Abstract
This study characterized the electrical and mechanical activities of human colonic muscle strips obtained from either the ascending, descending or sigmoid colon of patient volunteers during elective colon resections.
Rhythmic contractile activity was observed in colonic circular muscle strips in the absence of external stimuli. This activity persisted in the presence of atropine, phentolamine, propranolol, tetrodotoxin and Nω-nitro-L-arginine but was abolished by nifedipine.
The activity of whole circular muscle (WCM) was compared with that of the myenteric half (MCM), the submucosal half (SCM) and the interior (ICM) of the circular muscle layer. WCM exhibited a prominent 2–4 contractions min−1 contractile pattern which was also present in strips of SCM. In contrast, MCM and ICM exhibited slow (0.3–0.6 contractions min−1), long duration contractions with superimposed higher frequency contractions (17–18 contractions min−1).
Resting membrane potential (Vm), recorded at various positions through the thickness of WCM strips did not differ and averaged −50 mV.
Slow waves were observed in 83 % of muscles. They averaged 12 mV in amplitude, 9.4 s in duration and had a frequency of 2–4 contractions min−1. Slow waves were greatest in amplitude near the submucosal edge and decreased with distance away from this edge. Each slow wave was associated with a transient contraction.
Near the myenteric edge, rapid fluctuations of Vm with a mean frequency of 18 contractions min−1 were recorded in 67 % of muscles. Spiking activity was common and was superimposed upon slow waves and rapid Vm fluctuations.
In summary, slow waves were identified in the human colonic circular muscle layer which arise at or near the submucosal edge. These electrical events give rise to a 2–4 contractions min−1 contractile rhythm which is characteristic of the intact muscle layer. Thus, the nature and spatial organization of pacemaker activity in the human colon bears significant resemblance to other animal models, such as the dog and pig.
The electrical activity that generates the motility patterns of the human colon is not well understood. Most of our knowledge concerning colonic electrophysiology comes from studies of animal models, such as the dog. In the canine model, pacemaker cells have been identified along the myenteric and submucosal edges of the circular muscle layer. Pacemaker cells in the submucosal region generate electrical slow waves (40−50 mV amplitude at 4-6 contractions min−1) which conduct into the interior of the muscle layer (Smith, Reed & Sanders, 1987a, b) and these are associated with transient contractions of the muscle. Slow waves have also been recorded from colonic muscles of other animal models like the pig (Huizinga, Diamant & El-Sharkawy, 1983; Huizinga, Chow, Diamant & El-Sharkawy, 1987) and cat (Du & Conklin, 1989), but they have not been found in the smaller, more frequently used animal models such as the rat, mouse and guinea-pig. In dog, an additional rapid frequency pacemaker potential is observed near the myenteric edge. This electrical activity has been termed ‘myenteric potential oscillations’ (MPOs; Smith et al. 1987b) and averages 15-20 contractions min−1 in frequency and 10 mV in amplitude. MPOs and slow waves summate in the central region of the muscle layer producing a complex pattern of activity that regulates contractile amplitude and frequency. Electrophysiological studies of human colon performed in vitro have reported spiking behaviour and small amplitude, rapid fluctuations in membrane potential (Kirk, 1981; Kubota, Ito & Ikeda, 1983; Huizinga, Stern, Chow, Diamant & El-Sharkawy, 1985, 1986; Chow & Huizinga, 1987; Huizinga & Waterfall, 1988; Riezzo, Maselli, Pezolla, Thouvenot & Giorgio, 1992). However, spontaneous slow waves of the type observed in the canine colon have not been described, nor has the origin of pacemaker potentials in the human colon been investigated.
The present study was undertaken to characterize the spatial relationship of spontaneous electrical and contractile activity in the human colon. The pattern of contractile activity in the intact circular muscle layer was compared with that of strips containing only a portion of the circular muscle layer. Membrane potential was also recorded with intracellular microelectrodes from cells located at various positions through the thickness of the circular muscle layer. These studies revealed the presence of a pacemaker region near the submucosal edge of the circular muscle layer which gives rise to slow waves and a 2-4 contractions min−1 contractile rhythm.
METHODS
Fifty-five samples of sigmoid (45 %), descending (42 %) or ascending (13 %) colon were obtained, after obtaining informed written consent, from male and female patient volunteers ranging in age from 17 to 83 years during elective colon resections for non-obstructive neoplasms or diverticulitis. Each suitably healthy volunteer was approached on the day prior to the scheduled procedure by a medical doctor investigator who was not involved with the planned operation to elicit donation of superfluous tissue. Each volunteer signed a project-specific (B89/90-11) informed consent form previously approved by both the Biomedical Human Subjects Committee (DHHS Multiple Project Assurance M-1164-XM) and the Research and Development Committee. Signed, witnessed forms are maintained in the investigator's files. Coded specimens were stored in a saline solution at 2-4°C for 24 h prior to experimentation. All work in the present study was carried out in accordance with the standards set by the Declaration of Helsinki.
Tissue samples were pinned out in a dissecting dish containing oxygenated (95 % O2-5 % CO2) Krebs-Ringer-bicarbonate solution (KRB) of the following composition (mM): 118.5 NaCl, 4.7 KCl, 1.2 MgCl2, 23.8 NaHCO3, 1.2 KH2PO4, 11 dextrose, 2.5 CaCl2, pH 7.4. Strips (15 mm long) of the entire muscularis were cut parallel to the circular muscle fibres in regions between taenia with a knife consisting of a pair of parallel scalpel blades set 1.5 mm apart. The tissues used for this study had a compact tunica muscularis. Any region of the surgical sample which had a break or lesion in the muscularis or mucosa was discarded. To prevent any possible damage to the myenteric or submucosal plexi no attempt was made to remove either the thin covering of intra-taenial longitudinal muscle or the thin strip of submucosal connective tissue which was attached to the circular muscle layer.
Mechanical experiments
The following muscle strip preparations were used for contractile experiments. (1) Whole circular muscle (WCM) which contained the entire thickness of the circular muscle layer. (2) Myenteric circular muscle (MCM) consisting of the peripheral half of the circular muscle layer including the myenteric plexus region. (3) Submucosal circular muscle (SCM) which contained the luminal half of the circular muscle layer and the submucosal plexus region. (4) Interior circular muscle (ICM), from which both the myenteric and submucosal plexi and adjoining circular muscle were removed, leaving approximately one-third of the muscle layer (see Fig. 1). Eighty-eight strips were prepared from the colonic specimens of thirty-nine patients for mechanical experiments.
Figure 1. Schematic representation of the cross-sectional preparation of human colon.

Dashed lines indicate where cuts were made across the circular muscle layer to create the strips used for contractile measurements. Whole circular muscle (WCM) included a thin intra-taenial strip of longitudinal muscle at one edge and some submucosal connective tissue at the other edge. Myenteric circular muscle (MCM) strips also included the intra-taenial longitudinal muscle as well as half of the circular musle layer. Submucosal circular muscle (SCM) strips included half of the circular muscle layer as well as some submucosal connective tissue. Interior circular muscle (ICM) strips were devoid of both myenteric and submucosal edges and contained approximately one-third of the muscle layer.
The muscle strips were immersed in tissue baths containing KRB, maintained at 37°C and attached to isometric strain gauges (Gould). A resting force of 0.5 g was applied to all strips except WCM, to which 1 g resting force was applied (due to its greater cross-sectional area) after which the tissues were allowed to equilibrate for between 1-3 h, until a steady contractile rhythm appeared, with fresh KRB added to the bath every 15 min. Tissues which did not contract in response to 80 mM KCl and 10 μM acetylcholine were discarded. Contractile activity was recorded on a chart recorder (Western Graphtec).
Spontaneous contractions were observed in intact (WCM) and partial thickness muscle strips (MCM, SCM and ICM) but the pattern of activity in each region differed. In order to compare the patterns of activity, spontaneous contractions were combined by frequency into three groups: (i) ‘slow’ contractions, with a frequency of less than 1.5 contractions min−1; (ii) ‘intermediate’ contractions occurring between 1.5 and 5 contractions min−1; and (iii) ‘fast’ contractions occurring at frequencies greater than 5 contractions min−1.
Electrophysiological experiments
Intracellular recordings were made from thirty muscle strips obtained from twenty patients. Muscle strips were prepared for intracellular recording as previously described (see Keef, Murray, Sanders & Smith, 1997). Briefly, WCM strips were pinned in cross section to the floor of an electrophysiological chamber, exposing the full thickness of the tunica muscularis. This orientation facilitated impalement of cells at any point through the thickness of the circular muscle layer. One end of the tissue was attached to a tension transducer for recording contractile activity. After pinning, tension was re-adjusted to 1 g.
Tissues were perfused with oxygenated KRB (37°C) solution and allowed to equilibrate for approximately 2 h prior to experimentation unless otherwise stated. Once a constant contractile pattern developed a submaximal concentration of wortmannin (5 μM), the myosin light chain kinase inhibitor (Nakanishi et al. 1992), was added to facilitate the impalement of cells, without disrupting electrical events in the tissue (Burke, Gerthoffer, Sanders & Publicover, 1996).
Muscle cells were impaled with glass microelectrodes filled with 3 M KCl and having resistances ranging from 40 to 80 MΩ. Membrane potential was measured with a high input impedance electrometer (WPI Duo 773), with outputs displayed on an oscilloscope (Nicolet 3091). Analogue electrical and mechanical signals were digitized and recorded on both video tape (Vetter 875), and chart paper (Gould 2200). Since the thickness of muscles varied, the point of impalement within the circular muscle layer was expressed as percentage distance, with the submucosal edge considered 0 % and the myenteric edge considered as 100 %. Most recordings were made from cells in the 5, 50 and 95 % regions of the circular muscle layer.
Data analysis and statistics
Resting membrane potential was defined as the mean of the most negative potentials recorded between electrical oscillations. dV/dt values quoted in Results represent the maximum change in voltage with time during the upstroke of the action potential. Slow wave amplitude, where action potentials were superimposed on the slow waves, was determined as the mean of: (a) the pre-potential peak prior to the firing of the first action potential and (b) the nadirs of the small after-hyperpolarizations that occurred following the firing of each action potential. The potential reached during the slow wave was determined in the same manner except in this case the slow wave amplitude was subtracted from resting membrane potential.
Significance, calculated as the differences between means using Student's two-tailed paired or unpaired t test, was assumed for P < 0.05 and denoted thus *P < 0.05 and ***P < 0.001. Data were expressed as means ± s.e.m.N values represent the number of muscle strips (maximum two muscle strips per patient for any one measurement).
Drugs
Drugs used were apamin, atropine sulphate, L-NAME, propranolol hydrochloride, tetrodotoxin (TTX), wortmannin (all from Sigma) and nifedipine (from Calbiochem).
RESULTS
Contractile patterns in subsegments of the circular muscle layer
All isolated strips of whole circular muscle (WCM) contracted rhythmically in the absence of external stimuli. (peak stress 64 mN mm−2, n = 28). Rhythmic contractions persisted in the presence of a number of different blockers of enteric nerves and neurotransmitters including apamin (1 μM, n = 10), L-NAME (200 μM, n = 7), atropine, propranolol, phentolamine (all 1 μM, n = 10) and, of particular relevance, TTX (1 μM, n = 6) (Fig. 2) suggesting that the activity is non-neurogenic. In 89 % of WCM strips intermediate frequency contractions were observed which averaged 2.6 ± 0.1 contractions min−1 (n = 25, see Figs 2 and 3A). Slow (0.9 ± 0.1 contractions min−1, n = 9) longer duration contractions occurred in 32 % of muscles. In some of these, a mixed pattern of intermediate and longer duration contractions occurred while in others, only longer duration contractions were observed. Finally, smaller high frequency contractions, averaging 16.7 ± 0.7 contractions min−1 (n = 20) were often superimposed upon contractions of lower frequencies (see Table 1 for summary of frequencies).
Figure 2. Effect of various antagonists on the contractile activity of human colonic muscles.
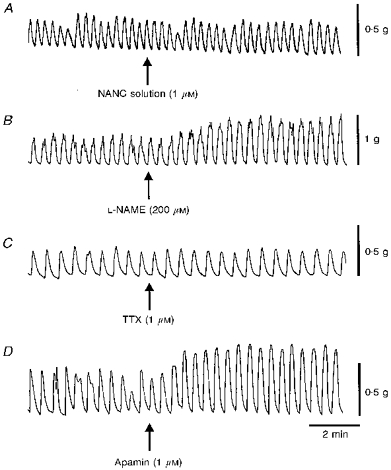
Shown in this figure are rhythmic contractions recorded from strips of whole circular muscle (WCM) obtained from four different patients. In each case a 2-4 contractions min−1 rhythm predominates. A, the contractile activity before and after addition of a ‘NANC’ solution containing atropine, phentolamine and propranolol (all 1 μM). The NANC solution did not change contractile amplitude or pattern in this muscle or in nine others tested. B, the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME; 200 μM) was added in the presence of NANC solution. L-NAME increased contractile amplitude in this and in six other muscles tested (mean increase 54 ± 12 %, n = 7) but the 2-4 contractions min−1 contractile rhythm persisted. C, the fast sodium channel blocker tetrodotoxin (TTX, 1 μM) was applied in the presence of NANC solution. In this case TTX had no effect on spontaneous contractions. Overall, in six muscles there was a small increase in contractile amplitude (i.e. 11 ± 4.6 %, n = 6) but the 2-4 contractions min−1 rhythm persisted. D, the small conductance calcium-activated K+ channel antagonist apamin (1 μM) was applied in the presence of NANC solution plus TTX. Apamin produced an increase in contractile amplitude in this and nine other muscles (i.e. 132 ± 36 %, n = 10) but the 2-4 contractions min−1 contractile rhythm persisted.
Figure 3. Examples of the most common contractile patterns observed in strips of human whole circular muscle (WCM), myenteric circular muscle (MCM), interior circular muscle (ICM) and submucosal circular muscle (SCM).

WCM (A) and SCM (D) both displayed a 2-4 contractions min−1 contractile rhythm. In contrast, both MCM (B) and ICM (C) exhibited slower contractions with superimposed rapid frequency contractions. The inset below B shows a 30 s period of rapid frequency contractions of MCM displayed at a more rapid sweep speed. A and D were recorded from muscle strips of one patient while B and C were recorded from muscle strips of another patient. All muscle strips were from sigmoid colon.
Table 1.
Contractile frequencies observed in human colonic circular muscle strips
| Slow contractions | Intermediate contractions | Fast contractions | |||||
|---|---|---|---|---|---|---|---|
| Muscle strip | Proportion of muscles with spontaneous activity (%) | Frequency (contractions min−1) | Proportion of muscles (%) | Frequency (contractions min−1) | Proportion of muscles (%) | Frequency (contractions min−1) | Proportion of muscles (%) |
| WCM (n = 28) | 100 | 0.9 ± 0.1 | 32 | 2.5 ± 0.2 | 89 | 16.7 ± 0.7 | 71 |
| MCM (n = 17) | 94 | 0.6 ± 0.1 | 82 | 2.4 ± 0.3 | 18 | 17.8 ± 1.0 | 94 |
| ICM (n = 15) | 87 | 0.3 ± 0.1 | 67 | 1.6 | 7 | 16.7 ± 1.3 | 80 |
| SCM (n = 19) | 100 | 0.5 ± 0.2 | 21 | 2.7 ± 0.2 | 89 | 15.7 ± 2.1 | 53 |
WCM, whole circular muscle; MCM, myenteric circular muscle; ICM, interior circular muscle; SCM, submucosal circular muscle. All contractile frequencies were determined after tissues were fully equilibrated and had reached a steady-state pattern of contractile activity. Percentages listed in each row add up to more than 100 % because multiple rhythms were sometimes superimposed upon one another.
To determine the possible site of origin of the 2-4 contractions min−1 contractile rhythm observed in WCM the contractile pattern of specific regions of the muscle layer was investigated. The predominant contractile pattern of MCM (n = 18) consisted of long duration contractions (0.6 ± 0.1 contractions min−1, n = 15) with superimposed small, rapid frequency contractions (see Fig. 3B). Interestingly, a similar pattern of activity was observed in strips of ICM (n = 15,Fig. 3C). In contrast, 89 % of SCM strips (i.e. 17/19 muscle strips) exhibited relatively uniform amplitude contractions at an intermediate frequency of 2.7 ± 0.2 contractions min−1 (n = 17, see Fig. 3D). Many SCM strips (i.e. 10/19 muscle strips or 53 %) also exhibited small, rapid frequency contractions superimposed upon the intermediate frequency contractions. The mean contractile frequencies and amplitudes observed in each region and the relative numbers of muscles exhibiting these patterns are shown in Table 1. All spontaneous contractile activity of WCM was abolished by nifedipine (1 μM, n = 4, data not shown). There were no obvious differences observed in the pattern of contractile activity in the various regions of muscle tested (i.e., ascending, descending and sigmoid) or between segments of muscle from non-diseased patients versus patients with neoplasms or diverticulitis.
It should be noted that the spontaneous contractile activity of colonic muscles changed during the initial equilibration period. When WCM strips were initially mounted in the recording chamber contractile complexes consisting of fused high frequency contractions averaging 10-20 contractions min−1 were often observed (Fig. 4Aa). With time (1-3 h) this pattern usually settled into the slower, intermediate pattern of contractile activity (Fig. 4Ba and described above). The intermediate contractile pattern was stable under in vitro conditions and persisted for many hours.
Figure 4. Time-dependent changes in contractile and electrical activity of human colonic whole circular muscle.
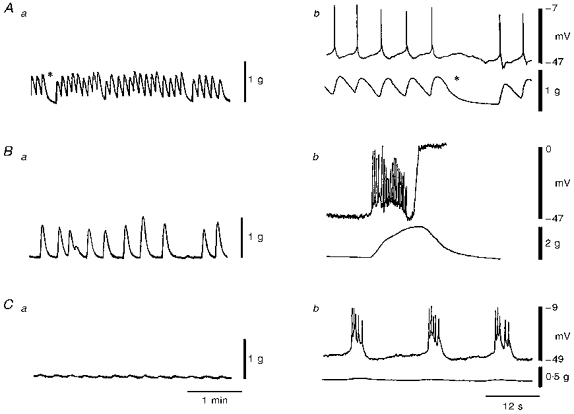
Aa shows the contractile pattern produced by a muscle strip 133 min after placing it in the recording chamber. This consisted of rapid phasic contractions at a frequency of 9 contractions min−1 with a few brief intermittent periods of relaxation (denoted *). In Ba the contractile activity of the same muscle strip as in A is shown at 207 min. At this time the frequency of contraction had declined to 3 contractions min−1 while the amplitude of contraction increased. This pattern continued for the next hour. Subsequent addition of wortmannin (5 μM) led to a marked reduction in contractile amplitude as shown in Ca obtained 36 min after exposure to wortmannin. Ab, Bb and Cb show examples of the electrical activity which accompanied contractions. Each panel includes an intracellular recording made near the submucosal edge (upper traces) and a contractile recording from the entire muscle layer (lower traces). Ab shows the same contractions as in Aa at a faster sweep speed. The asterisks in Aa and Ab represent the same moment in time. Phasic contractions were associated with actions potentials. Following development of the intermediate rhythm shown in Ba it was not possible to maintain impalements in cells. However, in another muscle strip a brief electrical recording was obtained as shown in Bb. This recording (made 226 min after placing a muscle strip in the recording chamber) reveals that contractions of the type shown in Ba were accompanied by slow waves. Due to the strength of contraction the impalement was lost. By including wortmannin in the superfusate it was possible to maintain impalements during contraction as shown in Cb. Cb shows the same contractions as in Ca at a faster sweep speed. This recording reveals that each phasic contraction was accompanied by a slow wave. Thus, time-dependent changes in contractile pattern were associated with the development of slow waves in the tissue. All recordings were made from strips of sigmoid colon.
Electrical activity of the circular muscle layer
The electrical activity of colonic muscles also changed during the equilibration period. The high frequency contractions observed initially were associated with spike potentials, recorded throughout the circular muscle (Fig. 4Ab). With time, the electrical activity at the submucosal edge changed to slow oscillations with superimposed spike potentials (Fig. 4Bb and 4Cb). Wortmannin (5 μM), by reducing the strong contractions of the tissues (Fig. 4Ca and 4Cb), facilitated maintenance of impalements without affecting electrical activity (Burke et al. 1996). Experiments usually began 20 min after addition of this agent, with all quantification of electrical activity coming from events following exposure to wortmannin. All intracellular recordings were made from WCM.
Activity of cells near the submucosal edge of the circular muscle
Cells near the submucosal edge of the circular muscle had resting potentials averaging -49.7 ± 0.6 mV (n = 24). Slow electrical oscillations (slow waves) were observed in 83 % of muscles. As shown in Fig. 5, slow waves were observed in the ascending, descending and sigmoid colon. These events averaged 12.1 ± 0.8 mV in amplitude and 9.4 ± 0.9 s in duration (n = 20). Slow waves occurred at a mean frequency of 3.2 ± 0.3 contractions min−1 (n = 20). Phasic contractions, when discernible following exposure to wortmannin, were accompanied by slow waves (Figs 4Cb and 5C and D) providing direct evidence that slow waves generate the 2-4 contractions min−1 rhythm. The membrane potential during the plateau phase of the slow wave averaged -37.6 ± 0.9 mV. In 75 % of muscles with slow waves, spikes were superimposed upon the slow waves averaging 26 ± 3 mV in amplitude (range 9−40 mV). The mean dV/dt of 938 spikes was 0.6 ± 0.02 V s−1 with a maximum value of 2.3 V s−1 for this group. The number of spikes per slow wave was relatively constant for a particular muscle strip (9 ± 1.5 spikes wave−1, n = 15), but varied substantially between preparations (range 1-22 spikes wave−1). Although the majority of cells displayed spiking activity, some cells exhibited slow waves without spikes suggesting that slow wave depolarizations are independent events and not merely a sustained depolarization during a burst of spikes. Contractions also accompanied slow waves without spikes (Fig. 5D). In 17 % of the muscles obvious slow waves were not observed. In these muscles spikes occurred, either continuously or organized into bursts (Fig. 5E), as described previously (Huizinga et al. 1986).
Figure 5. Electrical activity at the submucosal edge of whole circular muscle strips of the ascending (a), descending (B and D) or sigmoid (C and E) colon.

Records obtained from the tissue of five patients. Slow waves were recorded from all three regions examined and these typically had action potentials superimposed (A, B and C). In some preparations slow waves without spikes were observed (D). In a few muscles only spikes were recorded (E). Although all tissues contracted under control conditions, only C and D had discernible contractions in the presence of wortmannin (5 μM).
Activity of cells within the interior of the circular muscle
Membrane potential of cells midway through the thickness of the circular muscle layer averaged -46.1 ± 1.3 mV (n = 11). In 82 % of preparations slow waves were discernible but these were significantly smaller in amplitude than those recorded near the submucosal edge (6.9 ± 1.1 n = 9vs. 12.1 ± 0.8 mV, n = 20,P < 0.05). Examples of slow waves recorded at various distances from the submucosal edge in the various regions of colon studied are shown in Fig. 6A-C and mean slow wave amplitudes are plotted in Fig. 6D. Spikes recorded during the slow wave in this region had a mean of 33.7 ± 1.5 mV (n = 3) in amplitude. The mean dV/dt of fifty-eight spikes was 0.4 ± 0.03 V s−1 with a maximum value of 0.8 V s−1 for this group. MPOs were not observed in these cells.
Figure 6. Effect of distance from the submucosal edge on slow wave amplitude.

A, B and C show examples of the electrical activity recorded from cells near the submucosal edge (5 %), middle (50 %) and myenteric edge (95 %) of muscle strips isolated from the ascending (A), descending (B), and sigmoid colon (C) of three patients. D plots the mean amplitude (± s.e.m.) of slow waves recorded at various distances from the submucosal edge in fifteen muscle strips in which slow waves were present at the submucosal edge. N values for 50 % and 95 % were 9 and 8, respectively. All recordings were obtained in the presence of wortmannin (5 μM). *P < 0.05 and ***P < 0.001, significant differences in slow wave amplitude (i.e. 5 vs. 50 % and 50 vs. 95 %, respectively).
Activity of cells near the myenteric edge
Membrane potential near the myenteric edge of the circular muscle layer averaged -49.5 ± 1.6 mV (n = 22). This value was not significantly different from the values recorded from submucosal and interior circular muscle cells (P > 0.05). A highly variable pattern of activity was recorded at the myenteric edge, such that 18 % of muscles were electrically quiescent in this region even though normal activity was recorded elsewhere within the strips (e.g. see Fig. 6A) and inhibitory junction potentials could be evoked by electrical field stimulation (Fig. 7A). In 45 % of muscles oscillatory activity resembling the MPOs previously described in canine colon (Smith et al. 1987b) was observed (Fig. 7B). This activity varied in amplitude (1−15 mV) between preparations and had a mean frequency of 17.8 ± 0.8 contractions min−1 (n = 10). The MPOs also tended to ‘wax and wane’ in amplitude and gave rise to variable amplitude action potentials (15−30 mV, Fig. 7C). The mean dV/dt of 215 spikes was 0.3 ± 0.01 V s−1 with a maximum value of 0.7 V s−1 for this group. In 38 % of muscles oscillatory activity was absent but slow waves averaging 3.5 ± 0.9 mV (n = 8) could be detected (Fig. 7D). These slow waves were significantly smaller than the ones recorded from the interior (i.e. 3.5 ± 0.9, n = 8,vs. 6.9 ± 1.1 mV, n = 9, respectively, P < 0.05). Since MPOs were not observed when slow waves were present at the myenteric edge it suggests that slow waves tend to suppress other electrical activity within the muscle. A similar observation and conclusion was made in studies of the canine colon (Smith et al. 1987b). There was no clear separation of electrical patterns into colonic region (i.e. ascending vs. descending vs. sigmoid).
Figure 7. Electrical activity observed in cells near the myenteric edge of whole circular muscle.

Records obtained from tissues of four patients. A shows a recording from a muscle strip (sigmoid colon) which did not have any oscillating electrical activity at the myenteric edge. Cell impalement was confirmed by electrically evoking an inhibitory junction potential (IJP, denoted by n.s.; single pulse, 0.3 ms, 15 V). Note the rebound excitation following the IJP. B shows a recording from another muscle strip (descending colon) which exhibited ongoing MPOs (21contractions min−1) which were relatively constant in amplitude. C shows a recording from a third muscle strip (descending colon) in which MPOs gave rise to variable amplitude action potentials. D shows a recording from a fourth muscle strip (descending colon) which exhibited slow waves with superimposed action potentials. All recordings obtained in the presence of wortmannin (5 μM).
Spontaneous transient hyperpolarizations
In addition to MPOs and slow waves, another electrical event was observed in muscle strips from 50 % of patients (fifteen muscle strips). This activity consisted of spontaneous transient hyperpolarizations which typically occurred in bursts at unpredictable intervals. These events were observed in cells near both the myenteric (n = 8,Fig. 8A) and submucosal edges (n = 8,Fig. 8B) of the human colon. Transient hyperpolarizations were similar in time course to the inhibitory junction potentials that could be elicited with electrical field stimulation of nerves (Fig. 8C). Due to the highly unpredictable occurrence of these events we were unable to perform experiments to determine the origin of these events. However, the transient hyperpolarizations may have been due to spontaneous release of inhibitory transmitters, as observed in other colonic muscles (Smith, Reed & Sanders, 1989; Lyster, Bywater & Taylor, 1995; Keef et al. 1997). There was no consistent correlation between the occurrence of transient hyperpolarizations and nerve stimulation.
Figure 8. Spontaneous transient hyperpolarizations within whole circular muscle strips.

Records obtained from tissues of two patients. A shows an intracellular recording from a cell near the myenteric edge of a muscle strip (descending colon). Small (≈1 mV) MPOs are apparent in the recording. IJPs were evoked when nerves were electrically stimulated (NS) with a single pulse (first arrow; 0.3 ms, 15 V) or 5 pulses at 5 Hz (second and third arrows; 0.3 ms, 15 V). Following the third stimulus pulse a burst of transient hyperpolarizations was observed which led to a peak hyperpolarization to −80 mV. B shows an intracellular recording from a cell near the submucosal edge of the muscle strip (sigmoid colon). Slow waves (3 contractions min−1) which occasionally gave rise to action potentials are apparent in the recording. An IJP was elicited at the arrow with a single pulse (NS). This was followed by a period of rebound excitation. One minute later a series of transient hyperpolarizations appeared superimposed upon slow waves which led to a peak hyperpolarization to −76 mV. In C IJPs (upper traces) and transient hyperpolarizations (lower traces) observed during the same impalement have been plotted on an expanded time scale to show that these events were similar in time course. Left traces: electrical events recorded near the myenteric edge. Right traces: electrical events recorded near the submucosal edge. All recordings obtained in the presence of wortmannin (5 μM).
DISCUSSION
A number of previous studies have described the electrical activity of human colon (see inter aliaKirk, 1981; Kubota et al. 1983; Huizinga et al. 1985, 1986; Chow & Huizinga, 1987; Huizinga & Waterfall, 1988; Riezzo et al. 1992). It is difficult to relate the electrical and mechanical activities reported in these studies to the activities of common animal models of similar size, and the question has been raised as to whether the organization of the electrical activity of the human colon differs fundamentally from these other animal models. The present study investigated the spatial characteristics of pacemaking in the human colonic circular muscle layer. Our results suggest that the human colon shares some important similarities in the organization of electrical activity and electromechanical coupling with other animal models such as dog (see Sanders & Smith, 1989), pig (Huizinga et al. 1983, 1987) and cat (Du & Conklin, 1989).
Human colonic circular muscle strips were spontaneously active in the presence of TTX, atropine, L-NAME, phentolamine and propranolol suggesting that the activity is non-neurogenic (Huizinga et al. 1985; Keef, Du, Ward, McGregor & Sanders, 1993 and the present study). Three basic electrical events were observed: (i) slow wave activity with a mean frequency of 2-4 contractions min−1; (ii) ‘pre-potential’ oscillations similar to the MPOs described in canine colonic muscles (Smith et al. 1987b) with a mean frequency of 18 contractions min−1; and (iii) action potentials superimposed upon slow waves and MPOs. Slow waves were associated with phasic contractions which also occurred at a frequency of 2-4 contractions min−1. A similar 2-4 contractions min−1 rhythm has been recorded in vivo in human colon with barostat, manometric and extracellular electrical recording techniques (Taylor, Duthie, Smallwod & Linkens, 1975; Snape, Carlson & Cohen, 1977; Latimer, Sarna, Campbell, Latimer, Waterfall & Daniel, 1981; Frieri, Parisi, Corazziari & Caprilli, 1983; Narducci, Bassotti, Gaburri & Morelli, 1987; Steadman, Phillips, Camilleri, Haddad & Hanson, 1991; Ford, Camilleri, Wiste & Hanson, 1995).
An important difference between the electrical recordings made in the present study versus previous in vitro studies of the human colon is that muscles were positioned such that the entire thickness of the circular muscle layer was exposed making it possible to direct the microelectrode accurately to specific sites within this layer. Using this approach we found that slow waves were greatest in amplitude in cells at the submucosal edge and declined as a function of distance away from this edge. This observation suggested that slow waves arose near the submucosal edge. This conclusion was further supported by contractile studies which demonstrated that contractions occurring at the slow wave frequency (i.e. 2-4 contractions min−1) predominated in submucosal strips. A different contractile rhythm was present in strips of MCM and ICM which lacked the submucosal edge. There is substantial evidence to suggest that the cells responsible for generating slow waves in animal models are interstitial cells of Cajal (ICC; Berezin, Huizinga & Daniel, 1988; Langton, Ward, Carl, Norell & Sanders, 1989). ICCs have also been identified at the submucosal edge of the human colonic circular muscle layer (Rumessen, Peters & Thuneberg, 1993), and it is reasonable to suggest that these cells also serve a similar pacemaker function in the human colon.
In this study slow waves were recorded in 83 % of preparations near the submucosal edge as well as from each region of the colon studied (i.e. ascending, descending and sigmoid). Similar spontaneous slow wave activity was not reported in previous studies of the human colon (Kirk, 1981; Kubota et al. 1983; Huizinga et al. 1985, 1986; Chow & Huizinga, 1987; Huizinga & Waterfall, 1988; Riezzo et al. 1992). There are a number of possible reasons why these events were not detected in previous studies. First, most studies have utilized either sucrose gap or suction electrode techniques. Since human colonic slow waves are much smaller in amplitude than those of preparations such as canine colon (i.e. 12 vs. 45 mV) they may be more difficult to detect with these recording methods. Second, we found that slow waves required some time to develop. This may be due to a variety of factors including the trauma of surgery and dissection as well as the re-establishment of ionic gradients following prolonged storage at 4°C. Shortly after dissection and mounting tissues in the chamber, we often observed continuous spiking activity (see Fig. 4). However, once slow waves and the 2-4 contractions min−1 contractile pattern developed, this behaviour persisted for many hours, suggesting that it was a normal characteristic of the tissue rather than a transient stage of run-down. The presence of a similar frequency of contractions in vivo (Taylor et al. 1975; Narducci et al. 1987; Steadman et al. 1991; Ford et al. 1995) further supports the notion that slow waves are a physiological feature of human colonic electrical activity. The forceful contractions (averaging 64 mN mm−2 for strips of WCM) associated with slow waves and action potentials produce an additional problem limiting the fidelity of recording from human colonic muscles. This activity usually dislodges microelectrodes from cells, making long-term recording extremely difficult. Wortmannin, an inhibitor of myosin light chain kinase (Nakanishi et al. 1992), reduced the force of contractions, making an intracellular characterization of electrical events possible. Wortmannin reduces contractile amplitude without affecting underlying electrical events in other gastro-intestinal (GI) muscles (Burke et al. 1996). In a few instances we were able to record slow waves in the absence of wortmannin, confirming that slow waves are not an artifact of wortmannin treatment in the human colon.
Resting membrane potential throughout the human colonic circular muscle layer averaged −50 mV and no gradient of potential was observed from one edge to the other. A similar resting membrane potential has been reported for human colonic circular muscle cells impaled through the submucosal surface (Huizinga et al. 1985). Although slow waves averaged only 12 mV in amplitude the resulting depolarization was still sufficient to bring membrane potential into the range previously described as the ‘mechanical threshold’ (Szurszewski, 1987). This is the potential range at which significant Ca2+ entry occurs through L-type Ca2+ channels (Langton, Burke & Sanders, 1989; Xiong, Sperelakis, Noffsinger & Fenoglio-Preiser, 1995). Thus, it is likely that slow waves couple to contraction via Ca2+ entry through voltage-dependent Ca2+ channels. Action potentials super-imposed upon slow waves would tend to further enhance this Ca2+ entry. This hypothesis was supported by our observation that all spontaneous contractions were abolished by the L-type Ca2+ channel blocker nifedipine.
In addition to slow waves, another electrical oscillation was observed which resembled the MPOs previously described in the canine colon (Smith et al. 1987b). Interestingly, MPOs were only recorded near the myenteric edge of WCM strips. A plexus of ICCs is located in this region in human colon (Faussone-Pellegrini, Pantalone & Cortesini, 1990) and it is possible that these ICCs are responsible for generating the MPOs observed in this study. However, the situation is complicated by the fact that most strips of ICM (i.e. 87 %) also contracted spontaneously. Furthermore, the pattern of contractile activity in these interior strips was the same as that observed in MCM strips. This suggests that some cell types present within the interior of the muscle layer are also capable of generating spontaneous contractile and electrical activity. One possibility is that this activity is due to ICCs which penetrate into the interior of the circular muscle layer along septal structures (Rumessen et al. 1993). Previous studies in the canine colon have shown that slow waves can be recorded near septal structures in the circular muscle layer following removal of the submucosal pacemaker region (Ward & Sanders, 1990). Another possibility is that the phasic contractions observed in strips of MCM and ICM reflect spontaneous activity of smooth muscle cells. Isolated human colonic smooth muscle cells exhibit substantial voltage-dependent Ca2+ channel current (Xiong et al. 1995), and the relatively depolarized level of human circular muscles may allow spontaneous spiking to be generated directly by the smooth muscle.
The pattern of contraction of WCM was not a simple summation of the patterns observed in sub-segments of this muscle layer. In particular, the slow rhythm typical of MCM and ICM strips was under-represented in WCM strips (see Table 1) while the intermediate 2-4 contractions min−1 rhythm was represented in equal proportion in both WCM and SCM (i.e.89 %). This suggests that electrical activity emanating from the submucosal region may in some way predominate over the myenteric region of the intact human colon.
In summary, human colonic muscles were spontaneously active and displayed a prominent 2-4 contractions min−1 contractile rhythm which was associated with slow waves. Our results suggest that slow waves arise from cells located at, or near, the submucosal edge of the muscle layer. An additional electrical oscillation was observed in recordings made near the myenteric edge. These resembled the MPOs previously described in the canine model. However, the nature and site of origin of these potential oscillations remains unclear. Our results suggest that pacemaking in the human colon bears more similarities to other animal models, such as the dog, than previously thought.
Acknowledgments
This study was supported by NIH grants DK45376 to K. D. K. and D. K., DK41315 to K.M.S. and by postdoctoral fellowship money from the National Science Foundation, Nevada Experimental Program to Stimulate Competitive Research (EPSCoR) Award EPS 9353227, and with a Women in Science and Engineering (WiSE) component.
References
- Burke EP, Gerthoffer WT, Sanders KM, Publicover NG. Wortmannin inhibits contraction without altering electrical activity in canine gastric smooth muscle. American Journal of Physiology. 1996;270:C1405–1412. doi: 10.1152/ajpcell.1996.270.5.C1405. [DOI] [PubMed] [Google Scholar]
- Berezin I, Huizinga JD, Daniel EE. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. Journal of Comparative Neurology. 1988;273:42–51. doi: 10.1002/cne.902730105. [DOI] [PubMed] [Google Scholar]
- Chow E, Huizinga JD. Myogenic electrical control activity in longitudinal muscle of human and dog colon. The Journal of Physiology. 1987;392:21–34. doi: 10.1113/jphysiol.1987.sp016767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Conklin JL. Origin of slow waves in the isolated proximal colon of the cat. Journal of the Autonomic Nervous System. 1989;28:167–178. doi: 10.1016/0165-1838(89)90089-1. 10.1016/0165-1838(89)90089-1. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Pantalone D, Cortesini C. Smooth muscle cells, interstitial cells of Cajal and myenteric plexus interrelationships in the human colon. Acta Anatomica. 1990;139:31–44. doi: 10.1159/000146975. [DOI] [PubMed] [Google Scholar]
- Ford MJ, Camilleri M, Wiste JA, Hanson RB. Differences in colonic tone and phasic response to a meal in the transverse and sigmoid human colon. Gut. 1995;37:264–269. doi: 10.1136/gut.37.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieri G, Parisi F, Corazziari E, Caprilli R. Colonic electromyography in chronic constipation. Gastroenterology. 1983;84:737–740. [PubMed] [Google Scholar]
- Huizinga JD, Chow E, Diamant NE, El-Sharkawy TY. Coordination of electrical activities in muscle layers of the pig colon. American Journal of Physiology. 1987;252:G136–142. doi: 10.1152/ajpgi.1987.252.1.G136. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Diamant NE, El Sharkawy TY. Electrical basis of contractions in the muscle layers of the pig colon. American Journal of Physiology. 1983;245:G482–491. doi: 10.1152/ajpgi.1983.245.4.G482. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Stern HS, Chow E, Diamant NE, El-Sharkawy TY. Electrophysiologic control of motility in the human colon. Gastroenterology. 1985;88:500–511. doi: 10.1016/0016-5085(85)90513-x. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Stern HS, Chow E, Diamant NE, El-Sharkawy TY. Electrical basis of excitation and inhibition of human colonic smooth muscle. Gastroenterology. 1986;90:1197–1204. doi: 10.1016/0016-5085(86)90385-9. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Waterfall WE. Electrical correlate of circumferential contractions in human colonic circular muscle. Gut. 1988;29:10–16. doi: 10.1136/gut.29.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef KD, Du C, Ward SM, McGregor DB, Sanders KM. Enteric inhibitory neural control of human colon: role of nitric oxide. Gastroenterology. 1993;105:1009–1016. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- Keef KD, Murray DC, Sanders KM, Smith TK. Basal nitric oxide induces an oscillatory motor pattern in canine colon. The Journal of Physiology. 1997;499:773–786. doi: 10.1113/jphysiol.1997.sp021968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef KD, Ward SM, Stevens RJ, Frey BW, Sanders KM. Electrical and mechanical effects of acetylcholine and substance P in sub-regions of colonic muscle. American Journal of Physiology. 1992;262:G298–307. doi: 10.1152/ajpgi.1992.262.2.G298. [DOI] [PubMed] [Google Scholar]
- Kirk D. An electrophysiological study of the smooth muscle of the human colon. Annals of the Royal College of Surgery. 1981;63:393–398. [PMC free article] [PubMed] [Google Scholar]
- Kubota M, Ito Y, Ikeda K. Membrane properties an innervation of smooth muscle cells in Hirschsprung's disease. American Journal of Physiology. 1983;244:G406–415. doi: 10.1152/ajpgi.1983.244.4.G406. [DOI] [PubMed] [Google Scholar]
- Langton PD, Burke EP, Sanders KM. Participation of Ca currents in colonic electrical activity. American Journal of Physiology. 1989;257:C451–460. doi: 10.1152/ajpcell.1989.257.3.C451. [DOI] [PubMed] [Google Scholar]
- Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proceedings of the National Academy of Sciences of the USA. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer P, Sarna S, Campbell D, Latimer M, Waterfall W, Daniel EE. Colonic motor and myoelectrical activity. A comparative study of normal subjects, psychoneurotic patients and patients with irritable bowel syndrome. Gastroenterology. 1981;80:893–901. [PubMed] [Google Scholar]
- Lyster DJK, Bywater RAR, Taylor GS. Neurogenic control of myoelectric complexes in the mouse isolated colon. Gastroenterology. 1995;108:1371–1378. doi: 10.1016/0016-5085(95)90684-3. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Kakita S, Takahashi I, Kawahara K, Tsukuda E, Sano T, Yamada K, Yoshida M, Kase H, Matsuda Y, Hashimoto Y, Nonomura Y. Wortmannin, a microbial product inhibitor of myosin light chain kinase. Journal of Biological Chemistry. 1992;267:2157–2163. [PubMed] [Google Scholar]
- Narducci F, Bassotti G, Gaburri M, Morelli A. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut. 1987;28:17–25. doi: 10.1136/gut.28.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezzo G, Maselli MA, Pezzolla F, Thouvenot J, Giorgio I. In vitro electromechanical activity of the human colon. Simultaneous recording of the electrical patterns of the two muscle layers. Archives Internationales de Physiologie, de Bichimie et de Biophysique. 1992;100:93–100. doi: 10.3109/13813459209035266. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ, Peters S, Thuneberg L. Light and electron microsopical studies of interstitial cells of Cajal (ICC) and muscle cells at the submucosal border of human colon. Laboratory Investigation. 1993;68:481–495. [PubMed] [Google Scholar]
- Sanders KM, Smith TK. Electrophysiology of colonic smooth muscle. In: Schultz SG, Wood JD, Rauner BB, editors. Handbook of Physiology, The Gastrointestinal System. I. Bethesda, MD, USA: American Physiological Society; 1989. pp. 251–271. section 6. [Google Scholar]
- Sanders KM. Ionic mechanisms of electrical rhythmicity in gastrointestinal smooth muscles. Annual Review of Physiology. 1992;54:439–453. doi: 10.1146/annurev.ph.54.030192.002255. 10.1146/annurev.ph.54.030192.002255. [DOI] [PubMed] [Google Scholar]
- Sarna SK. Myoelectric correlates of colonic motor complexes and contractile activity. American Journal of Physiology. 1986;250:G213–220. doi: 10.1152/ajpgi.1986.250.2.G213. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. American Journal of Physiology. 1987a;252:C215–224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB, Sanders KM. Interaction of two electrical pacemakers in muscularis of canine proximal colon. American Journal of Physiology. 1987b;252:C290–299. doi: 10.1152/ajpcell.1987.252.3.C290. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB, Sanders KM. Electrical pacemakers of canine proximal colon are functionally innervated by inhibitory motor neurons. American Journal of Physiology. 1989;256:C466–477. doi: 10.1152/ajpcell.1989.256.3.C466. [DOI] [PubMed] [Google Scholar]
- Snape WJ, Carlson GM, Cohen S. Human colonic myoelectric activity in response to prostigmine and the gastrointestinal hormones. American Journal of Digestive Diseases. 1977;22:881–887. doi: 10.1007/BF01076164. [DOI] [PubMed] [Google Scholar]
- Steadman CJ, Phillips SF, Camilleri M, Haddad AC, Hanson RB. Variation of muscle tone in the human colon. Gastroenterology. 1991;101:373–381. doi: 10.1016/0016-5085(91)90014-c. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 2. New York: Raven Press; 1987. pp. 383–423. chap. 12. [Google Scholar]
- Taylor I, Duthie HL, Smallwood R, Linkens D. Large bowel myoelectrical activity in man. Gut. 1975;16:808–814. doi: 10.1136/gut.16.10.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Pacemaker activity in septal structures of canine colonic circular muscle. American Journal of Physiology. 1990;259:G264–273. doi: 10.1152/ajpgi.1990.259.2.G264. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Ca2+ currents in human colonic smooth muscle cells. American Journal of Physiology. 1995;269:G378–385. doi: 10.1152/ajpgi.1995.269.3.G378. [DOI] [PubMed] [Google Scholar]


