Abstract
The effects of sympathetic nerve stimulation on the motility of the circular and longitudinal muscle of the large intestine were investigated in vitro, and the involvement of various adrenoceptor subtypes determined. A comparison between the sympathetic supply arising from the prevertebral and pelvic ganglia was also made.
In the longitudinal muscle of the distal colon, sympathetic nerve stimulation caused responses which were contractile (0.1–2 Hz), biphasic (5–10 Hz) or purely inhibitory (20–30 Hz). All contractile responses were removed with phentolamine (3 μM), whereas the inhibitory responses were significantly diminished by propranolol (0.1 μM) and completely abolished by alprenolol (3 μM) or nadolol (300 μM).
In the longitudinal muscle of the proximal colon, the effects of sympathetic nerve stimulation were predominantly inhibitory. Some of this inhibition was removed by propranolol (0.1 μM), but was largely unaffected by alprenolol (3 μM). The remainder of the inhibitory response was probably non-noradrenergic as it was not removed by a combination of phentolamine (3 μM) and alprenolol (3 μM).
In the circular muscle of both the proximal and distal colon, sympathetic stimulation caused a strong contractile response which was completely removed by phentolamine (3 μM) to reveal an inhibitory response. This inhibitory response was unchanged by propranolol (0.1 μM) but was removed by alprenolol (3 μM), following which a weak non-noradrenergic contractile response was unmasked.
Stimulation of the hypogastric nerve to activate pelvic sympathetic pathways had no effect on the motility of the longitudinal muscle, but caused a contractile response in the circular muscle which was completely removed by phentolamine (3 μM).
We conclude that sympathetic nerves innervate adrenoceptors of different types in the various muscle layers and regions of the colon. They innervate a mixture of α-, and β3-adrenoceptors in the longitudinal muscle of the proximal colon, α-, classical β- and β3-adrenoceptors in the distal colon, and primarily α-adrenoceptors with a few β3-adrenoceptors in the circular muscle. In addition, the pelvic sympathetic innervation of the rectum differs from the prevertebral supply by innervating only excitatory α-adrenoceptors.
Sympathetic activation in the intestines commonly causes inhibition of intestinal motility, secretion and blood flow (see review of Furness & Costa, 1987). Due to the preponderance of sympathetic terminals in the myenteric plexus and the comparative paucity of sympathetic fibres in the non-sphincteric musculature of many gut regions (Furness & Costa, 1987), the mechanism of this sympathetic inhibition of motility is considered to be primarily by decreasing the activity of myenteric motor neurons. This ‘indirect’ action of sympathetic nerves is well documented (for review see Burnstock & Wong, 1981). However, although less densely innervated than the myenteric plexus, both the longitudinal and circular muscle layers do receive some sympathetic terminals (Furness & Costa, 1987). These are most prominent in the large intestine (Norberg, 1964; Furness, 1970), particularly in the rectum (Furness & Costa, 1973). Concomitantly, the noradrenergic innervation of the myenteric plexus in the large intestine in the rat is less extensive than that in the small intestine (Schultzberg et al. 1980). An investigation of this ‘direct’ innervation of the intestinal muscle, particularly in the colon and rectum, is lacking in the literature and is the focus of this study.
There are many adrenoceptors present in the smooth muscle of the gut, which have been heavily investigated with various pharmacological tools (see review of McIntyre & Thompson, 1992; Manara, Croci & Landi, 1995). The non-sphincteric regions of gut smooth muscle are typically inhibited by β-adrenoceptor agonists. Numerous studies have demonstrated that this inhibitory effect of catecholamines on motility in most of the rat gastrointestinal tract, guinea-pig ileum and taenia caecum is mediated solely or predominantly via β3-adrenoceptors, and in human colon is mediated by a combination of β-adrenoceptor subtypes (see review of Arch & Kaumann, 1993; Howe, 1993; Manara et al. 1995). In contrast to the sympathetic inhibition of non-sphincteric muscle, sphincters are excited by α-adrenoceptor agonists (Furness & Costa, 1987). However, there is growing evidence that excitatory α-adrenoceptors are also present on the muscle of many non-sphincteric regions of gut (see review of Burnstock & Wong, 1981; Krier, 1989; McIntyre & Thompson, 1992) and particularly in the large intestine of the cat (Venkova & Krier, 1993, 1995), human (Gagnon, Devroede & Belisle, 1972; Jacob, Brandt, Farkas & Frishman, 1983) and rat (Gagnon & Belisle, 1970).
Any consideration of the physiological role of adrenoceptor subtypes in the regulation of gut motility must take into account whether these receptors are innervated by sympathetic axons. The innervation of excitatory α-adrenoceptors has only been investigated in the cat colon (Rostad, 1973; Hedlund, Fasth & Hultén, 1984; Carlstedt, Fasth, Hultén & Nordgren, 1988; Venkova & Krier, 1993) and dog stomach (Nakazato, Saito & Ohga, 1970). In particular, in the circular muscle of the cat colon, stimulation of sympathetic nerves produces an increase in motility rather than the expected inhibition (Venkova & Krier, 1993). It is not known how widespread these sympathetic excitatory effects are within the digestive tract. The potential role of β3-adrenoceptors has been explored even less with only one study to date investigating their innervation (in the guinea-pig small intestine; Taneja & Clarke, 1992). The present paper aims to determine which adrenoceptors in the rat large intestine are innervated.
Finally, there has been no systematic comparison of the effects of sympathetic nerve stimulation (SNS) either in different intestinal regions or of the sympathetic supplies arising from different sympathetic ganglia. Strikingly, not even a comparison of the prevertebral innervation of the large intestine with the sympathetic innervation provided by the pelvic ganglia has been made. From histochemical and electrophysiological differences between the neurons of these ganglia (see reviews of Jänig & McLachlan, 1987; Keast, 1995), it is likely that their effects on motility will also differ. We have chosen to make this comparison in the rat where the pelvic ganglia and their connections with the gut are anatomically simple and where some of the characteristics of these pathways are already known (Keast, 1995). In particular, the majority of pelvic neurons lie in a pair of large ganglia, the major pelvic ganglia, which send their supply to the gut via the rectal nerves.
Our experiments have combined selective nerve activation with application of specific adrenoceptor antagonists to investigate the innervation of the circular and longitudinal muscle of the rat proximal colon, distal colon and rectum in vitro. Shorter series of experiments observing the effects of stimulation of paravascular mesenteric nerves on ileal muscle were also performed. The aim of these studies was to determine: (i) whether sympathetic stimulation in these regions produces an inhibition or excitation of motility, (ii) which adrenoceptor subtypes are innervated and contribute to the sympathetic response, (iii) whether the control of motility provided by the prevertebral ganglia (to the proximal and distal colon) differs from that supplied by the pelvic sympathetic neurons (to the rectum), and (iv) whether there are any differences between the small and large intestines, or the circular and longitudinal muscle layers, in the effects of sympathetic stimulation or the receptors activated. These experiments form the first comprehensive examination of the effects of sympathetic stimulation along the length of the large intestine in any one species. Hence, it is determined for the first time whether the sympathetic innervation of this organ is uniform between regions and between ganglia providing the supply.
METHODS
Male Wistar rats (100–230 g) were anaesthetized with sodium pentobarbitone (48 mg kg−1i.p.) for the removal of various intestinal regions with their corresponding sympathetic ganglia or with mesenteric nerves attached (Fig. 1). These were: proximal colon with colic arteries and nerves, distal colon with the inferior mesenteric artery and ganglion, upper rectum with a major pelvic ganglion attached via the rectal nerve, and the distal ileum with paravascular arcades (not shown). Animals were then exsanguinated. Each intestinal preparation was placed into an organ bath filled with physiological saline (mM: 114 NaCl, 25 NaHCO3, 11.7 glucose, 1.2 KH2PO4, 1.14 ascorbic acid, 1.2 MgSO4, 2.5 CaCl2) which was continuously bubbled with 95% O2-5% CO2 and maintained at 37°C.
Figure 1. Sites of electrical stimulation of sympathetic nerves and their connections with the large intestine.
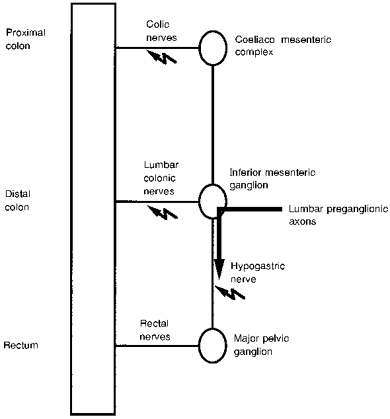
Electrical stimuli were applied to the nerves connecting the prevertebral and pelvic ganglia with the gut at the sites indicated by the jagged arrows. The proximal colon was removed with the colic nerves intact, but not including the coeliaco-mesenteric complex. The distal colon was removed with the inferior mesenteric ganglion attached via the lumbar colonic nerves. For stimulation of sympathetic pathways to the muscle of the rectum, one major pelvic ganglion was left attached via the rectal nerves and the hypogastric nerve was stimulated. Stimulation of the hypogastric nerve activates the sympathetic preganglionic axons, but not the parasympathetic preganglionic axons which enter the major pelvic ganglia via the pelvic nerves (not shown). The major pelvic ganglia also supply the caudal rectum via the cavernous nerves (not shown), but these pathways were not investigated. Effects of stimulating the paravascular nerves on the ileum were also investigated although not shown in this figure.
Preparations were connected to an isometric force transducer (Grass FT03; range, 0–55 g) and a MacLab (version 3.3 with bridge amplifier, Chart version 3.3.6), to measure contractions of either longitudinal (15 ml bath volume) or circular muscle (20 ml). Circular muscle preparations were placed under a larger load (3–4 g) than longitudinal muscle (1–1.5 g), as the circular muscle was considerably thicker. Furthermore, in the circular muscle, carbachol precontraction was not performed (see below), and inhibitory responses were larger and more consistent when using a larger load. The larger load did not interfere with the size of contractile responses. The bath fluid was replaced every 10–15 min while obtaining frequency response curves (FRCs) to sympathetic nerve stimulation (SNS).
Experimental protocol
All tissues were allowed to equilibrate for at least 30 min until tone and spontaneous activity became stable. Following this a non-cumulative FRC was obtained using 30 s SNS periods, each followed by a wash and 10 min stabilization period before the next stimulation. The stimulus was a 10 or 20 V square pulse with 1 ms duration at 0.1, 0.25, 0.5, 1, 2, 5, 10 and 20 Hz.
The effect of SNS on both the spontaneous tone and precontracted activity was tested in all preparations. Precontraction with sub-maximal doses of carbachol (0.5–2 μM) was carried out 1–3 min prior to SNS. Carbachol doses were selected to give a consistent increase in tone and contractile activity, which quickly returned to control levels following its washout after each SNS period. On precontracted tissues, nerve-evoked responses were compared with the carbachol activity immediately prior to SNS, while in untreated tissues the control was taken as the baseline spontaneous activity prior to SNS.
The effect of including desipramine (0.5 μM) in the bath was investigated only in the distal colon longitudinal muscle preparation. It was demonstrated that desipramine potentiated the effects of SNS, significantly increasing the amplitude, but not the time course or frequency dependence of the response (see Results). Desipramine was therefore included in the organ bath for the duration of all further experiments for all preparations. No run-down of the SNS response was observed with desipramine in the bath for up to 4 h from the time of tissue removal from the animal.
Administration of adrenoceptor antagonists
In most preparations, two FRCs were generated to allow a paired analysis of the responses in the presence and absence of an adrenoceptor antagonist. Antagonists equilibrated with the tissue for 30 min between FRCs. In some experiments a control FRC was compared with a second FRC with phentolamine (3 μM) in the bath. For all remaining experiments, 3 μM phentolamine remained in the bath throughout, while 0.01, 0.03, 0.1 or 1 μM propranolol, 3 μM alprenolol or 300 μM nadolol was present for the second FRC. For all FRCs tested in the presence of antagonists, frequencies of 0.5, 1, 2, 5, 10, and 20 Hz SNS were used.
Phentolamine (3 μM) removes all α-adrenoceptor mediated effects (Bauer, 1982; Pelckmans, van Maercke, de Maeyer, Herman & Verbeuren, 1990) and has a pA2 (a measure of drug antagonism) of 6.8 at postjunctional excitatory α1-adrenoceptors in the guinea-pig ileum (Fagbemi & Salako, 1980). Propranolol has a pA2 of 8.8 at β1-adrenoceptors and of 9.1 at β2-adrenoceptors (Bianchetti & Manara, 1990). In contrast, at β3-adrenoceptors propranolol has a pA2 of 6.2–6.5 (determined in the rat colon; Arch & Kaumann, 1993). Thus, 0.1 μM propranolol would effectively block almost 100% of classical β-adrenoceptors (i.e. occupying 98.4% of β1- and 99.2% of β2-adrenoceptors), but leave the sympathetic activation of β3-adrenoceptors largely unaffected (where it occupies only 13.7–24.0% of the receptors; Kaumann & Molenaar, 1996). The pA2 of alprenolol at β3-adrenoceptors is always greater than that of propranolol and is 6.8–7.6 in the rat colon (Bianchetti & Manara, 1990; Arch & Kaumann, 1993; Manara et al. 1996). Hence, 3 μM alprenolol would block almost all (96.0–99%) β-adrenoceptors, including β3-adrenoceptors. Thus, the difference, if any, between responses to SNS in the presence of 0.1 μM propranolol and 3 μM alprenolol would demonstrate a β3-adrenoceptor mediated component of sympathetic inhibition (see Results and Discussion). Finally, nadolol (10 μM) has recently been shown to cause blockade of isoprenaline-induced inhibition equivalent to the specific β3-adrenoceptor antagonists, the aryloxypropanolaminotetralins (rat distal colon; Manara, et al. 1996). Nadolol (300 μM) would be expected to block all three types of β-adrenoceptors, as it has a pA2 of 4.7 at β3-adrenoceptors in the guinea-pig ileum and would block 93.7% of this receptor type (Bond &Clarke, 1988).
Data analysis
Muscle activity was measured as the integrated activity (i.e. the area under the curve) over a given time period. The control activity was integrated over either 20 or 40 s immediately preceding the SNS period. The shorter period was used for all experiments except those in the circular muscle where carbachol induced large contractions; here the control activity was measured over 40 s to ensure an accurate representation of the baseline. All values for the integrated activity are expressed in grams, representing the average tone that the preparation had maintained over the time period (i.e. mean amount of work performed).
Muscle activity was measured over two 10 or 15 s periods within the 30 s SNS period (examples shown in Fig. 2). The first measurement, called the ‘first reading’, assessed the rapid response of the preparation to SNS and recorded the activity within the first 15 s of the stimulation period. The ‘second reading’ measured the activity in the remaining 15 s of SNS. This was particularly important to capture accurately the biphasic responses to SNS observed at certain frequencies of stimulation (see Results) or where the effect of SNS developed very slowly (e.g. hypogastric nerve- circular muscle, and colic nerves-circular muscle in the presence of alprenolol).
Figure 2. Examples of the effects of stimulating sympathetic nerves on large intestine motility.
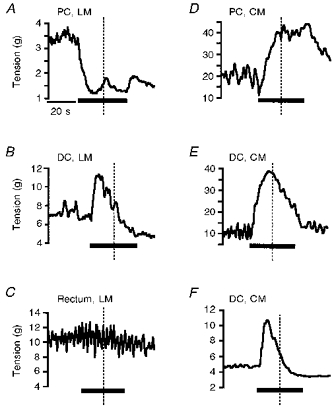
In each trace the region and layer of intestine is indicated, along with the period of sympathetic nerve stimulation (SNS). A, proximal colon (PC) longitudinal muscle (LM) stimulated at 20 Hz; B, distal colon (DC) longitudinal muscle stimulated at 2 Hz; C, rectum longitudinal muscle stimulated at 20 Hz; D, proximal colon circular muscle (CM) stimulated at 1 Hz; E, distal colon circular muscle stimulated at 10 Hz; F, distal colon circular muscle stimulated at 10 Hz. The activity was integrated for 10–15 s in the first and second halves of the stimulus period (i.e. either side of the dotted line, referred to as the first and second reading in subsequent figures). The time scale is the same for all traces and is indicated in the first trace. All traces are from tissues precontracted with carbachol (0.5–1.5 μM) except F. In most preparations, high frequency SNS resulted in inhibition of activity (A), a mid-range SNS caused a biphasic response (B) and low frequency SNS resulted in excitation of activity (D). Stimulation of the hypogastric nerve had no effect on the longitudinal muscle of the rectum (C). In the circular muscle carbachol attenuated the inhibition observed in the second half of the stimulus period (E and F from the same animal). Note the carbachol induced activity before the stimulus period in A-E. A lack of spontaneous activity in the circular muscle is observed in F.
In all experiments on the longitudinal muscle the change in integrated activity produced by SNS was expressed as a percentage of the size of the carbachol response. When carbachol was used on circular muscle preparations, the inhibitory responses were also expressed as a proportion of the carbachol response, while the contractile responses were normalized to the maximum response seen in that tissue (because of the much greater variability of excitatory responses). In the absence of carbachol, all responses were normalized.
All FRCs were carried out on at least four animals for each intestinal region, muscle layer and antagonist combination. Effects of antagonists on the FRCs were identified by Student's paired t test (two-way when analysing the effects of phentolamine and one-way for analysis of all β-adrenoceptor antagonists). For comparison of the effects of propranolol, alprenolol and nadolol, an unpaired one-way analysis of variance was used. Where the analysis of variance showed a significant difference between groups, a Student-Newmann-Keuls post hoc test was performed to identify disparate groups. For all statistical tests, significance was assumed when P < 0.05.
Tests of nerve stimulation procedure
A number of experiments were carried out to verify that the effects of SNS were mediated via activation of extrinsic nerve fibres and were not due to electrical spread through the bath fluid or along the connective tissue associated with the nerve bundles. These were performed by cutting or crushing the nerves between the stimulating electrodes and the gut before SNS. These were performed in longitudinal and circular muscle organ baths and for all intestinal regions at several frequencies of stimulation. For proximal and distal colon longitudinal muscle preparations, when the lumbar colonic or colic nerves were cut or crushed, a 20 V stimulus at any frequency failed to cause changes in either the spontaneous or carbachol precontracted activity. Consequently, all proximal or distal colon longitudinal muscle experiments were performed with 20 V SNS. In the circular muscle bath, the insulation of the electrodes from the gut was less efficient and a 20 V, 20 Hz stimulus produced small motility changes. All circular muscle experiments were therefore performed with a 10 V stimulus (which did not spread through the bath). For hypogastric nerve stimulation of the longitudinal muscle, the stimulus was also reduced to 10 V.
Drugs
The following drugs were purchased from Sigma: atropine sulphate, phentolamine hydrochloride, propranolol hydrochloride, desipramine hydrochloride, alprenolol hydrochloride, nadolol and carbachol. All drugs were prepared in aqueous solution except nadolol, for which a 1 mM stock solution was prepared in N,N-dimethylformamide. However, when added to the bath in the same volumes as required for nadolol administration, the solvent did not alter the affects of SNS on motility (n = 2, data not shown).
RESULTS
The effects of SNS on different gut regions and muscle layers are shown in Figs 2 and 3 and summarized in Table 1. Stimulation of the sympathetic nerves supplying the longitudinal muscle of the ileum caused a frequency-graded inhibition of precontracted activity, reaching a maximum at 20 Hz (Fig. 3A). However, in the proximal colon longitudinal muscle, nerve stimulation at 2–10 Hz caused a biphasic response of contraction followed by inhibition (Fig. 3B). Only with higher frequency stimulation (20 Hz) was an entirely inhibitory response evoked (Fig. 2A). In the distal colon, the contractile effects of SNS became even more pronounced (Fig. 3C). At low frequencies of stimulation (< 2 Hz) this contractile effect comprised the entire response, whereas at higher frequencies either a biphasic response (2–10 Hz; Fig. 2B) or a purely inhibitory response (20 Hz) was evoked. In contrast to these effects of stimulating prevertebral sympathetic pathways, activation of the pelvic sympathetic pathways to the rectum longitudinal muscle had no effect (Figs 2C and 3D).
Figure 3. Control frequency response curves for nerve stimulation of each gut region.
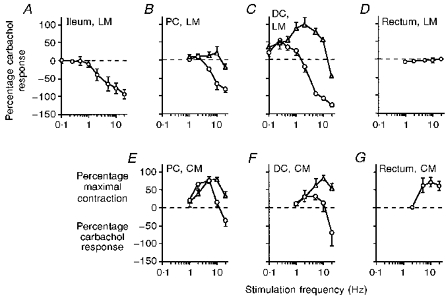
In the longitudinal muscle (LM) the effects of sympathetic nerve stimulation (SNS) are shown as a percentage of the carbachol precontraction, with a positive value representing a contraction in addition to the carbachol response and a negative value representing an inhibition. The first reading (▵) and second reading (^) are the SNS-evoked changes in activity recorded in the first and second halves of the stimulus period, respectively (see individual traces in Fig. 2). In the circular muscle (CM), inhibitory responses are also expressed as a percentage of the carbachol response. Contractile responses in the CM were very variable in size between animals and so were normalized such that the largest SNS-evoked contraction in each animal represented 100%. All points show the mean ±s.e.m. from 4–9 animals. PC, proximal colon; DC, distal colon.
Table 1.
Maximal contractions and inhibitions evoked by sympathetic nerve stimulation
| Maximal contraction | Maximal inhibition with phentolamine* | Maximal non-NA response† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Mean ±s.e.m. | Range | n | Mean ±s.e.m. | Range | n | Mean ±s.e.m. | Range | n |
| PC, LM | 1.0 ± 0.3 | 0.5–2.1 | 5 | 3.1 ± 0.2 | 1.9–4.7 | 13 | -1.5 ± 0.5 | -0.8–2.8 | 4 |
| PC, CM | 23.0 ± 3.9 | 17.1–34.6 | 4 | 3.1 ± 0.6 | 1.6–4.4 | 4 | |||
| 9.3 ± 1.4 | 6.3–12.6 | 4‡ | 1.5 ± 0.1 | 0.7–2.0 | 12‡ | +3.7 ± 0.3 | 2.9–4.1 | 4‡ | |
| DC, LM | 2.6 ± 0.4 | 0.6–4.0 | 9 | 4.5 ± 0.2 | 2.2–6.9 | 33 | nil | ||
| DC, CM | 25.8 ± 5.0 | 8.8–41.9 | 6 | 6.4 ± 1.5 | 2.7–9.7 | 4 | |||
| 11.2 ± 3.3 | 1.8–21.3 | 5‡ | 2.1 ± 0.2 | 1.3–3.8 | 12‡‡ | +1.8 ± 0.3 | 1.2–2.3 | 4‡ | |
| Rect, CM | 5.8 ± 2.0 | 1.3–12.3 | 5‡ | 0.2 ± 0.1 | 0.1–0.4 | 5‡ | nil | ||
Maximal inhibition was taken as the largest inhibition produced by SNS in a given preparation when phentolamine (3 μM) was present to remove any competing contractile effects.
Response to SNS when phentolamine (3 μM) and alprenolol (3 μM) were in the bath to block all α- and β-adrenoceptors; +, contraction; -, inhibition.
Values from SNS on carbachol-free preparations. n, number of animals. CM, circular muscle; DC, distal colon; LM, longitudinal muscle; NA, noradrenaline; PC, proximal colon; Rect, rectum
The effects of SNS on the circular muscle of the proximal colon and distal colon were quite different from those on the longitudinal muscle of the same regions (Figs 2D, E and F, and 3E and F). In particular, a predominantly contractile effect was evoked, with much larger contractions than those observed in the longitudinal muscle (Table 1). Inhibition was only observed at 20 Hz, where it always followed a contractile response. Activation of pelvic sympathetic pathways also caused a contractile effect in the circular muscle of the rectum (Fig. 3G).
Carbachol-induced activity
The spontaneous activity of preparations varied very little during the course of each experiment, although a gradual increase in tone of up to 1 g was common in the circular muscle. Carbachol caused an immediate increase in the tone and generated contractions in all preparations. Usually the response peaked within 1 min before falling to a consistent level of activity by 2 min, which was then maintained for up to 7 min. SNS was always performed after the carbachol tone had stabilized. The size of the average carbachol response varied between regions and muscle layers. In the ileum longitudinal muscle, the response to carbachol (1–2 μM) was the smallest, giving an increase in integrated activity of only 1.5–2 g. The response to the same dose of carbachol increased anally (along with the thickness of the longitudinal muscle layer) to reach a maximum of 8–10 g in the rectum (with 1–1.5 μM). The circular muscle in all regions was more sensitive to carbachol and lower concentrations (0.5 μM) were used to produce typically a response of 10–20 g.
In all longitudinal muscle preparations, SNS responses at all frequencies were comparable between untreated and carbachol precontracted tissues. However, in the circular muscle preparations of the proximal colon and distal colon, carbachol seemed to slightly potentiate the contractile response to SNS (Fig. 2E and F), as described previously (Ek, 1985). This effect was best observed at 10 Hz SNS. Because of this slightly attenuated inhibition, all circular muscle experiments investigating inhibitory responses to SNS were performed in the absence of carbachol. Experiments where the hypogastric nerve was stimulated and the circular muscle studied also omitted carbachol precontraction, as the responses were purely excitatory.
Effects of desipramine on responses to sympathetic nerve stimulation
The results of SNS in the presence and absence of desipramine (0.5 μM) was only investigated in the distal colon longitudinal muscle following stimulation of the lumbar colonic nerves. In the presence of desipramine, SNS-evoked responses had the same general features (i.e. contractile, biphasic or inhibitory), but were larger in size. In particular, the maximum inhibitory response observed for each experiment was 2.6 ± 0.3 g in control tissues, compared with 4.0 ± 0.3 g in the presence of desipramine (P < 0.05; n = 4). Similarly, the maximum contraction resulting from SNS in the presence of desipramine (2.8 ± 0.3 g) was significantly larger than in control tissues (1.4 ± 0.3 g; n = 4, P < 0.05). Because desipramine potentiated but did not otherwise alter the SNS-evoked responses, it was included in the bath for all other preparations.
α-Adrenoceptor mediated effects of prevertebral and pelvic SNS
The effects of phentolamine (3 μM) on the FRC of the proximal colon, distal colon and rectum are shown in Fig. 4 and Table 1. In the longitudinal muscle of the proximal and distal colon, phentolamine removed all contractile responses caused by SNS and facilitated inhibition (Fig. 4A and B). This suggests that stimulation of the colic and lumbar colonic nerves activated excitatory α-adrenoceptors, which slightly masked the inhibitory effects of SNS. Following the removal of the competing excitatory α-adrenoceptor effect with phentolamine, a 20 Hz stimulus caused an inhibition of 97.5 ± 4.1% (n = 5) of the carbachol contraction in the proximal colon (Fig. 4A) and 121.0 ± 2.2% of the carbachol response in the distal colon (data taken from all experiments with phentolamine in the bath; Figs 4B and 5; n = 33).
Figure 4. Frequency response curves of different gut regions treated with phentolamine.
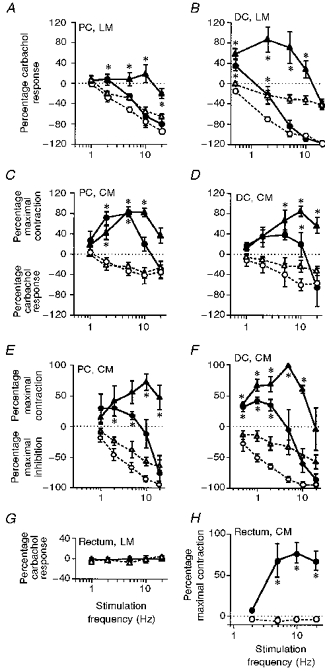
Nerve-evoked responses are expressed as a percentage of the carbachol response or, in the circular muscle, as a percentage of the maximum response. Continuous lines and filled symbols represent responses without adrenoceptor antagonists and the dotted lines with open symbols represent responses in the presence of phentolamine (3 μM). A-D and G were obtained following precontraction with carbachol (0.5–1.5 μM), while E, F and H were obtained without carbachol. The first and second readings are represented by ▴ and •, respectively. Note that phentolamine removed all contractile effects of sympathetic nerve stimulation and in the colon, but not in the rectum, revealed a sympathetic inhibition. Phentolamine does not change the response to stimulation of the hypogastric nerve in the longitudinal muscle of the rectum. Carbachol delays the onset of SNS-evoked inhibition (e.g. the response to 10 Hz stimulation in C and D is purely contractile, but is biphasic in E and F). Data are means ±s.e.m. from 4–9 animals. * Responses in the presence of phentolamine differ from control (P < 0.05). PC, proximal colon; DC, distal colon, CM, circular muscle; LM, longitudinal muscle.
Figure 5. Effects of β-adrenoceptor antagonists on the sympathetic nerve-evoked responses in the distal colon longitudinal muscle.
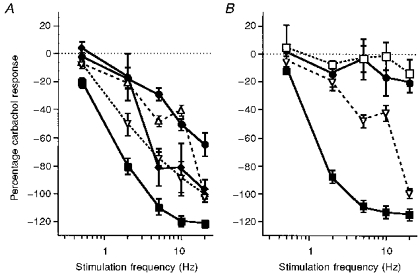
Nerve-evoked responses are expressed as a percentage of the carbachol induced activity and phentolamine (3 μM) was present in all experiments. A, the effects of different concentrations of propranolol on the second reading are shown: 0, ▪; 0.01 μM, ▿; 0.03 μM, ♦; 1 μM, •. For all stimuli of 2–20 Hz, each concentration of propranolol significantly decreased the effects of nerve stimulation when compared with those responses obtained with phentolamine alone (P < 0.05; except for 0.01 μM propranolol, 10 Hz stimuli). B, the effects of nadolol (300 μM, •) and alprenolol (3 μM, □), which block β3-adrenoceptors in addition to classical β-adrenoceptors, are compared with the effects of 0.1 μM propranolol (▿), which blocks only classical β-adrenoceptors. At 5, 10 and 20 Hz both nadolol and alprenolol removed significantly more of the sympathetic inhibition than propranolol (P < 0.05). All points represent means ±s.e.m. of 4 animals.
Similarly, in the circular muscle of the proximal and distal colon, phentolamine removed all contractile effects of SNS and revealed an inhibition of motility (Fig. 4C and D, and Table 1). SNS decreased the carbachol response by only 51.8 ± 7.8% (proximal colon) and 71.6 ± 10.7% (distal colon). In the absence of carbachol, effects of phentolamine were similar, removing all of the contractile responses but leaving the inhibitory effects of SNS intact (Figs 4E and F).
Stimulation of the hypogastric nerve to activate the pelvic sympathetic pathways had no effect on the longitudinal muscle in the presence or absence of adrenoceptor antagonists (Fig. 4G). However, in 7/9 rectal circular muscle preparations, hypogastric nerve stimulation caused a contraction which was abolished by phentolamine (Fig. 4H). The maximum effect of hypogastric nerve stimulation in the presence of phentolamine was an inhibition of 0.2 g (Table 1), not regarded as a significant change from baseline tone. Therefore, unlike the effects of prevertebral stimulation, pelvic sympathetic stimulation did not contain an inhibitory component.
Effect of β-adrenoceptor blockade on colonic SNS-evoked responses
Several methods of removing the β-adrenoceptor mediated effects of SNS were utilized in the preparation where the inhibitory response was the largest, the distal colon longitudinal muscle. The effects of various concentrations of propranolol (0.01, 0.03, 0.1 and 1 μM) on the FRC are shown in Fig. 5A. All concentrations of propranolol resulted in a significant reduction of the SNS inhibitory component at 2–20 Hz stimulation. Blockade of classical but not β3-adrenoceptors (0.1 μM propranolol, see Methods) removed most of the SNS-evoked inhibition (particularly at 2 Hz where only 20.3 ± 5.9% of the carbachol-induced activity remained; Fig. 5A). Both nadolol and alprenolol blocked a greater percentage of the SNS-induced inhibition than 0.1 μM propranolol at 5, 10 and 20 Hz, indicating that at these frequencies β3-adrenoceptors were being activated (Fig. 5B).
In the other colonic preparations, only 0.1 μM propranolol (to block classical but not β3-adrenoceptors) and alprenolol (having a higher affinity for β3-adrenoceptors than nadolol; see Methods) were used. The data from experiments using these antagonists on the distal colon longitudinal muscle are also shown in Fig. 6B to allow ready comparison with the effects of the same antagonists on SNS-evoked activity in the other colonic preparations (Fig. 6A,C and D).
Figure 6. Effects of β-adrenoceptor antagonists on nerve-evoked activity in all colonic preparations.
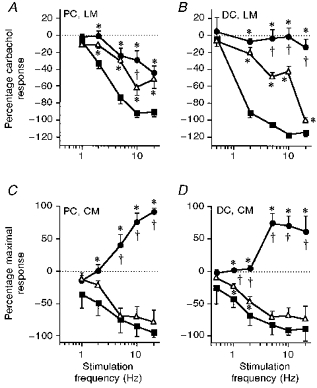
Nerve-evoked effects are expressed as a percentage of the carbachol response in the longitudinal muscle (LM) and as a percentage of the maximum contractile or inhibitory response in the circular muscle (CM). The frequency-response curves were obtained in the presence of phentolamine alone (3 μM, ▪) or in combination with propranolol (0.1 μM, ▵) or alprenolol (3 μM, •). Data are means ±s.e.m. from 4 animals. * Effects with propranolol or alprenolol differed from those with phentolamine alone (P < 0.05). † Points at which alprenolol removed more of the sympathetic inhibition than propranolol (P < 0.05). PC, proximal colon, DC, distal colon.
In the proximal colon longitudinal muscle, propranolol removed a small but significant component of the SNS-evoked inhibition at all frequencies greater than 1 Hz (Fig. 6A), suggesting that at least part of the colic nerve response is due to an activation of classical β-adrenoceptors. In contrast, the action of alprenolol differed from that of propranolol only at 10 Hz stimulation, suggesting that there may be very little or no innervation of β3-adrenoceptors in this tissue. In the presence of phentolamine and alprenolol, a sizeable inhibition was evoked by SNS (Table 1). The transmitter responsible for this was not investigated. It may be a non-noradrenergic response or due to activation of atypical adrenoceptors (i.e. not α-, β1-, β2- or β3-adrenoceptors).
The β-adrenoceptor mediated responses in the circular muscle of the colon were quite different to those seen in the longitudinal muscle. In the proximal colon, propranolol had no effect on the inhibitory component of the SNS response (Fig. 6C) and in the distal colon blocked only a very small proportion of the inhibition at 1 and 2 Hz SNS (Fig. 6D). In contrast, alprenolol removed all of the inhibitory effects of SNS in both the proximal and distal colon and unmasked a contraction (Table 1; Fig. 6C and D). Hence, the sympathetic inhibition of the circular muscle is mediated almost solely via β3-adrenoceptors, while contractile effects are mediated via α-adrenoceptors and an unknown transmitter or adrenoceptor subtype.
DISCUSSION
This study provides the first comprehensive investigation of sympathetic nerve-evoked responses in the large intestine. Furthermore, these experiments detail a novel comparison of the receptors activated by sympathetic axons originating from the prevertebral and pelvic ganglia. Finally, the only demonstration to date of innervated β3-adrenoceptors in the large intestine is provided.
Nerve-evoked responses are due to the activation of innervated receptors
Several observations suggest that the nerve-evoked responses recorded in these experiments are due to the activation of innervated (junctional) receptors. First, if the responses were due to the activation of both junctional and extra-junctional adrenoceptors, they would be expected to mimic the effects of exogenous noradrenaline. This causes a pure inhibition of motility in rat proximal colon and distal colon longitudinal muscle mediated predominantly by β3-adrenoceptors (McLaughlin & MacDonald, 1990; Manara et al. 1996); a contractile effect is only seen after β-adrenoceptors have been blocked (Gagnon & Belisle, 1970). In contrast, SNS-evoked responses in the proximal colon and particularly in the distal colon longitudinal muscle contained excitatory responses in the absence of β-adrenoceptor blockade, and SNS-evoked inhibition in proximal colon longitudinal muscle is due to an activation of β1/β2-adrenoceptors but not of β3-adrenoceptors (present study). The disparity in action between exogenous and nerve-released noradrenaline suggests that the latter activates a specific subset of adrenoceptors present on the muscle (i.e. junctional receptors; see review of Brock & Cunnane, 1992).
Second, nerve-evoked responses following activation of pelvic projections were observed in all preparations except the rectal longitudinal muscle. This tissue is not innervated by pelvic axons (Luckensmeyer & Keast, 1997). The lack of an SNS-evoked response in a tissue devoid of sympathetic axons suggests that the responses observed in other preparations (which contain sympathetic axons) are due to the specific activation of innervated receptors rather than ‘spillover’ of transmitter released from more distant axons.
Third, blockade of the neuronal uptake of catecholamines with desipramine potentiated SNS-evoked responses in the longitudinal muscle of the distal colon, but did not change the time course or type of response observed. This potentiation suggests that SNS-evoked responses were caused by the activation of junctional receptors. Other studies have also used this line of reasoning to identify innervated receptors (Marino, Marcoli, de Ponti, Cosentino, Lecchini & Frigo, 1994).
Differences between regions and muscle layers of the intestines in the innervation by prevertebral ganglia
These studies have demonstrated that the sympathetic innervation of the intestines differs between gut regions and muscle layers. For example, the excitatory sympathetic innervation of the longitudinal muscle is absent in the ileum, small in the proximal colon and dominant at most stimulation frequencies in the distal colon. Moreover, the inhibitory component of nerve-evoked responses was much smaller in the circular muscle and was caused by activation of different β- adrenoceptors.
There are also differences in the transmitters released by SNS between the different colonic preparations. In particular, while all the effects of SNS in the distal colon longitudinal muscle were noradrenergic, responses resistant to α- and β-adrenoceptor blockade were observed in the other colonic preparations. These responses may be due to actions of noradrenaline at a novel adrenoceptor subtype or to the release of a co-transmitter from sympathetic nerves. For example, both ATP and neuropeptide Y have been implicated in sympathetic motor responses in other preparations (Hellström, Olerup & Tatemoto, 1985; Venkova & Krier, 1993; Venkova, Milne & Krier, 1994; Ulman, Potter & McCloskey, 1995). It is also possible that the responses are due to the release of substances from the peripheral endings of primary sensory axons, which were also stimulated with sympathetic axons in all of the preparations.
Finally, this study provides the first investigation of the pelvic sympathetic innervation of the rectal musculature and demonstrates that it differs from that provided by the prevertebral ganglia. Previous studies investigating stimulation of the hypogastric nerve have largely been performed on the cat. However, unlike the rat hypogastric nerve which primarily contains preganglionic axons innervating pelvic neurons (Baron & Jänig, 1991), the cat hypogastric nerve predominantly consists of postganglionic axons (Baron, Jänig & McLachlan 1985). Hence, hypogastric nerve stimulation experiments in the cat would not be limited to pelvic sympathetic effects. In the rat, the pelvic sympathetic innervation is purely contractile and mediated solely via α-adrenoceptors and is therefore much simpler than the prevertebral innervation. This is in keeping with the observations that the rat pelvic ganglia may function largely as relay stations (see review of Keast, 1995). The weaker contractile action of pelvic sympathetic pathways to the rectal circular muscle compared with prevertebral pathways can be explained by the relative paucity of pelvic axons to this tissue (Luckensmeyer & Keast, 1997). This difference in function between the pelvic and prevertebral pathways means that our understanding of the function of the pelvic sympathetic innervation of target organs cannot be extrapolated exclusively from our knowledge of the prevertebral innervation.
Potential consequences of excitatory sympathetic effects
These studies have demonstrated that SNS at lower frequencies produced an excitatory response in the muscle, while inhibitory responses were limited to the higher frequencies of stimulation. One possible function of sympathetic contraction of the large intestine may be to improve absorption of substances from faecal material, providing vigorous mixing of the increasingly hard luminal contents. A second possibility is that sympathetic excitatory effects may be co-ordinated with the parasympathetic defecatory reflexes to facilitate colonic emptying. It is first necessary to know the circumstances during which the excitatory effects dominate the inhibitory effects in vivo before the sympathetic control of motility can be correctly understood.
Another consequence of the present results is that viscerofugal reflexes through the inferior mesenteric ganglion may result in an excitation rather than the commonly accepted inhibition of colon smooth muscle (see reviews of Szurszewski, 1981; Furness & Costa, 1987; Miolan & Niel, 1996). To date, the effects of activating viscerofugal reflexes have not been investigated in the rat, rabbit or cat large intestine, where excitatory responses to SNS have been observed. They are more commonly investigated in the small intestine (where SNS results in inhibition) or in the guinea-pig colon (which has not been thoroughly investigated for an α-adrenoceptor mediated contractile effect). In addition, those viscerofugal reflexes mediated via neurons of the major pelvic ganglion (Luckensmeyer & Keast, 1996) are likely to result in an excitation of motility.
Sympathetic excitation of gut motility is not limited to the present observations, although it is not generally acknowledged. One of the earliest observations of the motor effects of SNS was made by Langley & Anderson (1895), who showed that SNS evoked both contractile and inhibitory responses in both the cat and rabbit intestine. Excitation of motility following SNS has also been demonstrated in the cat colon (via α1-adrenoceptors and P2X-purinoceptors; Rostad, 1973; Hedlund et al. 1984; Carlstedt et al. 1988, Venkova & Krier, 1993, Venkova, et al. 1994) and in the dog stomach (Nakazato, Saito & Ohga, 1970). Furthermore, the excitatory action of the sympathetic nerves in the cat bowel is usually dominant and tonically active as section of the lumbar colonic or the hypogastric nerve results in an inhibition of the motility of the rectum (Hedlund et al. 1984). In addition, the presence of excitatory α1-adrenoceptors on the smooth muscle has been demonstrated pharmacologically in many species (guinea-pig, cat, dog, rabbit, ruminants, horse and man) and gut regions (see reviews of Burnstock & Wong, 1981; Krier, 1989; McIntyre & Thompson, 1992). It remains to be determined whether these receptors are innervated.
Inhibition: a primary action of the sympathetic supply
Despite the new focus on sympathetic excitation, the present studies have also demonstrated that sympathetic inhibition is important in some preparations and at certain frequencies of activation. This inhibition is mediated predominantly by β3-adrenoceptors in the circular muscle of the colon, by a mixture of β-adrenoceptors in the longitudinal muscle of the distal colon, and by β3-adrenoceptors and possibly a non-noradrenergic transmitter(s) in the longitudinal muscle of the proximal colon. The proportions of the different types of β-adrenoceptors activated are also known to differ between species, as well as between gut regions and muscle layers (e.g. β3-adrenoceptors are more common in the guinea-pig ileum, whereas β1- and β2-adrenoceptors are present in the rabbit ileum; Greenwood, Davison & Dodds, 1990; Taneja & Clarke, 1992).
In most previous in vivo experiments in the colon, where nerves have been sectioned to determine the tonic neuronal effects, sympathetic nerves have been demonstrated to have an inhibitory action (see review of Krier, 1989). Even in the cat, where sympathetic excitatory effects have been demonstrated by nerve stimulation both in vivo (Rostad, 1973; Carlstedt et al. 1988) and in vitro (Hedlund et al. 1984) and where these excitatory pathways have been demonstrated to be tonically active (Hedlund et al. 1984), a tonically active sympathetic inhibition of motility can still be demonstrated (e.g. by section of the lumbar colonic nerve; Rostad, 1973; Carlstedt et al. 1988). The administration of β-adrenoceptor antagonists also reveals tonic sympathetic inhibition in many species, including humans (Jacob et al. 1983). It is now important to study the spinal or supra-spinal mechanisms involved in determining whether the net sympathetic action under different circumstances is excitatory or inhibitory.
Conclusions
These studies have significantly advanced our understanding of the actions of sympathetic axons innervating smooth muscle in the large intestine and the receptors by which these effects occur. In particular, four conclusions regarding this innervation can now be drawn. (i) The action of sympathetic nerves on the large intestine differs from that on the small intestine. Most importantly, sympathetic nerves supplying the large intestine have an α-adrenoceptor mediated excitatory action, in addition to the inhibitory action observed in the small intestine. (ii) The pelvic sympathetic innervation differs from that supplied by the prevertebral ganglia. (iii) There are differences in the receptors innervated by sympathetic nerves supplying the two muscle layers. (iv) There may be non-noradrenergic sympathetic actions or atypical adrenoceptors on the circular muscle of both the proximal and distal colon and on the longitudinal muscle of the proximal colon. Together these conclusions highlight the necessity of investigating the sympathetic innervation of each gut region and muscle layer independently in order to acquire an accurate understanding of the action of sympathetic nerves on gastrointestinal motility.
Acknowledgments
We would like to thank Mark Kepper and Mandy Bauer for their technical assistance. This work was supported by the National Health and Medical Research Council of Australia.
References
- Arch JRS, Kaumann AJ. β3 and atypical β-adrenoceptors. Medical Research Reviews. 1993;13:663–729. doi: 10.1002/med.2610130604. [DOI] [PubMed] [Google Scholar]
- Baron R, Jänig W. Afferent and sympathetic neurons projecting into lumbar visceral nerves of the male rat. Journal of Comparative Neurology. 1991;314:429–436. doi: 10.1002/cne.903140302. [DOI] [PubMed] [Google Scholar]
- Baron R, Jänig W, McLachlan EM. The afferent and sympathetic components of the lumbar spinal outflow to the colon and pelvic organs in the cat. I. The hypogastric nerve. Journal of Comparative Neurology. 1985;238:135–146. doi: 10.1002/cne.902380202. [DOI] [PubMed] [Google Scholar]
- Bauer V. Distribution and types of adrenoceptors in the guinea-pig iluem: the action of α- and β-adrenoceptor blocking agents. British Journal of Pharmacology. 1982;76:569–578. doi: 10.1111/j.1476-5381.1982.tb09256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchetti A, Manara L. In vitro inhibition of intestinal motility by phenylethanolaminotetralines: evidence of atypical β-adrenoceptors in rat colon. British Journal of Pharmacology. 1990;100:831–839. doi: 10.1111/j.1476-5381.1990.tb14100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond RA, Clarke DE. Agonist and antagonist characterization of a putative adrenoceptor with distinct pharmacological properties from the α- and β-subtypes. British Journal of Pharmacology. 1988;95:723–734. doi: 10.1111/j.1476-5381.1988.tb11698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC. Electrophysiology of neuroeffector transmission in smooth muscle. In: Burnstock G, Hoyle CHV, editors. Neuromodulation. Chur, Switzerland: Harwood Academic Publishers; 1992. pp. 121–213. [Google Scholar]
- Burnstock G, Wong H. Systemic pharmacology of adrenergic agonists and antagonists: effects on the digestive system. In: Szekeres L, editor. Neuromodulation. New York: Springer-Verlag; 1981. pp. 129–159. [Google Scholar]
- Carlstedt A, Fasth S, Hultén L, Nordgren S. The sympathetic innervation of the internal anal sphincter and rectum in the cat. Acta Physiologica Scandinavica. 1988;133:423–431. doi: 10.1111/j.1748-1716.1988.tb08425.x. [DOI] [PubMed] [Google Scholar]
- Ek BA. Acta Physiologica Scandinavica. suppl. 546. 1985. Studies on mechanisms for beta-adrenoceptor mediated inhibition of colon motility; pp. 1–39. [PubMed] [Google Scholar]
- Fagbemi SO, Salako LA. The effect of prazosin on the guinea-pig ileum. British Journal of Pharmacology. 1980;70:395–402. doi: 10.1111/j.1476-5381.1980.tb08715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB. The origin and distribution of adrenergic nerve fibres in the guinea-pig colon. Histochemie. 1970;21:295–306. doi: 10.1007/BF00280899. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. The ramifications of adrenergic nerve terminals in the rectum, anal sphincter, and anal accessory muscles of the guinea-pig. Zeitschrift fuer Anatomie und Entwicklungsgeshichte. 1973;140:109–128. doi: 10.1007/BF00520721. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. The Enteric Nervous System. Edinburgh: Churchill Livingstone; 1987. [Google Scholar]
- Gagnon DJ, Belisle S. Stimulatory effects of catecholamines on isolated rat colon after beta-adrenergic blockade with oxzprenolol and propranolol. European Journal of Pharmacology. 1970;12:303–309. doi: 10.1016/0014-2999(70)90082-8. 10.1016/0014-2999(70)90082-8. [DOI] [PubMed] [Google Scholar]
- Gagnon DJ, Devroede G, Belisle S. Excitatory effects of adrenaline upon isolated preparations of human colon. Gut. 1972;13:654–657. doi: 10.1136/gut.13.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B, Davison JS, Dodds WJ. Influence of selective α- and β-adrenoceptor antagonists on the control of motor activity and transmural potential differences in the rabbit ileum in vitro. Gastrointestinal Motility. 1990;2:47–52. [Google Scholar]
- Hedlund H, Fasth S, Hultén L. Efferent sympathetic nervous control of rectal motility in the cat. Acta Physiologica Scandinavica. 1984;121:317–324. doi: 10.1111/j.1748-1716.1984.tb07462.x. [DOI] [PubMed] [Google Scholar]
- Hellström PM, Olerup O, Tatemoto K. Neuropeptide Y may mediate effects of sympathetic nerve stimulations on colonic motility and blood flow in the cat. Acta Physiologica Scandinavica. 1985;124:613–624. doi: 10.1111/j.1748-1716.1985.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Howe R. β3-Adrenergic agonists. Drugs of the Future. 1993;18:529–549. [Google Scholar]
- Jacob H, Brandt LJ, Farkas P, Frishman W. Beta-adrenergic blockade and the gastrointestinal system. American Journal of Medicine. 1983;74:1042–1051. doi: 10.1016/0002-9343(83)90813-6. 10.1016/0002-9343(83)90813-6. [DOI] [PubMed] [Google Scholar]
- Jänig W, McLachlan EM. Organization of lumbar spinal outflow to distal colon and pelvic organs. Physiological Reviews. 1987;67:1332–1404. doi: 10.1152/physrev.1987.67.4.1332. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Molenaar P. Differences between the third cardiac beta-adrenoceptor and the colonic beta 3-adrenoceptor in the rat. British Journal of Pharmacology. 1996;118:2085–2098. doi: 10.1111/j.1476-5381.1996.tb15648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast JR. Pelvic ganglia. In: McLachlan EM, editor. Neuromodulation. Chur, Switzerland: Harwood Academic Publishers; 1995. pp. 445–480. [Google Scholar]
- Krier J. Motor function of anorectum and pelvic floor musculature. In: Schultz SG, Wood JD, Rauner BB, editors. Neuromodulation. Bethesda, MD, USA: American Physiological Society; 1989. pp. 1025–1053. [Google Scholar]
- Langley JN, Anderson HK. On the innervation of the pelvic and adjoining viscera. I The lower portion of the intestine. The Journal of Physiology. 1895;18:67–105. doi: 10.1113/jphysiol.1895.sp000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckensmeyer GB, Keast JR. Immunohistochemical characterisation of viscerofugal neurons projecting to the inferior mesenteric and major pelvic ganglia in the male rat. Journal of the Autonomic Nervous System. 1996;61:6–16. doi: 10.1016/0165-1838(96)00056-2. [DOI] [PubMed] [Google Scholar]
- Luckensmeyer GB, Keast JR. Projections of pelvic autonomic neurons within the lower bowel of the male rat: An anterograde labelling study with DiI. Neuroscience. 1997 doi: 10.1016/s0306-4522(97)89502-4. in the Press. [DOI] [PubMed] [Google Scholar]
- McIntyre AS, Thompson DG. Adrenergic control of motor and secretory function in the gastrointestinal tract. Alimentary Pharmacology and Therapeutics. 1992;6:125–142. doi: 10.1111/j.1365-2036.1992.tb00257.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin DP, MacDonald A. Evidence for the existense of ‘atypical’β-adrenoceptors (β3-adrenoceptors) mediating relaxation in the rat distal colon in vitro. British Journal of Pharmacology. 1990;101:569–574. doi: 10.1111/j.1476-5381.1990.tb14122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manara L, Badone D, Baroni M, Boccardi G, Cecchi R, Croci T, Giudice A, Guzzi U, Landi M, Le Fur G. Functional identification of rat atypical β-adrenoceptors by the first β3-selective antagonists, aryloxypropanolaminotetralins. British Journal of Pharmacology. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manara L, Croci T, Landi M. β3-Adrenoceptors and intestinal motility. Fundamentals of Clinical Pharmacology. 1995;9:332–342. doi: 10.1111/j.1472-8206.1995.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Marino F, Marcoli M, De Ponti F, Cosentino M, Lecchini S, Frigo GM. Effect of desipramine-induced blockade of neuronal uptake mechanisms on adrenoceptor-mediated responses in the guinea-pig colon. Naunyn-Schmiedeberg's Archives of Pharmacology. 1994;350:499–506. doi: 10.1007/BF00173019. [DOI] [PubMed] [Google Scholar]
- Miolan JP, Niel JP. The mammalian sympathetic prevertebral ganglia: integrative properties and role in the nervous control of digestive tract motility. Journal of the Autonomic Nervous System. 1996;58:125–138. doi: 10.1016/0165-1838(95)00128-x. 10.1016/0165-1838(95)00128-X. [DOI] [PubMed] [Google Scholar]
- Nakazato Y, Saito K, Ohga A. Gastric motor and inhibitor response to stimulation of the sympathetic nerve in the dog. Japanese Journal of Pharmacology. 1970;20:131–141. doi: 10.1254/jjp.20.131. [DOI] [PubMed] [Google Scholar]
- Norberg K-A. Adrenergic innervation of the intestinal wall studied by fluorescence microscopy. International Journal of Neuropharmacology. 1964;3:379–382. doi: 10.1016/0028-3908(64)90067-x. 10.1016/0028-3908(64)90067-X. [DOI] [PubMed] [Google Scholar]
- Pelckmans PA, Van Maercke YM, de Maeyer MH, Herman AG, Verbeuren TJ. Cholinergic and adrenergic contractile properties of the canine ileocolonic junction. Journal of Pharmacology and Experimental Therapeutics. 1990;254:158–164. [PubMed] [Google Scholar]
- Rostad H. Colonic motility in the cat II. Extrinsic nervous control. Acta Physiologica Scandinavica. 1973;89:91–103. doi: 10.1111/j.1748-1716.1973.tb05500.x. [DOI] [PubMed] [Google Scholar]
- Schultzberg M, Hökfelt T, Nilsson G, Terenius L, Rehfeld JF, Brown M, Elde R, Goldstein M, Said S. Distribution of peptide- and catecholamine-containing neurons in the gastrointestinal tract of rat and guinea-pig: Immunohistochemical studies with antisera to substance P, vasoactive intestinal peptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine β-hydroxylase. Neuroscience. 1980;5:689–744. doi: 10.1016/0306-4522(80)90166-9. 10.1016/0306-4522(80)90166-9. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH. Physiology of mammalian prevertebral ganglia. Annual Review of Physiology. 1981;43:53–68. doi: 10.1146/annurev.ph.43.030181.000413. 10.1146/annurev.ph.43.030181.000413. [DOI] [PubMed] [Google Scholar]
- Taneja DT, Clarke DE. Evidence for a noradrenergic innervation to ‘atypical’ beta adrenoceptors (or putative beta-3 adrenoceptors) in the ileum of guinea pig. Journal of Pharmacology and Experimental Therapeutics. 1992;260:192–200. [PubMed] [Google Scholar]
- Ulman LG, Potter EK, McCloskey DI. Inhibition of vagally induced gastric contractions by sympathetic stimulation, neuropeptide Y and galanin. Journal of the Autonomic Nervous System. 1995;55:193–197. doi: 10.1016/0165-1838(95)00047-2. 10.1016/0165-1838(95)00047-2. [DOI] [PubMed] [Google Scholar]
- Venkova K, Krier J. Stimulation of lumbar sympathetic nerves evokes contractions of cat colon circular muscle mediated by ATP and noradrenaline. British Journal of Pharmacology. 1993;110:1260–1270. doi: 10.1111/j.1476-5381.1993.tb13951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkova K, Krier J. Postjunctional α1- and β-adrenoceptor effects of noradrenaline on electrical slow waves and phasic contractions of cat colon circular muscle. British Journal of Pharmacology. 1995;116:3265–3273. doi: 10.1111/j.1476-5381.1995.tb15134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkova K, Milne A, Krier J. Contraction mediated by α1-adrenoceptors and P2-purinoceptors in a cat colon circular muscle. British Journal of Pharmacology. 1994;112:1237–1243. doi: 10.1111/j.1476-5381.1994.tb13216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson N, Reddy H, Eglen RM. Characterization of muscarinic receptor and β-adrenoceptor interactions in guinea-pig oesophageal muscularis mucosae. European Journal of Pharmacology. 1995;294:779–785. doi: 10.1016/0014-2999(95)00656-7. 10.1016/0014-2999(95)00656-7. [DOI] [PubMed] [Google Scholar]


