanus force (50 impulses s−1). B, last surface action potentials recorded during the 1st (a) and 60th contractions (b) in control conditions (i) and after 7 days of dry immersion (ii).
Loss of muscle force may be explained by a decrease in electrical activity. We therefore analysed the maximal belly-tendon EMG. Figure 3B compares the muscle SAP measurements recorded during intermittent contractions separated by 1 s intervals, and shows differences between the control and disused muscles. After immersion the amplitude of the SAP first phase was reduced by 77.3 ± 10.7 % and the duration increased by 70.2 ± 3.6 % during 121 s intermittent contraction in comparison with the first 1 s. In control muscles, the amplitude was reduced by 45.0 ± 3.1 % and duration was increased by only 52.2 ± 12.2 % during an identical fatigue test.
In Fig. 4 the changes in SAP are examined in more detail. In both tests, SAP duration decreased significantly during the first 3 s and the corresponding amplitude and area increased. Thereafter, the duration of the negative SAP phase increased throughout the test but the time courses of SAP amplitude and area were quite different. The initial increases in these two parameters, observed during the first 150 and 250 responses, respectively, were followed not only by a drop to the initial level, but also by inversion of the measured parameters.
Figure 4. Comparison of the time courses of surface action potential parameters during the fatiguing series of intermittent contractions.
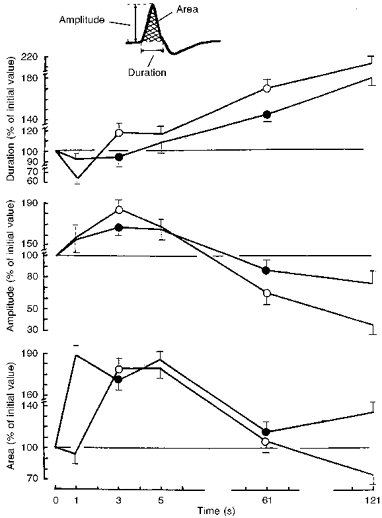
Changes in the SAP parameters (shown in the inset): duration (top), amplitude (middle) and area (bottom) are expressed as the percentage of their initial value. ○, after immersion; •, control.
The differences between changes in force and electrical activity during the fatiguing protocols are demonstrated by calculating the E:C (Fig. 5). During the initial 5 s of the fatiguing protocol there was a relatively greater decrease in the contraction force than in the electrical M-waves. With increasing duration, there was a relatively more marked decrease of the muscle electric responses.
Figure 5.
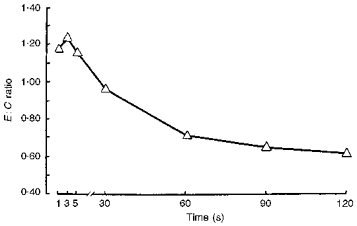
Changes in the ratio of electrical response to muscle contraction (the E:C ratio) during the fatiguing series of intermittent contractions after 7 days of ‘dry’ immersion.
DISCUSSION
The effects of reduced motor activity and/or amount of movement on the characteristics of muscular contraction have been studied in relation to morphological, biochemical and physiological changes (MacDougall et al. 1977; Booth & Seider, 1979; Witzmann, Kim & Fitts, 1982a; Witzmann et al. 1982b; Sale, McComas, MacDougal & Upton, 1982; Koryak, 1995a,b). Muscle adaptation to experimental conditions may occur for a number of reasons and whether the changes in contractile response of the muscle can be explained only by peripheral processes or whether the neural (motor) command is modified during disuse remains unclear (Moritani & DeVries, 1979; Sale et al. 1982).
The most important effect of the dry immersion found in our study was the decreased force of the voluntary contraction of the extensor muscles of the foot (Fig. 1A). Muscle disuse causes fibre atrophy (MacDougall et al. 1977) accompanied by a decline in the synthesis of contractile protein and an increase in its catabolism (Booth & Seider, 1979). However, atrophy is an unlikely explanation for the present results since Po, the force elicited by tetanic stimulation, did not decrease and the potentiating effect of doublet stimulation was, if anything, increased (see Fig. 1C). The most likely explanation for the differential loss of voluntary force lies in the fact that the neural control was changed under the influence of reduced functional requirements. This possibility is supported by reports that the amplitude and interference pattern of the voluntary EMG is changed by disuse (Duchateau & Hainaut, 1990). It has been suggested that a decrease in maximal firing rate could be explained by changes in proprioceptive afferent feedback to motoneurons (Mayer, Burke, Toop, Hodgson, Kanda & Walmsley, 1981) and/or with slowing of the capacity for activation of the motor units (Sale et al. 1982). The increased force deficit (Fig. 1B) following immersion found in this study is consistent with these suggestions and agrees with previous observations (Koryak, 1994, 1995a,b).
Immersion caused changes in the characteristics of the twitch. Time to peak tension (TPT) did not change whereas dP/dt decreased. This dissociation has been observed in many models simulating both intensified use of muscles (Roy, Meadows, Baldwin & Edgerton, 1982) and disuse created by denervation (Roy, Sacks, Baldwin, Short & Edgerton, 1984) and may be a non-specific response of sarcoplasmic reticulum (SR) to functional changes in the muscle. There is speculation that TPT and ½RT are mainly determined by changes in the SR and the capacity of the Ca2+ pump (Salviati, Sorenson & Eastwood, 1982) whereas dP/dt is mainly a function of myosin ATPase (Reiser, Moss, Guilian & Greaser, 1985). Thus there may be evidence of change both in calcium handling and cross-bridge function.
The time course of force development during electrically evoked tetani (Fig. 2B) showed only minor changes with immersion and this is in agreement with the observations of relatively constant mechanics of tetanic contraction (Witzmann et al. 1982a). The small increase in dP/dt would be consistent with the finding that myosin ATPase activity and maximal velocity of shortening are enhanced during immersion (Unsworth, Witzmann & Fitts, 1982).
Mechanical failure (fatigue) during contraction is probably one of the most intriguing phenomena of muscle physiology. In the present study, the possible effects of a change in the central motor drive were excluded by using intermittent electrical stimulation to activate the muscle. Our results show the characteristic loss of force during activity (Fig. 3a), but with no differences between control and disused muscle, which is in good agreement with previous observations (St-Pierre & Gardiner, 1985).
There were interesting, and differing, changes in force and the shape and size of the SAP, leading to major changes in the E:C ratio during the course of the fatiguing series of contractions. Overall there were similar changes in the control and disused muscles although, after immersion, there was not such a rapid increase in area of the SAP at the start of the series of contractions as that seen in the control muscles (Fig. 4, bottom).
In both conditions, during the first 3 s the SAP duration decreased whilst the amplitude and area increased. These changes imply that presynaptic and/or endplate potentials are facilitated and that the propagation velocity of the action potential along the fibres increases (Stalberg, 1966) and dispersion between fibre action potentials is reduced (Desmedt, Emeryk, Renoirte & Hainaut, 1968). The subsequent increase in SAP area and duration without a decrease in amplitude must be due mainly to slowing of the conduction velocity along membranes of muscle fibres (Bigland-Ritchie et al. 1979) so that the SAP broadens in shape (see Fig. 3B). The reduction in SAP amplitude in the second half of the series of intermittent contractions is clearly associated with a loss of SAP amplitude, although the duration continues to increase. The loss of amplitude can be due to either pre- or post-synaptic failure. It has been found that failure of propagation of action potentials may occur (i) along the terminal branches of motor nerves (Krnjevi´c & Miledi, 1958), (ii) at the neuromuscular junction (Krnjevi´c & Miledi, 1958) or (iii) along the surface of muscle fibres (Bigland-Ritchie et al. 1979) and along the T-tubules (Bezanilla, Caputo, Gonzalez-Serratos & Venosa, 1972). Changes in configuration of the action potential have been associated with a depletion of extracellular [Na+], increased [K+] and an increase in [H+] in the muscle fibres (Bigland-Ritchie et al. 1979; Lannergren & Westerblad, 1982). An additional factor may be that disuse significantly impairs electrolyte homeostasis, as suggested by Noskov, Kozyrevskaya, Morukov, Artamasova & Rustamyan (1995), thus contributing to the reduction of muscle membrane excitability described in fatigue (Lindstrom et al. 1977; Bigland-Ritchie et al. 1979; Edwards, 1981).
Although the relationship between EMG changes and fatigue remains unclear, the changes in the E:C ratio (Fig. 5) indicate that the peripheral fatigue process may be separated into electrogenic and contractile phases. At the start of the series of contractions there is clearly a decrease in force, whilst electrical activity is constant, or the size of the SAP is even increasing. This implies that the initial phase of fatigue is dominated by changes within the muscle fibres while in the later phases, EMG activity declines, suggesting an increasing role for electrogenic factors.
In conclusion, the results presented here show that 7 days of simulated space flight, achieved with the technique of dry immersion, significantly reduces voluntary muscle strength. This is thought to be a consequence of reduced motor drive since the electrically induced tetanic forces were not reduced. Fatiguability of the muscle was not affected by the period of disuse, although there were differences in the electrical responses of the control and disused muscles. The results also illustrate the dissociation that occurs between changes in contractile properties and electrical activity during the course of a series of fatiguing contractions.
Acknowledgments
The author gratefully acknowledges the six subjects that endured 7 days of confinement. He is especially grateful to Miss Lyudmila Prokopenkova and also very thankful to Mr Anatoli Dotsenko for the technical design of the work. The referees are thanked for their valuable comments and suggestions. Finally, the author wishes to express his appreciation to all persons who contributed to the successful performance of the experiment. This work was supported by the Institute of Biomedical Problems.
References
- Bezanilla F, Caputo C, Gonzalez-Serratos H, Venosa RA. Sodium dependence of the inward spread of activation in isolated twitch muscle fibres of the frog. The Journal of Physiology. 1972;223:507–523. doi: 10.1113/jphysiol.1972.sp009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Jones DA, Woods JJ. Excitation frequency and muscle fatigue: electrical responses during human voluntary and stimulated contraction. Experimental Neurology. 1979;64:414–427. doi: 10.1016/0014-4886(79)90280-2. [DOI] [PubMed] [Google Scholar]
- Booth FW. Effect of limb immobilization on skeletal muscle. Journal of Applied Physiology. 1982;52:1113–1118. doi: 10.1152/jappl.1982.52.5.1113. [DOI] [PubMed] [Google Scholar]
- Booth FW, Seider MJ. Recovery of skeletal muscle after 3 months of hindlimb immobilization in rats. Journal of Applied Physiology. 1979;47:974–977. doi: 10.1152/jappl.1979.47.2.435. [DOI] [PubMed] [Google Scholar]
- De Luca CJ. Physiology and mathematics of myoelectric signals. IEEE Transactions on Biomedical Engineering. 1979;26:313–325. doi: 10.1109/tbme.1979.326534. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Emeryk B, Renoirte P, Hainaut K. Disorder of contraction processes in sex-linked (Duchenne) muscular dystrophy, with correlative electromyographic study of myopathic involvement in small hand muscles. American Journal of Medicine. 1968;45:853–872. doi: 10.1016/0002-9343(68)90184-8. 10.1016/0002-9343(68)90184-8. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. The Journal of Physiology. 1990;422:55–65. doi: 10.1113/jphysiol.1990.sp017972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RHT. Human muscle function and fatigue. In: Porter J, Whelan J, editors. Human Fatigue: Physiological Mechanisms. London: Pitman Medical; 1981. pp. 1–18. [Google Scholar]
- Edwards RHT, Hill DK, MacDonnell M. Myothermal and intermuscular pressure measurement during isometric contractions of the human quadriceps muscle. The Journal of Physiology. 1972;224:58–59. P. [PubMed] [Google Scholar]
- Fitts RH, Courtright JB, Kim DH, Witzmann FA. Muscle fatigue with prolonged exercise: contractile and biochemical alterations. American Journal of Physiology. 1982;242:C65–73. doi: 10.1152/ajpcell.1982.242.1.C65. [DOI] [PubMed] [Google Scholar]
- Koryak YA. Methods of Investigation of Neuromuscular System of Athletes. Moscow: IMBP; 1992. [Google Scholar]
- Koryak Y. Contractile properties of the tibialis anterior and the triceps surae: a comparison of the effects of two training patterns. 1st Congress on Physical Education & Sport. 1993:18. [Google Scholar]
- Koryak Y. Contractile characteristics of the triceps surae muscle in healthy males during 120-days head-down tilt (HDT) and countermeasures. Journal of Gravitational Physiology. 1994;1:P141–143. [PubMed] [Google Scholar]
- Koryak Y. Contractile properties of the human triceps surae muscle during simulated weightlessness. European Journal of Applied Physiology and Occupational Physiology. 1995a;70:344–350. doi: 10.1007/BF00865032. [DOI] [PubMed] [Google Scholar]
- Koryak Y. Mechanical and electrical adaptation of skeletal muscle to gravitational unloading. Journal of Gravitational Physiology. 1995b;2:P76–79. [PubMed] [Google Scholar]
- Koryak Y. Influences of 120-days 6 degree head-down tilt bed rest on the functional properties of neuromuscular system in man. Aviation, Space and Environmental Medicine. 1998;98 in the Press. [PubMed] [Google Scholar]
- Koryak YA, Polyakov VW, Potsepaev AI, Martyanov VA. The research of dynamic capacity for work of peripheral neuromuscular system of athlete. In: Korobkov AV, editor. Physiological Grounds of Movement Control. Moscow: Academic Press; 1975. pp. 73–74. [Google Scholar]
- Krnjevi´c K, Miledi R. Failure of neuromuscular propagation in rats. The Journal of Physiology. 1958;140:440–461. [PMC free article] [PubMed] [Google Scholar]
- Lannergren J, Westerblad H. Action potential fatigue in single skeletal muscle fibres of Xenopus. Acta Physiologica Scandinavica. 1982;129:311–318. doi: 10.1111/j.1748-1716.1987.tb08074.x. [DOI] [PubMed] [Google Scholar]
- Lindstrom L, Kadefors R, Petersen I. An electromyographic index for localized muscle fatigue. Journal of Applied Physiology. 1977;43:750–754. doi: 10.1152/jappl.1977.43.4.750. [DOI] [PubMed] [Google Scholar]
- Lindstrom J, Magnusson RT. Interpretation of myoelectric power spectra: a model and its applications. Proceedings of the IEEE. 1977;65:653–662. [Google Scholar]
- MacDougall JD, Ward BR, Sale DG, Sutton JB. Biochemical adaptation of human skeletal muscle to heavy resistance training and immobilization. Journal of Applied Physiology. 1977;43:700–703. doi: 10.1152/jappl.1977.43.4.700. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Meadows JS, Merton PA. Isolated single motor unit in human muscle and their rate of discharge during maximal voluntary effect. The Journal of Physiology. 1971;217:12–13. P. [PubMed] [Google Scholar]
- Martin TP, Edgerton VR, Grindeland RE. Influence of spaceflight on rat skeletal muscle. Journal of Applied Physiology. 1988;65:2318–2325. doi: 10.1152/jappl.1988.65.5.2318. [DOI] [PubMed] [Google Scholar]
- Mayer RF, Burke RE, Toop J, Hodgson JA, Kanda K, Walmsley B. The effect of long-term immobilization on the motor unit population of the cat medial gastrocnemius muscle. Neuroscience. 1981;6:725–739. doi: 10.1016/0306-4522(81)90156-1. [DOI] [PubMed] [Google Scholar]
- Moritani T, De Vries HR. Neural factors versus hypertrophy in the time course of muscle strength gain. American Journal of Physical Medicine. 1979;58:115–130. [PubMed] [Google Scholar]
- Moritani T, Nagata A, De Vries HD, Muro M. Critical power as a measure of physical work capacity and anaerobic threshold. Ergonomics. 1981;24:339–350. doi: 10.1080/00140138108924856. [DOI] [PubMed] [Google Scholar]
- Noskov VB, Kozyrevskaya GI, Morukov BV, Artamasova EM, Rustamyan LA. Body position during hypokinesia and fluid-electrolyte metabolism. Kosmicheskaya Biologiya and Aviakosmicheskaya Meditsina. 1985;19:31–34. [PubMed] [Google Scholar]
- Reiser PJ, Moss RL, Guilian GG, Greaser ML. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. Journal of Biological Chemistry. 1985;260:9077–9080. [PubMed] [Google Scholar]
- Riley DA, Ilyina-Kakueva EI, Ellis S, Bain JLM, Slocum GR, Sedlak FR. Skeletal muscle fiber, nerve, and blood vessel breakdown in space-flown rats. FASEB Journal. 1990;4:84–91. doi: 10.1096/fasebj.4.1.2153085. [DOI] [PubMed] [Google Scholar]
- Roy RR, Meadows ID, Baldwin KM, Edgerton VR. Functional significance of compensatory overloaded rat fast muscle. Journal of Applied Physiology. 1982;52:473–478. doi: 10.1152/jappl.1982.52.2.473. [DOI] [PubMed] [Google Scholar]
- Roy RR, Sacks RD, Baldwin KM, Short M, Edgerton VR. Interrelationships of contraction time, Vmax and myosin ATPase after transection. Journal of Applied Physiology. 1984;56:1594–1601. doi: 10.1152/jappl.1984.56.6.1594. [DOI] [PubMed] [Google Scholar]
- St-Pierre D, Gardiner PF. Effect of ‘disuse’ on mammalian fast-twitch muscle: joint fixation compared with neurally applied tetrodotoxin. Experimental Neurology. 1985;90:635–651. doi: 10.1016/0014-4886(85)90161-x. [DOI] [PubMed] [Google Scholar]
- Sale DG, McComas AJ, MacDougall JD, Upton AR. Neuromuscular adaptation in human thenar muscles following strength and immobilization. Journal of Applied Physiology. 1982;53:419–424. doi: 10.1152/jappl.1982.53.2.419. [DOI] [PubMed] [Google Scholar]
- Salviati G, Sorenson MM, Eastwood AB. Calcium accumulation by the sarcoplasmic reticulum in two populations of chemically skinned human muscle fibers. Journal of General Physiology. 1982;79:603–632. doi: 10.1085/jgp.79.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulzhenko EV, Vil-Villiams IF. Possibility of a long-term water immersion by the method of ‘dry’ plunging. Kosmicheskaya Biologiya and Aviakosmicheskaya Meditsina. 1976;10:82–84. [PubMed] [Google Scholar]
- Sica REP, McComas AJ. An electrophysiological investigation of limb-girdle and facioscapulohumeral dystrophy. Journal of Neurology, Neurosurgery and Psychiatry. 1971;34:469–474. doi: 10.1136/jnnp.34.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard CP, Spector SA, Edgerton VR. Contractile properties of rat hind limb muscles immobilized at different lengths. Experimental Neurology. 1982;77:467–482. doi: 10.1016/0014-4886(82)90221-7. [DOI] [PubMed] [Google Scholar]
- Stalberg E. Propagation velocity in human muscle fibers in situ. Acta Physiologica Scandinavica. 1966;70(suppl. 287) [PubMed] [Google Scholar]
- Unsworth BR, Witzmann FA, Fitts RH. A comparison of rat myosin from fast and slow skeletal muscle and the effect of disuse. Journal of Biological Chemistry. 1982;25:15127–15136. [PubMed] [Google Scholar]
- Witzmann FA, Kim DH, Fitts RH. Recovery time course in contractile function of fast and slow skeletal muscle after limb immobilization. Journal of Applied Physiology. 1982a;52:677–682. doi: 10.1152/jappl.1982.52.3.677. [DOI] [PubMed] [Google Scholar]
- Witzmann FA, Kim DH, Fitts RH. Hindlimb immobilization: length-tension and contractile properties of skeletal muscle. Journal of Applied Physiology. 1982;53:335–345. doi: 10.1152/jappl.1982.53.2.335. [DOI] [PubMed] [Google Scholar]


