Abstract
Denervated fast extensor digitorum longus (EDL) muscles of adult rats were stimulated electrically for up to 4 months with a ‘slow’ pattern resembling the activity in soleus (Sol) motor units and examined with antibodies against myosin heavy chains (MHCs).
The normal EDL contained, on average, 45 % type IIB, 29 % type IIX, 23 % type IIA and 3 % type I fibres. All type IIB and almost all type IIX fibres disappeared during the first 3 weeks of stimulation. They were replaced by type IIA and type I fibres, whose percentages increased to about 75 and 15, respectively. Type IIA fibres remained at 75 % for nearly 2 months and were then gradually replaced by type I fibres during the next 2 months. The transformation occurred sequentially in the order IIB/IIX → IIA → I, the first step (IIB/IIX → IIA) occurring after a short delay (2 weeks) and the last step (IIA → I in originally IIB or IIX fibres) after a long delay (> 2 months). During the transformation coexpression of MHCs occurred.
It appears that the transformation to type I fibres occurred in pre-existing type II fibres since no signs of fibre damage or regeneration were observed.
Normal EDL was also stimulated through an intact nerve with the same pattern for up to 37 days. The effects on fibre type distributions were identical to those observed in the denervated EDL. The result indicated that the Sol-like pattern of evoked muscle activity, rather than nerve-derived trophic influences or denervation per se, was primarily responsible for the fast to slow transformation.
Hindlimb skeletal muscles of most adult mammals, including rodents, consist of up to four main types of muscle fibres: IIB, IIX (or IID), IIA and I (or β/slow). These fibre types contain correspondingly named myosin heavy chains (MHCs) encoded by separate MHC genes and occur in different combinations in individual muscles (for reviews see Pette & Staron, 1990; Schiaffino & Reggiani, 1996). Muscle fibres adapt to different usage by undergoing transformation from one type to another and such transformations can occur after cross-reinnervation (Buller, Eccles & Eccles, 1960; Close, 1969), during electrical stimulation of peripheral nerves or muscles (Salmons & Vrbová, 1969; Lømo, Westgaard & Dahl, 1974; Salmons & Sréter, 1976; for reviews see Pette & Vrbová, 1992; Schiaffino & Reggiani, 1996; Gundersen, 1998) and, to a smaller degree, during exercise (Schantz & Henriksson, 1983) and postnatal development (Kugelberg, 1976).
Several questions remain unresolved with respect to the mechanisms underlying these transformations. One question concerns whether the transformation can be complete in the sense that, for example, a fast type IIB fibre in the adult extensor digitorum longus (EDL) muscle can acquire all the properties of slow type I fibre in the adult soleus (Sol) muscle and vice versa. The question is important because if the answer is no, adult fast and slow muscle fibres would probably be intrinsically different and belong to differently determined cell lineages. Existing data suggest that transformations can be complete for some properties but not for others. It has therefore been proposed that contractile properties adjust to different usage within intrinsically different adaptive ranges that may overlap to different degrees for different properties (Westgaard & Lømo, 1988). For example, the Sol may become as fast as the EDL with respect to the isometric twitch time to peak but not with respect to isotonic intrinsic maximum shortening velocity (Vmax) or IIB MHC expression after reinnervation by EDL motoneurones or direct electrical stimulation with an EDL-like pulse pattern (Close, 1969; Gutmann & Carlson, 1975; Eken & Gundersen, 1988; Schiaffino, Ausoni, Gorza, Saggin, Gundersen & Lømo, 1988; Westgaard & Lømo, 1988; Ausoni, Gorza, Schiaffino, Gundersen & Lømo, 1990; Hämäläinen & Pette, 1996). The EDL, on the other hand, may become as slow as the Sol with respect to MHC expression (type I) and intrinsic Vmax after reinnervation with Sol motoneurons but not with respect to the isometric twitch time to peak, which remains considerably faster even 3 months to more than a year later (Close, 1969; Gutmann & Carlson, 1975; Mira, Janmot, Couteaux & D'Albis, 1992). Intermittent slow-pattern electrical stimulation of the denervated or innervated EDL for up to two months similarly induces an intermediate twitch speed (Eken & Gundersen, 1988; Westgaard & Lømo, 1988) but fails to induce type I MHC expression or a Sol-like intrinsic Vmax (Eken & Gundersen, 1988; Termin, Staron & Pette, 1989; Ausoni et al. 1990; Delp & Pette, 1994). On the other hand, continuous low frequency stimulation of the nerve to the EDL over a comparable time does induce variable amounts of type I MHC expression (Mayne, Mokrusch, Jarvis, Gilroy & Salmons, 1993). At present, it is not clear whether such differences and signs of incomplete transformation are related to the presence or absence of innervation, pattern of stimulation, or duration of experiment.
A second question dates back to Buller et al. (1960) and asks whether non-junctional properties, such as extrajunctional acetylcholine receptor (AChR) expression or contractile proteins, are controlled by nerve-derived trophic factors and/or electrical muscle activity. Evidence for trophic factors acting independently of electrical muscle activity has been reported (e.g. Salviati, Biasia & Aloisi, 1986; Witzemann, Brenner & Sakmann, 1991) but so has evidence against (e.g. Pasino, Buffelli, Arancio, Busetto, Salviati & Cangiano, 1996). To separate the effects of nerve-derived trophic factors and electrical muscle activity one may stimulate denervated muscles electrically but this approach has been questioned because the effects may reflect more the denervation per se than the stimulation (Al-Amood, Finol & Lewis, 1986). To use indirect stimulation through the nerve may also be questioned because nerve stimulation may affect the production and delivery of trophic factors to the muscle.
The present work addresses both these questions with respect to MHC expression in adult rat EDL muscles. In one series of experiments we denervated the EDL to remove influences derived from the nerve and stimulated the EDL directly with a pulse pattern comparable to that generated by Sol motoneurones in freely moving rats (Hennig & Lømo, 1985). Since Sol motoneurones appear to fire approximately normally after innervating antagonistic muscles (O'Donovan, Pinter, Dum & Burke, 1985; Gordon, Stein & Thomas, 1986), cross-reinnervated and electrically stimulated EDL muscles would be expected to undergo equivalent transformation if activity pattern is the responsible factor. To find out if the failure to induce type I MHC expression reported earlier (Termin et al. 1989; Ausoni et al. 1990; Delp & Pette, 1994) was due to stopping the stimulation too early, we extended the duration of stimulation from 2 to 4 months. We also examined the time course of the effects of stimulation to see if the changes in MHC expression occur sequentially in directly stimulated denervated muscles as they do in muscles stimulated through the nerve (Pette & Vrbová, 1992). Finally, we stimulated the EDL indirectly through the peroneal nerve with an identical stimulus pattern to see if the effects of stimulation obtained after denervation could be accounted for by an effect of denervation per se.
The results show that long-term electrical stimulation of innervated and denervated EDL muscles has similar effects and that transformation from type IIB to type I MHC can occur sequentially in the absence of innervation, indicating that pattern of electrical muscle activity rather than nerve-derived trophic factors is responsible for fast to slow transformation in the adult rat EDL.
METHODS
Young adult male Wistar rats were used, obtained from Møllegaards Breeding Centre Ltd (Skensved, Denmark) and weighing 250–300 g at the beginning of the experiment. All operations described below were carried out under full anaesthesia with Equithesin (42.5 mg chloral hydrate and 9.7 mg pentobarbitone in 1 ml solution, 0.4 ml (100 g body wt)−1, i.p.), as assessed by the absence of reflex muscle contractions and limb withdrawal to squeezing the skin during surgery. The muscles were removed under full anaesthesia as above and the animals given additional Equithesin and killed by cervical dislocation.
Denervation and chronic stimulation
The sciatic nerve was cut in the thigh and its proximal end reflected and sutured to the subcutis to prevent reinnervation. A longitudinal opening through skin and fascia was made along the lateral aspect of the tibialis anterior muscle (TA) and the TA belly moved medially with small hooks to expose the underlying EDL. The ends of two flexible, multistranded steel wires (AS 632, Cooner Sales Wire Co., Chatsworth, CA, USA) with their Teflon insulation removed for the distal 20–25 mm were placed obliquely across the EDL in a proximo-distal direction, one wire on the anterior side, the other wire on the posterior side distal to the first, making sure that the uninsulated wires touched muscle fibres and not tendons as they ran across the entire muscle without possibility of contact between them. Each wire was fixed to the tissue a few millimetres away from the EDL with a thin supramid thread which was placed just above a small knot on the insulated part of the wire to prevent the wires from being pulled away from the EDL at later times. TA was then moved back to its normal position. Through openings in the skin on the back and head the insulated wires were pulled under the skin to the head and from there through protective plastic tubes (o.d., 0.6 mm) to rotating contacts fixed about 0.5 m above the rat. Along the back the wires made a loop, several centimetres long, to slacken the wires and prevent their distal ends from being pulled away from the EDL as the animal grew during the course of the experiments (up to 4 months). On the head the connective tissue over the bone was removed and three stainless steel bone screws (no. 40–77-8, Frederick Haer & Co, Brunswick, ME, USA) inserted such that there was just room for the protective tube between them. The exposed bone was cleaned of blood, allowed to dry, and covered with a thin film of dental glue (Scotchbond, 3M Dental Products Division, St Paul, MN, USA). Dental cement (Simplex Rapid, Austenal Dental Products Ltd, Harrow, UK) was then placed on the bone with the screws and the protective tube pushed into the cement before it cured. The distal end of the tube contained two short pins which had been pushed through its wall. These pins became lodged in the cement and prevented the tube from being pulled out of the cement. By keeping the protective tube relatively slack, the rat could move freely within the cage while being unable to reach the tube and break the connection to the stimulator placed on a shelf above.
Stimulation started on the day of denervation in thirty animals and lasted from 6 to 124 days. The stimulus was bipolar with a duration of 0.2 ms and an intensity of 10–15 mA in each direction. The stimulation pattern was 20 Hz for 10 s every 20 s. A master stimulator delivered the stimulus pattern to a series of custom-built operational amplifiers through which the current intensity could be adjusted individually for each rat. The stimulus output from each of these amplifiers went through a corresponding number of 100 Ω resistances, each resistance in series with one of the two stimulating electrodes in each rat. By measuring the potential drop across this resistance for each rat the current intensity passing through each EDL was monitored daily on an oscilloscope screen and adjusted to an intensity (10–15 mA) above the apparent maximum palpable contraction in the anterior muscle group. By reading the potential across one resistance after another it was easy to check regularly the stimulation circuit for each rat and often to repair it if breaks occurred. Contralateral innervated or denervated EDL muscles served as controls.
Indirect stimulation via the nerve
Electrodes identical to those used for direct muscle stimulation (see above) were fixed by sutures on each side of the common peroneal nerve at the level of the knee. The electrodes did not touch the nerve. Stimulation and monitoring were as described above except that the intensity was much lower, usually around 0.5 mA. Nine animals were stimulated in this way from 8 to 37 days.
Fibre typing
Serial transverse cryosections of control and stimulated muscles were incubated with monoclonal antibodies against different MHCs and bound antibody was visualized by immunoperoxidase staining as previously described (Gorza, Sartore, Thornell & Schiaffino, 1986). The following monoclonal antibodies (a gift from S. Schiaffino) were used: BF-F3 against type IIB MHC, dilution 1:100; BA-71 against type IIA MHC, 1:10; BF-35 against type IIB, IIA and I MHC, 1:10; BA-D5 against type I MHC, 1:10; and BF-G6 specific for embryonic MHC, 1:20 (Schiaffino, Gorza, Sartore, Saggin & Carli, 1986; Schiaffino et al. 1989). BF-35, which labels all fibre types in EDL except IIX, was used to identify pure IIX fibres. Percentages of fibre types were determined either by counting all of the fibres in whole muscle cross-sections stained with a particular antibody, or by counting fibres in randomly selected fields from about 30 % of the cross-section. The antibody against embryonic MHC was used to identify regenerating muscle fibres (Sartore, Gorza & Schiaffino, 1982). The hematoxylin/eosin and Van Gieson reactions were used to assess the histological appearance of the muscle tissue.
The experiments involving chronic muscle and nerve stimulations had been inspected and permitted by the Norwegian Experimental Board and Ethical Committee for Animal Experiments and were overseen by the veterinarian responsible for the animal house. During the experiments the animals were checked daily and did not behaviourally respond to the stimulation or appear to suffer any pain. The animals moved about in the cage and appeared to have the same eating, drinking and sleeping behaviour as unstimulated animals.
RESULTS
Denervated EDL muscles: effects of direct stimulation
Long-term effects
Figure 1A shows an example of the end result after 4 months of denervation plus stimulation. All the fibres reacted with the type I antibody, except for some fibres along the top edge of muscle which reacted with the type IIA antibody. In contrast, both the denervated (80 days, Fig. 1B) and the normal (Fig. 1C) EDL contained only a few scattered type I fibres, the others being type II. The denervated muscle was very atrophied and contained type I fibres that were considerably larger in diameter than the type II fibres, as previously described (Niederle & Mayr, 1978).
Figure 1. Long-term 20 Hz stimulation of denervated EDL muscle induces appearance of slow type I fibres.
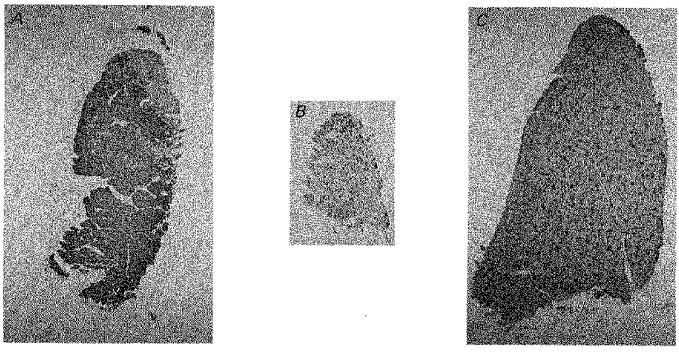
Cross-sections of EDL muscles that had been denervated plus stimulated for 124 days (A), denervated for 80 days (B), or was normal (C). The sections were stained with the BA-D5 antibody against type I MHC.
After 4 months of denervation plus stimulation the fibres appeared histologically normal, as illustrated by a representative field at larger magnification in Fig. 2. All the fibres in this field were labelled with the antibody against type IIA + IIB + I MHC (Fig. 2C) and unlabelled with the antibody against type IIB MHC (Fig. 2D). Therefore, these fibres were neither solely type IIX nor IIB. Three fibres were positive only for type IIA MHC (Fig. 2B). All the other fibres were positive for type I MHC (Fig. 2A) and were therefore pure type I fibres.
Figure 2. Denervated EDL contains mainly slow type I fibres after 4 months of 20 Hz stimulation.
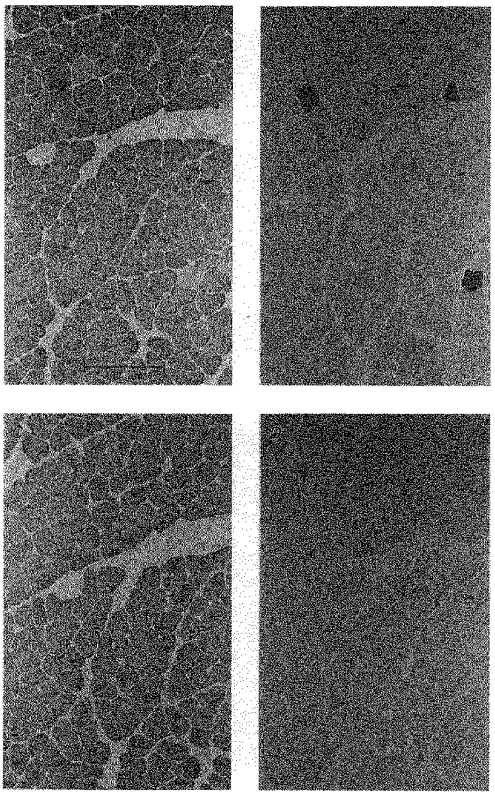
Serial cross-sections of an EDL muscle that had been denervated and stimulated for 124 days and then stained with antibodies against type I (A), IIA (B) and IIB (D) MHCs. The section in C was stained with the BF-35 antibody which reacts with types I, IIA, and IIB, but not IIX MHCs.
Sequential changes in fibre type composition
The muscle shown in Fig. 3A had been denervated and stimulated for 10 days. The majority of fibres (89 %) were labelled with the antibody against type IIA + IIB + I MHC (not shown). The remaining non-labelled fibres were therefore type IIX. Many of the labelled fibres (25 %) were also strongly labelled with the antibody specific for type IIA MHC (Fig. 3A) but not with the antibodies specific for type IIB or I (not shown). These fibres were therefore probably pure type IIA fibres. Other fibres labelled by the antibody against type IIA + IIB + I MHC were only weakly labelled by the antibody against type IIA MHC and not by the antibodies against type IIB and I MHCs. Therefore, these fibres probably contained type IIX MHC in addition to relatively small amounts of type IIA MHC. Figure 3B and C illustrates that after intermediate and long periods of denervation plus stimulation (43 and 124 days, respectively) the EDL contained very many (Fig. 3B) and very few (Fig. 3C) type IIA positive fibres, respectively. The fibres negative for IIA were instead labelled by the antibody against type I MHC (not shown).
Figure 3. Transient appearance of type IIA fibres in denervated and 20 Hz stimulated EDL muscles.
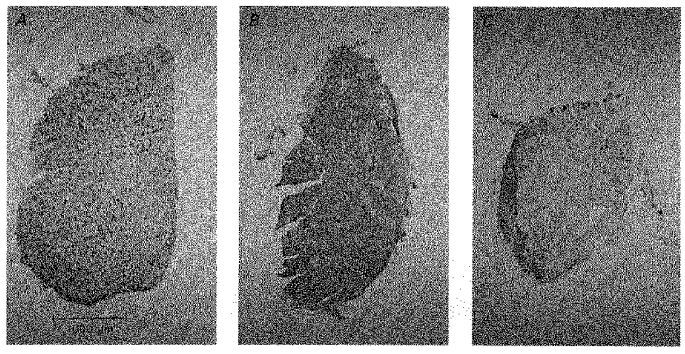
Cross-sections of EDL muscles after 10 (A), 43 (B), and 124 (C) days of denervation plus direct stimulation. The sections were stained with the BA-71 antibody against type IIA MHC.
Fibres labelled by both type I and IIA MHC (type II C fibres) were not observed in the normal EDL but appeared at low percentage (3–8 %) from the second week until the end of the experiment in both denervated and denervated plus stimulated muscles. Fibres labelled by both type IIB and IIA antibodies were not observed.
Figure 4 summarizes the effects of denervation and denervation plus stimulation on the distribution of type IIB, IIX, IIA and I fibres. After denervation alone, the percentage of IIB fibres slowly declined to a low value (< 10 %) over a period of about 2 months, while IIX disappeared between 3 and 8 weeks. Corresponding to these declines, the percentage of IIA fibres gradually increased from about 25 to 90 % and then stayed at this high level, while the percentage of type I fibres remained low (< 5 %). In contrast, within 3 weeks after denervation and onset of stimulation, the type IIB fibres disappeared, while the percentages of type IIA and type I fibres increased to around 75 and 20 %, respectively. Subsequently, type IIA fibres dominated until type I fibres started to replace them after 2 months of stimulation, in sharp contrast to the absence of changes in type IIA and type I fibres between 2 and 4 months after denervation alone. With respect to IIX fibres, denervation alone and denervation plus stimulation had similar effects except that their disappearance appeared to be somewhat faster during stimulation.
Figure 4. Time course of changes in fibre types during 20 Hz stimulation of denervated EDL muscles.
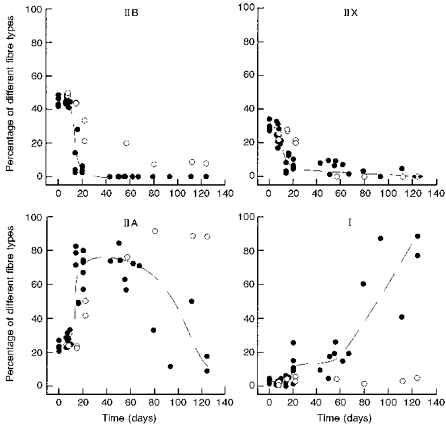
Percentage of different fibre types in EDL plotted against the length of time of denervation (^) or denervation plus direct stimulation (•). Each symbol represents one muscle. Curves fitted by eye are added.
Transformation of pre-existing fibres
All the muscles were inspected for signs of fibre damage, degeneration or regeneration, as indicated by accumulation of interstitial cells in sections treated with hematoxylin and eosin, presence of necrotic fibres as indicated by ragged outlines and invasion of mononuclear, presumably phagocytotic cells, and presence of small fibres reacting with the antibody against embryonic myosin and containing central nuclei. At no time after denervation and onset of stimulation were such signs of degeneration observed (Fig. 2) except along restricted parts of the surface in some muscles where, presumably, the stimulating electrodes had been lying. Furthermore, at no time did the fibres react with the antibody against embryonic MHC, except, again, along restricted parts of the surface of some muscles, as previously described for the denervated and directly stimulated rat Sol muscle (Gorza, Gundersen, Lømo, Schiaffino & Westgaard, 1988). Denervation and denervation plus stimulation did not change the number of fibres per muscle significantly and the number did not change during treatment. The mean number of fibres ± 95 % confidence limits was 3929 ± 165 (n = 30) in denervated plus stimulated muscles, 3913 ± 209 (n = 10) in denervated unstimulated muscles, and 3148 ± 573 (n = 6) in normal muscles. The variability was large for the normal group because one muscle had an unusually low number.
Innervated EDL muscles: effects of indirect stimulation
EDL muscles with intact innervation were stimulated through the peroneal nerve for 8–37 days. During this period the effects on fibre type distribution (Fig. 5) were indistinguishable from those observed during direct stimulation of the denervated muscle (Fig. 4). As in denervated plus stimulated muscles, fibres coexpressing type I and IIA MHCs were rare (3–8 %). There was no reaction with the antibody against embryonic MHC and histological signs of fibre damage were absent, indicating that the transformation occurred in pre-existing fibres in muscles stimulated through the nerve, in agreement with earlier reports (Delp & Pette, 1994). There were 3642 ± 267 fibres per muscle (n = 9), which is similar to that in denervated and denervated plus stimulated muscles.
Figure 5. Time course of changes in fibre types during 20 Hz stimulation of the nerve to EDL muscles.
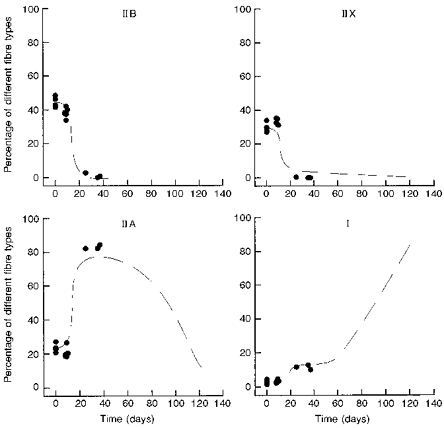
Percentage of different fibre types in innervated EDL muscles plotted against the length of time of stimulation through the peroneal nerve. The broken line is the same curve as in Fig. 4 and represents the effects of stimulating denervated EDL muscles.
DISCUSSION
The present results show that electrical stimulation of the denervated EDL with a pattern comparable to that generated by slow Sol motoneurones transformed the EDL into a muscle containing predominantly slow type I muscle fibres. In the absence of stimulation, no such upregulation of type I MHC occurred. If a pattern comparable to that generated by EDL motoneurones is applied, type IIB expression (Ausoni et al. 1990) and fast contractile properties (Eken & Gundersen, 1988) are maintained. Similar transformation from type IIB to type I MHC occurs in EDL muscles after cross-reinnervation by Sol motoneurones (Mira et al. 1992). After self-reinnervation by EDL motoneurones, on the other hand, the EDL retains its normal fast properties (Close, 1969). These similarities between direct muscle stimulation with slow and fast stimulus patterns on the one hand and reinnervation by slow and fast motoneurones on the other indicate that the pattern of muscle impulse activity and not putative nerve-derived trophic factors is responsible for fast to slow transformation in the EDL of the rat.
Slow type I fibres in normal EDL and Sol muscles have different isometric twitch times to peak, the time to peak of slow fibres in EDL being intermediate between that of slow Sol and fast EDL fibres (Close, 1967; Eken & Gundersen, 1988; Westgaard & Lømo, 1988). EDL muscles cross-reinnervated with the Sol nerve also express an intermediate isometric twitch time to peak although they acquire type I MHC (Close, 1969; Mira et al. 1992). Thus, cross-reinnervation by the Sol may induce complete fast to slow transformation of the EDL if comparison is made with type I fibres in the normal EDL but not if comparison is made with type I fibres in the normal Sol. Electrical stimulation of denervated rat EDL muscles for 2 months slows the twitch time to peak to the same intermediate value (∼21 ms) as in normal slow and cross-reinnervated EDL muscle fibres (Close, 1969; Eken & Gundersen, 1988; Westgaard & Lømo, 1988). Whether longer periods of stimulation would have slowed the twitch to values typical of the normal Sol (37–40 ms) is not known but appears unlikely since reinnervation with the Sol nerve fails to do so within 1.5 years when the EDL stays in its own bed (Close, 1969) or within at least 3 months when the EDL is transplanted to the bed of the Sol (Gutmann & Carlson, 1975). Failure to induce a slow, Sol-like twitch in EDL is an example of a property that is difficult to induce in one muscle but is normally present in the other. Another example is the difficulty to induce IIB MHC expression and an EDL-like intrinsic Vmax by electrical stimulation or cross-reinnervation of the Sol (Close, 1969; Eken & Gundersen, 1988; Schiaffino et al. 1988; Ausoni et al. 1990; Hämäläinen & Pette, 1996). In the present experiments on the EDL, type I MHC started to appear in the majority of the fibres only after 2 months of slow-pattern stimulation. Thus, many EDL fibres showed marked resistance to expressing type I MHC, which explains why earlier experiments based on up to 2 months of slow-pattern stimulation of the EDL failed to show transformation to type I MHC (Termin et al. 1989; Ausoni et al. 1990; Delp & Pette, 1994). Available evidence, therefore, supports the concept that rat EDL and Sol muscles are intrinsically different muscles that adjust their properties to different types of activity within adaptive ranges that may fully overlap with respect to some of these properties but not with respect to others (Westgaard & Lømo, 1988).
It is important to know whether transformation of fibre types occurs in original fibres that maintain their integrity throughout the period of transformation or is the result of generation of new fibres. In the present work, signs of fibre degeneration or regeneration were not observed at any time during the period of transformation, except occasionally in superficial regions near the stimulating electrodes. This finding strongly suggests that the fast to slow transformation occurred in pre-existing fibres and was not the result of formation of new fibres. In agreement with this conclusion previous work has shown no histological evidence of fibre degeneration and regeneration in the rat EDL during chronic low frequency stimulation (5–20 Hz) (Mayne et al. 1993; Delp & Pette, 1994), whereas such evidence is reported for the fast-twitch tibialis anterior muscle in the rabbit (Maier, Gambke & Pette, 1986).
The transformation from type IIB and IIX fibres to type I fibres occurred in two stages, an early rapid stage to type IIA fibres and a late stage to type I fibres starting after about 2 months. The early stage took place during the third week when type IIA MHC replaced type IIB and IIX MHCs. Given a half-life for IIB MHC of about 15 days (Termin et al. 1989), the rapid disappearance of type IIB and IIX fibres suggests that the stimulation blocked the expression of IIB and IIX MHCs very rapidly, if not immediately. In the absence of specific antibodies against IIX MHC it was not possible to demonstrate coexpression of IIB/IIX or IIX/IIA MHCs. During the transformation, however, many fibres that did not stain for type IIB or type I MHCs stained only very weakly for IIA MHC, suggesting that they coexpressed type IIX MHCs. Coexpression of IIX and IIB MHC was more uncertain because the antibody specific against IIB MHC stained IIB fibres relatively weakly. However, coexpression of type IIB/IIX and IIX/IIA MHCs is well documented both in normal and indirectly stimulated fast-twitch muscles (Termin et al. 1989; DeNardi et al. 1993; Delp & Pette, 1994). Coexpression of type IIB and IIA MHCs was not observed, in agreement with earlier reports.
The percentage of type I fibres increased from about 3 % to 15 % during the third week of stimulation and again towards 100 % after 2 months. We interpret the early increase as occurring in original type IIA fibres and the late increase as occurring in type IIA fibres that originally were type IIB or IIX. If this is correct, fast to slow transformation occurs along the sequence IIB/IIX → IIA → I in such a way that the switch from the originally active gene to the next originally inactive gene in the sequence is rapid, while activation of the last gene in the sequence is much delayed. For IIB fibres it could not be determined whether the transition to IIA fibres passed through a stage of IIX coexpression after the stimulation had started. If a less rapidly acting slow stimulation pattern is applied to intact EDL axons (see below), original IIB fibres do coexpress the IIX gene (Delp & Pette, 1994).
The present results are in general agreement with previous reports of sequential activation of MHC genes during stimulation-induced fast to slow transformation in the rat (Termin et al. 1989; Delp & Pette, 1994) but differ in degree and speed of transformation. The present transformation occurred faster than in the work by Delp & Pette (1994) and, with respect to type I MHC, later than in the work by Mayne et al. (1993). For example, in Delp & Pette (1994) the number of IIX fibres first increased and then slowly declined, whereas in the present work there was only an early rapid decline. The effects on IIA fibres were also different, the relative content of IIA fibres increasing gradually over a 2 month period in the work by Delp & Pette (1994) but rapidly to maximum levels during the third week in the present work. In Mayne et al. (1993) biochemical analysis showed significant amounts of type I MHC in some muscles after 2 months of stimulation, whereas in the present work and that by Delp & Pette (1994) only a modest increase in type I fibres was observed after 2 months. We attribute these results to differences in stimulus patterns. Mayne et al. (1993) stimulated continuously at 10 or 20 Hz, which may be more efficient than intermittent 20 Hz. Delp & Pette (1994) stimulated at 10 Hz for 10 h each day so that 14 h of inactivity separated each period of stimulation compared with 10 s in the present experiments. Increasing duration of inactive periods markedly reduces the efficiency of electrical stimulation on extrajunctional ACh sensitivity in denervated rat Sol muscles even when the total amount of stimulation per day is kept constant (Lømo & Westgaard, 1975). The efficiency of the effect of stimulation on myosin expression may be similarly dependent on activity pattern.
The innervated EDL muscles were stimulated for only up to 37 days but this was sufficient to compare the effects of stimulating denervated and innervated muscles with respect to the disappearance of IIB and IIX fibres and the appearance of a major component of IIA fibres and a smaller component of type I fibres. In all these respects the effects were identical. This may appear surprising since the two types of muscles received different pulse patterns, the innervated EDL receiving an unknown pattern from intact EDL motoneurones in addition to the low frequency stimulation. Amount of activity rather than frequency is the main activity factor controlling MHC expression and shortening velocity (Kernell, Eerbeek, Verhey & Donselaar, 1987; Gundersen & Eken, 1992). Any pattern, regardless of frequency, which imposes large amounts of activity on a muscle results in fast to slow transformation. Therefore, if the large amount of activity imposed by stimulation already saturates the mechanism for fast to slow transformation, then any additional activity will have no further effect. That electrical stimulation of denervated and innervated muscles had the same effects argues against the possibility that denervation per se accounts for the observed transformation of fibre types.
Denervation alone increased the percentage of type IIA fibres at the expense of type IIB and IIX fibres. This change in the slow direction may also be caused by altered muscle activity, which is strongly affected by denervation. After denervation, the EDL no longer receives the high-frequency, low-amount activity typical of many EDL motor units (Hennig & Lømo, 1985) but instead becomes subject to low-frequency fibrillatory activity, which can be substantial (Purves & Sakmann, 1974). In contrast to the effects of denervation alone, denervation coupled to slow-pattern stimulation (1) markedly accelerated the denervation-induced decrease in IIB MHC, (2) eventually suppressed IIA MHC expression, which remained up-regulated after denervation alone, and (3) resulted in upregulation of type I MHC, which remained at a low level after denervation alone. The stimulation also appeared to accelerate a denervation-induced decrease in type IIX fibres.
In conclusion, it appears (1) that the effects of electrical muscle stimulation after denervation are specifically different from those of denervation alone, (2) that denervation alone leads to a partial fast to slow transformation that may be reasonably attributed to the altered muscle activity caused by the denervation, and (3) that the effects of stimulating the denervated EDL with a Sol-like pattern are very similar to the effects of stimulating the innervated EDL with the same pattern, which again are very similar to the effects of cross-reinnervating the EDL with the Sol nerve. It thus appears that neither denervation per se nor neurotrophic factors can account for the transformation to type I fibres observed during electrical stimulation of the denervated EDL with a Sol-like stimulus pattern. Instead, the evidence obtained in this and previous experiments on denervated and electrically stimulated muscles suggests that most, if not all, of the changes induced by cross-reinnervation can be attributed to differences in the electrical activity of fast and slow motoneurones. Finally, electrical stimulation of denervated muscle in vivo appears to be a suitable model both for distinguishing between nerve-derived trophic factors and electrical impulse activity in the control of muscle contractile properties and for studying how these properties depend on patterns of electrical muscle activity.
Acknowledgments
We are grateful to Dr Stefano Schiaffino for the gift of antibodies and to Ms Sigrid Schaller for expert technical assistance. The work was supported by the Norwegian Research Council.
References
- Al-Amood WS, Finol HJ, Lewis DM. Chronic stimulation modifies the isotonic shortening velocity of denervated rat slow-twitch muscle. Proceedings of the Royal Society B. 1986;228:43–58. doi: 10.1098/rspb.1986.0039. [DOI] [PubMed] [Google Scholar]
- Ausoni S, Gorza L, Schiaffino S, Gundersen K, Lømo T. Expression of myosin heavy chain isoforms in stimulated fast and slow rat muscles. Journal of Neuroscience. 1990;10:153–160. doi: 10.1523/JNEUROSCI.10-01-00153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller JA, Eccles JC, Eccles RM. Interaction between motoneurons and muscles in respect of the characteristic speeds of their responses. The Journal of Physiology. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. The Journal of Physiology. 1967;193:45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Dynamic properties of fast and slow skeletal muscles of the rat after nerve cross-union. The Journal of Physiology. 1969;204:331–346. doi: 10.1113/jphysiol.1969.sp008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Pette D. Morphological changes during fiber type transitions in low-frequency-stimulated rat fast-twitch muscle. Cell and Tissue Research. 1994;277:363–371. doi: 10.1007/BF00327784. 10.1007/s004410050163. [DOI] [PubMed] [Google Scholar]
- DeNardi C, Ausoni S, Moretti P, Gorza L, Velleca M, Buckingham M, Schiaffino S. Type 2X myosin heavy chain is coded by a muscle fiber type-specific and developmentally regulated gene. Journal of Cell Biology. 1993;123:823–835. doi: 10.1083/jcb.123.4.823. 10.1083/jcb.123.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eken T, Gundersen K. Electrical stimulation resembling normal motor-unit activity: effects on denervated fast and slow rat muscles. The Journal of Physiology. 1988;402:651–669. doi: 10.1113/jphysiol.1988.sp017227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Stein RB, Thomas CK. Innervation and function of hind-limb muscles in the cat after cross-union of the tibial and peroneal nerves. The Journal of Physiology. 1986;374:429–441. doi: 10.1113/jphysiol.1986.sp016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorza L, Gundersen K, Lømo T, Schiaffino S, Westgaard RH. Slow-to-fast transformation of denervated soleus muscles by chronic high-frequency stimulation in the rat. The Journal of Physiology. 1988;402:627–649. doi: 10.1113/jphysiol.1988.sp017226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorza L, Sartore S, Thornell LE, Schiaffino S. Myosin types and fiber types in cardiac muscle. III. Nodal conduction tissue. Journal of Cell Biology. 1986;102:1758–1766. doi: 10.1083/jcb.102.5.1758. 10.1083/jcb.102.5.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen K. Determination of muscle contractile properties: The importance of the nerve. Acta Physiologica Scandinavica. 1998 doi: 10.1046/j.1365-201X.1998.0336e.x. in the Press. [DOI] [PubMed] [Google Scholar]
- Gundersen K, Eken T. The importance of frequency and amount of electrical stimulation for contractile properties of denervated rat muscles. Acta Physiologica Scandinavica. 1992;145:49–57. doi: 10.1111/j.1748-1716.1992.tb09335.x. [DOI] [PubMed] [Google Scholar]
- Gutmann E, Carlson BM. Contractile and histochemical properties of regenerating cross-transplanted fast and slow muscles in the rat. Pflügers Archiv. 1975;353:227–239. doi: 10.1007/BF00584286. [DOI] [PubMed] [Google Scholar]
- Hämäläinen N, Pette D. Slow-to-fast transitions in myosin expression of rat soleus muscle by phasic high-frequency stimulation. FEBS Letters. 1996;399:220–222. doi: 10.1016/s0014-5793(96)01325-7. [DOI] [PubMed] [Google Scholar]
- Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Kernell D, Eerbeek O, Verhey BA, Donselaar Y. Effects of physiological amounts of high- and low-rate chronic stimulation on cat's fast muscle. 1. Speed- and force-related properties. Journal of Neurophysiology. 1987;58:598–613. doi: 10.1152/jn.1987.58.3.598. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. Journal of the Neurological Sciences. 1976;27:269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Lømo T, Westgaard RH. Further studies on the control of ACh sensitivity by muscle activity in the rat. The Journal of Physiology. 1975;252:603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lømo T, Westgaard RH, Dahl HA. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proceedings of the Royal Society B. 1974;187:99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- Maier A, Gambke B, Pette D. Degeneration-regeneration as a mechanism contributing to the fast to slow conversion of chronically stimulated fast-twitch rabbit muscle. Cell and Tissue Research. 1986;244:635–643. doi: 10.1007/BF00212544. [DOI] [PubMed] [Google Scholar]
- Mayne CN, Mokrusch T, Jarvis JC, Gilroy SJ, Salmons S. Stimulation-induced expression of slow muscle myosin in a fast muscle of the rat: Evidence of an unrestricted adaptive capacity. FEBS Letters. 1993;327:297–300. doi: 10.1016/0014-5793(93)81008-n. [DOI] [PubMed] [Google Scholar]
- Mira JC, Janmot C, Couteaux R, D'Albis A. Reinnervation of denervated extensor digitorum longus of the rat by the nerve of the soleus does not induce the type I myosin synthesis directly but through a sequential transition of type II myosin isoforms. Neuroscience Letters. 1992;141:223–226. doi: 10.1016/0304-3940(92)90899-i. [DOI] [PubMed] [Google Scholar]
- Niederle B, Mayr R. Course of denervation atrophy in type I and type II fibres of rat extensor digitorum longus muscle. Anatomy and Embryology. 1978;153:9–21. doi: 10.1007/BF00569846. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ, Pinter MJ, Dum RP, Burke RE. Kinesiological studies of self- and cross-reinnervated FDL and Soleus muscles in freely moving cats. Journal of Neurophysiology. 1985;54:852–866. doi: 10.1152/jn.1985.54.4.852. [DOI] [PubMed] [Google Scholar]
- Pasino E, Buffelli M, Arancio O, Busetto G, Salviati A, Cangiano A. Effects of long-term conduction block on membrane properties of reinnervated and normally innervated rat skeletal muscle. The Journal of Physiology. 1996;497:457–472. doi: 10.1113/jphysiol.1996.sp021780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D, Staron S. Cellular and molecular diversities of mammalian skeletal muscle fibers. Reviews of Physiology, Biochemistry and Pharmacology. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbová G. Adaptation of mammalian skeletal muscle fibers to chronic electrical stimulation. Reviews of Physiology, Biochemistry and Pharmacology. 1992;120:115–202. doi: 10.1007/BFb0036123. [DOI] [PubMed] [Google Scholar]
- Purves D, Sakmann B. The effect of contractile activity on fibrillation and extrajunctional acetylcholine-sensitivity in rat muscle maintained in organ culture. The Journal of Physiology. 1974;237:157–182. doi: 10.1113/jphysiol.1974.sp010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons S, Sréter FA. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976;263:30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Salmons S, Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. The Journal of Physiology. 1969;210:535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati G, Biasia E, Aloisi M. Synthesis of fast myosin induced by fast ectopic innervation of rat soleus muscle is restricted to the ectopic endplate region. Nature. 1986;322:637–639. doi: 10.1038/322637a0. [DOI] [PubMed] [Google Scholar]
- Sartore S, Gorza L, Schiaffino S. Fetal myosin heavy chains in regenerating muscle. Nature. 1982;298:294–296. doi: 10.1038/298294a0. [DOI] [PubMed] [Google Scholar]
- Schantz P, Henriksson J. Increases in myofibrillar ATPase intermediate human skeletal muscle fibers in response to endurance training. Muscle and Nerve. 1983;6:553–556. doi: 10.1002/mus.880060803. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Ausoni S, Gorza L, Saggin L, Gundersen K, Lømo T. Myosin heavy chain isoforms and velocity of shortening of type 2 skeletal muscle fibres. Acta Physiologica Scandinavica. 1988;134:575–576. doi: 10.1111/j.1748-1716.1998.tb08539.x. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. Journal of Muscle Research and Cell Motility. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Carli M. Embryonic myosin heavy chain as a differentiation marker of developing human skeletal muscle and rhabdomyosarcoma. A monoclonal antibody study. Experimental Cell Research. 1986;163:211–220. doi: 10.1016/0014-4827(86)90574-4. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiological Reviews. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Termin A, Staron RS, Pette D. Changes in myosin heavy chain isoforms during chronic low-frequency stimulation of rat fast hindlimb muscles - A single fiber study. European Journal of Biochemistry. 1989;186:749–754. doi: 10.1111/j.1432-1033.1989.tb15269.x. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, Lømo T. Control of contractile properties within adaptive ranges by patterns of impulse activity in the rat. Journal of Neuroscience. 1988;8:4415–4426. doi: 10.1523/JNEUROSCI.08-12-04415.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzemann V, Brenner HR, Sakmann B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular junctions. Journal of Cell Biology. 1991;114:125–141. doi: 10.1083/jcb.114.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]


